Abstract
The most consistent and persistent biochemical characteristic of prostate cancer (PCa) is the marked decrease in zinc and citrate levels in the malignant cells. This relationship provides compelling evidence that the lost ability of the malignant cells to accumulate zinc is an important factor in the development and progression of prostate malignancy. In addition, this relationship provides a rational basis for the concept that restoration of high zinc levels in malignant cells could be efficacious in the treatment and prevention of PCa. Epidemiological studies regarding dietary zinc effects on PCa have been conflicting and confusing. The purpose of this presentation is to present a current state of information regarding zinc relationships in the pathogenesis and treatment of PCa. We also hope to bring more attention to the medical and research community of the critical need for concerted clinical and basic research regarding zinc and PCa.
Keywords: zinc, prostate cancer, zinc transporters, prostate epithelial cells, apoptosis, citrate oxidation
Introduction
In previous publications,1–3 we reviewed and detailed the compelling evidence and basis for the concept that zinc is involved in the pathogenesis of prostate cancer (PCa); and that zinc could be efficacious in the prevention and treatment of PCa. Since those earlier reviews, significant additional information has become available regarding the zinc relationship in prostate. We can best frame the discussion by addressing the following questions: ‘Is altered zinc metabolism an important factor in the pathogenesis of prostate cancer?’ Can zinc be efficacious against the development and progression of prostate cancer? Our intention in this presentation is to provide a comprehensive update of current information, to address contemporary issues and conflicting reports, and to expand the awareness of the scientific and clinical community regarding the implications of zinc in prostate cancer. For details of earlier related studies, which will be minimized in this presentation, we refer the reader to our previous reviews.1–5
The major function of zinc in the normal human prostate
For the following presentation, the human prostate gland must be anatomically defined. In this discussion, we will consider the prostate to be comprised of the peripheral zone (about 70%), the central zone (about 25%), and the transition zone (about 5%). In regard to zinc relationships and the development of malignancy, the peripheral zone is the major component. The central gland normally contains much lower zinc levels than in peripheral zone. Therefore, the normal glandular secretory epithelial cells of the peripheral zone, but not the central gland, are zinc-accumulating cells. In benign prostate hyperplasia (glandular BPH) the zinc levels in the central gland are dramatically increased and often exceed the concentration that exists in normal peripheral zone. The transition zone is believed to be the origin of BPH. The following presentation focuses on the zinc relationships to prostate cancer; and, unless otherwise defined, relates to the peripheral zone.
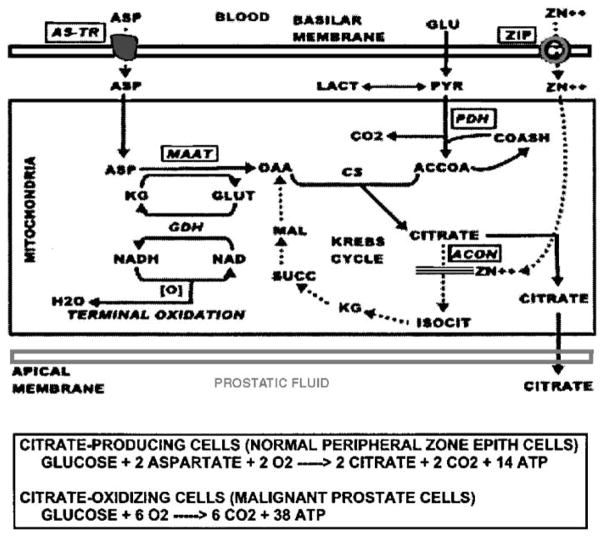
A major function of the peripheral zone glandular epithelium is the production and secretion of extraordinarily high levels of citrate (Table 1). The peripheral zone glandular secretory epithelium also accumulates high levels of zinc; three to 10-fold higher than other soft tissues. The uniqueness of this function becomes more apparent when one recognizes that mammalian cells generally possess mechanisms that prevent the accumulation of high zinc levels; particularly mobile reactive zinc that can have toxic effects. Why then does this function exist in these highly specialized secretory epithelial cells? The answer is that a high level of zinc in mitochondria is essential to inhibit m-aconitase activity.6 This prevents the oxidation of citrate, which accumulates and is secreted (Figure 1). In other mammalian cells, the inhibition of m-aconitase and citrate oxidation is lethal. This emphasizes the uniqueness of the highly specialized prostate secretory epithelial cells.
Table 1.
Representative citrate and zinc levels is prostate
| Citrate | Zinc | |
|---|---|---|
| Normal (mixed tissue) | 8000 | 209 |
| Normal (central zone) | 4000 | 121 |
| Normal (peripheral zone) | 13 000 | 295 |
| BPH | 8000–15 000 | 589 |
| PCa (mixed tissue) | 1000–2000 | 55 |
| PCa (malignant tissue) | 500 | — |
| Other soft tissue | 150–450 | 30 |
| Blood plasma | 90–110 | 1 |
| Prostatic fluid | 40 000–150 000 | 590 |
Citrate values are nmol/g wet weight. Zinc values are μg/g wet weight.
Figure 1.
Role of zinc in the pathway of net citrate production.
The apoptogenic effect of zinc
In addition to its major function and metabolic effect described above, the accumulation of high zinc levels can have other consequences. One such effect is the inhibition of cell growth, which results from its induction of mitochondrial apoptogenesis.7–9 This effect has been identified in human prostate cell lines (PC-3, LNCaP, BPH-1) and in normal rat ventral prostate epithelium, all of which are capable of accumulating high zinc levels when exposed to zinc-supplemented medium. In contrast, the human prostate HPr-1 cell line and the squamous carcinoma cell line, which are not zinc-accumulating cells, do not exhibit an apoptogenic response to zinc treatment. In most mammalian cells, zinc reportedly prevents apoptosis. Therefore, the apoptogenic effect of zinc is specific for zinc-accumulating cells. This effect results from a direct action of zinc on the mitochondria, which results in the release of cytochrome c that leads to apoptotic cascading events (Figure 2). The cell specificity is dependent upon two factors. (1) the ability of the cells to take up and accumulate zinc, and (2) the ability of the mitochondria to respond to the increased cytosolic level of zinc.
Figure 2.
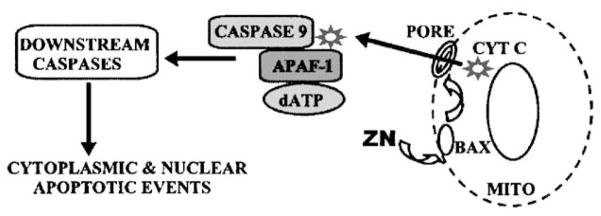
Mechanism of zinc-induced mitochondrial apoptogenesis in prostate cells.
The mechanism of zinc accumulation
Why are these prostate epithelial cells capable of accumulating cellular levels of zinc that are several-fold higher than most other mammalian cells although they share the same interstitial fluid environment? Presently, the mechanisms responsible for the high cellular zinc level are unknown. Recent studies10–12 have shown that the zinc transporter, ZIP1, is important in the uptake and accumulation of zinc by prostate cells. Upregulation of ZIP1 in prostate cells increases zinc accumulation, which inhibits cell growth and increases net citrate production. This reveals that a significant component of the accumulated cellular zinc is retained as mobile reactive zinc. Correspondingly, downregulation of ZIP1 decreases zinc accumulation in prostate cells. The net accumulation of zinc would also be dependent upon the export of zinc out of the cell. Although, a number of zinc export transporters have been identified, their functional relationship in cellular zinc accumulation has not been established.
Zinc levels in prostate cancer
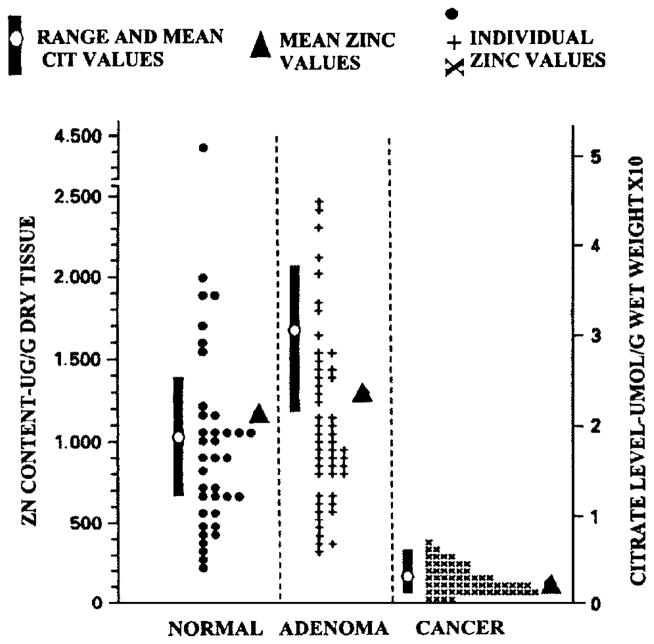
As represented in Table 1 many studies (reviewed and cited in Costello and Franklin1) have consistently demonstrated a marked decrease in prostate tissue zinc levels in PCa vs normal prostate or BPH samples. Clearly, the uniquely high zinc levels that characterize the normal glandular epithelium of the peripheral zone are greatly reduced (generally 70–80% lower) in the malignant tissue. Figure 3 compares the zinc values from the study of Zaichick et al13 with the citrate values from the study of Liney et al.14 Most importantly, there exists no individual case where the malignant tissue has retained either the high zinc level or citrate level of the normal peripheral zone. This consistent relationship exists despite the variations in patient populations, in stage of prostate cancer, in tissue sampling, in assay procedures, and other variables. Also important is the observation that the decrease in zinc and in citrate occurs early in malignancy.15,16 This is confirmed by the in situ magnetic resonance spectroscopy identification of decreased citrate in malignant loci in the peripheral zone of cancer subjects (for review, see Costello et al4,5). Decreased citrate is now the most specific and consistent marker for malignant loci in the prostate gland. This decrease occurs by the time that any histopathological evidence of malignancy is apparent. Since zinc is the cause of citrate production, its decrease precedes the decrease in citrate, and this metabolic transformation is initiated prior to the overt appearance of malignancy; that is, in a premalignant stage (Figure 4).
Figure 3.
Composite results of zinc and citrate levels in prostate cancer, benign hyperplasia, and normal individuals. The citrate values were determined by in situ magnetic resonance spectroscopy of the prostate.14 The zinc values were determined by energy dispersive X-ray fluorescence of resected prostate tissue.13
Figure 4.
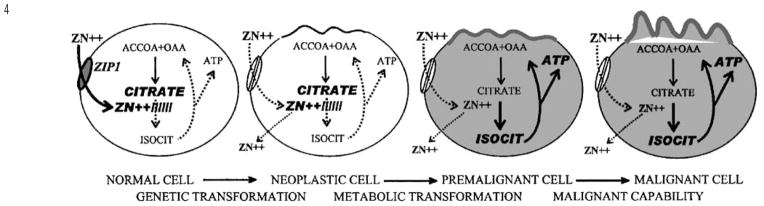
Concept of pathogenesis of prostate malignancy. The normal cell possesses the zinc-accumulating apparatus (ZIP1). The high zinc levels in mitochondria inhibit m-aconitase resulting in the inability to oxidize citrate and the accumulation of citrate. The neoplastic cell has lost the ability to accumulate zinc. As the cellular zinc levels are decreased in the premalignant cell, citrate oxidation occurs with the concurrent increased production of ATP. The malignant cell is now metabolically and energetically capable of proceeding with its malignant process. Another effect (not shown in this figure) of the decrease in zinc is the removal of its apoptogenic effect, which then permits the proliferation of the malignant cells.
The impact of decreased zinc level in malignancy
We have identified two important effects of decreased zinc levels; a metabolic effect, and a growth effect. The metabolic effect results from the release of zinc inhibition of m-aconitase, which then permits the oxidation of citrate via the Krebs cycle (Figure 1). This has a major effect on the bioenergetics of the cell. The inhibition of citrate oxidation at the aconitase step eliminates the coupled energy (ATP) production that normally occurs from Krebs cycle oxidation. Under such conditions, the aerobic oxidation of glucose results in the production 14 ATP/glucose, as contrasted with 38 ATP/glucose that results when citrate oxidation exists (Figure 1). Thus, the malignant cells become energy-efficient cells in contrast to the energy-inefficient, specialized citrate-producing secretory epithelial cell. This provides the additional energy production that is required for the malignant cell to perform its potential malignant activities. The growth effect results from the elimination of the apoptogenic influence of zinc, which, in combination with the metabolic effect, permits the proliferation of the malignant cells. These events will not occur if the malignant cell retains a high zinc accumulation, which explains why zinc-accumulating, citrate-producing malignant cells are not found in prostate cancer. However, this concept does not suggest or imply that the lost ability of peripheral zone epithelial cells to accumulate zinc is the cause of the development of malignant cells. A genetic mutation to a neoplastic cell with malignant potential is essential. Once such a neoplastic cell develops, the zinc-associated metabolic transformation is essential for the manifestation of its malignant activities. Figure 4 is a representation of this concept of the pathogenesis of prostate malignancy.
What is the cause of the decrease in zinc accumulation?
The elucidation of the cause of the lost ability of the malignant cells to accumulate zinc will be critical in understanding the pathogenesis of PCa. A possible reason is the involvement of ZIP1 since it is now known to be important in the uptake and accumulation of zinc in prostate cells.10,11 We recently showed that the downregulation of ZIP1 is accompanied by a decrease in the uptake of zinc by prostate cells. Most importantly, Rishi et al12 reported that ZIP1 and ZIP2 expression was downregulated in malignant loci of the peripheral zone as compared to its expression in adjacent normal tissue. Moreover, expression of both transporters is lower in normal peripheral zone from black males as compared to white males, which coincides with the race-associated higher incidence of PCa in African-Americans. Correspondingly, we have identified (unpublished information) that the level of ZIP1 protein (immunocytochemistry with ZIP1 antibody) is markedly decreased in the malignant loci of peripheral zone as compared with high ZIP1 level in the adjacent normal glandular epithelium. Consequently, evidence is evolving that downregulation of expression of the ZIP transporters is a cause of decreased zinc uptake and accumulation in malignant cells; and this altered gene expression might be a critical factor in the development of PCa. This leads to the future possibility that restoration of ZIP transporter gene expression could prevent prostate malignancy.
In situ zinc effects in peripheral zone vs effects in prostate cells in vitro: a conundrum?
The relationships described herein evolved from the combination of in vitro studies and in situ studies. The issue always arises as to the applicability of in vitro studies to the ‘true’ in situ conditions. This is especially relevant to the zinc relationships. While it is clearly established that the malignant cells in situ in PCa (ie ‘true’ malignant cells) have lost the ability to accumulate zinc, malignant cell lines such as LNCaP and PC-3 have retained the ability to accumulate zinc under in vitro conditions. The ability of these cells to accumulate zinc is not evidence that the ‘true’ malignant cells have not lost the ability to accumulate zinc. It is evidence that the ‘true’ malignant cells retained the potential ability to accumulate zinc; but, under the in situ conditions, either the zinc-accumulating apparatus is suppressed or not expressed, and/or zinc export mechanisms prevent the cellular retention of imported zinc. Nor does this difference demonstrate that the cell lines are inappropriate models for representation of ‘true’ malignant cells. It is essential to recognize these circumstances in order to employ appropriate experimental conditions and the limitations imposed by these circumstances.
Another important issue relates to the apoptogenic effect of zinc. We identified this effect in in vitro studies with prostate cell lines and freshly isolated rat ventral prostate cells. Then the question is raised, ‘Why don’t the normal secretory epithelial cells of the peripheral zone (ie ‘true’ normal epithelial cells), which accumulate very high zinc levels, exhibit a high rate of apoptogenesis and die?’ If they do not die, is it likely that zinc does not have an apoptogenic effect on the ‘true’ normal secretory epithelial cells? The answer does not reside in a conclusion that the zinc effect on apoptogenesis that occurs in vitro is not an effect of zinc in situ. In fact, the apoptogenic effect does occur in vivo as shown in the tumorigenic study described below (Figure 5). The explanation resides in the in situ conditions of the ‘true’ secretory epithelial cells, which must modulate the apoptogenic effect of zinc. Indeed, this should be expected since these are highly specialized cell that evolved for the function of zinc accumulation; and, therefore must have adapted mechanisms to protect the cells from the toxic influences of zinc.
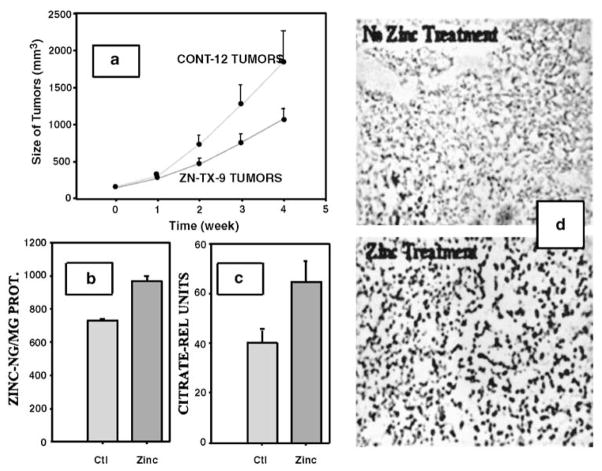
Figure 5.
Effect of zinc treatment of mice on PC-3 tumor growth. (a) Tumor growth. (b) Tumor zinc level. (c) Tumor citrate level. (d) Tumor cell apoptosis.
These currently unresolved relationships are not conundrums. There exist appropriate answers. They signal the necessity to elucidate the factors and conditions that represent the natural environment and in situ operation of the malignant cells and the normal epithelial cells from which they are derived.
The case for zinc treatment in prostate cancer
It is apparent that the malignant prostate cells virtually never exist as zinc-accumulating citrate-producing cells. The metabolic transformation to neoplastic cells that have lost the ability to accumulate zinc and are citrate-oxidizing cells is essential for the manifestation of their malignant activities. Therefore, it is plausible to apply this relationship to the treatment of PCa, and perhaps to the prevention of clinically manifested PCa. An approach is to create conditions that will enhance the uptake and accumulation of zinc in the malignant cell (for treatment) or in the premalignant cell (for prevention). The simplest regimen is to increase the circulating level of zinc to enhance the uptake of zinc. In this regard it is important to note that zinc bioavailability (plasma zinc and tissue levels) generally decline with aging.17 There is some evidence, albeit still inconclusive, that decreased plasma zinc level might be associated with PCa;18–20 but no reports that increased plasma zinc is associated with PCa. Nevertheless, a significant increase in circulating zinc, especially in a form that is available for cellular uptake, could be efficacious. The obvious mode for increasing the circulatory level of zinc would be by oral ingestion of supplemental zinc; but some problems exist with this approach as described below.
Effective cellular uptake of zinc does not occur by simple diffusion. The lost ability to extract zinc from circulation is due to the downregulation of the uptake transport process (eg ZIP transporters). Therefore, another approach is to restore or enhance the uptake transport process of the malignant cells. In this regard, we have shown that testosterone and prolactin can increase zinc uptake in prostate cells;21 and these hormones upregulate ZIP1 expression.10 Therefore, the application of an appropriate hormonal regimen to enhance zinc uptake in the malignant cells could be successful. A combination of increased circulating zinc and hormonal (such as prolactin) stimulation of zinc uptake could provide the most efficacious approach.
To test this concept, we recently conducted preliminary zinc treatment studies in an experimentally induced human prostate tumorigenic model; that is PC-3 cell-induced tumors in nude mice.22 Subcutaneous-implanted minipumps containing zinc chloride solution provided a sustained release of zinc. This route was selected to provide a more direct and better-controlled source of zinc to circulation than oral ingestion. The success of this treatment is revealed by a significant increase in the plasma zinc level. Over a five-week period, the zinc-treated animals exhibited a 50% reduction in tumor growth (Figure 5). Coinciding with this was an increase in the zinc and citrate levels of the tumors from the zinc-treated group, which reveals an increase in mobile reactive zinc. Consequently, zinc treatment metabolically altered the tumor cells to citrate-producing cells. Also apparent is a marked increase in apoptosis in the tumors from zinc-treated animals; thereby, demonstrating that the apoptogenic effect occurs in vivo as well as in vitro. Consequently, in this experimental model, all these effects are compatible with the concept described above. The critical question is whether or not this will translate into corresponding effects of zinc treatment in PCa in humans.
Epidemiological studies: what have they revealed?
Several case–control epidemiological studies that involved the efficacy of dietary zinc against PCa have been reported;23–29 (for reviews see Thomas,30 Platz and Helzlsouer,31 and Kolonel32). Most reports26–29 indicated that dietary zinc had no apparent beneficial or harmful effect on PCa risk. Kristal et al24 reported a significant reduction in the risk of PCa by zinc supplement; and Key et al25 also concluded that zinc might be beneficial against PCa. In contrast, Leitzmann et al23 reported an increase in the risk of PCa by excessive zinc supplement. Obviously, no unanimity or consensus regarding the effects of dietary zinc on PCa can be derived from these epidemiological studies. Indeed, the dominant conclusion of most of these studies is that further studies are essential and warranted.
It is unfortunate and disturbing that the conclusion of Leitzmann et al23 has been echoed in public announcements. The Washington Post (July 1, 2003, Reuters) announced, ‘Men who take too much zinc may be raising their prostate cancer risk, U.S. researchers said yesterday.’ HealthDay News (July 2, 2003) recounts, ‘Men who overdose on zinc supplements more than double their risk of prostate cancer, a government study finds.’ Excluded from the report of Leitzmann et al is any reference to or discussion of any previously reported epidemiologic studies; none of which are corroborative of their conclusions. The omission of such highly relevant information and lack of explanation for the apparent diverse observations lead the reader to an erroneous supposition that their report is the sole and incontrovertible study. In our view, this conclusion is unfounded, and the message now in the public domain is not warranted. Indeed, we hope that our presentation will provide a balanced assessment of the current state of the available scientific/medical information regarding this important issue.
One must ask why no consistency or consensus has been derived from these epidemiological studies. In some instances the database, the patient selection, confounding factors, and the application of statistical analyses are problematic. However, the major explanation resides within the complexity of interacting multiple dietary factors that affect the intestinal absorption and assimilation of zinc (for reviews see Lonnerdal,33 Krebs,34 and King et al35). For example the level of phytate, iron, calcium, and numerous other ingested nutrients can inhibit the absorption of zinc. Also, the absorption of zinc varies with the ligand form of zinc. An important factor that has not been considered in these epidemiological studies is the effect of zinc concentration on the efficiency of its absorption. The intestinal uptake of zinc provides a homeostatic mechanism that regulates the systemic level of zinc. A low-intestinal zinc level upregulates zinc absorption, and an excessive intestinal zinc level downregulates zinc absorption. The supposition of most of the epidemiological studies has been that the amount of ingested zinc is approximated by the amount of zinc that is absorbed and assimilated. However, when all these interacting factors are considered, this supposition becomes untenable. Any direct effect of zinc on normal or malignant prostate cells is dependent on the circulating level of zinc and its uptake by the cells; not on the intestinal level of zinc. The most important information is the effect of the ingested level of zinc on the systemic level of zinc; and that information is lacking in these epidemiological studies. It is interesting to note that several reports have demonstrated that plasma zinc levels were either normal or decreased, but not increased, in prostate cancer subjects;18–20 which further argues against a deleterious effect of elevated systemic zinc. It should be apparent that such epidemiological studies have not and likely will not establish the role and effectiveness of zinc in PCa.
Summary
Prostate malignancy involves the metabolic transformation of normal zinc-accumulating citrate-producing cells to citrate-oxidizing malignant cells that have lost the ability to accumulate zinc. Malignant cells in PCa virtually never contain high zinc levels. The lost ability to accumulate zinc is an essential metabolic transformation that is required for the manifestation of the malignant activities of the neoplastic malignant prostate cell.
Downregulation of ZIP (zinc uptake) transporter gene expression might be responsible for the lost ability to accumulate zinc; and, therefore, could be a significant genetic factor in PCa.
Overwhelming evidence supports the concept that restoration of high zinc levels in the malignant or premalignant cells could be efficacious against the development of overt malignancy or the progression of malignancy.
Epidemiological studies regarding the effectiveness of dietary zinc in the risk of PCa have been conflicting and inconclusive.
Well-controlled studies of the efficacy of zinc alone and in combination with other supplements are required, along with basic research studies to establish the mechanisms of regulation of zinc and its effects on normal and malignant prostate.
Acknowledgments
The studies of the authors contained in this presentation were supported by NIH Grants DK42839, CA71207, CA79903, CA93443 and by DOD Grant DAMD 17-01-1-0072.
References
- 1.Costello LC, Franklin RB. The novel role of zinc in the intermediary metabolism of prostate epitelial cells and its implications in prostate malignancy. Prostate. 1998;35:285–296. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Costello LC, Franklin RB. The intermediary metabolism of the prostate: a key to understanding the pathogenesis and progression of prostate malignancy. Oncology. 2000;59:269–282. doi: 10.1159/000012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello LC, Franklin RB. The metabolism of prostate malignancy: insights into the pathogenesis of prostate cancer and new approaches for its diagnosis and treatment. Oncol Spectr. 2001;2:452–457. [Google Scholar]
- 4.Costello L, Franklin RB, Kurhanewicz J. Encyclopedia of Cancer. 2. Vol. 3. Academic Press; New York: 2002. The metabolic characterization of prostate malignancy by magnetic resonance spectroscopy; pp. 167–177. [Google Scholar]
- 5.Costello LC, Franklin RB, Narayan P. Citrate in the diagnosis of prostate cancer. Prostate. 1999;38:237–245. doi: 10.1002/(sici)1097-0045(19990215)38:3<237::aid-pros8>3.0.co;2-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costello LC, Liu Y, Franklin RB, Kennedy MC. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J Biol Chem. 1997;272:28875–28881. doi: 10.1074/jbc.272.46.28875. [DOI] [PubMed] [Google Scholar]
- 7.Feng P, et al. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate. 2002;52:311–318. doi: 10.1002/pros.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng P, et al. Zinc induces mitochondria apoptogenesis in prostate cells. Mol Urol. 2002;4:31–35. [PubMed] [Google Scholar]
- 9.Liang JY, et al. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40:200–207. doi: 10.1002/(sici)1097-0045(19990801)40:3<200::aid-pros8>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costello LC, Liu Y, Zou J, Franklin RB. Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J Biol Chem. 1999;274:17499–17504. doi: 10.1074/jbc.274.25.17499. [DOI] [PubMed] [Google Scholar]
- 11.Franklin RB, et al. hZIP1 is a major zinc uptake transpoter for the accumulation of zinc in prostate cells. J Inorg Biochem. 2003;96:435–442. doi: 10.1016/s0162-0134(03)00249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rishi I, et al. Prostate cancer in African-American men is associated with down-regulation of zinc transporters. App Immunohistochem Mol Morph. 2003;11:253–260. doi: 10.1097/00129039-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Zaichick VY, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic, cancerous. Int Urol Nephr. 1997;29:565–574. doi: 10.1007/BF02552202. [DOI] [PubMed] [Google Scholar]
- 14.Liney GP, et al. In vitro quantification of citrate concentration and water T2 relaxation time of the pathologic prostate gland using 1H MRS and MRI. Mag Reson Imag. 1997;15:1177–1186. doi: 10.1016/s0730-725x(97)00182-3. [DOI] [PubMed] [Google Scholar]
- 15.Cooper JE, Farid I. The role of citric acid in the physiology of the prostate. Lactic/citrate ratios in benign and malignant prostatic homogenates as an index of prostatic malignancy. J Urol. 1964;92:533–536. doi: 10.1016/S0022-5347(17)64003-5. [DOI] [PubMed] [Google Scholar]
- 16.Habib FK, Mason MK, Smith PH, Stitch SR. Cancer of the prostate: early diagnosis by zinc and hormone analysis. Br J Cancer. 1979;39:700–704. doi: 10.1038/bjc.1979.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mocchegiani E, Muaaioli M, Giacconi R. Zinc, metallothioneins, immune responses, survival and ageing. Biogeront. 2000;1:133–143. doi: 10.1023/a:1010095930854. [DOI] [PubMed] [Google Scholar]
- 18.Feustel A, Wenrich R. Zinc and cadmium plasma and erythrocyte levels in prostatic carcinoma, BPH, urological malignancies, and inflammation. Prostate. 1986;8:75–79. doi: 10.1002/pros.2990080109. [DOI] [PubMed] [Google Scholar]
- 19.Whelan P, Walker BE, Kelleher J. Zinc, vitamin A, and prostate cancer. Br J Urol. 1983;55:525–528. doi: 10.1111/j.1464-410x.1983.tb03362.x. [DOI] [PubMed] [Google Scholar]
- 20.Ogunlewe JO, Osegbe DN. Zinc and cadmium concentrations in indigenous blacks with normal, hypertrophic, and malignant prostate. Cancer. 1989;63:1388–1392. doi: 10.1002/1097-0142(19890401)63:7<1388::aid-cncr2820630725>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Costello LC, Franklin RB. Prolactin and testosterone regulation of mitochondrial zinc in prostate epithelial cells. Prostate. 1997;30:26–32. doi: 10.1002/(sici)1097-0045(19970101)30:1<26::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Feng P, et al. Effect of zinc on prostatic tumorigenicity in nude mice. Ann N Y Acad Sci. 2003;1010:316–320. doi: 10.1196/annals.1299.056. [DOI] [PubMed] [Google Scholar]
- 23.Leitzmann MF, et al. Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst. 2003;95:1004–1007. doi: 10.1093/jnci/95.13.1004. [DOI] [PubMed] [Google Scholar]
- 24.Kristal AR, et al. Vitamin and mineral supplement use is associated with reduced risk of prostate cancer. Cancer Epidemiol Biomark Prevent. 1999;8:887–892. [PubMed] [Google Scholar]
- 25.Key TJA, et al. A case–control study of diet and prostate cancer. Br J Cancer. 1997;76:678–687. doi: 10.1038/bjc.1997.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West DW, et al. Adult dietary intake and prostate cancer risk in Utah: a case–control study with special emphasis on aggressive tumors. Cancer Causes Control. 1991;2:85–94. doi: 10.1007/BF00053126. [DOI] [PubMed] [Google Scholar]
- 27.Andersson SO, et al. Energy, nutrient intake and prostate cancer risk: a population-based case–control study in Sweden. Int J Cancer. 1996;68:716–722. doi: 10.1002/(SICI)1097-0215(19961211)68:6<716::AID-IJC4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Vlajinac HD, Marinkovic JM, Ilic MD, Kocev NI. Diet and prostate cancer: a case–control study. Eur J Cancer. 1997;33:101–107. doi: 10.1016/s0959-8049(96)00373-5. [DOI] [PubMed] [Google Scholar]
- 29.Kolonel LN, Yoshizawa CN, Hankin JH. Diet and prostatic cancer: a case–control study in Hawaii. Am J Epidemiol. 1988;127:999–1012. doi: 10.1093/oxfordjournals.aje.a114903. [DOI] [PubMed] [Google Scholar]
- 30.Thomas JA. Diet, micronutrients and the prostate gland. Nutr Rev. 1999;4:95–103. doi: 10.1111/j.1753-4887.1999.tb06932.x. [DOI] [PubMed] [Google Scholar]
- 31.Platz EA, Helzlsouer KJ. Selenium, zinc, and prostate. Epidemiol Rev. 2001;23:93–101. doi: 10.1093/oxfordjournals.epirev.a000801. [DOI] [PubMed] [Google Scholar]
- 32.Kolonel LN. Nutrition and prostate cancer. Cancer Causes Control. 1996;7:83–94. doi: 10.1007/BF00115640. [DOI] [PubMed] [Google Scholar]
- 33.Lonnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000;130:1378S–1383S. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- 34.Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr. 2000;130:1374S–1377S. doi: 10.1093/jn/130.5.1374S. [DOI] [PubMed] [Google Scholar]; J Nutr. 2000;130:1360S–1366S. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]
- 35.King JC, Shames DM, Woodhouse LB. Zinc homeostasis in humans. J Nutr. 2000;130:1360S–1366S. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]