Abstract
Neurocysticercosis, an infection of the central nervous system with the larval stage of the cestode Taenia solium, is common in developing countries but its occurrence and management in allogeneic hematopoietic stem cell transplantation (HSCT) has not been reported previously, to our knowledge. We report the case of an immigrant female patient who underwent a matched-related allogeneic HSCT for acute lymphoblastic leukemia and was incidentally found to have a solitary viable neurocysticercosis lesion. However, despite severe immunosuppression, the size of the cyst did not increase. More importantly, restoration of the immune system did not induce significant inflammation or seizures. Subsequent follow-up demonstrated complete resolution of the neurocysticercosis lesion. Thus, in the setting of HSCT, an asymptomatic patient with a single neurocysticercosis lesion was successfully managed without the use of anthelmintics, steroids, or anti-epileptics.
Keywords: allogeneic hematopoietic stem cell transplantation, neurocysticercosis, parasite, tapeworm, anthelmintics, Taenia solium
Neurocysticercosis is an infection of the central nervous system (CNS) caused by the larval stage of the cestode Taenia solium, commonly known as the pork tapeworm. Neurocysticercosis is endemic in Central and Latin America, Africa and Asia, although transmission of the infection has been reported on all continents (1). Neurocysticercosis ranks highest among CNS infections caused by helminth parasites worldwide, and as much as 29% of adult-acquired epilepsy may be attributable to this disease (1–6). Infection is acquired by ingestion of gravid segments of the adult worm, called proglottids, either from the environment because of soil contamination of the food chain, or by infection following direct hand-to-mouth transfer. Once exposed to gastric acid and bile, infective oncospheres are released in the upper small intestines of the human host, penetrate the intestinal wall, and disseminate throughout the body, including the CNS (7–10).
Immunocompromised patients, such as those undergoing hematopoietic stem cell transplantation (HSCT), are predicted to be at increased risk for reactivation and dissemination of some infections, such as toxoplasmosis and tuberculosis (11, 12). However, the natural history of neurocysticercosis in the setting of transplant recipients is not well understood. The few available reports have been published in the context of solid organ transplantation. One renal transplant recipient and 1 liver transplant patient presented acutely after acquiring neurocysticercosis (13–15), and in 1 case, a liver transplant patient, who likely acquired infection years before, the disease appeared to reactivate after transplantation (13). To the best of our knowledge, no cases of neurocysticercosis have been reported in the context of HSCT. Given that conventional treatment for neurocysticercosis with live parasites includes co-administration of relatively high-dose steroids with anthelmintics (16), which would potentially interfere with HSCT outcomes, little experience and no guidelines exist for the management of neurocysticercosis in this setting.
We report a case of a 35-year-old Hispanic woman with acute lymphoblastic leukemia (ALL) who underwent matched-related donor HSCT and was subsequently found to have had asymptomatic neurocysticercosis prior to her transplant. We found that conservative management, without the use of anti-parasitic agents or corticosteroids, resulted in complete resolution of the infection within 8 months of her transplant, without apparent sequelae.
Case report
A 35-year-old Hispanic woman, who emigrated to the U.S. from Mexico 10 years earlier, was diagnosed with pre-B cell ALL when she presented to her primary care physician with gum bleeding, headache, fever, and lymphadenopathy for 2 weeks. Past medical history was significant for diabetes mellitus type II. Her white blood cell count was 304,700, with 98% blasts, an absolute neutrophil count of 300 cells/uL, hemoglobin 6.3 gm/dL, and platelet count of 21,000/uL. She had a complex karyotype at diagnosis with the presence of the Philadelphia chromosome translocation t(9;22), deletion of 8p and 9p. She underwent emergent leukapheresis. Lumbar puncture showed 36% blasts in the cerebrospinal fluid (CSF) and she had leukemic retinopathy on examination; magnetic resonance imaging (MRI) of the brain and orbits did not show any evidence of leptomeningeal or parenchymal involvement by ALL.
She underwent induction therapy similar to Children’s Oncology Group ALL protocol (vincristine, idarubicin, prednisone, and peg-asparaginase) and CNS therapy with intrathecal cytarabine and methotrexate. She achieved first complete remission on day 28 and subsequent bone marrow biopsy did not show any evidence of disease. She completed 2 cycles of consolidation therapy and was then started on maintenance dasatinib. Her chemotherapy course was complicated with Clostridium difficile pancolitis and probable fungal pneumonia.
She was referred for matched sibling HSCT after 3 months of consolidation therapy in a molecular complete remission. She consented to our Institutional Review Board-approved protocol (13-H-0144). Pre-transplant conditioning utilized fludarabine 125 mg/m2, cyclophosphamide 120 mg/kg, and total body irradiation 1200 cGy with lung shielding to 600 cGy. She also received a cranial radiation boost with 1200cGy for her history of CNS involvement. Graft-versus-host disease prophylaxis was instituted with single-agent cyclosporine (levels 100–200 ng/mL) from day −6 to day +21. The graft source was CD34+ selected (4-log ex vivo T lymphocyte-depleted) peripheral blood progenitor cells from her human leukocyte antigen-identical brother. Antimicrobial prophylaxis included ivermectin (15 mg orally daily for 2 doses on day −8) as empiric Strongyloides prophylaxis, given routinely to all patients with a geographic predisposition at our institute, and post-transplant acyclovir, trimethoprim-sulfamethoxazole, and voriconazole. She engrafted fully and achieved complete donor CD3+ lymphoid and myeloid chimerism at 3 weeks post transplant.
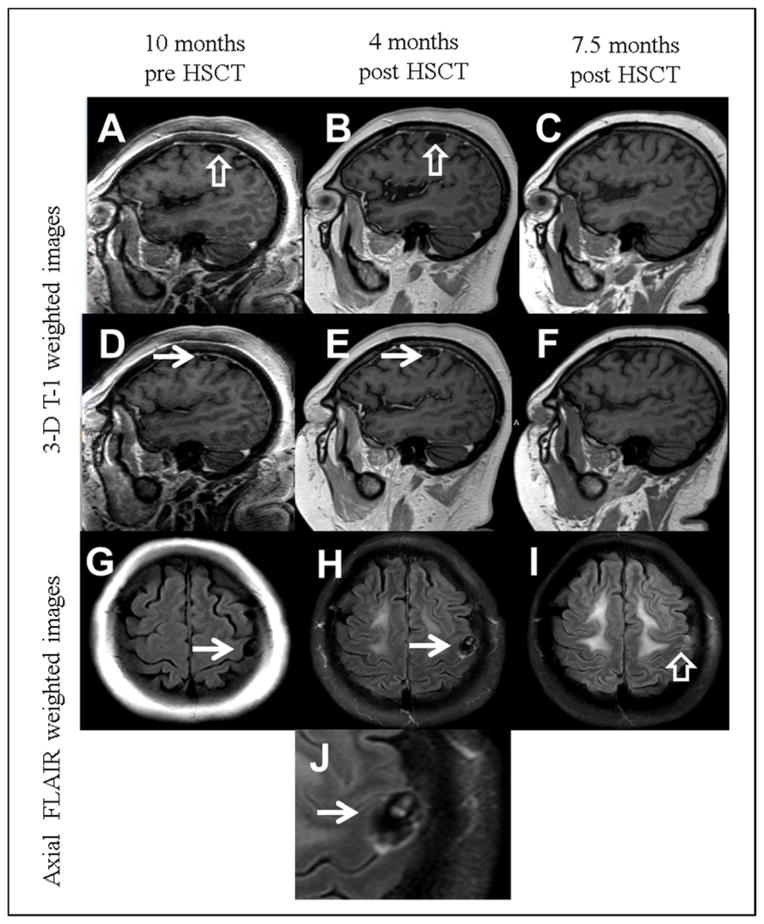
The post-transplant course was complicated by C. difficile colitis, cytomegalovirus reactivation, BK virus hemorrhagic cystitis, and Klebsiella pneumoniae urosepsis. She developed an altered mental status on day +130 associated with blurring of vision. These symptoms resolved spontaneously the next day, and were later attributed to corticosteroids administered as treatment for a rash the previous day. During the evaluation of her altered mental status, an MRI of the brain with contrast (Fig. 1B) showed a small extra-axial cystic structure (1.2 cm x 2 cm) overlying the left precentral gyrus with a thin irregularly enhancing rim. Minimal mass effect on the adjacent gyri was observed, but no edema or parenchymal invasion were noted. The cyst contents were isointense to CSF both on T1- and T2-weighted MRI images (Fig. 1B, E). A small eccentric T1 hyperintense structure was identified in the cyst, forming an incomplete circle. The overall appearance was highly suggestive of a neurocysticercosis cyst with an identifiable scolex, or “head” of the parasite (Fig. 1H, J). No calcifications were noted on computed tomography scan examination performed at the same time as the second MRI scan.
Fig. 1.
Magnetic resonance imaging of the neurocysticercosis lesion. Three-D T1-weighted images of the brain obtained 10 months before hematopoietic stem cell transplantation (HSCT) (A, D), 4 months after transplantation (B, E), and 7.5 months after transplantation (C, F). A small extra-axial cystic structure is identified adjacent to the left precentral gyrus (open white arrows), slightly increasing in size between the first and second examination (A and B). An eccentric hyperintensity within the cyst (solid white arrows) is suggestive of a scolex. The imaging is highly suggestive of subarachnoid neurocysticercosis. The last examination (C and F) show resolution of the cystic structure and internal scolex. (G–J) Axial FLAIR weighted images across the frontal lobes show a cystic structure with fluid signal intensity (white arrows) in G (pretransplant) and H (4 months after transplant). The last examination (I) shows slight residual hyperintensity at the location of the resolved cyst, suggestive of gliosis. (J) is a detail from H showing a clear appearance of a scolex.
At that point, careful review of an initial MRI performed 10 months before HSCT showed that the cystic structure with scolex was present, but was smaller in size (Fig. 1A, D, G). Lumbar puncture revealed mild pleocytosis upon CSF analysis (red blood cells 4/mm3, white blood cells 13/mm3, lymphocytes 87%, other cells 13%, protein 31 mg/dL, and glucose 60 mg/dL) with no evidence of a leukemic, bacterial, or viral infection. Serum and CSF immunoblot assay (an enzyme-linked immunoelectrotransfer blot [EITB] assay performed at the Centers for Disease Control and Prevention [CDC], Atlanta, Georgia, USA) were positive for T. solium antibodies.
Ophthalmologic evaluation ruled out retinal cysticercosis, revealing only non-proliferative diabetic retinopathy. Because of concern for a potential negative impact of corticosteroids on elimination of residual leukemia, conventional anthelmintic therapy and corticosteroids, the latter required to suppress post-treatment pericystic inflammation, were withheld and a strategy of watchful observation was adopted. Anti-epileptic therapy was deferred because of the subarachnoid location of the cyst and the absence of parenchymal lesions.
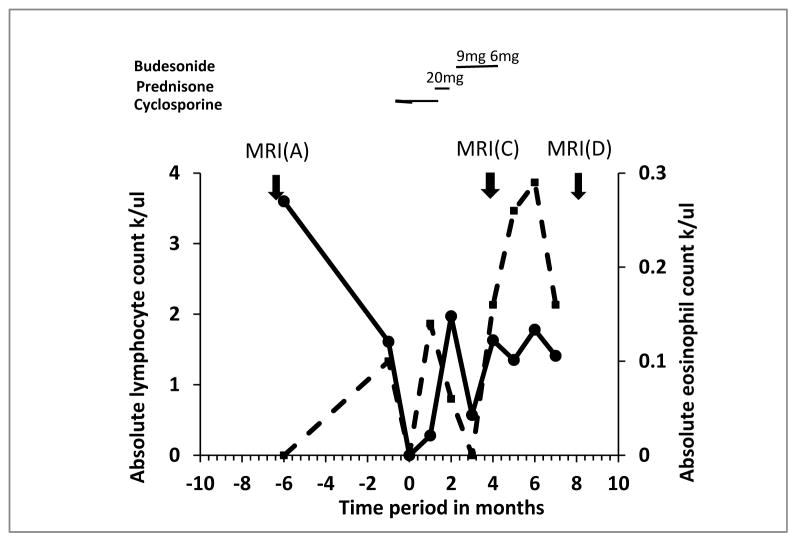
In the following weeks, trends in absolute lymphocyte counts and eosinophil counts, indicated successful immune reconstitution (Fig. 2). Repeat MRI 4 months after initial diagnosis of neurocysticercosis demonstrated interval spontaneous resolution of the previously identified cyst and scolex (Fig. 1C, F), leaving a small amount of residual signal abnormality on T2 and FLAIR weighted images in the adjacent gyrus, suggestive of residual gliosis (Fig. 1I).
Fig. 2.
Trend of absolute lymphocyte and eosinophil counts in pre- and post-transplant time points. MRI, magnetic resonance imaging.
Discussion
Although the true prevalence of neurocysticercosis is unknown, it is the most common helminthic infection of the brain and a major cause of seizures worldwide (17). Neurocysticercosis is highly endemic in areas of Latin America, Asia, and Africa, and increased immigration from these areas has resulted in an increased frequency of neurocysticercosis in developed countries (18). The prevalence of neurocysticercosis both in the US and worldwide is very likely underestimated, given the number of asymptomatic infected individuals, lack of availability of standard diagnostic tests in endemic countries, and reluctance of patients to seek medical attention because of the stigma associated with epilepsy in many societies.
Neurocysticercosis can mimic a large variety of neurologic syndromes, depending on the region of the CNS affected. Patients with parenchymal disease most frequently present with epilepsy (18–20). Patients with subarachnoid or ventricular neurocysticercosis, may develop hydrocephalus because of ventricular outlet obstruction (21), and frequently have spinal cord involvement (22). Vascular compromise and strokes have also been reported in subarachnoid neurocysticercosis located in close proximity to major intracranial vessels (23). Cysts in the Sylvian fissures can grow to large sizes (>10 cm), because of pressure than with parenchymal cysts (24).
The diagnosis of neurocysticercosis is based on clinical presentation, cross-sectional imaging, and evidence of anti-parasite antibodies or parasitic antigens (8, 19, 20, 25). Computed tomography scan and MRI provide information on the location, number, and viability of cysts (using alteration in fluid density and calcification as a marker of cyst death), as well as the severity of the inflammatory host reaction against the parasite (6, 26). Diagnostic immunologic assays include the EITB assay, developed by the CDC and used to detect antibodies against the parasite, and antigen-capture enzyme-linked immunosorbent assay (ELISA) to detect parasite antigens. EITB can be performed on serum or CSF with a reported sensitivity of 98% and specificity of 100% (27). However, EITB is more likely to be positive in those with viable, non-calcified, and enhancing cysts/lesions than in those with calcified cysts. Only 5 of 15 subjects with single enhancing cysts and none of 3 with a single calcified lesion had a positive EITB result (28). The antigen-detection ELISA performs better for CSF than for serum samples, but is less sensitive than the EITB assay. In general, antigen detection has been reported to be most useful for diagnosis and possibly for following efficacy of treatment in subarachnoid neurocysticercosis, but is insufficiently sensitive for similar application in parenchymal disease (29). Nevertheless, the antigen-capture ELISA, unlike the EITB, is able to differentiate active from resolved or prior infection (30), a characteristic that has made it useful tool.
In the past, little consensus existed on the standard treatment for neurocysticercosis. However, recent data from endemic regions have led to the emergence of a growing body of literature to guide anthelmintic therapy in neurocysticercosis, even in the setting of HSCT. The location, viability, and number of cysts in the CNS influence the treatment course (7, 31, 32). For viable parenchymal cysts, conventional treatment comprises the anthelmintics albendazole or praziquantel in combination with corticosteroids to suppress post-treatment edema that occurs as a response to the dying parasite (33). Anthelmintics are not indicated for inactive or dead parasites (i.e., calcified or solid lesions without capsules or surrounding inflammation) (31). Likewise, some studies suggest that asymptomatic patients with a single parenchymal lesion do not necessarily require anthelmintic therapy, as it has not been shown to significantly change the natural history (34, 35). Anti-epileptics are recommended for all neurocysticercosis patients with seizures (3), and also for patients at increased risk of seizures (31). Seizure risk is highest in the setting of multiple parenchymal lesions, especially with degenerating parasites and lesions with associated inflammation (36–40). Asymptomatic patients with only calcified lesions do not routinely require prophylactic anti-epileptic therapy, in the absence of perilesional edema. Where skilled neurosurgical services are available, minimally invasive endoscopy is now used frequently for lateral and third ventricular cysts (41), and open microscopic surgery is an option, when indicated, for fourth ventricular cysts (25, 31, 32, 42). In all cases of subarachnoid (extraventricular) neurocysticercosis, prolonged courses of albendazole and corticosteroids are required, and consensus has not been achieved on the best endpoints of therapy. In any case, experience and clinical trial evidence are insufficient to determine the best therapy in immunocompromised patients.
While no published data are available to guide treatment of neurocysticercosis in a transplant or immunocompromised patient, conventional management generally suggests that a combination of high-dose corticosteroids with anthelmintics should be effective (43). Corticosteroids are used to control post-treatment inflammation surrounding dying cysts (16). However, high-dose steroids can have a negative impact on a curative graft-versus-leukemia effect of a recovering immune system in the host, a process that may be necessary for eradication of the leukemia. Given the very slow enlargement of the cyst over a period of 14 months, the non-critical location with regards to CSF flow, and the asymptomatic nature of the lesion, careful observation was the consensus for management in our patient.
Altered mental status in this case was ultimately attributed to a different etiology (steroids), and therefore the neurocysticercosis was an incidental, non-symptomatic finding. She did not have any history of symptoms attributable to neurocysticercosis. Coming from a region endemic for neurocysticercosis, it is likely that she acquired the infection well before the development of ALL. The stability in size of the lesion through the course of chemotherapy, radiation, and consequent immunosuppression, indicates that the course of infection was not greatly influenced by the immunosuppression or corticosteroid therapy she received as a part of her transplant. It is very unlikely that the prophylactic dose of ivermectin (200 μg/kg x 2 doses) had significant clinical activity against the neurocysticercosis, as a previous report of efficacy utilized 10 mg per day for much longer durations (15–30 days) to treat albendazole/praziquantel-resistant neurocysticercosis with uncertain efficacy (44).
Very few double-blind trials have compared cysticidal drugs versus placebo in patients with parenchymal cysts in the vesicular stage (live parasites). One of the early trials (a suboptimal study, in the opinion of many experts, because of the heterogeneity of cysts in the study population) showed no benefit of albendazole over placebo in 29 patients with multiple cysts (45). Another study found no difference in proportions of patients free of seizures with or without treatment with albendazole (46). A study from India, in which patients with multiple parenchymal cysts were treated either with anthelmintics, corticosteroids and anti-epileptics, or with anti-epileptics alone, reported a higher rate of cyst resolution and fewer seizures in the follow-up period in patients receiving anti-epileptics alone (47). Reviewing these and similar studies (48, 49), a Cochrane Database review on drugs for treatment of neurocysticercosis concluded that insufficient evidence existed to ascribe beneficial effects to anthelmintics in the treatment of parenchymal neurocysticercosis (50).
An important caveat to many of these studies is that most of the study populations had single enhancing parenchymal lesions, a stage and type of neurocysticercosis that is thought to represent late degenerative stages of parasites with inflammatory reactions (6), which differs from the live cyst stage without surrounding inflammation (51). The best randomized placebo-controlled treatment trial for live parenchymal cysts did demonstrate clinical benefit a long-term reduction in generalized seizures (31, 38). Furthermore, in a meta-analysis of 11 clinical studies in which albendazole or praziquantel were used to treat cystic parenchymal disease, the authors concluded that cysticidal drugs significantly increased the rate of complete cyst resolution (69% in treated vs. 55% in untreated, after exclusion of an outlier study) (52). However, their data also indicate that almost a third of the patients did not show resolution of cysts, irrespective of pathological stage (clear vs. colloidal) with anthelmintic treatment.
Given the ambiguity of results from available studies in terms of efficacy of anthelmintics, many experts would concede that withholding anthelmintic therapy is a viable strategy in selected cases, for example, in patients without neurological symptoms, with single lesions that are stable over a period of observation, when other co-morbidities may enhance the risks of treatment. In the present case, the decision to treat with watchful observation and not to use anthelmintics or corticosteroids was based on concern about the corticosteroid-induced loss of a graft-versus-leukemia effect. This conservative strategy was successful, with involution of the cyst on follow-up (Fig. 1C, F, I) and no neurologic symptoms. The residual increased FLAIR signal on MRI in the precentral brain parenchyma was the only evidence of residual gliosis associated with the subarachnoid neurocysticercosis in our patient.
It is tempting to speculate that the resolution of the cyst could be related to post transplant immune reconstitution. Notably, our patient did not receive any immunosuppressive drugs after day +21. Another potential reason for lack of growth and involution may be the use of radiation during the induction phase of treatment prior to transplantation. However, the radiosensitivity of this parasite is unknown.
Our experience offers several generalizable insights applicable to the management of neurocysticercosis in the setting of transplantation and applicable to single lesions – the management of multiple lesions and subarachnoid disease differs, and would likely require a more aggressive therapeutic approach. Neurocysticercosis must always be considered as a part of the differential diagnosis in transplant patients with cystic lesions found on neuroimaging. Careful and exhaustive review of previous imaging is mandatory and may reveal a pre-existing lesion. Conservative management without anthelmintics, steroids, or anti-epileptics can be a valid strategy in selected cases (asymptomatic, pre-existing, stable, solitary lesions affecting noncritical areas of the brain). For other situations in the setting of an immunocompromised state, the benefits of treating the disease, which includes lower frequency of seizures and faster resolution of cysts, do appear to be significant, but must be carefully balanced against the risk of toxicity from anthelmintics, anti-epileptics, and corticosteroids, along with the possible mitigation of a graft-versus-malignancy effect. Even when anthelmintic therapy is indicated, the slow growth of the parasite allows therapy to be safely deferred to a time when treatment will be less risky; in the case of acute leukemia, this might be as long as 3 years, when the risk of relapse is greatly reduced.
This case represents the first ever report, to our knowledge, of neurocysticercosis in an HSCT patient. The paucity of reports related to neurocysticercosis management in HSCT is probably a result of geographic and socio-economic barriers to access to transplantation. However, more cases of neurocysticercosis complicating HSCT might be expected to present in future, owing to emigration from endemic areas, better access to healthcare, and extension of HSCT globally to highly endemic under-developed regions of the world.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH (National Heart, Lung, Blood Institute, National Institutes of Health; National Institute of Neurological Disorders and Stroke, Clinical Center; National Institute of Allergy and Infectious Diseases).
References
- 1.Medina MD, Degiorgio CM. Introduction to neurocysticercosis: a worldwide epidemic. Neurosurg Focus. 2002;12(6):1. [Google Scholar]
- 2.Commission on Tropical Diseases of the International League Against Epilepsy. Relationship between epilepsy and tropical diseases. Epilepsia. 1994;35(1):89–93. [PubMed] [Google Scholar]
- 3.Carpio A. Neurocysticercosis: an update. Lancet Infect Dis. 2002;2(12):751–762. doi: 10.1016/s1473-3099(02)00454-1. [DOI] [PubMed] [Google Scholar]
- 4.Del Brutto OH, Santibanez R, Noboa CA, Aguirre R, Diaz E, Alarcon TA. Epilepsy due to neurocysticercosis: analysis of 203 patients. Neurology. 1992;42(2):389–392. doi: 10.1212/wnl.42.2.389. [DOI] [PubMed] [Google Scholar]
- 5.Flisser A. Taeniasis and cysticercosis due to Taenia solium. Prog Clin Parasitol. 1994;4:77–116. [PubMed] [Google Scholar]
- 6.Garcia HH, Del Brutto OH. Imaging findings in neurocysticercosis. Acta Tropica. 2003;87(1):71–78. doi: 10.1016/s0001-706x(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 7.Del Brutto OH. Neurocysticercosis: up-dating in diagnosis and treatment. Neurologia. 2005;20(8):412–418. [PubMed] [Google Scholar]
- 8.Garcia HH, Del Brutto OH. Taenia solium cysticercosis. Infect Dis Clin North Amer. 2000;14(1):97–119. ix. doi: 10.1016/s0891-5520(05)70220-8. [DOI] [PubMed] [Google Scholar]
- 9.Sinha S, Sharma BS. Neurocysticercosis: a review of current status and management. J Clin Neurosci. 2009;16(7):867–876. doi: 10.1016/j.jocn.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Sotelo J, Del Brutto OH. Review of neurocysticercosis. Neurosurg Focus. 2002;12(6):e1. doi: 10.3171/foc.2002.12.6.2. [DOI] [PubMed] [Google Scholar]
- 11.Bumbacea D, Arend SM, Eyuboglu F, et al. The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur Respir Jl. 2012;40(4):990–1013. doi: 10.1183/09031936.00000712. [DOI] [PubMed] [Google Scholar]
- 12.Meers S, Lagrou K, Theunissen K, et al. Myeloablative conditioning predisposes patients for Toxoplasma gondii reactivation after allogeneic stem cell transplantation. Clin Infect Dis. 2010;50(8):1127–1134. doi: 10.1086/651266. [DOI] [PubMed] [Google Scholar]
- 13.Barra Valencia V, Moreno Elola-Olaso A, Fundora Suarez Y, et al. Second case of neurocysticercosis in a patient with liver transplantation (first case in Spain): a case report. Transplant Proc. 2007;39(7):2454–2457. doi: 10.1016/j.transproceed.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 14.Gordillo-Paniagua G, Munoz-Arizpe R, Ponsa-Molina R. Unusual complication in a patient with renal transplantation: cerebral cysticercosis. Nephron. 1987;45(1):65–67. doi: 10.1159/000184074. [DOI] [PubMed] [Google Scholar]
- 15.Hoare M, Gelson WT, Antoun N, Alexander GJ. Early recurrence of neurocysticercosis after orthotopic liver transplant. Liver Transplant. 2006;12(3):490–491. doi: 10.1002/lt.20643. [DOI] [PubMed] [Google Scholar]
- 16.Nash TE, Mahanty S, Garcia HH Cysticercosis Group in P. Corticosteroid use in neurocysticercosis. Expert Review Neurother. 2011;11(8):1175–1183. doi: 10.1586/ern.11.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ndimubanzi PC, Carabin H, Budke CM, et al. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4(11):e870. doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alarcon F. Neurocysticercosis: its aetiopathogenesis, clinical manifestations, diagnosis and treatment. Revista Neurol. 2006;43(Suppl 1):S93–100. [PubMed] [Google Scholar]
- 19.Del Brutto OH. Neurocysticercosis. Semin Neurol. 2005;25(3):243–251. doi: 10.1055/s-2005-917661. [DOI] [PubMed] [Google Scholar]
- 20.Del Brutto OH, Rajshekhar V, White AC, Jr, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57(2):177–183. doi: 10.1212/wnl.57.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelesidis T, Tsiodras S. Extraparenchymal neurocysticercosis in the United States. Am J Med Sci. 2012;344(1):79–82. doi: 10.1097/MAJ.0b013e31823e6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callacondo D, Garcia HH, Gonzales I, Escalante D, Nash TE Cysticercosis Working Group in P. High frequency of spinal involvement in patients with basal subarachnoid neurocysticercosis. Neurology. 2012;78(18):1394–1400. doi: 10.1212/WNL.0b013e318253d657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marquez JM, Arauz A. Cerebrovascular complications of neurocysticercosis. The Neurologist. 2012;18(1):17–22. doi: 10.1097/NRL.0b013e31823d7a80. [DOI] [PubMed] [Google Scholar]
- 24.Garcia HHCC, White AC, Jr, Guerrant RLWD, Weller PF. Tropical Infectious Diseases: Principles, Pathogens, and Practice. Philaselphia: Churchil-Livingstone; 2011. Cysticercosis; p. 815. [Google Scholar]
- 25.Hawk MW, Shahlaie K, Kim KD, Theis JH. Neurocysticercosis: a review. Surg Neurol. 2005;63(2):123–132. doi: 10.1016/j.surneu.2004.02.033. discussion 132. [DOI] [PubMed] [Google Scholar]
- 26.Garcia HH, Del Brutto OH, Nash TE, White AC, Jr, Tsang VC, Gilman RH. New concepts in the diagnosis and management of neurocysticercosis (Taenia solium) The Am J Trop Med Hyg. 2005;72(1):3–9. [PubMed] [Google Scholar]
- 27.Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) J Infect Dis. 1989;159(1):50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 28.Wilson M, Bryan RT, Fried JA, et al. Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J Infect Dis. 1991;164(5):1007–1009. doi: 10.1093/infdis/164.5.1007. [DOI] [PubMed] [Google Scholar]
- 29.Garcia HH, Rodriguez S, Gilman RH, Gonzalez AE, Tsang VC Cysticercosis Working Group in P. Neurocysticercosis: is serology useful in the absence of brain imaging? Trop Med Internat Health. 2012;17(8):1014–1018. doi: 10.1111/j.1365-3156.2012.03037.x. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez S, Dorny P, Tsang VC, et al. Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J Infect Dis. 2009;199(9):1345–1352. doi: 10.1086/597757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nash TE, Singh G, White AC, et al. Treatment of neurocysticercosis: current status and future research needs. Neurology. 2006;67(7):1120–1127. doi: 10.1212/01.wnl.0000238514.51747.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serpa JA, Yancey LS, White AC., Jr Advances in the diagnosis and management of neurocysticercosis. Expert review of anti-infective therapy. 2006;4(6):1051–1061. doi: 10.1586/14787210.4.6.1051. [DOI] [PubMed] [Google Scholar]
- 33.Garcia HH, Gilman RH, Horton J, et al. Albendazole therapy for neurocysticercosis: a prospective double-blind trial comparing 7 versus 14 days of treatment. Cysticercosis Working Group in Peru Neurology. 1997;48(5):1421–1427. doi: 10.1212/wnl.48.5.1421. [DOI] [PubMed] [Google Scholar]
- 34.Singhi P, Ray M, Singhi S, Khandelwal N. Clinical spectrum of 500 children with neurocysticercosis and response to albendazole therapy. J Child Neurol. 2000;15(4):207–213. doi: 10.1177/088307380001500401. [DOI] [PubMed] [Google Scholar]
- 35.Gulati S, Yoganathan S, Chakrabarty B. Epilepsy, cognition and behavior. Indian J Pediatr. 2014 doi: 10.1007/s12098-014-1530-4. [DOI] [PubMed] [Google Scholar]
- 36.Carpio A, Hauser WA. Prognosis for seizure recurrence in patients with newly diagnosed neurocysticercosis. Neurology. 2002;59(11):1730–1734. doi: 10.1212/01.wnl.0000036320.69823.ea. [DOI] [PubMed] [Google Scholar]
- 37.Del Brutto OH. Prognostic factors for seizure recurrence after withdrawal of antiepileptic drugs in patients with neurocysticercosis. Neurology. 1994;44(9):1706–1709. doi: 10.1212/wnl.44.9.1706. [DOI] [PubMed] [Google Scholar]
- 38.Garcia HH, Pretell EJ, Gilman RH, et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med. 2004;350(3):249–258. doi: 10.1056/NEJMoa031294. [DOI] [PubMed] [Google Scholar]
- 39.Rajshekhar V, Jeyaseelan L. Seizure outcome in patients with a solitary cerebral cysticercus granuloma. Neurology. 2004;62(12):2236–2240. doi: 10.1212/01.wnl.0000130471.19171.d8. [DOI] [PubMed] [Google Scholar]
- 40.Verma A, Misra S. Outcome of short-term antiepileptic treatment in patients with solitary cerebral cysticercus granuloma. Acta Neurol Scand. 2006;113(3):174–177. doi: 10.1111/j.1600-0404.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- 41.Rajshekhar V. Surgical management of neurocysticercosis. Internati J Surg. 2010;8(2):100–104. doi: 10.1016/j.ijsu.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Rangel-Castilla L, Serpa JA, Gopinath SP, Graviss EA, Diaz-Marchan P, White AC., Jr Contemporary neurosurgical approaches to neurocysticercosis. Am J Trop Med Hyg. 2009;80(3):373–378. [PubMed] [Google Scholar]
- 43.Garcia HH, Gonzales I, Lescano AG, et al. Enhanced steroid dosing reduces seizures during antiparasitic treatment for cysticercosis and early after. Epilepsia. 2014 doi: 10.1111/epi.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diazgranados-Sanchez JA, Barrios-Arrazola G, Costa JL, Burbano-Pabon J, Pinzon-Bedoya J. Ivermectin as a therapeutic alternative in neurocysticercosis that is resistant to conventional pharmacological treatment. Revista de neurologia. 2008;46(11):671–674. [PubMed] [Google Scholar]
- 45.Padma MV, Behari M, Misra NK, Ahuja GK. Albendazole in neurocysticercosis. Nat Med J India. 1995;8(6):255–258. [PubMed] [Google Scholar]
- 46.Carpio A, Kelvin EA, Bagiella E, et al. Effects of albendazole treatment on neurocysticercosis: a randomised controlled trial. J Neurol Neurosurg Psych. 2008;79(9):1050–1055. doi: 10.1136/jnnp.2008.144899. [DOI] [PubMed] [Google Scholar]
- 47.Das K, Mondal GP, Dutta AK, Mukherjee B, Mukherjee BB. Awareness of warning symptoms and risk factors of stroke in the general population and in survivors stroke. J Clin Neurosci. 2007;14(1):12–16. doi: 10.1016/j.jocn.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 48.Baranwal AK, Singhi PD, Khandelwal N, Singhi SC. Albendazole therapy in children with focal seizures and single small enhancing computerized tomographic lesions: a randomized, placebo-controlled, double blind trial. Pediatr Infect Dis J. 1998;17(8):696–700. doi: 10.1097/00006454-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Gogia S, Talukdar B, Choudhury V, Arora BS. Neurocysticercosis in children: clinical findings and response to albendazole therapy in a randomized, double-blind, placebo-controlled trial in newly diagnosed cases. Transact Royal Soc Trop Med Hyg. 2003;97(4):416–421. doi: 10.1016/s0035-9203(03)90075-7. [DOI] [PubMed] [Google Scholar]
- 50.Salinas R, Prasad K. Drugs for treating neurocysticercosis (tapeworm infection of the brain) Cochrane Database System Rev. 2000;(2):CD000215. doi: 10.1002/14651858.CD000215. [DOI] [PubMed] [Google Scholar]
- 51.Garcia HH, Del Brutto OH Cysticercosis Working Group in Peru. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005;4(10):653–661. doi: 10.1016/S1474-4422(05)70194-0. [DOI] [PubMed] [Google Scholar]
- 52.Del Brutto OH, Roos KL, Coffey CS, Garcia HH. Meta-analysis: cysticidal drugs for neurocysticercosis: albendazole and praziquantel. Ann Intern Med. 2006;145(1):43–51. doi: 10.7326/0003-4819-145-1-200607040-00009. [DOI] [PubMed] [Google Scholar]