Abstract
The olfactory bulb is one of the most vulnerable brain regions in age-related proteinopathies. Proteinopathic stress is mitigated by the heat shock protein (Hsp) family of chaperones. Here we describe age-related decreases in Hsc70 in the olfactory bulb of the female rat and higher levels of Hsp70 and Hsp25 in middle and old age than at 2-4 months. In order to model proteotoxic and oxidative stress in the olfactory bulb, primary olfactory bulb cultures were treated with the proteasome inhibitors lactacystin and MG132 or the pro-oxidant paraquat. Toxin-induced increases were observed in Hsp70, Hsp25, and Hsp32. In order to determine the functional consequences of the increase in Hsp70, we attenuated Hsp70 activity with two mechanistically distinct inhibitors. The Hsp70 inhibitors greatly potentiated the toxicity of sublethal lactacystin or MG132 but not of paraquat. Although ubiquitinated protein levels were unchanged with aging in vivo or with sublethal MG132 in vitro, there was a large, synergistic increase in ubiquitinated proteins when proteasome and Hsp70 functions were simultaneously inhibited. Our study suggests that olfactory bulb cells rely heavily on Hsp70 chaperones to maintain homeostasis during mild proteotoxic, but not oxidative insults, and that Hsp70 prevents the accrual of ubiquitinated proteins in these cells.
Keywords: olfaction, proteostasis, Hsp60, GRP78, HO1, CHIP, Hop, Hsp90
Introduction
Disruptions in the sense of smell adversely affect health and safety and are surprisingly common in a large number of age-related proteinopathies, such as Alzheimer's disease, Parkinson's disease, dementia with Lewy bodies, frontotemporal dementias, corticobasal syndrome, progressive supranuclear palsy, multiple system atrophy, amyotrophic lateral sclerosis, and Huntington's disease (Attems et al. 2014). Furthermore, individuals who will go on to develop Parkinson's and Alzheimer’ disease can become hyposmic or anosmic years before the onset of motor or cognitive deficits, suggesting that olfactory dysfunction has the potential to serve as an early biomarker of neurodegenerative disease (Ponsen et al. 2010, Morley & Duda 2010, Luzzi et al. 2007, Bohnen et al. 2010). The molecular basis of smell disruptions in the conditions listed above is likely due to the protein misfolding (i.e., proteotoxicity) that develops early in the olfactory system (Dickson 2009, Braak et al. 2003a, Daniel & Hawkes 1992, Pearce et al. 1995). For example, neurofibrillary tangles and Lewy pathology are thought to gain a foothold in the olfactory bulb and anterior olfactory nucleus long before similar protein aggregates appear deeper in the cerebral cortex or basal ganglia (Attems et al. 2014, Ohm & Braak 1987, Braak et al. 2003a, Daniel & Hawkes 1992, Pearce et al. 1995, Braak et al. 2003b, Hawkes et al. 2007). Furthermore, olfactory impairments in neurodegenerative disorders correlate with significant decreases in olfactory bulb volume (Brodoehl et al. 2012, ter Laak et al. 1994, Muller et al. 2002, Wang et al. 2011). With the recent appreciation of non-cardinal symptoms in Parkinson's and Alzheimer's disease, there is a growing interest to identify therapeutics against pathologies in understudied brain regions such as the olfactory bulb. However, there are few studies on the functional impact of proteinopathic stress on the olfactory bulb and whether it mounts any endogenous defenses that might be responsible for the delayed onset and slow progression of neurodegenerative disorders. Therefore, one goal of the present study was to determine whether olfactory bulb cells engage natural defenses against proteotoxic or oxidative stress.
Aging is the major risk factor for neurodegenerative disorders and is associated with proteotoxic and oxidative stress, which act in a positive feedback loop (Keller et al. 2000b, Cecarini et al. 2007). For example, aging is associated with an increase in protein inclusions in the olfactory bulb (Attems et al. 2005). One of the major endogenous defenses against the formation of such inclusions is the heat shock protein (Hsp) family of molecular chaperones. Hsps such as Hsp70 and heat shock cognate 70 (Hsc70) help refold misfolded proteins or guide irreparably damaged proteins to the proteasome or lysosome for clearance (Lanneau et al. 2010, Kalia et al. 2010). In addition, Hsp70 and a small Hsp, Hsp25, are both known to inhibit apoptosis (Stetler et al. 2009, Beere 2001). The impact of aging on Hsps has been reported primarily for regions affected in neurodegenerative disorders, such as the cortex, hippocampus, striatum, and ventral mesencephalon (Leak 2014). In contrast, the impact of aging on Hsps in the olfactory bulb is poorly understood. Thus, our second major goal was to characterize age-related changes in Hsps and co-chaperones in the olfactory bulb.
Chaperones and co-chaperones are both essential players in protein triage (Lanneau et al. 2010, Kalia et al. 2010, Hohfeld et al. 2001, Frydman & Hohfeld 1997). For example, Hsp70 proteins form complexes with Hip, Hop, Hsp40, and/or Hsp90 during substrate refolding (Esser et al. 2004, Frydman & Hohfeld 1997). When proteins must be degraded, Hsp70 complexes with Hsp90 and the E3 ubiquitin ligase CHIP (Esser et al. 2004, Ballinger et al. 1999). Resident members of these complexes help regulate Hsp70 activity: Hsp40 activates the ATPase activity of Hsp70 (Bukau & Horwich 1998, Minami et al. 1996), and Hsp90 stabilizes, folds, and activates numerous client proteins downstream of Hsp70 (Pearl & Prodromou 2006). Hip, also known as Hsp70-interacting protein, prolongs ADP-Hsp70 substrate complexes (Frydman & Hohfeld 1997, Hohfeld et al. 1995), whereas Hop, the Hsp70-organizing protein, transfers client proteins from Hsp70 to Hsp90 (Scheufler et al. 2000). A characterization of the impact of aging on these chaperones is warranted because loss of effective protein triage is the major hallmark of age-related neurodegenerative disorders (Leak 2014).
Our third goal was to examine the functional role of Hsp70/Hsc70 under conditions of cellular stress in olfactory bulb neurons. To this end, we developed a primary culture model of the olfactory bulb and elicited cellular stress with the proteasome inhibitors lactacystin and MG132 and the redox-active agent paraquat. Proteasome activity is impaired in the course of aging (Keller et al. 2002) and proteasome activity is reduced in both Parkinson's and Alzheimer's disease (Keller et al. 2000a, McNaught et al. 2002), providing justification for the use of proteasome inhibitors. In addition, neurodegenerative disorders and aging are both strongly linked to oxidative stress (Butterfield et al. 2010, Lovell & Markesbery 2007), justifying the use of paraquat. Paraquat has been used as an herbicide and is associated with an increased risk for developing Parkinson's disease (Tanner et al. 2011). Here we tested the hypothesis that loss of Hsp70 activity would potentiate the loss of olfactory bulb neurons in response to mild cellular stress. Low concentrations of proteasome inhibitors and paraquat that did not lead to significant loss of neuron numbers on their own were applied so that we could resolve a synergistic potentiation of cell loss in response to Hsp70/Hsc70 inhibition. As these concentrations of toxins raised Hsp70 levels, we were able to determine if inhibition of Hsp70 activity would exacerbate mild proteotoxic and oxidative stress. If this hypothesis were supported, the results would reinforce the idea that olfactory bulb cells lean on this chaperone to help preserve homeostasis.
Experimental Procedures
Additional methods can be found under Supplementary Information.
Animals
Animal use was approved by the Duquesne University Institutional Animal Care and Use Committee (protocol number 1306-21) and was in compliance with the principles outlined in the NIH Guide. For the aging study, we used female Sprague Dawley rats (Charles River Labs, Wilmington, MA) that formed part of a breeding colony designed to generate rat pups for our postnatal cultures (see below). Dams were all sacrificed together at ages spanning 2-22 months, as the aged females did not survive well past the 22-month mark. All animals had ad libitum access to food and water and were housed in a 12:12 light-dark cycle.
Primary Olfactory Bulb Cultures
Olfactory bulb cells were harvested from day 1-2 postnatal rat pups with modifications to previously described methods (Posimo et al. 2013). Dissociated olfactory bulb cells were plated in Neurobasal-A medium supplemented with 2% v/v B27 (Life Technologies, Grand Island, NY) and 4 mM L-glutamine. Cultures were treated with the proteasome inhibitors MG132 and lactacystin and with the oxidative toxin paraquat (see Supplementary Methods) on day-in-vitro 5 (DIV5) and assayed on DIV7 with three independent viability assays.
Viability Assays and Immunocytochemistry
We used three assays for structure and function to quantify viability in a blind and unbiased manner: the infrared DRAQ5 stain for nuclei, infrared immunocytochemistry for the neuronal marker microtubule associated protein 2 (MAP2), and a luminescent assay for ATP levels, as previously described (Posimo et al. 2013, Posimo et al. 2014). For high-magnification visualization of neurons, synapses, and astrocytes, olfactory bulb cultures were immunostained with mouse anti-MAP2 in conjunction with rabbit anti-synaptophysin or rabbit anti-glial fibrillary acidic protein (GFAP). Antibodies are listed in Supplementary Tables 1 and 2. Antibody binding was visualized with fluorescent secondary goat/donkey anti-mouse IgGs (488 nm) and secondary goat anti-rabbit IgGs (555 nm). Nuclei were stained with the Hoechst reagent (Sigma-Aldrich). As a negative control for the immunostaining, primary antibodies were omitted in all assays to ensure loss of fluorescent signal. Two blinded observers counted MAP2+ and Hoechst+ profiles from images captured on an epifluorescent microscope (EVOS, Life Technologies).
Statistical Analyses
Data are presented as the mean ± SEM from 3-6 animals per age group for the in vivo arm of the experiments. The Grubb's outlier test was performed once on all the data. For the infrared Western blotting, protein bands with fluorescent lint or air bubbles during transfer were also excluded from further analysis. Statistical significance for the in vivo Western blotting data was determined by one-way ANOVA followed by the LSD post hoc correction (IBM SPSS Statistics, Version 20, Armonk, NY). The in vitro Western blotting data were analyzed by the two-tailed t test. The remaining in vitro viability data were analyzed by one-, two-, or three-way ANOVA followed by the Bonferroni post hoc correction. With the exception of immunoblotting, all in vitro experiments were performed in triplicate wells. Data from these triplicate wells was averaged to generate an “n” of 1. Each experiment was then repeated on at least 3 completely independent occasions. Differences were deemed significant only when p ≤ 0.05.
Results
Age-related changes in chaperones and chaperone-associated proteins in the olfactory bulb
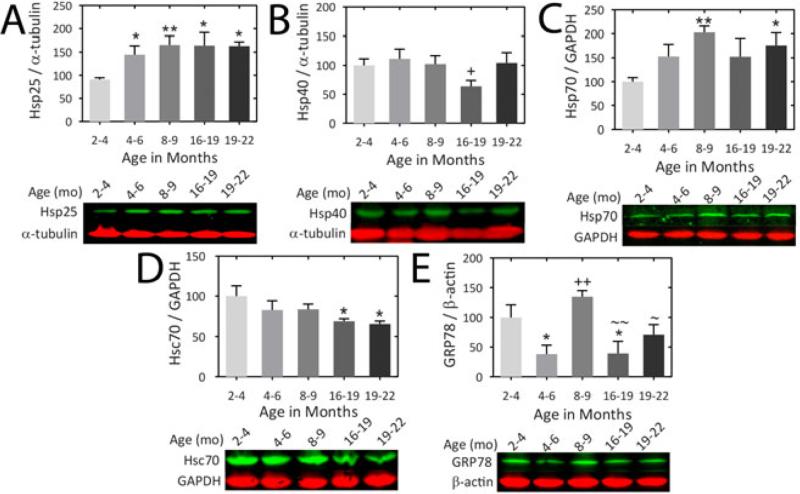
We assessed the impact of age on several Hsps and co-chaperones in olfactory bulb tissue from female rats (Fig.1). An increase in Hsp25 was observed when animals transitioned from early youth (2-4 months) to 4-6 months of age and high Hsp25 levels were sustained into old age (19-22 months). A transient decrease in Hsp40 levels was observed in the 16-19 month group. A modest trend towards a rise in Hsp40 levels was also observed from 16-19 to 19-22 months of age (p = 0.090). In contrast, Hsp70 levels rose at 8-9 months and at 19-22 months relative to the youngest, 2-4 month-old group. Relative to the 2-4 month-old group, there was a significant decrease in Hsc70 from 16 months of age onwards. Glucose-regulated protein 78 (GRP78), an endoplasmic reticular Hsp70 family member (Otero et al. 2010), exhibited high levels of expression only in 2-4 and 8-9 month old animals.
Figure 1. Impact of aging on Hsps and co-chaperones in the olfactory bulb of the female rat.
(A-E) Infrared Western immunoblots are shown for the indicated Hsps and co-chaperones. Olfactory bulb tissue was harvested from female rats at 2.0-3.9 months, 4.0-6.0 months, 8.0-9.0 months, 16.0-18.9 months, and 19.0-22.0 months of age. GAPDH, α-tubulin, or β-actin was used as a protein loading control, depending on the species of the primary antibodies and the expected molecular weights. n = 3-6 rats per group. * p ≤ 0.05, ** p ≤ 0.01, versus 2-4 month old, + p ≤ 0.05, ++ p ≤ 0.01 versus 4-6 month old, ~ p ≤ 0.05, ~~ p ≤ 0.01 versus 8-9 month old, LSD post hoc following one-way ANOVA.
No age-related changes were observed in the co-chaperone CHIP or the heat shock inducible protein heme oxygenase 1 (HO1; also known as Hsp32; see Fig.S1), a protein important for antioxidant defense (Motterlini & Foresti 2014). The chaperone-associated proteins Hip and Hop also remained unaffected by age, as were levels of Hsp60, which facilitates the folding of select proteins in eukaryotes (Horwich et al. 1993, Ryabova et al. 2013). However, there was a trend towards a decline in Hop levels between 4-6 and 19-22 months of age (p = 0.062; Fig.S1). Mitochondrial Hsp70 (mtHsp70) and Hsp90 remained unchanged with age, although a trend towards lower expression of mtHsp70 was evident in the oldest group relative to the 8-9 month old animals (p = 0.077).
Modeling proteotoxic and oxidative stresses in primary olfactory bulb cultures
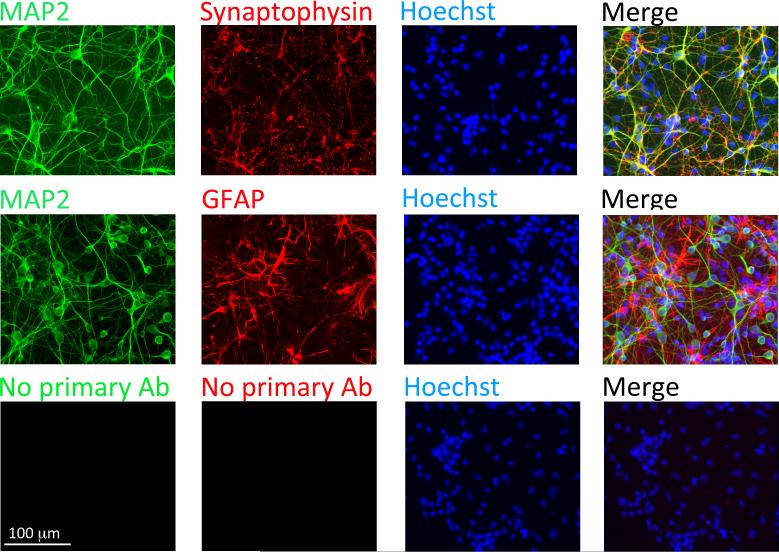
We next developed primary cultures of the olfactory bulb to conduct mechanistic analyses of the Hsps in this model system. Blinded counts from two independent observers indicated that ~67% of Hoechst+ cells in these cultures also expressed the neuronal phenotypic marker MAP2 (first observer: 66.9%; second observer: 67.1%; n = 3 independent experiments). The synaptic protein synaptophysin was also densely expressed in olfactory bulb cultures, especially in puncta abutted against the MAP2+ postsynaptic dendrites (Fig.2). The remaining, MAP2− cells appeared to be largely astrocytic as many expressed the glial marker GFAP. These findings are consistent with our previous work on postnatal cultures (Posimo et al. 2013), and with the observation that astrocyte births peak neonatally (Bayer & Altman 1991, Miller & Gauthier 2007).
Figure 2. Primary postnatal cultures of the olfactory bulb.
Dissociated primary olfactory bulb cells from day 1-2 neonatal rat pups were stained on day-in-vitro 5 (DIV5) for the neuronal marker MAP2, the synaptic protein synaptophysin, the astrocyte marker GFAP, and the Hoechst nuclear stain. Control wells (bottom panels) were exposed to all solutions except the primary antibodies.
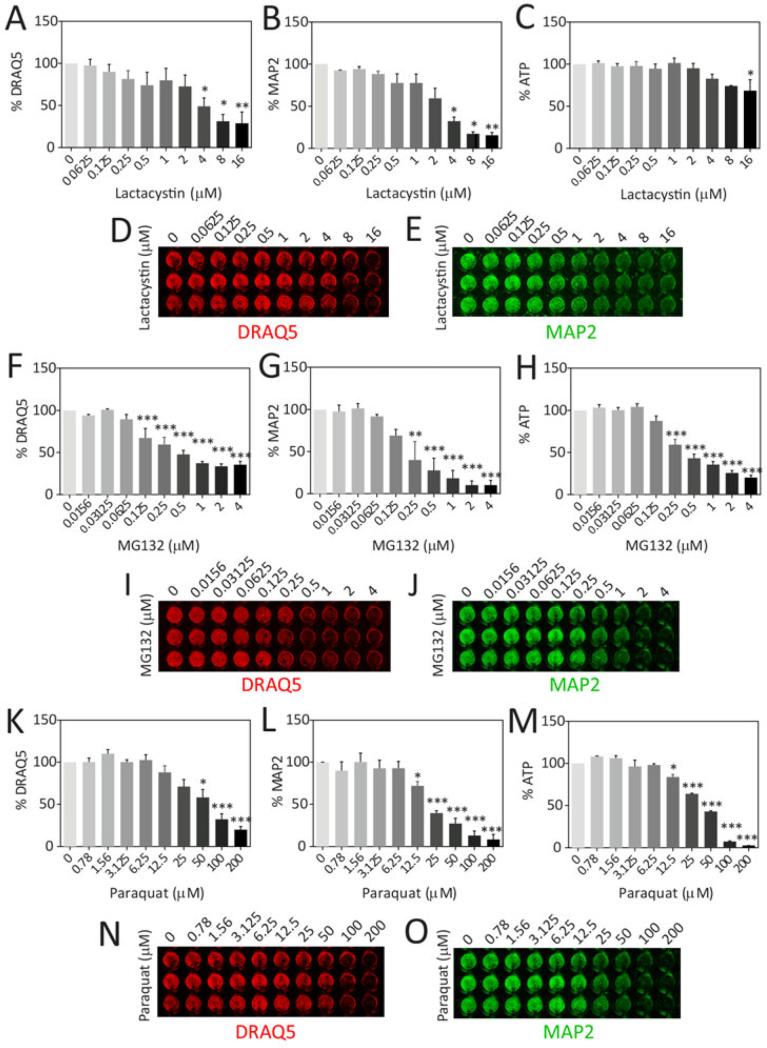
In order to model proteotoxic and oxidative stress, primary olfactory bulb cultures were treated with increasing concentrations of lactacystin, MG132, and paraquat for 48h and assayed by three independent, unbiased viability measures (Fig.3). The strengths and weaknesses of these relatively high-throughput viability assays have been discussed before (Posimo et al. 2013, Posimo et al. 2014, Unnithan et al. 2012). The In-Cell Western analysis of the neuronal marker MAP2 was the most sensitive of the three measures, consistent with previous studies (Posimo et al. 2013, Posimo et al. 2014). Notably, lethal concentrations of MG132 elicited greater loss of ATP than lethal concentrations of lactacystin. Thus, the two proteasome inhibitors elicited somewhat different biochemical responses, which may arise from distinct compensatory responses (Mu et al. 2008). These divergent patterns support the use of distinct assays to gain a more comprehensive view of cellular integrity as well as the use of two independent classes of proteasome inhibitors to induce proteotoxic stress.
Figure 3. In vitro model of proteotoxic and oxidative stress in olfactory bulb cultures.
Primary olfactory bulb cultures were treated with the proteasome inhibitors lactacystin (A-E) and MG132 (F-J), and the redox-active herbicide paraquat (K-O) on DIV5 and assayed by three independent assays for cell viability on DIV7: the infrared DRAQ5 stain for nuclei (A, D, F, I, K, N), infrared In-Cell Western analyses for the neuronal marker MAP2 (B, E, G, J, L, O), and the Cell Titer Glo assay for ATP (C, H, M). Representative DRAQ5 and MAP2 images were pseudocolored red and green, respectively. Shown are the mean and S.E.M of 3 independent experiments, each performed in triplicate. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 versus vehicle-treated groups, Bonferroni post hoc following one-way ANOVA.
To ensure that MAP2+ neurons were killed, a blinded observer counted MAP2+ cell numbers after toxin treatments (Fig.S2). A comparison of Fig.3 and Fig.S2 reveals that the In-Cell Western analyses exhibited slightly greater loss of signal than the cell count data in response to all three compounds. The microscopic images showed that this difference was not attributable to a loss of MAP2 expression per cell, but possibly due to a loss of dendritic profiles in the treated groups (Fig.S2B). However, the differences between the two assays were slight and the loss of MAP2+ signal in the In-Cell Western assay was largely attributable to a loss in neuron numbers, as expected.
Mild proteotoxic and oxidative stress increase Hsp levels in primary olfactory bulb cells
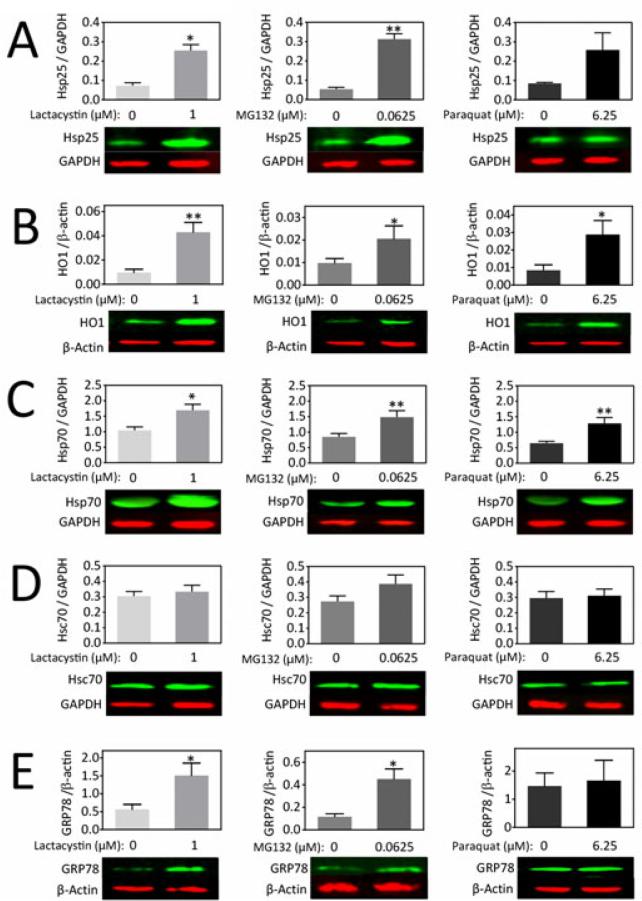
Next, we measured the levels of various Hsps by immunoblotting following mild proteotoxic and oxidative stress (Fig.4 and Fig.S3). To accomplish this goal, we chose concentrations of lactacystin (1 μM), MG132 (0.0625 μM), and paraquat (6.25 μM) that were half of the lowest concentration to elicit loss of MAP2 in the curves from Figure 3. HO1 and Hsp70 levels were increased by all three compounds, whereas Hsc70 was unaffected. Hsp25, Hop, and GRP78 were significantly increased by low concentrations of the two proteasome inhibitors but were not significantly affected by paraquat treatment. Hsp40 and Hsp60 levels were only increased by MG132. CHIP was only significantly increased by lactacystin. MG132 and paraquat both increased Hsp90 levels. Hip and mtHsp70 were not detectable in the in vitro model. These findings indicate toxin-dependent adaptive changes in Hsps and chaperone cofactor levels in the olfactory bulb culture model.
Figure 4. Mild proteotoxic and oxidative stress increases Hsps in olfactory bulb cultures.
(A-E) Primary olfactory bulb cultures were treated with low concentrations of lactacystin, MG132, and paraquat on DIV5 and harvested for measurements of Hsps on DIV6 by infrared Western blotting. GAPDH or β-actin was used as a protein loading control. Shown are the mean and S.E.M of 3-6 independent experiments. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 versus vehicle-treated groups, two-tailed t-test.
Inhibition of Hsp70 exacerbates proteotoxicity in primary olfactory bulb cultures challenged with proteasome inhibitors
Our Western blotting data confirmed that Hsp70 was increased in vitro by low concentrations of MG132, lactacystin, and paraquat. If Hsp70 was responding to these insults in a compensatory fashion, inhibition of Hsp70 under mild proteotoxic and oxidative conditions should exacerbate cell loss. In order to test this hypothesis, we treated olfactory bulb cultures with low concentrations of lactacystin, MG132, and paraquat in conjunction with the previously characterized Hsp70/Hsc70 inhibitor VER155008 (Schlecht et al. 2013, Massey et al. 2010, Saykally et al. 2012, Chatterjee et al. 2013, Williamson et al. 2009, Macias et al. 2011). As expected, the toxicity of low concentrations of lactacystin and MG132 was potentiated when Hsp70/Hsc70 activity were inhibited with VER155008 (Fig.5A-H). That is, the DRAQ5 and MAP2 assays were in agreement that both proteasome inhibitors were significantly more toxic when Hsp70/Hsc70 activity was reduced. However, the cells were more sensitive to MG132 than lactacystin. VER155008 was also slightly toxic by itself, as revealed by the ATP and MAP2 assays. Furthermore, VER155008 only potentiated ATP loss in the MG132-treated cells, suggesting again that olfactory bulb cells were more sensitive to MG132 than lactacystin when Hsp70 activity was inhibited.
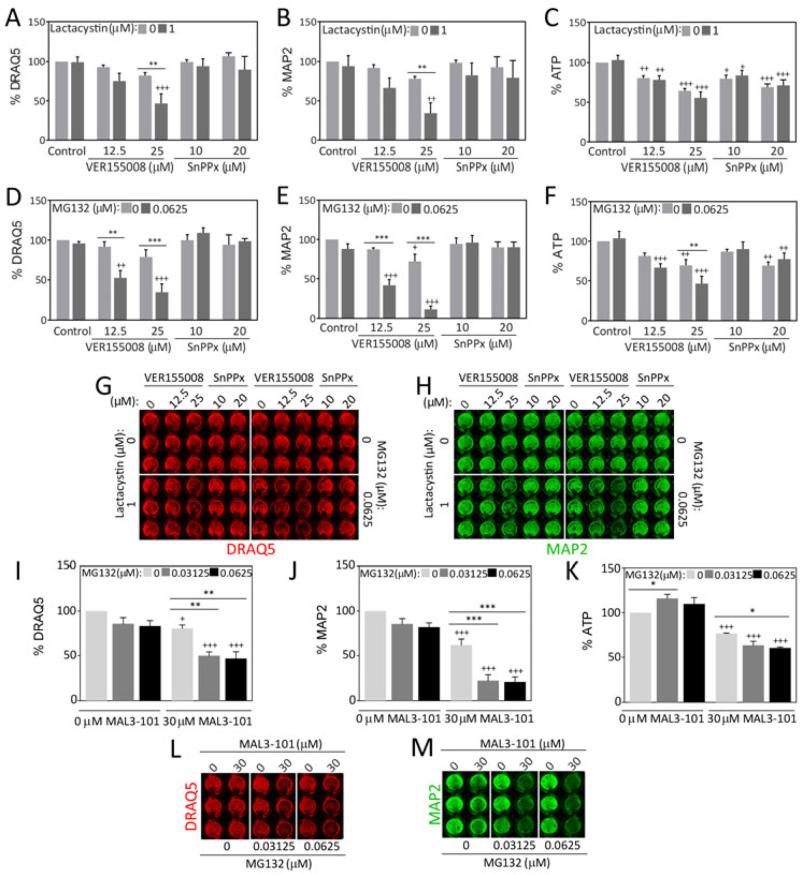
Figure 5. Effect of Hsp70/Hsc70 and HO1 inhibition on proteotoxic stress in olfactory bulb cultures.
Primary olfactory bulb cultures were treated on DIV5 with low concentrations of lactacystin (A-C) or MG132 (D-F) in conjunction with the Hsp70/Hsc70 inhibitor VER155008 or the HO1 inhibitor tin protoporphyrin (SnPPx) and three independent viability assays were performed on DIV7. Representative infrared images are shown in G and H. (I-M) Primary olfactory bulb cultures were treated on DIV5 with low concentrations of MG132 in conjunction with the Hsp70/Hsc70 inhibitor MAL3-101 and three independent viability assays were performed on DIV7. Shown are the mean and S.E.M of 3-6 independent experiments. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 versus 0 μM MG132 or lactacystin; + p ≤ 0.05, ++ p ≤ 0.01, +++ p ≤ 0.001 versus 0 μM VER155008, MAL3-101, or SnPPx, Bonferroni post hoc following two-way ANOVA.
As with Hsp70, the Western blotting data had shown that HO1 levels were increased by all three compounds. Therefore, we also applied the HO1 inhibitor tin protoporphyrin (SnPPx) (Drummond & Kappas 1981) to olfactory bulb cells in conjunction with low concentrations of lactacystin and MG132 (Fig.5A-H). SnPPx failed to modify the toxicity of either proteasome inhibitor, although it was significantly toxic under basal conditions and elicited ATP loss. These experiments support the view that HO1 induction by MG132 and lactacystin is not as protective as Hsp70 induction in olfactory bulb cells.
We next delivered the selective Hsp70/Hsc70 inhibitor MAL3-101 in conjunction with two low concentrations of MG132 in order to validate the VER155008 findings. MAL3-101 inhibits Hsp40-stimulated ATP hydrolysis, thereby interfering with Hsp70/Hsc70 chaperone activity (Huryn et al. 2011, Braunstein et al. 2011, Hatic et al. 2012, Kilpatrick et al. 2013, Adam et al. 2014, Fewell et al. 2004). In contrast, because VER15508 binds to the ATP binding site in the chaperone, it will inhibit all Hsp70/Hsc70-mediated activities. This includes ATP binding and hydrolysis, peptide binding, and possibly the interaction with Hsp40 co-chaperones and chaperone-specific nucleotide exchange factors. In contrast, some chaperone activities specifically require the action of the Hsp40 co-chaperones, which are able to bind to select polypeptide substrates and activate Hsp70/Hsc70 ATPase activity (Kampinga & Craig 2010). Therefore, if MAL3-101 exerts a similar effect as VER15508, one may assume that it is this more specific activity of Hsp70/Hsc70 that is disabled and consequently leads to cellular toxicity. As expected, MAL3-101 potentiated MG132 toxicity according to all three viability assays (Fig.5I-M). Similar to VER155008, MAL3-101 was also toxic on its own.
In order to test the hypothesis that the paraquat-triggered increase in Hsp70 also protects olfactory bulb cells against oxidative damage, we delivered paraquat in conjunction with the two Hsp70/Hsc70 inhibitors. Neither VER155008 nor MAL3-101 significantly increased the toxicity of paraquat (Fig.S4). Paraquat toxicity was also unchanged in the presence of the HO1 inhibitor SnPPx. These data suggest that the paraquat-induced increases in Hsp70 or HO1 do not significantly prevent loss of homeostasis, or that the increase in Hsps is overwhelmed by other cytotoxic effects of this electrophilic compound.
Hsp70 inhibition potentiates an increase in ubiquitinated proteins during mild proteotoxic stress
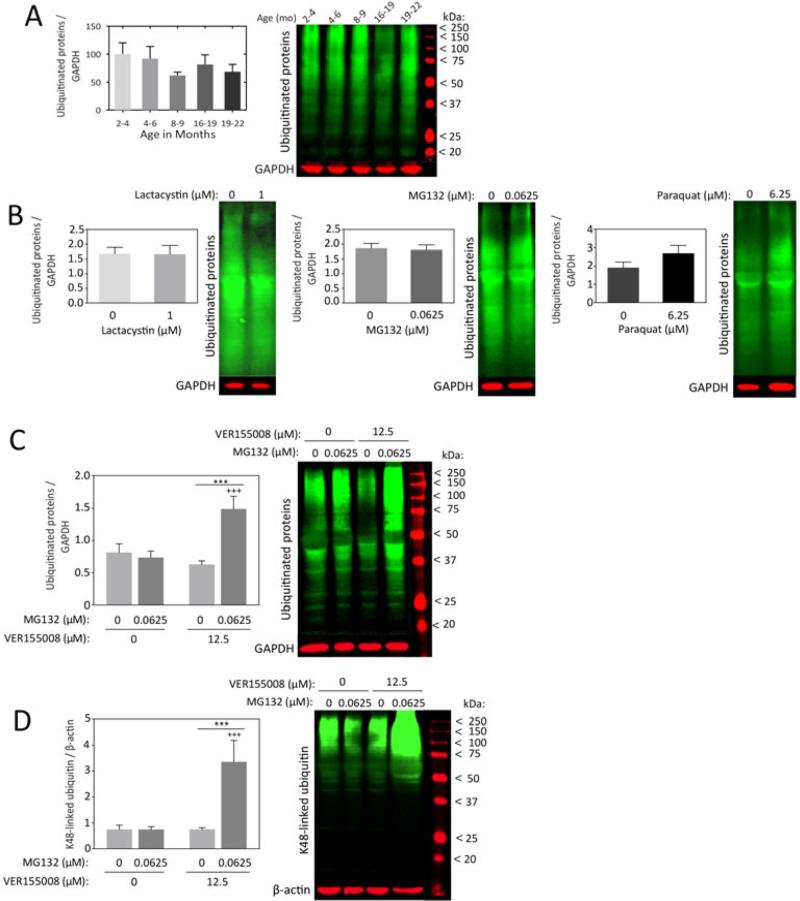
Under conditions of severe proteotoxic stress that overwhelm the proteasome, the levels of ubiquitinated proteins will rise because they cannot be degraded efficiently. However, if the proteotoxic stress is mild, compensatory increases in chaperones may help refold damaged proteins or accelerate their removal (for example, see Mu et al. 2008). Thus, under mildly stressed, homeostatic conditions one would not expect a net increase in ubiquitinated proteins. To examine which of these scenarios was evident in our in vivo and in vitro models, we visualized ubiquitinated protein levels in olfactory bulb tissue from aged female rats and in olfactory bulb cultures treated with low concentrations of lactacystin, MG132, and paraquat (Fig.6A-B). No net change in ubiquitinated proteins was observed, confirming that protein homeostasis was not severely compromised.
Figure 6. Impact of mild stress and Hsp70 activity on ubiquitinated protein levels in olfactory bulb cells.
(A) Olfactory bulb tissue was harvested from female rats at 2.0-3.9 months, 4.0-6.0 months, 8.0-9.0 months, 16.0-18.9 months, and 19.0-22.0 months of age and probed for ubiquitin-conjugated proteins by infrared Western blotting. n = 3-6 rats per group. (B) Primary olfactory bulb cultures were treated with low concentrations of lactacystin, MG132, and paraquat on DIV5 and harvested for immunoblots of ubiquitinated proteins on DIV6. Shown are the mean and S.E.M from 5-6 independent experiments. (C-D) Primary olfactory bulb cultures were treated on DIV5 with MG132 in the absence or presence of the Hsp70/Hsc70 inhibitor VER155008 and assayed for pan-ubiquitinated proteins (C) or K48-linked ubiquitin (D) on DIV6. GAPDH or β-actin was used as the loading control. Shown are the mean and S.E.M from 5-6 independent experiments. *** p ≤ 0.001 versus 0 μM MG132; +++ p ≤ 0.001 versus 0 μM VER155008, Bonferroni post hoc following two-way ANOVA.
We showed above that Hsp70 inhibition potentiated the toxicity of lactacystin and MG132. The magnitude of the effect was higher when the Hsp70/Hsc70 inhibitor was co-administered with MG132, as opposed to lactacystin. Therefore, we tested the hypothesis that Hsp70 inhibition in MG132-treated cells would elicit a net increase in ubiquitinated proteins due to severely compromised homeostasis. In support of this hypothesis, ubiquitinated protein levels greatly increased when MG132-treated cells were also exposed to VER155008 (Fig.6C,D). Two different ubiquitin antibodies confirmed the robustness of these effects. The first antibody recognizes pan-ubiquitinated proteins (Fig.6C), and the second recognizes only K48-linked ubiquitin (Fig.6D), which specifically targets proteins for proteasomal degradation (Sadowski & Sarcevic 2010). These findings suggest that there is sufficient protein misfolding to overwhelm the proteasome when Hsp70/Hsc70 activity is inhibited in olfactory bulb cultures.
Taken together, our studies reveal the natural resilience of olfactory bulb cells to proteotoxic insults, but also indicate that the magnitude and type of a specific stress can overwhelm cellular homeostasis and lead to cell death.
Discussion
This report represents the first characterization of Hsps- and chaperone-associated proteins in the olfactory bulb of female rats as a function of age and in response to specific stressors. We discovered that Hsp25 and Hsp70 protein levels are higher at middle and old age than at 2-4 months, and that aging decreases the levels of constitutive Hsc70 and transiently decreases the co-chaperone Hsp40. GRP78 levels were higher in young and middle-aged animals, perhaps magnified by endoplasmic reticulum stress during development and at the peak of middle age. Indeed, endoplasmic reticulum stress is known to occur as a natural outcome during specific developmental stages (Rutkowski & Hegde 2010). No other age-related changes in Hsps were observed in vivo. Next, to examine the functional role of Hsp70 in olfactory bulb cells, we developed a primary culture model from dissociated tissue. By applying low concentrations of lactacystin, MG132, and paraquat, we discovered that Hsp70 and HO1 levels were both significantly increased by mild proteotoxic and oxidative stress in olfactory bulb cells. Furthermore, Hsp25, CHIP, Hsp40, Hsp60, Hop, and GRP78 levels increased when proteotoxic, but not oxidative stress was triggered, whereas no change in Hsc70 was observed after treatment with any of our tested compounds. These findings show an imperfect overlap between the stress of aging in vivo and proteotoxic and oxidative stress in vitro. On the other hand, the in vitro model may mimic aspects of age-related proteinopathies, as several of the Hsps whose levels rose in vitro (e.g., HO1, Hsp70, Hsp25) are similarly increased in postmortem tissue from Alzheimer's and/or Parkinson's patients (Hauser et al. 2005, Renkawek et al. 1999, Lee et al. 2008, Di Domenico et al. 2010, Hoozemans et al. 2005, Braak et al. 2001, Schipper et al. 2006).
In our in vitro model, synergistic exacerbations were evident when mild proteotoxic stressors (i.e., low concentrations of MG132 or lactacystin) and Hsp70/Hsc70 inhibitors were combined. These data strongly suggest that the chaperones mitigate toxic effects when select stress conditions are applied. Because constitutive Hsc70 protein levels were unaffected, the chaperone inhibitors most likely potentiate cell loss via inhibition of Hsp70. In addition, the level of K48-linked ubiquitinated proteins was increased by low concentrations of MG132 only when Hsp70 activity was simultaneously inhibited. These data suggest further that Hsp70 prevents a toxic buildup of misfolded proteins under conditions of mild injury and are consistent with Hsp70 being one of many contributors to protein homeostasis, or “proteostasis” (Balch et al. 2008). If these findings are generalized to the in vivo condition, they support the idea that Hsp70 may preserve proteostasis in the olfactory bulb, perhaps even at middle and old age. We also speculate that disease-related increases in this protein may delay the onset of cell loss in neurodegenerative disorders. Accordingly, this model may explain the slow, progressive nature of many neurodegenerative disorders. Future studies to modulate Hsp activity or levels in vivo and determine the effects of Hsp70 on age- or disease-related neurodegeneration and loss of olfaction are warranted.
The literature on Hsp expression in aging and in neurodegenerative conditions is mixed and the effects depend upon the Hsp in question, the brain region, and the underlying condition, among other factors (Leak 2014). For example, Hsp90 is decreased in the temporal cortex in Alzheimer's disease (Yokota et al. 2006) but increased in the cingulate cortex in Parkinson's disease (Uryu et al. 2006). GRP78 is reportedly increased in Alzheimer's disease in the temporal cortex (Hoozemans et al. 2005) and the substantia nigra with aging (Alladi et al. 2010). However, other studies have shown a decrease in GRP78 in the cortex, striatum, and hippocampus with aging (Arumugam et al. 2010, Paz Gavilan et al. 2006, Hussain & Ramaiah 2007). In contrast, Hsp27 expression is consistently increased in neurodegenerative conditions and with aging (Zhang et al. 2005, Renkawek et al. 1999, Shinohara et al. 1993, Dickey et al. 2009, Gupte et al. 2010). Although we did not observe any change in several Hsps, including Hsp90, the present study did show similar stress-induced increases in Hsp25 as in the abovementioned literature.
The present study reveals that Hsp70 expression is higher in olfactory bulb cells mildly stressed with lactacystin, MG132, and paraquat. Hsp70 levels were also higher in 8-9- and 19-22-month old animals than in the youngest group. Some investigators have reported increases in Hsp70 expression with age in the cortex, striatum, hippocampus, and cerebellum (Calabrese et al. 2004), whereas others have reported losses in Hsp70 with aging in the cortex and striatum (Arumugam et al. 2010), in olfactory neurons (Getchell et al. 1995), and in the inferior colliculus (Helfert et al. 2002). An increase in the expression of the major genes that encode Hsp70 proteins (HSPA1A and HSPA1B) has been found in the substantia nigra in patients with Parkinson's disease, progressive supranuclear palsy, and frontotemporal dementia with Parkinsonism (Hauser et al. 2005). Furthermore, Hsp70 is more abundant in the inferior parietal lobule of patients with mild cognitive impairment, a potential precursor to Alzheimer's dementia (Di Domenico et al. 2010). Notably, Hsp70/Hsc70 colocalizes with protein inclusions in both Alzheimer's and Parkinson's diseases, perhaps in an endogenous attempt to ameliorate these protein aggregations (Sherman & Goldberg 2001, Kalia et al. 2010). Furthermore, Hsp70 decreases β-amyloid and MPTP toxicity and is protective in numerous experimental models of neurodegeneration (Kumar et al. 2007, Muchowski & Wacker 2005, Magrane et al. 2004, Dong et al. 2005). Our data are consistent with these findings and agree with other reports showing that Hsp70 induction is a “common response to mitigate the toxic effects of misfolded protein” (Hauser et al. 2005).
We previously found an age-related loss in Hsc70 in the striatum of female rats (Gleixner et al. 2014), consistent with the present study. Hsc70 levels have also been reported to fall in the temporal cortex in Alzheimer's patients (Yoo et al. 2001), particularly in the entorhinal cortex and hippocampus (Silva et al. 2014), and in the substantia nigra in Parkinson's patients (Alvarez-Erviti et al. 2010, Chu et al. 2009, Mandel et al. 2005). Unlike Hsp70, Hsc70 levels were unaffected by low concentrations of MG132, lactacystin, or paraquat in the present study. Furthermore, aged animals had higher Hsp70 levels but lower Hsc70 levels than the very youngest group. These findings suggest that our in vitro model does not capture all the sequelae of aging or development in vivo, or that the concentrations of these reagents were too low to elicit loss of Hsc70. The contrast between Hsp70 and Hsc70 in the present study may reflect the classic distinction between stress-inducible (Hsp70) and constitutively expressed (Hsc70) proteins. On the other hand, it must be noted that the Hsc70 response to stress is quite variable; some studies have shown increases in Hsc70 with age and proteinopathic disease states. For example, Hsc70 mRNA levels are higher in blood samples from Parkinson's and Alzheimer's patients than from control subjects (Molochnikov et al. 2012). Furthermore, studies in male rats indicate an increase in the basal level of Hsc70 in the pons, medulla, striatum, and thalamus (Unno et al. 2000). Others have also reported an age-related increase in Hsc70 in the striatum and substantia nigra of male rats (Calabrese et al. 2004). Finally, Hsc70 mRNA-expressing neurons become denser in the human hippocampus with aging (Tohgi et al. 1995). These discrepancies could reflect differences in gender, rodent strain, cell type, brain region, and/or species.
Only few studies to date have examined Hsps in the olfactory system. A proteomics study of the olfactory bulb demonstrated an increase in age-related protein oxidation in this structure and a 2.3-fold increase in Hsc70 levels in the olfactory bulb of aged male mice (Vaishnav et al. 2007). Again, the difference between our in vivo experiments and the previous study might reflect gender differences. A subset of olfactory receptor neurons expresses high levels of Hsp70/Hsc70 (Carr et al. 1994, Carr et al. 1999). Furthermore, Getchell and colleagues reported a down-regulation of Hsp70/Hsc70 in olfactory sensory neurons in older individuals and in patients with Alzheimer's disease (Getchell et al. 1995). As might be expected for a stress-inducible protein, Hsp70 is increased in olfactory bulb tissue in response to hypothermia, heat shock, and water deprivation (Frenkel et al. 2008, Kaneko & Kibayashi 2012). Following damage to olfactory receptor neurons, Hsp25 is highly expressed in astrocytes of olfactory bulb glomeruli (Hirata et al. 2008). As in the present study, these reports reveal dynamic changes in Hsps in the stressed olfactory system. However, our study is the first mechanistic exploration of the functional consequences of this stress response in cells from this structure.
A number of caveats in the interpretation of our collective data are worth noting. First, the in vitro cultures are mostly neuronal, whereas astrocytes are the major cell type in the brain (Sofroniew & Vinters 2010). However, some astrocytes remained in our postnatal cultures, perhaps better reflecting the in vivo milieu than pure, neuronal embryonic cultures. Second, the in vitro model involves dissociated, neonatal cells and can never fully represent an adult or aged intact brain. Postnatal neurons are acutely injured and do not suffer from a lifetime of accumulated insults. Third, some of the changes elicited with MG132 were more robust than with lactacystin. MG132 can inhibit cellular cathepsins and calpains in addition to its effect on the chymotrypsin-like activity of the proteasome (Lee & Goldberg 1998), and different effects on proteostasis have been observed with these compounds (Mu et al. 2008). Although lactacystin is generally held to be more specific, it can also inhibit cathepsin A (Aikawa et al. 2006, Kisselev & Goldberg 2001). One might add that autophagic stress, through interference with cathepsins, may be viewed as desirable when modeling proteinopathic diseases (Nixon & Yang 2012). Fourth, Hsp70 ATPase activity could not be measured directly in our model because numerous ATPases are present in cellular lysates. On the other hand, VER155008 and MAL3-101 are well-characterized inhibitors of Hsp70/Hsc70. VER155008 is an adenosine-derived ATP-competitive inhibitor that arrests the nucleotide-binding domain of Hsp70/Hsc70 in a half-open conformation (Schlecht et al. 2013). The downstream effect of Hsp70/Hsc70 inhibition with VER155008 includes autophagy inhibition, caspase-dependent and independent apoptosis, and Hsp90 client protein degradation (Massey et al. 2010, Budina-Kolomets et al. 2013). MAL3-101 is a pyrimidinone that inhibits Hsp40-stimulated Hsp70/Hsc70 ATPase activity (Fewell et al. 2004). Our findings on the impact of MAL3-101 on MG132 toxicity are consistent with previous reports that MAL3-101 increases α-synuclein aggregation in neuroglioma cells (Kilpatrick et al. 2013).
Despite a number of limitations, the strengths of the present study include the validation of multiple findings with more than one technique: 1) use of three independent, unbiased viability assays, 2) use of microscopy to verify the MAP2 In-Cell Western data, 3) two independent classes of proteasome inhibitors with unique modes of action, 4) two mechanistically independent Hsp70/Hsc70 inhibitors, 5) measurements of both pan-ubiquitinated proteins as well as K48-linked ubiquitinated proteins, and 6) two independent observers to determine the neuronal purity of olfactory bulb cultures. It is also worth mentioning that although all pharmacological inhibitors exert some non-specific effects, it is very unlikely that two inhibitors with unique modes of action have precisely the same non-specific effects. Therefore, if both inhibitors show the same effects (i.e. both potentiate proteotoxic stress), it is likely that these effects can indeed be attributed to the one mechanism that they share in common: Hsp70/Hsc70 inhibition. Additional strengths of the present study include verification of the specificity of the Hsp70 effects on proteotoxicity by including paraquat as an oxidative toxin as well as an inhibitor of an entirely different class of Hsps, the HO1 inhibitor SnPP. Paraquat toxicity was not exacerbated with the Hsp70/Hsc70 inhibitors and SnPP was not as effective as the Hsp70/Hsc70 inhibitors in olfactory bulb cells.
In conclusion, our study identifies a protective role for Hsp70 in olfactory bulb cells, in agreement with the notion that this chaperone is a natural defense against loss of proteostasis. These findings support attempts to boost levels of Hsp70 in elderly patients at risk for neurodegeneration with pharmacological tools or lifestyle interventions, such as daily exercise (Noble & Shen 2012). Future studies to measure the impact of Hsp70 inhibition on olfactory bulb structure and function in vivo and to examine age-related changes in the expression of Hsp70 within the layers of the olfactory bulb are justified.
Supplementary Material
Acknowledgements
RKL designed the experiments, generated some figures, and wrote the paper. TSC, AMG, and DMM collected and analyzed the data and generated the figures. JMP conducted some in vitro experiments. MTB and SDH performed cell counts. PW and JLB provided the Hsp70 inhibitor MAL3-101 and provided feedback on the manuscript. We are indebted to Mary Caruso, Deb Willson, and Jackie Farrer for excellent administrative support. We are also grateful to Denise Butler-Buccilli and Christine Close for outstanding animal care. This study was supported by the Hillman foundation (RKL), a C.U.R.E. Research Award from the Pennsylvania State Department of Health (RKL), NIH grant DK79307 (JLB), and NIGMS grant GM067082 (PW).
Abbreviations
- CHIP
C-terminus of Hsc70-interacting protein
- GFAP
glial fibrillary acidic protein
- GRP78
glucose regulated protein
- Hsc70
heat shock cognate 70
- Hsp
heat shock protein
- HO1
heme oxygenase 1
- MAP2
microtubule associated protein 2
- mtHsp70
mitochondrial Hsp70
- SnPPx
tin protoporphyrin
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as an ‘Accepted Article’, doi: 10.1111/jnc.13041
We have no conflicts of interest to disclose.
References
- Adam C, Baeurle A, Brodsky JL, Wipf P, Schrama D, Becker JC, Houben R. The HSP70 Modulator MAL3-101 Inhibits Merkel Cell Carcinoma. PloS one. 2014;9:e92041. doi: 10.1371/journal.pone.0092041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa S, Matsuzawa F, Satoh Y, Kadota Y, Doi H, Itoh K. Prediction of the mechanism of action of omuralide (clasto-lactacystin beta-lactone) on human cathepsin A based on a structural model of the yeast proteasome beta5/PRE2-subunit/omuralide complex. Biochimica et biophysica acta. 2006;1764:1372–1380. doi: 10.1016/j.bbapap.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Alladi PA, Mahadevan A, Vijayalakshmi K, Muthane U, Shankar SK, Raju TR. Ageing enhances alpha-synuclein, ubiquitin and endoplasmic reticular stress protein expression in the nigral neurons of Asian Indians. Neurochemistry international. 2010;57:530–539. doi: 10.1016/j.neuint.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH. Chaperone-mediated autophagy markers in Parkinson disease brains. Archives of neurology. 2010;67:1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Annals of neurology. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J, Lintner F, Jellinger KA. Olfactory involvement in aging and Alzheimer's disease: an autopsy study. Journal of Alzheimer's disease. 2005;7:149–157. doi: 10.3233/jad-2005-7208. discussion 173-180. [DOI] [PubMed] [Google Scholar]
- Attems J, Walker L, Jellinger KA. Olfactory bulb involvement in neurodegenerative diseases. Acta neuropathologica. 2014;127:459–475. doi: 10.1007/s00401-014-1261-7. [DOI] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Molecular and cellular biology. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Neocortical Development. Raven Press; 1991. [Google Scholar]
- Beere HM. Stressed to death: regulation of apoptotic signaling pathways by the heat shock proteins. Sci STKE. 2001:RE1. doi: 10.1126/stke.2001.93.re1. 2001. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Muller ML, Kotagal V, Koeppe RA, Kilbourn MA, Albin RL, Frey KA. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson's disease. Brain. 2010;133:1747–1754. doi: 10.1093/brain/awq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of aging. 2003a;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Sandmann-Kiel D, Rub U, Schultz C. Nerve cells expressing heat-shock proteins in Parkinson's disease. Acta Neuropathol (Berl) 2001;102:449–454. doi: 10.1007/s004010100395. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. Journal of neural transmission. 2003b;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Braunstein MJ, Scott SS, Scott CM, et al. Antimyeloma Effects of the Heat Shock Protein 70 Molecular Chaperone Inhibitor MAL3-101. Journal of oncology. 2011;2011:232037. doi: 10.1155/2011/232037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodoehl S, Klingner C, Volk GF, Bitter T, Witte OW, Redecker C. Decreased olfactory bulb volume in idiopathic Parkinson's disease detected by 3.0-tesla magnetic resonance imaging. Movement disorders. 2012;27:1019–1025. doi: 10.1002/mds.25087. [DOI] [PubMed] [Google Scholar]
- Budina-Kolomets A, Balaburski GM, Bondar A, Beeharry N, Yen T, Murphy ME. Comparison of the activity of three different HSP70 inhibitors on apoptosis, cell cycle arrest, autophagy inhibition, and HSP90 function. Cancer biology & therapy. 2013;15:194–199. doi: 10.4161/cbt.26720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Bader Lange ML, Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer's disease. Biochimica et biophysica acta. 2010;1801:924–929. doi: 10.1016/j.bbalip.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Ravagna A, Colombrita C, Spadaro F, Butterfield DA, Giuffrida Stella AM. Increased expression of heat shock proteins in rat brain during aging: relationship with mitochondrial function and glutathione redox state. Mech Ageing Dev. 2004;125:325–335. doi: 10.1016/j.mad.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Carr VM, Morimoto RI, Farbman AI. Development and further characterization of a small subclass of rat olfactory receptor neurons that shows immunoreactivity for the HSP70 heat shock protein. The Journal of comparative neurology. 1999;404:375–386. [PubMed] [Google Scholar]
- Carr VM, Murphy SP, Morimoto RI, Farbman AI. Small subclass of rat olfactory neurons with specific bulbar projections is reactive with monoclonal antibodies to the HSP70 heat shock protein. The Journal of comparative neurology. 1994;348:150–160. doi: 10.1002/cne.903480109. [DOI] [PubMed] [Google Scholar]
- Cecarini V, Ding Q, Keller JN. Oxidative inactivation of the proteasome in Alzheimer's disease. Free radical research. 2007;41:673–680. doi: 10.1080/10715760701286159. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Andrulis M, Stuhmer T, et al. The PI3K/Akt signaling pathway regulates the expression of Hsp70, which critically contributes to Hsp90-chaperone function and tumor cell survival in multiple myeloma. Haematologica. 2013;98:1132–1141. doi: 10.3324/haematol.2012.066175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson's disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35:385–398. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Daniel SE, Hawkes CH. Preliminary diagnosis of Parkinson's disease by olfactory bulb pathology. Lancet. 1992;340:186. doi: 10.1016/0140-6736(92)93275-r. [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Sultana R, Tiu GF, Scheff NN, Perluigi M, Cini C, Butterfield DA. Protein levels of heat shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic mild cognitive impairment: an investigation on the role of cellular stress response in the progression of Alzheimer disease. Brain Res Brain Res Protoc. 2010;1333:72–81. doi: 10.1016/j.brainres.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey C, Kraft C, Jinwal U, et al. Aging analysis reveals slowed tau turnover and enhanced stress response in a mouse model of tauopathy. The American journal of pathology. 2009;174:228–238. doi: 10.2353/ajpath.2009.080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. Neuropathology of non-Alzheimer degenerative disorders. International journal of clinical and experimental pathology. 2009;3:1–23. [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Wolfer DP, Lipp HP, Bueler H. Hsp70 gene transfer by adeno-associated virus inhibits MPTP-induced nigrostriatal degeneration in the mouse model of Parkinson disease. Molecular therapy. 2005;11:80–88. doi: 10.1016/j.ymthe.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Drummond GS, Kappas A. Prevention of neonatal hyperbilirubinemia by tin protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:6466–6470. doi: 10.1073/pnas.78.10.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser C, Alberti S, Hohfeld J. Cooperation of molecular chaperones with the ubiquitin/proteasome system. Biochimica et biophysica acta. 2004;1695:171–188. doi: 10.1016/j.bbamcr.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Fewell SW, Smith CM, Lyon MA, Dumitrescu TP, Wipf P, Day BW, Brodsky JL. Small molecule modulators of endogenous and co-chaperone-stimulated Hsp70 ATPase activity. The Journal of biological chemistry. 2004;279:51131–51140. doi: 10.1074/jbc.M404857200. [DOI] [PubMed] [Google Scholar]
- Frenkel L, Dimant B, Portiansky EL, Maldonado H, Delorenzi A. Both heat shock and water deprivation trigger Hsp70 expression in the olfactory lobe of the crab Chasmagnathus granulatus. Neuroscience letters. 2008;443:251–256. doi: 10.1016/j.neulet.2008.07.072. [DOI] [PubMed] [Google Scholar]
- Frydman J, Hohfeld J. Chaperones get in touch: the Hip-Hop connection. Trends Biochem Sci. 1997;22:87–92. doi: 10.1016/s0968-0004(97)01005-0. [DOI] [PubMed] [Google Scholar]
- Getchell TV, Krishna NS, Dhooper N, Sparks DL, Getchell ML. Human olfactory receptor neurons express heat shock protein 70: age-related trends. The Annals of otology, rhinology, and laryngology. 1995;104:47–56. doi: 10.1177/000348949510400108. [DOI] [PubMed] [Google Scholar]
- Gleixner AM, Pulugulla SH, Pant DB, Posimo JM, Crum TS, Leak RK. Impact of aging on heat shock protein expression in the substantia nigra and striatum of the female rat. Cell and tissue research. 2014;357:43–54. doi: 10.1007/s00441-014-1852-6. [DOI] [PubMed] [Google Scholar]
- Gupte AA, Morris JK, Zhang H, Bomhoff GL, Geiger PC, Stanford JA. Age-related changes in HSP25 expression in basal ganglia and cortex of F344/BN rats. Neuroscience letters. 2010;472:90–93. doi: 10.1016/j.neulet.2010.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatic H, Kane MJ, Saykally JN, Citron BA. Modulation of transcription factor Nrf2 in an in vitro model of traumatic brain injury. Journal of neurotrauma. 2012;29:1188–1196. doi: 10.1089/neu.2011.1806. [DOI] [PubMed] [Google Scholar]
- Hauser MA, Li YJ, Xu H, et al. Expression profiling of substantia nigra in Parkinson disease, progressive supranuclear palsy, and frontotemporal dementia with parkinsonism. Archives of neurology. 2005;62:917–921. doi: 10.1001/archneur.62.6.917. [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathology and applied neurobiology. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfert RH, Glatz FR, 3rd, Wilson TS, Ramkumar V, Hughes LF. Hsp70 in the inferior colliculus of Fischer-344 rats: effects of age and acoustic stress. Hearing research. 2002;170:155–165. doi: 10.1016/s0378-5955(02)00487-2. [DOI] [PubMed] [Google Scholar]
- Hirata K, Kanemaru T, Minohara M, Togo A, Kira J. Accumulation of stress-related proteins within the glomeruli of the rat olfactory bulb following damage to olfactory receptor neurons. Archives of histology and cytology. 2008;71:265–277. doi: 10.1679/aohc.71.265. [DOI] [PubMed] [Google Scholar]
- Hohfeld J, Cyr DM, Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO reports. 2001;2:885–890. doi: 10.1093/embo-reports/kve206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohfeld J, Minami Y, Hartl FU. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, Eikelenboom P, Scheper W. The unfolded protein response is activated in Alzheimer's disease. Acta neuropathologica. 2005;110:165–172. doi: 10.1007/s00401-005-1038-0. [DOI] [PubMed] [Google Scholar]
- Horwich AL, Low KB, Fenton WA, Hirshfield IN, Furtak K. Folding in vivo of bacterial cytoplasmic proteins: role of GroEL. Cell. 1993;74:909–917. doi: 10.1016/0092-8674(93)90470-b. [DOI] [PubMed] [Google Scholar]
- Huryn DM, Brodsky JL, Brummond KM, et al. Chemical methodology as a source of small-molecule checkpoint inhibitors and heat shock protein 70 (Hsp70) modulators. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6757–6762. doi: 10.1073/pnas.1015251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SG, Ramaiah KV. Reduced eIF2alpha phosphorylation and increased proapoptotic proteins in aging. Biochemical and biophysical research communications. 2007;355:365–370. doi: 10.1016/j.bbrc.2007.01.156. [DOI] [PubMed] [Google Scholar]
- Kalia SK, Kalia LV, McLean PJ. Molecular chaperones as rational drug targets for Parkinson's disease therapeutics. CNS & neurological disorders drug targets. 2010;9:741–753. doi: 10.2174/187152710793237386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nature reviews. Molecular cell biology. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Kibayashi K. Mild hypothermia facilitates the expression of cold-inducible RNA-binding protein and heat shock protein 70.1 in mouse brain. Brain Res Brain Res Protoc. 2012;1466:128–136. doi: 10.1016/j.brainres.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Keller JN, Gee J, Ding Q. The proteasome in brain aging. Ageing research reviews. 2002;1:279–293. doi: 10.1016/s1568-1637(01)00006-x. [DOI] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR. Impaired proteasome function in Alzheimer's disease. Journal of neurochemistry. 2000a;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR. Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech Ageing Dev. 2000b;113:61–70. doi: 10.1016/s0047-6374(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Kilpatrick K, Novoa JA, Hancock T, Guerriero CJ, Wipf P, Brodsky JL, Segatori L. Chemical induction of Hsp70 reduces alpha-synuclein aggregation in neuroglioma cells. ACS chemical biology. 2013;8:1460–1468. doi: 10.1021/cb400017h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol. 2001;8:739–758. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- Kumar P, Ambasta RK, Veereshwarayya V, Rosen KM, Kosik KS, Band H, Mestril R, Patterson C, Querfurth HW. CHIP and HSPs interact with beta-APP in a proteasome-dependent manner and influence Abeta metabolism. Human molecular genetics. 2007;16:848–864. doi: 10.1093/hmg/ddm030. [DOI] [PubMed] [Google Scholar]
- Lanneau D, Wettstein G, Bonniaud P, Garrido C. Heat shock proteins: cell protection through protein triage. The Scientific World Journal. 2010;10:1543–1552. doi: 10.1100/tsw.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak RK. Heat shock proteins in neurodegenerative disorders and aging. Journal of cell communication and signaling. 2014;8:293–310. doi: 10.1007/s12079-014-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- Lee KS, Chung JH, Oh BH, Hong CH. Increased plasma levels of heat shock protein 70 in patients with vascular mild cognitive impairment. Neuroscience letters. 2008;436:223–226. doi: 10.1016/j.neulet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic acids research. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45:1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Macias AT, Williamson DS, Allen N, et al. Adenosine-derived inhibitors of 78 kDa glucose regulated protein (Grp78) ATPase: insights into isoform selectivity. Journal of medicinal chemistry. 2011;54:4034–4041. doi: 10.1021/jm101625x. [DOI] [PubMed] [Google Scholar]
- Magrane J, Smith RC, Walsh K, Querfurth HW. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. The Journal of neuroscience. 2004;24:1700–1706. doi: 10.1523/JNEUROSCI.4330-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel S, Grunblatt E, Riederer P, Amariglio N, Jacob-Hirsch J, Rechavi G, Youdim MB. Gene expression profiling of sporadic Parkinson's disease substantia nigra pars compacta reveals impairment of ubiquitin-proteasome subunits, SKP1A, aldehyde dehydrogenase, and chaperone HSC-70. Annals of the New York Academy of Sciences. 2005;1053:356–375. doi: 10.1196/annals.1344.031. [DOI] [PubMed] [Google Scholar]
- Massey AJ, Williamson DS, Browne H, et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer chemotherapy and pharmacology. 2010;66:535–545. doi: 10.1007/s00280-009-1194-3. [DOI] [PubMed] [Google Scholar]
- McNaught KS, Belizaire R, Jenner P, Olanow CW, Isacson O. Selective loss of 20S proteasome alpha-subunits in the substantia nigra pars compacta in Parkinson's disease. Neuroscience letters. 2002;326:155–158. doi: 10.1016/s0304-3940(02)00296-3. [DOI] [PubMed] [Google Scholar]
- Miller FD, Gauthier AS. Timing is everything: making neurons versus glia in the developing cortex. Neuron. 2007;54:357–369. doi: 10.1016/j.neuron.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Minami Y, Hohfeld J, Ohtsuka K, Hartl FU. Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. The Journal of biological chemistry. 1996;271:19617–19624. doi: 10.1074/jbc.271.32.19617. [DOI] [PubMed] [Google Scholar]
- Molochnikov L, Rabey JM, Dobronevsky E, et al. A molecular signature in blood identifies early Parkinson's disease. Molecular neurodegeneration. 2012;7:26. doi: 10.1186/1750-1326-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JF, Duda JE. Olfaction as a biomarker in Parkinson's disease. Biomarkers in medicine. 2010;4:661–670. doi: 10.2217/bmm.10.95. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Foresti R. Heme oxygenase-1 as a target for drug discovery. Antioxidants & redox signaling. 2014;20:1810–1826. doi: 10.1089/ars.2013.5658. [DOI] [PubMed] [Google Scholar]
- Mu TW, Ong DS, Wang YJ, Balch WE, Yates JR, 3rd, Segatori L, Kelly JW. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008;134:769–781. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nature reviews. Neuroscience. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- Muller A, Mungersdorf M, Reichmann H, Strehle G, Hummel T. Olfactory function in Parkinsonian syndromes. Journal of clinical neuroscience. 2002;9:521–524. doi: 10.1054/jocn.2001.1071. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Yang DS. Autophagy and neuronal cell death in neurological disorders. Cold Spring Harbor perspectives in biology. 2012;4:pii: a008839. doi: 10.1101/cshperspect.a008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EG, Shen GX. Impact of exercise and metabolic disorders on heat shock proteins and vascular inflammation. Autoimmune diseases. 2012;2012:836519. doi: 10.1155/2012/836519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm TG, Braak H. Olfactory bulb changes in Alzheimer's disease. Acta neuropathologica. 1987;73:365–369. doi: 10.1007/BF00688261. [DOI] [PubMed] [Google Scholar]
- Otero JH, Lizak B, Hendershot LM. Life and death of a BiP substrate. Semin Cell Dev Biol. 2010;21:472–478. doi: 10.1016/j.semcdb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz Gavilan M, Vela J, Castano A, Ramos B, del Rio JC, Vitorica J, Ruano D. Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiology of aging. 2006;27:973–982. doi: 10.1016/j.neurobiolaging.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Pearce RK, Hawkes CH, Daniel SE. The anterior olfactory nucleus in Parkinson's disease. Movement disorders. 1995;10:283–287. doi: 10.1002/mds.870100309. [DOI] [PubMed] [Google Scholar]
- Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- Ponsen MM, Stoffers D, Wolters E, Booij J, Berendse HW. Olfactory testing combined with dopamine transporter imaging as a method to detect prodromal Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry. 2010;81:396–399. doi: 10.1136/jnnp.2009.183715. [DOI] [PubMed] [Google Scholar]
- Posimo JM, Titler AM, Choi HJ, Unnithan AS, Leak RK. Neocortex and allocortex respond differentially to cellular stress in vitro and aging in vivo. PloS one. 2013;8:e58596. doi: 10.1371/journal.pone.0058596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posimo JM, Unnithan AS, Gleixner AM, Choi HJ, Jiang Y, Pulugulla SH, Leak RK. Viability assays for cells in culture. Journal of visualized experiments. 2014;83:e50645. doi: 10.3791/50645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawek K, Stege GJ, Bosman GJ. Dementia, gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson's disease. Neuroreport. 1999;10:2273–2276. doi: 10.1097/00001756-199908020-00009. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. The Journal of cell biology. 2010;189:783–794. doi: 10.1083/jcb.201003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabova NA, Marchenkov VV, Marchenkova SY, Kotova NV, Semisotnov GV. Molecular chaperone GroEL/ES: unfolding and refolding processes. Biochemistry. 2013;78:1405–1414. doi: 10.1134/S0006297913130038. [DOI] [PubMed] [Google Scholar]
- Sadowski M, Sarcevic B. Mechanisms of mono- and poly-ubiquitination: Ubiquitination specificity depends on compatibility between the E2 catalytic core and amino acid residues proximal to the lysine. Cell division. 2010;5:19. doi: 10.1186/1747-1028-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykally JN, Rachmany L, Hatic H, Shaer A, Rubovitch V, Pick CG, Citron BA. The nuclear factor erythroid 2-like 2 activator, tert-butylhydroquinone, improves cognitive performance in mice after mild traumatic brain injury. Neuroscience. 2012;223:305–314. doi: 10.1016/j.neuroscience.2012.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Bennett DA, Liberman A, Bienias JL, Schneider JA, Kelly J, Arvanitakis Z. Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiology of aging. 2006;27:252–261. doi: 10.1016/j.neurobiolaging.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Schlecht R, Scholz SR, Dahmen H, et al. Functional analysis of Hsp70 inhibitors. PloS one. 2013;8:e78443. doi: 10.1371/journal.pone.0078443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29:15–32. doi: 10.1016/s0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- Shinohara H, Inaguma Y, Goto S, Inagaki T, Kato K. Alpha B crystallin and HSP28 are enhanced in the cerebral cortex of patients with Alzheimer's disease. Journal of the neurological sciences. 1993;119:203–208. doi: 10.1016/0022-510x(93)90135-l. [DOI] [PubMed] [Google Scholar]
- Silva PN, Furuya TK, Braga IL, et al. Analysis of HSPA8 and HSPA9 mRNA expression and promoter methylation in the brain and blood of Alzheimer's disease patients. Journal of Alzheimer's disease. 2014;38:165–170. doi: 10.3233/JAD-130428. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta neuropathologica. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler RA, Gao Y, Signore AP, Cao G, Chen J. HSP27: mechanisms of cellular protection against neuronal injury. Current molecular medicine. 2009;9:863–872. doi: 10.2174/156652409789105561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, et al. Rotenone, Paraquat and Parkinson's Disease. Environ Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Laak HJ, Renkawek K, van Workum FP. The olfactory bulb in Alzheimer disease: a morphologic study of neuron loss, tangles, and senile plaques in relation to olfaction. Alzheimer disease and associated disorders. 1994;8:38–48. [PubMed] [Google Scholar]
- Tohgi H, Utsugisawa K, Yoshimura M, Yamagata M, Nagane Y. Heat-shock cognate 70 messenger RNA expression in postmortem human hippocampus: regional differences and age-related changes. Neuroscience letters. 1995;196:89–92. doi: 10.1016/0304-3940(95)11854-p. [DOI] [PubMed] [Google Scholar]
- Unnithan AS, Choi HJ, Titler AM, Posimo JM, Leak RK. Rescue from a two hit, high-throughput model of neurodegeneration with N-acetyl cysteine. Neurochemistry international. 2012;61:356–368. doi: 10.1016/j.neuint.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Unno K, Asakura H, Shibuya Y, Kaiho M, Okada S, Oku N. Increase in basal level of Hsp70, consisting chiefly of constitutively expressed Hsp70 (Hsc70) in aged rat brain. The journals of gerontology. 2000;55:B329–335. doi: 10.1093/gerona/55.7.b329. [DOI] [PubMed] [Google Scholar]
- Uryu K, Richter-Landsberg C, Welch W, et al. Convergence of heat shock protein 90 with ubiquitin in filamentous alpha-synuclein inclusions of alpha-synucleinopathies. The American journal of pathology. 2006;168:947–961. doi: 10.2353/ajpath.2006.050770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnav RA, Getchell ML, Poon HF, Barnett KR, Hunter SA, Pierce WM, Klein JB, Butterfield DA, Getchell TV. Oxidative stress in the aging murine olfactory bulb: redox proteomics and cellular localization. Journal of neuroscience research. 2007;85:373–385. doi: 10.1002/jnr.21130. [DOI] [PubMed] [Google Scholar]
- Wang J, You H, Liu JF, Ni DF, Zhang ZX, Guan J. Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. AJNR. American journal of neuroradiology. 2011;32:677–681. doi: 10.3174/ajnr.A2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DS, Borgognoni J, Clay A, et al. Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. Journal of medicinal chemistry. 2009;52:1510–1513. doi: 10.1021/jm801627a. [DOI] [PubMed] [Google Scholar]
- Yokota T, Mishra M, Akatsu H, et al. Brain site-specific gene expression analysis in Alzheimer's disease patients. European journal of clinical investigation. 2006;36:820–830. doi: 10.1111/j.1365-2362.2006.01722.x. [DOI] [PubMed] [Google Scholar]
- Yoo BC, Kim SH, Cairns N, Fountoulakis M, Lubec G. Deranged expression of molecular chaperones in brains of patients with Alzheimer's disease. Biochemical and biophysical research communications. 2001;280:249–258. doi: 10.1006/bbrc.2000.4109. [DOI] [PubMed] [Google Scholar]
- Zhang Y, James M, Middleton FA, Davis RL. Transcriptional analysis of multiple brain regions in Parkinson's disease supports the involvement of specific protein processing, energy metabolism, and signaling pathways, and suggests novel disease mechanisms. American journal of medical genetics. Part B, Neuropsychiatric genetics. 2005;137B:5–16. doi: 10.1002/ajmg.b.30195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.