Abstract
Introduction
The selective serotonin reuptake inhibitor (SSRI), fluoxetine, leads to sexual dysfunction in a substantial proportion of women. In studies with the Fischer inbred rat, the 5-HT1A receptor has been implicated in this sexual dysfunction. Whether this association with 5-HT1A receptors holds for other rat strains is not known.
Aim
The effects of acute fluoxetine on sexual behavior in two strains of rats that differ in their response to a 5-HT1A receptor agonist were examined. Whether the strain difference is comparable in naturally cycling and hormonally primed, ovariectomized rats was determined.
Main Outcome Measures
Lordosis to mount ratios, lordosis quality, and proceptive behaviors were quantified. Sprague-Dawley and Fischer females were compared on each of these measures. The IC50 for inhibition of lordosis behavior was determined.
Methods
Proestrous rats and ovariectomized rats, hormonally primed with estradiol benzoate and progesterone, were treated with varying doses of fluoxetine. Sexual behavior was examined before and after treatment with the SSRI.
Results
In both the intact and the hormonally-primed, ovariectomized model, Sprague-Dawley females were less sensitive to the effects of fluoxetine on sexual behavior. In both groups, fluoxetine showed dose-dependency in behavioral inhibition, but a higher dose was required for Sprague-Dawley than for Fischer females. Naturally cycling, proestrous rats required a higher dose of fluoxetine than hormonally-primed ovariectomized rats to produce significant inhibition of sexual behavior. Thus, the strain difference in the response to fluoxetine does not parallel strain differences in the response to a 5-HT1A receptor agonist.
Conclusions
Acute treatment with fluoxetine inhibits lordosis behavior in both Fischer and Sprague-Dawley females and the strain difference cannot be explained by reported strain differences in the response to a 5-HT1A receptor agonist. Fluoxetine’s inhibition of female rat sexual behavior may involve effects of the SSRI in addition to activation of the 5-HT1A receptor.
Keywords: fluoxetine, rat strains, female sexual behavior, SSRI, estrogen, progesterone
Introduction
After its original marketing in the 1980s, Prozac® (fluoxetine) became one of the most frequently prescribed medications for the treatment of depression and other disorders of special significance to women [1, 2]. Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) thought to exert its therapeutic effect, in part, through blocking of the serotonin transporter (SERT) and consequent reduction of serotonin reuptake into the nerve terminal [3–6]. However, in spite of the clinical effectiveness of SSRIs for the treatment of mood disorders, the 2 to 3 week delay between onset of treatment and clinical effectiveness, coupled with the emergence of sexual side effects, can lead patients to discontinue treatment prior to relief from their original clinical symptoms [7–9].
Approximately 30–50% of women taking antidepressants experience some kind of sexual dysfunction [7, 10] and there is a higher probability of such sexual side effects with SSRI treatments leading to suggestions that the sexual dysfunction may involve the drugs’ effects on the serotonergic system [7, 11, 12]. However, compared to research on male sexual dysfunction, investigation of the mechanisms responsible for SSRI-induced female sexual dysfunction has been limited and it is not clear why some females experience such sexual side effects while others do not [13]. From animal models, it is well established that manipulations, including fluoxetine, that elevate CNS serotonin have inhibitory effects on female rat sexual behavior [14–16] and activation of the serotonin 1A (5-HT1A) receptor has been implicated in 5-HT-mediated sexual inhibition [15]. Activation of the 5-HT1A receptor also contributes to the acute effect of fluoxetine on female rat sexual behavior [17]. However, this association may not hold for the SSRI, paroxetine [18], and whether or not this relationship holds across rat strains has not been determined. In fact, the possibility of rat strain differences in fluoxetine’s effect on sexual behavior has received little investigation.
There is, though, evidence that rat and mouse strains vary in their response to several effects of fluoxetine [19–22] and fluoxetine-induced disruption of the female rat’s estrous cycle is more prevalent in the Fischer inbred strain than the Sprague-Dawley strain [23, 24]. In contrast, Sprague-Dawley females show greater sensitivity than Fischer females to the disruptive effects of a 5-HT1A receptor agonist on sexual behavior [25]. This is surprising since the Sprague-Dawley strain that is more sensitive to the 5-HT1A receptor agonist would have been expected to exhibit the greater vulnerability to sexual side effects of fluoxetine. However, it is important to note that effects of a 5-HT1A receptor agonist were investigated in hormonally-primed ovariectomized rats while strain differences in the effects of fluoxetine on estrous cyclicity were investigated in naturally cycling female rats. Therefore, it is possible that the direction of the strain difference in the female’s response to fluoxetine is not the same in the naturally cycling and hormonally-primed, ovariectomized model. The following experiments were designed to investigate this possibility. Portions of these studies were previously reported at the Annual Meeting of the Society for Neuroscience [26].
Materials and Methods
Materials
Estradiol benzoate (EB), progesterone (P), sesame seed oil, and the selective serotonin reuptake inhibitor, methyl [3-phenyl-3–4-(trifluoromethyl)-phenoxy] propyl] ammonium chloride (fluoxetine), were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Isoflurane (AErrane®) and suture materials were purchased from Butler Schein Animal Health (Dublin, OH). Food (Rodent Lab Diet 5001) was purchased from Lab Animal Supply (Highland Village, Texas). All other supplies were purchased from Fischer Scientific (Houston, TX).
General Methods
All procedures were in accordance with the NIH Guide for the Care and Use of Animals in Research and were approved by the Institutional Animal Care and Use Committee at Texas Woman’s University.
Animals and Housing Conditions
Female Fischer and Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) and housed 2 per cage in standard shoebox caging (45.72 × 24.13 × 2.59 cm). The housing area was maintained at 25 °C and 55% humidity with lights on from 12:00 midnight to 12:00 noon. Food and water were available ad lib. Age of the animals varied within experiments but was always matched between Fischer and Sprague-Dawley rats and was counterbalanced across treatment conditions.
Behavioral Testing Procedures
On the day of testing, rats were pretested for sexual behavior during the dark portion of the light/dark cycle (between 2 and 4 pm) as previously described [27]. Females were sexually naïve prior to testing and were used only a single time. Rats were placed into the home cage of a sexually active Sprague-Dawley male and behavior was monitored until 10 mounts had occurred. Females were then injected with fluoxetine and tested 30 min later for Experiments 1, 2, and 4 or after 5 min for Experiment 3. Sexual receptivity was measured as the lordosis/mount (L/M) ratio (number of lordosis reflexes by the female divided by the number of mounts by the male). Lordosis quality was measured on a scale of 1 to 4 as previously described [27] and was computed as the sum of individual lordosis quality scores divided by the number of lordosis responses. Rats with only minor arching of the back received a score of 1.0; an intermediate arch without head elevation was scored as 2.0; a standard arch with elevation of the head was scored as 3.0; and an exaggerated arch with elevation of the front legs was scored as 4.0. If the female failed to lordose, no quality score was given. The number of mounts by the male was recorded to assure that females received comparable stimuli. Proceptivity was measured by the presence of hopping and darting behavior. For L/M ratios, lordosis quality, and mounts, data were recorded for the pretest and consecutive intervals after fluoxetine. For proceptivity, the presence or absence of the behavior was recorded and females were categorized as either proceptive or not proceptive.
Statistical Procedures
For Experiments 1 and 2, L/M ratios, lordosis quality scores and number of mounts were computed for the pretest and the 15 min test period after fluoxetine. Data were evaluated by repeated measures ANOVA with strain and dose as independent factors and before or after fluoxetine as the repeated factor. Data for experiments 3 and 4 were divided into consecutive 5 min (Experiment 4) or 10 min (Experiment 3) intervals and were evaluated by repeated measures ANOVA with time relative to the fluoxetine injection as the repeated factor and strain as the independent factor. Proceptivity data were compared by Chi-Square or Fisher’s Exact Test procedures. In the first 2 experiments, L/M data were subjected to regression on dose for determination of the relationship between dose and L/M ratios and for estimation of the IC50. IC50, the dose that leads to 50% inhibition of the maximum effect [28], was defined as an L/M ratio of 0.5. SPSS versions 15.0 (for PC) or 17.0 (for Macintosh) were used for data analysis. Post-hoc Tukey test comparisons were performed manually and were limited a priori to (a) comparisons of data before and after fluoxetine (within treatment) and (b) comparison of strains (within dose and test interval) [29]. An alpha level of 0.05 was required for rejection of the null hypothesis. The statistical reference was Zar [29].
Specific Methods
Experiment 1: Intact, proestrous rats
Fischer and Sprague-Dawley rats were age-matched for the experiment and were 17–20 weeks of age at the time of testing. Approximately 2 weeks after their arrival at TWU, vaginal cyclicity was monitored daily as previously described [27]. Females showing at least two consecutive 4 to 5 day estrous cycles were tested on the afternoon of proestrus. Proestrus was defined as a relative scarcity of leukocytes and a high density of nucleated and cornified cells. Animals were selected on each test day on the basis of their prior vaginal smear history and their vaginal smear on the day of testing with the additional requirement that Fischer and Sprague-Dawley females be represented on every testing day.
On the day of testing, the means ± S.E. body weights of intact Fischer and Sprague-Dawley rats were 160.1 ± 1.8 and 264.9 ± 4.6 g, respectively. On the afternoon of proestrus, females were pretested for sexual behavior in the home cage of a sexually active male and immediately injected intraperitoneally (ip) with 10, 15, 20 or 30 mg/kg of fluoxetine hydrochloride. Thirty min later, females were again tested in the home cage of a sexually active male. Testing continued for 15 consecutive min. Data were compared for the pretest (before fluoxetine) and a 15 min test period 30 through 45 min after fluoxetine.
Experiment 2: Hormonally primed, ovariectomized rats
Approximately two weeks after their arrival, females were ovariectomized under AErrane® anesthesia as previously described [27]. Ten to 14 days later, rats were hormonally primed with estradiol benzoate (EB) followed 48 hr later with progesterone (P). Because Fischer and Sprague-Dawley females differ in body weight, hormones were administered by body weight (0.067 μg/g EB and 3.333 μg/g P) and were the same doses as previously used in the strain comparison with a 5-HT1A receptor agonist [25]. These doses are roughly equivalent to 10 μg EB and 500 μg P for a 150 g Fischer female. EB and P were dissolved in sesame seed oil and administered subcutaneously (sc) in a volume of 0.1 ml/150 g body weight. On the day of testing, Fischer and Sprague-Dawley females were 15–19 weeks of age and weighed 168.8 ± 1.4 and 276.2 ± 3.4 g, respectively.
Immediately following the pretest, females were injected with 5, 10, 15 or 20 mg/kg fluoxetine. A lower dose range was chosen for ovariectomized females because of an expectation that the ovariectomized rats would be more sensitive to the effects of fluoxetine. This expectation was derived from prior work demonstrating that sexual behavior of naturally cycling females is more resistant to disruption by both chemical and experimental factors than is that of hormonally-primed ovariectomized rats [15, 25, 30]. Although exogenous hormonal treatment is effective in priming for sexual behavior, it does not replicate the endogenous pattern of hormonal secretion that is probably optimal for maintenance of reproductive activity. Thirty min after fluoxetine, rats were retested for sexual behavior as in Experiment 1. Data were compared before and for 15 consecutive min of testing 30 through 45 min after fluoxetine.
Experiment 3: Rapid effects of fluoxetine in hormonally primed, ovariectomized rats
Females were ovariectomized and hormonally primed as for Experiment 2. Rats fell into two different age groups (14–15 weeks or 20 weeks) and all treatment conditions were represented in both age groups. Fischer and Sprague-Dawley females weighed 160.2 ± 3.07 and 257.2 ± 7.94, respectively, on the day of testing. After the pretest for sexual behavior, females were injected with 15 mg/kg fluoxetine. Five min later, rats were tested for sexual behavior for 40 consecutive min (to allow overlap with rats tested 30 min after fluoxetine). Data were computed for the pretest and for the 4 continuous 10 min intervals after initiation of testing. Sexual behavior and motor disturbances were monitored. Females were judged to exhibit motor disturbance when they showed immobility, disordered gait, or difficulty with ambulation. L/M ratios and lordosis quality were compared by repeated measures ANOVA with test interval as the repeated factor and strain as the independent factor. Motor disturbance was evaluated by Chi-Square procedures.
Experiment 4: Effect of hormonal priming per rat
In Experiments 2 and 3, hormonal priming was based on body weight so that the larger Sprague-Dawley females received higher absolute amounts of hormones. Both estradiol and progesterone can influence the serotonergic system [31] and progesterone can reduce effects of fluoxetine on lordosis behavior [17]. Therefore, in the final study, rats were treated as described for Experiment 2 but doses of 10 μg EB and 500 μg P per rat were given to both strains. EB and P were dissolved in sesame seed oil and administered subcutaneously (sc) in a volume of 0.1 ml/rat. At the time of testing, rats were 18–20 weeks of age and weighed 166.8 ± 2.23 and 280.1 ± 5.47, respectively, for Fischer and Sprague-Dawley females. After the pretest for sexual behavior, rats were injected with 15 mg/kg fluoxetine. Behavioral testing was initiated 30 min after progesterone and continued for 15 consecutive min. Data were computed for the pretest and 3 continuous 5-min intervals after fluoxetine and were analyzed by repeated measures ANOVA as for Experiment 3.
Outcome measures
Lordosis to mount ratios, lordosis quality, and proceptive behaviors were quantified. Hormonally-primed and naturally-cycling Sprague-Dawley and Fischer females were compared on each of these measures. The IC50 for inhibition of lordosis behavior was determined.
Results
Experiment 1: Intact, proestrous females
In intact, proestrous females, there was a significant main effect of strain (F1, 40 = 10.71, p ≤ 0.002) and dose on L/M ratios after fluoxetine (Figure 1; F3, 40 = 6.77, p ≤ 0.001). Strains did not differ in L/M ratios before fluoxetine. L/M ratios declined after fluoxetine leading to a significant effect of time (e.g. before or after fluoxetine) that was due primarily to effects in Fischer females (F1, 40 = 45.68, p ≤ 0.001). There was a significant time by strain interaction (F1,40 = 11.55, p ≤ 0.002) as well as a significant time by dose interaction (F3,40 = 7.23, p ≤ 0.001). L/M ratios of Fischer rats were significantly different from their pretest after 15, 20 and 30 mg/kg fluoxetine (q40,4, respectively, = 4.03, 6.59 and 8.99, p ≤ 0.05). In contrast, L/M ratios of Sprague-Dawley females differed from their pretest only after 30 mg/kg (q40,4 = 4.64, p ≤ 0.05). L/M ratios of Fischer rats were significantly lower than those of Sprague-Dawley rats at the 20 and 30 mg/kg doses (respectively, q40,4 = 5.50 and 4.35, p ≤ 0.05).
Fig 1. Fluoxetine dose-dependently decreased L/M ratios in intact rats.

Proestrous Fischer (F) and Sprague-Dawley (S-D) females were pretested for sexual behavior and then injected ip with 10, 15, 20 or 30 mg/kg fluoxetine. Thirty min later, sexual behavior was again monitored for 15 consecutive min. Data are the mean ± S.E. lordosis/mount (L/M) ratios before (PRE) and for the 15 min after fluoxetine. Ns for Fischer rats at 10, 15, 20 and 30 mg/kg fluoxetine, respectively, were 6, 7, 6 and 6; Ns, respectively, for Sprague-Dawley rats were 6, 6, 5, and 6. * indicates significant difference between Fischer and Sprague-Dawley rats within dose of fluoxetine; † indicates significant difference from the pretest, within strain.
There was a significant linear relationship between dose and L/M ratios for both strains (for Fischer and Sprague-Dawley, respectively, r = 0.78 and 0.52, p ≤ 0.001). The IC50 for Fischer and Sprague-Dawley females, respectively, was 26.0 and 64.4 mg/kg.
Females that failed to show lordosis behavior were omitted from the analysis for lordosis quality. For the remaining rats, fluoxetine had relatively minor effects (data not shown). There was a significant effect of dose (F3,38 = 3.43, p ≤ 0.026) and an interaction between strain and dose (F3,38 = 7.14, p ≤ 0.001) due to a lower lordosis quality in Fischer females after 15 mg/kg fluoxetine and in Sprague-Dawley females after 30 mg/kg; but even after 30 mg/kg, Fischer and Sprague-Dawley rats, respectively, showed relatively high lordosis quality (average scores of 2.79 ± 0.09 and 2.27 ± 0.20).
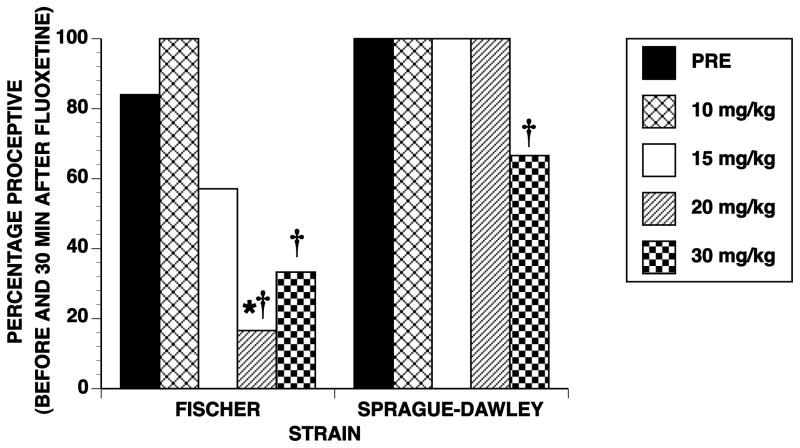
Prior to injection with fluoxetine, 84% of Fischer and 100% of Sprague-Dawley females showed proceptive (hopping and darting) behavior. In both strains, fluoxetine dose-dependently reduced proceptivity (Chi Square values for Fischer and Sprague-Dawley, respectively, were 28.72 and 40.62, df = 4, p ≤ 0.001; see Figure 2). However, Fischer females were affected at a lower dose than Sprague-Dawley females. Fischer females were significantly different from their pretest after 20 and 30 mg/kg fluoxetine while Sprague-Dawley females differed only after 30 mg/kg. As a consequence, the strains differed significantly only at 20 mg/kg fluoxetine (Chi Square = 7.63, df = 1, p ≤ 0.006).
Fig 2. Dose-dependent effects of fluoxetine on proceptivity of intact rats.
Data are for the same rats shown in Figure 1 and are the percentage of rats that showed evidence of proceptivity before (PRE) and after fluoxetine treatment. * indicates significant strain differences within dose of fluoxetine. † indicates significant difference from the pretest, within strain.
The strain difference in lordosis behavior was not due to differential mounting by the males (p > 0.05). The mean ± S.E. number of mounts during the 15 min after injection for Fischer and Sprague-Dawley rats, respectively, for 10, 15, 20 and 30 mg/kg fluoxetine, were: 14.83 ± 2.52, 19.28 ± 3.03, 24.3 ± 3.91, 18.5 ± 3.62 and 15.16 ± 1.51, 17.16 ± 2.27, 21.0 ± 2.9, 17.66 ± 4.57.
Experiment 2: Hormonally primed, ovariectomized females
Strain differences in hormonally-primed, ovariectomized rats were similar to those of intact females with Fischer females showing the greater decline in L/M ratios after fluoxetine (ANOVA for strain, F1, 54 = 5.27, p ≤ 0.026; see Figure 3). There was also a significant main effect of dose (F3, 54 = 9.39, p ≤ 0.001) and a strain by dose interaction (F3, 54 = 3.08, p ≤ 0.035). The time-dependent decline (before versus after fluoxetine) in L/M ratios (F3, 54 = 94.59, p ≤ 0.001) interacted significantly with both strain (F1, 54 = 14.65, p ≤ 0.001) and dose (F3, 5 = 14.11, p ≤ 0.001), and there was a significant 3-way interaction (F3, 54 = 2.82, p ≤ 0.048). L/M ratios of Fischer females were significantly different from their pretest after 10, 15 and 20 mg/kg fluoxetine (all q54,4 ≥ 4.97, p ≤ 0.05) while L/M ratios of Sprague-Dawley females differed from their pretest only after 20 mg/kg (q54,4 = 5.39, p ≤ 0.05). L/M ratios of the two strains differed significantly after 15 and 20 mg/kg (respectively, q54,4 = 4.15 and 8.16, p ≤ 0.05).
Fig 3. Fluoxetine dose-dependently decreased L/M ratios in hormonally primed, ovariectomized rats.

Ovariectomized rats, hormonally primed with 0.067 μg EB/g body weight and 3.33 μg P/g body weight were pretested for sexual behavior 4 to 6 hr after P. Rats were then injected with 5, 10, 15, or 20 mg/kg of fluoxetine. Thirty minutes later, rats were again tested for 15 consecutive min. Data are the mean ± SE L/M ratios for the pretest (PRE) and 15 min after fluoxetine. The Ns for Fischer (F) and Sprague-Dawley (S-D) rats for 5, 10, 15 and 20 mg/kg fluoxetine, respectively, were 8, 8, 9 and 7 and 8, 7, 7 and 8. * indicates significant strain differences within dose of fluoxetine. † indicates significant difference from the pretest, within strain.
As for intact females, there were significant linear regressions between dose and L/M ratios (respectively, for Fischer and Sprague-Dawley rats, r = 0.82 and 0.56, p ≤ 0.001). The IC50 was 16.93 and 39.5 mg/kg for Fischer and Sprague-Dawley females, respectively.
Females that did not show lordosis after fluoxetine were omitted from the data analysis. For the remaining rats, lordosis quality decreased after fluoxetine (ANOVA for pre versus post, F1, 54 = 49.58, p ≤ 0.001; data not shown) and there was a significant interaction between time and dose of fluoxetine (F3, 54 = 4.57, p ≤ 0.006). No other effects were significant. Lordosis quality after 20 mg/kg fluoxetine ranged from a high of 2.4 ± 0.198 to a low of 2.1 ± 0.75 in Fischer and Sprague-Dawley rats, respectively.
Before injection with fluoxetine, 65.62% of Fischer females and 80% of Sprague-Dawley females showed proceptive behavior which declined after fluoxetine (Chi Square = 9.44 and 12.65, respectively, for Fischer and Sprague-Dawley females, df = 4, p ≤ 0.05; see Figure 4). Proceptivity of Fischer females was lower than their pretest at every dose of fluoxetine but, because of relatively low proceptivity in the pretest, showed a significant difference only after 15 and 20 mg/kg of fluoxetine (Fisher’s Exact Test, p ≤ 0.03). Proceptivity of Sprague-Dawley females declined at 10, 15 and 20 mg/kg fluoxetine but was only significantly different from their pretest at 10 mg/kg (Fischer’s Exact Test, p ≤ 0.014). Since proceptivity declined in both strains, only after 5 mg/kg was there a significant strain difference in proceptivity (Chi Square = 5.33, df = 1, p ≤ 0.02) and this primarily reflected the 100% incidence of proceptivity in Sprague-Dawley females at this dose.
Fig 4. Dose-dependent effects of fluoxetine on proceptivity in hormonally primed, ovariectomized rats.
Data are for the same rats shown in Figure 3 and are the percentage of rats that showed evidence of proceptivity before (PRE) and after fluoxetine treatment. * indicates significant strain differences within dose of fluoxetine. † indicates significant difference from the pretest, within strain.
Strain differences in lordosis behavior did not result from differences in number of mounts from the male. The mean ± S.E. number of mounts during the 15 min after injection for Fischer and Sprague-Dawley rats, respectively, for 5, 10, 15 and 20 mg/kg fluoxetine, were 27.25 ± 2.84, 16.62 ± 1.51, 20.44 ± 1.56, 27.25 ± 4.33 and 19.37 ± 2.18, 25.57 ± 4.11, 23.28 ± 2.66, 18.75 ± 2.45. None of the main effects were significant but there was a significant strain by dose interaction (F3, 54= 3.58 ≤ 0.020) due to slightly fewer mounts of Sprague-Dawley females at 5 mg/kg and slightly more mounts at 10 mg/kg.
Ovariectomized rats appeared to be more sensitive than intact rats to the lordosis-inhibiting effects of fluoxetine. Therefore, a post facto comparison of the two data sets was performed on the L/M ratios after injection with 10, 15 or 20 mg/kg fluoxetine (the doses present in both Experiments 1 and 2). There was the expected main effect of strain (F1,70 = 19.98, ≤ 0.001), dose (F2,70 = 8.32, p ≤ 0.001) and their interaction (F2,70 = 5.03, p ≤ 0.009). As anticipated from the dose response analyses for Experiments 1 and 2, type of animal (e.g. ovariectomized or intact) was also a significant factor (F1,70 = 14.86, p ≤0.001)
Experiment 3: Rapid effects of fluoxetine in hormonally primed, ovariectomized rats
The next experiment was designed to determine if strain differences examined 30 min after fluoxetine resulted from different rates of recovery from the immediate effects of fluoxetine on motor function. Continuous behavioral monitoring began 5 min after injection with 15 mg/kg fluoxetine. As expected, females showed motor disturbances, characterized primarily by immobility, early after treatment (see Table 1). Surprisingly, a greater proportion of Sprague-Dawley females showed evidence of motor disturbance than did Fischer females; but this difference was not significant (Chi Square, p > 0.05). Probably because of this immobility (especially 10 to 20 min after injection), males expressed low interest in the females so that mounting did not occur for every female in every 5-min interval. In particular, 4 Sprague-Dawley and 1 Fischer female had no mounts during at least one 5-min test interval after treatment with fluoxetine so the data were collapsed into 10-min intervals for analysis.
Table 1.
Early effects of fluoxetine on the percentage of females showing motor disturbance and proceptivity
| Pretest | 10–15 minutes | 20–25 minutes | 30–35 minutes | 40–45 minutes | |
|---|---|---|---|---|---|
| Motor disturbance | |||||
| Fischer | 0 | 38.4 | 30.7 | 15.3 | 7.6 |
| Sprague-Dawley | 0 | 63.6 | 45.5 | 36.4 | 27.3 |
| Proceptivity | |||||
| Fischer | 76.9 | 18.18 | 0* | 23.07 | 8.3* |
| Sprague-Dawley | 100 | 20 | 36.36 | 45.45 | 54.45 |
Indicates significant difference between strains, within time interval
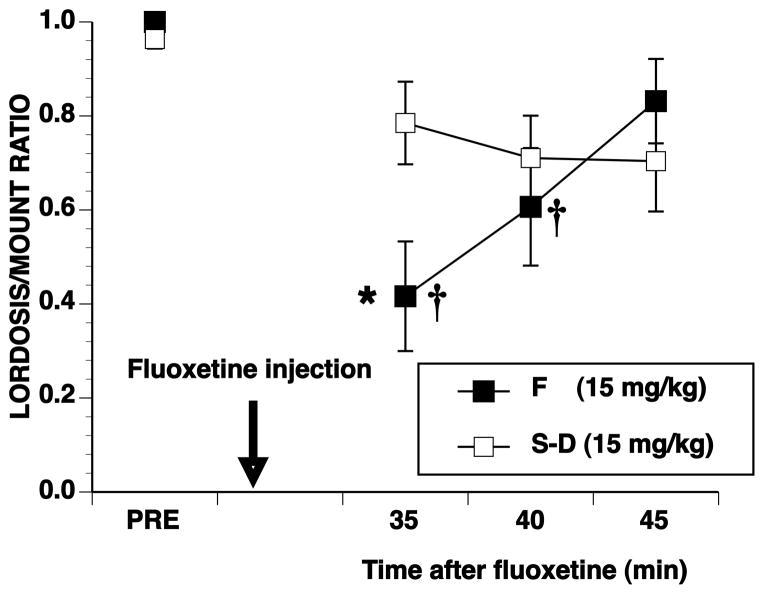
The rapid effects of fluoxetine on L/M ratios are shown in Figure 5. Compared to Experiment 2 when testing was delayed for 30 min after injection (Figure 3), Sprague-Dawley females had lower L/M ratios when testing began 5 min after fluoxetine. Both strains, therefore, showed robust declines in L/M ratios within the first 15–20 min after fluoxetine, and the main effect of strain was not significant (F1, 17 = 0.44, p ≤ 0.05). There was a significant effect of time (F4, 68 = 24.44, p ≤ 0.001) but not a significant interaction between time and strain (F4, 68 = 0.48, p ≤ 0.05). In contrast to behavior tested 30 min after fluoxetine, with the immediate initiation of testing, L/M ratios of both strains were significantly different from the pretest at every test interval (all q5.68 ≥ 3.97, p ≤ 0.05). For both strains, L/M ratios gradually increased after the early nadir to reach L/M ratios at the end of testing that were similar in Fischer females to those seen in Experiment 2 but lower in Sprague-Dawley females.
Fig 5. Rapid effects of fluoxetine on lordosis behavior.
Ovariectomized rats, hormonally primed with 0.067 μg EB/g body weight and 3.33 μg P/g body weight were pretested for sexual behavior 4 to 6 hr after P. Rats were then injected with 15 mg/kg of fluoxetine and 5 min later were tested for 40 consecutive min. Data are the mean ± SE L/M ratios for the pretest (PRE) and consecutive 10-min intervals initiated 5 min after fluoxetine injection. The Ns for Fischer (F) and Sprague-Dawley (S-D) rats, respectively were 12 and 7. † indicates significant difference from the pretest, within strain.
For lordosis quality, there were several missing intervals due to L/M ratios of zero so data were evaluated by repeated measures ANOVA with lordosis quality before and after fluoxetine as the repeated factors. There was a significant decline in lordosis quality after injection (ANOVA for time F1, 21 = 84.76, p ≤ 0.001) but neither the strain (F1, 21 = 0.46, p > 0.05) nor the strain by time interaction (F1, 21 = 1.25, p > 0.05) was significant. The means ± S.E. lordosis quality for Fischer females before and after fluoxetine, respectively, were 2.9 ± 0.05 and 1.69 ± 0.17; for Sprague-Dawley, lordosis quality before and after fluoxetine were 2.7 ± 0.05 and 1.7 ± 0.18, respectively.
Proceptivity was reduced by the SSRI and Fischer rats were more severely affected and for a longer time interval than Sprague-Dawley females (Table 1) [strain differences were present at the 20–25 min and 40–45 min intervals (Fischer’s Exact test, p < 0.05)]. When the relationship between immobility and proceptivity was examined, the two variables were inversely correlated in Sprague-Dawley females at the 10 to 15, 20 to 25, and 40 to 45 min intervals (respectively, r = −0.74, −0.69, and −0.67; p ≤ 0.05). For Fischer females, a significant correlation was present only at the 10 to 15 min interval (r = −0.47, p ≤ 0.05).
Experiment 4: Effects of hormonal priming per rat
In a final experiment, ovariectomized Fischer and Sprague-Dawley females were primed with 10 μg EB and 500 μg P per rat to determine if strain differences resulted from the greater amount of hormones received by Sprague-Dawley females when hormones had been administered relative to body weight. As shown in Figure 6, strain differences were still evident with Sprague-Dawley females being less sensitive to fluoxetine than Fischer females. However, in contrast to Experiment 2, the strain difference was present only early during testing.
Fig 6. Strain differences when given a constant hormonal priming.
Ovariectomized rats, hormonally primed with 10 μg EB and 500 μg P per rat were pretested for sexual behavior 4 to 6 hr after P. Rats were then injected with 15 mg/kg of fluoxetine and 30 min later were tested for 15 consecutive min. Data are the mean ± SE L/M ratios for the pretest (PRE) and consecutive 5-min intervals 30 min after fluoxetine injection. The Ns for Fischer (F) and Sprague-Dawley (S-D) rats, respectively were 11 and 11. * indicates significant strain differences within dose of fluoxetine. † indicates significant difference from the pretest, within strain.
There was a significant effect of time (F3,60 = 11.48, p ≤ 0.001) and a significant strain by time interaction (F3,60 = 4.76, p ≤ 0.005) for L/M ratios. Ratios for Fischer females were significantly different from their pretest during the 35 and 40 min intervals after fluoxetine (q60,8 ≥ 4.36, p ≤ 0.05) while ratios for Sprague-Dawley females were never significantly different from their pretest. The apparent differences between Experiment 2 and Experiment 4 resulted from a slightly lower L/M ratio of Sprague-Dawley females in Experiment 4 and the unusually high L/M ratio of Fischer females during the last test interval.
For lordosis quality, 1 Sprague-Dawley and 4 Fischer females had L/M of zero during at least one test interval. For remaining rats, there was a decrease in lordosis quality (data not shown) after fluoxetine (F3,45 = 2.87, p ≤ 0.046) but no other effects were significant. Similarly, the only significant effect for mounts was for time (F3,66 = 7.96, p ≤ 0.001). The average numbers of mounts per 5 min test interval after fluoxetine were 5.44 ± 0.96 and 7.5 ± 0.96 for Fischer and Sprague-Dawley females, respectively.
Discussion
Three major observations were made in these studies: (1) both lordosis behavior and proceptivity were reduced by fluoxetine; (2) hormonally-primed, ovariectomized females were more sensitive to the antidepressant than naturally cycling, intact females; and (3) both hormonally-primed, ovariectomized and intact Sprague-Dawley females were less sensitive to the disruptive effects of fluoxetine on sexual behavior than were Fischer females. These studies were originally initiated because Sprague-Dawley females show greater sensitivity to the lordosis-inhibiting effects of a 5-HT1A receptor agonist [25] and 5-HT1A receptors have been implicated in fluoxetine’s antidepressant actions [32, 33] and in the SSRI’s reduction of female rat sexual behavior [17]. It was, therefore, expected that Sprague-Dawley females might also be more sensitive to the effects of fluoxetine on sexual behavior. This clearly was not the case and challenges the role of 5-HT1A receptors in fluoxetine’s effect on sexual behavior.
However, relative to Sprague-Dawley or some other rat strains, Fischer rats are reported to have more SERT mRNA in the dorsal raphe nucleus [34]. If this translates into greater SERT activity in dorsal raphe of Fischer females, then fluoxetine would be expected to (a) increase extracellular 5-HT to a lesser extent in the vicinity of the dorsal raphe and (b) lead to a lesser activation of somatodendritic 5-HT1A autoreceptors that function to reduce firing of 5-HT neurons. Consequently, extracellular 5-HT in brain areas such as the mediobasal hypothalamus that are important in the control of female sexual behavior could be elevated more by fluoxetine in Fischer than in Sprague-Dawley females. In addition, because of a putative hyperfunctional 5-HT system in Fischer rats [34, 35], a lower dose of fluoxetine might be expected to reduce lordosis behavior. Such differences in SERT activity might account for the reversal of strain difference profiles found here after fluoxetine compared to that previously reported for a 5-HT1A receptor agonist.
This possibility is even more interesting since Sprague-Dawley females appeared to have greater motor disturbance than Fischer females. Serotonin’s effect on several indices of motor function is thought to result from effects on the descending 5-HT system [36, 37] while sexual behavioral effects may result from effects on ascending 5-HT systems [15]. A potential dissociation between fluoxetine effects on sexual behavior and motor function was seen in the current study where Sprague-Dawley females showed more motor effects than Fischer females.
The hyperfunctional HPA axis of Fischer rats could also contribute to the strain difference in the response to fluoxetine. Relative to several other rat strains, Fischer rats are thought to be more HPA responsive [35, 38], have a higher [39] and longer duration [40] corticosterone response to stress, and are regarded to be a highly emotionally reactive strain [41]. Acute treatment with fluoxetine is associated with increased activation of the hypothalamic-pituitary-adrenal axis [42] and adrenal corticosterone has been implicated in some effects of fluoxetine [20]. Therefore, fluoxetine’s acute anxiogenic action may be more disruptive for Fischer than for Sprague-Dawley females.
The observation that ovariectomized females were more sensitive than intact females to the effects of fluoxetine is not surprising since the endogenous sequence of hormonal priming is probably optimal for both facilitation of sexual behavior and for resistance to its disruption. Relative to other stages of the estrous cycle, proestrous females show a blunted response to SSRIs as measured either by microdialysis of the mediobasal hypothalamus [43] or by in vivo chronoamperometry of the hippocampus [44]. In the naturally cycling female, both estrogen and progesterone likely contribute to the smaller response to SSRIs by their modulation of SERT activity and/or by modulation of serotonin receptor number and/or function [31]. Although such hormonal modulation also occurs in ovariectomized, hormonally-primed females, the temporal characteristics of the priming mimic but do not replicate that of the naturally cycling animal.
In ovariectomized rats, both EB and P alter the behavioral and neurochemical responses to fluoxetine [45–48]. In EB-primed ovariectomized Fischer rats, P was reported to attenuate the lordosis-inhibiting effects of fluoxetine by shifting fluoxetine’s dose response curve to the right [17] and fluoxetine’s reduction of sexual behavior can be reduced by the progesterone metabolite, allopregnanolone [49]. Therefore, potential strain differences in gonadal steroids could influence the strain-dependent response to fluoxetine. It is important to note that Fischer and Sprague-Dawley females were age-matched for these experiments so that Sprague-Dawley females weighed approximately 1.5 times that of Fischer females. In Experiments 2 and 3, with ovariectomized rats, both EB and P were administered per body weight so that Sprague-Dawley females received greater absolute amounts of the hormones than their Fischer counterparts. It is possible that the greater amount of hormones contributed to the lower effect of fluoxetine in Sprague-Dawley rats. Indirect evidence for hormonal modulation of SERT activity has been reported for both Fischer [43] and Sprague-Dawley [44] females and effects of gonadal hormones on serotonin receptor function have been reported for both strains [50, 51]. In the current experiment, when both strains received the same dose of hormones (10 μg/rat EB and 500 μg/rat P), the strain difference was present but was not persistent throughout the 15 min testing. The absence of a strain difference in the latter parts of the 15 min testing was a reflection of lower L/M ratios in Sprague-Dawley females given the lower concentrations of hormonal priming while L/M ratios of Fischer females were slightly higher than in prior experiments. Although this outcome could evidence a slight protective effect of the higher hormonal priming in Sprague-Dawley females, the fact that both the naturally-cycling and hormonally-primed, ovariectomized Fischer rats showed heightened sensitivity to fluoxetine makes it unlikely that differential responses to exogenous hormonal priming is the only explanation for the strain difference. In the few studies in which endogenous levels of hormones have been compared in Fischer and Sprague-Dawley females, there has been little evidence of consistent strain differences in plasma levels of both estradiol and progesterone [52, 53]. Nevertheless, such consideration deserves further examination since there is some evidence that chronic treatment with SSRIs may reduce serum levels of gonadal hormones [54, 55].
Estradiol is essential for facilitation of lordosis behavior while progesterone is not required but is more important for the occurrence of proceptive behavior [56]. The hormonal priming used in the present study is sufficient to elicit high levels of lordosis responding in Fischer females but is not adequate for facilitation of proceptivity [27]. The fact that both lordosis behavior and proceptivity were reduced by fluoxetine challenges a simple hormonal explanation for the strain difference seen in the current study. Nevertheless, we cannot rule out the possibility that the strains differ in the degree to which the gonadal hormones modulate their serotonergic and/or other neuronal system.
Regardless of the ultimate explanation for the strain differences, the current findings illustrate the potential importance of genetic differences in the vulnerability for development of SSRI-induced sexual dysfunction. It is increasingly recognized that genetic differences contribute to both the vulnerability for development of mood disorders and for the therapeutic efficacy of SSRIs [57–59]. Similarly, differences in the behavioral and neurochemical responses to SSRIs have been reported for a variety of mouse and rat strains [60, 61]. Less attention has been focused on the possibility of genetic contributions for vulnerability to SSRI-induced sexual side effects. Although some 30–50% of females that are treated with SSRIs may develop some form of sexual dysfunction, the remaining 50–70% do not. The factors responsible for resistance to these sexual side effects is not known. However, Bishop and colleagues have described evidence that genetic polymorphisms in the promoter regions of the 5-HT2A receptor gene or in the SERT gene may influence vulnerability to SSRI-associated sexual dysfunction [62, 63] but not all reports agree [64]. Other investigators have implicated individual differences in the effect of SSRIs on the P450 2D6 isoenzyme leading to accumulation of higher concentrations of the drugs [65]. Clearly, the ideal would be to differentiate those factors that are required for the therapeutic efficacy of SSRIs from those which lead to sexual side effects. The differential vulnerability of Fischer and Sprague-Dawley females to SSRI-induced inhibition of female sexual behavior could, therefore, be valuable in identifying these factors.
Conclusions
Fischer inbred female rats are more sensitive than Sprague-Dawley females to the acute sexual side effects of fluoxetine. This difference appears to be independent of gonadal hormones.
Acknowledgments
Research supported by NIH HD28419 and TWU REP
References
- 1.Simpson K, Noble S. Fluoxetine: a review of its use in women’s health. CNS Drugs. 2000;14:301–328. [Google Scholar]
- 2.Grigoriadis S, Robinson GE. Gender issues in depression. Ann Clin Psychiatry. 2007;19:247–255. doi: 10.1080/10401230701653294. [DOI] [PubMed] [Google Scholar]
- 3.Blier P. The pharmacology of putative early-onset antidepressant strategies. Eur Neuropsychopharmacol. 2003;13:57–66. doi: 10.1016/s0924-977x(02)00173-6. [DOI] [PubMed] [Google Scholar]
- 4.Haenisch B, Bonisch H. Depression and antidepressants: insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol Ther. 2010;129:352–368. doi: 10.1016/j.pharmthera.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Auerbach SB, Minzenberg MJ, Wilkinson LO. Extracellular serotonin and 5-hydroxyindoleacetic acid in hypothalamus of the unanesthetized rat measured by in vivo dialysis coupled to high-performance liquid chromatography with electrochemical detection: dialysate serotonin reflects neuronal release. Brain Res. 1989;499:281–290. doi: 10.1016/0006-8993(89)90776-2. [DOI] [PubMed] [Google Scholar]
- 6.Dawson LA, Nguyen HQ, Smith DI, Schechter LE. Effects of chronic fluoxetine treatment in the presence and absence of (+/−)pindolol: a microdialysis study. Br J Pharmacol. 2000;130:797–804. doi: 10.1038/sj.bjp.0703378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segraves RT. Sexual dysfunction associated with antidepressant therapy. Urol Clin North Am. 2007;34:575–579. vii. doi: 10.1016/j.ucl.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Clayton AH. Female sexual dysfunction related to depression and antidepressant medications. Curr Womens Health Rep. 2002;2:182–187. [PubMed] [Google Scholar]
- 9.Morehouse R, Macqueen G, Kennedy SH. Barriers to achieving treatment goals: a focus on sleep disturbance and sexual dysfunction. J Affect Disord. 2011;132 (Suppl 1):S14–20. doi: 10.1016/j.jad.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 10.Clayton A, Keller A, McGarvey EL. Burden of phase-specific sexual dysfunction with SSRIs. J Affect Disord. 2006;91:27–32. doi: 10.1016/j.jad.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29:259–266. doi: 10.1097/JCP.0b013e3181a5233f. [DOI] [PubMed] [Google Scholar]
- 12.Sidi H, Asmidar D, Hod R, Guan NC. Female Sexual Dysfunction in Patients Treated with Antidepressant-Comparison between Escitalopram and Fluoxetine. J Sex Med. 2012;5:976–988. doi: 10.1111/j.1743-6109.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- 13.Snoeren EM, Chan JS, de Jong TR, Waldinger MD, Olivier B, Oosting RS. A new female rat animal model for hypoactive sexual desire disorder; behavioral and pharmacological evidence. J Sex Med. 2011;8:44–56. doi: 10.1111/j.1743-6109.2010.01998.x. [DOI] [PubMed] [Google Scholar]
- 14.Mendelson SD. A review and reevaluation of the role of serotonin in the modulation of lordosis behavior in the female rat. Neurosci Biobehav Rev. 1992;16:309–350. doi: 10.1016/s0149-7634(05)80204-0. [DOI] [PubMed] [Google Scholar]
- 15.Uphouse L. Female gonadal hormones, serotonin, and sexual receptivity. Brain Res Brain Res Rev. 2000;33:242–257. doi: 10.1016/s0165-0173(00)00032-1. [DOI] [PubMed] [Google Scholar]
- 16.Adams S, Heckard D, Hassell J, Uphouse L. Factors influencing fluoxetine-induced sexual dysfunction in female rats. Behav Brain Res. 2012;235:73–81. doi: 10.1016/j.bbr.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guptarak J, Sarkar J, Hiegel C, Uphouse L. Role of 5-HT(1A) receptors in fluoxetine-induced lordosis inhibition. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snoeren EM, Refsgaard LK, Waldinger MD, Olivier B, Oosting RS. Chronic paroxetine treatment does not affect sexual behavior in hormonally sub-primed female rats despite 5-HT(A) receptor desensitization. J Sex Med. 2011;8:976–988. doi: 10.1111/j.1743-6109.2010.02192.x. [DOI] [PubMed] [Google Scholar]
- 19.Ivarsson M, Paterson LM, Hutson PH. Antidepressants and REM sleep in Wistar-Kyoto and Sprague-Dawley rats. Eur J Pharmacol. 2005;522:63–71. doi: 10.1016/j.ejphar.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 20.Alahmed S, Herbert J. Strain differences in proliferation of progenitor cells in the dentate gyrus of the adult rat and the response to fluoxetine are dependent on corticosterone. Neuroscience. 2008;157:677–682. doi: 10.1016/j.neuroscience.2008.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz JM, Hallas BH, Torres G. Rat strain differences to fluoxetine in striatal Fos-like proteins. Neuroreport. 2002;13:2463–2467. doi: 10.1097/00001756-200212200-00018. [DOI] [PubMed] [Google Scholar]
- 23.Uphouse L, Hensler JG, Sarkar J, Grossie B. Fluoxetine disrupts food intake and estrous cyclicity in Fischer female rats. Brain Res. 2006;1072:79–90. doi: 10.1016/j.brainres.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 24.Maswood N, Sarkar J, Uphouse L. Modest effects of repeated fluoxetine on estrous cyclicity and sexual behavior in Sprague Dawley female rats. Brain Res. 2008;1245:52–60. doi: 10.1016/j.brainres.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uphouse L, Maswood S, Jackson A, Brown K, Prullage J, Myers T, Shaheen F. Strain differences in the response to the 5-HT1A receptor agonist, 8-OH-DPAT. Pharmacol Biochem Behav. 2002;72:533–542. doi: 10.1016/s0091-3057(02)00714-1. [DOI] [PubMed] [Google Scholar]
- 26.Miryala CS, Hassell J, Hiegel C, Uphouse L. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011. Female Fischer and Sprague-Dawley rats differ in fluoxetine-induced sexual dysfunction. 714/15/VV85. ONline. [Google Scholar]
- 27.White S, Uphouse L. Estrogen and progesterone dose-dependently reduce disruptive effects of restraint on lordosis behavior. Horm Behav. 2004;45:201–208. doi: 10.1016/j.yhbeh.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Neubig RR, Spedding M, Kenakin T, Christopoulos A International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol Rev. 2003;55:597–606. doi: 10.1124/pr.55.4.4. [DOI] [PubMed] [Google Scholar]
- 29.Zar J. Biostatistical Analysis. Englewood Cliffs, New Jersey: Prentice Hall; 1999. [Google Scholar]
- 30.Uphouse L, White S, Harrison L, Hiegel C, Majumdar D, Guptarak J, Truitt WA. Restraint accentuates the effects of 5-HT2 receptor antagonists and a 5-HT1A receptor agonist on lordosis behavior. Pharmacol Biochem Behav. 2003;76:63–73. doi: 10.1016/s0091-3057(03)00194-1. [DOI] [PubMed] [Google Scholar]
- 31.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- 32.Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- 33.Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- 34.Burnet PW, Michelson D, Smith MA, Gold PW, Sternberg EM. The effect of chronic imipramine administration on the densities of 5-HT1A and 5-HT2 receptors and the abundances of 5-HT receptor and transporter mRNA in the cortex, hippocampus and dorsal raphe of three strains of rat. Brain Res. 1994;638:311–324. doi: 10.1016/0006-8993(94)90664-5. [DOI] [PubMed] [Google Scholar]
- 35.Rosecrans JA, Robinson SE, Johnson JH, Mokler DJ, Hong JS. Neuroendocrine, biogenic amine and behavioral responsiveness to a repeated foot-shock-induced analgesia (FSIA) stressor in Sprague-Dawley (CD) and Fischer-344 (CDF) rats. Brain Res. 1986;382:71–80. doi: 10.1016/0006-8993(86)90112-5. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs BL, Klemfuss H. Brain stem and spinal cord mediation of a serotonergic behavioral syndrome. Brain Res. 1975;100:450–457. doi: 10.1016/0006-8993(75)90500-4. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol. 1997;7:820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- 38.Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- 39.Armario A, Gavalda A, Marti J. Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology. 1995;20:879–890. doi: 10.1016/0306-4530(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 40.Sarrieau A, Mormede P. Hypothalamic-pituitary-adrenal axis activity in the inbred Brown Norway and Fischer 344 rat strains. Life Sci. 1998;62:1417–1425. doi: 10.1016/s0024-3205(98)00080-0. [DOI] [PubMed] [Google Scholar]
- 41.van der Staay FJ, Blokland A. Behavioral differences between outbred Wistar, inbred Fischer 344, brown Norway, and hybrid Fischer 344 x brown Norway rats. Physiol Behav. 1996;60:97–109. doi: 10.1016/0031-9384(95)02274-0. [DOI] [PubMed] [Google Scholar]
- 42.Robert G, Drapier D, Bentue-Ferrer D, Renault A, Reymann JM. Acute and chronic anxiogenic-like response to fluoxetine in rats in the elevated plus-maze: modulation by stressful handling. Behav Brain Res. 2011;220:344–348. doi: 10.1016/j.bbr.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 43.Maswood S, Truitt W, Hotema M, Caldarola-Pastuszka M, Uphouse L. Estrous cycle modulation of extracellular serotonin in mediobasal hypothalamus: role of the serotonin transporter and terminal autoreceptors. Brain Res. 1999;831:146–154. doi: 10.1016/s0006-8993(99)01439-0. [DOI] [PubMed] [Google Scholar]
- 44.Benmansour S, Piotrowski JP, Altamirano AV, Frazer A. Impact of ovarian hormones on the modulation of the serotonin transporter by fluvoxamine. Neuropsychopharmacology. 2009;34:555–564. doi: 10.1038/npp.2008.23. [DOI] [PubMed] [Google Scholar]
- 45.Schneider T, Popik P. Attenuation of estrous cycle-dependent marble burying in female rats by acute treatment with progesterone and antidepressants. Psychoneuroendocrinology. 2007;32:651–659. doi: 10.1016/j.psyneuen.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Charoenphandhu J, Teerapornpuntakit J, Nuntapornsak A, Krishnamra N, Charoenphandhu N. Anxiety-like behaviors and expression of SERT and TPH in the dorsal raphe of estrogen- and fluoxetine-treated ovariectomized rats. Pharmacol Biochem Behav. 2011;98:503–510. doi: 10.1016/j.pbb.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 47.Bertrand PP, Paranavitane UT, Chavez C, Gogos A, Jones M, van den Buuse M. The effect of low estrogen state on serotonin transporter function in mouse hippocampus: a behavioral and electrochemical study. Brain Res. 2005;1064:10–20. doi: 10.1016/j.brainres.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 48.Eser D, Baghai TC, Schule C, Nothdurfter C, Rupprecht R. Neuroactive steroids as endogenous modulators of anxiety. Curr Pharm Des. 2008;14:3525–3533. doi: 10.2174/138161208786848838. [DOI] [PubMed] [Google Scholar]
- 49.Frye CA, Rhodes ME. Fluoxetine-induced decrements in sexual responses of female rats and hamsters are reversed by 3alpha,5alpha-THP. J Sex Med. 2010;7:2670–2680. doi: 10.1111/j.1743-6109.2010.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson A, Uphouse L. Dose-dependent effects of estradiol benzoate on 5-HT1A receptor agonist action. Brain Res. 1998;796:299–302. doi: 10.1016/s0006-8993(98)00238-8. [DOI] [PubMed] [Google Scholar]
- 51.Jackson A, Etgen AM. Estrogen modulates 5-HT(1A) agonist inhibition of lordosis behavior but not binding of [(3)H]-8-OH-DPAT. Pharmacol Biochem Behav. 2001;68:221–227. doi: 10.1016/s0091-3057(00)00455-x. [DOI] [PubMed] [Google Scholar]
- 52.Kacew S, Ruben Z, McConnell RF. Strain as a determinant factor in the differential responsiveness of rats to chemicals. Toxicol Pathol. 1995;23:701–714. doi: 10.1177/019262339502300608. discussion 714–705. [DOI] [PubMed] [Google Scholar]
- 53.Wetzel LT, Luempert LG, 3rd, Breckenridge CB, Tisdel MO, Stevens JT, Thakur AK, Extrom PJ, Eldridge JC. Chronic effects of atrazine on estrus and mammary tumor formation in female Sprague-Dawley and Fischer 344 rats. J Toxicol Environ Health. 1994;43:169–182. doi: 10.1080/15287399409531913. [DOI] [PubMed] [Google Scholar]
- 54.Rehavi M, Attali G, Gil-Ad I, Weizman A. Suppression of serum gonadal steroids in rats by chronic treatment with dopamine and serotonin reuptake inhibitors. Eur Neuropsychopharmacol. 2000;10:145–150. doi: 10.1016/s0924-977x(00)00066-3. [DOI] [PubMed] [Google Scholar]
- 55.Taylor GT, Farr S, Klinga K, Weiss J. Chronic fluoxetine suppresses circulating estrogen and the enhanced spatial learning of estrogen-treated ovariectomized rats. Psychoneuroendocrinology. 2004;29:1241–1249. doi: 10.1016/j.psyneuen.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Blaustein JD. Neuroendocrine regulation of feminine sexual behavior: lessons from rodent models and thoughts about humans. Annu Rev Psychol. 2008;59:93–118. doi: 10.1146/annurev.psych.59.103006.093556. [DOI] [PubMed] [Google Scholar]
- 57.Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- 58.Anttila S, Huuhka K, Huuhka M, Rontu R, Hurme M, Leinonen E, Lehtimaki T. Interaction between 5-HT1A and BDNF genotypes increases the risk of treatment-resistant depression. J Neural Transm. 2007;114:1065–1068. doi: 10.1007/s00702-007-0705-9. [DOI] [PubMed] [Google Scholar]
- 59.Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15:473–500. doi: 10.1038/mp.2008.116. [DOI] [PubMed] [Google Scholar]
- 60.Pitychoutis PM, Pallis EG, Mikail HG, Papadopoulou-Daifoti Z. Individual differences in novelty-seeking predict differential responses to chronic antidepressant treatment through sex- and phenotype-dependent neurochemical signatures. Behav Brain Res. 2011;223:154–168. doi: 10.1016/j.bbr.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 61.Razzoli M, Carboni L, Andreoli M, Michielin F, Ballottari A, Arban R. Strain-specific outcomes of repeated social defeat and chronic fluoxetine treatment in the mouse. Pharmacol Biochem Behav. 2011;97:566–576. doi: 10.1016/j.pbb.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 62.Bishop JR, Moline J, Ellingrod VL, Schultz SK, Clayton AH. Serotonin 2A -1438 G/A and G-protein Beta3 subunit C825T polymorphisms in patients with depression and SSRI-associated sexual side-effects. Neuropsychopharmacology. 2006;31:2281–2288. doi: 10.1038/sj.npp.1301090. [DOI] [PubMed] [Google Scholar]
- 63.Bishop JR, Ellingrod VL, Akroush M, Moline J. The association of serotonin transporter genotypes and selective serotonin reuptake inhibitor (SSRI)-associated sexual side effects: possible relationship to oral contraceptives. Hum Psychopharmacol. 2009;24:207–215. doi: 10.1002/hup.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strohmaier J, Wust S, Uher R, Henigsberg N, Mors O, Hauser J, Souery D, Zobel A, Dernovsek MZ, Streit F, Schmal C, Kozel D, Placentino A, Farmer A, McGuffin P, Aitchison KJ, Rietschel M. Sexual dysfunction during treatment with serotonergic and noradrenergic antidepressants: clinical description and the role of the 5-HTTLPR. World J Biol Psychiatry. 2011;12:528–538. doi: 10.3109/15622975.2011.559270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zourkova A, Hadasova E. Relationship between CYP 2D6 metabolic status and sexual dysfunction in paroxetine treatment. J Sex Marital Ther. 2002;28:451–461. doi: 10.1080/00926230290001565. [DOI] [PubMed] [Google Scholar]