Abstract
Objective
To better understand why immunosuppressed individuals with systemic lupus erythematosus (SLE) fail to receive influenza and pneumococcal vaccines.
Methods
These cross-sectional data derive from the 2009 cycle of the Lupus Outcomes Study (LOS), an annual longitudinal telephone survey of individuals with confirmed SLE. Respondents were included in the analysis if they had taken immunosuppressive medications in the past year. We assessed any prior receipt of pneumococcal vaccine and receipt of influenza vaccine in the past year, then elicited reasons for not receiving vaccination. We used bivariate statistics and multivariate logistic regression to assess frequency and predictors of reported reasons for not obtaining influenza or pneumococcal vaccines.
Results
Among 508 respondents who received immunosuppressants, 485 reported whether they had received vaccines. Among the 175 respondents who did not receive an influenza vaccine, the most common reason was lack of doctor recommendation (55%), followed by efficacy or safety concerns (21%), and lack of time (19%). Reasons for not receiving pneumococcal vaccine (N=159) were similar: lack of recommendation (87%), lack of time (7%), and efficacy or safety concerns (4%). Younger, less-educated, non-white patients with shorter disease duration, as well as those immunosuppressed with steroids alone, were at greatest risk for not receiving indicated vaccine recommendations.
Conclusions
The most common reason why individuals with SLE did not receive pneumococcal and influenza vaccines was that physicians failed to recommend them. Data suggest that increasing vaccination rates in SLE will require improved process quality at the provider level, as well as addressing patient concerns and barriers.
Keywords: systemic lupus erythematosus, preventive care, vaccine, quality of care
BACKGROUND
Vaccine-preventable diseases remain common causes of morbidity and mortality in the United States. Nevertheless, in 2011-2012 only 50% of children and 40% of adults received an influenza vaccine (1). Therefore, improving vaccination rates in the general population has become a national health care priority, targeted by initiatives such as Healthy People 2020 and performance measurement programs such as the Physician Quality Reporting System and Meaningful Use.
As estimated 5-year survival in systemic lupus erythematosus (SLE) has improved from <50% to >95% over the past 50 years, preventive care has become increasingly important (2). Infection is now the third-leading cause of death in individuals with SLE in developed countries. Nearly half of those deaths are attributed to pneumonia, making vaccination against influenza and pneumococcus critical to prevention of mortality (3). Recent literature also suggests that hospitalizations for pneumonia among individuals with SLE are common and may be preventable (4). Currently, vaccination against pneumococcus and influenza is recommended for all immunosuppressed SLE patients (5).
Nonetheless, previous work has shown that only 50-60% of SLE patients receive indicated influenza and pneumococcus vaccinations, and only 40% are up-to-date on both vaccines (6). This is similar to findings in other chronic diseases such as rheumatoid arthritis, inflammatory bowel disease and diabetes (7, 8). Predictors of receiving vaccinations in previous studies have included older age, college education, increased physician visits, and lower disease activity. However, reasons why individuals with SLE fail to receive vaccines have not been previously explored. Causes may include lack of knowledge about vaccination recommendations, competing demands of complex SLE-related care, concerns about vaccine safety in immunocompromised hosts, lack of coordination among providers, and lack of access to vaccines.
The goal of this study was to explore provider-based (e.g. recommendation of vaccines), patient-based (e.g. vaccine beliefs) and health system-based (e.g. vaccine availability) reasons why immunosuppressed individuals with SLE fail to receive influenza and pneumococcal vaccines.
METHODS
Data Source
The study cohort consisted of 814 individuals participating in the 2009 Lupus Outcomes Study (LOS) survey, an ongoing longitudinal study of persons with SLE from the United States. Details regarding eligibility and enrollment of participants have been described elsewhere (9). Briefly, respondents were recruited from an existing cohort, the UCSF Lupus Genetics Project (10), developed from a combination of academic rheumatology clinics, community rheumatologists, and various non-clinical sources (e.g., support groups, conferences, newsletters, websites). All participants had a confirmed diagnosis of SLE according to chart review supervised by a rheumatologist. Respondents participated in annual structured telephone interviews containing validated items pertaining to demographic and socioeconomic characteristics, SLE disease activity and manifestations, medications, general health, mental health, cognition, employment, health care utilization, and health insurance coverage. Interviews are conducted throughout the year. The study was approved by the UCSF Committee on Human Research, and all participants provided written informed consent.
Measures
The primary outcome measure was patient-reported reason for failure to receive influenza or pneumococcal vaccine. We assessed any prior receipt of pneumococcal vaccine and receipt of influenza vaccine in the past year. Respondents who did not receive a vaccine were asked whether their physician had recommended it (Figure 1). If the vaccine was recommended but not received, interviewers elicited reasons for not receiving vaccination using categorical responses (efficacy or safety concerns, lack of time or motivation, vaccine availability, cost or access to care, and allergy). Responses not represented in the categories were recorded as free text and later categorized by two reviewers (EL, LT), who resolved differences by consensus.
Figure 1.
Survey questions assessing vaccination status in the 2009 wave of the Lupus Outcomes Study
Sociodemographic predictor variables included age, sex, race/ethnicity (non-Hispanic white vs. all other) and education (bachelor's degree or higher vs. lower education, given overall high educational attainment in the cohort).
Health insurance status was categorized as employer-sponsored, Medicare, Medicaid, or no insurance. We assessed whether respondents had visited a generalist MD (internist, internal medicine specialist, family doctor, or general practice doctor) in the past year, and whether they had visited a rheumatologist in the past year. Respondents were categorized as immunosuppressed if they reported use of steroid medications (oral or IV glucocorticoids), DMARD medications (azathioprine, mycophenolate mofetil, mycophenolic acid, methotrexate, tacrolimus, cyclosporine, leflunomide, cyclophosphamide), or biologic medications (etanercept, adalimumab, infliximab, abatacept or rituximab) in the past year. Based on reported use of immunosuppressive medications, we created three categories of immunosuppression for our primary analysis: 1) steroids alone, 2) DMARD with or without steroids, and 3) biologic with or without DMARD or steroids. Additional variables used to explore the effect of the intensity of immunosuppression included current steroid dose, low-intensity immunosuppression (prednisone ≤ 5 mg daily without use of other immunosuppressive drugs), high steroid dose (>10mg for >90 days), and high-intensity immunosuppression (high steroid dose with concomitant DMARD or biologic use).
Disease-related predictor variables included disease duration and disease activity assessed with a validated, self-report measure, the Systemic Lupus Assessment Questionnaire (SLAQ) (11). The influence of renal disease was explored but was not found to significantly alter the likelihood of vaccine recommendation in bivariate or multivariate analysis, and therefore was not included in the final models.
Study Sample
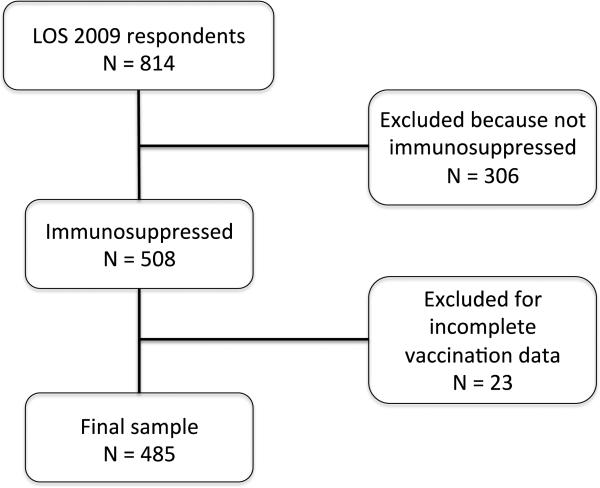
Respondents were included in analyses if they had taken any immunosuppressive medications in the past year (N=508), and therefore qualified for receipt of both influenza and pneumococcal vaccinations. We excluded those who failed to report whether they had received influenza and pneumococcal vaccines. Survey questions used to assess vaccination status and vaccine recommendation are displayed in Figure 2. Reasons for not receiving a vaccination were assessed among all individuals who reported having not received a vaccination (N=175 for influenza, N=159 for pneumococcal). When assessing predictors of receiving a vaccine recommendation, all individuals who had received a vaccination or reported physician recommendation of vaccination were included in the vaccine-recommended group (N=389 for influenza, N=346 for pneumococcal). These respondents were compared with individuals who did not receive vaccination and reported that their physician had not recommended vaccination (N=96 for influenza, N=139 for pneumococcal).
Figure 2.
Study sample from 2009 wave of the Lupus Outcomes Study
Statistical Analysis
Characteristics of the cohort and reasons for failure to receive vaccinations were assessed using summary statistics. We used bivariate statistics (Student's t-test, rank sum and chi-square test) to compare respondents who reported physician recommendation of vaccination with those who reported no recommendation. Multivariate logistic regression analysis, adjusted for age, gender, education, race, disease duration, disease activity, immunosuppression category (steroids alone, DMARD with our without steroids, and biologic with or without steroids or DMARD), employer-sponsored insurance, generalist MD visit in the past year and rheumatologist visit in the past year, was used to assess predictors of not receiving physician recommendation of influenza or pneumococcal vaccines. In sensitivity analyses, we examined alternate models for the steroid variables, including current steroid dose (also adjusting for DMARD and biologic use), low-intensity immunosuppression (prednisone ≤ 5 mg daily without use of other immunosuppressive drugs), high steroid dose (>10mg for >90 days, also adjusting for DMARD and biologic use), and high-intensity immunosuppression (high steroid dose with concomitant DMARD or biologic use). Models were assessed for colinearity, excessive influence of individual observations, and goodness of fit. All statistical analyses were performed using STATA 11.0.
RESULTS
Demographics and immunosuppression
The study included 485 respondents with SLE who had taken immunosuppressive medications in the past year. Mean age was 50 years, 93% were female, 60% were Caucasian, and mean disease duration was 18 years (Table 1). Oral or IV steroids had been taken by 88% of respondents in the past year (86% receiving oral steroids and 14% receiving IV steroids). Median current oral prednisone dose was 5 mg (range 0.5-100 mg). Only 2% had received IV steroids without any oral steroids. DMARDs had been taken by 55%, and 9% had taken biologic medications. Respondents excluded for missing data (N=23, 5%) were younger (mean age 39 v. 50) and more likely to be insured via Medicaid (20% v. 5%). There were no significant differences between groups with regard to gender, ethnicity, education, disease duration and health care utilization.
Table 1.
Respondent characteristics among immunosuppressed individuals in the 2009 wave of the Lupus Outcomes Study
| Variable | N = 485 N (%) unless noted |
|---|---|
| Demographics | |
| Age in years, mean ± SD | 50 ± 12 |
| Female | 451 (93) |
| Ethnicity | |
| White | 292 (60) |
| Latino | 50 (10) |
| African American | 56 (12) |
| Asian | 60 (12) |
| Other | 27 (6) |
| Bachelor's degree or higher attained | 199 (41) |
| Poverty^ | 74 (16) |
| SLE characteristics | |
| Age at diagnosis in years, median (range) | 31 (7-67) |
| Disease duration in years, median (range) | 16 (0-47) |
| Renal disorder | 152 (31) |
| SLAQ+, median (range) | 12 (0-46) |
| Immunosuppressant medications | |
| Any steroid & | 427 (88) |
| Current steroid dose, median (range) | 5 (0-100) |
| High-dose oral steroids~ | 47 (10) |
| Steroids alone | 202 (42) |
| Low dose steroids alone¢ | 110 (23) |
| DMARD# | 268 (55) |
| Azathioprine | 68 (14) |
| Mycophenolate mofetil | 126 (26) |
| Methotrexate | 75 (15) |
| Cyclosporine, tacrolimus or leflunomide | 55 (11) |
| Cyclophosphamide | 19 (4) |
| Biologic£ | 45 (9) |
| Rituximab | 16 (3) |
| Abatacept | 4 (1) |
| Etanercept | 28 (6) |
| Combination immunosuppression∞ | 25 (5) |
| Health care utilization characteristics | |
| Health insurance | |
| Employer-sponsored | 256 (53) |
| Medicare | 193 (40) |
| Medicaid | 25 (5) |
| None | 11 (2) |
| Generalist physician+ | 393 (81) |
| Rheumatologist* | 408 (84) |
| Influenza vaccination in past year | 310 (64) |
| Pneumococcal vaccination ever | 326 (67) |
Income below 125% of the U.S. poverty level based on household size.
Use of IV or oral steroids in the past year.
≥ 10 mg prednisone daily (or equivalent) for ≥ 90 days.
≤ 5 mg prednisone daily (or equivalent) without other immunosuppressant
Use of azathioprine, mycophenolate mofetil or mycophenolic acid, methotrexate, tacrolimus, cyclosporine or leflunomide in the past year.
Use of rituximab, abatacept or any TNF inhibitor in the past year.
Use of both high dose oral steroids and a DMARD or biologic medication in the past year.
Seen by an internist, internal medicine specialist, family doctor or general practice doctor in the past year.
Seen by a rheumatologist in the past year.
Reasons for failure to receive vaccination
For both influenza and pneumococcal vaccines, the most common reason for failure to receive a vaccination was lack of health care provider recommendation, N=96/175=55% for influenza vaccine and N=139/159=87% for pneumococcal vaccine (Figure 3). Among the 175 individuals who did not receive influenza vaccine, N=37/175=21% cited concerns about vaccine efficacy or safety, and N=33/175=19% cited lack of time or motivation as primary reasons for lack of vaccination. Among the 159 individuals who did not receive pneumococcal vaccine, only N=7/159=4% cited efficacy or safety concerns, and N=11/159=7% cited lack of time or motivation. For both vaccines, few individuals attributed not receiving the vaccination to lack of availability, cost, or allergy.
Figure 3.
Reasons for not receiving influenza and pneumococcal vaccines among immunosuppressed individuals with SLE*
Predictors of influenza vaccine recommendation
Respondents who reported a recommendation for influenza vaccine had longer disease duration (OR 1.04 per year, 95% CI 1.01-1.08, Table 2). They were also more likely to have a 4-year college degree (OR 1.5, 95% CI 0.9-2.5), although this difference did not reach statistical significance in the multivariable model. Individuals who were immunosuppressed with DMARD or biologic medications were more likely to receive a flu vaccine recommendation as compared to those receiving steroids alone, though in multivariate analysis this finding reached statistical significance only among individuals receiving DMARDs without a biologic (OR 1.9, 95% CI 1.1-3.3). However, in sensitivity analyses there was no difference in the likelihood of influenza vaccine recommendation based on current steroid dose, treatment with low-intensity immunosuppression, or treatment with high-intensity immunosuppression. There was also a greater likelihood of having seen a rheumatologist in the past year (86%, OR 1.8, 95% CI 1.0-3.4) among individuals who received an influenza vaccine recommendation.
Table 2.
Bivariate and multivariate predictors of physician recommendation of influenza and pneumococcal vaccines among immunosuppressed individuals with SLE
| Influenza Vaccine | Pneumococcal Vaccine | |||||
|---|---|---|---|---|---|---|
| Vaccine recommended (N = 389) |
Vaccine not recommended (N = 96) |
Adjusted odds ratio# |
Vaccine recommended (N=346) |
Vaccine not recommended (N=139) |
Adjusted odds ratio# |
|
| Variable | N (%) |
OR (95% CI) |
N (%) |
OR (95% CI) |
||
| Age, mean ± SD | 50 ± 12 | 48 ± 12 | 1.00 (0.98-1.03) | 51 ± 12* | 45 ± 12* | 1.04 (1.02-1.06)* |
| Female | 360 (92) | 91 (94) | 0.7 (0.3-2.0) | 346 (93) | 128 (92) | 1.2 (0.5-2.8) |
| Non-white ethnicity | 156 (40) | 37 (39) | 1.1 (0.7-1.8) | 124 (35)* | 69 (50)* | 0.7 (0.4-1.0) |
| Education | 166 (42) | 33 (34) | 1.5 (0.9-2.5) | 143 (41) | 56 (40) | 1.5 (0.9-2.3) |
| Disease duration in years, median (range) | 16 (0-47)* | 14 (1-37)* | 1.04 (1.01-1.08)* | 17 (0-47)* | 14 (1-43)* | 1.00 (0.97-1.03) |
| SLAQ, median (range) | 11 (0-46) | 12 (0-33) | 0.99 (0.96-1.02) | 12 (0-46) | 11 (0-33) | 1.00 (0.97-1.03) |
| Immunosuppress ant medications in past year | ||||||
| Glucocorticoids only | 152 (39) | 50 (52) | ref | 137 (40)* | 65 (47)* | ref |
| DMARDs +/− glucocorticoids + | 198 (51) | 37 (39) | 1.9 (1.1-3.1)* | 166 (48)* | 69 (49)* | 1.7 (1.1-2.6)* |
| Biologic +/− glucocorticoids DMARDs∞ | 36 (9) | 9 (9) | 1.5 (0.7-3.4) | 40 (12)* | 5 (4)* | 4.9 (1.8-13.8)* |
| Employer-sponsored insurance | 199 (51) | 57 (59) | 0.6 (0.4-1.1) | 47 (47)* | 67 (93)* | 0.5 (0.3-0.8)* |
| Generalist physician^ | 315 (81) | 78 (81) | 1.1 (0.6-2.0) | 289 (84)* | 104 (75)* | 1.3 (0.7-2.2) |
| Rheumatologist^ | 332 (86) | 76 (79) | 1.8 (1.0-3.4) | 290 (84) | 118 (84) | 1.1 (0.6-1.9) |
P < 0.05
Odds ratios adjusted for all variables shown.
~ Use of IV or oral steroid in the past year.
+ Use of azathioprine, mycophenolate mofetil or mycophenolic acid, methotrexate, tacrolimus, cyclosporine, leflunomide, or cyclophosphamide in the past year.
Use of a TNF inhibitor, abatacept or rituximab in the past year.
Visit to provider in the past year.
Predictors of pneumococcal vaccine recommendation
As expected because of age-based national recommendations, older individuals were more likely to receive a pneumococcal vaccine recommendation in both adjusted and unadjusted analysis (mean age 51 v. 45 years, OR 1.04 per year, 95% CI 1.02-1.06). Individuals who received a pneumococcal vaccine recommendation were also more likely to be of white ethnicity (OR 1.5, 95% CI 1.0-2.4), though this finding only approached statistical significance..With regard to immunosuppression, there was a clear trend toward increasing likelihood of vaccine recommendation among individuals receiving DMARDs (OR 1.6, 95% CI 1.1-2.6) and biologics (OR 4.9, 95% CI 1.8-13.8) as compared to those taking steroids alone. In sensitivity analyses, individuals treated with low-intensity immunosuppression were significantly less likely to receive a pneumococcal vaccine recommendation as compared to respondents receiving more intense immunosuppression (OR 0.4, 95% CI 0.2-0.8). There was no difference in the likelihood of pneumococcal vaccine recommendation based on current steroid dose or treatment with high-intensity immunosuppression.
CONCLUSION
Among immunosuppressed individuals with SLE who did not receive recommended influenza and pneumococcal vaccines, the most common reason was lack of health care provider recommendation. While some patients cited concerns about vaccine efficacy and safety, or lacked time to obtain vaccination, these were far less common causes. Difficulty accessing vaccines was also not a common obstacle. Previous work done by our group assessing vaccine receipt in the LOS as compared to the general population and a sample with non-rheumatic chronic conditions showed similar vaccination rates among groups (6). Predictors of failure to receive vaccine recommendation in the current study were parallel to previously identified predictors of receipt of influenza and pneumococcal vaccination among individuals with SLE: younger, less-educated, non-white patients with shorter disease duration, as well as those immunosuppressed with steroids in the absence of DMARD or biologic medications, were at greatest risk for not receiving indicated recommendations for vaccination.
There are several potential explanations for these findings. Providers may not communicate the need for vaccination effectively to their patients. SLE care is complex, and vaccination may not be addressed when other issues take the forefront during a busy clinic visit. Primary care providers may not be aware of the need for all immunosuppressed lupus patients to be vaccinated against pneumococcus and influenza, regardless of patient age, which is supported by our finding that individuals who had seen a rheumatologist in the past year were more likely to have received an influenza vaccine recommendation. Perceived degree of immunosuppression also appears to influence the likelihood of vaccine recommendation, even though CDC recommendations do not differ based on degree of immunosuppression. We found that individuals immunosuppressed with steroids alone (regardless of dosage) were less likely to receive a vaccine recommendation as compared to those receiving DMARDs or biologic medications, which may be due to a perception that the former group are at less risk for infection. While individuals receiving low-dose steroids may be at less risk for infection as compared to those receiving therapy with DMARDs, biologics, or a combination, research shows that steroid therapy, even at low doses, can increase risk of serious infections among lupus patients, and in fact may put patients at greater risk compared to treatment with other DMARD medications (12, 13). Further research is needed to better elucidate the impact of different immunosuppressive drugs and dosages on infection risk, in order to provide evidence to evaluate the practice identified in this study: more frequent recommendation of vaccination for patients immunosuppressed with DMARDs and biologics as compared to those treated with steroids alone.
Concerns have been raised in the past over the safety and efficacy of vaccinations in SLE patients (14), but based on numerous studies over the past 30 years, vaccinations against influenza and pneumococcus appear to be safe and efficacious in this population (14). Post-vaccination antibody titers are generally reduced in SLE patients as compared to healthy controls, but are still adequate for protection in a significant majority of patients. Specifically, studies have shown that approximately 80% of SLE patients vaccinated with the 23-valent polysaccharide pneumococcal vaccine reach protective specific antibody levels. There appears to be greater variation in response to influenza vaccine, with some studies reporting a significantly lower rate of seroconversion as compared to healthy controls, and others reporting a similar response between SLE patients and controls (15). Given this potential for reduced response to vaccination, patients should be vaccinated prior to starting or increasing immunosuppression when possible.
Since SLE patients are known to have compromised cellular and humoral immune responses, or functional immunosuppression, even in the absence of pharmacologic immunosuppression, universal vaccination regardless of medication usage may be warranted (15). However, it is worth noting that while adequate antibody titers to influenza and pneumococcus have been found in vaccinated SLE patients, vaccination has not been proven to reduce death in this population specifically.
Finally, physicians may not discuss vaccination if they expect another provider to take responsibility for this aspect of care. For example, rheumatologists may expect the patient's primary care providers to take responsibility for indicated preventive care, while primary care providers may expect the prescribing rheumatologists to take responsibility for preventive care associated with lupus treatment (16, 17). The use of preventive care algorithms that assign responsibility for vaccination, or improved collaboration between primary care and specialty providers via shared electronic task dashboards or other technology-based solutions, could also improve vaccination rates among lupus patients.
Addressing these complex barriers to vaccination will require a systems-based approach. Electronic medical record (EMR) technologies may be a useful tool to assure that indicated vaccines are recommended in spite of the complexity of lupus care. Automated EMR alerts can notify nurses or physicians of the need for vaccination in immunosuppressed patients. After-visit summaries, a required component of the Centers for Medicare & Medicaid Services’ “Meaningful Use” criteria governing the receipt of incentive payments for using EMR technology, can easily provide all lupus patients with information about the need for vaccination(18). EMR systems can also improve communication between primary care and specialty providers, particularly if providers are able to route communications to each other in a reliable way through the system. However, EMR systems do depend on the accurate and reliable recording of information about vaccination, which may require time-intensive vaccine record retrieval and also requires the training and diligence of staff.
Finally, having vaccinations available for immediate administration in clinic can decrease the complexity of care for patients, eliminating the need to remember to get vaccinated or make an additional visit to a health care provider. However, substantial barriers to administration of vaccines in rheumatology clinics do exist, including high costs and short shelf life of vaccines. The increasing availability of influenza and pneumococcal vaccinations in U.S. pharmacies can help to address this challenge when it is not economically feasible to administer vaccinations in a provider's office (19).
This study has several important limitations. First, while data is respondent-reported, methods used to assess vaccination by self-report in this study have been validated as part of the original study assessing vaccine receipt in this cohort, as well as other work (6, 20, 21). Nonetheless, it is likely that some of the respondents reporting not receiving vaccine recommendation merely did not remember the recommendation. Second, while all vaccinated respondents were assumed to have received a vaccine recommendation from their health care provider, some patients may have been vaccinated at local pharmacies without provider recommendation. However, since the most relevant outcomes for these patients are understanding of the need for vaccination and receipt of vaccination, patient perception of vaccine recommendation may be a more important measure than actual physician recommendation. Finally, by assessing whether respondents have ever received a vaccination against pneumococcus, this study design over-estimates the number of respondents appropriately vaccinated. Immunosuppressed individuals who have received an initial dose of pneumococcal vaccine will require an additional dose 5 years later to remain adequately protected. However, given the complexity of the recommendations and the limitations of respondent recall, we were not able to fully assess history of appropriate vaccination against pneumococcus.
In summary, our findings demonstrate an opportunity to improve influenza and pneumococcal vaccination rates among immunosuppressed individuals with SLE, and subsequently decrease morbidity and mortality, through improved communication of the need for vaccination. EMR technology offers an important opportunity to address these challenges through systems-based approaches(22, 23).
Acknowledgments
Financial Support:
(Arthritis Foundation PDF 6111, NICHD T32-HD044331)
(NIAMS P60-AR053308)
(NIAMS P60-AR053308)
(NIAMS K23 AR060259)
There was no financial support or other benefit from commercial sources for the work reported on in the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any financial interests that could create a potential conflict of interest or the appearance of a conflict of interest with regard to this work.
REFERENCES
- 1.Centers for Disease Control and Prevention Seasonal Infuenza. 2013 [cited; Available from: http://www.cdc.gov/flu/professionals/vaccination/coverage_1112estimates.htm.
- 2.Borchers AT, Keen CL, Shoenfeld Y, Gershwin ME. Surviving the butterfly and the wolf: mortality trends in systemic lupus erythematosus. Autoimmunity reviews. 2004;3(6):423–53. doi: 10.1016/j.autrev.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54(8):2550–7. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 4.Ward MM. Avoidable hospitalizations in patients with systemic lupus erythematosus. Arthritis Rheum. 2008;59(2):162–8. doi: 10.1002/art.23346. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Vaccine Recommendation of the Advisory Committee for Immunization Practices. 2013 [cited; Available from: http://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
- 6.Yazdany J, Tonner C, Trupin L, Panopalis P, Gillis JZ, Hersh AO, et al. Provision of preventive health care in systemic lupus erythematosus: data from a large observational cohort study. Arthritis research & therapy. 2010;12(3):R84. doi: 10.1186/ar3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kremers HM, Bidaut-Russell M, Scott CG, Reinalda MS, Zinsmeister AR, Gabriel SE. Preventive medical services among patients with rheumatoid arthritis. J Rheumatol. 2003;30(9):1940–7. [PubMed] [Google Scholar]
- 8.Selby L, Kane S, Wilson J, Balla P, Riff B, Bingcang C, et al. Receipt of preventive health services by IBD patients is significantly lower than by primary care patients. Inflamm Bowel Dis. 2008;14(2):253–8. doi: 10.1002/ibd.20266. [DOI] [PubMed] [Google Scholar]
- 9.Yelin E, Trupin L, Katz P, Criswell L, Yazdany J, Gillis J, et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum. 2007;57(1):56–63. doi: 10.1002/art.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorburn CM, Prokunina-Olsson L, Sterba KA, Lum RF, Seldin MF, Alarcon-Riquelme ME, et al. Association of PDCD1 genetic variation with risk and clinical manifestations of systemic lupus erythematosus in a multiethnic cohort. Genes Immun. 2007;8(4):279–87. doi: 10.1038/sj.gene.6364383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlson EW, Daltroy LH, Rivest C, Ramsey-Goldman R, Wright EA, Partridge AJ, et al. Validation of a Systemic Lupus Activity Questionnaire (SLAQ) for population studies. Lupus. 2003;12(4):280–6. doi: 10.1191/0961203303lu332oa. [DOI] [PubMed] [Google Scholar]
- 12.Danza A, Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. 2013;22(12):1286–94. doi: 10.1177/0961203313493032. [DOI] [PubMed] [Google Scholar]
- 13.Luijten RK, Cuppen BV, Bijlsma JW, Derksen RH. Serious infections in systemic lupus erythematosus with a focus on pneumococcal infections. Lupus. 2014;23(14):1512–6. doi: 10.1177/0961203314543918. [DOI] [PubMed] [Google Scholar]
- 14.Murdaca G, Orsi A, Spano F, Puppo F, Durando P, Icardi G, et al. Influenza and pneumococcal vaccinations of patients with systemic lupus erythematosus: Current views upon safety and immunogenicity. Autoimmun Rev. 2013 doi: 10.1016/j.autrev.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Murdaca G, Orsi A, Spano F, Puppo F, Durando P, Icardi G, et al. Influenza and pneumococcal vaccinations of patients with systemic lupus erythematosus: current views upon safety and immunogenicity. Autoimmun Rev. 2014;13(2):75–84. doi: 10.1016/j.autrev.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Rickert D, Santoli J, Shefer A, Myrick A, Yusuf H. Influenza vaccination of high-risk children: what the providers say. Am J Prev Med. 2006;30(2):111–8. doi: 10.1016/j.amepre.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Jessop AB, Dumas H, Moser CA. Delivering influenza vaccine to high-risk adults: subspecialty physician practices. American journal of medical quality : the official journal of the American College of Medical Quality. 2013;28(3):232–7. doi: 10.1177/1062860612456236. [DOI] [PubMed] [Google Scholar]
- 18.Brownfield E, Marsden JE, Iverson PJ, Zhao Y, Mauldin PD, Moran WP. Point of care experience with pneumococcal and influenza vaccine documentation among persons aged >/=65 years: high refusal rates and missing information. American journal of infection control. 2012;40(7):672–4. doi: 10.1016/j.ajic.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Taitel M, Cohen E, Duncan I, Pegus C. Pharmacists as providers: targeting pneumococcal vaccinations to high risk populations. Vaccine. 2011;29(45):8073–6. doi: 10.1016/j.vaccine.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 20.Mac Donald R, Baken L, Nelson A, Nichol KL. Validation of self-report of influenza and pneumococcal vaccination status in elderly outpatients. Am J Prev Med. 1999;16(3):173–7. doi: 10.1016/s0749-3797(98)00159-7. [DOI] [PubMed] [Google Scholar]
- 21.Mangtani P, Shah A, Roberts JA. Validation of influenza and pneumococcal vaccine status in adults based on self-report. Epidemiol Infect. 2007;135(1):139–43. doi: 10.1017/S0950268806006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollack AH, Kronman MP, Zhou C, Zerr DM. Automated Screening of Hospitalized Children for Influenza Vaccination. Journal of the Pediatric Infectious Diseases Society. 2014;3(1):7–14. doi: 10.1093/jpids/pit044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makam AN, Lanham HJ, Batchelor K, Samal L, Moran B, Howell-Stampley T, et al. Use and satisfaction with key functions of a common commercial electronic health record: a survey of primary care providers. BMC medical informatics and decision making. 2013;13:86. doi: 10.1186/1472-6947-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]