INTRODUCTION
The endocrine system plays a crucial role in maintaining human homeostasis and is often affected by exogenous stimuli. A range of synthetic as well as naturally occurring agents have been identified as interacting with the endocrine system. If the interaction of these exogenous substances with the endocrine system leads to adverse health effects in an intact organism or its progeny or (sub) populations, these substances are referred to as “endocrine disrupting chemicals” (EDCs) [1].
The group of molecules identified as EDCs is highly heterogeneous and includes synthetic drugs, pesticides, compounds used in industry and in consumer products, industrial by-products and pollutants, including some metals. Natural chemicals found in foods for humans as well as animals (e.g. phytoestrogens, including genistein and coumestrol or the mycotoxin zearalenone) can also act as EDCs. The disrupting activity can occur by altering normal hormone levels, inhibiting or stimulating the production and metabolism of hormones, or changing the way hormones circulate through the body, thus affecting the functions that these hormones control [2]. There is growing evidence that EDCs can function at very low doses in a tissue-specific manner [3]. EDCs may also exert non-monotonic dose-responses due to the complicated dynamics of hormone receptor occupancy and saturation [4].
The correlation between the accumulation of persistent chemicals and an increase in reproductive disorders, such as infertility of males and females, i.e. endocrine disorders in humans and wild animals, has raised concern that there may be a causal link between hormone-dependent cancers and exposure to endocrine disruptors [5]. In both humans and rodent models, EDCs have been shown to disrupt normal mammary development and lead to adverse lifelong consequences, especially when exposures occur during early life [6]. EDCs can act directly or indirectly on mammary tissue to increase sensitivity to chemical carcinogens or enhance development of hyperplasia, beaded ducts, or tumors [6]. Animal studies demonstrate that early life exposure to hormonally active agents can lead to effects on mammary gland (MG) development, impaired lactation, and increased susceptibility to breast cancer [7]. However, the influence of environmental exposures on breast development outcomes is poorly understood, as is the relationship between breast development, lactation deficits, and breast cancer. The female mammary glands undergo most of their development post-natally , achieving a fully differentiated state late in pregnancy. During this time, the gland prepares itself for functional lactation. Interruption of this process can lead to mortality or malnutrition of the offspring. Impaired lactation may be associated with altered MG development (decreased or unresponsive breast tissue) and/or endocrine disruption (improper hormonal support for lactation) [6].
In the present study we have chosen three EDCs widely used in personal care products i.e. Diethylphthalate (DEP), Methylparaben (MPB), and Triclosan (TCS). Considering that humans are likely to be exposed to these chemicals simultaneously either due to the chemicals being used as ingredients in the same personal care product or to people's use of many products with these chemicals as ingredients, we examined the biological effects of these EDCs as a mixture. It is unclear whether the effects of environmental toxicant mixtures on mammary tumor development occur at concentrations below the no-effect level for the individual components of the mixture. Moreover, results from in vitro studies suggest that environmental toxicant mixtures can interact in an additive, synergistic, or inhibitory manner to modify the risk of breast cancer by altering cancer-cell proliferation and estrogen signaling [8-11]. Animal studies have also indicated that mixtures of chemicals produce synergistic effects [12, 13]. Thus hundreds of chemicals each at levels below toxicity could interact together to cause health problems.
Pregnancy is considered as a protective factor for breast cancer in women [14-16]. Like humans, rats exhibit parity induced protection against breast cancer. It has been demonstrated that early and complete pregnancy is associated with reduced risk of breast cancer in humans, and manystudies have compared gene expression profiles in mammary glands from nulliparous and parous rats. Shan et al. [17, 18] demonstrated that normal mammary glands from virgin, pregnant and lactating Sprague-Dawley (SD) rats exhibit marked differences in gene expression.
The aim of this study was to determine whether low doses of DEP, MPB, TCS, and a mixture of the three EDCs administered at a dose level comparable to human exposure, starting from Post Natal Day (PND) 1 until PND 180, affect the developmental pattern and the proliferative activity of the mammary gland in adult parous and nulliparous female rats. However, we observed macroscopic hypotrophy of the milk lines after parturition as well as a higher mortality of pups generated by treated animals; in particular, the latter effect was more evident in TCS treated animals compared to the control group. For this reason, to gain molecular insights, we also profiled the transcriptome with a focus on lactation-related genes in mammary glands during lactation, investigating the periods around weaning and following weaning when the mammary gland involutes. The purpose of this further investigation was to examine the temporal coordination of changes in gene expression that take place during secretory activation, and to generate hypotheses about the molecular control mechanism involved.
Materials and methods
1. Test compounds
Diethylphtalate (DEP, CAS # 84-66-2, lot # STBB0862V, 99% purity), Methylparaben (MPB, CAS # 99-76-3, lot # BCBG0852V, 99% purity) and Triclosan (TCS, CAS # 3380-34-5, lot # 1412854V, 97% purity) were supplied by Sigma Aldrich (Milan, Italy). Olive Oil (Montalbano Agricola Alimentare Toscana, Florence, lot # 111275, Italy) was used as the vehicle in preparing all dosing solutions. The olive oil was analyzed to exclude the presence of the three studied compounds as contaminants. During the experiment, each compound was stored at room temperature (20 °C) and in the dark. The solutions were prepared on the first day of treatment for the whole duration of the experiment and were continuously stirred throughout the study; the stability of the solutions was confirmed by gas chromatography-mass spectrometry (GC-MS) (Neotron Laboratory, Modena, Italy). In order to minimize any plastic contamination, the compounds were administered using a 5 ml glass syringe; biological samples were collected in polypropylene vials.
2. Experimental Animals
The experiment used female Sprague-Dawley (SD) rats which belong to the colony used in the laboratory of the Cesare Maltoni Cancer Research Center of the Ramazzini Institute (CMCRC/RI) for over 40 years. The breeder animals were distributed into ten groups and randomized so as to have no sisters in the same group. Animals were housed in makrolon cages (41×25×15 cm) 2 or 3 per cage, with a stainless steel wire top and a shallow layer of white wood shavings as bedding. Cages were identified by a card indicating the experiment, the group and the experimental number/pedigree number of each animal. All the animals used in the experiment were kept in a single room at 23 ± 3°C and at 40-60% relative humidity. The light/dark cycle was 12 hours. Before starting treatment, animals were weighed and the dose (mg/kg b.w.) of the various EDCs was calculated on the basis of the average weight. Rat feed (Dr. Piccioni Laboratory, Milan, Italy) and tap water were available ad libitum. Each lot of feed and tap water was periodically analyzed for biological (bacteria) and chemical contaminants (mycotoxins, pesticides, arsenic, lead, mercury, selenium). The day on which parturition occurs was indicated as lactation day 0 (LD 0) for the dam and post natal day 0 (PND 0) for the offspring. The experimental animals (F1) received the treatment from PND 1 by lactation; the dams (F0) were exposed to EDCs from LD 0. After weaning (LD 28), the animals (F1) were separated from their mothers (F0), divided into two groups, “nulliparous” and “parous”, and exposed through gavage until the final sacrifice at PND 181.
“Nulliparous” female rats (F1): ten female rats per group (treated and controls) were exposed from PND 1 until PND 181. At PND 146, three nulliparous rats from each group, age-matched with parous at LD 28, were sacrificed and selected for macroscopic (Whole Mount preparation –WM) and microscopic examination of mammary glands. At PND 181, 7 animals from each group were sacrificed and their mammary glands histopathologically examined.
“Parous” female rats (F1): Ten female rats per group (treated and controls) were exposed from PND 1 until PND 181. At PND 97 these animals were mated (outbred) and the exposure to the test substances continued for the pregnant F1 dams through delivery of pups (F2) and lactation. At the end of lactation (LD 28), 3 dams from each group were sacrificed and selected for macroscopic (WM) and microscopic examination of mammary glands (animals referred to as “dams at LD 28”). At PND 181, the remaining 7 dams in each group were sacrificed and their mammary glands histopathologically examined.
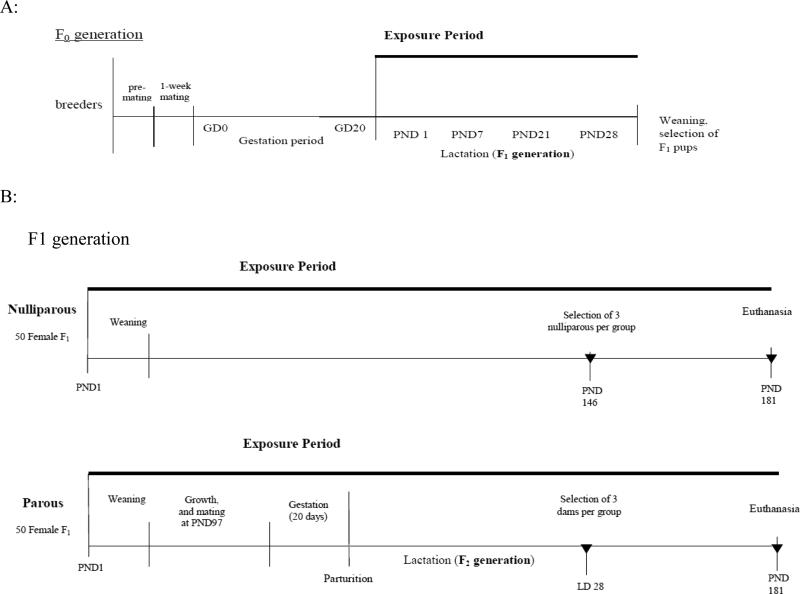
The generation of the experimental animals is shown in Figure 1; the plan of the experiment is outlined in Table 1. During the experiment mean daily water and feed consumption were measured per cage; body weights were individually measured once a week for the first 13 weeks and every two weeks thereafter. Animal procedures were performed in accordance with the rules of Italian law for Animal Welfare [19], following the principles of Good Laboratory Practices and the Standard Operating Procedures of the CMCRC/RI facility, which include authorization by an Ethical Committee.
FIGURE 1.
Outline of generation and treatment of experimental animals. A: F0 generation corresponds to breeders of the studied animals (F1), treated from delivery until weaning of the pups. B: The F1 generation was divided into two groups: nulliparous females treated from PND 1 until PND 181and parous females treated from PND 1 until PND 181, mated at PND 97 and observed during their pregnancy and lactation of their offspring. the F2 generation. For each group, at LD 28 (PND 146), corresponding to the end of lactation for parous rats, three animals per experimental group were sacrificed and mammary glands analyzed. At PND 181 all females were sacrificed and mammary gland examined. Solid triangles (▼) represent conclusion of exposure and sacrifice of animals.
Table 1.
Experimental plan of EDC treatment for “nulliparous” and “parous” female Sprague-Dawley rats. Animals are treated from PND 1 to PND 181.
| Group | Compounda | Animals |
Treatmentb |
|
|---|---|---|---|---|
| Status | No. | Dose (mg/Kg b.w.) | ||
| I | DEP | Nulliparous/virgin | 10 | 0.1735 |
| II | Parous | 10 | ||
| III | MPB | Nulliparous/virgin | 10 | 0.1050 |
| IV | Parous | 10 | ||
| V | TCS | Nulliparous/virgin | 10 | 0.050 |
| VI | Parous | 10 | ||
| VII | MIX | Nulliparous/virgin | 10 | Mixture of DEP+MPB+TCS |
| VIII | Parous | 10 | ||
| IX | OILc | Nulliparous/virgin | 10 | - |
| X | Parous | 35d | ||
| Total | 125 | |||
DEP (Diethylphtalate, CAS 84-66-2), MPB (Methylparaben, CAS 99-76-3), TCS (Triclosan, CAS 3380-34-5), MIX (mixture of DEP+MPB+TCS in equal quantity), OIL (olive oil control, vehicle alone)
Animals were treated from PND1 to PND181. Three dams for each parous group are sacrificed at the end of lactation at LD 28 and 3 nulliparous age-matched ones are sacrificed at the same time (PND 146). Each compound was administered in olive oil as vehicle by gastric intubation (gavage), starting with 0.5 ml of olive oil from 4 to 9 weeks of age, and then with 1 ml once adult.
Control animals from different control groups
The animals serve as concurrent control groups, in common with other studies
3. Dosing
The doses used in the present study are in the range of those to which women may be exposed; the doses were obtained by comparing the urine concentration of compound/metabolite in rats and women [20]. The doses selected were: NOAEL/10,000 for DEP and MPB; NOAEL/1,000 for TCS (NOAEL in mg/Kg/day; DEP =1,735; MPB=1,050; TCS=50). The mixture solutions were prepared using the same quantities of the three EDCs mentioned above. The control group received gavage with olive oil alone (vehicle).
4. Necropsy
All the experimental animals were given a macroscopic post-mortem examination following euthanasia via carbon dioxide inhalation.
Mammary glands
axillary mammary glands (MGs) were dissected, placed on cardboard and fixed in alcohol 70% for 48 h for histopathological examination [21]. Perineal MGs were snap frozen and stored at −80 C until analyzed. Three age-matched animals per treatment group were sacrificed at PND 146 as well as at LD 28 in the case of parous rats. All the alcohol-fixed MGs were processed, embedded in paraffin, sectioned at 4 microns, and mounted on glass slides. The sections were stained with hematoxylin and eosin (HE) for histopathological examination.
MG whole-mount preparations (WMs) were performed as follows: inguinal MGs were excised at necropsy and left and right chains were fixed in 10% neutral buffered formalin and kept in plastic bags, then sent to the University of Chieti for whole-mount examination. WMs were stained as previously reported [21-23]. Briefly, the fixed mammary glands (left and right) were immersed in acetone overnight, rehydrated, stained in ferric hematoxylin (Sigma Aldrich), dehydrated in increasing concentrations of alcohol, cleared with Bioclear (Bio-Optica) and stored in methyl salicylate (Sigma Aldrich). Digital photos were acquired with a Nikon Coolpix 995 (Nital SpA, Turin, Italy) mounted on a stereoscopic microscope (MZ6, Leica Microsystems, Milan, Italy).
Hematoxylin-eosin sections of the axillary (right and left) mammary glands and WMs of the inguinal (right and left) mammary glands of 3 rats per group were examined by two pathologists. The lobular development was classified by different grades: from complete, to medium and poor; a number was attributed to each grade (3 = complete, 2 = medium, 1= poor) and a score was given to each treatment group based on the sum of the values assigned to each gland.
5. Statistical analyses
A General Linear Model (GLM) was used to compare the difference of outcome among the different chemical groups, adjusted by litter size. All statistical tests were two-sided, and p < 0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.3 for Windows (Cary, NC, USA).
6. Gene expression profiling in mammary tissues
Right caudal mammary glands of TCS-treated (n = 3) and OIL-treated animals (n = 3) at PND 146 (LD 28) and PND 181 were collected in cryovials on ice and stored at −80°C. Total RNA was extracted using the Maxwell 16 LEV simplyRNA Blood Kit according to the manufacturer's protocol (Promega, WI), and quantified using Nanodrop (Thermo Scientific, MA). The RNA quality was assessed using a 2100 Bioanalyzer (Agilent Technologies, CA). Transcriptome profiling was carried out using GeneChip Rat Gene 2.0 ST Arrays (Affymetrix, CA) at the Yale Center for Genome Analysis (Yale School of Medicine, CT) according to the manufacturer's protocol. All arrays passed QC. After removing control probes, we filtered each dataset to reduce the number of multiple hypothesis tests prior to differential gene expression (DGE) analysis. Probe sets were first filtered by intensity (the signal intensity of a probe set should be at least log2(100) in at least 50 percent of samples) [24] followed by variance (retain top 50 percent of the probe sets with the highest interquartile range) [25] using the genefilter package [26] in R Studio (R version 3.0.2). We also carried out focused analyses on lactation/milk-production genes, which were selected according to the following criteria: the gene should have a log2 intensity value of 4.5 in at least half the samples assessed and the gene should be related to the Gene Ontology Biological Process (GOBP). The term‘lactation’ on the array should contain the symbol ‘Csn’ (Casein kinase), given that caseins are among the most abundant proteins in milk [27]. In addition, the genes lactalbumin (LALBA) and whey acidic protein (WAP) were chosen since they are commonly present in milk and have previously been found to be modified by exposure to organic chemicals [28]. Epidermal growth factor receptor (EGFR) was also assessed, since it was identified as being the top hub in a bioinformatics-driven approach to identifying lactation gene networks [29]. In sum, the expression profiles of 59 genes were investigated. Overall DGE between TCS and OIL at PND 146 (LD 28) and at PND 181 was carried out using 1-way ANOVA, where genes were considered to be significantly different at a false discovery rate (FDR) of < 25% using the Benjamini Hochberg correction [30]. DGE was visualized by hierarchical clustering using Partek Genomics Suite 6.6 (Partek Inc., MO) and analyzed by Ingenuity Pathway Analysis (IPA) for enrichment of canonical pathways (Ingenuity Systems, www.ingenuity.com), where P < 0.05 was considered statistically significant (Fisher's exact test). To assess the differences in expression of lactation genes between TCS and OIL at PND146 (LD 28) and at PND 181, two-sided t-tests were performed using the gene filter package [27] in R Studio (R version 3.0.2). P < 0.05 was considered statistically significant.
Results
The fertility rate was 100% for MPB, TCS and MIX (10/10 females were pregnant), while for DEP the fertility rate was 90% (9/10). There were 31 pregnant rats out of 35 in the OIL control group (88.6%). No significant differences in pregnancy rates were observed among F1 dams from treated and control groups.
1. Litter Size and Mortality
The details of litter size, mortality and birth weight are shown in Table 2. For the control group, the average litter size at delivery was 12.8. Treatment with DEP and MPB resulted in a significantly higher number of pups per litter at delivery with a litter size of 15.6 and 15.2, respectively, while TCS and MIX groups did not show any significant difference. We also observed increased mortality in all treated groups at PND 7 and onwards. Compared to the average mortality of ~3% in the oil control group, EDC treatment resulted in mortality rates >20% as early as PND 7. However, there is little indication of changes in the weight of the pups across treatment groups except the lower birth weight in DEP-treated rats at PND 7 and PND 14.
Table 2.
Parous female rats : distribution and comparison of number of pups per litter among 5 chemicals by age of pupsa
| Age of pups in days | Group | Litter (dams) |
N. pups/litter |
N. dead pups/litter |
Mortailty |
Average weight/pup |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | with dead pups (%) | Mean | Range | Estimateb | Mean | Range | Estimateb | Mean | Range | Estimateb | Mean | Range | Estimateb | ||
| 1 (delivery) | OIL | 31 | - | 12.8 | 6-18 | Ref. | - | - | - | - | - | - | - | - | - |
| DEP | 9 | - | 15.6 | 12-17 | 2.72* | - | - | - | - | - | - | - | - | - | |
| MPB | 10 | - | 15.2 | 13-18 | 2.36* | - | - | - | - | - | - | - | - | - | |
| TCS | 10 | - | 14.0 | 11-18 | 1.16 | - | - | - | - | - | - | - | - | - | |
| MIX | 10 | - | 12.5 | 7-18 | −0.34 | - | - | - | - | - | - | - | - | - | |
| 7 | OIL | 31 | 7(22.6) | 12.4 | 6-17 | Ref. | 0.4 | 0-4 | Ref. | 3.1 | 0-28.6 | Ref. | 12.6 | 9.8-15.8 | Ref. |
| DEP | 9 | 8(88.8) | 11.7 | 6-17 | −3.24** | 3.9 | 0-10 | 3.25** | 24.6 | 0-62.5 | 22.21** | 11.6 | 9.9-14.3 | −0.2 | |
| MPB | 10 | 8(80.0) | 11.9 | 3-16 | −2.68** | 3.3 | 0-11 | 2.68** | 21.7 | 0-78.6 | 19.28** | 11.0 | 5.0-12.8 | −0.94 | |
| TCS | 10 | 7(70.0) | 10.9 | 5-14 | −2.58** | 3.1 | 0-8 | 2.58** | 20.8 | 0-61.5 | 18.03* | 11.9 | 10.4-13.6 | −0.44 | |
| MIX | 10 | 8(80.0) | 10.1 | 0-17 | −2.01* | 2.4 | 0-7 | 2.01* | 22.8 | 0-100 | 19.63** | 11.9 | 9.7-13.6 | −0.68 | |
| 14 | OIL | 31 | 7(22.6) | 12.4 | 6-17 | Ref. | 0.5 | 0-4 | Ref. | 3.3 | 0-28.6 | Ref. | 22.6 | 16.9-29.0 | Ref. |
| DEP | 9 | 8(88.8) | 11.6 | 6-17 | −3.31** | 4.0 | 0-10 | 3.31** | 25.3 | 0-62.5 | 22.66** | 22.5 | 18.2-26.6 | 1.63** | |
| MPB | 10 | 8(80.0) | 11.6 | 0-16 | −2.94** | 3.6 | 0-14 | 2.94** | 23.9 | 0-100 | 21.18** | 22.1 | 18.4-24.2 | 1.13 | |
| TCS | 10 | 7(70.0) | 10.8 | 5-14 | −2.65** | 3.2 | 0-8 | 2.65** | 21.4 | 0-61.5 | 18.39* | 22.5 | 20.6-26.6 | 0.7 | |
| MIX | 10 | 8(80.0) | 10.1 | 0-17 | −1.98* | 2.4 | 0-7 | 1.98* | 22.8 | 0-100 | 19.40** | 22.4 | 18.1-25.3 | 0.06 | |
Statistical evaluation was performed excluding any relationship between the mortality rate and the number of pups born
GLM: general linear model, adjusted by number of pups per litter at delivery
P < 0.05
P < 0.01
2. Histopathology of mammary glands
All animals from each treated group (10) and the first ten from controls were microscopically examined; in all groups frozen samples were collected from 5 of them. WM preparation was performed from 3 of them sacrificed at PND 146 (LD 28).
Nulliparous rats
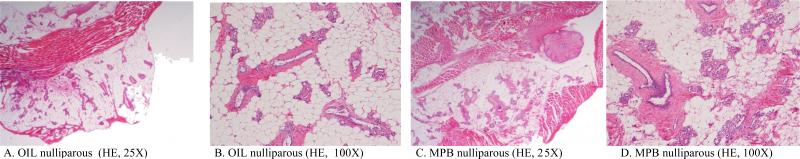
The development of MGs had a normal tissue morphology in both treated and control groups. In these virgin females, the duct system was the most prominent component and the lobules of alveoli were sparse and small. The ducts consisted of an inner layer of cuboidal/columnar cells which were surrounded by a layer of elongated myoepithelial cells with scant cytoplasm (Fig. 2).
FIGURE 2.
Representative morphological and histological features of hematoxylin and eosin-stained paraffin sections of nulliparous female Sprague Dawleys rats, sacrificed at PND 181. Both the vehicle control (A,B) and treated (in this case with MPB) groups (C, D) showed normal ducts, epithelia, and stroma
Parous rats
a) Dams at the end of lactation (LD 28)
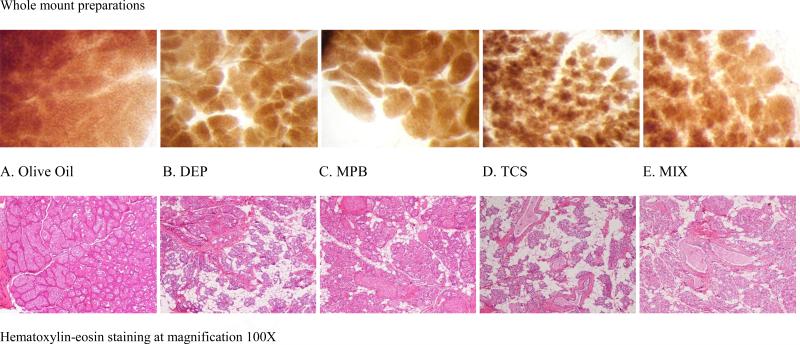
during lactation, under normal conditions, the breast was composed almost exclusively of alveoli strongly dilated with milk; interlobular connective tissue was reduced to thin septa between lobules. The alveoli were filled with secretions containing lipid vacuoles, epithelial cells were flattened and acini were filled with secretions. At this stage of development, the MGs of control animals appeared morphologically normal (Fig. 3A).
FIGURE 3.
Mammary gland from “Dams at the end of lactation-LD 28” Sprague-Dawley rat, 21-weeks old, sacrificed at LD 28 (after the weaning of their pups). Olive oil control (A,100X), DEP (B, 100X), MPB (C, 100X), TCS (D, 100X), MIX (E, 100X).
Generally, treated animals showed evident histological differences from controls: the alveoli were not always milk-filled and an increase in adipose tissue was noted (Fig. 3B-E). The collapsed alveolar and duct structures showed residual secretory content.
Whole-mount preparations (WM) confirmed the same morphological pattern (Fig. 3A-E) showing differences in the lobular development among control and treated groups. Figure 3A illustrates the WM appearance of a normal lactating mammary gland with complete development of lobular structures, totally filling the mammary fat pad (Olive oil, controls).
Figure 3B-E show that all the treatments induced a marked decrease in the size of the lobular structures which also appear darker and denser, due to the lower dilatation of the secretory alveoli. The effect of TCS is particularly evident with much smaller lobuli composed of a lower number of alveoli, often empty. A semi quantitative evaluation of the changes in lobular development observed during examination of HE slides and WM preparations is reported in Table 3.
Table 3.
Lactating mammary gland development in dams at LD 28.
| N. of casea | Treatmentb |
|||||
|---|---|---|---|---|---|---|
| OIL | DEP | MPB | TRC | MIX | ||
| Rat 1 | left | 2.5 | 3 | 3 | 2 | 3 |
| right | 3 | 3 | 3 | 2 | 2.5 | |
| Rat 2 | left | 3 | 2 | 2.5 | 1 | 1.5 |
| right | 3 | 2 | 2 | 1 | 1 | |
| Rat 3 | left | 2.5 | 2 | 1.5 | 1.5 | 1.5 |
| right | 3 | 2 | 1 | 1.5 | 1.5 | |
| Total score | 17 | 14 | 13 | 9 | 11 | |
Mammary glands of three cases per each type of treatment were examined for morphological lobular development based on HE stained sections (axillary) and WM preparations (inguinal)
Lobular development was classified in different grades: from 3 = complete to 1 = poor development. The total score for each group of treatment was based on the sum of values assigned to each gland.
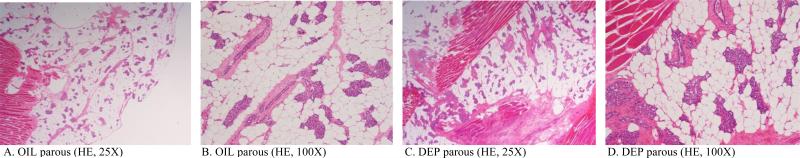
b) Dams at final sacrifice (PND 181)
no histopathological differences were observed among treated and control groups. A three-fold reduction in volume of MGs, compared to those during lactation, was observed. An increased number of terminal alveolar buds and lobules, scattered inside the stromal fat pad, was observed as compared to the morphological features of nulliparous rats (Fig.4).
FIGURE 4.
Representative morphological and histological features of hematoxylin and eosin-stained paraffin sections of parous female Sprague Dawleys rats, sacrificed at PND 181. Both the vehicle control (A, B) and treated (in this case with DEP) groups (C,D) showed residual secretory content in the collapsed alveolar and ducts structures
3. Gene expression of mammary glands
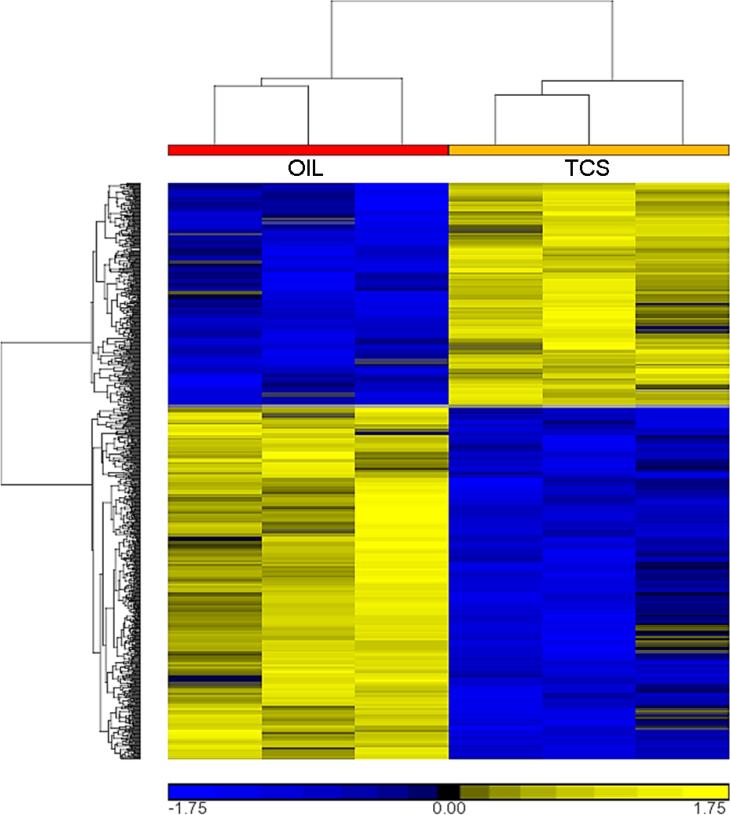
The results obtained through histologic and WM evaluations showed that TCS had a marked effect on the development of alveoli in the lactating mammary glands compared to the other EDC-treated animals and controls. We therefore started our study by profiling the mammary transcriptome in order to identify genes that were differentially expressed in TCS vs. OIL treated rats at PND 146 (LD 28) and PND 181. DGE analysis revealed that 909 probe sets were differentially expressed at PND 146 (LD 28), but none at PND 181. Of the 909 probe sets, 557 were down- and 352 were up-regulated in TCS-treated animals compared to OIL controls. Of the 557 down-regulated probe sets, 40 were changed more than two-fold and of the 352 up-regulated probe sets, 21 were changed more than two-fold. Hierarchical clustering of the 909 differentially expressed probe sets revealed overall reproducibility among the three animals in each group (Fig. 5).
FIGURE 5.
Hierarchical clustering of significantly differentially expressed probe sets between TCS and OIL at PND 146 (LD 28) (FDR < 0.25, 1-way ANOVA). Yellow corresponds to up-regulated gene expression, blue to down-regulated gene expression.
We used Ingenuity Pathway Analysis (IPA) to determine the top five canonical pathways represented by the 557 down- and 352 up-regulated probe sets (Table 4). Our results indicated that cholesterol biosynthetic pathways were strongly down-regulated in TCS-treated animals compared to controls (P < 0.001, Fisher's Exact Test). Squalene epoxidase (SQLE) and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), which were among the genes that were down-regulated by more than two-fold, contributed to the enrichment of cholesterol biosynthetic pathways. While cAMP and nitric oxide signaling were among the top up-regulated IPA canonical pathways (P < 0.02, Fisher's Exact Test), these top five pathways shared little overlap compared to the top five down-regulated pathways.
Table 4.
Top 5 canonical pathways from ingenuity pathway analysis (IPA) up- and down-regulated in the TCS group compared to OIL at PND 146 (LD 28) (P < 0.05, Fisher's exact test).
| Up-regulated in TCS | Down-regulated in TCS |
|---|---|
| cAMP-mediated signaling | Superpathway of Cholesterol Biosynthesis |
| Nitric Oxide Signaling in the Cardiovascular System | Cholesterol Biosynthesis I |
| Granulocyte Adhesion and Diapedesis | Cholesterol Biosynthesis II (via 24,25-dihydrolanosterol) |
| eNOS Signaling | Cholesterol Biosynthesis III (via Desmosterol) |
| Sorbitol Degradation I | Zymosterol Biosynthesis |
Based on the morphological findings of mammary tissues from dames at weaning, we also performed targeted analyses on expression profiles of lactation/milk-related genes (Table 5). Consistently with our overall DGE analysis of the transcriptome from PND 146 (LD 28) and PND181, we observed changes in expression of lactation/milk-related genes only at PND 146 (LD 28) (Table 6). Specifically, seven genes were down- and three were up-regulated in TCS-treated animals as compared to controls. Of these ten genes, SLC30A4 was depleted by more than two-fold and eight were among the 909 differentially expressed probe sets identified by overall DGE analysis at PND 146 (LD 28) (Fig. 5).
Table 5.
List of 59 genes related to lactation and/or milk from the microarray datasets at PND 146 (LD 28) and PND 181.
| GO Biological Process term ‘lactation’ |
| Agpat6, Aprt, Atp2b2, Atp7a, Atp7b, Bcat2, Cad, Cav1, Ccnd1, Cdo1, Chuk, Creb1, Csn1s2b, Csn3, Ddr1, Dhodh, Eif2ak3, Erbb4, Foxb1, Hif1a, Hk2, Med1, Met, Muc4, Ncoa1, Ncor1, Ncor2, Nme1, Oxt, Pam, Ppat, Prlr, Slc29a1, Slc29a2, Slc30a4, Socs2, Stat5a, Stat5b, Umps, Uprt, Usf2, Vdr, Vegfa, Xdh |
| Other Casein Kinase Genes |
| Csn1s1, Csn1s2a, Csnk1a1, Csnk1d, Csnk1e, Csnk1g1, Csnk1g2, Csnk1g3, Csn2, Csnk2a1, Csnk2a2, Csnk2b |
| Extra |
| Egfr, Lalba, Wap |
Table 6.
Lactation/milk genes differentially expressed in the TCS group compared to OIL at PND 146 (LD 28) (P < 0.05, t-test).
| Gene | Entrez gene name | P | Fold change | Expression in TCS relative to OIL |
|---|---|---|---|---|
| Slc30a4 | solute carrier family 30 (zinc transporter), member 4 | 0.035 | 2.494 | Down |
| Csnk1g1 | casein kinase 1, gamma 1 | 0.000 | 1.422 | Down |
| Uprt | uracil phosphoribosyltransferase (FUR1) homolog (S. cerevisiae) | 0.047 | 1.383 | Down |
| Csnk2a2 | casein kinase 2, alpha prime polypeptide | 0.002 | 1.309 | Down |
| Csnk1g3 | casein kinase 1, gamma 3 | 0.038 | 1.300 | Down |
| Ppat | phosphoribosyl pyrophosphate amidotransferase | 0.005 | 1.289 | Down |
| Chuk | conserved helix-loop-helix ubiquitous kinase | 0.033 | 1.249 | Down |
| Stat5b | signal transducer and activator of transcription 5B | 0.028 | 0.849 | Up |
| Aprt | adenine phosphoribosyl transferase | 0.032 | 0.776 | Up |
| Cav1 | caveolin 1, caveolae protein | 0.031 | 0.590 | Up |
Discussion
There are many examples of EDCs that have been shown to affect the development of the MG [7]. Exposure timing and dose influence the pattern of MG changes [31]. Few guideline studies for testing environmental chemicals include prenatal or early life dosing, and MG endpoints are limited primarily to indirect or surrogate observation during lactation and to clinical and pathological evaluation of adult mammary tissue. Effects of early life EDC exposure can lead to altered developmental programming in the breast and have been reported neonatally, at puberty, and well into adulthood, when effects on lactation or mammary tumorigenesis become evident [32]. Many animal studies are conducted with exposure doses that would result in a body burden higher than is found in humans; we used the general US population urinary concentration as a reference which is in the range of typical human exposure [33]. Our animal model, the SD rat from our colony, could be considered a human equivalent model especially for breast lesions (non-neoplastic, pre-neoplastic and neoplastic) allowing us to translate rodent data on mammary gland effects to humans. Our results support the hypothesis that a critical period of MG development, significantly affected by EDC exposure, is the development of the lactating gland. We chose LD 28, which was end of lactation-day, as time-point for the collection of lactating mammary glands. At this point in time, the process of involution of mammary glands is not immediate and histologically the gland appears similar to what is observed during the entire process of lactation. Only after 2 days from the end of lactation does the gland begin the irreversible sequence of cell death and remodelling, with a peak at day 4 of termination [34]. Furthermore, the same period of LD 28 was used for mammary gland monitoring both in treated and control dams, and similarly in nulliparous females at the same age (PND 146). The altered functional differentiation of the lactating mammary gland may be a direct effect of EDCs on the gland or may indicate that offspring are not thrifty enough to stimulate normal lactational development [35]. In our study there were no changes in pup weight between treated and control groups in the first week after birth (PND 1-7) indicating that EDC exposure did not affect neonatal growth which may impact on the ability of offspring to suckle and sufficiently stimulate lactation.
One notes that the altered MG development (decreased or unresponsive breast tissue) appeared to be a transient effect which was evident only during lactation. Indeed, for parous dams sacrificed at PND 181, no histopathological differences among treated and control groups were observed. Although numerous studies have shown persistent effects on the MG, few have evaluated whether the changes could be reversible. For example, in utero exposure to dioxin, Ziracin, PFOA or BPA leads to permanent changes in the adult MG. In contrast, the effects of genistein and ethinyl estradiol in male MG appear to reverse after treatment is withdrawn [36]. Transient or permanent effects may be due to gene imprinting, altered gene expression, modified endogenous MG signaling or changes in hormonal milieu [32]. No treatment-related effects were observed in the age-matched nulliparous rats, where the glands were undergoing only minimal cycle-related changes in epithelial growth. All virgin rats, regardless of treatment, demonstrated a similar degree of growth and branching.The differences in histopathology in the mammary glands of ED-treated animals compared to controls at PND 146 (LD 28), especially in the TCS group (Fig. 3D), motivated us to investigate the underlying molecular mechanisms associated with these changes. Transcriptome profiles revealed that hundreds of genes were differentially expressed in TCS-treated animals but only at PND 146 and not at PND 181, corroborating the histopathology results. Specifically, cholesterol biosynthetic pathways were down-regulated in TCS-treated animals (Table 4). TCS has been shown to reduce serum cholesterol levels with a concomitant reduction in the synthesis of androgens in treated male rats [37]. In our experiment, two key enzymes in the cholesterol biosynthesis pathway were down-regulated by more than two-fold in TCS-treated animals at PND 146 (LD 28): HMGCR, which catalyzes the rate-limiting step of cholesterol biosynthesis, and SQLE, which is the rate-limiting enzyme in the sterol biosynthesis pathway [38, 39]. Cholesterol constitutes an important component of milk, being secreted in large quantities by lactating rats [40]. HMGCR has in turn been shown to play a pivotal role in cholesterol biosynthesis in the rat mammary gland [41]. The synthesis of fatty acids and lipids is increased during lactation since fatty acids are the major constituent of the triglyceride pool in mammalian milk [42-44]. In SD rats, TCS has been shown to inhibit the activity of fatty acid synthase (FAS) a key enzyme in lipogenesis and the production of long chain fatty acids [45]. When we inputted the top 40 probe sets that were more than two-fold down-regulated in TCS-treated animals into IPA, fatty acid activation and fatty acid β oxidation were among the top five canonical pathways (data not shown). Taken together, our data suggest that lactating/weaning mammary glands at LD 28 may be more susceptible to the possible cholesterol- and lipid-disrupting properties of TCS than their later stage counterparts at PND 181 and may at least in part support the findings of the abnormal histology of TCS-treated animals at PND 146 (LD 28) (Fig. 3D).
Previous studies have found that exposure to organic chemicals such as perfluorooctanoic acid (PFOA) results in abnormal lactational development and changes in milk protein gene expression in mice, resulting in delayed development in exposed pups [28]. We tested whether TCS-treated animals at PND 146 and at PND 181 exhibited changes in lactation/milk-specific genes (Table 5). Consistent with the overall transcriptome pattern, significant changes among the lactation/milk-specific genes (10 out of 59) were only present at PND146 (LD 28) but not at PND 181. The top IPA network connecting 10 genes was ‘cell-cell signaling and interaction, drug metabolism and small molecule biochemistry’ (data not shown). Specifically SLC30A4, which belongs to a family of zinc transporters, was the only gene down-regulated by more than two-fold (Table 3). Zinc transporters play key roles in the mammary gland during lactation, as several processes in the secretory system rely on zinc[46]. The localization of SLC30A4 and other members in the family close to the luminal membrane of secreting mammary epithelial cells in mice suggests that they are important in the transport of zinc into milk [47, 48] Additionally, mice deficient in SLC30A4 were found to have lower mammary gland weight and milk volume [48]. Our observation that TCS exposure was associated with lower levels of not only SLC30A4 but also changes in other genes with possible roles in lactation (Table 3) may explain in part the observation that the alveoli of TCS-treated animals were smaller and often empty compared to the milk-filled alveoli of controls (Fig. 3D).
The treatment did not impact on the ability of the dams to carry their litters to term; however, the impaired gland development observed in the EDC-treated rats is likely to result in impaired lactation and reduce offspring survival. Necropsies performed on the dead pups revealed no evidence of gross defects in organ formation, but did show that their stomachs were empty.
However, the processes which regulate these stages of development differ, and therefore EDCs may not mediate the observed changes by the same mechanism. The impact of EDCs on breast milk production in women is not known. Once the mechanisms which mediate these alterations in the rodent mammary gland are further elucidated, the influence of DEP, MPB, TCS and the mixture of them on the human breast and ultimately on functional lactation, may be better estimated.
In conclusion, in using human equivalent exposures to three EDCs and a mixture of them, this animal study highlights the heightened sensitivity to EDCs of the MGs during pregnancy and lactation, suggesting an impact on pup survival. These results will provide a foundation for future studies of DEP, MPB and TCS that may evaluate their biological effects in an experimental setting so that information can be used in risk assessment for human exposure. Further investigation will be performed to better understand the mode of action mediating the lactation defects seen in the current study.
Highlights.
We studied low doses of three Endocrine Disrupting chemicals and their mixture.
We used parous and nulliparous female Sprague Dawley rats treated from birth.
Treatment resulted in higher mortality rates in pups compared to control group.
Morphological changes were observed in the mammary glands at the end of lactation.
The transcriptome profile showed that gene expression changes are also present at the end of lactation.
An increased sensitivity of the MGs to EDCs during pregnancy and lactation was demonstrated.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (U01 ES019451), Mount Sinai Children's Environmental Health Center Pilot Fund, Ramazzini Institute Fund for Special Projects.
We would like to thank Dr. Marianna Mariotti from the Immuno-Oncology Laboratory, Aging Research Center (CeSI), G. D'Annunzio University Foundation, Department of Medicine and Aging Sciences, G. D'Annunzio University of Chieti-Pescara for her precious technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World health Organization (WHO) Global Assessment of the State-of-the-Science of Endocrine Disruptors. International Programme on Chemical Safety (IPCS) 2002 [Google Scholar]
- 2.Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127:204–15. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr., Lee DH, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brisken C. Endocrine Disruptors and Breast Cancer. Chimia. 2008;62:406–9. [Google Scholar]
- 6.Macon MB, Fenton SE. Endocrine disruptors and the breast: early life effects and later life disease. J Mammary Gland Biol Neoplasia. 2013;18:43–61. doi: 10.1007/s10911-013-9275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147:S18–24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 8.Du K, Chu S, Xu X. Stimulation of MCF-7 cell proliferation by low concentrations of Chinese domestic polychlorinated biphenyls. J Toxicol Environ Health A. 2000;61:201–7. doi: 10.1080/00984100050131341. [DOI] [PubMed] [Google Scholar]
- 9.Payne J, Rajapakse N, Wilkins M, Kortenkamp A. Prediction and assessment of the effects of mixtures of four xenoestrogens. Environ Health Perspect. 2000;108:983–7. doi: 10.1289/ehp.00108983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Payne J, Scholze M, Kortenkamp A. Mixtures of four organochlorines enhance human breast cancer cell proliferation. Environ Health Perspect. 2001;109:391–7. doi: 10.1289/ehp.01109391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki T, Ide K, Ishida M. Response of MCF-7 human breast cancer cells to some binary mixtures of oestrogenic compounds in-vitro. J Pharm Pharmacol. 2001;53:1549–54. doi: 10.1211/0022357011777927. [DOI] [PubMed] [Google Scholar]
- 12.Rider CV, Furr JR, Wilson VS, Gray LE., Jr. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int J Androl. 2010;33:443–62. doi: 10.1111/j.1365-2605.2009.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hass U, Scholze M, Christiansen S, Dalgaard M, Vinggaard AM, Axelstad M, et al. Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environ Health Perspect. 2007;115(Suppl 1):122–8. doi: 10.1289/ehp.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelsey JL, Gammon MD, Jhon EM. Reproductive factors and breast cancer. Epidemiol Rev. 15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 15.Lanfranchi A. “Normal breast physiology: the reasons hormonal contraceptives and induced abortion increase breast-cancer risk”. Issues Law Med. 2014;29(1):135–46. Spring. [PubMed] [Google Scholar]
- 16.Valentini A, Lubinski J, Byrski T, Ghadirian P, Moller P, Lynch HT, et al. Hereditary Breast Cancer Clinical Study Group. The impact of pregnancy on breast cancer survival in women who carry a BRCA1 or BRCA2 mutation. Breast Cancer Res Treat. 2013 Nov;142(1):177–85. doi: 10.1007/s10549-013-2729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan L, He M, Yu M, Qiu C, Lee NH, Liu ET, et al. cDNA microarray profiling of rat mammary gland carcinomas induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 2002;23:1561–8. doi: 10.1093/carcin/23.10.1561. [DOI] [PubMed] [Google Scholar]
- 18.Chan MM, Lu X, Merchant FM, Iglehart JD, Miron PL. Gene expression profiling of NMU-induced rat mammary tumors: cross species comparison with human breast cancer. Carcinogenesis. 2005;26(8):1343–53. doi: 10.1093/carcin/bgi100. [DOI] [PubMed] [Google Scholar]
- 19.Decreto Legislativo Decreto Legislativo 116, 1992. Attuazione della direttiva. 86/609/CEE in materia di protezione degli animali utilizzati a fini sperimentali o ad altri fini scientifici [in Italian]. Gazzetta Ufficiale. 1992;(Supplemento ordinario):5–25. [Google Scholar]
- 20.Teitelbaum S, Lambertini L, Li Q, Belpoggi F, Falcioni L, Bua L, et al. Identification of endocrine disrupting chemical doses in rats to reproduce human urinary metabolite concentrations. Environment and Health –Bridging South, North, East and West. Basel, Switzerland 19–23 August, 2013 Oral presentation and ISEE Seattle Washington 24-28 August, 2014. Abstract number: 5107; ID: P-1-13-10. [Google Scholar]
- 21.Hvid H, Thorup I, Oleksiewicz MB, Sjogren I, Jensen HE. An alternative method for preparation of tissue sections from the rat mammary gland. Exp Toxicol Pathol. 2011;63:317–24. doi: 10.1016/j.etp.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. Journal of Anatomy. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- 23.Enoch R, Stanko J, Greiner S, Youngblood G, Rayner J, Fenton SE. Mammary Gland Development as a Sensitive End Point after Acute Prenatal Exposure to an Atrazine Metabolite Mixture in Female Long-Evans Rats. EHP. 2007;115(4):541–547. doi: 10.1289/ehp.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Heydebreck A, Huber W, Gentleman R. Encyclopedia of Genetics, Genomics, Proteomics and Bioinformatics. John Wiley & Sons, Ltd; 2004. Differential expression with the Bioconductor Project. [Google Scholar]
- 25.Bourgon R, Gentleman R, Huber W. Independent filtering increases detection power for high-throughput experiments. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9546–51. doi: 10.1073/pnas.0914005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gentleman R, Carey V, Huber W. F. H. Genefilter: Methods for filtering genes from microarray experiments. R package 1.46.1 ed2014 [Google Scholar]
- 27.Lemay DG, Lynn DJ, Martin WF, Neville MC, Casey TM, Rincon G, et al. The bovine lactation genome: insights into the evolution of mammalian milk. Genome biology. 2009;10:R43. doi: 10.1186/gb-2009-10-4-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicological sciences : an official journal of the Society of Toxicology. 2007;96:133–44. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- 29.Lemay DG, Neville MC, Rudolph MC, Pollard KS, German JB. Gene regulatory networks in lactation: identification of global principles using bioinformatics. BMC systems biology. 2007;1:56. doi: 10.1186/1752-0509-1-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 31.Warri A, Saarinen NM, Makela S, Hilakivi-Clarke L. The role of early life genistein exposures in modifying breast cancer risk. Br J Cancer. 2008;98:1485–93. doi: 10.1038/sj.bjc.6604321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudel RA, Fenton SE, Ackerman JM, Euling SY, Makris SL. Environmental exposures and mammary gland development: state of the science, public health implications, and research recommendations. Environ Health Perspect. 2011;119:1053–61. doi: 10.1289/ehp.1002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CDC. (US Centers for Disease Control and Prevention) Fourth report on human exposure to environmental chemicals. 2012 [Google Scholar]
- 34.Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia. 2000 Apr;5(2):227–41. doi: 10.1023/a:1026499523505. Review. [DOI] [PubMed] [Google Scholar]
- 35.White SS, Stanko JP, Kato K, Calafat AM, Hines EP, Fenton SE. Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environ Health Perspect. 2011;119:1070–6. doi: 10.1289/ehp.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latendresse JR, Bucci TJ, Olson G, Mellick P, Weis CC, Thorn B, et al. Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats. Reprod Toxicol. 2009;28:342–53. doi: 10.1016/j.reprotox.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Kumar V, Chakraborty A, Kural MR, Roy P. Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reprod Toxicol. 2009;27:177–85. doi: 10.1016/j.reprotox.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–30. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 39.Hidaka Y, Satoh T, Kamei T. Regulation of squalene epoxidase in HepG2 cells. Journal of lipid research. 1990;31:2087–94. [PubMed] [Google Scholar]
- 40.Clarenburg R, Chaikoff IL. Origin of milk cholesterol in the rat: dietary versus endogenous sources. Journal of lipid research. 1966;7:27–37. [PubMed] [Google Scholar]
- 41.Gibbons GF, Pullinger CR, Munday MR, Williamson DH. Regulation of cholesterol synthesis in the liver and mammary gland of the lactating rat. The Biochemical journal. 1983;212:843–8. doi: 10.1042/bj2120843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hachey DL, Silber GH, Wong WW, Garza C. Human lactation. II: Endogenous fatty acid synthesis by the mammary gland. Pediatric research. 1989;25:63–8. doi: 10.1203/00006450-198901000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Mohammad MA, Haymond MW. Regulation of lipid synthesis genes and milk fat production in human mammary epithelial cells during secretory activation. American journal of physiology Endocrinology and metabolism. 2013;305:E700–16. doi: 10.1152/ajpendo.00052.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu S, Archer MC. Fatty acid synthase is a potential molecular target for the chemoprevention of breast cancer. Carcinogenesis. 2005;26:153–7. doi: 10.1093/carcin/bgh278. [DOI] [PubMed] [Google Scholar]
- 45.Flavin R, Peluso S, Nguyen PL, Loda M. Fatty acid synthase as a potential therapeutic target in cancer. Future oncology (London, England) 2010;6:551–62. doi: 10.2217/fon.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelleher SL, Velasquez V, Croxford TP, McCormick NH, Lopez V, MacDavid J. Mapping the zinc-transporting system in mammary cells: molecular analysis reveals a phenotype-dependent zinc-transporting network during lactation. Journal of cellular physiology. 2012;227:1761–70. doi: 10.1002/jcp.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelleher SL, Lonnerdal B. Zn transporter levels and localization change throughout lactation in rat mammary gland and are regulated by Zn in mammary cells. The Journal of nutrition. 2003;133:3378–85. doi: 10.1093/jn/133.11.3378. [DOI] [PubMed] [Google Scholar]
- 48.McCormick NH, Kelleher SL. ZnT4 provides zinc to zinc-dependent proteins in the trans-Golgi network critical for cell function and Zn export in mammary epithelial cells. American journal of physiology Cell physiology. 2012;303:C291–7. doi: 10.1152/ajpcell.00443.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]