Sadly, mistakes of nature are often our greatest learning tools. Such is the case with the recent description by three independent groups of the molecular etiology of leukocyte adhesion deficiency (LAD) syndrome III.1–3 The discovery that mutations in kindlin-3 are responsible for this rare genetic disorder teaches us a great deal about how leukocytes regulate adhesion and trafficking through their primary adhesive receptors—the β1-, β2- and β3-integrin receptors. This story also teaches us how mistakes can be made (and avoided) in science.
There are three types of LAD syndromes, LADI, II and III (also referred to as variant LADI).4 As the name implies, these diseases are characterized by an inability of leukocytes to adhere and migrate during inflammatory and host defense reactions. As a result, patients with these autosomal recessive syndromes present with repeated bacterial and fungal infections in the absence of pus formation. Hundreds of LADI patients have been described worldwide, all with mutations in the gene encoding the β2 leukocyte integrin. Less than 10 patients have been described with LADII, which is a clinically milder form of the disease, and is caused by mutations in a fucose transporter protein leading to poor formation of Sialyl Lewis X (CD15), the ligand for L-selectin. LADIII is also rare (approximately 20 patients worldwide, with the largest kindred of Turkish origin), but presents with both the immunodeficiency of LADI as well as severe bleeding disorders resembling Glanzman’s thrombasthenia—a known deficiency of the platelet αIIbβ3-integrin. Unlike LADI, the characterized LADIII patients have normal cell surface expression of all the major leukocyte integrins; however, these receptors fail to become activated to mediate leukocyte and platelet adhesion and cell spreading. Hence, these patients must have a defect in the intracellular signaling pathways that regulate integrin activation.
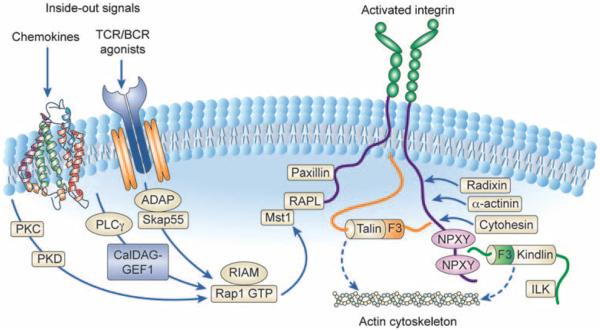
The signaling networks that regulate β1-, β2- and β3-integrin function are complex.5 In resting leukocytes and platelets, the integrins are held in a bent/inactive conformation that limits their ability to interact with ligands (endothelial ICAMs or extracellular matrix proteins such as fibrinogen or collagen). This conformation is maintained by a salt bridge between the cytoplasmic tails of the α and β chains of the integrin heterodimer, and through association with proteins such as talin. Following cellular activation (by agonists including chemokines, growth factors, antigens or selectin ligands), the cytoplasmic tails of the integrins separate, transmitting a conformational change throughout the integrin heterodimer that results in unfolding of the extracellular domains to allow high-affinity ligand binding. The processes that mediate integrin activation are referred to as ‘inside–out’ signaling (detailed in Figure 1).
Figure 1.
Integrin inside–out signaling. The figure outlines the key signaling pathways that have been implicated in integrin inside–out signaling downstream of chemokine and B- or T-cell receptor stimulation. In their inactive state, integrins adopt a bent conformation that is unable to bind ligand at high affinity. Initiation of most signaling pathways that lead to integrin activation involve the recruitment of Rap1 GTPase. Rap1 activation is controlled by upstream GEFs, in particular CalDAG-GEF1, which has been implicated in regulating cell adhesion and migration.6 Following Rap1 activation, its effectors RIAM and RapL form a complex with the cytoplasmic tails of integrins. This leads to a critical change in the association of talin with the integrin cytoplasmic tail, and binding of a number of other proteins to the integrin tail, including paxillin, radixin, cytohesin, α-actinin and the kindlins. Altogether, these proteins facilitate separation of the cytoplasmic tails of the integrins, leading to full conformational changes required for high-affinity ligand binding and coupling of the integrin to the actin cytoskeleton. The precise sequence of events from Rap1 activation to integrin tail separation and protein associations are unclear; however, this overall theme is likely common between the β1-, β2- and β3-integrins.
The fact that LADIII patients fail to activate high-affinity integrin ligand binding led Alon et al.7 to focus on each of the molecules involved in the inside–out pathway. Initial reports suggested that Rap1 failed to become activated in leukocytes from several patients. The group investigated the upstream regulator of Rap1, CalDAG-GEF1, and in fact they identified a potential mutation in a splice acceptor site for exon16 of the CalDAG-GEF1 gene in two of the LADIII patients.6 Leukocytes from these patients had low levels of CalDAG-GEF1 mRNA and the protein was not detected in cell lysates. The patients showed complete failure to activate β1 and β2-integrins on leukocytes, as well as β3-integrins on platelets. Altogether, this explains the full clinical spectrum of infections and bleeding suffered by these children.8 The fact that CalDAG-GEF1-deficient mice manifest an overall syndrome extremely similar to human LADIII9 buttressed this group’s assertion that LADIII is caused by loss of CalDAG-GEF1 leading to impaired Rap1 activation and failure to activate leukocyte integrins. This view was reflected in a number of reviews of integrin signaling, including our own.5
Yet, when more LADIII patients were examined, in particular patients from other families, impairment of Rap1 activation and reduced CalDAG-GEF1 expression was not observed.3,10 Whereas some of these patients had the splice acceptor ‘mutation’ described by Alon’s group, others did not. These disparate results initiated the controversy as to the molecular nature of LADIII syndrome.
To identify the genetic defect in these patients Kuijpers et al.1 carried out a complete homozygosity mapping experiment on a larger number of LADIII samples, using single nucleotide polymorphism oligonucleotide arrays. Although these experiments localized the causative genetic mutation to a region containing the CalDAG-GEF1 gene, not all patients had the splice acceptor mutation and no defects in CalDAG-GEF1 expression were found. Instead mutations were mapped to the FERMT3 gene, which encodes kindlin-3, located a mere 500 kb away from the CalDAG-GEF1 gene. Mutations creating premature stop codons (Arg509X, Arg573X and Trp229X) were found in the different families. Leukocytes from patients with these mutations lacked kindlin-3 protein by immunoblotting. These results appear to rule out CalDAG-GEF1 and instead suggest that the loss of kindlin-3 underlies LADIII.
The real proof came from more complete studies by two groups.2,3 Describing different patient sets, these groups ruled out mutations in CalDAG-GEF1 as causative, even in patient samples from individuals with the putative splice site mutation. At best, this polymorphism caused variable or slightly reduced CalDAG-GEF1 mRNA in lymphoblast cell lines established from some of the Turkish LADIII patients. The definitive experiment was carried out by Svensson et al.,3 who reexpressed ‘wild-type’ CalDAG-GEF1, in cell lines established from LADIII patients and found that this did not rescue the adhesion and migration defects in these cells. Both groups focused on the neighboring KIN-DLIN-3 (FERMT3) gene; Svensson et al.3 found the same Arg509X mutation in their Turkish samples, but a new exon 14 splice acceptor site mutation in the Maltese sample, whereas Malinin et al.2 found a premature stop codon (Trp16X) in two siblings. All mutations (summarized in Table 1) lead to reduced expression of kindlin-3 mRNA and protein. Lymphocytes from patients with the Trp16X mutation produced a truncated form of kindlin-3, presumably due to reinitiation at an alternative ATG transcription start site at codon 181. Most importantly, both groups re-expressed wild-type kindlin-3 protein in immortalized lymphoblast cell lines derived from patients and restored their normal adhesive and migratory defects. In summary, these three reports provide definitive evidence that LADIII results from loss-of-function mutations in the KINDLIN-3 (FERMT3) gene.
Table 1.
Mutations in kindlin-3 found in LADIII patients
| Mutation | Mutational result | Protein expression | Number of patients |
Reference |
|---|---|---|---|---|
| G→A at nucleotide 48 of exon 2 resulting in Trp16X |
Premature stop codon close to N terminus of kindlin-3 |
No (potential truncated protein from alternative initiation codon that lacks N terminus) |
2 | 2 |
| G→A at nucleotide 687 of exon 6 resulting in Trp229X |
Premature stop codon at N terminus of FERM domain |
No | 1 | 1 |
| C→T at nucleotide 1525 of exon 12 resulting in Arg509Xa |
Premature stop codon within FERM F2 subdomain | No | 14 | 1,3,11 |
| A→G in splice acceptor site of exon 14 | Joining of exons 13 and 14 affected, loss of FERM F3 subdomain |
No | 1 | 3 |
| C→T at nucleotide 1717 of exon 14 resulting in Arg573X |
Premature stop codon within FERM F3 subdomain |
No | 1 | 1 |
The kindlin family consists of three proteins: kindlin-1 and -2 are widely expressed in many tissues, whereas kindlin-3 is restricted to hematopoietic cells.12 Kindlins contain a FERM domain that is most closely related to talin, but is unusual due to the insertion of a PH domain. Kindlins bind to the membrane distal NPXY motif in the tails of β1- and β3-integrins where they are believed to act as coactivators with talin to mediate integrin subunit tail separation. Mutations within the β3-integrin membrane distal NPXY motif lead to Glanzmann’s thrombasthenia suggesting that the impaired integrin activation in this syndrome could be caused by failure to recruit kindlin-3 to the integrin.9 Mutations within the KINDLIN-1 gene produce Kindler’s syndrome, characterized by skin blistering and oral mucosal inflammation. Keratinocytes from these patients show defects in cell–cell adhesion properties. Mice lacking kindlin-1 also show skin defects, but die soon after birth from loss of the intestinal epithelial barrier.13 Kindlin-2-deficient mice die early in gestation due to an implantation defect, but ES cells lacking kindlin-2 show decreased adhesion on a variety of integrin ligands. An elegant study by Moser et al.,14 published alongside the human KINDLIN-3 studies, shows that loss of kindlin-3 in mice blocks leukocyte β2-integrin activation, leading to defective neutrophil adhesion and activation—exactly as seen in the LADIII patients. Furthermore, kindlin-3-deficient mice die early in life due to severe platelet dysfunction, again mirroring the LADIII phenotype.
These studies show that kindlin-3 plays a central role in the activation of hematopoietic integrins allowing leukocyte adhesion/migration in response to infection, and platelet activation for normal thrombosis. Kindlin-1 and -2 appear to serve the same function to mediate cell adhesion in a wide variety of non-hematopoietic cells. These studies also show us the importance of re-expressing wild-type proteins in mutant cells to validate that the mutation we are studying is really causing the phenotype observed. It’s not just reviewers being picky! It is only when such rescue or re-expression studies were carried out that the controversy over the etiology of LADIII was truly solved.
References
- 1.Kuijpers TW, van de Vijver E, Weterman MA, de Boer M, Tool AT, van den Berg TK, et al. LAD-1/variant syndrome is caused by mutations in FERMT3. Blood. (e-pub ahead of print 8 December 2008; doi:10.1182/blood-2008-10-182154) [DOI] [PubMed]
- 2.Malinin NL, Zhang L, Choi J, Ciocea A, Razorenova O, Ma YQ, et al. A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat Med. 2009;15:313–318. doi: 10.1038/nm.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svensson L, Howarth K, McDowall A, Patzak I, Evans R, Ussar S, et al. Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat Med. 2009;15:306–312. doi: 10.1038/nm.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etzioni A. Leukocyte adhesion deficiencies: molecular basis, clinical findings, and therapeutic options. Adv Exp Med Biol. 2007;601:51–60. doi: 10.1007/978-0-387-72005-0_5. [DOI] [PubMed] [Google Scholar]
- 5.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Ann Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasvolsky R, Feigelson SW, Kilic SS, Simon AJ, Tal-Lapidot G, Grabovsky V, et al. A LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDAG-GEFI in lymphocytes, neutrophils, and platelets. J Exp Med. 2007;204:1571–1582. doi: 10.1084/jem.20070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alon R, Aker M, Feigelson S, Sokolovsky-Eisenberg M, Staunton DE, Cinamon G, et al. A novel genetic leukocyte adhesion deficiency in subsecond triggering of integrin avidity by endothelial chemokines results in impaired leukocyte arrest on vascular endothelium under shear flow. Blood. 2003;101:4437–4445. doi: 10.1182/blood-2002-11-3427. [DOI] [PubMed] [Google Scholar]
- 8.Kilic SS, Etzioni A. The clinical spectrum of leukocyte adhesion deficiency (LAD) III due to defective CalDAG-GEF1. J Clin Immunol. 2009;29:117–122. doi: 10.1007/s10875-008-9226-z. [DOI] [PubMed] [Google Scholar]
- 9.Bergmeier W, Goerge T, Wang HW, Crittenden JR, Baldwin AC, Cifuni SM, et al. Mice lacking the signaling molecule CalDAG-GEFI represent a model for leukocyte adhesion deficiency type III. J Clin Invest. 2007;117:1699–1707. doi: 10.1172/JCI30575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuijpers TW, van Bruggen R, Kamerbeek N, Tool AT, Hicsonmez G, Gurgey A, et al. Natural history and early diagnosis of LAD-1/variant syndrome. Blood. 2007;109:3529–3537. doi: 10.1182/blood-2006-05-021402. [DOI] [PubMed] [Google Scholar]
- 11.Mory A, Feigelson SW, Yarali N, Kilic SS, Bayhan GI, Gershoni-Baruch R, et al. Kindlin-3: a new gene involved in the pathogenesis of LAD-III. Blood. 2008;112:2591. doi: 10.1182/blood-2008-06-163162. [DOI] [PubMed] [Google Scholar]
- 12.Larjava H, Plow EF, Wu C. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 2008;9:1203–1208. doi: 10.1038/embor.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ussar S, Moser M, Widmaier M, Rognoni E, Harrer C, Genzel-Boroviczeny O, et al. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet. 2008;4:e1000289. doi: 10.1371/journal.pgen.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, Sixt M, et al. Kindlin-3 is required for β2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med. 2009;15:300–305. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]