Abstract
Electrophilic products of lipid peroxidation are important contributors to the progression of several pathological states. The prototypical α,β–unsaturated aldehyde, 4-hydroxynonenal (HNE), triggers cellular events associated with oxidative stress, which can be curtailed by the glutathione-dependent elimination of HNE. The glutathione transferases (GSTs) are a major determinate of the intracellular concentration of HNE and can influence susceptibility to toxic effects, particularly when HNE and GST levels are altered in disease states. In this article, we provide a brief summary of the cellular effects of HNE, followed by a review of its GST-catalyzed detoxification, with an emphasis on the structural attributes that play an important role in the interactions with alpha-class GSTs. Some of the key determining characteristics that impart high alkenal activity reside in the unique C-terminal interactions of the GSTA4-4 enzyme. Studies encompassing both kinetic and structural analyses of related isoforms will be highlighted, with additional attention to stereochemical aspects that demonstrate the capacity of GSTA4-4 to detoxify both enantiomers of the biologically relevant racemic mixture while generating a select set of diastereomeric products with subsequent implications. A summary of the literature that examines the interplay between GSTs and HNE in model systems relevant to oxidative stress will also be discussed to demonstrate the magnitude of importance of GSTs in the overall detoxification scheme.
Keywords: Oxidative stress, glutathione transferase A4-4, GSHNE, 4-hydroxynonenal-adducts
Introduction
Electrophilic products of oxidative stress are clearly associated with toxic effects, and some may have a causal role in several diseases, including Alzheimer’s disease (AD), Parkinson’s disease, diabetes, atherosclerosis, cancer, and aging (Butterfield et al., 2010a; Grimsrud et al., 2007; Leitinger, 2003; Nair et al., 2007; Zhou et al., 2008; Zimniak, 2008). Among the major products generated endogenously from the degradation of polyunsaturated fatty acids during lipid peroxidation (LPO) are 4-hydroxynonenal (HNE), malondialdehyde, and acrolein, which all share a reactive α,β–unsaturated aldehyde moiety (Esterbauer et al., 1991). Similarly, electrophilic metabolites of prostaglandins are also reactive and are potential therapeutic targets in as much as they regulate inflammatory responses (Straus and Glass, 2001). Additional products of oxidative stress include lipid peroxides, which are also electrophilic (Singhal et al., 1994).
Conjugation with the tripeptide, glutathione (GSH), can prevent each of these electrophiles from reacting with other crucial cellular nucleophiles and render them more hydrophilic to facilitate elimination. Although some compounds can spontaneously react with GSH to varying extents, the cytosolic glutathione transferases (GSTs) comprise a family of versatile enzymes with the capacity to catalyze the nucleophilic conjugation of GSH with a wide spectrum of electrophiles (Armstrong, 1997). Although the substrate selectivity of different GST classes and isoforms is broad and overlapping, the alpha-class GSTs are abundant in tissues exposed to high levels of reactive oxygen species (ROS) and are known to play a significant role in the detoxification of oxidative stress products (Coles and Kadlubar, 2005; Sharma et al., 2004; Yang et al., 2001; Morel et al., 2002). Despite high sequence similarities, the alpha-class GSTs display highly divergent substrate specificities. The GSTA4-4 isoform is known to be selective for the GSH conjugation of LPO products and is well-recognized for its catalytic efficiency with HNE (Board, 1998; Hubatsch et al., 1998). Structurally, this isoform consists of a rigid scaffold preorganized for HNE metabolism, whereas the structural homolog GSTA1-1, which has low activity toward HNE, utilizes a conformationally flexible scaffold to accomplish promiscuous catalysis with diverse substrates, such as 1-chloro-2,4-dinitrobenzene (CDNB) and Δ5-androstene-3,17-dione (Balogh et al., 2010; Bruns et al., 1999; Hou et al., 2007; Blikstad et al., 2008). However, in addition to directly metabolizing HNE, some of the alpha-class GSTs can also provide antioxidant protection by attenuating HNE formation. Both the GSTA1-1 and A2-2 isoforms can catalyze the reduction of lipid hydroperoxides through their GSH peroxidase activity, thereby decreasing the propagation of LPO that can ultimately generate HNE (Awasthi et al., 2004; Yang et al., 2003; Zhao et al., 1999).
Collectively, the cytosolic GSTs are part of key detoxification mechanisms that play a pivotal role in the clearance of lipid derivatives generated during oxidative stress. Furthermore, to the extent that GSTs regulate the intracellular concentration of HNE, they can influence susceptibility to the effects elicited by HNE, particularly when HNE and GST levels are altered in disease states (Coles and Kadlubar, 2005, LoPachin et al., 2008). This review aims to summarize studies concerning the underlying role of cytosolic GSTs in these processes. Because HNE represents a major toxicological link between oxidative stress and disease, it will be the focus of this work.
Relevance of HNE
Although LPO has historically been associated with toxicity, and earlier studies had already begun the characterization of alkenals and hydroxyalkenals, the link between activated alkenals and the harmful effects associated with oxidative stress garnered further attention when HNE was designated as the most abundant, cytotoxic aldehyde produced from the peroxidation of rat liver microsomes (Benedetti et al., 1980). Based on estimates calculated with physiological concentrations of GSH and the forward rate constant previously determined for HNE (Esterbauer et al., 1975), the aforementioned study approximated the half-life of HNE at 2.5 minutes, and suggested this could explain the effects elicited by oxidative stress on cellular targets far removed from the site of origin. These effects had previously been difficult to comprehend, given the short half-lives of free radicals involved in the initiation and propagation of LPO. HNE and HNE adducts have subsequently become common biomarkers for detecting the occurrence and extent of oxidative stress (Petersen and Doorn, 2004; Uchida, 2007; Zarkovic, 2003b).
The consequence of HNE adduction on structure/function and the corresponding toxicological significance still remain to be fully characterized for many modifications. However, numerous investigations have collectively generated an extensive list of proteins that are covalently modified by HNE, many of which are correlated with exposure to various pro-oxidant treatments intended as models of oxidative stress. A brief list of some of the prominent biological molecules known to be modified by HNE include albumin (Aldini et al., 2006; Szapacs et al., 2006), the amyloid, β peptide (Liu et al., 2008; Siegel et al., 2007), α-synuclein (Qin et al., 2007), apolipoprotein B in oxidized low-density lipoprotein (Bolgar et al., 1996), adipocyte fatty acid–binding protein (Grimsrud et al., 2007), Keap1 (Levonen et al., 2004), and several GST isoforms (Mitchell et al., 1995; Shireman et al., 2010; van Iersel et al., 1997). A recent study that tackled a global analysis of protein damage by HNE provides an excellent perspective on the systems-level view of the impact of protein damage with respect to intricate networks involved with oxidative stress (Codreanu et al., 2009). HNE has also been described to covalently modify p53 DNA (Hu et al., 2002) and protein, with a significant increase in p53 adducts observed in the inferior parietal lobule of samples from AD patients, leading to speculation that HNE may influence the pro-apoptotic activity of p53 during advanced stages of neurodegeneration (Cenini et al., 2008).
The ability of HNE to exert a number of toxicological effects has been attributed to its electrophilic α,β–unsaturated carbonyl moiety that can react through 1,2- and 1,4-additions with nucleophiles, such as cysteine, histidine, and lysine residues in proteins (Carini et al., 2004; LoPachin et al., 2008). The electrophilicity of HNE is enhanced by polarization due to the hydroxyl substituent on C-4 that increases the reactivity of hydroxyalkenals with nucleophiles, such as GSH, when compared with their alkenal counterparts (Esterbauer et al., 1975, 1991). Although LPO generates both enantiomers of HNE, the stereochemical configuration at C-4 may be of biological significance, and studies pertaining to this aspect will be highlighted below.
HNE is also involved in modulating proliferation, differentiation, and apoptosis and has been shown to generate concentration-dependent alterations in a variety of signaling cascades (Awasthi et al., 2004, 2005b; Uchida, 2003). The direct and indirect actions of HNE are numerous and appear to simultaneously influence multifactorial pathways, making effects complex to deconvolute. Nonetheless, microarray analyses aimed at examining global alterations in gene expression have demonstrated that the network of signaling events influenced by HNE eventually affect multiple genes known to be regulated within the scope of antioxidant, heat shock, and ER stress responses (Jacobs and Marnett, 2010; West and Marnett, 2005).
Both the electrophilic reactivity and the involvement in signaling cascades have been invoked in mechanisms to explain the physio- and pathological roles of HNE. Overall, HNE can be considered a second messenger that originates from and augments early free radical events that initiate LPO and, eventually, alters gene expression and cell viability. A survey of the literature indicates the range of HNE concentrations in various mammalian tissues and plasma is 0.5–6 μM, with 3–10-fold increases observed in patients under oxidative stress (Awasthi et al., 2005b). It has also been approximated that HNE accumulates in membranes at concentrations of 10 μM–4.5 mM in response to oxidative insults (Esterbauer et al., 1991). Given the toxic nature of HNE and its roles in oxidative stress, factors controlling its cellular concentration are of significant interest, as they likely influence an organism’s susceptibility to toxic effects and represent a further step in understanding the relationship between oxidative stress and disease states.
Detoxification of HNE
Spontaneous reaction with GSH
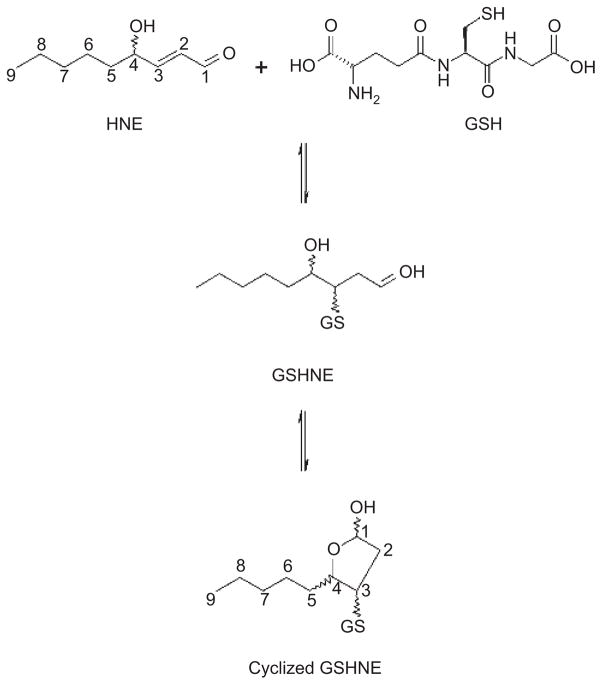
Conjugation to GSH is the primary component in the defense strategy used in the detoxification of reactive electrophiles (Hayes and McLellan, 1999). HNE spontaneously reacts with GSH to form a conjugate through a 1,4-addition reaction (Esterbauer et al., 1975, 1991). The nucleophilic addition of the thiolate to C-3 of HNE initially yields an enolate tautomer that reacts with a proton donor to produce glutathionyl 4-hydroxynonanal (GSHNE). Equilibrium favors the cyclic hemiacetal structure that is generated from an intramolecular reaction of the 4-hydroxyl and carbonyl groups (Figure 1).
Figure 1.
Reaction of GSH with racemic HNE. 1,4-addition reaction, followed by an intramolecular cyclization..
Whether generated by the spontaneous reaction or enzyme-mediated catalysis, as reviewed below, characterization of the GSHNE conjugates represents an important step not only in understanding the routes of HNE elimination, but also in cell-signaling cascades, as compelling evidence indicates that GSHNE is also involved in modulating effects previously attributed to HNE itself. An insightful study conducted in aortic smooth muscle cells indicated that a subsequent metabolite, generated by the aldose reductase-catalyzed aldehyde reduction of GSHNE, is a mediator of the cell signaling associated with HNE that was found to stimulate protein kinase C, nuclear factor-κB (NF-κB), and activator protein-1 (Ramana et al., 2006). Moreover, in addition to the buildup of proteins excessively adducted by HNE, GSHNE and its secondary metabolites have been found to accrue in neurodegenerative disease states (Selley, 1998; Volkel et al., 2006) and models of oxidative stress (Volkel et al., 2005), thereby suggesting a dependence on the adequate functioning of this detoxification pathway to combat oxidative stress.
GST-catalyzed conjugation
The accumulation of HNE is also countered by a collection of enzymes that can contribute to detoxification reactions (Alary et al., 2003; Alin et al., 1985; Amunom et al., 2007; Awasthi et al., 2005b; Boon et al., 1999). Although the spontaneous reaction with GSH presumably provides some level of protection against HNE, results with various cell lines suggest that many cells in general respond in initial stages of stress by upregulating the mechanisms for detoxification and exclusion of HNE (Cheng et al., 2001a). Mercapturic acids constitute the major portion of HNE metabolites identified in urine, and GSTs, being a major determinant of the intracellular concentration of HNE, are believed to play a central role in the overall detoxification scheme (Alary et al., 1998).
Based principally upon sequence homology, cytosolic GSTs are categorized into seven classes in mammals that are further divided into subclasses identified numerically by their monomer composition (Sheehan et al., 2001). Beyond characterization of the spontaneous reaction with thiols, early studies established that products of LPO were, indeed, substrates for several GST isoforms, thereby implicating the GSTs in an important physiological role with respect to these endogenous compounds (Alin et al., 1985; Danielson et al., 1987). Since these initial studies, GSTA4-4 has emerged as the alpha-class GST enzyme distinguished for its high catalytic efficiency with toxic LPO products, such as HNE (Board, 1998; Hubatsch et al., 1998; Liu et al., 1998; Stenberg et al., 1992; Zimniak et al., 1992). The kinetic parameters (KM = 34 μM, kcat = 100 s−1), as determined by a liquid chromatography/mass spectrometry (LC/MS)-based product formation assay in our laboratory (Balogh et al., 2008), are consistent with previously reported constants for racemic HNE and human GSTA4-4 (hGSTA4-4) (Cheng et al., 2001b, Hubatsch et al., 1998). Based on these parameters and the rate constant provided for the corresponding spontaneous reaction (Esterbauer et al., 1975), the catalytic proficiency (as defined by the ratio: (kcat/KM)/knon; Miller and Wolfenden, 2002) of hGSTA4-4 is calculated to be 2.7 × 106 and could be indicative of a significant contribution to HNE detoxification—orders of magnitude above the spontaneous conjugation rate in vivo. For comparison, the catalytic efficiencies reported for the hGSTA1-1 (Zhao et al., 1999) and P1-1 (Singhal et al., 1994) isoforms would yield proficiencies 50- and 400-fold lower, respectively, relative to hGSTA4-4. Although these other GSTs in the alpha, pi, and mu classes exhibit significantly reduced activity with HNE, they may also contribute to the detoxification, to some extent, given these isoforms can constitute the bulk of GST protein in some tissues (Eaton and Bammler, 1999). This concept is exemplified by the mGsta4 null mouse, which retains as much as 64% of conjugation activity in liver tissue (Engle et al., 2004).
Rationally engineered mutants of GSTA1-1, including the elegantly redesigned GSTA1-1 “GIMFhelix” mutant, which contains amino-acid substitutions representing 6% of the sequence (A12G/L107I/L108M/V111F/A1 208–222 A4), have also uncovered structural elements important within the context of high activity toward alkenals (Blikstad et al., 2008; Nilsson et al., 2000; Babbitt, 2000). Although it is still less efficient than GSTA4-4, the GIMFhelix mutant is remarkably more active with HNE than GSTA1-1. The mutant displayed 20- and >300-fold increases in catalytic efficiency with HNE and nonenal, respectively. Interestingly, over a 10-fold decrease in the catalytic efficiency for the characteristic aromatic substitution reaction with CDNB also emerged, indicating that the effects of the particular substitutions are correlated with a shift in specificity toward the Michael-like additions performed by GSTA4-4.
Stereochemical considerations
Among the studies that explore the stereochemical course of GST catalysis are the conjugation reactions of prostaglandin A2 by GSTA1-1 and P1-1 (Bogaards et al., 1997) and the stereoselective GSH conjugation of 13-oxooctadeca-9,11-dienoic acid by GSTM1-1, M2-2, and allelic variants of P1-1 (Bull et al., 2002). HNE exists in two enantioisomeric forms, whereas the cyclized form of GSHNE contains three chiral centers in the hemiacetal moiety. Results described by others (Ji et al., 2002) and our laboratory (Balogh et al., 2008) both indicate that the spontaneous reaction of racemic HNE with GSH produces a high-performance liquid chromatography (HPLC) or LC/MS chromatogram with four major diastereomeric peaks. Taking into account the chiral centers inherent in GSH, there are a total of eight potential GSHNE diastereomers, with the remaining possibility that each of the four peaks constitute a pair of unresolved diastereomers. The significance of the chirality of HNE and GSHNE with respect to kinetic and structural attributes of GSTA4-4 has been a point of interest in studies involving both the rat and human isoforms.
Substrate stereoselectivity
The relative preference for R- versus S-HNE as initial substrates could play an important role in the removal of these electrophiles, as both enantiomers are reactive toward cellular nucleophiles and are presumed to be toxic (West et al., 2004). Although LPO generates both enantiomers of HNE, the unique intracellular localization of R- versus S-HNE histidine adducts in the renal cortex of rats exposed to ferric nitrilotriacetate emphasizes the potential for stereoselective toxicological interactions (Hashimoto et al., 2003). HNE can also form adducts with DNA, and the ability of the exocyclic HNE-deoxyguanosine adduct to form interstrand cross-links was found to be contingent upon stereochemistry (Huang et al., 2008). One of the HNE-deoxyguanosine adducts derived from S-HNE was observed to form interstrand cross-links, whereas adducts derived from R-HNE did not. Although initial adducts are formed with both enantiomers, only the adduct derived from S-HNE was positioned to facilitate cross-link formation when the reactive aldehyde is unmasked during the opening of the cyclic hemiacetal structure, thereby increasing its propensity to ultimately block DNA replication.
Given the stereoselectivity outlined above, it is important to understand what determines the relative concentrations of HNE enantiomers. The S-HNE enantiomer was found to irreversibly inactivate rabbit glyceraldehyde-3-phosphate dehydrogenase (GAPDH) at a greater rate than R-HNE (Hiratsuka et al., 2000), and a recent review highlights how the modification of this enzyme is now attracting attention due to roles in neurodegeneration (Butterfield et al., 2010b). Interestingly, the previous study also described a corresponding stereoselective substrate preference by rat GSTA4-4 in the order of S- > racemic > R-HNE. Likewise, our studies conducted with the human isoform determined that GSTA4-4 is modestly substrate selective, with a preference for S-HNE in the presence of both enantiomers (Balogh et al., 2008). The apparent KM and the relative contributions to kcat ultimately result in a 1.5-fold greater apparent catalytic efficiency for S-HNE in the GSTA4-4-mediated GSH conjugation. However, despite the small preference for S-HNE as a substrate, GSTA4-4 still exhibits high apparent activities with either enantiomer, as expected for an enzyme that may have evolved to detoxify a racemic substrate.
Even though it matched, or even surpassed, hGSTA4-4 with other alkenal substrates, the hGSTA1-1 GIMFhelix mutant does not quite exhibit maximal catalytic efficiency with HNE (Nilsson et al., 2000). Based upon the ratio of the apparent catalytic efficiencies (kcat/KM) in our studies, the GIMFhelix mutant exhibits a preference for S- versus R-HNE of 1.6, comparable to that of wild-type GSTA4-4 (Balogh et al., 2010). However, it is notable that the GIMFhelix mutant does not show any major differences in the kinetic parameters between nonenal and HNE (Nilsson et al., 2000), whereas GSTA4-4 shows a reduction and increase in the KM and kcat values, respectively, for HNE, as opposed to nonenal as a substrate. This can be interpreted to suggest that the GSTA1-1 GIMFhelix mutant cannot exploit the hydroxyl group, in either configuration, as efficiently as GSTA4-4. This rationalization, which is supported by our recent structure determinations discussed below, implies that GSTA4-4 exhibits low substrate stereoselectivity because it exploits the hydroxyl group of both substrates, whereas GSTA1-1 GIMFhelix exhibits low substrate stereoselectivity because it effectively exploits neither.
Stereoselectivity of product formation
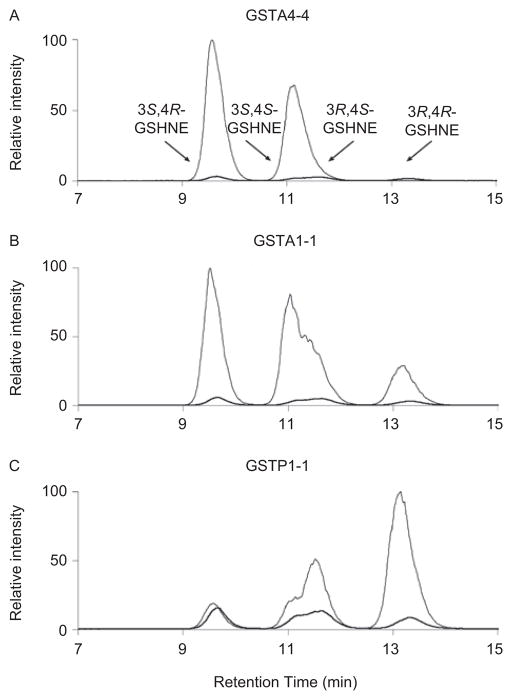
Although crystal structures have yet to depict HNE bound to the GST active site, predictions derived from the structure for apo hGSTA4-4 and hGSTA4-4 in complex with the inhibitor, S-(2-iodobenzyl)-GSH (Bruns et al., 1999), suggested either enantiomer of HNE could fit into the binding pocket, but anticipated that the nucleophilic attack by the GSH sulfur would generate only the S-configuration at the site of conjugation in the GSHNE diastereomers. As described above, the spontaneous reaction produces an LC/MS spectrum with four major diastereomeric peaks. However, our studies confirmed the crystal structure-based proposal concerning product stereoselectivity within the active site by explicitly demonstrating that only two of the diastereomeric peaks are observed in the catalytic reaction (Figure 2A), wherein hGSTA4-4 conjugates GSH to HNE in a stereoselective manner that is not maintained in the spontaneous reaction (Balogh et al., 2008). Moreover, LC/MS and nuclear magnetic resonance (NMR) experiments, in combination with simulated annealing structure determinations, were also consistent with a catalyzed nucleophilic attack that produces only the S-configuration at the site of conjugation, regardless of initial substrate chirality. The product stereoselectivity of GSTA4-4, as compared with other GST isoforms (Figure 2A–C), can be rationalized on structural grounds (reviewed below), and, overall, these comparisons illustrate how GSTA4-4 has a unique stereoselectivity profile that yields only a select set of GSHNE diastereomers.
Figure 2.
Comparison of the stereoselectivity of product formation for the different GST isoforms. The GSHNE diastereomers (gray line) were prepared by incubating GSH and (A) hGSTA4-4, (B) hGSTA1-1, and (C) hGSTP1-1 with racemic HNE and analyzed by LC/MS (electrospray positive ion mode, selected ion monitoring, m/z 464). The maximum contribution possible from the spontaneous reaction (black line), which produces small, roughly equivalent amounts of all four peaks, is also shown for comparison.
In addition to regulating any other biological effects produced by GSHNE itself (Ramana et al., 2006), the efficient export of GSHNE presumably allows GSTs to sustain maximal HNE detoxification that would otherwise be hindered by product inhibition (Alin et al., 1985). Hence, in conjunction with GSTs, transporters are also thought to play an important role in regulating the intracellular concentrations of HNE and GSHNE and their subsequent effects in cells (Awasthi et al., 2005b; Cheng et al., 2001a; Sharma et al., 2000). The stereochemical configuration at the site of conjugation could also impact GSHNE bioactivity and further elimination. For example, it was hypothesized that product stereoselectivity of GSTs could contribute to the unequal distribution of GSHNE diastereomers observed in rat liver cytosol (Boon et al., 1999). Similarly, an unequal distribution of the GSHNE diastereomers in the apical compartment was reported for multidrug resistance protein 2 Madin-Darby canine kidney cells (Ji et al., 2002). Such distributions could, indeed, originate from stereoselective GSHNE production, but the potential for stereoselective contributions by transporters should also be acknowledged. Interestingly, though it was not explicitly discussed, the distribution pattern of the different GSHNE diastereomers accumulated in the liver tissue of Ral-binding protein (RLIP76)−/− mice versus RLIP76+/+ mice (Singhal et al., 2008), alludes to a stereoselective preference of the RLIP76 transporter for the diastereomers selectively produced by hGSTA4-4. Collectively, these results underscore a role for the product stereoselectivity exhibited by hGSTA4-4, and it is conceivable that the overall HNE detoxification scheme, including metabolism and transport, could be coordinated with respect to stereochemistry. Further research into the biological implications concerning GSHNE by utilizing direct transport assays with the individual diastereomers will help to elucidate the significance of chirality in the transport process.
Structural characterizations
Alpha-class GST structure
All members of the cytosolic GSTs are functionally dimeric proteins that contain one active site per subunit. In general, the N-terminal domain of each subunit creates a GSH-binding site (G-site), whereas an area in between the bundle of α-helices in the C-terminal domain essentially creates the substrate-binding site (H-site), both of which have been extensively discussed in previous reviews (Armstrong, 1997; Dirr et al., 1994; Dourado et al., 2008; Hayes et al., 2005; Sheehan et al., 2001). The alpha-class GSTs are commonly known to play a significant role in the detoxification of oxidative stress products (Sharma et al., 2004; Yang et al., 2001; Zhao et al., 1999). One of the unique features with particular relevance to the binding of HNE is the extra α9-helix in the C-terminal domain that participates in the H-site of the alpha-class GSTs (Sinning et al., 1993). Another distinguishing feature of alpha-class GSTs is the incorporation of the Arg15 residue, which helps to stabilize the GSH thiolate and lower the pKa value of the well-recognized Tyr9 residue (Armstrong, 1997; Bjornestedt et al., 1995a; Dourado et al., 2010; Gildenhuys et al., 2010). This Arg15 residue has also been proposed to donate a proton or activate a water molecule during catalysis as well as interact with the hydroxyl group of HNE, as discussed below (Bruns et al., 1999).
Although GSTA4-4 and GSTA1-1 have similar overall topologies, dimer interactions, and conserved G-sites, the specificity of GSTA4-4 is highly distinguished from that of GSTA1-1 (Hou et al., 2007; Hubatsch et al., 1998). GSTA1-1 is a promiscuous enzyme, whereas GSTA4-4 has much higher substrate specificity and catalytic efficiency toward alkenal substrates, such as HNE, although it also maintains negligible stereoselectivity toward the individual enantiomers. The role of numerous interactions involving regions, such as the C-terminus or the domain-domain interface, have been explored with respect to enzyme stability and dynamics as well as the relationship to catalytic function (Adman et al., 2001; Balchin et al., 2010; Dirr et al., 2005; Dirr and Wallace, 1999; Gustafsson et al., 1999; Ibarra et al., 2001; Kuhnert et al., 2005; Mosebi et al., 2003; Nieslanik and Atkins, 2000; Nieslanik et al., 1999, 2001; Nilsson et al., 2002). Both isoforms utilize three substrate recognition regions (the β1-α1 region, the end of the α4-helix, and the C-terminus) to construct the H-sites in the folded protein. However, though the studies cited above, as well as additional structural characterizations (Cameron et al., 1995; Grahn et al., 2006; Le Trong et al., 2002; Zhan and Rule, 2004), indicate the C-terminus of GSTA1-1 is a highly disordered helix that is transformed to an ordered helix in a ligand-dependent manner, the first crystal structures of hGSTA4-4 (PDB entries 1GUM and 1GUL) helped to reveal how its readily positioned C-terminal helix creates an H-site ideally shaped for alkenals (Bruns et al., 1999).
Additional studies with the aforementioned GIMFhelix mutant further established the relationship between the structural determinants that contribute to the distinct specificities of alpha-class GSTs (Balogh et al., 2009; Blikstad et al., 2008; Nilsson et al., 2000). Because a number of the targeted residues comprise the α9-helix, the entire C-terminus (residues 208–222) was exchanged with that of GSTA4-4 in the final GSTA1-1 GIMFhelix mutant designed by Mannervik and co-workers Nilsson et al., 2000. One well-recognized feature underscored by these studies is the simultaneous presence of Tyr212 in the α9-helix and Gly12 in the β1-α1 loop that is critical for binding and activating HNE (Bjornestedt et al., 1995b; Bruns et al., 1999; Nilsson et al., 2000). Although it is the Tyr212 residue that is proposed to interact with the aldehyde of alkenals, this area is occupied by the Cβ of Ala12 in hGSTA1-1, and the lack of a side-chain at residue 12 was found to be necessary for Tyr212 to adopt the optimal position for binding and catalysis. Furthermore, the pKa value of Tyr9 was decreased from 8.1 in hGSTA1-1 to a value of 7.3 in the GIMFhelix mutant, which shifts it closer to the anomalously low value of 6.7 measured in hGSTA4-4 (Hubatsch and Mannervik, 2001). Although the precise basis of the different pKa values in these isoforms is not entirely clear, the trend suggests a correlation between a low pka value and structural elements that contribute to high alkenal activity.
In light of these comparisons, characterization of the GSTA1-1 and GSTA4-4 isoforms was extended to examine the relationship between functional promiscuity and conformational heterogeneity (Hou et al., 2007). The hGSTA4-4 enzyme contains an edge-to-face aromatic-aromatic interaction between Phe111 and Tyr217 in the α4–α5 helix-turn-helix “tower” that is not present in hGSTA1-1 (Figure 3). The interaction between these two residues was speculated to contribute to a closer association between the tower and C-terminal regions of hGSTA4-4 that increases core packing and preorganization of the active site for HNE binding. Although GSTA1-1 was found to be more conformationally heterogeneous, the tower mutants that eliminate and incorporate the aromatic-aromatic interaction into GSTA4-4 and GSTA1-1, respectively, were shown to exhibit substrate specificity between those of the two wild-type proteins and were sufficient to change local, as well as global, dynamics. Altogether, these observations suggest that a conserved scaffold with a relatively small number of substitutions is able to convey not only differences in local dynamics, but also differences in conformational heterogeneity that span the folded protein structure and, ultimately, result in unique specificities embodied by these GST isoforms.
Figure 3.
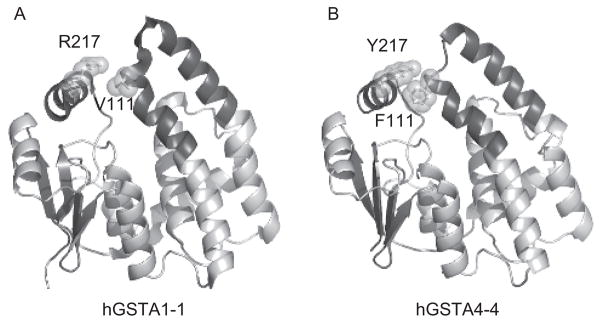
Comparison of key C-terminal domain interactions in alpha-class GSTs. Ribbon diagrams of a (A) hGSTA1-1 (PDB entry 1K3Y) and (B) hGSTA4-4 (PDB entry 1GUL) subunit as viewed perpendicular to the 2-fold axis of symmetry for the corresponding dimer. The tower region and α9-helix are emphasized in dark gray. The aromatic-aromatic interaction between F111 in the #4-turn-#5 tower region and Y217 in the #9-helix is depicted for hGSTA4-4. This interaction is not present in hGSTA1-1. The ligands are not shown for clarity.
Substrate stereoselectivity
Enzymologically, GSTA4-4 demonstrates an interesting combination of promiscuity and specificity, which is opposite from the expected parallel between high chemospecificity and high catalytic efficiency. The stereoselectivity of product formation observed for hGSTA4-4 is contingent upon the consistent maintenance of the relative orientation between GSH and the 2,3-double bond of both HNE enantiomers. As a result, the hydroxyl group of each enantiomeric substrate is positioned on opposite sides of the active site. Consequently, in order to also attain the low level of substrate stereoselectivity, the hydroxyl group is either not exploited for binding or else there are comparable interactions, such as enantiospecific interactions with different residues, or interactions with a symmetrically placed residue that generates energetically degenerate diastereomeric transistion states (Balogh et al., 2008). As previously mentioned, the difference in the kinetic parameters between nonenal and HNE suggest that the hydroxyl group is exploited within the GSTA4-4 active site, and hence, the latter situation is the likely strategy (Bruns et al., 1999; Hubatsch and Mannervik, 2001; Hubatsch et al., 1998; Nilsson et al., 2000). Murine GSTA4-4 has been cocrystallized with GSHNE (PDB entry 1B48), but extrapolation of the observed interactions to initial HNE binding is difficult, considering the cyclized structure of the final conjugate (Xiao et al., 1999). We recently described the first crystal structures of hGSTA4-4 (PDB entry 3IK7) and the hGSTA1-1 GIMFhelix mutant (PDB entry 3IK9), cocrystallized with the open-chain form of the 3S-GSHNE conjugate, 3S-glutathionyl 1,4-dihydroxynonanol (3S-GSDHN), which serves as an important model for the uncyclized and stereochemically relevant ternary substrate complex (Balogh et al., 2010). As expected, the hydroxyl group derived from reduction of the aldehyde is positioned at the bottom of the H-site near Tyr212, whereas the alkyl chain extends into a hydrophobic cavity delineated by Ile107, Met108, Phe111 in the α4-helix, Tyr212, Val216, and Tyr217 in the α9-helix, in a manner that turns out to illustrate how the hydroxyl group of either 4R- or 4S-HNE can be positioned near Arg15 without any surrounding residues to discriminate between either enantiomer (Figure 4). Furthermore, consistent with the modest stereoselective preference of GSTA4-4, occasional binding in a proposed additional binding mode that is still compatible within the confines of the structure would, in fact, favor an interaction with S-HNE.
Figure 4.
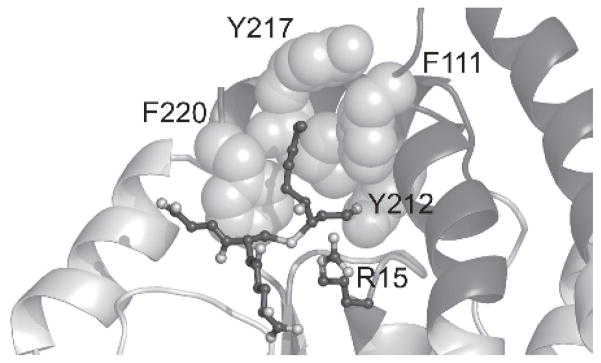
Human GSTA4-4 active site. GSTA4-4 is shown in complex with 3S,4R-GSDHN (PDB entry 3IK7) as a model for the ternary complex formed with GSH and HNE. The 4-hydroxyl group is in proximity of R15, whereas the aldehyde-derived oxygen is near Y212 at the bottom of the H-site. The alkyl chain extends into the hydrophobic groove lined with other key active site residues shown as spheres.
Stereoselectivity of product formation
The extra C-terminal helix clearly plays an important role in the different specificities exhibited by the structurally related alpha-class GSTs, and the incorporation of the Phe111-Tyr217 tower region interaction into GSTA1-1 created a mutant with a less dynamic C-terminus and increased HNE selectivity (Hou et al., 2007). In line with this trend, our studies have also shown that conformational heterogeneity is inversely correlated with product stereoselectivity in these isoforms (Balogh et al., 2008, 2010). Specifically, the GSTA4-4 and A1-1 tower mutants exhibit stereoselectivity intermediate between the wild-type templates, whereas the GIMFhelix mutant, which also incorporates the aromatic-aromatic interaction, as well as other key GSTA4-4 interactions, displays intermediate stereoselectivity of product formation similar to that observed with the GSTA1-1 tower mutant alone. In light of the catalytic properties of the GIMFhelix and tower mutants (Blikstad et al., 2008; Hou et al., 2007; Nilsson et al., 2000), it appears that although the other H-site mutations present in GIMFhelix are also imperative for alkenal activity, simply the Phe111-Tyr217 interaction is sufficient to obtain the level of stereoselectivity observed. This finding is consistent with a role in limiting local and global dynamics, which evidently allows for a higher level of steric control within the active site.
The different GSTA1-1 GIMFhelix mutant structures (PDB entries 3I69, 3I6A, and 3IK9) have also detailed the β1-α1 loop, α4-helix, and C-terminal regions that are coupled to high alkenal activity and provided a fortuitous crystallographic look that helped to explain why GSTA4-4 is completely stereoselective in product formation. Despite similarities in the binding of the 3S-GSDHN ligand, comparisons of residues comprising the active site identified an aromatic network of interactions resulting from the simultaneous presence of the A1-1-derived Phe10 and A4-4-derived Tyr212 residues within the context of the GIMFhelix active site (Balogh et al., 2010). The Phe10 residue, which is replaced by a proline in hGSTA4-4, ultimately resulted in the displacement of Phe220, a conserved residue in the C-terminal helix that is thought to assist in guiding the reacting substrates to the transition state (Nilsson et al., 2002). In total, we propose these interactions sterically hinder the optimal preorganization of the C-terminus and, inevitably, the maintenance of a consistent HNE orientation with respect to GSH. In turn, this demonstrates how these fundamental residues are linked to the high substrate specificity and transition-state interactions, as well as the complete steric control, that yield only the S-configuration at the site of conjugation in hGSTA4-4.
A decrease in stereoselectivity was also found with GSTP1-1 (Balogh et al., 2008). Curiously, relative to the alpha-class GSTs, this isoform actually catalyzes a partially preferential formation of the diastereomers with the opposite configuration at the site of conjugation. The physiological relevance of this is not clear, given that GSTP1-1 has a different tissue distribution and a 400-fold lower catalytic efficiency with HNE (Eaton and Bammler, 1999; Singhal et al., 1994). Regardless, it is interesting to note that the GSTA4-4 α9-helix, containing the critical Tyr212 residue, resides across the active site from the end of the α4-helix, which does not contain any Tyr residues in the alpha-class GST H-sites discussed herein. Although GSTP1-1 completely lacks the extended C-terminal helix, it does contain two Tyr residues near the end of the α4-helix, one of which, Tyr108, has already been implicated in the active site of GSTP1-1 (Oakley et al., 1997). This Tyr residue could, speculatively, play an analogous role to Tyr212, resulting in an orientation of the aldehyde group toward the opposing side of the active site, which could, in turn, favor the opposite stereoselectivity of product formation, compared with the alpha-class GSTs. However, in the absence of a GSTP1-1 crystal structure with HNE bound or rigorous docking studies to facilitate the identification of residues, it is difficult to predict precisely what structural attributes account for the observed differences. Nevertheless, because GSTP1-1 lacks the C-terminal helix, these results complement the evidence supporting a role for this region in the stereoselectivity of GSTA4-4.
GST adduction by HNE
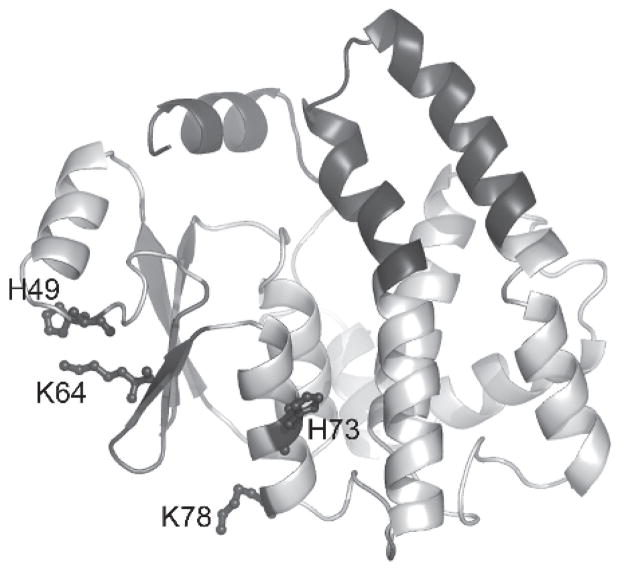
The modification of macromolecules by HNE has received considerable attention. Of particular relevance to this review is the adduction of the very enzymes responsible for the detoxification of HNE. Initial studies conducted using various mouse GST isozymes demonstrated that GST-HNE adducts were indeed formed, and that the reactivity with HNE was most strongly correlated with the number of Cys residues in the protein sequence (Mitchell et al., 1995; van Iersel et al., 1997). GSTs were also among the protein adducts identified in a more recent study that featured a global analysis of adducted proteins in colon carcinoma cells treated with HNE (Codreanu et al., 2009). The exposure of GSTA4-4 to HNE, as necessitated by its role in detoxification of this endogenous electrophile, could render the enzyme particularly susceptible to adduction and enhance the intracellular concentration and hence toxicity of HNE, if the enzyme were to be inactivated. Speculatively, if GSTA4-4 has evolved to clear HNE, then it would likely be resistant either to adduction by HNE or to deleterious functional affects of adduction by HNE. Indeed, a direct comparison of the kinetics of adduction of GSTA1-1, A4-4, and P1-1 revealed that GSTA4-4 is completely resistant under conditions that yield moderate or extensive adduction of the other isoforms (Shireman et al., 2010). The structural basis for this is not completely clear, but a qualitative inspection of the constellation of nucleophilic residues on the surface of each suggests that GSTA4-4 has fewer exposed sites for adduction. It is also likely that the protein dynamics are important, and the more dynamic nature of GSTA1-1, compared to A4-4, may contribute to the greater accessibility of its surface nucleophiles. Along these lines, it is interesting to note that, although GSTA4-4 is highly resistant to adduction, the modified residues that were identified are not part of the H-site (Figure 5).
Figure 5.
HNE adduction of GSTA4-4. Ribbon diagram illustrating the location of the residues covalently modified by HNE within the context of the hGSTA4-4 subunit (PDB entry 1GUL). Identification of the adducted residues reveals that adduction does not occur in the H-site region. The view is aligned perpendicular to the 2-fold axis of symmetry for the corresponding dimer, with the tower region and the α9-helix emphasized in dark gray.
Biological implications
HNE is now implicated in numerous pathological states associated with the consequences of oxidative stress that have been the subject of many publications. To the extent that GSTs regulate the concentration of HNE, they are poised to play an important role in modulating the effects elicited by HNE. Conversely, oxidative stress and, more specifically, HNE have been found to induce the expression of phase II enzymes (Zhang and Forman, 2009), including GSTA4-4 (Raza and John, 2006). A collection of studies have shown that GSTs offer protection against HNE-mediated damage and enhance survival (Gallagher et al., 2007; Xie et al., 1998; Sharma et al., 2004). An elevation of hGSTA4-4 has been documented in early atherosclerotic plaques from aortic samples that suggests an initial defensive role of this enzyme (Yang et al., 2004). This concept is supported by subsequent studies demonstrating the protection afforded to endothelial cells transfected with murine GSTA4 that found an associated upregulation of inducible nitric oxide synthase conveyed through the translocation of NF-κB, which is suggested to be beneficial with respect to atherosclerotic processes (Yang et al., 2008). Additional evidence for the protective role of GSTs, with potential implications concerning osteoarthritis, is illustrated by the finding that GSTA4-4 overexpression protects human chondrocytes from HNE-mediated cell death, whereas transfection with GSTA4-4 siRNA enhances the toxicity derived from HNE (Vaillancourt et al., 2008). The ability of superoxide dismutase (SOD) to scavenge superoxide anions has general relevance to pathological states related to oxidative stress. Although adult superoxide dismutase-1 (SOD1) knockout mice are known to have a reduced lifespan, young SOD1 knockout mice actually exhibit few abnormalities. Investigations examining whether this could be related to compensatory mechanisms that counter oxidative stress led to the discovery that elevated levels of GSTA4-4 are present in these young mice deficient in SOD1 and, presumably, contribute to the maintenance of normal HNE levels (Yoshihara et al., 2009).
The GSTA4-4 isoform exhibits remarkable chemoselectivity and high catalytic efficiency toward HNE, and expression of GSTA4-4 is a critical determinant of an organism’s susceptibility to disease and aging (Zarkovic, 2003a; Zimniak, 2008). Consequently, HNE involvement has been extensively documented in a number of the components associated with AD, wherein elevated levels of free and protein-bound HNE have been detected in both early and late stages of AD (Butterfield et al., 2006; Reed et al., 2009; Volkel et al., 2006; Williams et al., 2006). Although an increase in the magnitude of LPO products is a hallmark feature of neurodegeneration, notable studies provide evidence that GST activity and protein levels are altered, as well. The cytotoxic effects of HNE are known to be replicated by the administration of exogenous HNE, whereas increased GST is known to protect against HNE toxicity. Accordingly, a decline in both total GST activity and GST protein (as estimated by 4-chloro-7-nitro-2,1,3-benzoxadiazole activity and a GSTM1-1 antibody, respectively) was observed in many brain regions of short-postmortem-interval autopsy patients with AD (Lovell et al., 1998). A related AD study that also found a decrease in GST activity (measured using CDNB) uncovered a greater extent of HNE-modified GST protein and alluded to a loss in function due to altered protein structure (Sultana and Butterfield, 2004). The levels of alpha-class GSTs were actually increased, rather than decreased, and the discrepancy in protein levels between classes/studies is not entirely understood; however, isoform-specific induction and inhibition may both be contributing factors during disease progression. Furthermore, recent results highlighted above dissect the adduction of different GSTs and provide insight necessary to begin deconvoluting isoform-specific effects (Shireman et al., 2010). Although it is beyond the scope of this review, in light of the potential coordination between GSTs and transporters, it is interesting to note that multidrug resistance protein 1, which is known to transport GSH-conjugates (Renes et al., 2000), was also found to be modified by HNE in patients with AD (Sultana and Butterfield, 2004). Increased brain levels of GSHNE have also been observed (Volkel et al., 2006), suggesting that a decrease in the transport or further metabolism of GSHNE may conceivably occur during the progression of AD and further contribute to the accumulation of HNE by disrupting the flow of the detoxification process.
Continuing within the context of biological implications, evaluation of the expression and activity of antioxidant enzymes identified the alteration of several GST isoform levels in nonalcoholic fatty liver disease (Hardwick et al., 2010). Increases in GST mRNA (A1, A2, A4, M3, and P1) and protein levels assessed for each isoform family paralleled disease progression, with the exception of GSTM protein, which decreased. Conversely, GST activity, as deduced by CDNB-conjugating activity, declined with disease progression. The researchers attribute this phenomenon to a complicated mechanism of GST regulation potentially exacerbated by decreased GSH levels in vivo, which, overall, is consistent with a reduced ability to curtail oxidative stress in advanced disease states. The researchers also speculate that the HNE adduction of specific GSTs offers a plausible explanation to the discrepancy in enzyme expression and activity. In light of the recent GST-adduct characterization that indicates GSTA4-4 is highly resilient with respect to adduction (Shireman et al., 2010), it would be of interest to include HNE when monitoring GST activity in many of these studies. Shifts in the distribution of activity during different stages may not be adequately deduced by CDNB-conjugating activity alone, given a hypothetical circumstance where the small increase in CDNB conjugation provided by increased GSTA4-4 (which has poor activity with this “universal” substrate) may not compensate for the reduction in GSTA1-1 activity due to enzyme adduction and inhibition, despite elevated levels of the isoform.
Oxidative stress is also linked with the development of insulin resistance and diabetes. Consistent with the recurrent theme of alteration in GST levels coupled with HNE-protein adduction, adipose proteins from obese insulin-resistant mice were detected to have an increase in HNE modifications, whereas GSTA4-4 protein levels exhibited a decrease in the adipose tissue of obese, insulin-resistant mice and humans (Curtis et al., 2010; Grimsrud et al., 2007). In order to explore the impact of the interaction between GSTA4-4 and HNE in an intact organism, researchers have been exploiting a mGsta4 null mouse model (Engle et al., 2004). These mice have a reduced capacity to metabolize HNE and were found to show an earlier onset of degenerative declines, in addition to lower survival rates when presented with oxidative challenges. Notably, these mice did retain between 23 and 64% of HNE-conjugating activity, depending on the tissue, which attests to the importance of other GSTs with respect to redundancy in HNE detoxification. Complicating the situation is the observation that the biochemical phenotype of this Gsta4 null mouse is strain dependent and hence must be considered when interpreting outcomes (Singh et al., 2008). Nonetheless, these mice serve as a useful model, and subsequent studies derived from null mice of the 129/sv genetic background have contributed to the establishment of the relationship between increased levels of HNE and obesity. In contrast, Gsta4 null mice of the C57BL/6 background were generally not found to have elevated levels of HNE and remained lean in this study. Expanding upon this counterintuitive result is the finding that these mice have an increased lifespan (Singh et al., 2010). This is particularly interesting, in light of model studies concerning Caenorhabditis elegans that demonstrated mGsta4 transgeneic strains were accompanied by an increase in HNE-conjugating activity and had increased stress resistance with an extended lifespan (Ayyadevara et al., 2005). This finding is consistent with additional studies that identified GSTs among the detoxification enzymes with elevated transcripts in long-lived forms of C. elegans (McElwee et al., 2004, 2007) whereas RNAi knockdown of related GSTs and disruption of HNE detoxification significantly reduced longevity (Ayyadevara et al., 2005a, 2007). Although these results merit future clarification, the manipulation of organisms that ultimately alter HNE concentrations attest to the relevance of HNE in various physio- and pathological processes, wherein GSTs can make important contributions to a detoxification scheme that coordinates the complex system.
Summary
GSTs clearly play a central role in the detoxification of electrophilic aldehydes. Collectively, the available data stress that although GSTA4-4 is not the most abundant isoform, its presence can play a crucial role in the detoxification of HNE. A decrease in this protective mechanism may partially underlie the pronounced effects of HNE and thus the pathogenesis of several disease states. Within the context of accommodating a racemic alkenal, the available studies demonstrate that GSTA4-4 achieves a high chemospecificity and an optimal stereoselectivity profile without undergoing enantiospecific induced fit, but rather by utilizing a preorganized alkenal-template and the active site residue Arg15, which is ideally located to interact with the 4-hydroxyl group of either HNE enantiomer. The data further suggest that hGSTA4-4 may optimize its biological utility by combining high catalytic efficiency and low substrate stereoselectivity with strict stereoselectivity of product formation. This strategy allows for the acceptance of both enantiomers as substrates, which provides protection from the harmful consequences of oxidative stress products while producing only a select set of GSHNE diastereomers with potential biological implications for stereoselective bioactivity and transport. Furthermore, though, undoubtedly, the result of multiple complex phenomena, the results described with model systems reviewed herein provide a comprehensive view of the outcomes associated with altered GST and HNE levels in intact organisms and offer important insights regarding the interaction of GSTs and HNE that may be extended to human biology.
Acknowledgments
The authors would like to thank Dr. Abhinav Nath for his insightful suggestions in the review of the manuscript.
Footnotes
Declaration of interest
This work was supported by a National Institutes of Health (Bethesda, Maryland, USA) grant (GM62284; to W.M.A.).
References
- Adman ET, Le Trong I, Stenkamp RE, Nieslanik BS, Dietze EC, Tai G, et al. Localization of the C-terminus of rat glutathione S-transferase A1-1: crystal structure of mutants W21F and W21F/F220Y. Proteins. 2001;42:192–200. doi: 10.1002/1097-0134(20010201)42:2<192::aid-prot60>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Alary J, Debrauwer L, Fernandez Y, Cravedi JP, Rao D, Bories G. 1,4-dihydroxynonene mercapturic acid, the major end metabolite of exogenous 4-hydroxy-2-nonenal, is a physiological component of rat and human urine. Chem Res Toxicol. 1998;11:130–135. doi: 10.1021/tx970139w. [DOI] [PubMed] [Google Scholar]
- Alary J, Gueraud F, Cravedi JP. Fate of 4-hydroxynonenal in vivo: disposition and metabolic pathways. Mol Aspects Med. 2003;24:177–187. doi: 10.1016/s0098-2997(03)00012-8. [DOI] [PubMed] [Google Scholar]
- Aldini G, Gamberoni L, Orioli M, Beretta G, Regazzoni L, Maffei Facino R, et al. Mass spectrometric characterization of covalent modification of human serum albumin by 4-hydroxy-trans- 2-nonenal. J Mass Spectrom. 2006;41:1149–1161. doi: 10.1002/jms.1067. [DOI] [PubMed] [Google Scholar]
- Alin P, Danielson UH, Mannervik B. 4-hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985;179:267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- Amunom I, Stephens LJ, Tamasi V, Cai J, Pierce WM, Jr, Conklin DJ, et al. Cytochromes P450 catalyze oxidation of alpha,beta-unsaturated aldehydes. Arch Biochem Biophys. 2007;464:187–196. doi: 10.1016/j.abb.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RN. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem Res Toxicol. 1997;10:2–18. doi: 10.1021/tx960072x. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Singhal SS, Yadav S, Singhal J, Drake K, Nadkar A, et al. RLIP76 is a major determinant of radiation sensitivity. Cancer Res. 2005a;65:6022–6028. doi: 10.1158/0008-5472.CAN-05-0968. [DOI] [PubMed] [Google Scholar]
- Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Li J, et al. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic Biol Med. 2004;37:607–619. doi: 10.1016/j.freeradbiomed.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Dandapat A, Singh SP, Benes H, Zimniak L, Shmookler Reis RJ, et al. Lifespan extension in hypomorphic daf-2 mutants of Caenorhabditis elegans is partially mediated by glutathione transferase CeGSTP2-2. Aging Cell. 2005a;4:299–307. doi: 10.1111/j.1474-9726.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Dandapat A, Singh SP, Siegel ER, Shmookler Reis RJ, Zimniak L, et al. Life span and stress resistance of Caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2- enal. Mech Ageing Dev. 2007;128:196–205. doi: 10.1016/j.mad.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyadevara S, Engle MR, Singh SP, Dandapat A, Lichti CF, Benes H, et al. Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4- hydroxynonenal. Aging Cell. 2005b;4:257–271. doi: 10.1111/j.1474-9726.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- Babbitt PC. Reengineering the glutathione S-transferase scaffold: a rational design strategy pays off. Proc Natl Acad Sci U S A. 2000;97:10298–10300. doi: 10.1073/pnas.97.19.10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi YC, Ansari GA, Awasthi S. Regulation of 4-hydroxynonenal mediated signaling by glutathione S-transferases. Meth Enzymol. 2005;401:379–407. doi: 10.1016/S0076-6879(05)01024-4. [DOI] [PubMed] [Google Scholar]
- Balchin D, Fanucchi S, Achilonu I, Adamson RJ, Burke J, Fernandes M, et al. Stability of the domain interface contributes towards the catalytic function at the H-site of class alpha glutathione transferase A1-1. Biochim Biophys Acta. 2010;1804:2228–2233. doi: 10.1016/j.bbapap.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Balogh LM, Le Trong I, Kripps KA, Shireman LM, Stenkamp RE, Zhang W, et al. Substrate specificity combined with stereopromiscuity in glutathione transferase A4-4-dependent metabolism of 4-hydroxynonenal. Biochemistry. 2010;49:1541–1548. doi: 10.1021/bi902038u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh LM, Le Trong I, Kripps KA, Tars K, Stenkamp RE, Mannervik B, et al. Structural analysis of a glutathione transferase A1-1 mutant tailored for high catalytic efficiency with toxic alkenals. Biochemistry. 2009;48:7698–7704. doi: 10.1021/bi900895b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh LM, Roberts AG, Shireman LM, Greene RJ, Atkins WM. The stereochemical course of 4-hydroxy-2- nonenal metabolism by glutathione S-transferases. J Biol Chem. 2008;283:16702–16710. doi: 10.1074/jbc.M801725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980;620:281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- Bjornestedt R, Stenberg G, Widersten M, Board PG, Sinning I, Jones TA, et al. Functional significance of arginine 15 in the active site of human class alpha glutathione transferase A1-1. J Mol Biol. 1995;247:765–773. doi: 10.1016/s0022-2836(05)80154-8. [DOI] [PubMed] [Google Scholar]
- Bjornestedt R, Tardioli S, Mannervik B. The high activity of rat glutathione transferase 8-8 with alkene substrates is dependent on a glycine residue in the active site. J Biol Chem. 1995b;270:29705–29709. doi: 10.1074/jbc.270.50.29705. [DOI] [PubMed] [Google Scholar]
- Blikstad C, Shokeer A, Kurtovic S, Mannervik B. Emergence of a novel highly specific and catalytically efficient enzyme from a naturally promiscuous glutathione transferase. Biochim Biophys Acta. 2008;1780:1458–1463. doi: 10.1016/j.bbagen.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Board PG. Identification of cDNAs encoding two human alpha class glutathione transferases (GSTA3 and GSTA4) and the heterologous expression of GSTA4-4. Biochem J. 1998;330:827–831. doi: 10.1042/bj3300827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaards JJ, Venekamp JC, van Bladeren PJ. Stereoselective conjugation of prostaglandin A2 and prostaglandin J2 with glutathione, catalyzed by the human glutathione S-transferases A1-1, A2-2, M1a-1a, and P1-1. Chem Res Toxicol. 1997;10:310–317. doi: 10.1021/tx9601770. [DOI] [PubMed] [Google Scholar]
- Bolgar MS, Yang CY, Gaskell SJ. First direct evidence for lipid/protein conjugation in oxidized human low density lipoprotein. J Biol Chem. 1996;271:27999–28001. doi: 10.1074/jbc.271.45.27999. [DOI] [PubMed] [Google Scholar]
- Boon PJ, Marinho HS, Oosting R, Mulder GJ. Glutathione conjugation of 4-hydroxy-trans-2,3-nonenal in the rat in vivo, the isolated perfused liver, and erythrocytes. Toxicol Appl Pharmacol. 1999;159:214–223. doi: 10.1006/taap.1999.8742. [DOI] [PubMed] [Google Scholar]
- Bull AW, Seeley SK, Geno J, Mannervik B. Conjugation of the linoleic acid oxidation product, 13-oxooctadeca-9,11-dienoic acid, a bioactive endogenous substrate for mammalian glutathione transferase. Biochim Biophys Acta. 2002;1571:77–82. doi: 10.1016/s0304-4165(02)00216-7. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Bader Lange ML, Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer’s disease. Biochim Biophys Acta. 2010a;1801:924–929. doi: 10.1016/j.bbalip.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Hardas SS, Lange ML. Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer’s disease: many pathways to neurodegeneration. J Alzheimers Dis. 2010;20:369–393. doi: 10.3233/JAD-2010-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Perluigi M, De Marco C, Coccia R, Cini C, et al. Elevated protein-bound levels of the lipid peroxidation product, 4-hydroxy-2-nonenal, in brain from persons with mild cognitive impairment. Neurosci Lett. 2006;397:170–173. doi: 10.1016/j.neulet.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Cameron AD, Sinning I, L’Hermite G, Olin B, Board PG, Mannervik B, et al. Structural analysis of human alpha-class glutathione transferase A1-1 in the apo-form and in complexes with ethacrynic acid and its glutathione conjugate. Structure. 1995;3:717–727. doi: 10.1016/s0969-2126(01)00206-4. [DOI] [PubMed] [Google Scholar]
- Carini M, Aldini G, Facino RM. Mass spectrometry for detection of 4-hydroxy-trans- 2-nonenal (HNE) adducts with peptides and proteins. Mass Spectrom Rev. 2004;23:281–305. doi: 10.1002/mas.10076. [DOI] [PubMed] [Google Scholar]
- Cenini G, Sultana R, Memo M, Butterfield DA. Elevated levels of pro-apoptotic p53 and its oxidative modification by the lipid peroxidation product, HNE, in brain from subjects with amnestic mild cognitive impairment and Alzheimer’s disease. J Cell Mol Med. 2008;12:987–994. doi: 10.1111/j.1582-4934.2008.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JZ, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, et al. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J Biol Chem. 2001;276:41213–41223. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- Cheng JZ, Yang Y, Singh SP, Singhal SS, Awasthi S, Pan SS, et al. Two distinct 4-hydroxynonenal metabolizing glutathione S-transferase isozymes are differentially expressed in human tissues. Biochem Biophys Res Commun. 2001b;282:1268–1274. doi: 10.1006/bbrc.2001.4707. [DOI] [PubMed] [Google Scholar]
- Codreanu SG, Zhang B, Sobecki SM, Billheimer DD, Liebler DC. Global analysis of protein damage by the lipid electrophile 4-hydroxy-2-nonenal. Mol Cell Proteomics. 2009;8:670–680. doi: 10.1074/mcp.M800070-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles BF, Kadlubar FF. Human alpha class glutathione S-transferases: genetic polymorphism, expression, and susceptibility to disease. Methods Enzymol. 2005;401:9–42. doi: 10.1016/S0076-6879(05)01002-5. [DOI] [PubMed] [Google Scholar]
- Curtis JM, Grimsrud PA, Wright WS, Xu X, Foncea RE, Graham DW, et al. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59:1132–1142. doi: 10.2337/db09-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson UH, Esterbauer H, Mannervik B. Structure-activity relationships of 4-hydroxyalkenals in the conjugation catalysed by mammalian glutathione transferases. Biochem J. 1987;247:707–713. doi: 10.1042/bj2470707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirr H, Reinemer P, Huber R. X-ray crystal structures of cytosolic glutathione S-transferases. Implications for protein architecture, substrate recognition, and catalytic function. Eur J Biochem. 1994;220:645–661. doi: 10.1111/j.1432-1033.1994.tb18666.x. [DOI] [PubMed] [Google Scholar]
- Dirr HW, Little T, Kuhnert DC, Sayed Y. A conserved N-capping motif contributes significantly to the stabilization and dynamics of the C-terminal region of class alpha glutathione S-transferases. J Biol Chem. 2005;280:19480–19487. doi: 10.1074/jbc.M413608200. [DOI] [PubMed] [Google Scholar]
- Dirr HW, Wallace LA. Role of the C-terminal helix 9 in the stability and ligandin function of class alpha glutathione transferase A1-1. Biochemistry. 1999;38:15631–15640. doi: 10.1021/bi991179x. [DOI] [PubMed] [Google Scholar]
- Dourado DF, Fernandes PA, Mannervik B, Ramos MJ. Glutathione transferase A1-1: catalytic importance of arginine 15. J Phys Chem B. 2010;114:1690–1697. doi: 10.1021/jp908251z. [DOI] [PubMed] [Google Scholar]
- Dourado DF, Fernandes PA, Ramos MJ. Mammalian cytosolic glutathione transferases. Curr Prot Pept Sci. 2008;9:325–337. doi: 10.2174/138920308785132677. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Bammler TK. Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol Sci. 1999;49:156–164. doi: 10.1093/toxsci/49.2.156. [DOI] [PubMed] [Google Scholar]
- Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, Ceci JD, et al. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4- hydroxynonenal: generation and analysis of mGsta4 null mouse. Toxicol Appl Pharmacol. 2004;194:296–308. doi: 10.1016/j.taap.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde, and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Zollner H, Scholz N. Reaction of glutathione with conjugated carbonyls. Z Naturforsch [C] 1975;30:466–473. doi: 10.1515/znc-1975-7-808. [DOI] [PubMed] [Google Scholar]
- Gallagher EP, Huisden CM, Gardner JL. Transfection of HepG2 cells with hGSTA4 provides protection against 4-hydroxynonenal-mediated oxidative injury. Toxicol In Vitro. 2007;21:1365–1372. doi: 10.1016/j.tiv.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildenhuys S, Dobreva M, Kinsley N, Sayed Y, Burke J, Pelly S, et al. Arginine 15 stabilizes an S(N)Ar reaction transition state and the binding of anionic ligands at the active site of human glutathione transferase A1-1. Biophys Chem. 2010;146:118–125. doi: 10.1016/j.bpc.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Grahn E, Novotny M, Jakobsson E, Gustafsson A, Grehn L, Olin B, et al. New crystal structures of human glutathione transferase A1-1 shed light on glutathione binding and the conformation of the C-terminal helix. Acta Crystallogr D Biol Crystallogr. 2006;62:197–207. doi: 10.1107/S0907444905039296. [DOI] [PubMed] [Google Scholar]
- Grimsrud PA, Picklo MJ, Sr, Griffin TJ, Bernlohr DA. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol Cell Proteomics. 2007;6:624–637. doi: 10.1074/mcp.M600120-MCP200. [DOI] [PubMed] [Google Scholar]
- Gustafsson A, Etahadieh M, Jemth P, Mannervik B. The C-terminal region of human glutathione transferase A1-1 affects the rate of glutathione binding and the ionization of the active-site Tyr9. Biochemistry. 1999;38:16268–16275. doi: 10.1021/bi991482y. [DOI] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Lake AD, Cherrington NJ. Diversity in antioxidant response enzymes in progressive stages of human non-alcoholic fatty liver disease. Drug Metab Dispos. 2010;38:2293–2301. doi: 10.1124/dmd.110.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Sibata T, Wasada H, Toyokuni S, Uchida K. Structural basis of protein-bound endogenous aldehydes. Chemical and immunochemical characterizations of configurational isomers of a 4-hydroxy-2-nonenal-histidine adduct. J Biol Chem. 2003;278:5044–5051. doi: 10.1074/jbc.M210129200. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- Hiratsuka A, Hirose K, Saito H, Watabe T. 4-hydroxy-2(E)-nonenal enantiomers: (S)-selective inactivation of glyceraldehyde-3-phosphate dehydrogenase and detoxification by rat glutathione S-transferase A4-4. Biochem J. 2000;349:729–735. doi: 10.1042/bj3490729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Honaker MT, Shireman LM, Balogh LM, Roberts AG, Ng KC, et al. Functional promiscuity correlates with conformational heterogeneity in A-class glutathione S-transferases. J Biol Chem. 2007;282:23264–23274. doi: 10.1074/jbc.M700868200. [DOI] [PubMed] [Google Scholar]
- Hu W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, et al. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23:1781–1789. doi: 10.1093/carcin/23.11.1781. [DOI] [PubMed] [Google Scholar]
- Huang H, Wang H, Qi N, Lloyd RS, Rizzo CJ, Stone MP. The stereochemistry of trans-4-hydroxynonenal-derived exocyclic 1,N2-2′-deoxyguanosine adducts modulates formation of interstrand cross-links in the 5′-CpG-3′ sequence. Biochemistry. 2008;47:11457–11472. doi: 10.1021/bi8011143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubatsch I, Mannervik B. A highly acidic tyrosine 9 and a normally titrating tyrosine 212 contribute to the catalytic mechanism of human glutathione transferase A4-4. Biochem Biophys Res Commun. 2001;280:878–882. doi: 10.1006/bbrc.2000.4230. [DOI] [PubMed] [Google Scholar]
- Hubatsch I, Ridderstrom M, Mannervik B. Human glutathione transferase A4-4: an alpha class enzyme with high catalytic efficiency in the conjugation of 4-hydroxynonenal and other genotoxic products of lipid peroxidation. Biochem J. 1998;330:175–179. doi: 10.1042/bj3300175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra C, Nieslanik BS, Atkins WM. Contribution of aromatic-aromatic interactions to the anomalous pK(a) of tyrosine-9 and the C-terminal dynamics of glutathione S-transferase A1-1. Biochemistry. 2001;40:10614–10624. doi: 10.1021/bi010672h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AT, Marnett LJ. Systems analysis of protein modification and cellular responses induced by electrophile stress. Acc Chem Res. 2010;43:673–683. doi: 10.1021/ar900286y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Ito K, Suzuki H, Sugiyama Y, Horie T. Multidrug resistance-associated protein2 (MRP2) plays an important role in the biliary excretion of glutathione conjugates of 4-hydroxynonenal. Free Radic Biol Med. 2002;33:370–378. doi: 10.1016/s0891-5849(02)00906-1. [DOI] [PubMed] [Google Scholar]
- Kuhnert DC, Sayed Y, Mosebi S, Sayed M, Sewell T, Dirr HW. Tertiary interactions stabilise the C-terminal region of human glutathione transferase A1-1: a crystallographic and calorimetric study. J Mol Biol. 2005;349:825–838. doi: 10.1016/j.jmb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Le Trong I, Stenkamp RE, Ibarra C, Atkins WM, Adman ET. 1.3-A resolution structure of human glutathione S-transferase with S-hexyl glutathione bound reveals possible extended ligandin binding site. Proteins. 2002;48:618–627. doi: 10.1002/prot.10162. [DOI] [PubMed] [Google Scholar]
- Leitinger N. Cholesteryl ester oxidation products in atherosclerosis. Mol Aspects Med. 2003;24:239–250. doi: 10.1016/s0098-2997(03)00019-0. [DOI] [PubMed] [Google Scholar]
- Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, et al. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Komatsu H, Murray IV, Axelsen PH. Promotion of amyloid beta protein misfolding and fibrillogenesis by a lipid oxidation product. J Mol Biol. 2008;377:1236–1250. doi: 10.1016/j.jmb.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Liu S, Stoesz SP, Pickett CB. Identification of a novel human glutathione S-transferase using bioinformatics. Arch Biochem Biophys. 1998;352:306–313. doi: 10.1006/abbi.1998.0608. [DOI] [PubMed] [Google Scholar]
- Lopachin RM, Barber DS, Gavin T. Molecular mechanisms of the conjugated alpha,beta-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Toxicol Sci. 2008;104:235–249. doi: 10.1093/toxsci/kfm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer’s disease. Neurology. 1998;51:1562–1566. doi: 10.1212/wnl.51.6.1562. [DOI] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Piper MD, Thomas JH, Patel DS, et al. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol. 2007;8:R132. doi: 10.1186/gb-2007-8-7-r132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem. 2004;279:44533–44543. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- Miller BG, Wolfenden R. Catalytic proficiency: the unusual case of OMP decarboxylase. Annu Rev Biochem. 2002;71:847–885. doi: 10.1146/annurev.biochem.71.110601.135446. [DOI] [PubMed] [Google Scholar]
- Mitchell AE, Morin D, Lame MW, Jones AD. Purification, mass spectrometric characterization, and covalent modification of murine glutathione S-transferases. Chem Res Toxicol. 1995;8:1054–1062. doi: 10.1021/tx00050a009. [DOI] [PubMed] [Google Scholar]
- Morel F, Rauch C, Coles B, Le Ferrec E, Guillouzo A. The human glutathione transferase alpha locus: genomic organization of the gene cluster and functional characterization of the genetic polymorphism in the hGSTA1 promoter. Pharmacogenetics. 2002;12:277–286. doi: 10.1097/00008571-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Mosebi S, Sayed Y, Burke J, Dirr HW. Residue 219 impacts on the dynamics of the C-terminal region in glutathione transferase A1-1: implications for stability and catalytic and ligandin functions. Biochemistry. 2003;42:15326–15332. doi: 10.1021/bi035671z. [DOI] [PubMed] [Google Scholar]
- Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic Biol Med. 2007;43:1109–1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Nieslanik BS, Atkins WM. The catalytic Tyr-9 of glutathione S-transferase A1-1 controls the dynamics of the C terminus. J Biol Chem. 2000;275:17447–17451. doi: 10.1074/jbc.M002083200. [DOI] [PubMed] [Google Scholar]
- Nieslanik BS, Dabrowski MJ, Lyon RP, Atkins WM. Stopped-flow kinetic analysis of the ligand-induced coil-helix transition in glutathione S-transferase A1-1: evidence for a persistent denatured state. Biochemistry. 1999;38:6971–6980. doi: 10.1021/bi9829130. [DOI] [PubMed] [Google Scholar]
- Nieslanik BS, Ibarra C, Atkins WM. The C-terminus of glutathione S-transferase A1-1 is required for entropically-driven ligand binding. Biochemistry. 2001;40:3536–3543. doi: 10.1021/bi001869x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson LO, Edalat M, Pettersson PL, Mannervik B. Aromatic residues in the C-terminal region of glutathione transferase A1-1 influence rate-determining steps in the catalytic mechanism. Biochim Biophys Acta. 2002;1598:199–205. doi: 10.1016/s0167-4838(02)00362-x. [DOI] [PubMed] [Google Scholar]
- Nilsson LO, Gustafsson A, Mannervik B. Redesign of substrate-selectivity determining modules of glutathione transferase A1-1 installs high catalytic efficiency with toxic alkenal products of lipid peroxidation. Proc Natl Acad Sci U S A. 2000;97:9408–9412. doi: 10.1073/pnas.150084897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AJ, Rossjohn J, Lo Bello M, Caccuri AM, Federici G, Parker MW. The three-dimensional structure of the human pi class glutathione transferase P1-1 in complex with the inhibitor ethacrynic acid and its glutathione conjugate. Biochemistry. 1997;36:576–585. doi: 10.1021/bi962316i. [DOI] [PubMed] [Google Scholar]
- Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Qin Z, Hu D, Han S, Reaney SH, Di Monte DA, Fink AL. Effect of 4-hydroxy-2-nonenal modification on alpha-synuclein aggregation. J Biol Chem. 2007;282:5862–5870. doi: 10.1074/jbc.M608126200. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, et al. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation derived aldehyde 4-hydroxy-trans- 2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J Biol Chem. 2006;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- Raza H, John A. 4-hydroxynonenal induces mitochondrial oxidative stress, apoptosis and expression of glutathione S-transferase A4-4 and cytochrome P450 2E1 in PC12 cells. Toxicol Appl Pharmacol. 2006;216:309–318. doi: 10.1016/j.taap.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Reed TT, Pierce WM, Markesbery WR, Butterfield DA. Proteomic identification of HNE-bound proteins in early Alzheimer disease: Insights into the role of lipid peroxidation in the progression of AD. Brain Res. 2009;1274:66–76. doi: 10.1016/j.brainres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Renes J, De Vries EE, Hooiveld GJ, Krikken I, Jansen PL, Muller M. Multidrug resistance protein MRP1 protects against the toxicity of the major lipid peroxidation product 4-hydroxynonenal. Biochem J. 2000;350(Pt 2):555–561. [PMC free article] [PubMed] [Google Scholar]
- Selley ML. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson’s disease. Free Radic Biol Med. 1998;25:169–174. doi: 10.1016/s0891-5849(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Sharma R, Awasthi S, Zimniak P, Awasthi YC. Transport of glutathione-conjugates in human erythrocytes. Acta Biochim Pol. 2000;47:751–762. [PubMed] [Google Scholar]
- Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stressmediated apoptosis. Antioxid Redox Signal. 2004;6:289–300. doi: 10.1089/152308604322899350. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function, and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shireman LM, Kripps KA, Balogh LM, Conner KP, Whittington D, Atkins WM. Glutathione transferase A4-4 resists adduction by 4-hydroxynonenal. Arch Biochem Biophys. 2010;504:182–189. doi: 10.1016/j.abb.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel SJ, Bieschke J, Powers ET, Kelly JW. The oxidative stress metabolite 4- hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry. 2007;46:1503–1510. doi: 10.1021/bi061853s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Niemczyk M, Saini D, Awasthi YC, Zimniak L, Zimniak P. Role of the electrophilic lipid peroxidation product 4-hydroxynonenal in the development and maintenance of obesity in mice. Biochemistry. 2008;47:3900–3911. doi: 10.1021/bi702124u. [DOI] [PubMed] [Google Scholar]
- Singh SP, Niemczyk M, Saini D, Sadovov V, Zimniak L, Zimniak P. Disruption of the mGsta4 gene increases life span of C57BL mice. J Gerontol A Biol Sci Med Sci. 2010;65:14–23. doi: 10.1093/gerona/glp165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal J, Singhal SS, Yadav S, Suzuki S, Warnke MM, Yacoub A, et al. RLIP76 in defense of radiation poisoning. Int J Radiat Oncol Biol Phys. 2008;72:553–561. doi: 10.1016/j.ijrobp.2008.06.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal SS, Zimniak P, Awasthi S, Piper JT, He NG, Teng JI, et al. Several closely related glutathione S-transferase isozymes catalyzing conjugation of 4- hydroxynonenal are differentially expressed in human tissues. Arch Biochem Biophys. 1994;311:242–250. doi: 10.1006/abbi.1994.1233. [DOI] [PubMed] [Google Scholar]
- Sinning I, Kleywegt GJ, Cowan SW, Reinemer P, Dirr HW, Huber R, et al. Structure determination and refinement of human alpha class glutathione transferase A1-1, and a comparison with the mu and pi class enzymes. J Mol Biol. 1993;232:192–212. doi: 10.1006/jmbi.1993.1376. [DOI] [PubMed] [Google Scholar]
- Stenberg G, Ridderstrom M, Engstrom A, Pemble SE, Mannervik B. Cloning and heterologous expression of cDNA encoding class alpha rat glutathione transferase 8-8, an enzyme with high catalytic activity towards genotoxic alpha,beta-unsaturated carbonyl compounds. Biochem J. 1992;284(Pt 2):313–319. doi: 10.1042/bj2840313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med Res Rev. 2001;21:185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- Sultana R, Butterfield DA. Oxidatively modified GST and MRP1 in Alzheimer’s disease brain: implications for accumulation of reactive lipid peroxidation products. Neurochem Res. 2004;29:2215–2220. doi: 10.1007/s11064-004-7028-0. [DOI] [PubMed] [Google Scholar]
- Szapacs ME, Riggins JN, Zimmerman LJ, Liebler DC. Covalent adduction of human serum albumin by 4-hydroxy- 2-nonenal: kinetic analysis of competing alkylation reactions. Biochemistry. 2006;45:10521–10528. doi: 10.1021/bi060535q. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Uchida K. Future of toxicology—lipid peroxidation in the future: from biomarker to etiology. Chem Res Toxicol. 2007;20:3–5. doi: 10.1021/tx600304n. [DOI] [PubMed] [Google Scholar]
- Vaillancourt F, Fahmi H, Shi Q, Lavigne P, Ranger P, Fernandes JC, et al. 4-hydroxynonenal induces apoptosis in human osteoarthritic chondrocytes: the protective role of glutathione-S-transferase. Arthritis Res Ther. 2008;10:R107. doi: 10.1186/ar2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iersel ML, Ploemen JP, Lo Bello M, Federici G, Van Bladeren PJ. Interactions of alpha, beta-unsaturated aldehydes and ketones with human glutathione Stransferase P1-1. Chem Biol Interact. 1997;108:67–78. doi: 10.1016/s0009-2797(97)00096-3. [DOI] [PubMed] [Google Scholar]
- Volkel W, Alvarez-Sanchez R, Weick I, Mally A, Dekant W, Pahler A. Glutathione conjugates of 4-hydroxy-2(E)-nonenal as biomarkers of hepatic oxidative stress-induced lipid peroxidation in rats. Free Radic Biol Med. 2005;38:1526–1536. doi: 10.1016/j.freeradbiomed.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Volkel W, Sicilia T, Pahler A, Gsell W, Tatschner T, Jellinger K, et al. Increased brain levels of 4-hydroxy-2-nonenal glutathione conjugates in severe Alzheimer’s disease. Neurochem Int. 2006;48:679–686. doi: 10.1016/j.neuint.2005.12.003. [DOI] [PubMed] [Google Scholar]
- West JD, Ji C, Duncan ST, Amarnath V, Schneider C, Rizzo CJ, et al. Induction of apoptosis in colorectal carcinoma cells treated with 4-hydroxy-2-nonenal and structurally related aldehydic products of lipid peroxidation. Chem Res Toxicol. 2004;17:453–462. doi: 10.1021/tx034248o. [DOI] [PubMed] [Google Scholar]
- West JD, Marnett LJ. Alterations in gene expression induced by the lipid peroxidation product, 4-hydroxy-2-nonenal. Chem Res Toxicol. 2005;18:1642–1653. doi: 10.1021/tx050211n. [DOI] [PubMed] [Google Scholar]
- Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4- hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Xiao B, Singh SP, Nanduri B, Awasthi YC, Zimniak P, Ji X. Crystal structure of a murine glutathione S-transferase in complex with a glutathione conjugate of 4-hydroxynon-2-enal in one subunit and glutathione in the other: evidence of signaling across the dimer interface. Biochemistry. 1999;38:11887–11894. doi: 10.1021/bi990468i. [DOI] [PubMed] [Google Scholar]
- Xie C, Lovell MA, Markesbery WR. Glutathione transferase protects neuronal cultures against four hydroxynonenal toxicity. Free Radic Biol Med. 1998;25:979–988. doi: 10.1016/s0891-5849(98)00186-5. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cheng JZ, Singhal SS, Saini M, Pandya U, Awasthi S, et al. Role of glutathione S-transferases in protection against lipid peroxidation. Overexpression of hGSTA2-2 in K562 cells protects against hydrogen peroxide-induced apoptosis and inhibits JNK and caspase 3 activation. J Biol Chem. 2001;276:19220–19230. doi: 10.1074/jbc.M100551200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol. 2003;50:319–336. [PubMed] [Google Scholar]
- Yang Y, Trent MB, He N, Lick SD, Zimniak P, Awasthi YC, et al. Glutathione-S-transferase A4-4 modulates oxidative stress in endothelium: possible role in human atherosclerosis. Atherosclerosis. 2004;173:211–221. doi: 10.1016/j.atherosclerosis.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Yang Y, Xu Y, Lick SD, Awasthi YC, Boor PJ. Endothelial glutathione-S-transferase A4-4 protects against oxidative stress and modulates iNOS expression through NF-kappaB translocation. Toxicol Appl Pharmacol. 2008;230:187–196. doi: 10.1016/j.taap.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara D, Fujiwara N, Ookawara T, Kato S, Sakiyama H, Yokoe S, et al. Protective role of glutathione S-transferase A4 induced in copper/zinc-superoxide dismutase knockout mice. Free Radic Biol Med. 2009;47:559–567. doi: 10.1016/j.freeradbiomed.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Zarkovic K. 4-hydroxynonenal and neurodegenerative diseases. Mol Aspects Med. 2003a;24:293–303. doi: 10.1016/s0098-2997(03)00024-4. [DOI] [PubMed] [Google Scholar]
- Zarkovic N. 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol Aspects Med. 2003b;24:281–291. doi: 10.1016/s0098-2997(03)00023-2. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Rule GS. Glutathione induces helical formation in the carboxy terminus of human glutathione transferase A1-1. Biochemistry. 2004;43:7244–7254. doi: 10.1021/bi0363329. [DOI] [PubMed] [Google Scholar]
- Zhang H, Forman HJ. Signaling pathways involved in phase II gene induction by alpha, beta-unsaturated aldehydes. Toxicol Ind Health. 2009;25:269–278. doi: 10.1177/0748233709102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Singhal SS, Piper JT, Cheng J, Pandya U, Clark-Wronski J, et al. The role of human glutathione S-transferases hGSTA1-1 and hGSTA2-2 in protection against oxidative stress. Arch Biochem Biophys. 1999;367:216–224. doi: 10.1006/abbi.1999.1277. [DOI] [PubMed] [Google Scholar]
- Zhou C, Huang Y, Przedborski S. Oxidative stress in Parkinson’s disease: a mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci. 2008;1147:93–104. doi: 10.1196/annals.1427.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimniak P. Detoxification reactions: relevance to aging. Ageing Res Rev. 2008;7:281–300. doi: 10.1016/j.arr.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimniak P, Eckles MA, Saxena M, Awasthi YC. A subgroup of class alpha glutathione S-transferases. Cloning of cDNA for mouse lung glutathione S-transferase GST 5.7. FEBS Lett. 1992;313:173–176. doi: 10.1016/0014-5793(92)81438-r. [DOI] [PubMed] [Google Scholar]