Abstract
BACKGROUND
m-aconitase catalyzes the first step leading to the oxidation of citrate via the Krebs cycle. It is a constituitive enzyme in virtually all mammalian cells, found in excess, and is considered to be a regulatory or regulated enzyme. In contrast to these general relationships, prostate secretory epithelial cells possess a uniquely limiting mitochondrial (m-) aconitase which minimizes the oxidation of citrate. This permits the unique prostate function of accumulating and secreting extraordinarily high levels of citrate. Previous animal studies demonstrated that testosterone and prolactin regulate the level of m-aconitase specifically in citrate-producing prostate cells. The present studies were conducted to determine if testosterone and prolactin regulated the expression of the m-aconitase gene in prostate cells, and to determine the effect of the hormones on human prostate cells.
METHODS
The studies were conducted with freshly prepared rat ventral, rat lateral, and pig prostate epithelial cells, and with the human malignant cell lines LNCaP and PC-3. The effects of 1 nM testosterone and 3 nM prolactin on the level of m-aconitase mRNA and on the transcription rate of m-aconitase were determined.
RESULTS
The studies revealed that both prolactin and testosterone increase the levels of m-aconitase mRNA and the transcription rates of m-aconitase in rat ventral prostate cells, pig prostate cells, and human malignant prostate cells (LNCaP and PC-3). In contrast, both hormones decreased the level of m-aconitase mRNA and repressed m-aconitase gene transcription in rat lateral prostate cells. The hormonal regulation of m-aconitase corresponded with the levels of m-aconitase enzyme, m-aconitase activity, and citrate oxidation.
CONCLUSIONS
In addition to the constitutive expression of m-aconitase, the m-aconitase gene is testosterone- and prolactin-regulated in specifically targeted prostate cells. The hormonal regulation of m-aconitase gene expression and biosynthesis of m-aconitase provide a regulatory mechanism for the oxidation of citrate, and consequently, the level of net citrate production by prostate. The hormonally increased expression and biosynthesis of m-aconitase in human malignant cells might be involved in the increased citrate oxidation associated with the development of true malignant cells in prostate cancer.
Keywords: mitochondrial aconitase, prostate cells, aconitase gene, testosterone, prolactin, citrate oxidation, prostate cancer
INTRODUCTION
Mitochondrial (m-) aconitase is a constitutive enzyme which is expressed in all mammalian cells. Generally it is not considered to be a regulatory enzyme of intermediary metabolism, since its activity normally is in excess and not rate-limiting. This is evident from the fact that mammalian cells consistently maintain a relatively constant steady-state citrate/isocitrate ratio of about 9–10/1, which is determined by the equilibrium established by m-aconitase. Consequently, little attention has been directed to the possibility that m-aconitase might function as a regulatory and regulated enzyme.
These general relationships are not applicable to prostate epithelial cells. The prostate gland of humans and many other animals has the unique capability and function of producing and secreting extraordinarily high levels of citrate (for reviews of prostate citrate metabolism, see Costello and Franklin [1–3] and Costello et al. [4]). Normal human prostate contains about 15,000 nmol citrate/g tissue vs. 150–400 nmol/g for all other soft tissues; the citrate concentration in prostatic fluid is 10–150 mM vs. 0.1–0.2 mM for blood plasma. Normal prostate tissue exhibits a citrate/isocitrate ratio of 30–40/1. Unlike virtually all other cells in which citrate is an essential oxidizable intermediate of metabolism, in prostate cells citrate is an end-product of metabolism. To achieve these functional and metabolic relationships, prostate secretory epithelial cells possess a uniquely limiting m-aconitase activity which minimizes the oxidation of citrate and essentially truncates the operation of the Krebs cycle. Consequently, m-aconitase is a regulatory enzyme in the metabolism of normal prostate epithelial cells.
Moreover, in prostate cancer (PCa), malignant epithelial cells have lost the ability to accumulate citrate, i.e., m-aconitase is not limiting and these “true” malignant cells (as defined from immortalized malignant prostate cell lines) are “typical” citrate-oxidizing cells. Therefore, the regulation of m-aconitase is a key factor in normal prostate function and in the pathogenesis of PCa. These relationships raise important issues concerning the mechanism(s) of regulation of m-aconitase activity in normal and malignant prostate epithelial cells. One important factor is the unique accumulation of zinc by normal prostate cells, which functions as a specific inhibitor of m-aconitase activity [5].
Another important factor in the regulation of prostate m-aconitase is the role of testosterone and prolactin. The early studies of Humphrey and Mann [6], Grayhack and Lebowitz [7], and others (reviewed by Costello and Franklin [8,9]) established that these are the major hormones involved in the regulation of net citrate by production, i.e., the accumulation and secretion of citrate by prostate. However, the mechanisms of hormonal regulation of net citrate production have not been elucidated. Consequently, we postulated that prolactin and testosterone might be involved in the regulation of the biosynthesis and level of m-aconitase in targeted prostate cells. Previous in vivo and in vitro studies with rat and pig prostates demonstrated that both hormones regulate the level of m-aconitase enzyme in citrate-producing prostate epithelial cells [10–12]. The regulation is cell-specific in that other cells are not similarly affected by either hormone. Moreover, cytosolic aconitase is not regulated by these hormones. Thus, m-aconitase, specifically in citrate-producing prostate epithelial cells, is a hormonally-regulated enzyme. However, the regulation is complicated by the fact that the hormonal effects on m-aconitase enzyme levels can be stimulatory as in rat ventral prostate and pig prostate cells, or inhibitory as in rat lateral prostate cells, or not effective as in rat dorsal prostate (which is not a citrate-producing gland).
These animal studies raised the important question as to which prostate epithelial cell type is represented in human prostate epithelial cells. The present studies were conducted to determine if m-aconitase is hormonally regulated in human prostate epithelial cells, and if the mechanism of the hormonal effects on the biosynthesis of m-aconitase involves the regulation of the m-aconitase gene. The results reveal for the first time that the m-aconitase gene is hormonally (by testosterone and prolactin) regulated specifically in human and animal prostate epithelial cells. This represents the first report (to our knowledge) that provides direct evidence that the m-aconitase gene in any mammalian cells is hormonally regulated in addition to its constitutive expression.
MATERIALS AND METHODS
Freshly prepared rat prostate epithelial cells were obtained from Wistar rats weighing 275–350 g. Handling and treatment of rats were in accordance with National Institutes of Health guidelines. The resection of the ventral and lateral prostate lobes and the subsequent preparation of the epithelial cell suspensions have been previously described [11,13].
During the course of this investigation, we were fortunate enough to gain access to four normal mini-pigs provided by Dr. Vernon Pursel (Beltsville Agricultural Research Center, Beltsville, MD). The prostate glands were collected within several minutes, following the sacrifice of the animals. The preparation of epithelial cell suspensions was as previously described [12].
The human malignant prostate cell lines LNCaP and PC-3 were obtained from the American Type Culture Collection (Rockville, MD). The cells were routinely cultured and harvested, as earlier described [14]. Transient transfection of PC-3 cells with androgen receptor was achieved by the method of Juang et al. [15].
The level of m-aconitase enzyme was determined by Western blot [10–12], using an m-aconitase antibody which was raised against purified beef heart m-aconitase (kindly provided by Dr. Claire Kennedy) The antibody is specific for m-aconitase in that it does not react with the cytosolic isozyme. The antibody does cross-react with rat, pig, and human m-aconitase.
The extraction of RNA and Northern blot analysis were performed as previously described [14,16]. m-aconitase mRNA was probed with a full-length human cDNA (Genbank accession no. U80040) kindly provided by Dr. H. Juang. The transcription rate of m-aconitase was determined by nuclear runoff assay [17].
The effects of hormone treatment on m-aconitase mRNA levels and transcription of prostate cells was determined as follows. Generally, aliquots of the cell suspension were added to HBSS in a final volume of 5.0 ml. Testosterone (1 nM), prolactin (3 nM), or vehicle was added to the reaction system. These hormone concentrations were elected based on their effectiveness in altering the level of m-aconitase enzyme in prostate cells [10–12]. The reaction tubes were incubated at 37°C for varying time periods. At conclusion of the reaction period, the reaction tubes were centrifuged to harvest the cells. The cells were then washed with cold HBSS, harvested, and suspended in appropriate medium for RNA extraction or nuclei preparation.
In all experiments, duplicate reaction systems were employed. Most experiments were repeated at least twice. The results presented are representative of the replicated experiments, and we observed no experiments which deviated from the presented effects.
RESULTS
Rat Prostate Epithelial Cells
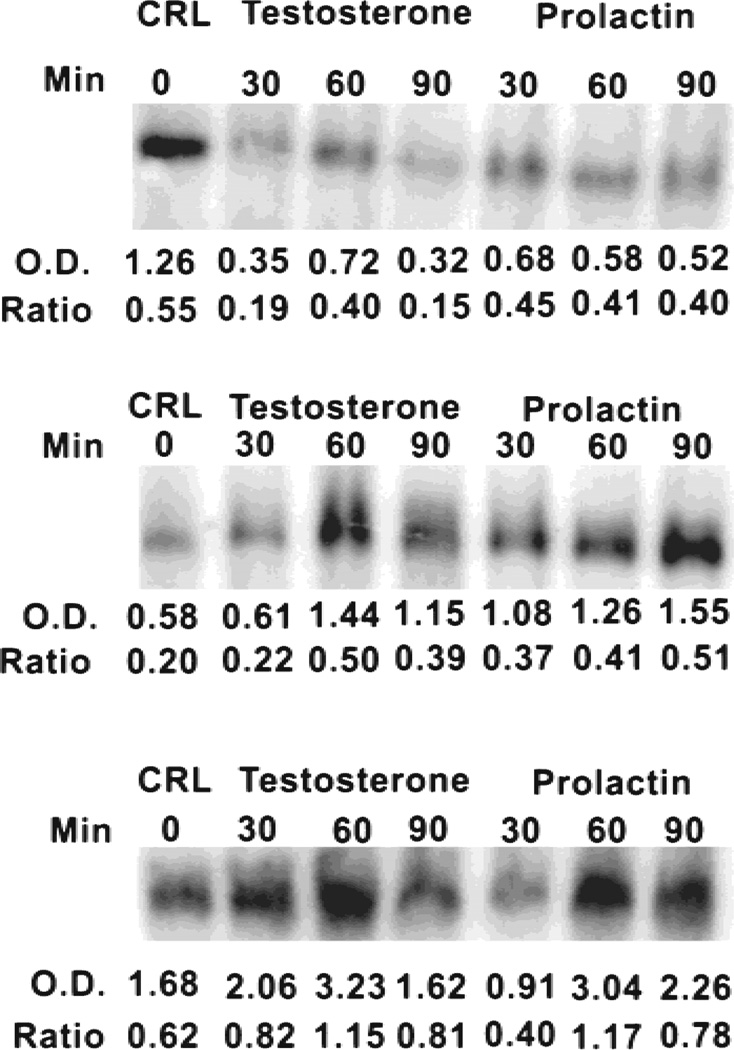
Our earlier studies [10,11] demonstrated that both prolactin and testosterone increased the level of m-aconitase enzyme in rat ventral prostates, while decreasing the level in rat lateral prostates. Consequently, we proceeded to determine hormonal effects on the level of m-aconitase mRNA (Fig. 1). Both prolactin and testosterone treatment of ventral prostate cells resulted in a significant increase in the cellular level of aconitase mRNA within 60 min following hormonal treatment. In the case of lateral prostate cells, both hormones decreased the level of m-aconitase mRNA. The decrease was evident by 30 min following hormonal exposure. This would suggest that m-aconitase mRNA has a short half-life, and this characteristic needs to be pursued in future studies.
Fig. 1.
Effects of prolactin (3 nM) and testosterone (1 nM) on levels of m-aconitase mRNA in rat lateral (top lane) and rat ventral prostate (middle lane) epithelial cells and pig prostate (bottom lane) epithelial cells. O.D. values are relative optical density values within each experiment. Ratio values are relative optical density values corrected for any differences in RNA loading.
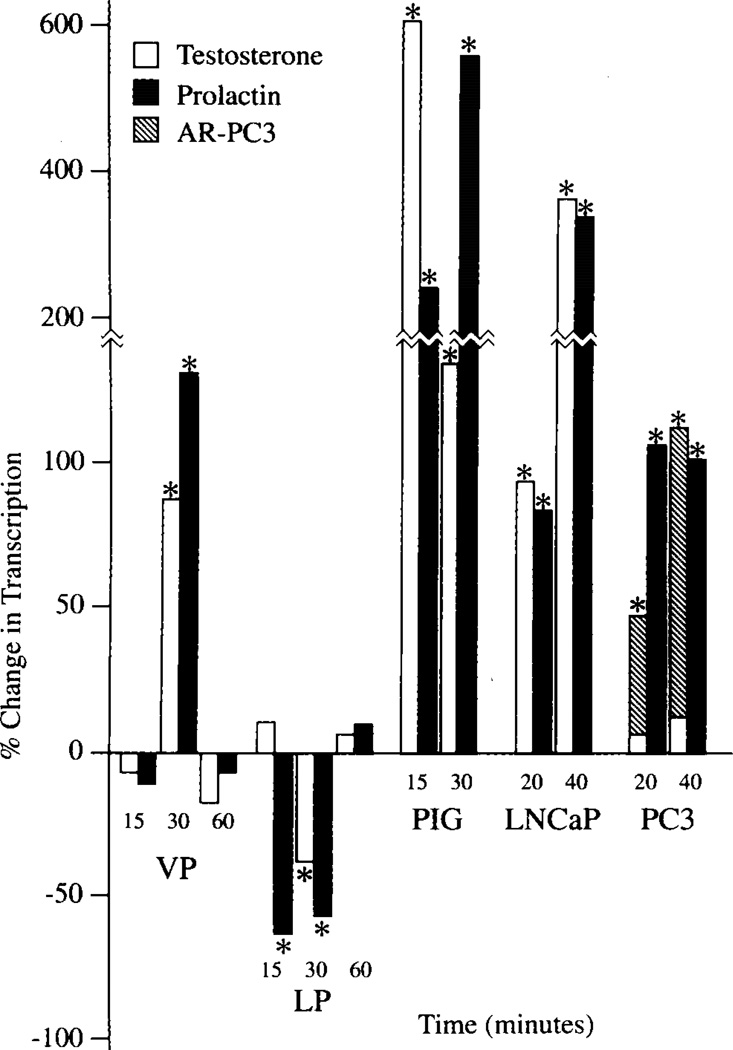
Based on these results and the earlier report that hormonal effects were inhibited by actinomycin and cycloheximide [10,11], we proceeded to determine directly if the hormonal effects were mediated via regulation of m-aconitase gene transcription. Figure 2 shows that both prolactin and testosterone markedly stimulated the transcription rate of nuclei from ventral prostate cells treated with hormone. The stimulatory effect was evident between 15–30 min following exposure to hormone. The effect was transient, with the transcription rate returning to contol rate at between 30–60 min. In contrast to the stimulatory effects on the ventral prostate, both hormones markedly inhibited the transcription of lateral prostate cells over the same hormonal exposure period. These results are consistent with the hormonal effects on the level of m-aconitase enzyme as earlier reported [10,11].
Fig. 2.
Effects of prolactin and testosterone on rates of transcription of m-aconitase in prostate epithelial cells. % Change in Transcription is relative transcription rate at each time course in relation to the initial 0-time transcription rate. *Significant change in transcription rate. VP, rat ventral prostate; LP, rat lateral prostate; AR-PC3, PC3 cells transfected with androgen receptor.
Pig Prostate Cells
We previously reported that both prolactin and testosterone stimulated the level of m-aconitase enzyme and m-aconitase activity of normal pig prostate epithelial cells [12]. During the course of this current investigation, we were again fortunate to gain access to a limited supply of normal pigs which provided a source of freshly prepared normal epithelial cells, as described in our earlier report. This allowed us to determine hormonal effects on m-aconitase gene expression and to relate these effects to the earlier study regarding m-aconitase enzyme level.
Treatment of pig prostate cells with either prolactin or testosterone resulted in a transient increase in the cellular level of m-aconitase mRNA (Fig. 1). With testosterone, the mRNA increase was evident by 30 min, while the prolactin effect was evident by 60 min. Therefore, we proceeded to determine if hormone treatment also increased the expression of the m-aconitase gene. Nuclei isolated from pig prostate cells preincubated with either testosterone or prolactin exhibited a significant increase in the transcription rate of m-aconitase (Fig. 2). The increase was apparent by 15 min following hormone treatment. Thus the hormonal stimulation of m-aconitase in pig prostate cells was identical to the hormonal regulation of rat ventral prostate cells.
LNCaP and PC-3 Cells
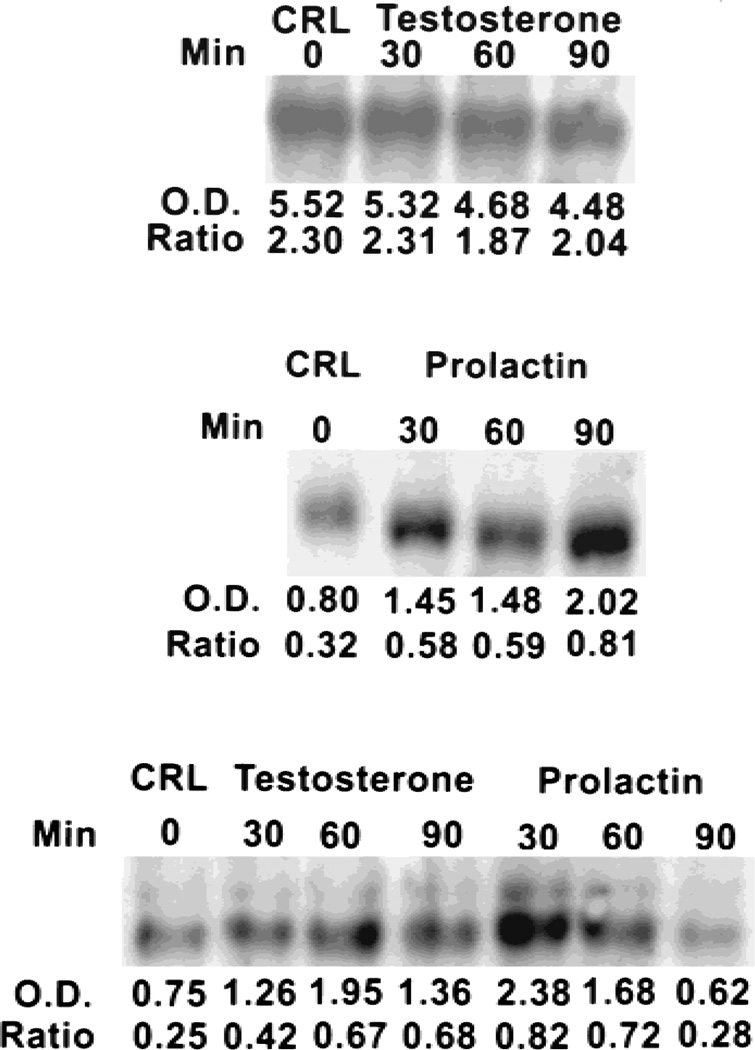
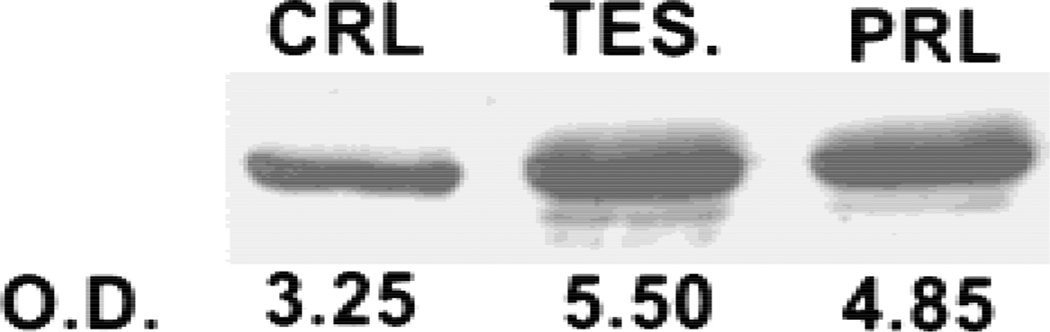
It is clear from the results above and our earlier reports [10–12] on animal studies that hormonal regulation of m-aconitase is dependent on the source of the prostate epithelial cells. The responses include hormonal stimulation (as in rat ventral prostate and pig prostate cells), hormonal inhibition (as in the case of rat lateral prostate cells), or no response to hormones (as in the case of rat dorsal prostate cells). An important issue now was which type of hormonal effects on m-aconitase existed in the human prostate. We previously reported that LNCaP cells were citrate-producing cells and exhibited low citrate oxidation [18]. In addition, prolactin and testosterone stimulated citrate oxidation by LNCaP cells. Therefore, we determined the effect of these hormones on the level of m-aconitase enzyme. Figure 3 shows that treatment of these cells with testosterone or prolactin resulted in an increase in the level of m-aconitase enzyme. This would be consistent with the hormonal stimulation of citrate oxidation. We then proceeded to determine hormonal effects on gene expression. Figure 4 reveals that treatment of LNCaP cells with either testosterone or prolactin resulted in a significant increase in the cellular level of m-aconitase mRNA within 30 min. Consistent with this effect, both hormones also stimulated the transcription of m-aconitase within 15 min of treatment. The results clearly establish that prolactin and testosterone are positive regulators of the expression and biosynthesis of m-aconitase in LNCaP cells.
Fig. 3.
Effects of prolactin and testosterone on levels of m-aconitase mRNA in human prostate cells. Top and middle lanes, PC-3 cells; bottom lane, LNCaP cells. See Figure 1 for further specifics.
Fig. 4.
Effects of prolactin and testosterone on level of m-aconitase enzyme in LNCaP cells. Cells were exposed to hormone for 2.5 hr.
In the case of PC-3 cells, prolactin markedly increased the level of m-aconitase mRNA, whereas testosterone had no effect (Fig. 3). Correspondingly, prolactin, but not testosterone, increased m-aconitase transcription of PC-3 cells (Fig. 2). The absence of an effect of testosterone on PC-3 cells is consistent with the absence of normal androgen receptor in these cells. However, when PC-3 cells were transfected with androgen receptor, testosterone markedly stimulated the transcription of m-aconitase (Fig. 2). The requirement for androgen receptor further supports the conclusion that hormonal effects on m-aconitase are mediated via gene regulation. Thus the hormonal effects on these human malignant prostate cell lines are similar to the effects observed with pig prostate and rat ventral prostate cells.
DISCUSSION
The results of this study coupled with previous reports [10–12] provide conclusive evidence that testosterone and prolactin regulate the transcription of the m-aconitase gene in human and animal prostate epithelial cells. The cell specificity of this regulation is revealed by the absence of any effect of either hormone on m-aconitase in normal cells which are not prostate citrate-producing cells [10–12]. Even the m-aconitase of citrate-producing pig seminal vesicle cells is unaffected by either hormone, although citrate-producing pig prostate cells are stimulated by both hormones [12]. Although both function as citrate-producing cells, they are not ontogenetically homologous in that seminal vesicles are derived from Wolffian duct, while the prostate arises from the urogenital sinus. Thus, in addition to the constitutive expression of m-aconitase in all mammalian cells, the m-aconitase gene is a prolactin- and androgen-responsive gene specifically in citrate-producing prostate epithelial cells. To our knowledge this is the first report which directly demonstrates that the m-aconitase gene can be a hormonally regulated gene.
In typical mammalian cell intermediary metabolism, the constitutive level of m-aconitase activity is in excess so that it is not a regulatory rate-limiting enzyme. Under such conditions, increased levels of the enzyme would have no metabolic effect on the oxidation of citrate and the operation of the Krebs cycle. Therefore, it is reasonable to inquire if the hormonal regulation of the expression and biosynthesis of m-aconitase has any significant effect on net citrate production and citrate oxidation in prostate epithelial cells. Our earlier studies [10–12] demonstrated that prolactin- and testosterone-induced alterations of the level of m-aconitase specifically in normal citrate-producing prostate epithelial cells (rat lateral and ventral prostate and pig prostate cells) were consistently accompanied by corresponding alterations in the oxidation of citrate. Coupled with other information such as the characteristic high citrate/isocitrate ratio of citrate-producing prostate and the insensitivity of prostate citrate oxidation to fluoroacetate [1,2,8,9], there can be little doubt that m-aconitase activity and citrate oxidation are uniquely limiting in citrate-producing prostate epithelial cells. Therefore, the alteration in m-aconitase activity via prolactin, and testosterone regulation of m-aconitase gene expression, and the biosynthesis of m-aconitase play an important role in the ability of citrate-producing cells to oxidize citrate, and in the prostatic function of high citrate production and secretion.
However, the regulation of m-aconitase activity also involves zinc [3], which is a specific inhibitor of m-aconitase activity in prostate cells [5]. Alterations in zinc accumulation were excluded as a factor in the present studies and in previous studies [10–12], so that we could establish the relationship of altered biosynthesis of m-aconitase to m-aconitase activity and citrate oxidation. Our recent studies with rat prostates [19] demonstrated that zinc accumulation was regulated by prolactin and testosterone. A perfect correlation existed in that hormonal alteration of zinc accumulation was inversely related to the hormonal effect on biosynthesis of m-aconitase and citrate oxidation. Thus, the regulation of m-aconitase activity and citrate oxidation occurs by hormonal regulation of m-aconitase gene expression and by hormonal regulation of zinc accumulation. We are now investigating the interrelationship of these two factors in normal and malignant prostate cells.
In normal prostate epithelial cells, net citrate production is determined by the relative rates of citrate synthesis and citrate oxidation. Because of the limiting m-aconitase, the rate of citrate synthesis is about 6–10 times greater than the rate of citrate oxidation [20,21]. One might conclude that hormonal stimulation of m-aconitase and citrate oxidation should necessarily result in decreased net citrate production in prostate cells. This would seem to be inconsistent with the fact that hormone treatment can increase both citrate oxidation and citrate accumulation, as in the case of testosterone effects on net citrate production in rat ventral prostate cells [20]. However, this is due to hormonal stimulation of citrate synthesis as well as citrate oxidation, as previously discussed [20]. In contrast, in the rat lateral prostate, hormones increase citrate accumulation predominantly by inhibiting m-aconitase biosynthesis and citrate oxidation. Thus one must take into consideration the effects of hormones on citrate synthesis and on citrate oxidation when determining the mechanism of hormonal control of net citrate production by normal prostate epithelial cells. Nevertheless, hormonal regulation of m-aconitase gene expression, leading to increased biosynthesis and level of m-aconitase enzyme and activity, is an important factor in determining the accumulation of citrate, and in the oxidation of citrate, which has important energetic (i.e., coupled ATP production) and metabolic implications associated with the functioning of the Krebs cycle.
It is now evident that m-aconitase is a regulatory and regulated enzyme in targeted prostate epithelial cells. Evidence is emerging that altered m-aconitase activity is associated with altered metabolism in other cells [22–24], but those studies provided no information concerning alterations in the level of m-aconitase enzyme, i.e., the biosynthesis of m-aconitase and the regulation of the m-aconitase gene. Melnick et al. [25] recently demonstrated that the levels of m-aconitase enzyme and activity of renal cortical cells were altered by acid-base changes in relation to citraturia. Thus, m-aconitase enzyme expression and biosynthesis might prove to be more regulated in mammalian cells than currently recognized.
The hormonal regulation of the m-aconitase gene is similar to the regulation of the mitochondrial aspartate aminotransferase (mAAT) gene by prolactin and testosterone specifically in prostate cells [14,15,17,26]. In prostate cells, mAAT is a key regulatory enzyme in the synthesis of citrate. The prolactin regulation of mAAT is mediated via protein kinase c (PKC); the mAAT gene contains a putative PKC response element [15,26] (also, our unpublished information). The mAAT gene also contains an androgen-response element which is essential for testosterone regulation [14,17] (also, our unpublished information). It will be important to determine if the m-aconitase gene also contains the PKC and androgen-response elements, and to establish the mechanism associated with the cell specificity of the hormonally-regulated prostate m-aconitase gene. Moreover, these studies raise the intriguing issue of the mechanism by which hormones stimulate gene expression in some prostate cells while repressing gene expression in other prostate cells, even within the same species (e.g., rat ventral vs. rat lateral prostate cells).
In sharp contrast to normal human prostate and benign prostatic hyperplasia, PCa is characterized by loss of ability of the malignant cells to accumulate citrate [1–4]. Thus, prostate malignancy involves the metabolic transformation of nonmalignant citrate-producing epithelial cells into malignant citrate-oxidizing cells. This metabolic transformation involves an alteration of m-aconitase activity. An important issue related to the pathogenesis of PCa is the mechanism(s) of increased m-aconitase activity in malignant prostate cells. It will be of paramount importance to establish the role of hormonal and genetic relationships in the normal and malignant human prostate. LNCaP and PC-3 cell lines were derived from prostate malignancies. However, the relationship of the characteristics of these established immortalized cell lines to the original malignant progenitor cells (i.e., “true” malignant prostate cells) is questionable. Nevertheless, under specific experimental conditions they do express genotypic and phenotypic characteristics of human prostate cells. In normal culture medium, LNCaP cells are citrate-producing cells and do not readily oxidize citrate. Treatment of LNCaP cells with testosterone or prolactin increases m-aconitase activity, increases citrate oxidation, increases oxygen consumption, and decreases citrate production [18] (also, our unpublished information). These effects are consistent with the present observations that both hormones stimulate expression of the m-aconitase gene. As discussed above, this is taken as additional supporting evidence that that the hormonal regulation of m-aconitase transcription functionally and metabolically alters the metabolism of citrate of these cells. Moreover, LNCaP cells exhibit a low tumorigenicity, which is consistent with their metabolic characteristic of being citrate-producing cells. In the presence of Matrigel, the respiration of cells is increased by 125% (our unpublished information), indicative of altered metabolism, and the cells become aggressively tumorigenic. These relationships provide a model which mimics the metabolic transformation associated with the development of citrate-oxidizing true malignant prostate cells in PCa from nonmalignant citrate-producing prostate cells. Unfortunately, the lack of availability of functional normal human peripheral zone secretory epithelial cells and true malignant prostate cells has impeded progress in resolving these important issues, which we can now begin to address.
ACKNOWLEDGMENTS
We express our appreciation to Dr. Vernon Pursel (Reproduction Laboratory, Livestock and Poultry Sciences Institute, Beltsville Agricultural Research Center, Beltsville, MD) for providing the pig prostate tissue employed in these studies. We are grateful to Dr. H.H. Juang (Department of Anatomy, Chang Gung College of Medicine and Technology, Taiwan) for making available the m-aconitase cDNA. Our thanks go to Dr. Mary Claire Kennedy (Department of Biochemistry, Medical College of Wisconsin) for providing the purified m-aconitase used in raising the antibody, and for her expert and valuable discussions relating to the m-aconitase enzyme.
Grant sponsor: NIH NCI; Grant number: CA 71207.
REFERENCES
- 1.Costello LC, Franklin RB. Citrate metabolism of normal and malignant prostate epithelial cells. Urology. 1997;50:3–12. doi: 10.1016/S0090-4295(97)00124-6. [DOI] [PubMed] [Google Scholar]
- 2.Costello LC, Franklin RB. Intermediary energy metabolism of normal and malignant prostate epithelial cells. In: Naz RK, editor. Prostate: basic and clinical aspects. New York: CRC Press; pp. 115–150. [Google Scholar]
- 3.Costello LC, Franklin RB. The novel role of zinc in the intermediary metabolism of prostate epithelial cells and its implications in prostate malignancy. Prostate. 1998;35:285–296. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Costello LC, Franklin RB, Narayan P. Citrate in the diagnosis of prostate cancer. Prostate. 1999;38:237–245. doi: 10.1002/(sici)1097-0045(19990215)38:3<237::aid-pros8>3.0.co;2-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello LC, Liu Y, Franklin RB, Kennedy MC. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J Biol Chem. 1997;272:28875–28881. doi: 10.1074/jbc.272.46.28875. [DOI] [PubMed] [Google Scholar]
- 6.Humphrey GF, Mann T. Studies on the metabolism of semen. 5. Citric acid in semen. Biochem J. 1949;44:97–105. [PMC free article] [PubMed] [Google Scholar]
- 7.Grayhack JT, Lebowitz A. Effect of prolactin on citric acid of lateral lobe of prostate of Sprague-Dawley rats. Invest Urol. 1967;5:87–94. [Google Scholar]
- 8.Costello LC, Franklin R. Concepts of citrate production and secretion by prostate. 1. Metabolic relationships. Prostate. 1991;18:25–46. doi: 10.1002/pros.2990180104. [DOI] [PubMed] [Google Scholar]
- 9.Costello LC, Franklin RB. Concepts of citrate production and secretion by prostate. 2. Hormonal relationships in normal and neoplastic prostate. Prostate. 1991;19:181–205. doi: 10.1002/pros.2990190302. [DOI] [PubMed] [Google Scholar]
- 10.Costello LC, Liu Y, Franklin RB. Testosterone stimulates the biosynthesis of m-aconitase in prostate epithelial cells. Mol Cell Endocrinol. 1995:45–51. doi: 10.1016/0303-7207(95)03582-r. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Costello LC, Franklin RB. Prolactin specifically regulates citrate oxidation and m-aconitase of prostate epithelial cells. Metabolism. 1996;45:442–449. doi: 10.1016/s0026-0495(96)90217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costello LC, Liu Y, Franklin RB. Testosterone and prolactin stimulation of m-aconitase in pig prostate epithelial cells. Urology. 1996;48:654–659. doi: 10.1016/S0090-4295(96)00217-8. [DOI] [PubMed] [Google Scholar]
- 13.Costello LC, Franklin RB, Liu Y. Testosterone regulates pyruvate dehydrogenase E1α in prostate. Endocr J. 1994;2:147–151. [Google Scholar]
- 14.Franklin RB, Zou J, Gorski E, Yang YH, Costello LC. Prolactin regulation of mAAT and PKC in human prostate cancer cells. Mol Cell Endocrinol. 1997;127:19–25. doi: 10.1016/s0303-7207(96)03972-x. [DOI] [PubMed] [Google Scholar]
- 15.Juang HH, Costello LC, Franklin RB. Androgen modulation of multiple transcription start sites of the mAAT gene in rat prostate. J Biol Chem. 1996;270:12629–12634. doi: 10.1074/jbc.270.21.12629. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi P. A single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Qian K, Franklin RB, Costello LC. Testosterone regulates mitochondrial aspartate aminotransferase gene expression and mRNA stability in prostate. J Steroid Biochem Mol Biol. 1993;44:13–19. doi: 10.1016/0960-0760(93)90146-n. [DOI] [PubMed] [Google Scholar]
- 18.Franklin RB, Juang HH, Zou J, Costello LC. Regulation of citrate metabolism by androgen in human prostate carcinoma cells. Endocrine. 1995;5:603–607. doi: 10.1007/BF02953026. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Franklin RB, Costello LC. Prolactin and testosterone regulation of mitochondrial zinc in prostate epithelial cells. Prostate. 1997;30:26–32. doi: 10.1002/(sici)1097-0045(19970101)30:1<26::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 20.Franklin RB, Khang MW, Akuffo V, Costello LC. The effect of testosterone on citrate synthesis and citrate oxidation and a proposed mechanism for regulation of net citrate production in prostate. Horm Metab Res. 1986;18:177–181. doi: 10.1055/s-2007-1012264. [DOI] [PubMed] [Google Scholar]
- 21.Franklin RB, Costello LC. Glutamate dehydrogenase and a proposed glutamate-aspartate pathway for citrate synthesis in rat ventral prostate. Urology. 1984;132:1239–1243. doi: 10.1016/s0022-5347(17)50113-5. [DOI] [PubMed] [Google Scholar]
- 22.Boquist L, Ericsson I, Lorentzon R, Nelson LA. Alterations in mitochondrial aconitase activity and respiration and in concentration of citrate in some organs of mice with experimental or genetic diabetes. FEBS Lett. 1985;183:173–176. doi: 10.1016/0014-5793(85)80979-0. [DOI] [PubMed] [Google Scholar]
- 23.Hernanz A, de la Fuente M. Characteristics of aconitate hydratase from mitochondria and cytoplasm of ascites tumor cells. Biochem Cell Biol. 1998;66:792–795. doi: 10.1139/o88-090. [DOI] [PubMed] [Google Scholar]
- 24.Hall R, Henrikson K. Mitochondrial myopathy with succinic dehydrogenase and aconitase deficiency. J Clin Invest. 1993;92:2660–2666. doi: 10.1172/JCI116882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melnick JM, Preisig PA, Moe OW, Srere P, Alpern RJ. Renal cortical mitochondrial aconitase is regulated in hypo- and hypercitarturia. Kidney Int. 1998;54:160–165. doi: 10.1046/j.1523-1755.1998.00974.x. [DOI] [PubMed] [Google Scholar]
- 26.Gorski E, Zou J, Costello LC, Franklin RB. Protein kinase C mediates prolactin regulation of mitochondrial aspartate aminotransferase gene expression in prostate cells. Mol Urol. 1999;3:17–23. [PubMed] [Google Scholar]