Abstract
Objective
To assess tThe efficacy and safety of early, non-invasive of inhaled nitric oxide (iNO) therapy in premature newborns who do not require mechanical ventilation.
Study design
We performed a multicenter randomized trial including 124 premature newborns who required non-invasive supplemental oxygen within the first 72 hours after birth. Newborns were stratified into 3 different groups by birth weight (500-749, 750-999, 1000-1250g) prior to randomization to iNO (10 ppm) or placebo gas (controls) until 30 weeks postmenstrual age (PMA). The primary outcome was a composite of death or BPD at 36 weeks PMA. Secondary outcomes included the need for and duration of mechanical ventilation, severity of bronchopulmonary dysplasia (BPD) and safety outcomes.
Results
There was no difference in the incidence of death or BPD in the iNO and placebo groups (42% vs. 40%, p=0.86, RR=1.06, 0.7-1.6). BPD severity was not different between the treatment groups. There were no differences between the groups in the need for mechanical ventilation (22% vs. 23%; p=0.89), duration of mechanical ventilation (9.7 days vs. 8.4 days; p=0.27), or safety outcomes including severe intracranial hemorrhage (3.4% vs. 6.2%, p=0.68).
Conclusions
We found that iNO delivered non-invasively to premature infants who have not progressed to early respiratory failure is a safe treatment, but does not decrease the incidence or severity of BPD, reduce the need for mechanical ventilation or alter the clinical course.
Keywords: Prematurity, respiratory failure, respiratory distress syndrome, chronic lung disease, bronchopulmonary dysplasia, mechanical ventilation, inhaled nitric oxide
Inhaled nitric oxide (iNO) is a safe and effective treatment for near–term and term newborns with acute hypoxemic respiratory failure and persistent pulmonary hypertension of the newborn (PPHN).1,2 However, whether iNO therapy is useful for the management of preterm infants remains controversial.
BPD is a major sequela of prematurity, occurring in 10-15,000 cases per year in the USA and leading to significant morbidities such as prolonged ventilation and hospitalization, and recurrent respiratory exacerbations with rehospitalizations during infancy.3 Endotracheal intubation and mechanical ventilation are associated with lung injury and promote lung inflammation, which increases the risk and severity of BPD in preterm infants.4 Early initiation of nasal CPAP might decrease the need for intubation and mechanical ventilation, thereby decreasing the risk of ventilator induced lung injury (VILI) and BPD, although the evidence is controversial.5,6,7
Inhaled NO can improve gas exchange and reduce pulmonary hypertension (PH) in premature infants, but clinical trials of iNO in premature newborns with hypoxemic respiratory failure have yielded conflicting results to date.8 However, improvement in pulmonary morbidity and neuroprotection in subsets of this population have been reported.9,10 Whether iNO delivered non-invasively in infants who have not progressed to respiratory failure will alter the clinical course and decrease the incidence and severity of (BPD) has not been studied.
Therefore, we hypothesized that early and prolonged treatment with non-invasive iNO with nCPAP or nasal cannula would reduce the need for endotracheal intubation and mechanical ventilation and the risk for BPD. To test this hypothesis, we performed a multicenter RCT to determine whether early non-invasive iNO would reduce the combined endpoint of mortality or BPD in premature newborns (500-1250 grams) who required oxygen by nasal cannula or CPAP at the time of randomization in the first 72 hours after delivery.
Methods
Five clinical centers with tertiary care neonatal intensive care units (NICU) and a study coordinating center participated in the trial. The study was approved by individual Institutional Review Boards and the Food and Drug Administration under an Investigational New Drug Exemption and monitored by an independent data and safety monitoring board (DSMB) appointed by the National Heart Lung and Blood Institute. Criteria for enrollment included: gestational age at or less than 34 weeks, birthweight between 500 and 1250 g, postnatal age less than 72 hours, on supplemental oxygen by nasal cannula or CPAP. Exclusion criteria were lethal congenital anomalies or congenital heart disease (including an atrial septal defect larger than 1 cm and ventricular septal defect larger than 2 mm). Patients were enrolled after written informed consent was obtained from parents.
Randomization was stratified by center and birth weight into one of 3 groups (500 – 749 g; 750 – 999; 1000 - 1250 g) balanced in blocks of 2 or 4 within strata based upon a planned enrollment of 124 patients. Randomization numbers were linked to masked iNO or placebo study gas cylinders identified only by sequence numbers.
After randomization, the non-invasive oxygen delivery circuit was configured to allow delivery of iNO at 10 ppm or nitrogen placebo through a shielded iNOVent device (INO Therapeutics, Inc). This shielding allowed visualization of the set NO dose, but not the read-out of the NO/NO2 analyzers. Study gas was delivered until 30 weeks postmenstrual age (minimum of 2 weeks).
The primary outcome measure was the combined endpoint of death or BPD at 36 weeks postmenstrual age. BPD was defined according to the National lnstitutes of Health (NIH) criteria by the need for supplemental oxygen or the use of respiratory support for positive airway pressure at 36 weeks PMA.11 Tests were performed by respiratory therapists who were blinded to treatment group.
Secondary outcomes included assessment of the severity of BPD as defined by the oxygen reduction test,11 the subsequent need for and duration of endotracheal intubation and mechanical ventilation. Safety outcomes were severe intracranial hemorrhage as assessed by cranial ultrasound at 7 and 28 days, necrotizing enterocolitis, the need for treatment of a patent ductus arteriosus, and retinopathy of prematurity (ROP) requiring treatment.
Treatment Strategies
Inhaled NO was initiated non-invasively at 10 ppm to yield a minimum of 5 ppm to the posterior pharynx. 12 Study gas was delivered through CPAP devices or nasal cannula using configurations designed to consistently deliver the set, blinded dose with proximal dose monitoring and integrated alarms. Study gas was delivered for a minimum of 2 weeks and until 30 weeks PMA. The dose was decreased to 5 ppm if intubation was required. To assure consistent iNO delivery through nasal cannula, the minimum blended gas flow rate was set at 0.75 L/min. Nitric oxide delivery was discontinued if the flow rate was reduced below this level as weaning progressed.
Nasal cannula and CPAP strategies were individualized to allow for local practices. CPAP was typically initiated at 5-8 cm H2O and high flow nasal cannula was delivered using flow rates of 2-8 L/min. Indications for intubation and mechanical ventilation were at the discretion of each participating center. In general, centers participating in this trial used refractory apnea (requiring bag-mask ventilation) or progressive respiratory failure with PCO2 greater than 70 torr and pH less than 7.2 as criteria for intubation.
Sample Size and Analyses
The planned sample size for this trial was 124 infants, which was based upon the estimate that a two group chi-square test would have 91% power to detect the difference between the placebo group proportion of 0.60 for the incidence of BPD and mortality and the iNO group proportion of 0.35 when the sample size in each group was 62. Safety analyses were conducted by the DSMB and through routine monitoring of all serious adverse events and a formal interim analysis midway through the trial. Preplanned subgroup analyses were performed according to birth weight stratum prior to randomization.
Binomial data were analyzed using Chi-square/Fisher Exact test, where appropriate. Continuous data were compared using the Student t-test, or the Wilcoxon test for data that were not normally distributed. Analyses controlling for birth weight strata, site and other covariates were conducted using generalized estimating equations (PROC GENMOD in SAS). The analysis plan adjusted for study site and randomization strata using Cochran-Mantel-Haenszel testing Generalized Estimating Equations were used to provide parametric model adjustment for these design effects. The level of statistical significance was set at p less than 0.05.
Results
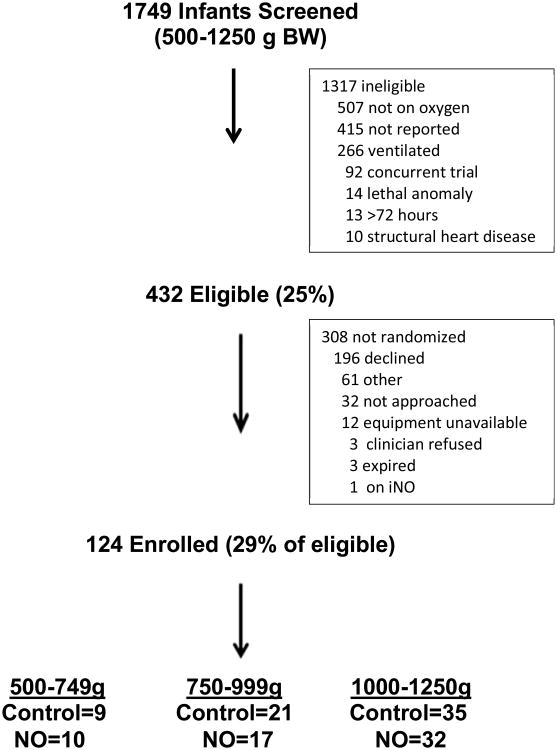
Between July, 2007 and February, 2012, 1749 newborns who met the birth weight criterion were screened at 5 clinical sites (Figure; available at www.jpeds.com). 432 infants met eligibility criteria and 124 newborns were randomized for this study. The most common reasons for ineligibility included the lack of an early need for supplemental oxygen (40%); and endotracheal intubation with mechanical ventilation (20%) during the first 72 hours after birth. For the 124 randomized patients, 59 (48%) were in the iNO group and 65 (52%) in the control group, including 19 newborns in the 500 to 749 g stratum, 38 newborns in the 750 to 999 g stratum, and 67 newborns in the 1000 to 1250 g stratum. There were no significant differences between groups in birth weight, gestational age, sex, ethnicity, the use of antenatal corticosteroids, chorioamnionitis, inborn status, Apgar scores, or respiratory status at enrollment (Table I).
Figure. Screening and Enrollments.
Table 1. Baseline Characteristics.
|
iNO N= 59 |
Control N= 65 |
P | |
|---|---|---|---|
|
| |||
| Birth weight – g | 961 ± 186 | 968 ± 159 | 0.82 |
| Birth weight strata: | |||
| 500-749 grams | 669.3 ± 68.8 | 698.3 ± 41.6 | 0.29 |
| 750-999 grams | 867.1 ± 71.5 | 878.6 ± 55.6 | 0.58 |
| 1000-1250 grams | 1102.1 ± 91.1 | 1091.1 ± 74.3 | 0.58 |
|
| |||
| Gestational Age | 27.5 ± 1.6 | 27.3 ± 1.8 | 0.59 |
|
| |||
| Male sex – no. (%) | 24 (40.7) | 31 (47.7) | 0.47 |
|
| |||
| Mother's race/ethnic group –no. (%) | |||
| White | 48 (81) | 48 (74) | 0.39 |
| Black | 8 (14) | 11 (17) | |
| Other | 3(5) | 6(9) | |
|
| |||
| Inborn – no. (%) | 49 (83) | 48 (88) | 0.61 |
|
| |||
| Antenatal corticosteroids – no. (%) | 47 (80) | 52 (80) | 0.96 |
|
| |||
| 1-minute Apgar score median (IQ range) | 2 (1-4) | 2 (1-3) | 0.16 |
|
| |||
| 5-minute Apgar score median (IQ range) | 7 (1-9) | 7 (2-9) | 0.10 |
|
| |||
| Maternal complications – no. (%) | |||
| Cesarean delivery | 31 (52) | 29 (45) | 0.47 |
| Chorioamnionitis | 10 (17) | 15 (23) | 0.50 |
| Preeclampsia | 8 (14) | 9 (14) | 0.99 |
| Multiple gestation | 21 (36) | 19 (29) | 0.56 |
| Diabetes | 3 (5) | 5 (8) | 0.72 |
|
| |||
| Age at randomization (hours) | 44.1±18.4 | 40.5 ±18.1 | 0.27 |
Mean ± SD, median-range or no. (%)
For the overall study population, the combined endpoint of death or BPD was not different between study groups (42% in the iNO group versus 40% in controls; p=0.86) (Table II). There were no differences in the individual variables of death or BPD between the study groups, and no differences in the main outcome measures for each birth weight stratum. The overall rates of BPD for the iNO and control groups were 41% and 39%, respectively. There were also no differences between the iNO and control groups for severity of BPD when categorized as none, mild, moderate or severe by respiratory support and oxygen reduction testing at 36 weeks PMA. (Table III; available at www.jpeds.com).
Table 2. Primary Outcomes: Death and BPD.
|
iNO No. (%) N= 59 |
Control No. (%) N= 65 |
P | RR (95% CI) | |
|---|---|---|---|---|
|
| ||||
| All patients: | ||||
| Death | 1 (1.7) | 2 (3.1) | 1 | 0.55 (0.1,5.5) |
| BPD | 24 (41) | 25 (39) | 0.86 | 1.02 (0.7,1.6) |
| Death/BPD | 25 (42) | 26 (40) | 0.86 | 1.06 (0.7,1.6) |
|
| ||||
| Stratum 1: 500-749 g | ||||
| Death | 0 | 2 (22) | 0.47 | - |
| BPD | 3 (30) | 4 (44) | 0.39 | 0.83 (0.2,3.5) |
| Death/BPD | 3 (30) | 5 (56) | 0.36 | 0.66 (0.2,2.6) |
|
| ||||
| Stratum 2: 750-999 g | ||||
| Death | 1 (6) | 0 | 0.13 | - |
| BPD | 8 (47) | 6 (29) | 0.29 | 1.47 (0.5,3.5) |
| Death/BPD | 9 (53) | 6 (29) | 0.16 | 1.65 (0.6,4.4) |
|
| ||||
| Stratum 3: 1000-1250 g | ||||
| Death | 0 | 0 | - | - |
| BPD | 13 (41) | 15 (44) | 0.77 | 0.95 (0.5,1.8) |
| Death/BPD | 13 (41) | 15 (44) | 0.77 | 0.95 (0.5,1.8) |
Table 3. BPD Severity by Treatment Group.
|
iNO N=59 |
Control N=62 |
||
|---|---|---|---|
| N% | N% | P | |
| None | 35 (59.3) | 37 (59.7) | 0.07 |
| Mild | 2 (3.4) | 9 (14.5) | |
| Moderate | 20 (33.9) | 12 (19.4) | |
| Severe | 2 (3.4) | 4 (6.5) |
During the study period, there were no significant differences between the groups in the rates of serious adverse events, including necrotizing enterocolitis, severe intracranial hemorrhage, need for medical or surgical treatment of patent ductus arteriosus, threshold ROP, or sepsis (Table IV). There were also no significant differences between groups in the need for mechanical ventilation after randomization (22% iNO vs. 23% control), number of days on mechanical ventilation or duration of hospitalization.
Table 4. Secondary Outcomes.
|
iNO N= 59 |
Control N= 65 |
P | RR (95% CI) | |
|---|---|---|---|---|
|
| ||||
| Mechanical Ventilation - no. (%) | 13 (22) | 15 (23) | 0.89 | 0.97 (0.62-1.52) |
| Total Ventilation Days. – M±SD | 9.7 (29) | 8.4 (12) | 0.27 | - |
|
| ||||
| Necrotizing Enterocolitis – no. (%) | 5 (9) | 10 (16) | 0.23 | 0.54 (0.20-1.49) |
|
| ||||
| Symptomatic PDA – no. (%) | ||||
| Medical treatment | 1 (2) | 2 (3) | 1 | 0.55 (0.1,5.5) |
| Surgical ligation | 3 (2) | 8 (12) | 0.21 | 0.41 (0.1,1.5) |
|
| ||||
| Threshold ROP* – no. (%) | 3 (5) | 4 (6) | 1 | 0.83 (0.2,3.5) |
|
| ||||
| Severe ICH** | 2 (3.4) | 4 (6.2) | 0.68 | 0.78 (0.13-4.47) |
|
| ||||
| Sepsis – no. (%) | 13 (22) | 14 (22) | 0.98 | 1.01 (0.52-1.96) |
|
| ||||
| Days in Hospital – M±SD | 75 (32) | 75 (29) | 0.72 | - |
Mean ± SD or no. (%)
Threshold ROP defined as requiring interventional therapy.
Intracranial hemorrhage grade 3-4
Discussion
In this multicenter, randomized, controlled trial of early iNO therapy in premature newborns receiving nasal cannula or nCPAP therapy for the prevention of BPD, we found that prolonged treatment with non-invasive iNO was safe but did not decrease the composite endpoint of death and BPD. We also report that early iNO therapy administered during non-invasive respiratory therapy did not reduce the subsequent need for endotracheal intubation and mechanical ventilation in newborns treated within 72 hours after birth.
Inhaled NO has proven to be a safe and effective therapy for PPHN in near-term and term newborns, and is an FDA approved standard of care for treating this high risk population due to its role in causing selective and sustained pulmonary vasodilation.1,2 The potential role of iNO therapy in premature newborns has been intensively studied over the last 15 years since the first report of improved oxygenation in a premature newborn with severe pulmonary hypertension13 and the first reported blinded, randomized trial.14 However, the emphasis changed from the treatment of pulmonary hypertension to reducing acute and chronic lung injury because of the putative benefits of iNO on inflammatory injury, surfactant function, and lung growth.15,16,17,18,19
Despite some promising findings, studies of iNO have not consistently shown decreased risks for BPD. The largest trials of iNO therapy in premature newborns reported to date include the single center study of Schreiber et al,20 and the multicenter trials of Van Meurs et al,21 Ballard et al,9 Kinsella et al10 and the Ikaria sponsored EUNO study.22 All of these studies were randomized, controlled and masked, and were focused on premature newborns with respiratory failure who required mechanical ventilation. These trials had important differences in patient population, disease severity, dose and duration of iNO therapy. Ballard et al randomized infants between 7 and 21 days of age and found that the incidence of survival without BPD was increased in the iNO treatment group compared with controls.9 Kinsella et al randomized mechanically ventilated infants in the first 48 hours after birth and reported a neuroprotective effect, and a reduction in BPD for infants with birthweight > 1000 grams.10 However, using a similar study design, the EUNO study group did not confirm these findings.22
When the current trial was designed, we hypothesized that the potential beneficial effects of iNO would be more pronounced in premature newborns who were stable without the need for mechanical ventilation in the first 72 hours after birth, potentially reducing the need for mechanical ventilator support and thus reducing the incidence of BPD. In this trial, the rate of intubation and mechanical ventilation was not decreased with iNO therapy, and although there were no differences in adverse events between the iNO and control groups, there was also no demonstrable short term pulmonary benefit from iNO treatment.
One limitation of this trial is that we used only one dose for non-invasive NO administration. This was based upon our early experience with iNO in ventilated newborns demonstrating safety,10 and the feasibility of conducting a trial with multiple treatment arms in this population. It is also possible that the delivered dose of iNO was variable due to dilution of the delivered gas when infants breathed through the mouth. We enrolled infants in the first week after birth, rather than selecting infants with evolving BPD as in the Ballard trial.9 However, another trial sponsored by Ikaria Inc. and designed to test the efficacy of iNO in a population similar to that of Ballard et al has now been completed and preliminary results were recently presented.23 Yoder et al randomized 451 mechanically ventilated premature newborns with birth weight less than 1250 grams at 7-21 days after birth to treatment with iNO or placebo gas. They found no differences in survival without BPD, severity of BPD, or adverse events between the two groups.
In conclusion, prolonged treatment with non-invasive iNO was safe but did not decrease the composite endpoint of death/BPD in newborns with birth weights of 500-1250 g treated within 72 hours after birth. Long-term follow-up studies of these infants are ongoing to determine later pulmonary and neuro-cognitive outcomes of early iNO therapy.
Acknowledgments
Administrative and regulatory support were provided by Carol Blaisdell, MD, Gail Weinmann, MD, and Tim Moore MD, (NHLBI), and the NHLBI LD SCCOR Data Safety Monitoring Board.
Funded by National Heart, Lung, and Blood Institute (HL084923) and the National Institutes of Health/National Center for Advancing Translational Sciences (Colorado CTSI Grant Number UL1 TR000154). INO Therapeutics Inc provided study gas and INOVent devices for this trial. The company remained masked to the results and was not involved in study design, data analysis or interpretation, or preparation of the manuscript.
Footnotes
The authors declare no conflicts of interest.
Registered with ClinicalTrials.gov: xx
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 2.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342:469–74. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 3.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006 Apr 29;:367, 1421–31. doi: 10.1016/S0140-6736(06)68615-7. [DOI] [PubMed] [Google Scholar]
- 4.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 5.Finer NN, Carlo WA, Duara S, Fanaroff AA, Donovan EF, Wright LL, et al. Delivery room continuous positive airway pressure/positive end-expiratory pressure in extremely low birth weight infants: a feasibility trial. Pediatrics. 2004;114:651–657. doi: 10.1542/peds.2004-0394. [DOI] [PubMed] [Google Scholar]
- 6.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB COIN Trial Investigators. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2006 Feb 14;:358, 700–8. doi: 10.1056/NEJMoa072788. [DOI] [PubMed] [Google Scholar]
- 7.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2006 May 27;:362, 1970–9. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Askie LM, Ballard RA, Cutter GR, Dani C, Elbourne D, Field D, et al. Meta-analysis of Preterm Patients on Inhaled Nitric Oxide Collaboration. Inhaled nitric oxide in preterm infants: an individual-patient data meta-analysis of randomized trials. Pediatrics. 2011 Oct;:128, 729–39. doi: 10.1542/peds.2010-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, et al. Inhaled nitric oxide in preterm infantsundergoing mechanical ventilation. N Engl J Med. 2006;205:343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 10.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;205:354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Pediatrics. 2006. Dec, National Institutes of Child Health and Human Development Neonatal Research Network Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia; pp. 116pp. 1353–60. [DOI] [PubMed] [Google Scholar]
- 12.Kinsella JP, Parker TA, Ivy DD, Abman SH. Noninvasive delivery of inhaled nitric oxide therapy for late pulmonary hypertension in newborn infants with congenital diaphragmatic hernia. J Pediatr. 2006 Apr;:142, 397–401. doi: 10.1067/mpd.2003.140. [DOI] [PubMed] [Google Scholar]
- 13.Abman SH, Kinsella JP, Schaffer MS, Wilkening RB. Inhaled nitric oxide in the management of a premature newborn with severe respiratory distress and pulmonary hypertension. Pediatrics. 1993;92:606–609. [PubMed] [Google Scholar]
- 14.Kinsella JP, Walsh WF, Bose CL, Gerstmann DR, Labella JJ, Sardesai S, et al. Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomised controlled trial. Lancet. 1999;354:1061–5. doi: 10.1016/s0140-6736(99)03558-8. [DOI] [PubMed] [Google Scholar]
- 15.Kinsella JP, Ivy DD, Abman SH. Inhaled nitric oxide lowers pulmonary vascular resistance and improves gas exchange in severe experimental hyaline membrane disease. Pediatr Res. 1994;36:402–408. doi: 10.1203/00006450-199409000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Issa A, Lappalainen U, Kleinman M, Bry K, Hallman M. Inhaled nitric oxide decreases hyperoxia-induced surfactant abnormality in preterm rabbits. Pediatr Res. 1999;45:247–54. doi: 10.1203/00006450-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Tang JR, Markham NE, Balasubramaniam V, McMurtry IF, Maxey A, Kinsella JP, et al. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol. 2004;287:L344–51. doi: 10.1152/ajplung.00291.2003. [DOI] [PubMed] [Google Scholar]
- 18.Mccurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol – Lung. 2005;288:450–459. doi: 10.1152/ajplung.00347.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinsella JP, Parker TA, Galan H, Sheridan BC, Halbower AC, Abman SH. Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease, Pediatr Res. 1997;41:457–63. doi: 10.1203/00006450-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349:2099–107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 21.Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med. 2005;353:13–22. doi: 10.1056/NEJMoa043927. [DOI] [PubMed] [Google Scholar]
- 22.Mercier JC, Hummler H, Durrmeyer X, Sanchez-Luna M, Carnielli V, Field D, et al. EUNO Study Group. Lancet. 2006 Jul 31;376(9738):346–54. doi: 10.1016/S0140-6736(10)60664-2. [DOI] [PubMed] [Google Scholar]
- 23.Yoder BA. Inhaled NO for prevention of BPD: Update on the NEWNO Trial. Presented at Hot Topics in Neonatology; Washington, D.C.. December 8, 2013. [Google Scholar]