Abstract
Despite the successes of tyrosine kinase inhibitors (TKIs) in improving outcomes in patients with chronic myeloid leukemia (CML) and Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL), allogeneic hematopoietic stem cell transplantation (HSCT) continues to be an important and potentially curative option for selected patients with either disease. After HSCT, TKIs are increasingly being used to treat or prevent disease relapse, and practice patterns suggest that these TKIs are often chosen empirically without regard to pre-HSCT mutation status. We investigated whether ABL kinase domain mutations persist after transplantation, and thus whether pre-HSCT mutation status should inform the selection of post-HSCT TKIs in these patients. We retrospectively analyzed adults who underwent allogeneic HSCT for CML and Ph+ ALL at our institution between 2000 and 2010, and identified subjects who had detectable BCR-ABL transcripts by polymerase chain reaction (PCR), as well as available RNA for Sanger sequencing of the ABL kinase domain, in both the pre- and post-HSCT settings. In total, 95 CML and 20 Ph+ ALL patients with positive PCR transcripts were identified, of which 10 (10.5%) and 4 (20.0%), respectively, were found to have pre-HSCT ABL kinase mutations known to confer TKI resistance. In 9 (64.2%) of these 14 patients, the same kinase mutation was also detectable at an average time of 191 days post-HSCT. Seven (50.0%) of the 14 harboring mutations had relapsed/refractory disease by last follow-up, of which, in retrospect, 6 had received a predictably ineffective TKI within the first 100 days following transplant, based on our mutation analysis. These data support the idea that pre-existing mutations in the ABL kinase domain, frequently associated with resistance to TKIs and prevalent in a transplant population, are persistently detectable in the majority of patients after transplant. We propose that such resistance patterns should be considered when selecting TKIs in the post-HSCT setting, including clinical trials of post-HSCT TKI prophylaxis.
Keywords: BCR-ABL kinase mutations, Chronic myeloid leukemia, Philadelphia-chromosome acute lymphoblastic leukemia, Allogeneic hematopoietic stem cell transplantation
INTRODUCTION
Tyrosine kinase inhibitors (TKIs) remain the front-line therapy for chronic myeloid leukemia (CML) and also improve outcomes when incorporated into induction and maintenance regimens for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Resistance to TKIs is commonly due to the emergence of clones containing point mutations in the ABL kinase domain of BCR-ABL, occurring in as many as 30-60% of CML patients with imatinib resistance,1,2 as well as in over one third of Ph+ ALL patients at the time of diagnosis.3 More than 100 ABL kinase domain mutations associated with TKI resistance have been described.4
In the current treatment approach for CML, allogeneic hematopoietic stem cell transplant (HSCT) is generally reserved for patients who fail or are intolerant of TKI therapy, or for those with advanced phase disease. Transplantation is also a potentially curative option for Ph+ ALL patients with HLA identically matched related or unrelated donors. Relapse after HSCT is fairly common in both advanced phase CML (30-40%)5,6 and Ph+ ALL (30-60%).7 Therapeutic strategies to treat post-HSCT relapse historically consisted of withdrawal of immunosuppression or donor lymphocyte infusions, though several studies have shown efficacy in treating relapsed CML or Ph+ ALL with TKIs.8-15 An increasing number of studies have also evaluated the prophylactic use of TKIs after transplant in high-risk patients, including those with detectable BCR-ABL at the time of transplant, though there is very limited data as to guide their selection and administration.16-19
In addition to cost, the selection of post-transplant TKIs is based, at least in part, on toxicity profiles of the available agents. The bulk of retrospective and prospective studies using imatinib indicate that it is generally well-tolerated after HSCT, even in the early post-transplant period.16-18,20,21 Data regarding the efficacy and safety of second-generation TKIs after HSCT is limited to small case series or individual case reports.13,14,22-26 In this limited experience, however, there is at least some indication that toxicity after HSCT may be more pronounced with the newer drugs. In one of the larger series reporting on the use of dasatinib in patients with relapsed CML or Ph+ ALL, all 7 patients taking dasatinib at the 70mg twice-daily dose, beginning at a mean of 9 months after HSCT, required interruption or dose reduction for gastrointestinal, hematologic, pulmonary or hepatic toxicity.13 Additional anecdotal evidence from an ongoing clinical trial at our institution increasingly suggests that nilotinib may be associated with considerably more hematologic toxicity than imatinib in the early post-transplant period (data unpublished). The second generation TKIs have also been associated with greater vascular toxicity compared to imatinib, which may be of concern in the post-transplant setting, given that cardiovascular risk factors, including diabetes, hypertension and hypertriglyceridemia, are already more prevalent in allogeneic transplant recipients.27
Considering that sub-populations of leukemic cells may exist within a patient, with some harboring a resistance mutation and others not, it is reasonable to suspect that the relative proportions of these sub-populations would be affected by the selective pressures of continued TKI exposure versus cessation of TKI exposure, as well as of the transplant conditioning regimen. The question arises whether pre-HSCT mutation status should guide the selection of post-HSCT TKI prophylaxis. Therefore, we sought to investigate if specific ABL kinase domain mutations persist in CML and Ph+ ALL patients after HSCT.
METHODS
Study Population
In this retrospective analysis, all patients who had undergone allogeneic HSCT at our Center between January 1, 2000 and July 15, 2010 for CML or Ph+ ALL, and who were at least 18 years of age at the time of transplantation, were screened for inclusion in the study. Due to logistical changes in the acquisition and processing of patient samples in 2010, we were not able to obtain RNA for laboratory testing after this time, and therefore this analysis was restricted to those patients undergoing transplantation within the specified time period. We limited chart review and laboratory analysis to those subjects with a history of any positive p210 or p190 BCR-ABL transcript by polymerase chain reaction (PCR) in both the pre- and post-HSCT settings. Patients without available records for chart review and those without available paired pre- and post-HSCT RNA were excluded. All clinical investigations were conducted according to Declaration of Helsinki principles.
Molecular Analysis
Total RNA was extracted from pre-HSCT and post-HSCT bone marrow or peripheral blood samples using TRIzol reagent (Invitrogen, CA, USA). An initial RT-PCR step with a nested PCR was used to amplify a 928-bp product spanning exons 4-9 (codons 199 – 507) of the ABL kinase domain in p210 and/or p190 transcripts. Bi-directional Sanger sequencing of the PCR product was performed using an Applied Biosystems 3730xl Analyzer (ABI, CA, USA). Pre-HSCT samples were first screened for mutations, and in patients with a mutation identified, the paired post-HSCT samples were amplified and then sequenced in a similar fashion.
RESULTS
Pre-Transplant Patient Characteristics
A total 252 adult patients underwent HSCT for CML during the specified study period. Of these, archived RNA samples were not available for 116 patients, and another 40 didn't have positive BCR-ABL transcripts both pre- and post-HSCT. One patient lacked sufficient available records for chart review. Therefore, we identified 95 CML patients for inclusion in the study. Similarly, out of a total 91 adult patients undergoing HSCT for Ph+ ALL during the study period, 45 lacked available RNA samples for testing, and 26 didn't have positive BCR-ABL transcripts both pre- and post-HSCT, leaving 20 subjects for inclusion in the study.
Clinical characteristics for both groups of patients are detailed in Table 1. History was notable for pre-HSCT TKI therapy in 61 (64.2%) of CML patients with a mean duration of pre-HSCT exposure of 12.3 months. Thirty-three (34.7%) of CML patients had demonstrated TKI resistance or failure prior to transplant, with the majority proceeding to transplant for reasons other than TKI failure. In fact, 34 (35.8%) were transplanted without prior TKI therapy, all in the early study period between January 2000 and July 2002; all patients transplanted since the latter half of 2002 were treated with at least one TKI prior to transplant. Thirty-three (34.7%) were transplanted because of a history of accelerated phase or blast crisis, of which 11 were in second or higher chronic phase at the time of HSCT. Eighteen (18.9%) were transplanted for “other” reasons, which included issues related to drug cost or availability, as well as patient/physician preference. Indications for HSCT were not mutually exclusive (for example, blast crisis and TKI failure).
Table 1.
Clinical characteristics of CML and Ph+ ALL Patients
| CML (n=95) | Ph+ ALL (n=20) | |||
|---|---|---|---|---|
| Mean age at HSCT [range], years | 41.1 [18 - 66] | 42.9 [22 - 63] | ||
| Gender | Male | 55.0% | 55.0% | |
| Female | 45.0% | 45.0% | ||
| Disease status prior to HSCT | CP1 | 60 (63.1%) | ||
| CP2+ | 13 (13.7%) | |||
| AP/BC | 22 (23.2%) | |||
| CR1 | 14 (70.0%) | |||
| CR2+ | 5 (25.0%) | |||
| Relapse | 1 (5.0%) | |||
| Indication for HSCTa | No TKI therapy | 34 (35.8%) | ||
| TKI failure | 33 (34.7%) | |||
| AP/BC | 33 (34.7%) | |||
| Otherb | 18 (18.9%) | |||
| Conditioning | Myeloablative | 84 (88.4%) | 13 (65.0%) | |
| Non-myeloablative | 11 (11.6%) | 7 (35.0%) | ||
| Pre-HSCT | Received TKI? | No | 34 (35.8%) | 2 (10.0%) |
| Yes | 61 (64.2%) | 18 (90.0%) | ||
| 1 TKI | 52 (54.7%) | 13 (65.0%) | ||
| 2 TKIs | 7 (7.4%) | 5 (25.0%) | ||
| 3 TKIs | 2 (2.1%) | .. | ||
| Type and durationc of TKI | Imatinib | 52 (54.7%) 11.3 mo |
16 (80.0)% 9.5 mo |
|
| Dasatinib | 7 (7.4%) 7.2 mo |
4 (20.0%) 4.5 mo |
||
| Nilotinib | 2 (2.1%) 5.0 mo |
.... | ||
| Post-HSCT | Received TKI? | No | 66 (69.5%) | 6 (30.0%) |
| Yes | 29 (30.5%) | 14 (70.0%) | ||
| Type and durationc of TKI | Imatinib | 25 (26.3%) 10.3+ mo |
12 (60.0%) 8.6+ mo |
|
| Dasatinib | 5 (5.3%) 24.0+ mo |
4 (20.0%) 6.5+ mo |
||
| Nilotinib | 3 (3.2%) 6.0+ mo |
2 (10.0%) 9.5 mo |
||
| Ponatinib | 1 (1.1%) 3.0 mo |
.... | ||
| Initial indication for post-HSCT | Relapsed / refractory | 10 (34.5%) | 0 (0.0%) | |
| Prophylaxis / molecular MRD | 19 (65.5%) | 14 (100.0%) | ||
Indications for HSCT are not mutually exclusive (e.g. blast crisis and TKI failure)
“Other” indications for HSCT include patient/physician preference.
Mean duration of TKI use prior to and after HSCT (for those who received the drug). At the time of last follow-up, post-HSCT TKI use was ongoing in some patients.
Among Ph+ ALL patients, 18 (90.0%) received a TKI prior to transplant, for a mean duration of 10.6 months. Nineteen (95.0%) of Ph+ ALL patients were transplanted in complete remission. Two Ph+ ALL patients had previously undergone a first allogeneic transplant, and had subsequently relapsed. The majority of patients (88.4% of CML and 65.0% of Ph+ ALL patients) received myeloablative conditioning.
Post-Transplant Therapy
By the time of last clinical follow-up at our institution, corresponding with a mean duration of 2.1 years post-transplant follow-up (range 0.2 – 11.1 years), a total of 29 (30.5%) of CML and 14 (70.0%) of Ph+ ALL patients had received a TKI at some point in the post-HSCT setting. All 14 Ph+ ALL patients receiving a TKI after transplant initially received the drug for prophylaxis, beginning at a mean of 1 month post-HSCT (range 0.5-2 months). Of these, 4 ultimately relapsed – two of which had pre-transplant BCR-ABL mutations and two of which did not.
Among the 29 CML patients receiving post-HSCT TKIs, 10 (34.5%) were initially started on TKI therapy for treatment of refractory/relapsed disease, beginning at a mean of 1.5 months post-HSCT for the 6 with refractory disease, and beginning at a mean of 5.3 months post-HSCT for the 4 with disease relapse following transplant. In addition, 19 (65.5%) received a post-HSCT TKI prophylactically, beginning at a mean of 11.0 months post-HSCT, overall. Most began the TKI between 0.8 and 16 months post-HSCT, although two outlier patients (both transplanted in 2001) started TKIs at 51 and 101 months post-HSCT, respectively, because of molecular positivity. Of the 19 CML patients receiving TKI prophylaxis, 6 patients ultimately developed cytogenetic or hematologic relapse – two of which had pre-transplant BCR-ABL mutations and four of which did not. The time to initiation of post-HSCT TKI was lower among the 6 CML patients who relapsed on prophylaxis (mean of 1.3 months post-HSCT) compared to the 10 without relapse (mean of 15.4 months post-HSCT), suggesting that clinical characteristics may have influenced the decision to begin TKI prophylaxis sooner.
Pre-Transplant Mutation Analysis
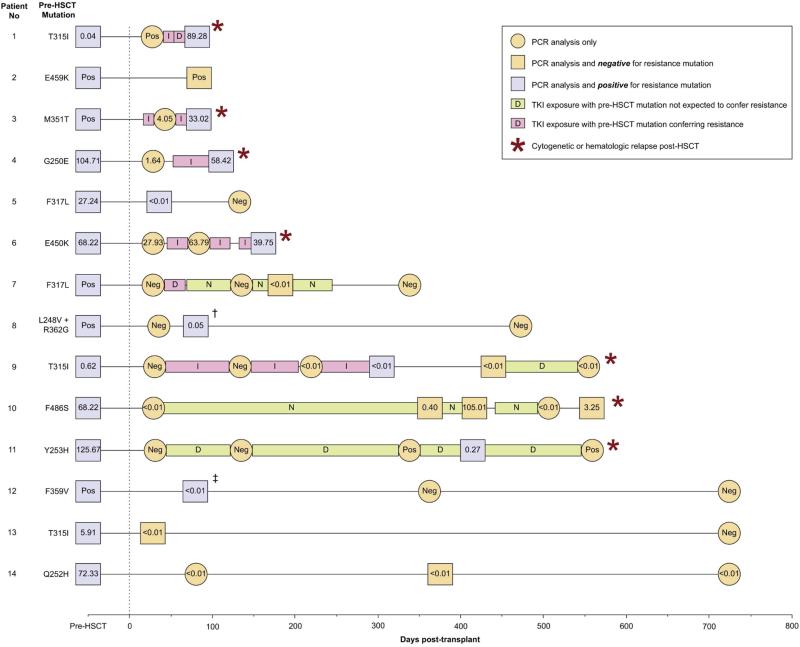
Sequencing revealed 12 different ABL kinase mutations in 10 (10.5%) of CML and in 4 (20.0%) of Ph+ ALL pre-HSCT samples. All 14 patients harbored at least one mutation previously reported to be associated with resistance to one or more TKIs.4,28-31 Mutations occurred across the kinase domain, including the TKI-binding site, P-loop and A-loop: L248V, G250E, Q252H, Y253H, T315I, F317L, M351T, F359V, R362G, E450K, E459K and F486S. Three patients were found to carry the T315I mutation. One patient (Pt. 8, Figure 1) had two mutations: L248V and R362G. Three additional patients were found to have a deletion in exon 7, a common splice variant not associated with imatinib resistance.32
Figure 1. BCR-ABL PCR status and BCR-ABL mutation analysis pre- and post-transplant in 14 patients with identified ABL domain kinase mutations.
Pre-transplant and post-transplant BCR-ABL transcript levels, as determined by PCR testing, are reported as the ratio of BCR-ABL: BCR, or as qualitatively negative (“Neg”). Transcript levels are alternatively reported as qualitatively positive (“Pos”) when quantitative results are not available. Samples are also identified as being positive for a BCR-ABL kinase domain mutation (purple square), negative for a BCR-ABL kinase domain mutation (orange square), or when mutation testing was not performed (orange circle). Post-transplant TKI exposure is indicated for each patient using a bar corresponding to imatinib (I), dasatinib (D), nilotinib (N) or ponatinib (P), and are shaded pink when the pre-existing mutation is known to confer resistance to the TKI administered, or green when the pre-existing mutation is not expected to confer resistance to the TKI.
†Patient 8 was determined to initially harbor two ABL mutations (L248V and R362G) prior to transplant, but only the L248V mutation was detectable after transplant.
‡Patient 12 was found to initially harbor a F359V mutation prior to transplant, but two mutations (F359V and D391G) were detectable after transplant.
Persistence of Mutations After Transplant
In 9 (64.2%) of the 14 patients with pre-HSCT mutations, the same mutation conferring TKI resistance was also detectable post-HSCT at an average time of 191 days following transplantation (range 25 to 559; Figure 1). Patient 8, who initially harbored two pre-HSCT ABL mutations (L248V and R362G), had only the L248V mutation detectable after transplant. Patient 12 initially harbored only a F359V mutation pre-HSCT, but was found to have two mutations (F359V and D391G) detectable post-HSCT. ABL mutations were persistently detectable in 7 of 11 (63.6%) patients receiving myeloablative conditioning, and in 2 of 3 (66.6%) patients receiving non-myeloablative conditioning.
Post-HSCT TKI Therapy and Outcomes in Patients with ABL Mutations
Among the 14 patients with pre-HSCT mutations, 8 (57.1%) received a TKI in the post-HSCT setting, and 7 (50.0%) demonstrated refractory disease and/or hematologic/cytogenetic relapse following transplant. Notably, of the 7 with relapsed/refractory disease, 5 had been given a predictably ineffective TKI within the first 100 days following HSCT, based on our retrospective mutation analysis (Pts. 1, 3, 4, 6 and 9). Three patients with refractory disease died of disease-related complications (Pts. 1, 3 and 4). Patients 6 and 9 both relapsed while receiving imatinib; however, in patient 9, the mutant clone was no longer detectable after cessation of imatinib and administration of reinduction chemotherapy, though the patient ultimately died in blast crisis with a BCR-ABL-negative clone. Patient 7 also initially received a predictably ineffective TKI (dasatinib) based on mutation analysis, but was switched to nilotinib and subsequently achieved a molecular remission. Two patients received a prophylactic TKI for which the pre-HSCT mutation is not expected to confer resistance; however it is notable that they both relapsed anyway (Pts. 10 and 11). Of the 6 patients who did not receive any TKI therapy after transplant, 3 tested negatively for the pre-existing mutation (Pts. 2, 13 and 14), whereas the other 3 tested positively for the pre-existing mutation but ultimately became PCR negative as of last follow-up. (Pts. 5, 8 and 12).
It should be noted that in all but one case (Pt. 10), patients who were PCR positive, but without the pre-transplant mutation, had very low BCR-ABL values (<0.01%); thus, these cases may be “wild-type” simply because of the relative lack of sensitive detection with Sanger sequencing. The noted exception is Pt. 10, who pre-transplant had the F386S mutation, and relapsed with wild-type ABL with very high BCR-ABL transcript levels (105%).
DISCUSSION
The majority of transplants for CML are now done for patients who are resistant to multiple TKI agents, and these cases often will have ABL point mutations1-3,33 After transplant, persistent leukemic clones may compete without the selective pressure of a TKI. Given the goal to prevent post-allograft relapse, and given the different toxicity spectrum of imatinib and the second generation TKIs, how should the clinician use the ABL mutation status to inform the use of prophylactic TKI use? We have demonstrated that the clones that persist after allografting are largely due to the persistence of the ABL mutated clone present pre-transplant, and thus, prophylactic TKI use aimed at targeting that ABL clone is a rational strategy.
Data regarding the prevalence or persistence of ABL mutations after HSCT is extremely limited. In two retrospective reviews of dasatinib for treatment of post-transplant relapse of CML or Ph+ ALL, authors report that 3 of 6 patients (50%) and 4 of 7 patients (55%), respectively, were identified as carrying resistance mutations; however, no information on pre-transplant mutation status or mutation-specific outcomes are available.13,14 In 2010, Jabbour et al reported on ABL kinase domain sequencing analysis in 7 CML patients with post-HSCT relapse. Among 4 patients harboring a pre-HSCT mutation, 2 relapsed with the same mutation, 1 relapsed with a new mutation, and 1 relapsed without a detectable mutation. Patient-specific TKI exposure was not reported.34 Analysis of our cohort of patients reveals similar findings: among the 7 patients with relapsed/refractory disease after transplant, 5 (71.4%) relapsed with the same mutation, and 2 relapsed without a detectable mutation. The overall rate of ABL mutation persistence after transplant, 64.2% in our cohort, has not previously been reported.
The emergence of TKI resistance is an example of Darwinian selection, where wild type and resistant clones compete under a potentially changing selective pressure. Jones et al demonstrated an association between TKI exposure and detection of mutations at the time of disease recurrence in patients with Ph+ ALL.35 In addition, Hanfstein et al showed that the relative expression of BCR-ABL mutant alleles within CML patients fluctuated in response to changes in TKI therapy.36 These findings demonstrate that TKI exposure directly exerts a selective pressure on specific clonal populations, and support our own observation that in patients with detectable resistance mutations after transplant the administration of TKIs might confer a selective advantage for any existing drug-resistant clones. Indeed, in our study, all three patients who received TKI prophylaxis in the setting of a resistance mutation ultimately relapsed.
Our study has practical implications for the design of studies of post-transplant TKI prophylaxis. The current methodology, as illustrated by four published prospective trials in this area, is that all patients receive an empirically selected TKI (imatinib in the case of the four prospective trials) regardless of mutation status or prior TKI exposure.16-19 One could alternatively suggest the empiric use of a second- or third-generation TKI. However, because the resistance profiles of the newer TKIs, while overlapping, are unique for each drug, the empiric selection of any one TKI will almost certainly result in predictable treatment failures in a subset of those with pre-existing mutations. Indeed, 5 of the 7 patients who relapsed in this cohort had been given a TKI in the presence of a corresponding mutation conferring resistance against that agent. The administration of effective anti-leukemic therapies may be of even greater importance in the early post-transplant period when the graft-versus-leukemia effect has yet to exert its influence. Therefore, in addition to considerations of cost and toxicity, we propose that pre-transplant mutation analysis should also be regarded when selecting a TKI for post-transplant prophylaxis, based on our observations that the majority of resistance mutations are still detectable after transplantation, and that patients often relapse with these mutant clones despite receiving TKI therapy.
While this is the largest paired analysis of ABL kinase domain mutations in a transplant population, our study was constrained by the degree with which patients, many of whom had been referred to our institution for transplant, remained in our system for clinical follow-up, and by the incomplete availability of RNA specimens for the entire transplant cohort (especially once patients left our Center and returned home). Our overall rate of ABL mutations in CML is lower than others have reported.1,2 though this is likely due to the large proportion of study subjects transplanted without demonstrated TKI failure. We had decided to begin the study period in 2000, in order to maximize the number of subjects with exposure to TKIs. However, this resulted in the inclusion of 34 subjects (35.8% of the study population) who proceeded to transplant without TKI therapy, all between 2000 and 2002, when long-term outcomes of imatinib were unknown. Because the sensitivity of Sanger sequencing for detection of mutant alleles is estimated to be 20%,37 it is also possible that low-level mutant clones were present but undetected either before or after transplant. Studies employing more sensitive screening methods for ABL mutations reveal a complex clonal architecture, consisting of a dynamic mix of compound and polyclonal mutations.38,39 However, it is not yet clear how such ultra-sensitive mutation analysis and population complexity should be interpreted, in regards to clinical decision-making.
In conclusion, we demonstrate that pre-existing mutations in the ABL kinase domain, frequently associated with resistance to TKIs and prevalent in a transplant population, are persistently detectable in the majority of patients after transplant, and that such resistance patterns need to be considered when selecting TKIs in the post-transplant setting and when designing clinical trials of post-transplant TKI prophylaxis. Further study, involving larger numbers of patients with resistance mutations and perhaps utilizing more sensitive screening methods, is required to more clearly understand patterns of clonal evolution and TKI resistance mutations after hematopoietic stem cell transplantation for CML and Ph+ ALL.
Highlights.
We retrospectively analyzed adults who underwent allogeneic HSCT for CML and Ph+ ALL.
Using RNA, we sequenced the BCR-ABL kinase domain to detect resistance mutations.
Pre-HSCT BCR-ABL mutations are often detectable in patients with post-HSCT relapse.
ACKNOWLEDGMENTS
This work was supported in part by the National Institutes of Health training grant 5-T32-CA009515-28/29.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE STATEMENT:
DNE and LB declare no competing financial interests. JPR has had consulting agreements with Novartis, BMS, and Ariad, and receives research contracts from Novartis.
Contribution: D.N.E. and L.B. performed sequencing experiments. D.N.E. performed chart review, analyzed data and wrote the manuscript. J.C.R. designed experiments and edited the manuscript.
REFERENCES
- 1.Jabbour E, Kantarjian H, Jones D, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006;20:1767–1773. doi: 10.1038/sj.leu.2404318. [DOI] [PubMed] [Google Scholar]
- 2.Lahaye T, Riehm B, Berger U, et al. Response and resistance in 300 patients with BCR-ABL-positive leukemias treated with imatinib in a single center: A 4.5-year follow-up. Cancer. 2005;103:1659–1669. doi: 10.1002/cncr.20922. [DOI] [PubMed] [Google Scholar]
- 3.Soverini S, Vitale A, Poerio A, et al. Philadelphia-positive acute lymphoblastic leukemia patients already harbor BCR-ABL kinase domain mutations at low levels at the time of diagnosis. Haematologica. 2011;96:552–557. doi: 10.3324/haematol.2010.034173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochhaus A, Kreil S, Corbin AS, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002;16:2190–2196. doi: 10.1038/sj.leu.2402741. [DOI] [PubMed] [Google Scholar]
- 5.Gratwohl A, Brand R, Apperley J, et al. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2006;91:513–521. [PubMed] [Google Scholar]
- 6.Barrett AJ, Horowitz MM, Ash RC, et al. Bone marrow transplantation for philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1992;79(11):3067–3070. [PubMed] [Google Scholar]
- 7.Copelan EA, Crilley PA, Szer J, et al. Late mortality and relapse following BuCy2 and HLA-identical sibling marrow transplantation for chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2009;15:851–855. doi: 10.1016/j.bbmt.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian HM, O'Brien S, Cortes JE, et al. Imatinib mesylate therapy for relapse after allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2002;100:1590–1595. [PubMed] [Google Scholar]
- 9.Olavarria E, Ottmann OG, Deininger M, et al. Response to imatinib in patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Leukemia. 2003;17:1707–1712. doi: 10.1038/sj.leu.2403068. [DOI] [PubMed] [Google Scholar]
- 10.DeAngelo DJ, Hochberg EP, Alyea EP, et al. Extended follow-up of patients treated with imatinib mesylate (gleevec) for chronic myelogenous leukemia relapse after allogeneic transplantation: durable cytogenetic remission and conversion to complete donor chimerism without graft-versus-host disease. Clin Cancer Res. 2004;10:5065–5071. doi: 10.1158/1078-0432.CCR-03-0580. [DOI] [PubMed] [Google Scholar]
- 11.Hess G, Bunjes D, Siegert W, et al. Sustained complete molecular remissions after treatment with imatinib-mesylate in patients with failure after allogeneic stem cell transplantation for chronic myelogenous leukemia: results of a prospective phase II open-label multicenter study. J Clin Oncol. 2005;23:7583–7593. doi: 10.1200/JCO.2005.01.3110. [DOI] [PubMed] [Google Scholar]
- 12.Palandri F, Amabile M, Rosti G, et al. Imatinib therapy for chronic myeloid leukemia patients who relapse after allogeneic stem cell transplantation: a molecular analysis. Bone Marrow Transplant. 2007;39:189–191. doi: 10.1038/sj.bmt.1705554. [DOI] [PubMed] [Google Scholar]
- 13.Atallah E, Kantarjian H, De Lima M, et al. The role of dasatinib in patients with Philadelphia (Ph) positive acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML) relapsing after stem cell transplantation (SCT). Blood (ASH Annual Meeting Abstracts) 2006;108 Abstract 4520. [Google Scholar]
- 14.Klyuchnikov E, Schafhausen P, Kroger N, et al. Second-generation tyrosine kinase inhibitors in the post-transplant period in patients with chronic myeloid leukemia or Philadelphia-positive acute lymphoblastic leukemia. Acta Haematol. 2009;122:6–10. doi: 10.1159/000228587. [DOI] [PubMed] [Google Scholar]
- 15.Wright MP, Shepherd JD, Barnett MF, et al. Response to tyrosine kinase inhibitor therapy in patients with chronic myelogenous leukemia relapsing in chronic and advanced phase following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:639–646. doi: 10.1016/j.bbmt.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Olavarria E, Siddique S, Griffiths MJ, et al. Posttransplantation imatinib as a strategy to postpone the requirement for immunotherapy in patients undergoing reduced-intensity allografts for chronic myeloid leukemia. Blood. 2007;110:4614–4617. doi: 10.1182/blood-2007-04-082990. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter PA, Snyder DS, Flowers ME, et al. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk philadelphia chromosome-positive leukemia. Blood. 2007;109:2791–2793. doi: 10.1182/blood-2006-04-019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wassmann B, Pfeifer H, Stadler M, et al. Early molecular response to posttransplantation imatinib determines outcome in MRD+ philadelphia-positive acute lymphoblastic leukemia (ph+ ALL). Blood. 2005;106:458–463. doi: 10.1182/blood-2004-05-1746. [DOI] [PubMed] [Google Scholar]
- 19.Pfeifer H, Wassmann B, Bethge W. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR–ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27:1254–1262. doi: 10.1038/leu.2012.352. [DOI] [PubMed] [Google Scholar]
- 20.Ram R, Storb R, Sandmaier BM, et al. Non-myeloablative conditioning with allogeneic hematopoietic cell transplantation for the treatment of high-risk acute lymphoblastic leukemia. Haematologica. 2011;96:1113–1120. doi: 10.3324/haematol.2011.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderlini P, Sheth S, Hicks K, et al. Re: Imatinib mesylate administration in the first 100 days after stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:883–884. doi: 10.1016/j.bbmt.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Kang BW, Moon JH, Chae YS, et al. Pre-emptive treatment with nilotinib after second allogeneic transplantation in a Philadelphia chromosome-positive acute lymphoblastic leukemia patient with high risk of relapse. Acta Haematol. 2010;123:242–247. doi: 10.1159/000314538. [DOI] [PubMed] [Google Scholar]
- 23.Merante S, Colombo AA, Calatroni S, et al. Nilotinib restores long-term full-donor chimerism in Ph-positive acute lymphoblastic leukemia relapsed after allogeneic transplantation. Bone Marrow Transplant. 2009;44:263–264. doi: 10.1038/bmt.2009.6. [DOI] [PubMed] [Google Scholar]
- 24.Tiribelli M, Sperotto A, Candoni A, et al. Nilotinib and donor lymphocyte infusion in the treatment of Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) relapsing after allogeneic stem cell transplantation and resistant to imatinib. Leuk Res. 2009;33:174–7. doi: 10.1016/j.leukres.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 25.Czyz A, Lewandowski K, Kroll R, Komarnicki M. Dasatinib-induced complete molecular response after allogeneic hematopoietic stem cell transplantation in Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to prior imatinib-containing regimen: a case report and discussion. Med Oncol. 2010;27:1123–1126. doi: 10.1007/s12032-009-9347-0. [DOI] [PubMed] [Google Scholar]
- 26.Caocci G, Vacca A, Ledda A, et al. Prophylactic and preemptive therapy with dasatinib after hematopoietic stem cell transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2012;18:652–654. doi: 10.1016/j.bbmt.2011.12.587. [DOI] [PubMed] [Google Scholar]
- 27.Tichelli A, Bucher C, Rovo A, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110:3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 28.Kang HY, Hwang JY, Kim SH, et al. Comparison of allele specific oligonucleotidepolymerase chain reaction and direct sequencing for high throughput screening of ABL kinase domain mutations in chronic myeloid leukemia resistant to imatinib. Haematol. 2006;91(5):659–662. [PubMed] [Google Scholar]
- 29.Hughes T, Saglio G, Branford S, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. 2009;27(25):4204–4210. doi: 10.1200/JCO.2009.21.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redaelli S, Piazza R, Rostagno R, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol. 2009;27(3):469–471. doi: 10.1200/JCO.2008.19.8853. [DOI] [PubMed] [Google Scholar]
- 31.Soverini S, De Benedittis C, Papayannidis C. Drug resistance and BCR-ABL kinase domain mutations in Philadelphia chromosome–positive acute lymphoblastic leukemia from the imatinib to the second-generation tyrosine kinase inhibitor era: the main changes are in the type of mutations, but not in the frequency of mutation involvement. Cancer. 2014;120:1002–1009. doi: 10.1002/cncr.28522. [DOI] [PubMed] [Google Scholar]
- 32.Gruber FX, Hjorth-Hansen H, Mikkola1 I, Stenke L, Johansen T. A novel Bcr-Abl splice isoform is associated with the L248V mutation in CML patients with acquired resistance to imatinib. Leukemia. 2006;20:2057–2060. doi: 10.1038/sj.leu.2404400. [DOI] [PubMed] [Google Scholar]
- 33.Pfeifer H, Wassmann B, Pavlova A, et al. Kinase domain mutations of BCR-ABL frequently precede imatinib-based therapy and give rise to relapse in patients with de novo Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood. 2007;110:727–734. doi: 10.1182/blood-2006-11-052373. [DOI] [PubMed] [Google Scholar]
- 34.Jabbour E, Cortes J, Santos FPS, et al. Results of allogeneic hematopoietic stem cell transplantation for chronic myelogenous leukemia patients who failed tyrosine kinase inhibitors after developing BCR-ABL1 kinase domain mutations. Blood. 2007;117:3641–3647. doi: 10.1182/blood-2010-08-302679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones D, Thomas D, Yin CC, et al. Kinase domain point mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia emerge after therapy with BCR-ABL kinase inhibitors. Cancer. 2008;113:985–994. doi: 10.1002/cncr.23666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanfstein B, Muller MC, Kriel S, et al. Dynamics of mutation BCR-ABL-positive clones after cessation of tyrosine kinase inhibitor therapy. Haematologica. 2011;96(3):360–366. doi: 10.3324/haematol.2010.030999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Branford S, Rudski Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2012;102(1):276–283. doi: 10.1182/blood-2002-09-2896. [DOI] [PubMed] [Google Scholar]
- 38.Khorashad JS, Kelley TW, Szankasi P, et al. BCR-ABL1 compound mutation in tyrosine kinase inhibitor-resistant CML: frequency and clonal relationships. Blood. 2013;121:489–498. doi: 10.1182/blood-2012-05-431379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soverini S, De Benedittis C, Polakova KM, et al. Unraveling the complexity of tyrosine kinase inhibitor-resistant populations by ultra-deep sequencing of the BCR-ABL kinase domain. Blood. 2013;122:1634–1648. doi: 10.1182/blood-2013-03-487728. [DOI] [PubMed] [Google Scholar]