Abstract
Identifying unknown drug interactions is of great benefit in the early detection of adverse drug reactions. Despite existence of several resources for drug-drug interaction (DDI) information, the wealth of such information is buried in a body of unstructured medical text which is growing exponentially. This calls for developing text mining techniques for identifying DDIs. The state-of-the-art DDI extraction methods use Support Vector Machines (SVMs) with non-linear composite kernels to explore diverse contexts in literature. While computationally less expensive, linear kernel-based systems have not achieved a comparable performance in DDI extraction tasks. In this work, we propose an efficient and scalable system using a linear kernel to identify DDI information. The proposed approach consists of two steps: identifying DDIs and assigning one of four different DDI types to the predicted drug pairs. We demonstrate that when equipped with a rich set of lexical and syntactic features, a linear SVM classifier is able to achieve a competitive performance in detecting DDIs. In addition, the one-against-one strategy proves vital for addressing an imbalance issue in DDI type classification. Applied to the DDIExtraction 2013 corpus, our system achieves an F1 score of 0.670, as compared to 0.651 and 0.609 reported by the top two participating teams in the DDIExtraction 2013 challenge, both based on non-linear kernel methods.
Keywords: drug-drug interaction, biomedical literature, linear kernel approach
1. Introduction
New drugs are generally studied on relatively small and homogeneous patient populations. As a result, pharmaceuticals often have side effects that remain unnoticed until they are already available to the public. This is especially true of side effects that emerge when two drugs are co-administered. A change in the effect of one drug in the presence of another drug is known as a drug-drug interaction (DDI) [1]. It is characterized as an increase or decrease in the action of either substance, or it may be an adverse effect that is not normally associated with either drug. Understanding these drug-drug interactions and their downstream effects is of significant importance, leading to reduced number of drug-safety incidents and reduced healthcare costs.
To address the DDI problem, a number of drug databases such as Drug- Bank [2] and Stockley’s Drug Interactions [1] have been created. Yet, they cover only a fraction of knowledge available. A large amount of up-to-date information is still hidden in the text of journal articles, technical reports and adverse event reporting systems, and this body of unstructured published literature is growing rapidly. MEDLINE®, for example, has doubled in size within the last decade and currently contains about 23 million documents. This creates an urgent need for text mining techniques to extract DDI information.
Using text mining techniques for DDI extraction has received less attention compared to other biomedical relation extraction tasks (e.g., proteinprotein interactions), possibly due to the lack of gold standard sets [3, 4, 5, 6]. The DDIExtraction challenges are the first community-wide competition addressing the DDI extraction problem [7, 8] and a series of studies have been reported at the 2011 and 2013 challenge workshops [9, 10, 11].
Top performing systems in the DDIExtraction challenges use Support Vector Machines (SVMs) with non-linear kernels [12, 13]. To handle structural representations of input instances, such as dependency graphs, nonlinear kernels directly calculate similarities between two graphs by comparing embedded subgraphs [14]. While non-linear kernels are theoretically capable of implicitly searching a high dimensional feature space of subgraphs, existing methods generally exploit only a partial feature space because of the exponential number of subgraphs [15]. In addition, non-linear kernels are frequently combined into composite kernels [12, 11]. Composite kernels, however, incur more computational cost because the complexity of the underlying kernels accumulates and additional learning is required to optimize the weights for individual kernels.
Despite the popularity of non-linear kernel methods, linear kernels are a good alternative for relation extraction tasks [16, 17, 18]. Linear kernels with word-level features alone provide a strong baseline performance [11, 12]. Moreover, they can explicitly include nodes, edges and path structures of the dependency graphs [17]. Also, the straightforward representation of linear kernels enables the intuitive interpretation of obtained results. Most importantly, when training large-scale datasets, it has been demonstrated that often linear kernels are the only practical choice [19, 20]. However, the performance of linear kernel systems in DDI extraction tasks has a noticeable gap from that of the top systems using non-linear kernels [7, 8, 21].
We conjecture that linear kernel-based systems may benefit from a rich set of lexical and syntactic features. With the goal to build a simple and scalable system, we develop a DDI extraction system based on a single linear SVM classifier. We define five types of features to capture the complexity of data: word features with position information, pairs of non-adjacent words, dependency relations, parse tree structures and tags for differentiating DDI pairs within the same noun phrase. Unlike other state-of-the-art systems [13, 21] which incorporate external, domain-specific resources, our features originate exclusively from training data.
We evaluate our system on the DDIExtraction 2013 corpus [22]. Consistent with other studies [11, 12, 13], we adopt a two-phase approach, where DDI pairs are identified first, and then classified into specific DDI types. The proposed method achieves an overall F-score of 67% which outperforms the best performing system by 1.9%. We believe that the strength of our method comes from using a diverse set of features. In addition, the one-against-one strategy [23] used in the DDI type classification contributes to the higher performance. As the first linear kernel method that achieves the state-of-the-art performance on both DDI detection and classification tasks, we consider it a strong alternative to the nonlinear, composite kernel-based approaches. The inherent simplicity of the method adds transparency to the overall system, which could be especially beneficial if the system is used as a part of a more complicated schema. The source code for generating the features proposed in this article is available at http://www.ncbi.nlm.nih.gov/IRET/DDI.
2. Methods
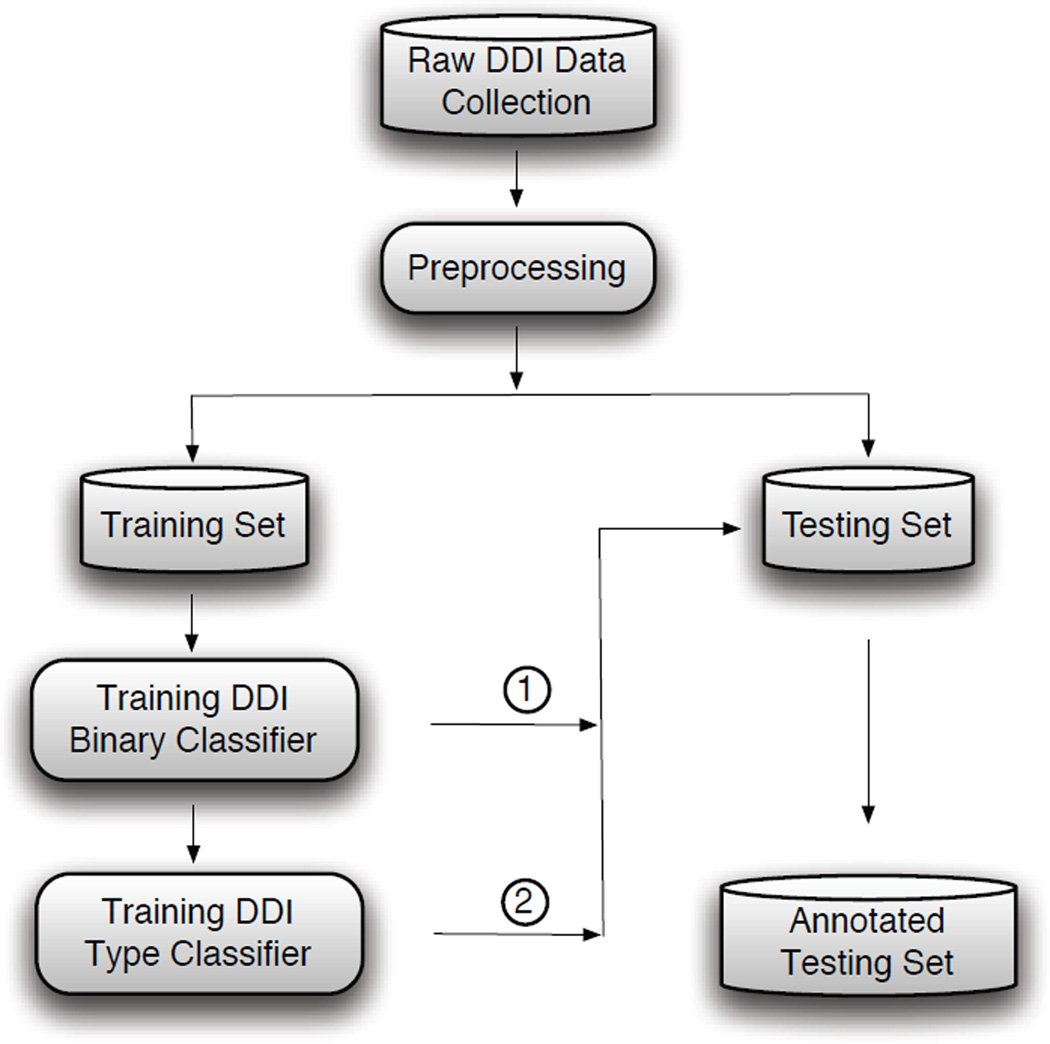
Figure 1 illustrates the overall architecture of our DDI extraction system. A binary classifier is trained first to extract interacting drug pairs from all candidate interactions. A DDI type classifier is then built to classify the interacting pairs into predefined relation categories. Our approach focuses on interactions expressed within the boundaries of a single sentence, and also assumes that drug entities involved in the target interactions have been annotated.
Figure 1.
Two-phase DDI extraction framework. DDI detection (①) decides whether a drug pair interacts. DDI type classification (②) assigns DDI types to interacting pairs.
In this section, we first elaborate the five types of features used, including two novel features proposed for the DDI problem: word pair and noun phrase-constrained coordination (NPC) features. Then, we briefly introduce the preprocessing steps completed on both training and test data. Next, we describe our linear SVM classifier with a modified Huber loss function [24]. In the end, we compare our method with existing DDI extraction systems.
2.1. Features
2.1.1. Word features
Word-level features such as individual words in a sentence and sequences of words have been demonstrated to provide a strong performance baseline in extracting relational knowledge [11, 17, 25]. Hence, in our system, we use n-gram features of size up to 3, i.e., unigrams, bigrams and trigrams. Including n-grams of larger size does not always lead to a performance increase due to the data sparseness problem [25]. Similar to the works of He et al. [11] and Giuliano et al. [26], the position information is appended to each word feature according to positions of words in a sentence relative to an investigated drug pair: before (BF), between (BE) and after (AF). For instance, “Interaction BF of BF ketamine and BE halothane in AF rats AF” where “ketamine” and “halothane” are two drug names.
2.1.2. Word pair features
While word features may capture repetitive expression patterns in neighboring words, they are not able to discover patterns involving distant words in a sentence. A simple solution to capture distant word patterns is to extract all possible word combinations from a training set. However, this approach increases the number of features considerably, and it also may degrade classification performance. To address this issue, we here propose a novel technique for selecting significant word pairs.
First, unigram word features are paired and only those pairs with a minimum frequency k are selected. Second, for selected word pairs, p-values are calculated using the hypergeometric distribution [27]. The p-value reflects how strongly a feature is represented in the positive set as compared to the negative set. It relates to the null hypothesis that the co-occurrence of two words is randomly distributed between positive and negative sets. If the co-occurrence is randomly distributed, the word pair will have a high p-value. If the p-value is low, this indicates a 1−p probability that the co-occurrence is not random and is likely indicative for positive DDIs.
To obtain the most useful word pairs, we need the least restrictive frequency and the most restrictive p-value. In this work, we set k = 200 and p-value = 0.01 based on F1 scores via 10-fold document-level cross validation on the training set. This significant p-value helps select 588 word pairs from a total of 449,826 pairs with k above 200. This feature set contains certain informative word pairs such as “drug1 ⋯ drug2 ⋯ increase ⋯ level” and “interaction ⋯ drug1 ⋯ with ⋯ drug2”, which provide a strong signal indicating DDIs.
2.1.3. Dependency graph features
Dependency graphs use nodes to represent words in a sentence and edges to describe governor-dependent relations between the words. Thus, they can capture long-range dependencies among sentential constituents by considerably narrowing the linear order distance between target entities [19]. Since the syntactic dependencies closely approximate the underlying semantic relationships, they have been effectively used by biomedical knowledge extraction systems [28, 16, 29, 30, 31].
While some approaches use an all-inclusive approach to explore paths of all possible lengths between any two nodes in a dependency graph [32, 15], the shortest path between two nodes is particularly likely to carry the most valuable information about their mutual relationship [33, 34, 35, 36]. Given the dependency graph of each sentence, therefore, the shortest dependency path connecting the target drugs in the undirected version of the graph is selected. If there exist multiple shortest paths, we randomly choose one. The extracted path is then transformed into an ordered sequence of individual dependency relations, in which original relation labels and edge orientations are appropriately preserved. For instance, a dependency path “ketamine interacts halothane” is encoded as “nsubj(interacts,ketamine); prep with(interacts,halothane)”. We further split a dependency path into n-grams of up to size 3. Compared to the vertex-walks based q-grams proposed by Kuboyama et al. [37], our dependency features are equivalent to q-bigrams in their work.
2.1.4. Parse tree features
We have observed in the training data of the DDIExtraction 2013 challenge [8] that the textual descriptions of more than 25% of the total 4,023 interacting drug-drug pairs involve different subordinate clauses of a sentence such as “If additional adrenergic drugs are to be administered by any route, they should be used with caution because the pharmacologically predictable sympathetic effects of BROVANA may be potentiated.”, or appear in the main sentence and its subordinate clause for instance “When carbamazepine is withdrawn from the combination therapy, aripiprazole dose should then be reduced.” Capturing these grammatical patterns is thus important to the successful extraction of these interactions. Compared to constituent parse trees which inherently retain phrasal and clausal structures, dependency graphs do not explicitly preserve this rich syntactic information.
It has been shown that systems exclusively relying on parse trees obtain inferior results to using dependency graphs in information extraction tasks [31, 16]. However, combining information from both representations improves the overall performance [38, 11]. To supplement our dependency features, we extracted the shortest path connecting the two investigated drugs in the parse tree. The resulting path is a sequence of grammatical tags such as “NP S VP VP SBAR S VP PP NP”, representing a concise syntactic traverse from one drug to the other. To capture frequent syntactic patterns, n-grams over individual tags of size 3 are used as our parse tree features. Unlike word features and dependency graph features, unigrams and bigrams are not used for parse tree features because these patterns are too short to represent syntactic structures.
2.1.5. Noun phrase-constrained coordination features
Linguistically, relationships are rarely discussed among entities in syntactic constituents where 3 or more target entities appear in a coordination. For instance, in the sentence “Clidinium may decrease the effect of phe- nothiazines, levodopa, and ketoconazole.”, the coordination structure “phenothiazines, levodopa, and ketoconazole” is used to enumerate a list of drugs that potentially interact with “Clidinium’, with no indication of interactions among the drugs inside the coordination.
In this work, we use base noun phrases to constrain the scope of the coordinated drug mentions, and propose a novel, noun phrase-constrained coordination feature to indicate if the target drugs are coordinated in a noun phrase. Suppose the total number of drug mentions in a base noun phrase NPb is n, the new feature fc for each candidate DDI pair (d1, d2) is defined as follows:
| (1) |
Because of the cascaded structure, candidate noun phrases are recursively extracted from the constituent parse trees. A base noun phrase NPb is defined to be the longest noun phrase that does not contain any prepositional phrases (PP), verb phrases (VP), subordinate clauses (SBAR) or sentences (S). We observed only 16 interacting drug-drug pairs (0.2%) among the total 8,045 pairs satisfying fc = 1 in the DDIExtraction 2013 training data.
2.2. Preprocessing
Several standard preprocessing steps are first completed on both training and test data. These include sentence segmentation and tokenization, Part-of-Speech (POS) tagging and syntactic parsing that produces constituent parse trees and dependency graphs for sentences [39, 40]. To ensure generalization of the features, drug mentions are anonymized using “DRUG” for target drugs and “DRUG OTHER” for other drugs. Numbers are replaced by a generic tag “NUM”, and other tokens normalized into their corresponding lemmas by the BioLemmatizer [41].
The same drug mentions can appear multiple times in a sentence. Considering that drugs are unlikely to interact with themselves, candidate pairs with both drugs referring to the same name are removed [42, 12]. This helps reduce candidate drug pairs. In addition, we notice that drug names are sometimes separated by a colon from the detailed description on their interactions with other drugs. For instance, “Morphine: Combination hormonal contra- ceptives may increase the clearance of morphine.” In such cases, as the description itself is an independent sentence, pairing “Morphine” on the left of the colon with drug mentions in the description may interfere with the narrative flow of the description. Thus, we remove the drug mentions on the left of the colon from further consideration.
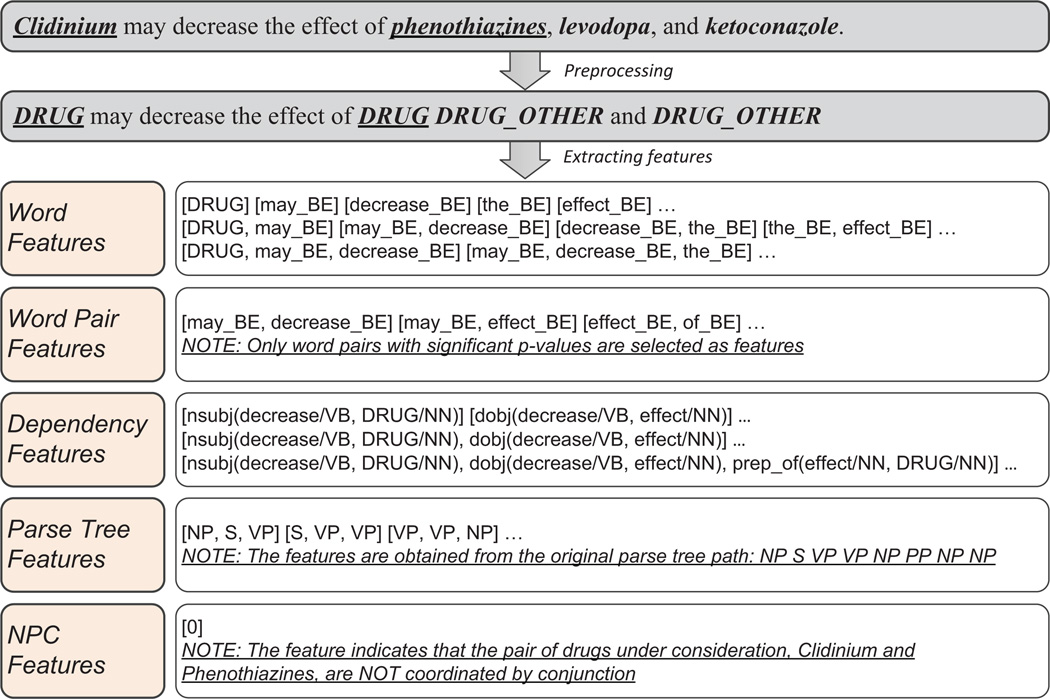
Figure 2 shows the preprocessing step and the feature vector obtained for an example sentence.
Figure 2.
An example of preprocessing and feature extraction. The underlined drug pair, clidinium and phenothiazines, is the candidate DDI. ‘NPC’ means noun phrase-constrained coordination and ‘BE’ denotes between candidate drugs.
2.3. SVM Classifier
For DDI detection and classification, we use an SVM classifier with the modified Huber loss function [24]. We have observed that the modified Huber loss function has consistently achieved better performance than the hinge loss function used in traditional SVMs for biomedical classification problems [43, 25, 44]. Let T denote the size of the training set. Let the binary feature vector of the ith pair in the training set be denoted by Xi. Let yi = 1 if the pair is annotated as positive and yi = −1 otherwise. Let w denote a vector of feature weights, of the same length as Xi. Let θ denote a threshold parameter, and let λ denote a regularization parameter. Then the cost function is given by:
| (2) |
where the function h is the modified Huber loss function defined as follows:
| (3) |
The values of the parameters, w and θ minimizing C are determined using a gradient descent algorithm. The regularization parameter λ is computed from the training set as follows:
| (4) |
where 〈|x|〉 is the average Euclidean norm of the feature vectors in the training set. The parameter λ′ is set to 0.00001 for the DDI task.
2.4. Comparison with existing DDI extraction systems
Our linear SVM classifier relies on a set of general features to achieve the state-of-the-art performance. Word-level features, dependency graphs and parse trees are commonly used by relation extraction systems [31, 17, 12, 11]. Compared to the implicit use in non-linear kernel systems [11, 12], features extracted from dependency graphs and parse trees are represented as linear n-grams in our system. As the only linear kernel system of the top 3 DDIExtraction 2013 teams, UTurku [21] makes intensive use of dependency graph features, but does not take advantage of the rich syntactic information in parse trees. In our system, both are used to complement each other for multiple aspects of structural analysis of sentences.
The noun phrase-constrained coordination and word pair features are novel in our DDI extraction approach. Chowdhury and Lavelli [11] aimed for general conjunction structures around investigated drugs by encoding relative distances into features. However, our coordination feature is able to explicitly capture the coordination structure of enumerated drugs, and semantically constrain the scope of the structure by noun phrases. Also, even though Bobić et al. [45] combined non-adjacent tokens across sections (“BF”, “BE” and “AF”), they used all the combinations without any feature selection. We find that the feature selection using p-values is effective for reducing the data complexity and for improving the DDI extraction performance.
In addition, Björne et al. [21] took advantage of domain knowledge derived from DrugBank [2] and MetaMap [46]. Besides domain-specific resources, He et al. [11] additionally asked domain experts to manually compile keyword and semantic type features. Considering that our features originate exclusively from training data and their extraction is domain independent, our feature types may be more generalizable to other relation extraction tasks.
3. Results and Discussion
3.1. Dataset
We train and evaluate the proposed approach on the DDI corpus from the DDIExtraction 2013 challenge [8]. The DDI corpus includes 905 manually annotated documents from the DrugBank database and MEDLINE abstracts, which are split into 714 and 191 documents for training and test sets, respectively. The DDI set provides examples by sentences and, for each sentence, all drug mentions and DDI pairs are annotated. There are four different types of DDI relationships in the set [22]; mechanism, effect, advice and int. Mechanism is used for DDIs that are described by their pharmacokinetic (PK) mechanism2. Effect is for DDIs describing an effect or a pharmacodynamic (PD) mechanism3. Advice is used when a recommendation or advice related to a DDI is given. Int is used when a DDI appears in a sentence without providing any additional information.
Non-interacting drug pairs are not explicitly provided in the DDI corpus. Hence, all drug pairs that do not overlap with positives are considered as negatives. Table 1 shows the number of positive and negative pairs before and after preprocessing. Removing pairs with the same drug mentions and the colon case described in Preprocessing filters out 29 positive and 3,972 negative pairs. The removed positive pairs constitute 0.58% of the positive set. We find the fraction to be negligible compared to the advantage of not showing nearly 14% negative pairs to SVM classifiers. In a basic setting where only word features are used, this step improves F1 by 4% using 10-fold document-level cross validation on the training set.
Table 1.
Number of positive and negative pairs in the dataset. 29 positive and 3972 negative pairs were removed through preprocessing.
| Original set | Preprocessed set | |||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| Training | 4023 | 23756 | 3996 | 20368 |
| Test | 979 | 4734 | 977 | 4150 |
| Total | 5002 | 28490 | 4973 | 24518 |
Table 2 shows the number of positive DDI pairs in the training and test sets for each DDI type. Mechanism and effect are dominant classes, while advice and int contain many fewer instances comprising about 25% of the positive set. This unbalanced size in the training data may be problematic, in particular, for a machine learning solution because it may lead to poor classification performance [47]. To address the issue, we apply the one-against-one approach for the DDI type classification task. Compared to the one-against-all strategy which takes negative examples from all non-positive classes, the one-against-one strategy uses only one negative class for each classifier; it alleviates the imbalance.
Table 2.
Positive drug pairs used for training and testing. The preprocessed set is compared with the original set.
| Class | Original set | Preprocessed set | ||
|---|---|---|---|---|
| Training | Test | Training | Test | |
| mechanism | 1321 | 302 | 1309 | 301 |
| effect | 1688 | 360 | 1675 | 359 |
| advice | 826 | 221 | 824 | 221 |
| int | 188 | 96 | 188 | 96 |
| Total | 4023 | 979 | 3996 | 977 |
3.2. Performance comparison
Eight teams participated in the DDIExtraction 2013 challenge, and the official performance ranged from 21.4% to 65.1% F1 [8]. Table 3 compares our method with the top three ranking teams in the DDIExtraction task based on F1 scores. Our approach achieves 67% F1 for detection and classification performance (‘CLA’), whereas FBK-irst, WBI and UTurku produced 65.1%, 60.9% and 59.4% F1, respectively. For DDI detection performance (DEC), i.e. before applying the one-against-one strategy, the proposed approach performs second best by achieving 77.5% F1.
Table 3.
Performance comparison between the proposed method and top-ranking approaches on the DDIExtraction 2013 test data. The performance is measured based on F1 scores. ‘CLA’ indicates detection and classification performance for all classes. ‘DEC’ indicates detection performance. ‘MEC’, ‘EFF’, ‘ADV’ and ‘INT’ are for mechanism, effect, advice and int types respectively.
| Method | CLA | DEC | MEC | EFF | ADV | INT |
|---|---|---|---|---|---|---|
| Our method | 0.670 | 0.775 | 0.693 | 0.662 | 0.725 | 0.483 |
| FBK-irst | 0.651 | 0.800 | 0.679 | 0.628 | 0.692 | 0.547 |
| WBI | 0.609 | 0.759 | 0.618 | 0.610 | 0.632 | 0.510 |
| UTurku | 0.594 | 0.696 | 0.582 | 0.600 | 0.630 | 0.507 |
FBK-irst [12] uses a hybrid kernel for combining linear features, shallow linguistic and path-enclosed tree kernels. WBI [13] utilizes an ensemble approach to combine outputs from other DDI prediction tools. UTurku [21] uses a linear kernel with domain knowledge from external resources as well as word and dependency graph features. For DDI type classification, FBK-irst uses binary SVMs with a one-against-all strategy. WBI and UTurku use a multi-class SVM, which does not require choosing either one-against-one or one-against-all. Our method, on the other hand, uses a simple binary SVM classifier with linear kernel for identifying DDIs and the one-against-one strategy for assigning DDI types. We choose the one-against-one strategy to reduce the negative effect of unbalanced classes. In Table 3, our approach performs best for mechanism, effect and advice types. In contrast, the same approach does not perform well for int. This is different from the 10-fold document-level cross validation results for the training set (Refer to 3.4). By definition, int contains DDIs which cannot be assigned to other three types. Thus, either the general description of int or insufficient evidence from the small number of training (188 examples) and testing (96 examples) sets may play a role.
Furthermore, Table 8 shows the separate performance of our system on DrugBank and MEDLINE test documents. While the DDI detection and classification (‘CLA’) performance on the DrugBank set shows 69.8% F1, the performance on the MEDLINE set is substantially lower (38.2% F1). This difference is consistent with the results from the DDIExtraction 2013 challenge [8]. It may be due to the small number of training examples provided for MEDLINE. The 232 DDI pairs in the MEDLINE training set constitute only 6% of the overall training data. In addition, the DrugBank and MEDLINE documents may have different characteristics [12].
Table 8.
Performance comparison (F1 scores) of the one-against-all and one-against-one strategies on the test set.
| Class | one-against-all | one-against-one |
|---|---|---|
| mechanism | 0.673 | 0.693 |
| effect | 0.671 | 0.662 |
| advice | 0.718 | 0.725 |
| int | 0.440 | 0.483 |
In the following subsections, we discuss the contribution of each feature type and the effect of the one-against-one strategy compared to the one-against-all strategy.
3.3. Feature analysis
Table 5 presents changes of DDI detection performance by adding each feature type to the baseline (word features). For the results, 10-fold document-level cross validation was performed ten times and the scores were averaged. Relative positions attached to word features improve the F1 performance by 24.1%. This significant improvement is understandable because relative position is a good indicator whether an individual word is used in describing DDIs.
Table 5.
Performance changes by varying feature types in DDI detection. The baseline performance was measured by using word features with position (‘pos’) information. ‘Change’ shows the F1 score difference between the baseline and the performance in each row. 10-fold document-level cross-validation was performed ten times for the training set and scores were averaged.
| Features | Precision | Recall | F1 | Change |
|---|---|---|---|---|
| Baseline (w/o pos) | 0.544 | 0.427 | 0.478 | −24.1% |
| Baseline | 0.774 | 0.670 | 0.719 | - |
| + Word pairs | 0.780 | 0.700 | 0.738 | +1.9% |
| + Dependency | 0.791 | 0.669 | 0.725 | +0.6% |
| + Parse trees | 0.783 | 0.688 | 0.733 | +1.4% |
| + NPC | 0.771 | 0.681 | 0.723 | +0.4% |
| All features | 0.798 | 0.711 | 0.752 | +3.3% |
Using word features with positions as a baseline, word pairs, dependency relations, parse trees and NPC are added and evaluated individually. From the table, word pairs and parse trees contribute the most by increasing F1 by 1.9% and 1.4%, respectively. Dependency relations and NPC have less impact on the performance, however, dependency relations help get higher precision and NPC helps the recall. While word features cover neighboring words, syntactic structure and word pair features seem to help with the overall picture of DDI sentences. It is understandable, yet remarkable that using words with relative positions alone achieves such high performance for identifying DDIs. Integrating position information into word features is important because one sentence often involves multiple drug mentions and the position information helps differentiate the context of interacting pairs from that of non-interacting ones. It would be interesting to see how the same strategy would work on other entity-entity relationship extraction tasks.
An advantage of using the linear kernel approach is that obtained results have an intuitive interpretation. Although Table 5 provides some information, it still lacks in explaining what features contribute to identify a particular DDI in a sentence. Figure 3 shows a simple visual aid, where significant words are highlighted based on the weights of word and word pair features from the SVM classifier. The features listed are the ones that classify the example as positive. The words with higher weights are emphasized by thicker lines and darker gray. From the highlighted words, one can understand that “DRUG with DRUG” and “not recommended” are the key elements for detecting a DDI in the sentence.
Figure 3.
A solution for presenting drug pairs with significant word and word pair features. Highly weighted words are highlighted in the sentence and emphasized according to all the weights they receive in the feature list. ‘BF’, ‘BE’ and ‘AF’ mean before, between and after, respectively. DRUG indicates a target drug.
3.4. DDI type detection
Our approach to DDI extraction has two steps. First, drug pairs are classified whether they interact or not. Second, one of four DDI types (mechanism, effect, advice and int) is assigned to interacting pairs.
Two popular ways to address multi-class classification using binary classifiers are the one-against-one and one-against-all strategies [23]. The one-against-all method builds a classifier for each class vs. all other classes. The one-against-one strategy, however, builds a binary classifier for each pair of classes, and the output of the classifiers is aggregated using majority voting. DDI type classification requires 4 and 12 classifiers for one-against-all and one-against-one, respectively.
A critical issue for the DDI type classification is that the number of training examples differs significantly among the four classes (Table 2). For the one-against-all strategy, this imbalance may lead to poor performance on the small classes. Therefore, we use the one-against-one strategy for the DDI classification task. Tables 6 and 7 present the performance difference between one-against-all and one-against-one. The performance is better and more balanced with the one-against-one strategy. Table 8 shows F1 scores for one-against-all and one-against-one on the test set. Although there is a slight performance decrease on effect, F1 scores increase on other DDI types.
Table 6.
Performance comparison on the one-against-all strategy. 10-fold document-level cross-validation was performed ten times for the training set and scores were averaged.
| Class | Precision | Recall | F1 |
|---|---|---|---|
| mechanism | 0.911 | 0.774 | 0.837 |
| effect | 0.885 | 0.839 | 0.861 |
| advice | 0.894 | 0.830 | 0.861 |
| int | 0.725 | 0.495 | 0.587 |
Table 7.
Performance comparison on the one-against-one strategy. 10-fold document-level cross-validation was performed ten times for the training set and scores were averaged.
| Class | Precision | Recall | F1 |
|---|---|---|---|
| mechanism | 0.941 | 0.964 | 0.952 |
| effect | 0.943 | 0.979 | 0.960 |
| advice | 0.921 | 0.960 | 0.940 |
| int | 0.912 | 0.953 | 0.932 |
3.5. Annotation inconsistency
The proposed method is completely data-driven. Even though SVM classifiers are robust to noisy training examples to some degree, our approach is sensitive to the quality of the training set.
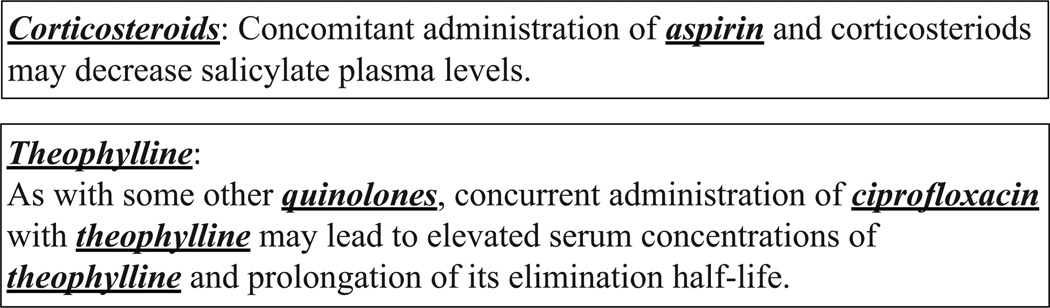
During algorithm development, we found that the DDI corpus contained irregularly formatted sentences. In one case, section titles are concatenated with the next sentence as shown in Fig. 4. A more severe problem can occur when tables are converted to text. Sentences derived from tables can cause false positive and false negative drug pairs. Positive drug pairs from a table are not useful because the proposed approach is designed for grammatically well-formed text. In our experiments, we kept the table-derived sentences because no rules could be found to remove them. We presume that DrugBank sentences were automatically extracted from HTML or XML data prior to annotation, or curators annotated the dataset in a structured form and it was later flattened by an automatic extraction process.
Figure 4.
Example sentences which start with “[drug name]:”. “[drug name]:” is a section title which is concatenated with the next sentence in the DDIExtraction set.
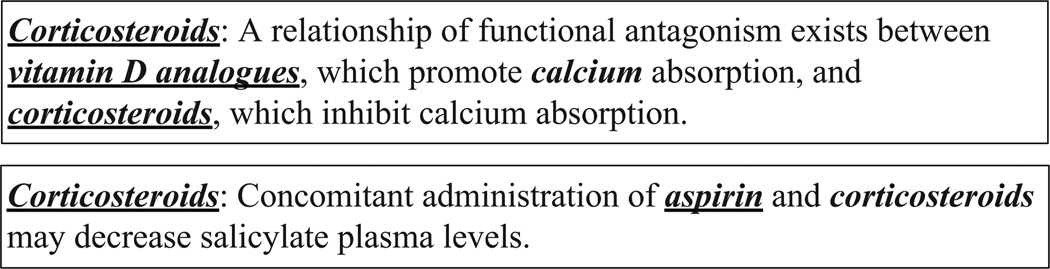
Another problem in the dataset is that not all drug mentions are annotated. In Figure 5, “calcium” in the first sentence and the second “corticosteroids” in the second sentence are not annotated as drug names. It is obvious that “corticosteroids” in the second example is overlooked by curators. However, it is difficult to decide whether “calcium” is a drug name here. While “calcium” is often annotated as a drug in the dataset, there are also exceptions where “calcium” is not considered a drug. We assume that it is either overlooked or not considered a drug.
Figure 5.
Example sentences, where some drug names are not annotated. “calcium” in the first sentence and the second “corticosteroids” in the second sentence are not annotated as drug names.
4. Conclusion
We present a two-step classification algorithm for identifying DDIs from biomedical literature. Unlike other state-of-the-art approaches, the proposed method focuses on word and syntactic features in a linear SVM. For assigning DDI types to drug pairs, positive DDI pairs are first identified by a single SVM classifier, and multiple SVM classifiers are used to decide DDI types through the one-against-one strategy in the second step. The features used in our approach are words with relative positions, pairs of non-adjacent words, dependency relations, syntactic structures and noun phrase-constrained co-ordination tags. Applied to the DDIExtraction corpus, the proposed method showed competitive performance to top-ranking teams in the DDIExtraction 2013 challenge by obtaining 67% F1.
The main contribution of the proposed method is the rich-feature based approach using linear SVMs. Non-linear, composite kernel approaches can directly use structural information. However, they tend to be complex and may not be readily applicable to a large-scale dataset. Our feature-based approach, on the other hand, is more flexible. Feature types can be easily evaluated, hence an intuitive interpretation is readily available. The linear kernel approach is also a practical alternative for large-scale problems. Moreover, the one-against-one strategy used in the DDI type classification is found to be useful for achieving higher classification performance. It addresses some difficulties of solving multi-class classification with binary classifiers. As future work, we plan to extend our system by integrating it with named entity recognition tools. We also would like to evaluate the end-to-end DDI extraction system for PubMed® abstracts in a large-scale setting.
Table 4.
Performance comparison between DrugBank and MEDLINE test sets in DDIExtraction 2013. The performance is measured based on F1 scores. ‘CLA’ indicates detection and classification performance for all classes. ‘DEC’ indicates detection performance. ‘MEC’, ‘EFF’, ‘ADV’ and ‘INT’ are for mechanism, effect, advice and int types respectively.
| Dataset | CLA | DEC | MEC | EFF | ADV | INT |
|---|---|---|---|---|---|---|
| DrugBank | 0.698 | 0.804 | 0.714 | 0.706 | 0.736 | 0.497 |
| MEDLINE | 0.382 | 0.471 | 0.455 | 0.352 | 0.429 | 0.250 |
Highlights.
An urgent need for developing text mining techniques in DDI extraction.
Linear kernel approaches have not achieved the state-of-the-art performance.
We propose an efficient and scalable system using a linear kernel for DDI extraction.
Linear SVMs are competitive when equipped with rich lexical and syntactic features.
One-against-one approach addresses an imbalance issue in multi-class classification.
Acknowledgments
The authors thank Isabel Segura-Bedmar for her help evaluating our preliminary results on the DDIExtraction 2013 dataset. Funding: This research was supported by the Intramural Research Program of the NIH, National Library of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
What the body does to the drug; absorption, distribution, metabolism, elimination.
What the drug does to the body.
Contributor Information
Sun Kim, Email: sun.kim@nih.gov.
Haibin Liu, Email: haibin.liu@nih.gov.
Lana Yeganova, Email: lana.yeganova@nih.gov.
W. John Wilbur, Email: wilbur@ncbi.nlm.nih.gov.
References
- 1.Baxter K, Preston CL, editors. Stockley’s Drug Interactions. London, UK: Pharmaceutical Press; 2013. [Google Scholar]
- 2.Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS. DrugBank 3.0: a comprehensive resource for ‘Omics’ research on drugs. Nucleic Acids Research. 2011;39(Suppl 1):D1035–D1041. doi: 10.1093/nar/gkq1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duda S, Aliferis C, Miller R, Statnikov A, Johnson K. Extracting drug-drug interaction articles from MEDLINE to improve the content of drug databases; AMIA Annual Symposium Proceedings; 2005. pp. 216–220. [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin DL, Thorn CF, Klein TE, Altman RB. A statistical approach to scanning the biomedical literature for pharmacogenetics knowledge. Journal of the American Medical Informatics Association. 2005;12(2):121–129. doi: 10.1197/jamia.M1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segura-Bedmar I, Martínez P, de Pablo-Sánchez C. A linguistic rule-based approach to extract drug-drug interactions from pharmacological documents. BMC Bioinformatics. 2011;12(Suppl 2):S1. doi: 10.1186/1471-2105-12-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segura-Bedmar I, Martínez P, de Pablo-Sánchez C. Using a shallow linguistic kernel for drugdrug interaction extraction. Journal of Biomedical Informatics. 2011;44(5):789–804. doi: 10.1016/j.jbi.2011.04.005. doi: http://dx.doi.org/10.1016/j.jbi.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Segura-Bedmar I, Martínez P, Sánchez-Cisneros D. The 1st DDIExtraction-2011 challenge task: Extraction of drug-drug interactions from biomedical texts. Proceedings of the 1st Challenge Task on Drug-Drug Interaction Extraction (DDIExtraction 2011) 2011:1–9. [Google Scholar]
- 8.Segura-Bedmar I, Martinez P, Herrero-Zazo M. SemEval-2013 Task 9: Extraction of drug-drug interactions from biomedical texts. Proceedings of the 7th International Workshop on Semantic Evaluation (SemEval 2013) 2013:341–350. [Google Scholar]
- 9.Thomas P, Neves M, Solt I, Tikk D, Leser U. Relation extraction for drug-drug interactions using ensemble learning. Proceedings of the 1st Challenge Task on Drug-Drug Interaction Extraction (DDIExtraction 2011) 2011:11–18. [Google Scholar]
- 10.Chowdhury MFM, Lavelli A. Drug-drug interaction extraction using composite kernels. Proceedings of the 1st Challenge Task on Drug- Drug Interaction Extraction (DDIExtraction 2011) 2011:27–33. [Google Scholar]
- 11.He L, Yang Z, Zhao Z, Lin H, Li Y. Extracting drug-drug interaction from the biomedical literature using a stacked generalization-based approach. PLoS One. 2013;8(6):e65814. doi: 10.1371/journal.pone.0065814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhury MFM, Lavelli A. FBK-irst: A multi-phase kernel based approach for drug-drug interaction detection and classification that exploits linguistic information. Proceedings of the 7th International Workshop on Semantic Evaluation (SemEval 2013) 2013:351–355. [Google Scholar]
- 13.Thomas P, Neves M, Rocktäschel T, Leser U. WBI-DDI: Drug-drug interaction extraction using majority voting. Proceedings of the 7th International Workshop on Semantic Evaluation (SemEval 2013) 2013:628–635. [Google Scholar]
- 14.Collins M, Duffy N. Convolution kernels for natural language. Advances in Neural Information Processing Systems (NIPS 2001) 2001:625–632. [Google Scholar]
- 15.Zhang Y, Lin H, Yang Z, Wang J, Li Y. A single kernel-based approach to extract drug-drug interactions from biomedical literature. PLoS One. 2012;7(11):e48901. doi: 10.1371/journal.pone.0048901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tikk D, Thomas P, Palaga P, Hakenberg J, Leser U. A comprehensive benchmark of kernel methods to extract protein–protein interactions from literature. PLoS Computational Biology. 2010;6:e1000837. doi: 10.1371/journal.pcbi.1000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Björne J, Salakoski T. Generalizing biomedical event extraction. Proceedings of BioNLP Shared Task 2011 Workshop; Association for Computational Linguistics; Portland, Oregon, USA. 2011. pp. 183–191. [Google Scholar]
- 18.Björne J, Salakoski T. TEES 2.1: Automated annotation scheme learning in the BioNLP 2013 shared task. Proceedings of the BioNLP Shared Task 2013 Workshop; Association for Computational Linguistics; Sofia, Bulgaria. 2013. pp. 16–25. [Google Scholar]
- 19.Björne J, Heimonen J, Ginter F, Airola A, Pahikkala T, Salakoski T. Extracting complex biological events with rich graph-based feature sets; Proceedings of the Workshop on BioNLP (BioNLP ’09), Association for Computational Linguistics; 2009. pp. 10–18. [Google Scholar]
- 20.Björne J, Ginter F, Pyysalo S, Tsujii J, Salakoski T. Scaling up biomedical event extraction to the entire PubMed. Proceedings of the 2010 Workshop on Biomedical Natural Language Processing (BioNLP ’10); ACL; 2010. pp. 28–36. [Google Scholar]
- 21.Björne J, Kaewphan S, Salakoski T. UTurku: Drug named entity recognition and drug-drug interaction extraction using SVM classification and domain knowledge. Proceedings of the 7th International Workshop on Semantic Evaluation (SemEval 2013) 2013:651–659. [Google Scholar]
- 22.Herrero-Zazo M, Segura-Bedmar I, Martnez P, Declerck T. The DDI corpus: An annotated corpus with pharmacological substances and drugdrug interactions. Journal of Biomedical Informatics. 2013;46(5):914–920. doi: 10.1016/j.jbi.2013.07.011. doi: http://dx.doi.org/10.1016/j.jbi.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Hsu C-W, Lin C-J. A comparison of methods for multiclass support vector machines. IEEE Transactions on Neural Networks. 2002;13(2):415–425. doi: 10.1109/72.991427. [DOI] [PubMed] [Google Scholar]
- 24.Zhang T. Solving large scale linear prediction problems using stochastic gradient descent algorithms; Proceedings of the 21st International Conference on Machine Learning (ICML 2004); 2004. pp. 919–926. [Google Scholar]
- 25.Kim S, Wilbur WJ. Classifying protein-protein interaction articles using word and syntactic features. BMC Bioinformatics. 2011;12(Suppl 8):S9. doi: 10.1186/1471-2105-12-S8-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuliano C, Lavelli A, Romano L. Exploiting shallow linguistic information for relation extraction from biomedical literature; Proceedings of the 11th Conference of the European Chapter of the Association for Computational Linguistics (EACL 2006); 2006. pp. 401–408. [Google Scholar]
- 27.Kim W, Wilbur WJ. Corpus-based statistical screening for content-bearing terms. Journal of the American Society for Information Science and Technology. 2001;52(3):247–259. [Google Scholar]
- 28.Fundel K, Küffner R, Zimmer R. Relex—relation extraction using dependency parse trees. Bioinformatics. 2007;23(3):365–371. doi: 10.1093/bioinformatics/btl616. [DOI] [PubMed] [Google Scholar]
- 29.McClosky D, Charniak E, Johnson M. Automatic domain adaptation for parsing; Proceedings of the 2010 Annual Conference of the North American Chapter of the Association for Computational Linguistics (NAACL 2010), Association for Computational Linguistics; 2010. pp. 28–36. [Google Scholar]
- 30.Sagae K, Miyao Y, Matsuzaki T, Tsujii J. Challenges in mapping of syntactic representations for framework-independent parser evaluation. Proceedings of the Workshop on Automated Syntatic Annotations for Interoperable Language Resources. 2008 [Google Scholar]
- 31.Miyao Y, Sagae K, Saetre R, Matsuzaki T, Tsujii J. Evaluating contributions of natural language parsers to protein-protein interaction extraction. Bioinformatics. 2009;25(3):394–400. doi: 10.1093/bioinformatics/btn631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Airola A, Pyysalo S, Björne J, Pahikkala T, Ginter F, Salakoski T. All-paths graph kernel for protein-protein interaction extraction with evaluation of cross-corpus learning. BMC Bioinformatics. 2008;9(Suppl 11):1–12. doi: 10.1186/1471-2105-9-S11-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunescu RC, Mooney RJ. A shortest path dependency kernel for relation extraction; Proceedings of the Conference on Human Language Technology and Empirical Methods in Natural Language Processing; 2005. pp. 724–731. [Google Scholar]
- 34.Rinaldi F, Schneider G, Kaljurand K, Clematide S, Vachon T, Romacker M. OntoGene in BioCreative II.5. IEEE/ACM Transactions on Computational Biology and Bioinformatics. 2010;7(3):472–480. doi: 10.1109/TCBB.2010.50. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Hunter L, Keselj V, Verspoor K. Approximate subgraph matching-based literature mining for biomedical events and relations. PLoS One. 2013;8(4):e60954. doi: 10.1371/journal.pone.0060954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H, Verspoor K, Comeau DC, MacKinlay A, Wilbur WJ. Generalizing an approximate subgraph matching-based system to extract events in molecular biology and cancer genetics. Proceedings of the BioNLP Shared Task 2013 Workshop. 2013:76–85. [Google Scholar]
- 37.Kuboyama T, Hirata K, Kashima H, Aoki-Kinoshita KF, Yasuda H. A spectrum tree kernel. Information and Media Technologies. 2007;2(1):292–299. [Google Scholar]
- 38.Qian L, Zhou G. Tree kernel-based protein-protein interaction extraction from biomedical literature. Journal of Biomedical Informatics. 2012;45(3):535–543. doi: 10.1016/j.jbi.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 39.McClosky D, Charniak E. Self-training for biomedical parsing; Proceedings of the 46th Annual Meeting of the Association for Computational Linguistics on Human Language Technologies, The Association for Computer Linguistics; 2008. pp. 101–104. [Google Scholar]
- 40.de Marneffe M-C, Manning CD. The Stanford typed dependencies representation; CrossParser ’08: Coling 2008: Proceedings of the Workshop on Cross-Framework and Cross-Domain Parser Evaluation, Association for Computational Linguistics; 2008. pp. 1–8. [Google Scholar]
- 41.Liu H, Christiansen T, Baumgartner WA, Verspoor K. Biolemmatizer: a lemmatization tool for morphological processing of biomedical text. Journal of Biomedical Semantics. 2012;3:3. doi: 10.1186/2041-1480-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chowdhury MFM, Lavelli A. Impact of less skewed distributions on efficiency and effectiveness of biomedical relation extraction; Proceedings of the 24th International Conference on Computational Linguistics (COLING 2012); 2012. pp. 205–216. [Google Scholar]
- 43.Smith LH, Wilbur WJ. Finding related sentence pairs in MEDLINE. Information Retrieval. 2010;13(6):601–617. doi: 10.1007/s10791-010-9126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeganova L, Comeau DC, Kim W, Wilbur WJ. Text mining techniques for leveraging positively labeled data; Proceedings of the 2011 Workshop on Biomedical Natural Language Processing (BioNLP ’11), Association for Computational Linguistics; 2011. pp. 155–163. [Google Scholar]
- 45.Bobić T, Fluck J, Hofmann-Apitius M. SCAI: Extracting drug-drug interactions using a rich feature vector; Proceedings of the 7th International Workshop on Semantic Evaluation (SemEval 2013); 2013. pp. 675–683. [Google Scholar]
- 46.Aronson AR. Effective mapping of biomedical text to the UMLS Metathesaurus: the MetaMap program; AMIA Annual Symposium Proceedings; 2001. pp. 17–21. [PMC free article] [PubMed] [Google Scholar]
- 47.Chawla NV. Data Mining for Imbalanced Datasets: An Overview. New York, USA: Springer; 2005. [Google Scholar]