Abstract
New genomic disorders associated with large, rare, recurrent copy number variations (CNVs) are being discovered at a rapid pace. Detailed phenotyping and family studies are rare, however, as are data on adult phenotypic expression. Duplications at 2q13 were recently identified as risk factors for developmental delay/autism and reported in the prenatal setting, yet few individuals (all children) have been extensively phenotyped. During a genome-wide CNV study of schizophrenia, we identified two unrelated probands with 2q13 duplications. In this study, detailed phenotyping and genotyping using high-resolution micro-arrays was performed for 12 individuals across their two families. 2q13 duplications were present in six adults, and co-segregated with clinically significant later-onset neuropsychiatric disorders. Convergent lines of evidence implicated GABAminergic dysfunction. Analysis of the genic content revealed promising candidates for neuropsychiatric disease, including BCL2L11, ANAPC1, and MERTK. Intrafamilial genetic heterogeneity and “second hits” in one family may have been the consequence of assortative mating. Clinical genetic testing for the 2q13 duplication and the associated genetic counseling was well received. In summary, large rare 2q13 duplications appear to be associated with variable adult neuropsychiatric and other expression. The findings represent progress toward clinical translation of research results in schizophrenia. There are implications for other emerging genomic disorders where there is interest in lifelong expression.
Keywords: chromosome 2q13, copy number variation, schizophrenia, obsessive-compulsive, genomic disorder, genetic counseling, GABA, SLC1A1, RHOA, microRNA, chromosome 16p13.11
INTRODUCTION
Rare copy number variations (CNVs) contribute to genetic vulnerability for many developmental and neuropsychiatric disorders [Miller et al., 2010; Pinto et al., 2010; Cooper et al., 2011; Lionel et al., 2011], including later-onset conditions like schizophrenia [Xu et al., 2008; Kirov et al., 2009; Levinson et al., 2011; Costain et al., 2013]. New genomic disorders associated with large, rare, recurrent CNVs, such as 2q13 duplications [Szatmari et al., 2007; Rudd et al., 2009; Cooper et al., 2011; Yu et al., 2012; Costain et al., 2013], are being discovered at a rapid pace. Detailed phenotyping and family studies are rare, however. Data on adult phenotypic expression would be of particular value for genetic counseling [Dolcetti et al., 2013].
The first report of rare recurrent ~1.7 Mb CNVs at the 2q13 locus involved 5 of 2,419 samples (2 duplications and 3 deletions) from patients referred to a clinical cytogenetic laboratory [Rudd et al., 2009]. In retrospect, a previous study of CNV in multiplex autism families had identified a 2q13 duplication in an affected parent–child dyad [Szatmari et al., 2007]. Duplications at this locus are increasingly accepted as risk factors for developmental disorders [Cooper et al., 2011; Yu et al., 2012]. The total number of individuals with 2q13 duplications with detailed phenotype data available remains two, however. Both were children with developmental delay, tooth abnormalities, and neurologic and other features [Rudd et al., 2009]. The recent prenatal clinical reporting of a 2q13 duplication (as a variant of uncertain significance) in a fetus with an abnormality detected by ultrasound [Wapner et al., 2012] underscores the need for additional information to inform genetic counseling.
Recently, we detected typical 2q13 duplications in 2 of 420 unrelated probands with schizophrenia of European ancestry [Costain et al., 2013]. Herein, we report the results of follow-up family studies to delineate the inheritance status, segregation pattern, and associated extended phenotype. A secondary goal was to document the outcome of clinical genetic testing and counseling for adults with schizophrenia and their family members.
MATERIALS AND METHODS
Recruitment of the probands with schizophrenia is described in detail elsewhere [Costain et al., 2013]. Available adult family members (≥18 years) were subsequently contacted with the permission of the probands. For the proband in Family A, we were able to recruit family members across three generations; for the proband in Family B, the sole full- and half-siblings, but no ancestors, were available for study (Fig. 1). After description of the study, written informed consent was obtained. The study was approved by local hospital and university institutional review boards. The possibility of the return of clinically relevant findings (e.g., the presence or absence of the 2q13 duplication) was discussed at the time of consent. Following standard practice, genetic counseling was provided in conjunction with the clinical confirmation and return of confirmed results for these variants.
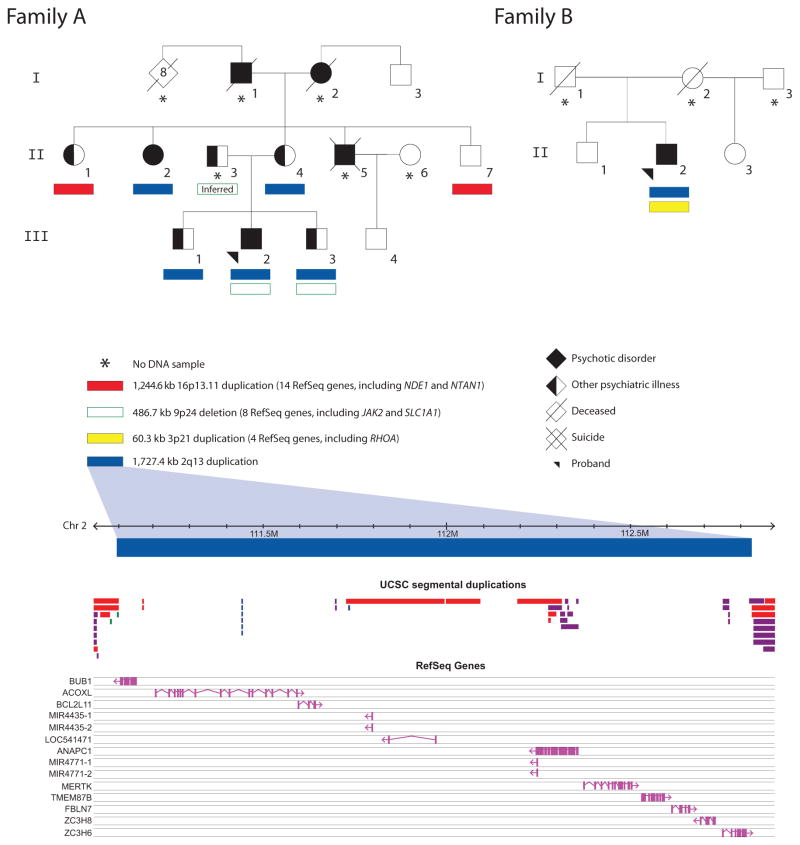
FIG. 1.
Familial segregation and genic content of 2q13 duplications and other rare CNVs. Solid and open colored bars represent gains (duplications) and losses (deletions), respectively. Start and stop coordinates for the four CNVs of interest were: chr2:111,105,101–112,832,463, chr3:49,382,869–49,443,186, chr9:4,527,834–5,014,492, and chr16:15,016,089–16,260,667. Genomic parameters are from NCBI Build 36 (hg18). All CNVs pictured were confirmed using a second method (see text). Notably, these 2q13 duplications are distal to another 2q13 locus for recurrent CNVs overlapping NPHP1 [Cooper et al., 2011]. Subject II-3 is a presumed carrier of the 9p24 deletion, given its presence in two of his offspring (III-2, III-3) and absence in their mother (II-4) and other maternal relatives. Psychiatric conditions in individuals with 2q13 duplications are outlined in Table I. In Family A, the psychiatric conditions in individuals without confirmed 2q13 duplications included: a history of untreated psychotic and paranoid features for subject I-1, lifelong “nerve problems” requiring treatment and psychotic features for subject I-2, trichotillomania and major depression for subject II-1, alcohol abuse with impulsive and violent behavior for subject II-3, and chronic paranoid schizophrenia (age at onset 28 years) and dyslexia with average IQ for subject II-5. Subject III-4 has no chronic psychiatric conditions, but has had two brief episodes of substance-induced psychosis. Of note, a distant maternal cousin (second cousin once removed) with schizophrenia (not pictured), a 6th degree relative to the proband in Family A, had neither the 2q13 duplication nor the 16p13.11 duplication, but did have a rare 1.4 Mb exonic loss overlapping five genes including LIN7A (Case 44 in [Costain et al., 2013]) that was not found in any of the individuals portrayed in this figure.
High quality genomic DNA was genotyped using the Affymetrix® Genome-Wide Human SNP Array 6.0 (n = 6) or Affymetrix® CytoScanHD Array (n = 6), at The Centre for Applied Genomics (Toronto, Ontario, Canada). An established multi-algorithm approach was used to maximize sensitivity and specificity of CNV calling, as previously described [Silversides et al., 2012; Costain et al., 2013]. Pair-wise identity by descent was calculated for every pair of individuals to confirm relatedness within families and non-relatedness between families (data not shown) [Costain et al., 2013]. The presence or absence of 2q13 duplications was clinically confirmed in a CLIA-approved laboratory [Costain et al., 2013]. All other rare CNVs of interest (Fig. 1) were validated using clinical microarray and/or qPCR [Costain et al., 2013]. Select CNVs present at a frequency of <0.1% in our initial 2,357 population-based adult controls (i.e., “rare” CNVs) were assessed in an additional 21,481 controls [Costain et al., 2013].
Phenotyping was done via direct one-on-one assessment, systems review, and review of lifetime medical records. Standard laboratory and imaging investigations were performed as indicated on a clinical basis for the individuals with 2q13 duplications. Detailed psychiatric assessments included use of a modified version of the Structured Clinical Interviews for DSM-III-R (SCID) for Axis I disorders. Neuropsychological testing was performed using the Wechsler Abbreviated Scale of Intelligence (WASI) and the Wide Range Achievement Test 3 (WRAT3). Collateral history was collected from participants regarding family members including those unavailable for study.
RESULTS
Genotype
Stringent [Costain et al., 2013] genome-wide CNV calls for all 12 adults with available DNA samples are included in Table SI. The 2q13 duplications confirmed in 6 of these individuals overlapped 10 known RefSeq genes and 4 microRNAs (Fig. 1; Table SII). There were only 3 such duplications in 23,838 controls (0.013%) [Costain et al., 2013]. No other rare CNVs were shared between families (Table SI). Of note, the 2 unrelated probands each had a (different) additional rare exonic CNV that was absent in these 23,838 controls using a 50% overlap criterion (Fig. 1): a 9p24 deletion (Family A) and a 3p21 duplication (Family B). The maternal aunt (II-1) and uncle (II-7) in Family A without 2q13 duplications had typical 16p13.11 duplications [Tropeano et al., 2013] that were present in 25 of the 23,838 controls (0.10%) (Fig. 1). None of the remaining CNVs were predicted to be of clinical significance based on ACMG guidelines (Table SI) [Kearney et al., 2011], although note was made of a recent report of a rare 9p24 deletion disrupting SLC1A1 co-segregating with psychosis in an extended multigenerational pedigree [Myles-Worsley et al., 2013].
Phenotype in Adults
The six adults with 2q13 duplications shared a number of related phenotypic features (Table I). The dental (ectopic teeth/dental crowding), otologic (hearing loss), and urogenital (cryptorchidism) abnormalities, and short stature, were previously reported in other cases with 2q13 duplications (reviewed in Table SIII). Most notable, however, was the presence of treatable later-onset psychiatric illness in all of these six individuals, albeit with two of the six ascertained because of schizophrenia. The highly variable neuropsychiatric expression appeared to encompass psychotic disorders, obsessive–compulsive spectrum disorders (including trichotillomania [Flessner et al., 2012]), major mood disorders, and disinhibitory psychopathology (including conduct disorder). The presentation and natural history of each psychiatric disorder, including age at onset and response to treatment, was generally unremarkable (Table I and data not shown). No individual had a childhood diagnosis of developmental delay, autism spectrum disorder, or multiple congenital anomalies. However, relative deficits in arithmetic were present, and the individual with the mildest neuropsychiatric phenotype (Family A, III-1) had one major (cryptorchidism) and one minor (bifid uvula) congenital anomaly. The psychiatric phenotypes of the six family members without 2q13 duplications (mean age 57.2 years; range 30–74 years) are shown in Figure 1; none had a major psychotic disorder, a history of developmental delay/intellectual disability, or a congenital abnormality.
TABLE I.
Summary of Phenotypes in Adults With 2q13 Duplicationsa
| Family
|
||||||
|---|---|---|---|---|---|---|
| A
|
B
|
|||||
| Proband (subject III-2) | Brother (subject III-1) | Brother (subject III-3) | Aunt (subject II-2) | Mother (subject II-4) | Proband (subject II-2) | |
| Age at last assessment (years) | 30 | 32 | 29 | 56 | 55 | 70 |
| Sex | Male | Male | Male | Female | Female | Male |
| Neuropsychiatric featuresb | ||||||
| Psychotic disorder | Yes | No | No | Yes | No | Yes |
| Age at onset of psychosis (years) | 20 | — | — | 19 | — | 22 |
| Major mood and/or anxiety disorder | — | Yes | Yes | — | Yes | — |
| Obsessive–compulsive disorder | Yes | Noc | No | No | Yes | Noc |
| Trichotillomania | Yes | No | Yes | Yes | No | No |
| Vertigo | Yes | Yes | No | No | No | No |
| Sleep disturbances/insomnia | Yes | Yes | Yes | Yes | Yes | Yes |
| Autism spectrum disorder | No | No | No | No | No | No |
| Seizures | No | No | No | No | No | No |
| Cognitive features | ||||||
| Global developmental delay | No | No | No | No | No | No |
| Learning difficulties | Yes | No | Yes | Yes | Yes | No |
| WASI Full Scale IQd,e | Avg | Avg | Aavg | Bl | Avg | Avgf |
| WASI Verbal IQd,e | Avg | Avg | Avg | Avg | Avg | Avgf |
| WASI Performance IQd,e | Avg | Aavg | Aavg | Mild | Avg | Avgf |
| WRAT-3 Reading (grade level)d | >HS | >HS | >HS | 6 | >HS | — |
| WRAT-3 Spelling (grade level)d | 12 | >HS | >HS | 4 | 12 | — |
| WRAT-3 Arithmetic (grade level)d | 6 | 6 | 12 | 4 | 6 | — |
| Other notable featuresg | ||||||
| Dental | Yes | No | Yes | Yes | Yes | No |
| Otolaryngologic | Yes | Yes | Yes | Yes | Yes | No |
| Renal/urogenital | No | Yes | No | Yes | No | No |
| Endocrine/metabolic/hematologic | Yes | Yes | Yes | Yes | Yes | Yes |
| Ophthalmologic | No | No | No | No | Yes | Yes |
| Cardiac | No | No | No | No | No | Yes |
| Respiratory | Yes | No | No | No | Yes | No |
| Gastrointestinal | Yes | No | No | Yes | No | Yes |
| Dermatologic | No | No | No | No | Yes | No |
| Musculoskeletal/rheumatologic | No | Yes | Yes | Yes | Yes | Yes |
| Immunologic | No | No | No | No | No | No |
| Multiple congenital abnormalities | No | No | No | No | No | No |
| Anthropometric measurements | ||||||
| Height (percentile)d | <5th | <5th | 15th–25th | <5th | <5th | 25th–50thh |
| Body-mass index (kg/m2) | 25–30 | 19–25 | 19–25 | 25–30 | >30 | 19–25h |
| Head circumference (percentile)d | 50th–75th | 3rd–10th | 25th–50th | 25th–50th | >97th | 25th–50thh |
HS, high school; WASI, Wechsler Abbreviated Scale of Intelligence; WRAT3, Wide Range Achievement Test 3.
Addition details are provided in Table SIII. Patient photos are available upon request.
All six subjects had additional neuropsychiatric features not listed here (see Table SIII).
Subclinical features only (see Table SIII).
Actual scores/values are provided in Table SIII.
Mild, mild intellectual disability (55–70); Bl, borderline intellectual disability (70–84); Avg, average intellect (85–115); Aavg, above average intellect (>115).
At age 39 years, using the Wechsler Adult Intelligence Scale.
Some features have been previously reported in other individual(s) with 2q13 duplication (see Table SIII).
At age 50 years (measurements at age 70 years also available).
In Family A, all three brothers in the proband’s sibship (III-1, III-2, III-3) reported multiple episodes of alcohol-induced blackouts in the absence of heavy consumption. III-1 and III-2 also had a history of vertigo. Their mother (II-4) and maternal aunt (II-2) with the 2q13 duplication have had limited exposure to alcohol, but the former reported a serious amnesic response to a benzodiazepine and the latter had a chronic tic disorder. The unrelated proband with schizophrenia (Family B, II-2) was previously reported to have elevated γ-aminobutyric acid (GABA) concentrations in the CSF [Perry et al., 1983]. Blackout propensity was unknown for him, with minimal alcohol exposure and benzodiazepine exposure only in the context of other medications. All six affected individuals also had chronic sleep disturbances/insomnia. None of the adults with 2q13 duplications had a history of seizures or EEG abnormalities, despite the presence of risk factors such as anti-psychotic medication use.
Outcomes of Clinical Genetic Testing and Counseling
All participants either displayed a passive curiosity or expressed a genuine psychological benefit as a result of clinical testing for the 2q13 duplication. No negative psychological consequences were reported by the study participants at time of disclosure nor at follow-up, up to two years later. During pre-test genetic counseling, the mother in Family A (II-4) endorsed blaming herself somewhat for her son’s psychotic illness because “it came from [her] side of the family,” and also questioned whether “something” might have happened during her pregnancy. She reported that all three of her children (III-1, III-2, III-3) had already decided not to have children because psychiatric illness is so common in her family (“poor genetic material”). Post-testing, she did not express any guilt at having “passed on” the 2q13 duplication to her three sons, but instead was appreciative of finally having a partial explanation for the family’s struggles. In Family A, the reproductive implications for III-1, III-2, and III-3 were discussed, as was the possibility of prenatal detection of the 2q13 duplication. Previously, III-2 had been considering a vasectomy because of a fear of transmitting his psychotic illness to any offspring. The current uncertainty surrounding these 2q13 duplications and their association with schizophrenia (and other phenotypes) was appropriately communicated in the course of genetic counseling. Probands and family members reported choosing to participate in the research in part because of an altruistic desire to help other (unrelated) individuals with these 2q13 duplications.
DISCUSSION
Understanding Expression Across the Lifespan
This is the first study of the adult phenotype associated with 2q13 duplications. Much of our knowledge of emerging genomic disorders comes from pediatric samples with limited phenotype data available to clinical diagnostic laboratories. Few adults, and almost none with schizophrenia, have yet benefitted from clinical micro-array testing [Costain et al., 2013]. While the majority of 2q13 duplications reported to date have been inherited, little information has been provided about the transmitting parents. Descriptions of parental phenotypes as “normal,” especially if (as is sometimes the case) used as a code word for “not assessed,” are unhelpful and may be counter-productive. In addition, ascertainment of transmitting parents is expected to bias toward those with milder phenotypes [Costain et al., 2011; Dolcetti et al., 2013; Costain, 2014]. The adult phenotype of most genomic disorders remains relatively understudied, and neuropsychiatric and other symptoms may appear over time and/or be present but not assessed. Our findings reinforce the importance of long-term follow-up of pediatric patients with genetic findings and of careful phenotyping of transmitting parents. Use of complementary strategies for population-wide genetic screening to identify individuals for clinical follow-up are helpful. For example, two typical 2q13 duplications were discovered in a unselected population cohort (Northern Finland 1966 Birth Cohort) and could now be phenotyped [Pietilainen et al., 2011]. The overall positive outcomes reported in this study are representative of our experiences to date in providing clinical genetics services to adults with developmental and/or neuropsychiatric disease. However, none of the study participants would have a priori met existing criteria for clinical genetic testing with chromosomal microarray analysis [Miller et al., 2010].
Genotype–Phenotype Correlations
Typical 2q13 duplications are contained within a much larger region on chromosome 2 that has shown linkage to both schizophrenia and disinhibitory phenotypes (conduct disorder and suicide attempts) [Lewis et al., 2003; Dick et al., 2010]. Also, a karyotypically visible unbalanced insertion leading to trisomy 2q11.2–q21.1 was previously found in a mother and daughter, both with psychotic illness and mild intellectual disability [Glass et al., 1998]. Recurrent microduplications at 2q13 implicate at least three promising candidate genes in this region (Table SII; Fig. 1): (i) the neurodevelopmental facilitator ANAPC1; (ii) the neuronal apoptosis regulator BCL2L11; and, (iii) the TAM receptor component and multiple sclerosis risk gene MERTK. Other apparent features associated with 2q13 duplications (e.g., dentition abnormalities) may be associated with these and/or other genes in the region (e.g., FBLN7). Dosage changes in the non-protein coding content of the 2q13 region may also play a role in disease; for example, predicted targets of the microRNAs mir-4435 and mir-4771 include many genes implicated in schizophrenia including the GABAA receptor GABRA1 (Table SII). That the reciprocal deletion at this locus may similarly predispose to developmental disorders including schizophrenia [Rudd et al., 2009; Cooper et al., 2011; Yu et al., 2012; Costain et al., 2013] is of interest with respect to understanding the pathway from genotype to phenotype. The adult phenotype associated with 2q13 deletions is thus deserving of separate study.
Evidence for GABAminergic Dysfunction
Knowledge of the extended neuropsychiatric phenotype allows speculation about effects of 2q13 dosage variation on GABAergic neurotransmission. Alcohol-induced blackouts (reported in Family A, III-1, III-2, and III-3, though not in the other affected individuals where alcohol exposure was limited) result in part from potentiation of GABAA-mediated inhibition [Nelson et al., 2004]. Blackout susceptibility is only indirectly associated with alcoholism risk [Nelson et al., 2004], and only one of the Family A members had a history of alcohol abuse. Instead, blackout susceptibility shows a stronger direct association with impaired judgment (e.g., greater levels of other alcohol-related risk-taking behavior or victimization), and with susceptibility to periods of anterograde amnesia associated with other drugs (e.g., benzodiazepines; reported in Family A, II-4) [Nelson et al., 2004]. The unrelated proband with the 2q13 duplication (Family B, II-2) had no history of either of these two potential symptoms of GABAminergic dysfunction, but was previously reported to have elevated concentrations of GABA in his CSF [Perry et al., 1983]. Additional evidence consistent with the potential for increased inhibitory tone in association with 2q13 duplications includes: (i) a well-recognized role for GABA-minergic dysfunction in schizophrenia, vertigo, tic disorders, and the other neurological/neuropsychiatric features present in Family A and B [Nishiike et al., 2001; Hines et al., 2012]; and, (ii) the absence of seizures in any of the study participants or other cases with 2q13 duplications reported to date (Table SIII). In contrast, seizures have been reported in several individuals with the reciprocal 2q13 deletion [Rudd et al., 2009; Cooper et al., 2011; Yu et al., 2012].
Intrafamilial Genetic Heterogeneity and Assortative Mating
Intrafamilial genetic heterogeneity and assortative mating are important considerations in multiplex families with neuropsychiatric disease. We previously reported an excess of individuals with two or more rare exonic CNVs in a schizophrenia cohort; this would have included these two unrelated probands with 2q13 duplications [Costain et al., 2013]. The second rare exonic CNV in each proband with a 2q13 duplication overlapped additional promising candidates for neuropsychiatric disease (Table SII): JAK2 and SLC1A1 (9p24 deletion in Family A), and RHOA (3p21 duplication in Family B). The segregation pattern suggested that the 9p24 deletion was inherited from the father (II-3) in Family A, unavailable for study but reported to have both impulsive and violent behavior, and a personal and family history of alcohol abuse (Fig. 1).
The 16p13.11 duplication identified in Family A is consistently found at a modestly elevated prevalence in cohorts with neuro-developmental diseases [Tropeano et al., 2013]. The occurrence of this CNV could be an incidental finding of limited significance in this family. However, the familial co-occurrence of the 16p13.11 and the 2q13 duplications could also be a consequence of assortative mating in the maternal grandparental generation, where I-1 and I-2 in Family A both reportedly had a major psychiatric illness and each could have had one of these CNVs. The maternal uncle with schizophrenia unavailable for study (II-5; Fig. 1) would thus have been at 75% risk of inheriting at least one of the 2q13 or 16p13.11 duplications. The notable familial recurrence of trichotillomania in Family A could be the result of shared genetic or non-genetic influences that act as modifiers of an initial CNS “insult” (the 16p13.11 duplication for II-1, and the 2q13 duplication for II-2, III-2, and III-3), or altogether different genetic variants segregating in the family. Whole genome sequencing is a logical future consideration.
CONCLUSIONS
Large rare duplications at 2q13 can be added to the growing list of recurrent CNVs associated with highly variable neuropsychiatric expression including schizophrenia. These findings represent progress toward clinical translation of research results with implications for emerging genomic disorders where there is interest in lifelong expression. Analysis of the genic content revealed novel candidate genes for neuropsychiatric disease, supporting the likelihood of multiple gene involvement within individuals. As for children with developmental disorders found to have pathogenic CNVs, clinical genetic testing and counseling could be provided for the benefit of adults with schizophrenia and their families [Costain et al., 2013, 2014a,b], regardless of the acknowledged need for a more complete understanding about key genetic parameters.
Supplementary Material
Acknowledgments
We thank the families for their participation in this study, the clinicians involved in their care, and students and staff at the Clinical Genetics Research Program and The Centre for Applied Genomics. K. Russell, R. Melvin, and L. Fitzpatrick deserve special mention. Dr. D. Baribeau provided helpful feedback on an early version of this manuscript. We thank A. Fiebig, A. Franke, and S. Schreiber at POPGEN (University of Kiel, Germany), and A. Stewart, R. McPherson, and R. Roberts of the University of Ottawa Heart Institute (University of Ottawa, Canada) for providing control data. In reviewing all existing phenotype information associated with 2q13 duplications (Table SIII), we made use of the International Standards for Cytogenomic Arrays (ISCA) Consortium database (www.iscaconsortium.org/), which generates information using NCBI’s database of genomic structural variation (dbVar, www.ncbi.nlm.nih.gov/dbvar/) with samples and associated phenotype data provided by ISCA Consortium member laboratories. We also made use of data generated by the DECIPHER Consortium. A full list of centers who contributed to the generation of these data is available from http://decipher.sanger.ac.uk and via email from decipher@sanger.ac.uk. Funding for the DECIPHER project was provided by the Wellcome Trust.
This work was supported by the Canadian Institutes of Health Research (CIHR) [MOP-89066 and MOP-111238 to A.S.B.] and a McLaughlin Centre Accelerator Grant. G.C. is supported by MD/PhD Studentships from the CIHR and McLaughlin Centre. A.C.L. was supported by a NeuroDevNet doctoral fellowship. A.S.B. holds the Canada Research Chair in Schizophrenia Genetics and Genomic Disorders, and the Dalglish Chair in 22q11.2 Deletion Syndrome.
Grant sponsor: Canadian Institutes of Health Research (CIHR), Grant numbers: MOP-89066, MOP-111238; Grant sponsor: McLaughlin Centre Accelerator Grant.
Footnotes
Conflict of interest: S.W.S. is on the Scientific Advisory Board of Population Diagnostics, Inc. and is a co-founder of YouNique Genomics. The other authors declare no conflicts of interest.
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, Baker C, Williams C, Stalker H, Hamid R, Hannig V, Abdel-Hamid H, Bader P, McCracken E, Niyazov D, Leppig K, Thiese H, Hummel M, Alexander N, Gorski J, Kussmann J, Shashi V, Johnson K, Rehder C, Ballif BC, Shaffer LG, Eichler EE. A copy number variation morbidity map of developmental delay. Nat Genet. 2011;43:838–846. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costain G. Parental expression is overvalued in the interpretation of rare inherited variants. Eur J Hum Genet. 2014 doi: 10.1038/ejhg.2014.64. E-published 23 April 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costain G, Chow EW, Silversides CK, Bassett AS. Sex differences in reproductive fitness contribute to preferential maternal transmission of 22q11.2 deletions. J Med Genet. 2011;48:819–824. doi: 10.1136/jmedgenet-2011-100440. [DOI] [PubMed] [Google Scholar]
- Costain G, Lionel AC, Merico D, Forsythe P, Russell K, Lowther C, Yuen T, Husted J, Stavropoulos DJ, Speevak M, Chow EW, Marshall CR, Scherer SW, Bassett AS. Pathogenic rare copy number variants in community-based schizophrenia suggest a potential role for clinical microarrays. Hum Mol Genet. 2013;22:4485–4501. doi: 10.1093/hmg/ddt297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costain G, Esplen MJ, Toner B, Hodgkinson KA, Bassett AS. Evaluating genetic counseling for family members of individuals with schizophrenia in the molecular age. Schizophr Bull. 2014a;40:88–99. doi: 10.1093/schbul/sbs124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costain G, Esplen MJ, Toner B, Scherer SW, Meschino WS, Hodgkinson KA, Bassett AS. Evaluating genetic counseling for individuals with schizophrenia in the molecular age. Schizophr Bull. 2014b;40:78–87. doi: 10.1093/schbul/sbs138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Meyers J, Aliev F, Nurnberger J, Jr, Kramer J, Kuperman S, Porjesz B, Tischfield J, Edenberg HJ, Foroud T, Schuckit M, Goate A, Hesselbrock V, Bierut L. Evidence for genes on chromosome 2 contributing to alcohol dependence with conduct disorder and suicide attempts. Am J Med Genet Part B. 2010;153B:1179–1188. doi: 10.1002/ajmg.b.31089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcetti A, Silversides CK, Marshall CR, Lionel AC, Stavropoulos DJ, Scherer SW, Bassett AS. 1q21.1 Microduplication expression in adults. Genet Med. 2013;15:282–289. doi: 10.1038/gim.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flessner CA, Knopik VS, McGeary J. Hair pulling disorder (trichotillomania): Genes, neurobiology, and a model for understanding impulsivity and compulsivity. Psychiatry Res. 2012;199:151–158. doi: 10.1016/j.psychres.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Glass IA, Stormer P, Oei PT, Hacking E, Cotter PD. Trisomy 2q11.2→q21.1 resulting from an unbalanced insertion in two generations. J Med Genet. 1998;35:319–322. doi: 10.1136/jmg.35.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines RM, Davies PA, Moss SJ, Maguire J. Functional regulation of GABAA receptors in nervous system pathologies. Curr Opin Neurobiol. 2012;22:552–558. doi: 10.1016/j.conb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney HM, Thorland EC, Brown KK, Quintero-Rivera F, South ST. American College of Medical Genetics standards and guidelines for interpretation and reporting of postnatal constitutional copy number variants. Genet Med. 2011;13:680–685. doi: 10.1097/GIM.0b013e3182217a3a. [DOI] [PubMed] [Google Scholar]
- Kirov G, Grozeva D, Norton N, Ivanov D, Mantripragada KK, Holmans P, Craddock N, Owen MJ, O’Donovan MC. Support for the involvement of large copy number variants in the pathogenesis of schizophrenia. Hum Mol Genet. 2009;18:1497–1503. doi: 10.1093/hmg/ddp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, Zhang N, Mowry BJ, Olincy A, Amin F, Cloninger CR, Silverman JM, Buccola NG, Byerley WF, Black DW, Kendler KS, Freedman R, Dudbridge F, Pe’er I, Hakonarson H, Bergen SE, Fanous AH, Holmans PA, Gejman PV. Copy number variants in schizophrenia: Confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 2011;168:302–316. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, Brzustowicz LM, Kaufmann CA, Garver DL, Gurling HM, Lindholm E, Coon H, Moises HW, Byerley W, Shaw SH, Mesen A, Sherrington R, O’Neill FA, Walsh D, Kendler KS, Ekelund J, Paunio T, Lonnqvist J, Peltonen L, O’Donovan MC, Owen MJ, Wildenauer DB, Maier W, Nestadt G, Blouin JL, Antonarakis SE, Mowry BJ, Silverman JM, Crowe RR, Cloninger CR, Tsuang MT, Malaspina D, Harkavy-Friedman JM, Svrakic DM, Bassett AS, Holcomb J, Kalsi G, McQuillin A, Brynjolfson J, Sigmundsson T, Petursson H, Jazin E, Zoega T, Helgason T. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionel AC, Crosbie J, Barbosa N, Goodale T, Thiruvahindrapuram B, Rickaby J, Gazzellone M, Carson AR, Howe JL, Wang Z, Wei J, Stewart AF, Roberts R, McPherson R, Fiebig A, Franke A, Schreiber S, Zwaigenbaum L, Fernandez BA, Roberts W, Arnold PD, Szatmari P, Marshall CR, Schachar R, Scherer SW. Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Sci Transl Med. 2011;3:95ra75. doi: 10.1126/scitranslmed.3002464. [DOI] [PubMed] [Google Scholar]
- Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, Church DM, Crolla JA, Eichler EE, Epstein CJ, Faucett WA, Feuk L, Friedman JM, Hamosh A, Jackson L, Kaminsky EB, Kok K, Krantz ID, Kuhn RM, Lee C, Ostell JM, Rosenberg C, Scherer SW, Spinner NB, Stavropoulos DJ, Tepperberg JH, Thorland EC, Vermeesch JR, Waggoner DJ, Watson MS, Martin CL, Ledbetter DH. Consensus statement: Chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749–764. doi: 10.1016/j.ajhg.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles-Worsley M, Tiobech J, Browning SR, Korn J, Goodman S, Gentile K, Melhem N, Byerley W, Faraone SV, Middleton FA. Deletion at the SLC1A1 glutamate transporter gene co-segregates with schizophrenia and bipolar schizoaffective disorder in a 5-generation family. Am J Med Genet Part B. 2013;162B:87–95. doi: 10.1002/ajmg.b.32125. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Heath AC, Bucholz KK, Madden PA, Fu Q, Knopik V, Lynskey MT, Whitfield JB, Statham DJ, Martin NG. Genetic epidemiology of alcohol-induced blackouts. Arch Gen Psychiatry. 2004;61:257–263. doi: 10.1001/archpsyc.61.3.257. [DOI] [PubMed] [Google Scholar]
- Nishiike S, Takeda N, Kubo T, Nakamura S. Noradrenergic pathways involved in the development of vertigo and dizziness—A review. Acta Otolaryngol Suppl. 2001;545:61–64. doi: 10.1080/000164801750388135. [DOI] [PubMed] [Google Scholar]
- Perry TL, Wright JM, Hansen S. Hyperasparaginemia in a schizophrenic patient. Biol Psychiatry. 1983;18:89–97. [PubMed] [Google Scholar]
- Pietilainen OP, Rehnstrom K, Jakkula E, Service SK, Congdon E, Tilgmann C, Hartikainen AL, Taanila A, Heikura U, Paunio T, Ripatti S, Jarvelin MR, Isohanni M, Sabatti C, Palotie A, Freimer NB, Peltonen L. Phenotype mining in CNV carriers from a population cohort. Hum Mol Genet. 2011;20:2686–2695. doi: 10.1093/hmg/ddr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Almeida J, Bacchelli E, Bader GD, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bolte S, Bolton PF, Bourgeron T, Brennan S, Brian J, Bryson SE, Carson AR, Casallo G, Casey J, Chung BH, Cochrane L, Corsello C, Crawford EL, Crossett A, Cytrynbaum C, Dawson G, de Jonge M, Delorme R, Drmic I, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Goldberg J, Green A, Green J, Guter SJ, Hakonarson H, Heron EA, Hill M, Holt R, Howe JL, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Korvatska O, Kustanovich V, Lajonchere CM, Lamb JA, Laskawiec M, Leboyer M, Le Couteur A, Leventhal BL, Lionel AC, Liu XQ, Lord C, Lotspeich L, Lund SC, Maestrini E, Mahoney W, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Merikangas A, Migita O, Minshew NJ, Mirza GK, Munson J, Nelson SF, Noakes C, Noor A, Nygren G, Oliveira G, Papanikolaou K, Parr JR, Parrini B, Paton T, Pickles A, Pilorge M, Piven J, Ponting CP, Posey DJ, Poustka A, Poustka F, Prasad A, Ragoussis J, Renshaw K, Rickaby J, Roberts W, Roeder K, Roge B, Rutter ML, Bierut LJ, Rice JP, Salt J, Sansom K, Sato D, Segurado R, Sequeira AF, Senman L, Shah N, Sheffield VC, Soorya L, Sousa I, Stein O, Sykes N, Stoppioni V, Strawbridge C, Tancredi R, Tansey K, Thiruvahindrapduram B, Thompson AP, Thomson S, Tryfon A, Tsiantis J, Van Engeland H, Vincent JB, Volkmar F, Wallace S, Wang K, Wang Z, Wassink TH, Webber C, Weksberg R, Wing K, Wittemeyer K, Wood S, Wu J, Yaspan BL, Zurawiecki D, Zwaigenbaum L, Buxbaum JD, Cantor RM, Cook EH, Coon H, Cuccaro ML, Devlin B, Ennis S, Gallagher L, Geschwind DH, Gill M, Haines JL, Hallmayer J, Miller J, Monaco AP, Nurnberger JI, Jr, Paterson AD, Pericak-Vance MA, Schellenberg GD, Szatmari P, Vicente AM, Vieland VJ, Wijsman EM, Scherer SW, Sutcliffe JS, Betancur C. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd MK, Keene J, Bunke B, Kaminsky EB, Adam MP, Mulle JG, Ledbetter DH, Martin CL. Segmental duplications mediate novel, clinically relevant chromosome rearrangements. Hum Mol Genet. 2009;18:2957–2962. doi: 10.1093/hmg/ddp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silversides CK, Lionel AC, Costain G, Merico D, Migita O, Liu B, Yuen T, Rickaby J, Thiruvahindrapuram B, Marshall CR, Scherer SW, Bassett AS. Rare copy number variations in adults with tetralogy of Fallot implicate novel risk gene pathways. PLoS Genet. 2012;8:e1002843. doi: 10.1371/journal.pgen.1002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, Feuk L, Qian C, Bryson SE, Jones MB, Marshall CR, Scherer SW, Vieland VJ, Bartlett C, Mangin LV, Goedken R, Segre A, Pericak-Vance MA, Cuccaro ML, Gilbert JR, Wright HH, Abramson RK, Betancur C, Bourgeron T, Gillberg C, Leboyer M, Buxbaum JD, Davis KL, Hollander E, Silverman JM, Hallmayer J, Lotspeich L, Sutcliffe JS, Haines JL, Folstein SE, Piven J, Wassink TH, Sheffield V, Geschwind DH, Bucan M, Brown WT, Cantor RM, Constantino JN, Gilliam TC, Herbert M, Lajonchere C, Ledbetter DH, Lese-Martin C, Miller J, Nelson S, Samango-Sprouse CA, Spence S, State M, Tanzi RE, Coon H, Dawson G, Devlin B, Estes A, Flodman P, Klei L, McMahon WM, Minshew N, Munson J, Korvatska E, Rodier PM, Schellenberg GD, Smith M, Spence MA, Stodgell C, Tepper PG, Wijsman EM, Yu CE, Roge B, Mantoulan C, Wittemeyer K, Poustka A, Felder B, Klauck SM, Schuster C, Poustka F, Bolte S, Feineis-Matthews S, Herbrecht E, Schmotzer G, Tsiantis J, Papanikolaou K, Maestrini E, Bacchelli E, Blasi F, Carone S, Toma C, Van Engeland H, de Jonge M, Kemner C, Koop F, Langemeijer M, Hijmans C, Staal WG, Baird G, Bolton PF, Rutter ML, Weisblatt E, Green J, Aldred C, Wilkinson JA, Pickles A, Le Couteur A, Berney T, McConachie H, Bailey AJ, Francis K, Honeyman G, Hutchinson A, Parr JR, Wallace S, Monaco AP, Barnby G, Kobayashi K, Lamb JA, Sousa I, Sykes N, Cook EH, Guter SJ, Leventhal BL, Salt J, Lord C, Corsello C, Hus V, Weeks DE, Volkmar F, Tauber M, Fombonne E, Shih A, Meyer KJ. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropeano M, Ahn JW, Dobson RJ, Breen G, Rucker J, Dixit A, Pal DK, McGuffin P, Farmer A, White PS, Andrieux J, Vassos E, Ogilvie CM, Curran S, Collier DA. Male-biased autosomal effect of 16p13.11 copy number variation in neurodevelopmental disorders. PLoS ONE. 2013;8:e61365. doi: 10.1371/journal.pone.0061365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapner RJ, Martin CL, Levy B, Ballif BC, Eng CM, Zachary JM, Savage M, Platt LD, Saltzman D, Grobman WA, Klugman S, Scholl T, Simpson JL, McCall K, Aggarwal VS, Bunke B, Nahum O, Patel A, Lamb AN, Thom EA, Beaudet AL, Ledbetter DH, Shaffer LG, Jackson L. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med. 2012;367:2175–2184. doi: 10.1056/NEJMoa1203382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet. 2008;40:880–885. doi: 10.1038/ng.162. [DOI] [PubMed] [Google Scholar]
- Yu HE, Hawash K, Picker J, Stoler J, Urion D, Wu BL, Shen Y. A recurrent 1.71 Mb genomic imbalance at 2q13 increases the risk of developmental delay and dysmorphism. Clin Genet. 2012;81:257–264. doi: 10.1111/j.1399-0004.2011.01637.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.