Abstract
Research suggests that maternal immune activation (MIA) during pregnancy increases the risk of neurodevelopmental disorders including schizophrenia and autism in the offspring. Current theories suggest that inflammatory mediators including cytokines and chemokines may underlie the increased risk of these disorders in humans. For example, elevated maternal interleukin-8 (IL-8) during pregnancy is associated with increased risk of schizophrenia in the offspring. Given this association, the present experiments examined ELR-CXC chemokines CXCL1 and CXCL2, rodent homologues of human IL-8, and activation of their receptors (CXCR1 and CXCR2) in an established rodent model of MIA. Pregnant Long Evans rats were treated with the viral mimetic polyinosinic–polycytidylic acid (polyI:C; 4 mg/kg, i.v.) on gestational day 15. Protein analysis using multiplex assays and ELISA showed that polyI:C significantly increased maternal serum concentrations of interleukin-1β, tumor necrosis factor, and CXCL1 3 h after administration. Subsequent experiments tested the role of elevated maternal CXCL1 on behavior of the offspring by administering a CXCR1/CXCR2 antagonist (G31P; 500 μg/kg, i.p.; 1 h before, 48 and 96 h after polyI:C treatment). The male offspring of dams treated with polyI:C demonstrated subtle impairments in prepulse inhibition (PPI), impaired associative and crossmodal recognition memory, and altered behavioral flexibility in an operant test battery. While G31P did not completely reverse the behavioral impairments caused by polyI:C, it enhanced PPI during adolescence and strategy set-shifting and reversal learning during young adulthood. These results suggest that while polyI:C treatment significantly increases maternal CXCL1, elevations of this chemokine are not solely responsible for the effects of polyI:C on the behavior of the offspring.
Keywords: Crossmodal memory, Interleukin-8, Polyi:C, Schizophrenia, Set-shifting
1. Introduction
Numerous studies have found evidence that maternal infection during pregnancy increases the offspring’s risk of developing neurodevelopmental psychiatric disorders such as schizophrenia and autism (Arias et al., 2012; Ashwood et al., 2011; Patterson, 2009). Given that a variety of pathogens are associated with increase d risk of psychiatric disorders, exposure of the developing fetus to maternal and/or fetal cytokines released during infection may alter normal development of the nervous system resulting in the behavioral and cognitive symptoms (Fineberg and Ellman, 2013; Gilmore et al., 2004; Watanabe et al., 2010). Chemokines, a sub-class of cytokines with chemotactic properties (Tran and Miller, 2003), and their associated G protein-coupled receptors are involved in brain development (Arrode-Bruses and Bruses, 2012; Brown et al., 2004; Cho and Miller, 2002; Deverman and Patterson, 2009; Garay et al., 2013; Ragozzino, 2002) and recent human epidemiological research has uncovered associations between numerous chemokines and neurodevelopmental disorders (Ashwood et al., 2011; Reale et al., 2011; Stuart and Baune, 2014). One study of a large birth cohort found correlations between elevated maternal serum levels of the chemokine interleukin-8 (IL-8) during pregnancy and: 1) the development of schizophrenia spectrum disorders in adult offspring (Brown et al., 2004); 2) structural brain alterations, including increased ventricular and decreased cortical volumes, in schizophrenia patients (Ellman et al., 2010). These findings strongly suggest that pre-natal exposure to maternal immune activation (MIA) alters the offspring’s development through a mechanism that is at least partially dependent on IL-8 signaling (Meyer et al., 2011).
Rodent models of MIA have high validity in terms of the underlying etiology, disease progression, and behavioral symptoms of human disorders including schizophrenia and autism (Meyer and Feldon, 2012; Meyer et al., 2009; Patterson, 2011; Piontkewitz et al., 2012). One MIA model involves systemic treatment of pregnant rats or mice with the viral mimetic polyinosinic–polycytidylic acid (polyI:C). In pregnant mice, polyI:C treatment increases levels of circulating cytokines including IL-1β, IL-6, IL-10, and tumor necrosis factor (TNF) in the maternal serum for several hours (Arrode-Bruses and Bruses, 2012; Meyer et al., 2006a,b) and a critical role for IL-6 in the behavioral effects of MIA on the offspring of mice has been demonstrated (Smith et al., 2007). Less information regarding the immune response to polyI:C in pregnant rats is available. One study using pregnant Sprague–Dawley rats showed that TNF and IL-10 were significantly increased 3 h following polyI:C treatment on gestational day (GD) 9 (Song et al., 2011). Given the lack of data in pregnant rats, we measured the effect of polyI:C treatment on 9 cytokines (IL-1β, IL-6, TNF, CXCL1 (GRO-α/KC), IFNγ, IL-1α, IL-2, IL-4 and IL-10) in the serum of pregnant Long–Evans rats, which have been used previously to assess the effects of MIA on behavior and cognition (Howland et al., 2012; Sangha et al., 2014; Zhang et al., 2012). Given the human epidemiological data reviewed above (Brown et al., 2004; Ellman et al., 2010), we were particularly interested in the profile of CXCL1, the rodent homologue of human IL-8.
As CXCL1 was significantly increased following polyI:C treatment (see the Results section), our second objective was to test whether blocking the activity of CXCL1 during MIA would protect against the long-term effects of polyI:C on the offspring. CXCL1 signals through two receptors (CXCR1 and CXCR2) which can be blocked by the CXCR1/R2 antagonist CXCL8(3–72)K11R/G31P (G31P), a synthetic, mutated form of human IL-8 that binds CXCR1/R2 with high affinity (Gordon et al., 2005; Zhao et al., 2009). The potent activity of G31P has been demonstrated in rodent models of airway inflammation (Wei et al., 2013; Zhao et al., 2009) and ischemia–reperfusion injury (Zhao et al., 2010). Thus, our hypothesis was that treating pregnant dams with G31P before and after polyI:C would prevent the effects of elevated CXCL1 on behavioral changes in the offspring.
We used a series of rodent tests related to the cognitive domains most consistently impaired in schizophrenia (Young et al., 2009) as tests related to the positive symptoms of the disorder have received considerable attention previously (reviewed in Meyer and Feldon, 2012; Meyer et al., 2009; Patterson, 2011; Piontkewitz et al., 2012). Prepulse inhibition (PPI), a measure of sensorimotor gating, is impaired in schizophrenia patients (Braff et al., 2001) and the offspring of rats and mice treated with polyI:C during pregnancy (Dickerson et al., 2010; Howland et al., 2012; Klein et al., 2013; Mattei et al., 2014; Meyer et al., 2010; Shi et al., 2003; Smith et al., 2007; Wolff and Bilkey, 2008; Wolff and Bilkey, 2010; see also Fortier et al., 2007; Li et al., 2009). Impaired visual learning and memory is a cognitive symptom of schizophrenia identified by the MATRICS initiative (Marder and Fenton, 2004). Therefore, we chose to assess associative visuospatial memory using the object-in-place (OIP) recognition memory test (Barker et al., 2007) given the impairments in associative visuospatial memory observed in schizophrenia (Wood et al., 2002) and previous impairments noted in the offspring of rats treated with polyI:C (Howland et al., 2012). Accumulating evidence also suggests that schizophrenia patients exhibit alterations in multisensory integration (Stekelenburg et al., 2013; Stone et al., 2011; Williams et al., 2010). To the best of our knowledge, multisensory integration has not been examined in rodent models of MIA. Therefore, we tested the effects of polyI:C on crossmodal recognition memory, a recently developed test of multisensory integration, which includes visual, tactile and crossmodal components (Jacklin et al., 2012; Winters and Reid, 2010). Finally, behavioral flexibility was examined as patients typically exhibit decreased flexibility on tests of attentional set-shifting and reversal learning (Leeson et al., 2009; Pantelis et al., 1999; Waltz and Gold, 2007). An operant test battery that assesses visual cue discrimination, strategy set-shifting, and reversal learning was used (Enomoto et al., 2011; Floresco et al., 2008, 2009). Using the polyI:C model in rats, Zhang et al. (2012) found that MIA impaired strategy set-shifting in the male, but not female, offspring. Findings on reversal learning are mixed with enhancements (Zhang et al., 2012; Zuckerman and Weiner, 2005) and impairments (Han et al., 2011; Savanthrapadian et al., 2013) observed using different task designs. We expected the offspring of polyI:C-treated dams to show impaired PPI, associative recognition memory, crossmodal recognition memory, and behavioral flexibility. The offspring whose mothers were treated with polyI:C and G31P were expected to perform similarly to controls on the tests.
2. Methods
2.1. Animals
Timed pregnant Long Evans dams (Charles River Laboratories, Que-bec, Canada) arrived at the laboratory on GD 7. They were singly housed with food (Purina Rat Chow) and water available ad libitum in a colony room (maintained at 21 °C). Experiments were conducted during the light phase (12 h light/dark cycle, lights on at 0700 h) and experimenters were blind to the treatments during testing. All experiments were performed in accordance with the Canadian Council on Animal Care and were approved by the University of Saskatchewan Animal Research Ethics Board.
2.2. Maternal treatment for behavioral experiments
On GD 15, dam weight and rectal temperature (Homeothermic Blanket System, Harvard Instruments, MA, USA) were recorded. Dams were anesthetized for approximately 10 min using isoflurane (5% induction and 2.5% maintenance) and given one intravenous (i.v.) tail vein injection of either saline or polyI:C (4.0 mg/kg, high molecular weight; InVivoGen, San Diego, CA, USA). Half of the dams from each treatment group also received three intraperitoneal (i.p.) injections of G31P (500 μg/kg; 1 h before, 48 h after, and 96 h after polyI:C or saline treatment). Previous studies using this dose and treatment protocol for G31P confirm the drug’s effectiveness at blocking CXCR1 and CXCR2 following systemic inflammatory challenges for at least 48 h (Gordon et al., 2005, 2009; Li et al., 2002; Zhao et al., 2009, 2010). Other than injections, weight, and temperature recordings (8, 24 and 48 h after treatment) dams were undisturbed until postnatal day (PND) 1, when litters were weighed and culled to a maximum of ten pups (6 males where possible). On PND 21 litters were weaned into same-sex sibling cages and male pups were randomly selected for behavioral testing.
2.3. Multiplex assays and ELISAs for cytokines
Dams for cytokine assays were treated the same as those whose offspring were used for behavioral experiments until 3 h after polyI:C treatment. At that time, they were deeply anesthetized with isoflurane, decapitated, and trunk blood samples were collected. Note that dams used for cytokine analysis received G31P treatment 1 h before polyI:C (i.e., 4 h before they were sacrificed). Trunk blood was allowed to clot for 60 min (room temperature) and then centrifuged at 12,000 rpm for 4 min. Serum was pipetted from the sample and flash frozen with liquid nitrogen. All samples were stored at −80 °C until analysis. Bio-Plex Pro Assay multiplex kits were used to quantify protein in the samples, as previously described (Garay et al., 2013). Levels of IL-1β, IL-6, TNF, CXCL1, IFN-γ, IL-1α, IL-2, IL-4 and IL-10 were first quantified in tissue samples from rats treated with either saline (n = 3) or polyI: C (n = 5). A subsequent experiment measured cytokines in samples from rats treated with either saline–saline (sal–sal), saline–polyI:C (sal–polyI:C), G31P–saline (G31P–sal), or G31P–polyI:C (n = 4 for each group). ELISAs were performed on maternal serum for CXCL1 (GROα/KC) and CXCL2 (GROβ/MIP-2; R&D Systems, Inc., Minneapolis, MN) to confirm the measurements from the multiplex assays for CXCL1 and to measure CXCL2. All procedures followed the manufacturer’s instructions.
2.4. Behavioral testing
Behavioral data were collected from four separate squads that included polyI:C and G31P treated dams. Behavioral tests were conducted according to published protocols (Floresco et al., 2008, 2009; Howland et al., 2012; Jacklin et al., 2012; Thai et al., 2013; Winters and Reid, 2010; Zhang et al., 2012). Testing occurred in the following order: PPI during puberty (PNDs 35–36) and young adulthood (PNDs 56–57), recognition memory (PNDs 60–80) and operant testing (PNDs 80–100). Rats were singly housed and food deprived to 85% of their free-feeding weight prior to operant testing.
2.4.1. PPI
Two SR-LAB startle boxes (San Diego Instruments, San Diego, CA, USA) were used. Each session had a constant background noise (70 dB) and began with a 5 min acclimatization, followed by six pulse-alone trials (120 dB, 40 ms). Pulse-alone (6), prepulse + pulse (72) and no stimulus (6) trials were then presented in a pseudorandom order, followed by 6 additional pulse-alone trials. Prepulse + pulse trials began with a 20 ms prepulse of 3, 6, or 12 dB above background (70 dB). Prepulse−pulse intervals were 30, 50, 80 or 140 ms between the onset of the prepulse and the onset of the 120 dB pulse. The inter-trial interval varied randomly from 3 to 14 s. The boxes were cleaned with 40% ethanol between rats.
2.4.2. Associative (object-in-place) recognition memory
The testing apparatus was a white open-field arena (60 × 60 × 60 cm) with one black wall (Fig. 4A). Three 10 min habituation sessions occurred prior to testing. During the first two sessions, two rats were simultaneously habituated in separate arenas. In the third session, rats were individually habituated. One day after the third habituation, rats explored four distinct objects for 5 min (sample phase). Following a 1 h delay, rats explored identical copies of the same four objects with the location of two of the objects switched (test phase). The OIP paradigm measures associative visuospatial memory as the rats must use both object identity and object location information to demonstrate preferential exploration of the pair of objects that were moved in the test trial (Barker et al., 2007; Howland et al., 2012).
2.4.3. Visual, tactile, and crossmodal recognition memory
The testing apparatus was Y-shaped, with one entrance arm and two object arms (10 × 27 cm) (Fig. 4C, E, G). Testing included three distinct tests: the visual, tactile, and crossmodal memory tests. Transparent plastic barriers were inserted in front of the objects during visual, but not tactile phases. One red light bulb (60 W) was illuminated during the tactile phases, preventing the rats from seeing the objects, but allowing recordings of the rats’ behavior. One paired and one individual habituation session (10 min) occurred prior to testing. White overhead lighting and the red light bulb were separately illuminated for half of each habituation, with the order of illumination counterbalanced. Testing began one day after the second habituation. The order of administration of the visual, tactile and crossmodal tests was counterbalanced. All tests included a 3 min sample phase and 2 min test phase with a 1 h delay between phases. The maze contained two identical copies of an object during the sample phase, and a third copy of the original object with a novel object during the test phase. In this paradigm, visual recognition memory is defined as a significantly greater time spent looking at a novel object when it is paired with a familiar object that had only been seen (but not touched) during a sample trial (Fig. 4C). Tactile recognition memory refers to a significantly greater tactile exploration of a novel object when it is paired with a familiar object that had been previously touched (but not seen) during a sample trial (Fig. 4E). Crossmodal recognition memory refers to a significantly greater time spent looking at a novel object when it is paired with an object that had been touched, but not seen, previously (Fig. 4G).
2.4.4. Operant test battery
Eight operant chambers (MedAssociates Systems, St. Albans, VT, USA) located within sound-attenuating cubicles were used. Chambers contained two retractable levers and stimulus lights, positioned on either side of a food receptacle used to deliver rewards. A 100 mA house light illuminated the chamber. Sessions began with levers retracted and the chamber in darkness (inter-trial state). Rats were tested once each day, with no break given between test types. Lever training. Rats were trained to press the levers as described previously and immediately after reaching criterion, side preference was determined. Visual-cue discrimination. Rats were trained to press the lever with a stimulus light illuminated above it to a criterion of 10 consecutive correct choices. Trials began with an illumination of one stimulus light, followed 3 s later by the house light and insertion of both levers. A correct press of the lever underneath the illuminated stimulus light caused retraction of both levers and the delivery of a reward pellet. An incorrect press returned the chamber to the inter-trial state. Strategy set-shift. Rats were trained to ignore the illuminated stimulus light and respond to a spatial-cue (the lever opposite to their side preference) to receive a reward pellet. Reversal learning. Rats were trained to press the lever opposite to the one rewarded during set-shifting.
2.5. Data analysis
All results are reported as group means ± standard error of the mean (SEM). Outlying values, greater than 2 standard deviations above or below the mean, were removed prior to analysis. Outlier criteria for PPI were determined using mean PPI on all long-interval (50, 80, 140 ms) trials. Outliers on the operant test battery were determined using trials to criterion (TTC). SPSS Statistics version 21 (IBM) was used to conduct all statistical tests, using a significance value of p < 0.05. Non-significant findings are reported as n.s. Two way analysis of variance (ANOVA) with polyI:C and G31P treatment as between-subjects factors were predominantly used for analysis. Corrections were made for violations of sphericity (Mauchly’s test) where appropriate. Post hoc analyses were performed separately using Tukey’s test. PPI. PPI was calculated by averaging the startle amplitudes for each trial type, and the percent PPI for each prepulse intensity was calculated using the formula: [100 − (100 × startle amplitude on prepulse + pulse trials) / (startle amplitude on pulse-alone trials)] (Howland et al., 2004, 2012). Recognition memory. Exploration was scored when a rat was judged to be actively exploring an object with its nose directed within 2 cm of the object and its head or vibrissae moving, but not when it was standing on top of the object or not directing attention towards it. A discrimination ratio (DR), calculated as the time spent exploring (novel-familiar) / (novel + familiar), was used to quantify memory (Cazakoff and Howland, 2011; Howland et al., 2012). Operant test battery. TTC and total errors were obtained from the raw data. Total errors were broken down into three subtypes (Floresco et al., 2008, 2009; Thai et al., 2013; Zhang et al., 2012): 1) perseverative errors were scored when a rat continued to use a previously relevant but currently irrelevant strategy. For strategy set-shift trials, eight out of every 16 consecutive trials (defined as a block) allowed the rat to respond this way. For each block, perseverative errors were counted when rats pressed the incorrect lever on 6 or more trials per block. For reversal learning trials, perseverative errors were scored when 10 or more errors were committed within a block; 2) regressive errors were scored once the rat had ceased perseverating as defined above for the set-shifting and reversal learning test days; and 3) never-reinforced errors were scored during the set-shifting test day when rats pressed the incorrect lever when the stimulus light was illuminated above the correct lever.
3. Results
3.1. Effects of polyI:C and G31P treatment on cytokine concentrations in maternal serum
Multiplex analysis of maternal serum samples (Fig. 1A) demonstrated that polyI:C treatment increased the concentration of proinflammatory cytokines including IL-1β, IL-6, TNF, and CXCL1 in the maternal serum 3 h after treatment. A significant main effect of polyI:C treatment for CXCL1 (F(1,20) = 7.61, p = 0.012) as well as significant main effects of G31P for IFNγ (F(1,20) = 6.30, p = 0.021), IL-1α (F(1,20) = 7.12, p = 0.015) and IL-2 (F(1,20) = 5.19, p = 0.034) were observed. No significant interactions were observed (n.s.). Analysis of the simple main effects of polyI:C treatment revealed significant increases for IL-1β (t(14) = −2.16, p = 0.049) and TNF (t(14) = −2.22, p = 0.044). The two-fold increase in IL-6 caused by polyI:C treatment failed to reach significance (t(14) = −1.56, p = 0.14).
Fig. 1.
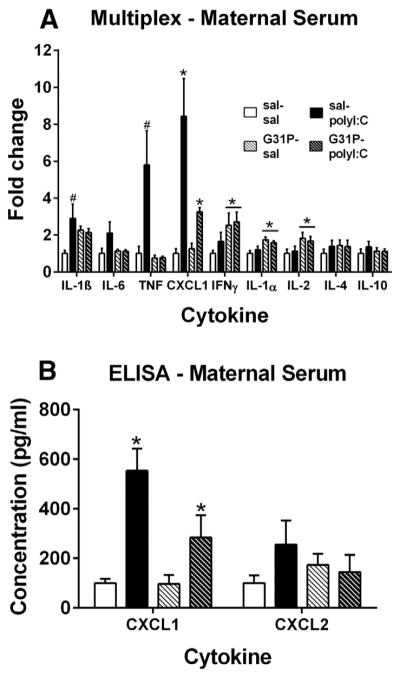
Effects of polyI:C and G31P treatment on cytokine concentrations in maternal serum. A. PolyI:C treatment significantly increased serum concentrations of CXCL1, IL-1β, TNF. G31P treatment significantly increased serum levels of INFγ, IL-1α, and IL-2. Group sizes were: sal–sal (n = 7), sal–polyI:C (n = 9), G31P–sal (n = 4), and G31P–polyI:C (n = 4). B. Serum concentration of CXCL1, but not CXCL2, was significantly increased by polyI:C treatment. Samples were the same as those in panel A except for that two were not available for testing from the sal–polyI:C group. Serum was collected 3 h after polyI:C treatment on gestational day 15. Multiplex data are presented as fold-change relative to the group mean of the sal–sal treated group as the concentrations of the cytokines in the saline-treated group varied. * indicates a significant main effect of either polyI:C or G31P. # indicates a significant difference between the sal–sal and sal–polyI:C groups.
To further characterize the changes in chemokine concentration 3 h after polyI:C and G31P treatment, ELISAs for CXCL1 (GROα/KC) and CXCL2 (GROβ/MIP-2) were performed on the serum samples (Fig. 1B). The results indicated that polyI:C treatment significantly increased the concentration of CXCL1 (F(1,18) = 20.25, p < 0.001) without altering the concentration of CXCL2 (n.s.). There were no significant main effects of G31P treatment or significant interactions between polyI:C and G31P for either chemokine (n.s.)
3.2. Effects of polyI:C and G31P on the pregnant dams and pups
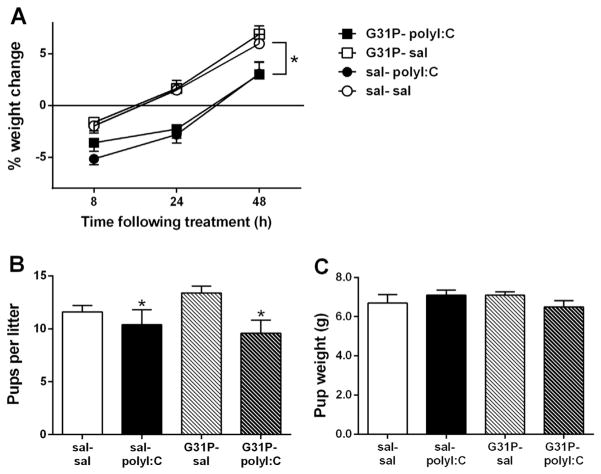
Similar to previous results from our laboratory (Howland et al., 2012; Sangha et al., 2014; Zhang et al., 2012), polyI:C treatment significantly decreased the weight of the dams for 48 h following treatment (Fig. 2A; main effect of polyI:C (F(1,45) = 17.49, p < 0.001). No significant effects of G31P treatment or an interaction between polyI:C and G31P were found. Neither polyI:C nor G31P significantly altered temperature during the 48 h following treatment (data not shown).
Fig. 2.
Effects of polyI:C and G31P treatment on maternal weight change (A), number of pups per litter (B), and average pup weight at birth (C). PolyI:C treatment caused a significant weight loss in the 48 h following treatment (A) and decreased the number of pups per litter (B), without affecting pup weight (C). Maternal weight change (A) was normalized to the weight of the dams immediately before the initial saline or G31P treatment on gestational day (GD) 15. G31P or saline (500 μg/kg, i.p.) treatments occurred on gestational days 15, 17, and 19. PolyI:C or saline (4 mg/kg, i.v.) treatments were administered on GD15, 60 min after G31P or saline. Group sizes were sal–sal (n = 15 dams), sal–polyI:C (n = 11 dams), G31P–sal (n = 11 dams), G31P–polyI:C (n = 12 dams). B, C. Number of litters assessed for pups per litter and average pup weight was: sal–sal (n = 15 litters), sal–polyI:C (n = 11 litters), G31P–sal (n = 11 litters), and G31P–polyI:C (n = 12 litters). * in panels A and B denote a significant main effect of polyI:C treatment. No significant effects of G31P were observed.
At birth, the number of pups per litter and the total weight of each litter were taken. PolyI:C treatment significantly decreased litter size (F(1,48) = 75.85, p = 0.016) from 12.35 ± 0.5 to 9.96 ± 0.9 pups (Fig. 2B). G31P treatment did not affect litter size, with n.s. effects of G31P and the polyI:C by G31P interaction. Average pup weights were not affected by either treatment (Fig. 2C) with all main effects and interactions n.s.
3.3. Effects of exposure to polyI:C and G31P on PPI in the offspring
3.3.1. PND 35. Startle amplitude (Fig. 3A)
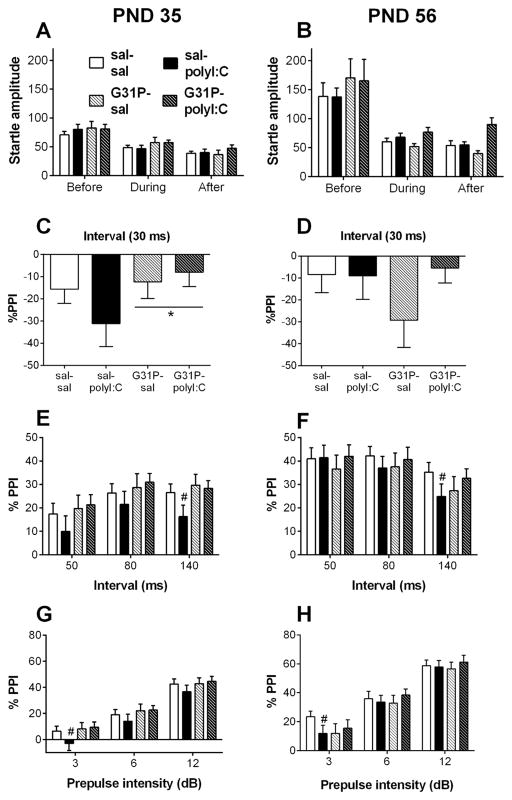
Fig. 3.
Acoustic startle responses and prepulse inhibition (PPI) of the offspring following maternal polyI:C and G31P treatment. A, B. Acoustic startle responses (startle amplitude, arbitrary units) for 120 dB pulse trials before, during, and after the PPI trials at postnatal days (PNDs) 35 (A) and 56 (B) (sal–sal, n = 27; sal–polyI:C, n = 21; G31P–sal, n = 21; and G31P–polyI:C, n = 23). C, D. % PPI for trials with a 30 ms prepulse–pulse interval at PNDs 35 (C) and 56 (D). Data are averaged for the 3, 6, and 12 dB prepulse intensities. Negative % PPI values reflect an increase in startle to trials with a prepulse. G31P-exposed rats showed less prepulse facilitation than other rats (main effect of G31P). E, F. Percent PPI averaged by prepulse intensity for the 50, 80, and 140 ms prepulse–pulse intervals at PNDs 35 (E) and 56 (F). G, H. Percent PPI averaged by prepulse–pulse interval for 3, 6 and 12 dB prepulse intensities at PNDs 35 (G) and 56 (H). Group sizes for C, E, G: sal–sal (n = 24), sal–polyI:C (n = 20), G31P–sal (n = 21), and G31P–polyI:C (n = 22). Group sizes for D, F, H: sal–sal (n = 26), sal–polyI:C (n = 21), G31P–sal (n = 19), and G31P–polyI:C (n = 22). Number of litters included in testing: sal–sal (n = 15), sal–polyI:C (n = 11), G31P–sal (n = 11), and G31P–polyI:C (n = 12). # in panels F and H show the significant decreases in PPI for sal–polyI:C rats compared with sal–sal rats for trials with a 140 ms interval (F) and for trials with a 3 dB prepulse (H).
The average startle amplitudes during blocks of pulse-alone trials (before, during and after prepulse + pulse trials) were analyzed to assess the habituation of startle during a PPI session. Significantly lower startle amplitudes were exhibited in the pulse blocks during and after PPI (F(1.40,116.29) = 91.65, p < 0.001; post hoc, p < 0.05). The effects of polyI:C, G31P, and all interactions were n.s.
3.3.2. PND 35. PPI: short interval (Fig. 3C)
As described previously, prepulse facilitation occurs for trials with a 30 ms prepulse–pulse interval (Howland et al., 2012) and these trials were analyzed separately from the other PPI trials. No effects of polyI: C treatment on responses to trials with a 30 ms interval were noted, whereas G31P-treated rats displayed significantly less facilitation on these trials (F(1,83) = 4.49, p = 0.037). The expected effect of increasing PPI with increasing prepulse intensity was also observed (data not shown, F(2,166) = 44.95, p < 0.001).
3.3.3. PND 35. PPI: long intervals (Fig. 3E, G)
Prepubescent rats displayed significant PPI at all three long prepulse–pulse intervals (50, 80, 140 ms). Regardless of treatment, rats exhibited decreased PPI at the 50 ms interval compared with the 80 and 140 ms intervals (Fig. 3E; main effect of interval: F(1.86,154.75) = 19.77, p < 0.001; post hoc p < 0.05). The expected main effect of prepulse intensity was also significant (F( 2,166) = 373.95, p < 0.001), as rats exhibited higher PPI at higher dB prepulses (Fig. 3G).
There was no main effect of polyI:C treatment (F(1,83) = 0.73, p = 0.40) while the G31P main effect was close to significant (F(1,83) = 3.41, p = 0.068). A three-way interaction between interval, prepulse intensity and polyI:C treatment (F(4,332) = 3.94, p = 0.004) was also significant. Simple main effects analysis of the effects of polyI:C on PPI revealed that sal–polyI:C rats demonstrated significantly lower PPI than sal–sal rats at the 140 ms interval (Fig. 3E; t(45) = 5.09, p = 0.029) and for the trials with 3 dB prepulses (Fig. 3G: t(45) = 4.43, p = 0.041).
3.3.4. PND 56. Startle amplitude (Fig. 3B)
Analysis of startle amplitude during the blocks of pulse-alone trials revealed a significant main effect of block (F(1.09,91.49) = 41.57, p < 0.001), as startle was higher on the first block of pulse-alone trials than the other pulse-alone trial blocks. The main effects of polyI:C, G31P, and all other interactions were n.s.
3.3.5. PND 56. PPI: short interval (Fig. 3D)
Similar to the results from PND 35, a significant effect of prepulse intensity was found (F(2,168) = 53.92, p < 0.001). However, at this age neither polyI:C or G31P caused significant alterations in PPI when a 30 ms prepulse–pulse interval was tested (n.s.).
3.3.6. PND 56. PPI: long intervals (Fig. 3F, H)
Data analysis for these trials revealed significant main effects of prepulse–pulse interval (F(2,168) = 21.24, p < 0.001) and prepulse intensity (F(1.82,152.80) = 213.20, p < 0.001). In contrast to PND 35, at PND 56 the effect of interval was caused by decreased PPI at the 140 ms interval. The main effects of polyI:C, G31P, and all interactions in this analysis were n.s. In an analysis of simple main effects, excluding animals exposed to G31P, the effects of prepulse–pulse interval (F(2,90) = 13.98, p < 0.001) and prepulse intensity (F(2,90) = 102.26, p < 0.001) remained significant and a prepulse–pulse interval by polyI: C interaction was close to significant (F(2,90) = 2.79, p = 0.067). A t-test comparing PPI on trials with the longest prepulse–pulse interval (140 ms) revealed a significantly lower PPI in sal–polyI:C rats than sal–sal control rats (Fig. 3F; t(45) = 2.21, p = 0.032). A comparison of PPI on long-interval trials with the 3 dB intensity prepulse revealed a significant difference (t(51) = 2.19, p = 0.033) between sal–polyI:C and sal–sal rats (Fig. 3H).
3.4. Effects of exposure to polyI:C and G31P on recognition memory
3.4.1. Exploration times (Table 1)
Table 1.
Total exploration time of the objects (s ± SEM) during the sample and test phases of the object-in-place (OIP), visual, tactile and crossmodal recognition memory tests. The total time for the sample phase is presented, whereas the time for the first minute is presented for the test phase. No significant differences in exploration were present, except for the increased exploration caused by polyI:C treatment during the sample phase of the crossmodal recognition test.
| Group | OIP | Visual | Tactile | Crossmodal |
|---|---|---|---|---|
| sal–sal | ||||
| Sample | 102.21 ± 2.1 | 3.84 ± 0.4 | 39.33 ± 2.6 | 31.36 ± 2.0 |
| Test | 20.03 ± 0.9 | 2.34 ± 0.3 | 15.19 ± 0.9 | 3.44 ± 0.3 |
| sal–polyI:C | ||||
| Sample | 105.20 ± 2.7 | 4.27 ± 0.3 | 36.47 ± 1.8 | 35.86 ± 3.5a |
| Test | 22.52 ± 1.4 | 1.98 ± 0.2 | 14.82 ± 1.0 | 2.71 ± 0.2 |
| G31P–sal | ||||
| Sample | 105.68 ± 3.7 | 3.94 ± 0.3 | 38.50 ± 3.1 | 29.60 ± 2.2 |
| Test | 21.08 ± 1.6 | 1.92 ± 0.2 | 14.65 ± 1.1 | 3.48 ± 0.3 |
| G31P–polyI:C | ||||
| Sample | 97.67 ± 3.1 | 4.20 ± 0.5 | 34.90 ± 1.9 | 36.91 ± 3.2a |
| Test | 22.78 ± 1.5 | 2.53 ± 0.4 | 14.99 ± 1.1 | 3.60 ± 0.5 |
Indicates the significant main effect of polyI:C treatment.
With one exception, all treatment groups explored the objects for similar amounts of time during both the sample and test phases of the memory tests, as two way between-subjects ANOVAs revealed n.s. effects of polyI:C, G31P and polyI:C by G31P interaction. During the sample phase of the crossmodal test, polyI:C exposure significantly increased exploration from 30.63 ± 1.5 s to 36.40 ± 2.4 s (F(1,73) = 4.96, p = 0.029).
3.4.2. Object-in-place memory (Fig. 4A, B)
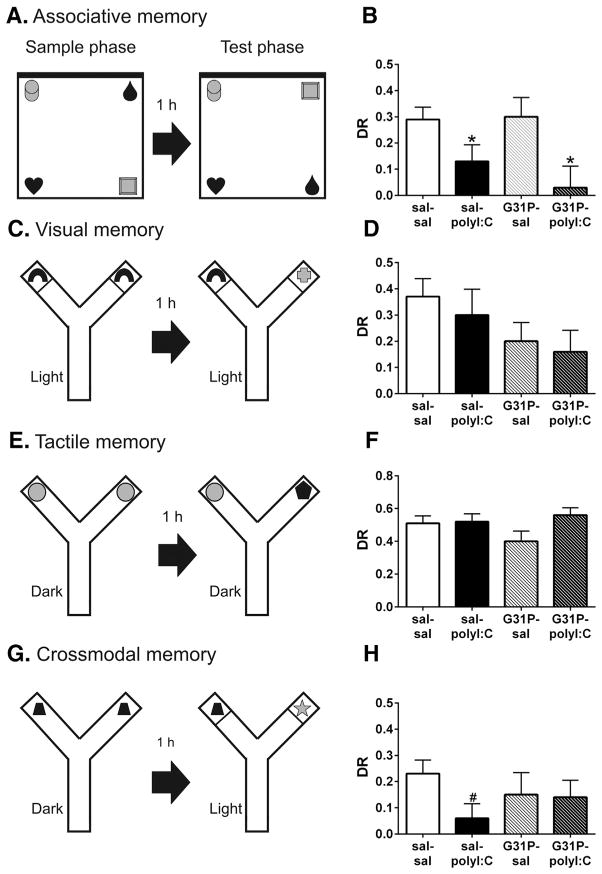
Fig. 4.
Associative (A, B), visual (C, D), tactile (E, F), and crossmodal (G, H) recognition memory of the offspring following maternal polyI:C and G31P treatment. Schematics using an overhead view of each test are shown in panels A, C, E and G. A 1 h delay was used between the sample and test phases of all tests. Note that the black wall of the open field chamber in panel A is indicated with a thicker black line for the north wall. In panels C and G, thin lines in the maze arms depict transparent Plexiglas walls that prevent the rats from touching the objects. Discrimination ratios (DR) by group are depicted in panels B, D, F, H; note: the y-axis varies among these panels. B. Rats from the polyI:C groups had significantly decreased associative memory, as assayed by the object-in-place test (sal–sal, n = 19; sal–polyI:C, n = 17; G31P–sal, n = 15; G31P–polyI:C, n = 17). Rats in the sal–polyI:C group also had significantly lower crossmodal memory (H). Group sizes for D: sal–sal, n = 18; sal–polyI:C, n = 14; G31P–sal, n = 19; and G31P–polyI:C, n = 13. Group sizes for F: sal–sal, n = 22; sal–polyI:C, n = 20; G31P–sal, n = 19; and G31P–polyI:C, n = 21. Group sizes for H: sal–sal, n = 23; sal–polyI:C, n = 20; G31P–sal, n = 17; and G31P–polyI:C, n = 19. Number of litters included in B: sal–sal (n = 12), sal–polyI:C (n = 11), G31P–sal (n = 11), and G31P–polyI:C (n = 12). Number of litters included in D,F,H: sal–sal (n = –polyI:C ( 15), sal n = 11), G31P–sal (n = 11), and G31P–polyI:C (n = 10). * represents main effects, # represents simple main effects comparing sal–sal and sal–polyI:C rats.
Analysis of discrimination ratios (DRs) showed that polyI:C-exposed rats were impaired on the OIP test, as evident from a main effect of polyI:C treatment (F(1,64) = 10.16, p = 0.002). G31P treatment did not alter memory performance, as the effects of G31P and polyI:C by G31P were n.s. One sample t-test comparing the DR to 0, showed that sal–sal (t(18) = 6.12, p < 0.001) and G31P–sal rats (t(14) = 4.035, p = 0.001) exhibited significantly more exploration of the object pair that was moved than would be predicted by chance. G31P–polyI:C rats failed to show more exploration of the object pair that was moved (t(16) = 0.323, p = 0.751) while sal–polyI:C rats showed a preference that was at the threshold of significance (t(16) = 2.107, p = 0.05).
3.4.3. Crossmodal recognition memory (Fig. 4C–H)
Analysis of DRs on the visual (Fig. 4C, D) and tactile memory (Fig. 4E, F) tests showed n.s. main effects of polyI:C, G31P, and polyI:C by G31P interactions, although the G31P-exposed rats tended to show lower DRs on the visual component of the test (Fig. 4D). An analysis using one sample t-test comparing with a DR of 0 found that G31P–polyI:C rats did not show significant memory compared with chance on the visual test (t(12) = 1.929, p = 0.078). All other groups showed a significant memory on both the tactile and visual tests (statistics not shown).
On the crossmodal portion of the test (Fig. 4H), rats were tested for their ability to use information gained from a tactile experience during the sample phase to guide the exploration of visual stimuli during the test phase. Sal–sal control rats showed evidence of significant memory (t(22) = 4.42, p < 0.001), similar to results described previously (Jacklin et al., 2012; Winters and Reid, 2010). Sal–polyI:C rats failed to show significant memory as reflected by a mean DR not different from chance (t(1 9) = 1.06, p = 0.30). G31P–sal rats showed nearly significant memory on the test (t(18) = 2.03, p = 0.057), while G31P–polyI:C rats did show a significant memory (t(18) = 2.18, p = 0.043). ANOVA failed to reveal significant main effects of polyI:C, G31P or a significant polyI:C by G31P interaction. However, a separate analysis of the simple main effect of polyI:C treatment showed that sal–polyI:C rats were significantly impaired on the crossmodal test compared with sal–sal rats (t(41) = 2.22, p = 0.032).
3.5. Behavioral flexibility following exposure to polyI:C and G31P
3.5.1. Visual cue discrimination (Fig. 5)
Fig. 5.
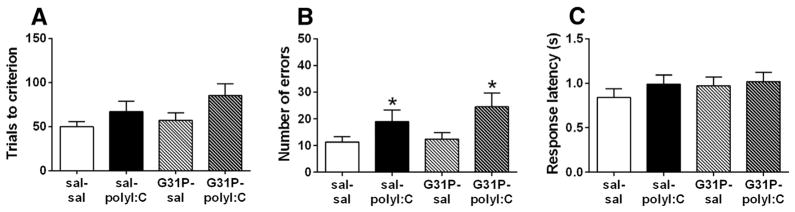
Visual cue discrimination of the offspring following maternal treatment with either polyI:C or G31P. (A) Trials to criterion, (B) total errors, and (C) average response latency. sal–sal, n = 23; sal–polyI:C, n = 20; G31P–sal, n = 17; and G31P–polyI:C, n = 20. Number of litters included in testing: sal–sal = 15, sal–poly = 11, G31P–sal = 10, and G31P–poly = 11. * indicates main effects of polyI:C treatment for the visual cue total errors.
After being trained to press the levers for food reward, rats were trained to press the lever located under a visual cue (a light) to receive food reward. PolyI:C treatment increased the number of trials to criterion (Fig. 5A) for this initial discrimination from ~55 to ~72 and the total number of errors (Fig. 5B) committed from ~13 to ~20. Analysis of the data revealed a significant effect for total errors (F(1,76) = 4.76, p = 0.032) but not trials to criterion (F(1,76) = 2.92, p = 0.091). The main effects of G31P and the polyI:C by G31P interactions were n.s. Importantly, latency to respond to the visual cue was not affected by either treatment (Fig. 5C, statistics not shown).
3.5.2. Strategy set-shifting (Fig. 6)
Fig. 6.
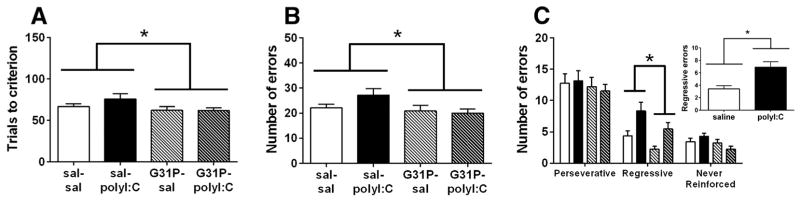
The effects of maternal polyI:C and G31P treatment on strategy set shifting from visual cue to response. (A) Trials to criterion, (B) total errors, and (C) analysis of error subtypes. The inset on panel C depicts the main effect of polyI:C on regressive errors. sal–sal, n = 23; sal–polyI:C, n = 21; G31P–sal, n = 17; and G31P–polyI:C, n = 20. Number of litters included in testing: sal–sal = 15, sal–poly = 11, G31P–sal = 10, and G31P–poly = 11. * indicates main effects of G31P treatment on trials to criterion, total errors, and regressive errors.
The day following visual cue discrimination, rats were required to respond to a lever on one side of the operant chamber, regardless of the location of the visual cue, for food reward. When trials to criterion (Fig. 6A) and total errors (Fig. 6B) were analyzed, polyI:C treatment increased both measures, although the effects were not significant (trials: F(1,77) = 2.56, p = 0.11; total errors: F(1,77) = 2.21, p = 0.14). G31P treatment significantly decreased the average number of trials to criterion (F(1,77) = 6.50, p = 0.013) and total errors (F(1,77) = 10.13, p = 0.002) during the strategy set-shift. There were no significant interactions between the treatments for either trials to criterion or total errors (statistics not shown).
Analysis of the error subtypes revealed significant main effects of both polyI:C and G31P with no interactions (Fig. 6C). The number of perseverative errors was not altered by either polyI:C (F(1,77) = 0.03, p = 0.86) or G31P treatment (F(1,77) = 1.31, p = 0.26); however, polyI:C treatment significantly increased (F(1,77) = 11.56, p < 0.001) while G31P treatment significantly reduced (F(1,77) = 5.74, p = 0.019) the number of regressive errors. In addition, G31P treatment significantly reduced never reinforced errors (F(1,77) = 6.86, p = 0.01) while polyI:C treatment had no effect (F(1,77) = 0.14, p = 0.71).
3.5.3. Reversal learning (Fig. 7)
Fig. 7.
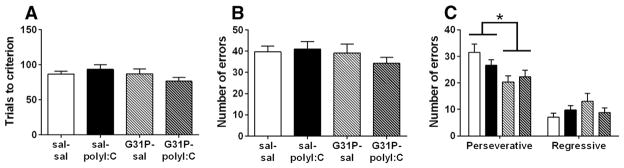
Performance of the reversal learning task following maternal polyI:C and G31P treatment. (A) Trials to criterion, (B) total errors, and (C) analysis of error subtypes. sal–sal, n = 24; sal–polyI:C, n = 20; G31P–sal, n = 18; and G31P–polyI:C, n = 21. Number of litters included in testing: sal–sal = 15, sal–poly = 11, G31P–sal = 10, and G31P–poly = 11. * indicates a main effect of G31P treatment on regressive errors.
On the final day of the operant battery, rats were required to perform a reversal and respond on the lever opposite to the one they were trained on the previous day. PolyI:C treatment had no effect on any measure of reversal learning (statistics not shown). G31P treatment did not significantly affect trials to criterion (Fig. 7A) or total (Fig. 7B). When perseverative and regressive error subtypes were analyzed separately (Fig. 7C), a significant reduction in the former subtype (F(1,79) = 8.80, p = 0.004) but not that latter (F(1,79) = 3.11, p = 0.081) was found as a result of the G31P treatment.
4. Discussion
In this study, we demonstrated novel effects of polyI:C and G31P treatments on the inflammatory profile of the dams and behavior of the offspring. Treating pregnant rats with polyI:C increased the concentration of circulating CXCL1, TNF, and IL-1β in the maternal serum 3 h after treatment (Fig. 1). In behavioral experiments, the offspring of polyI:C-treated dams displayed subtle impairments in PPI (Fig. 3), impaired associative and crossmodal recognition memory (Fig. 4), and impairments in the visual cue and set-shifting components of the operant task battery (Figs. 5, 6). G31P failed to consistently reverse the polyI: C-induced behavioral impairments; however, it did alter PPI during adolescence (Fig. 3) and enhance set-shifting and reversal learning during young adulthood (Figs. 6, 7).
4.1. Inflammatory response of pregnant rodents to polyI:C treatment
To our knowledge, this is the first study to use multiplex assays to examine the maternal immune response to polyI:C treatment in pregnant rats, in particular the Long–Evans strain. PolyI:C (4.0 mg/kg, i.v.) increased the levels of several pro-inflammatory cytokines including IL-1β, TNF, and CXCL1 in maternal serum. Treating mice with polyI:C (5 mg/kg, i.v.) dramatically increased the maternal serum levels of IL-1β, IL-6, TNF, and IL-10 3 h after treatment (Meyer et al., 2006a,b) and an array of cytokines 6 h after treatment (Meyer et al., 2006a,b). Of particular relevance is the analyses of chemokines in mice following polyI:C treatment (GD 16, 20 mg/kg, i.p.) performed by Arrode-Bruses and Bruses (2012). PolyI:C increased levels of several chemokines in maternal serum 6 h after treatment, including CXCL1 (KC, 276% increase) and CXCL2 (MIP-2, 108% increase). Although we failed to find a significant effect of polyI:C treatment on CXCL2 in rats, we observed a similar magnitude of response (154%, Fig. 1B). Previous research using Sprague–Dawley rats showed that polyI:C treatment on GD 9 significantly increases TNF and IL-10 (Song et al., 2011); however, we did not observe a significant effect of polyI:C on IL-10 levels in dams treated on GD 15. This discrepancy may be explained by the age of treatment as IL-10 levels 3 h after polyI:C treatment are significantly more enhanced at earlier (GD 9) than later (GD 17) stages of pregnancy in mice (Meyer et al., 2006b). Given the important role of IL-6 in the behavioral effects of polyI:C treatment in mice (Smith et al., 2007), it was surprising that the two-fold increase of IL-6 in the maternal serum was not significantly higher than the control levels in the present experiments. Male rats injected with polyI:C (0.75 mg/kg, i.p.) show elevated IL-6 in serum 2, but not 4, h after polyI:C (Fortier et al., 2004). Thus, our sampling time may not have been optimal to observe an increase in IL-6. Following subcutaneous injection of polyI:C (1.0 mg/kg) into the tail, Kamerman et al. (2011) showed increased CXCL1, but not IL-6, with a 3 h delay. Thus, the route of administration, dose of polyI:C used, timing of sample collection, and species tested may be important factors that affect the pattern of cytokine elevations observed. While statistically significant, the modest increases of IFNγ, IL-1α and IL-2 in maternal serum following G31P treatment were unanticipated and may not be biologically significant. Previous research in rats shows that G31P does not increase IL-1β, IL-6, or CXCL2 in rat lung or jejunum (Zhao et al., 2010), which is consistent with our findings from maternal serum. Therefore, systemic G31P treatment does not cause the release of proinflammatory mediators in rats.
PolyI:C and lipopolysaccharide increase cytokines including IL-1β, IL-6 and TNF in the placenta and fetus in the hours following MIA (Ashdown et al., 2006; Gilmore et al., 2005; Hsiao and Patterson, errors 2011; Meyer et al., 2006a,b). Low doses of lipopolysaccharide also increase CXCL1 in the placenta of mice (Carpentier et al., 2011); however, it is unknown whether increased CXCL1 in the maternal serum following polyI:C also enters the placenta and ultimately the fetus in rats. In mice, significant increases in chemokines in fetal brain were not as widespread 6 h after polyI:C treatment as in the maternal serum, although CXCL1 and CXCL2 could not be detected (Arrode-Bruses and Bruses, 2012). Interestingly, changes in CXCL1 during the postnatal period in the hippocampus but not cortex have been shown following polyI:C treatment of pregnant mice (Garay et al., 2013), thus raising the possibility that long-term dysregulation of the immune system may occur following MIA. As direct actions of cytokines on the placenta and fetal brain are hypothesized as critical for the long-term effects of MIA on cognition (Ashdown et al., 2006; Hsiao and Patterson, 2011; Smith et al., 2007), measurements of cytokine responses in these tissues will be conducted in future experiments.
4.2. Effects of polyI:C on behavior of the offspring
The behavioral experiments revealed an array of long-term changes in the offspring following polyI:C and G31P treatments. The disruptive effect of polyI:C on PPI was smaller in this study than previously reported by our laboratory in Long–Evans rats (Howland et al., 2012). Other groups have reported deficits in PPI in Sprague–Dawley and Wistar rats following polyI:C treatment (Dickerson et al., 2010; Klein et al., 2013; Mattei et al., 2014; Wolff and Bilkey, 2008; Wolff and Bilkey, 2010), although Fortier et al. (2007) failed to find disrupted PPI in the offspring following maternal polyI:C treatment in Sprague–Dawley rats.
The effects of polyI:C were more pronounced for recognition memory, as polyI:C exposure significantly impaired memory on the OIP test. Comparison of the animals’ DRs to chance performance (DR = 0) showed that sal–sal rats demonstrated significant memory, while sal–polyI:C rats did not, which replicates previous findings (Howland et al., 2012) and validates the polyI:C model for studying the associative recognition memory impairments seen in schizophrenia (Wood et al., 2002). PolyI:C treatment also selectively impaired multisensory integration, as sal–polyI:C rats showed significant memory on the visual and tactile tests, but were impaired on the crossmodal test. This finding compliments a recent report showing an impaired crossmodal recognition memory following acute treatment with an NMDA receptor antagonist (Jacklin et al., 2012), and indicates that the polyI:C model is useful for studying the developmental factors that may alter multisensory integration in schizophrenia (Stekelenburg et al., 2013; Stone et al., 2011; Williams et al., 2010). Similar to previous observations in Long–Evans rats (Howland et al., 2012), polyI:C treatment did not alter “simpler” forms of recognition memory in the visual or tactile tests. Thus, it is unlikely that alterations in perception or other non-specific factors underlie the impairments observed in the OIP or crossmodal tests.
The effects of polyI:C treatment on the operant test battery were different than those we reported previously (Zhang et al., 2012). In the present experiments, polyI:C treatment significantly increased the number of errors made during visual cue discrimination and increased the number of regressive errors made during the set-shifting phase of the battery. In our earlier study, we found no effect of polyI:C treatment on visual cue discrimination but a significant increase in trials to criterion and perseverative errors during set-shifting in the male offspring of polyI:C-treated dams (Zhang et al., 2012). Consideration of these data along with the PPI data suggests that this group of offspring was more mildly affected by polyI:C treatment. While the specific factors that led to this pattern of results are unclear, others have noted that body weight change in the dams following polyI:C treatment is predictive of the phenotype of the offspring (Bronson et al., 2011). Since the body weight changes observed in the dams following polyI:C treatment are of a similar initial magnitude between this data set and the other studies from our group (Howland et al., 2012; Sangha et al., 2014; Zhang et al., 2012), other non-specific factors may have contributed. For example, the timed pregnant dams were shipped from a remote supplier on GD 7. Prenatal stress in rodents induces behavioral and neurochemical changes in the offspring associated with psychiatric illness-like symptoms (Koenig et al., 2002; Meyer et al., 2009). While the potential effects of stress related to shipment on the dams and fetuses cannot be determined, similar performance of the same tasks by our saline-treated offspring and rats in other studies (Barker et al., 2007; Floresco et al., 2009; Howland et al., 2012; Winters and Reid, 2010; Zhang et al., 2012) suggest that transportation did not affect the offspring.
4.3. Effects of G31P on behavior of the offspring
G31P was administered during pregnancy to block the effects of maternal polyI:C administration on the offspring. G31P treatment did not affect maternal weight loss after polyI:C treatment, litter size, or average pup weight the day after birth (Fig. 2) suggesting that it did not cause gross alterations in pregnancy or pup development. However, G31P did not convincingly reverse the behavioral impairments in PPI, OIP recognition memory, or the operant battery caused by polyI:C. In the crossmodal task, memory significantly greater than chance was noted in the G31P–polyI:C treated rats, although the performance of this group was not significantly better than the sal–polyI:C-treated rats when they were compared directly. Thus, the effectiveness of G31P at blocking the effects of maternal polyI:C treatment should be characterized as mild at best. While our results do not support a role of maternal CXCR1/R2s in the effects of polyI:C on the offspring, an alternative possibility is that signaling of fetal CXCR1/R2s is critical for the behavioral effects observed. If elevated CXCL1 is detected in the fetus in future experiments, the transport of G31P through the placenta will also be tested as blocking fetal CXCR1/R2s may be necessary to prevent the behavioral phenotype observed. It is worth noting that human IL-8, the molecule on which G31P is based, does not travel across human placentas obtained from pregnancies at term (Aaltonen et al., 2005; Reisenberger et al., 1996), although its transport in rodents has not been determined.
Surprisingly, G31P enhanced PPI during adolescence and behavioral flexibility during the set-shifting and reversal learning tasks in the operant test battery. While the mechanism underlying these behavioral effects is not known, it is possible that the small increases in IFNγ, IL-1α and IL-2 observed in the maternal serum impacted behavior of the offspring. Cytokines are involved in behavioral flexibility as IL-6 signaling in the prefrontal cortex contributes to reversal learning (Donegan et al., 2014). However, in our experiments, the effects of CXCR1 and CXCR2 blockade following G31P administration would have to persist from in utero to young adulthood. Determining the reliability and specific mechanism underlying this effect will require future research.
4.4. General conclusions
The present experiments quantified the effects of maternal treatment with the viral mimetic polyI:C and the chemokine receptor antagonist G31P on the behavior of Long–Evans rat offspring. PolyI:C treatment triggered a significant acute inflammatory event, which included an elevation of the chemokine CXCL1 in maternal serum. The offspring of polyI: C-treated rats exhibited behavioral impairments consistent with the most prominent cognitive symptoms of schizophrenia including impairments of PPI, associative recognition memory, multisensory integration, and strategy set-shifting. Blocking the signaling of CXCR1/R2 failed to convincingly reverse the polyI:C-induced impairments but did alter PPI during adolescence and behavioral flexibility during young adulthood. Given the reported association between IL-8 levels in the maternal serum during pregnancy and schizophrenia in the offspring (Brown et al., 2004; Ellman et al., 2010), further investigation of the effects of ELR-CXC chemokine signaling during early development may increase the understanding of the environmental risk factors for the disorder.
Acknowledgments
This work was supported by an operating grant from the Canadian Institutes for Health Research (CIHR)-Saskatchewan Health Research Foundation Regional Partnership Program to JGH (125984). JGH is a CIHR New Investigator. SAB was supported by a CIHR Master’s Student Award and a Graduate Student Fellowship from the University of Saskatchewan. The authors would like to acknowledge the expert technical assistance of Brittany N. Cazakoff, Brittany D. Klischuk, Naila Kuhlmann, and Chester A. Thai for data collection during these experiments.
Abbreviations
- ANOVA
analysis of variance
- DR
discrimination ratio
- GD
gestational day
- IL
interleukin
- MIA
maternal immune activation
- OIP
object-in-place
- PND
postnatal day
- polyI:C
polyionosinic-polycytidylic acid
- PPI
prepulse inhibition
- SEM
standard error of the mean
- TNF
tumor necrosis factor
- TTC
trials to criterion
References
- Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A. Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol. 2005;106:802–7. doi: 10.1097/01.AOG.0000178750.84837.ed. [DOI] [PubMed] [Google Scholar]
- Arias I, Sorlozano A, Villegas E, de Dios LJ, McKenney K, Cervilla J, et al. Infectious agents associated with schizophrenia: a meta-analysis. Schizophr Res. 2012;136:128–36. doi: 10.1016/j.schres.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Arrode-Bruses G, Bruses JL. Maternal immune activation by poly I:C induces expression of cytokines IL-1beta and IL-13, chemokine MCP-1 and colony stimulating factor VEGF in fetal mouse brain. J Neuroinflammation. 2012;9:83. doi: 10.1186/1742-2094-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11:47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011;232:196–9. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–57. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Bronson SL, Ahlbrand R, Horn PS, Kern JR, Richtand NM. Individual differences in maternal response to immune challenge predict offspring behavior: contribution of environmental factors. Behav Brain Res. 2011;220:55–64. doi: 10.1016/j.bbr.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161:889–95. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Dingman AL, Palmer TD. Placental TNF-α signaling in illness-induced complications of pregnancy. Am J Pathol. 2011;178:2802–10. doi: 10.1016/j.ajpath.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazakoff BN, Howland JG. AMPA receptor endocytosis in rat perirhinal cortex underlies retrieval of object memory. Learn Mem. 2011;18:688–92. doi: 10.1101/lm.2312711. [DOI] [PubMed] [Google Scholar]
- Cho C, Miller RJ. Chemokine receptors and neural function. J Neurovirol. 2002;8:573–84. doi: 10.1080/13550280290101003. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Dickerson DD, Wolff AR, Bilkey DK. Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. J Neurosci. 2010;30:12424–31. doi: 10.1523/JNEUROSCI.3046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan JJ, Girotti M, Weinberg MS, Morilak DA. A novel role for brain interleukin-6: facilitation of cognitive flexibility in rat orbitofrontal cortex. J Neurosci. 2014;34:953–62. doi: 10.1523/JNEUROSCI.3968-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman LM, Deicken RF, Vinogradov S, Kremen WS, Poole JH, Kern DM, et al. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr Res. 2010;121:46–54. doi: 10.1016/j.schres.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2011;69:432–41. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol Psychiatry. 2013;73:951–66. doi: 10.1016/j.biopsych.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;287:R759–66. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–7. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age-and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun. 2013;31:54–68. doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Fredrik JL, Vadlamudi S, Lauder JM. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology. 2004;29:1221–9. doi: 10.1038/sj.npp.1300446. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal–fetal unit of the rat. J Neuroimmunol. 2005;159:106–12. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Gordon JR, Li F, Zhang X, Wang W, Zhao X, Nayyar A. The combined CXCR1/CXCR2 antagonist CXCL8(3–74)K11R/G31P blocks neutrophil infiltration, pyrexia, and pulmonary vascular pathology in endotoxemic animals. J Leukoc Biol. 2005;78:1265–72. doi: 10.1189/jlb.0805458. [DOI] [PubMed] [Google Scholar]
- Gordon JR, Zhang X, Li F, Nayyar A, Zhao X. Amelioration of pathology by ELR-CXC chemokine antagonism in a swine model of airway endotoxin exposure. J Agromed. 2009;14:235–41. doi: 10.1080/10599240902845047. [DOI] [PubMed] [Google Scholar]
- Han X, Li N, Meng Q, Shao F, Wang W. Maternal immune activation impairs reversal learning and increases serum tumor necrosis factor-alpha in offspring. Neuropsychobiology. 2011;64:9–14. doi: 10.1159/000322455. [DOI] [PubMed] [Google Scholar]
- Howland JG, Hannesson DK, Phillips AG. Delayed onset of prepulse inhibition deficits following kainic acid treatment on postnatal day 7 in rats. Eur J Neurosci. 2004;20:2639–48. doi: 10.1111/j.1460-9568.2004.03731.x. [DOI] [PubMed] [Google Scholar]
- Howland JG, Cazakoff BN, Zhang Y. Altered object-in-place recognition memory, prepulse inhibition, and locomotor activity in the offspring of rats exposed to a viral mimetic during pregnancy. Neuroscience. 2012;201:184–98. doi: 10.1016/j.neuroscience.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25:604–15. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacklin DL, Goel A, Clementino KJ, Hall AW, Talpos JC, Winters BD. Severe cross-modal object recognition deficits in rats treated sub-chronically with NMDA receptor antagonists are reversed by systemic nicotine: implications for abnormal multisensory integration in schizophrenia. Neuropsychopharmacology. 2012;37:2322–31. doi: 10.1038/npp.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerman P, Skosana M, Loram L, Mitchell B, Weber J. Fever and inflammatory cytokine response in rats injected subcutaneously with viral double-stranded RNA analog, polyinosinic:polycytidylic acid (Poly-I:C) J Therm Biol. 2011;35:397–402. [Google Scholar]
- Klein J, Hadar R, Gotz T, Manner A, Eberhardt C, Baldassarri J, et al. Mapping brain regions in which deep brain stimulation affects schizophrenia-like behavior in two rat models of schizophrenia. Brain Stimul. 2013;6:490–9. doi: 10.1016/j.brs.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27:309–18. doi: 10.1016/S0893-133X(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–93. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Zhang X, Mizzi C, Gordon JR. CXCL8(3–73)K11R/G31P antagonizes the neutrophil chemoattractants present in pasteurellosis and mastitis lesions and abrogates neutrophil influx into intradermal endotoxin challenge sites in vivo. Vet Immunol Immunopathol. 2002;90:65–77. doi: 10.1016/s0165-2427(02)00223-4. [DOI] [PubMed] [Google Scholar]
- Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U, et al. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PLoS One. 2009;4:e6354. doi: 10.1371/journal.pone.0006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder SR, Fenton W. Measurement and treatment research to improve cognition in schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72:5–9. doi: 10.1016/j.schres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Mattei D, Djodari-Irani A, Hadar R, Pelz A, de Cossio LF, Goetz T, et al. Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun. 2014;38:175–84. doi: 10.1016/j.bbi.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. To poly(I:C) or not to poly(I:C): advancing preclinical schizophrenia research through the use of prenatal immune activation models. Neuropharmacology. 2012;62:1308–21. doi: 10.1016/j.neuropharm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal–foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun. 2006a;20:378–88. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of pre-natal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006b;26:4752–62. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009;33:1061–79. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophr Bull. 2010;36:607–23. doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Weiner I, McAlonan GM, Feldon J. The neuropathological contribution of prenatal inflammation to schizophrenia. Expert Rev Neurother. 2011;11:29–32. doi: 10.1586/ern.10.169. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res. 1999;37:251–70. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–21. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17:389–94. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Tracing the development of psychosis and its prevention: what can be learned from animal models. Neuropharmacology. 2012;62:1273–89. doi: 10.1016/j.neuropharm.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Ragozzino D. CXC chemokine receptors in the central nervous system: role in cerebellar neuromodulation and development. J Neurovirol. 2002;8:559–72. doi: 10.1080/13550280290100932. [DOI] [PubMed] [Google Scholar]
- Reale M, Patruno A, De Lutiis MA, Pesce M, Felaco M, Di GM, et al. Dysregulation of chemo-cytokine production in schizophrenic patients versus healthy controls. BMC Neurosci. 2011;12:13. doi: 10.1186/1471-2202-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisenberger K, Egarter C, Vogl S, Sternberger B, Kiss H, Husslein P. The transfer of interleukin-8 across the human placenta perfused in vitro. Obstet Gynecol. 1996;87:613–6. doi: 10.1016/0029-7844(95)00473-4. [DOI] [PubMed] [Google Scholar]
- Sangha S, Greba Q, Robinson PD, Ballendine SA, Howland JG. Heightened fear in response to a safety cue and extinguished fear cue in a rat model of maternal immune activation. Front Behav Neurosci. 2014;8:168. doi: 10.3389/fnbeh.2014.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savanthrapadian S, Wolff AR, Logan BJ, Eckert MJ, Bilkey DK, Abraham WC. Enhanced hippocampal neuronal excitability and LTP persistence associated with reduced behavioral flexibility in the maternal immune activation model of schizophrenia. Hippocampus. 2013;23:1395–409. doi: 10.1002/hipo.22193. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Wenqiang L, Yongfeng Y, Zhao J, Jiang C, Li W, et al. The nuclear factor-κB inhibitor pyrrolidine dithiocarbamate reduces polyinosinic–polycytidilic acid-induced immune response in pregnant rats and the behavioral defects of their adult offspring. Behav Brain Funct. 2011;7:1–9. doi: 10.1186/1744-9081-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stekelenburg JJ, Maes JP, Van Gool AR, Sitskoorn M, Vroomen J. Deficient multisensory integration in schizophrenia: an event-related potential study. Schizophr Res. 2013;147:253–61. doi: 10.1016/j.schres.2013.04.038. [DOI] [PubMed] [Google Scholar]
- Stone DB, Urrea LJ, Aine CJ, Bustillo JR, Clark VP, Stephen JM. Unisensory processing and multisensory integration in schizophrenia: a high-density electrical mapping study. Neuropsychologia. 2011;49:3178–87. doi: 10.1016/j.neuropsychologia.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart MJ, Baune BT. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: A systematic review of biomarker studies. Neurosci Biobeha Rev. 2014;42:93–115. doi: 10.1016/j.neubiorev.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Thai CA, Zhang Y, Howland JG. Effects of acute restraint stress on set-shifting and reversal learning in male rats. Cogn Affect Behav Neurosci. 2013;13:164–73. doi: 10.3758/s13415-012-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–55. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci. 2010;64:217–30. doi: 10.1111/j.1440-1819.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- Wei J, Peng J, Wang B, Qu H, Wang S, Faisal A, et al. CXCR1/CXCR2 antagonism is effective in pulmonary defense against Klebsiella pneumoniae infection. Biomed Res Int. 2013;2013:720975. doi: 10.1155/2013/720975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Light GA, Braff DL, Ramachandran VS. Reduced multisensory integration in patients with schizophrenia on a target detection task. Neuropsychologia. 2010;48:3128–36. doi: 10.1016/j.neuropsychologia.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Reid JM. A distributed cortical representation underlies crossmodal object recognition in rats. J Neurosci. 2010;30:6253–61. doi: 10.1523/JNEUROSCI.6073-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behav Brain Res. 2008;190:156–9. doi: 10.1016/j.bbr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. The maternal immune activation (MIA) model of schizophrenia produces pre-pulse inhibition (PPI) deficits in both juvenile and adult rats but these effects are not associated with maternal weight loss. Behav Brain Res. 2010;213:323–7. doi: 10.1016/j.bbr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Proffitt T, Mahony K, Smith DJ, Buchanan JA, Brewer W, et al. Visuospatial memory and learning in first-episode schizophreniform psychosis and established schizophrenia: a functional correlate of hippocampal pathology? Psychol Med. 2002;32:429–38. doi: 10.1017/s0033291702005275. [DOI] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cazakoff BN, Thai CA, Howland JG. Prenatal exposure to a viral mimetic alters behavioural flexibility in male, but not female, rats. Neuropharmacology. 2012;62:1299–307. doi: 10.1016/j.neuropharm.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Town JR, Li F, Zhang X, Cockcroft DW, Gordon JR. ELR-CXC chemokine receptor antagonism targets inflammatory responses at multiple levels. J Immunol. 2009;182:3213–22. doi: 10.4049/jimmunol.0800551. [DOI] [PubMed] [Google Scholar]
- Zhao X, Town JR, Yang A, Zhang X, Paur N, Sawicki G, et al. A novel ELR-CXC chemokine antagonist reduces intestinal ischemia reperfusion-induced mortality, and local and remote organ injury. J Surg Res. 2010;162:264–73. doi: 10.1016/j.jss.2009.04.047. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–23. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]