Abstract
PURPOSE
To evaluate the effectiveness of artificial tears and corticosteroids on mitigating the acute ocular surface response to low humidity environments.
DESIGN
Single-group, crossover clinical trial.
METHODS
Twenty subjects with aqueous deficient dry eye were enrolled. Subjects meeting inclusion criteria at visit 1 and were exposed to a baseline 90-minute low humidity environment at visit 2. They then used artificial tears for 2 weeks prior to low humidity exposure at visit 3, followed by 0.1% dexamethasone for two weeks prior to the final low humidity exposure at visit 4. Outcome measures included corneal and conjunctival staining, blink rate and irritation symptoms before and after each low humidity exposure. Digital polymerase chain reaction (PCR) was performed to measure HLA-DR RNA transcripts in conjunctival cells taken by impression cytology at each visit.
RESULTS
There was significantly less corneal and conjunctival epitheliopathy after the low humidity exposure at visit 4 compared to after the low humidity exposure at visit 3 (p= 0.003). Subjects reported significantly less eye irritation during the low humidity exposure after using the dexamethasone (visit 4) compared to artificial tears (visit 3) (p=0.01). HLA-DR transcripts significantly decreased after the stress at visit 4 (post dexamethasone) compared to visit 2.
CONCLUSION
Our study demonstrates corticosteroid eye drops mitigate the acute adverse effects of an experimental low humidity challenge, likely due to suppression of stress-activated inflammatory pathways. While extended use of corticosteroids is not indicated, other anti-inflammatory therapies with activity against stress-activated pathways may prove as effective.
INTRODUCTION
Dry eye is a multifactorial disease of the tears and ocular surface. The tear-secreting glands and the ocular surface function as a complex integrated unit, interconnected by sensory and autonomic nerves.1 Dysfunction of any part of this unit results in an unstable and hyperosmolar tear film that no longer adequately supports the ocular surface.2 Inflammation is a consequence of this dysfunction and contributes to tear instability, corneal epithelial disease and ocular discomfort.3 Thus, dry eye disease is usually accompanied by symptoms of eye irritation, visual disturbances, tear film instability and damage to the ocular surface, which can impair quality of life.4, 5
Certain environmental factors, such as low humidity, extended computer usage, and air drafts have been shown to contribute to the development of dry eye symptoms in normal individuals, as well as worsen symptoms and signs in those with dry eye disease.6,7 This may be a result of the desiccating stress that air drafts, low humidity, and decreased blinking have on the ocular surface. This stress leads to increased tear instability, evaporation and osmolarity, which contributes to a downstream cascade of events that promote production of inflammatory mediators (cytokines and matrix metalloproteinases), epithelial apoptosis and loss of conjunctival goblet cells.3 Moreover, this desiccating stress has been shown to decrease tear production and the protective function of the tear film.8,9
We previously demonstrated using an environmentally controlled goggles system, that certain factors, such as low tear volume, render individuals with dry eye disease more susceptible to an ocular surface response to desiccating environmental stimuli.7 Thus, reduced tear production from aging, use of systemic medications with anticholinergic effects or autoimmune conditions such as Sjögren syndrome and rheumatoid arthritis may contribute to development of environmentally induced dry eye.10,11,12
Despite the adverse effects of low humidity environments on the ocular surface, many people spend the majority of their waking hours in air-conditioned, low humidity and drafty environments. Low humidity environments are ubiquitous. The 2009 Residential Energy Consumption Survey (RECS) performed by the United States Energy Information Administration found that 87% of households and almost all office buildings and commercial buildings in the United States are air-conditioned. Additionally, an ambient relative low humidity of 40-60% is recommended for office buildings. This is problematic because desiccation of the ocular surface due to low humidity is a potent inflammatory stress.13,14 Normal individuals have the ability to recover from the adverse environmental exposure, via reflex tearing from the lacrimal glands and goblet cells.15 Aqueous deficient dry eye patients, on the other hand, have decreased goblet cell number and mucus production, reduced tear volume and may have loss of reflex tearing due to decreased corneal sensitivity or lacrimal gland inflammation.16,17
Because exposure to low humidity environments is difficult to avoid, the use of therapies to mitigate the adverse effects of desiccating environmental stimuli is critical in preventing the development and/or progression of dry eye disease. Topically applied corticosteroid, but not saline, was reported to prevent corneal barrier disruption in response to experimental low humidity environmental challenge in mice.18 The efficacy of topical corticosteroid in preventing worsening ocular surface epithelial disease in dry eye patients subjected to a low humidity environment has not been previously evaluated. The purpose of this study was to evaluate the effectiveness of two therapies, artificial tears and corticosteroid eye drops, on mitigating the acute ocular surface response to low humidity environments. The study was designed to sequentially evaluate the effects of artificial tears followed by a corticosteroid because most dry eye patients initially treat environmentally induced irritation with over the counter (OTC) artificial tears.
METHODS
Study design
We performed a prospective, single-group, crossover clinical trial to determine the effectiveness of artificial tears, which hydrate and lubricate the ocular surface, and corticosteroid eye drops, which inhibit inflammation, on mitigating the adverse effects of a short-term low humidity desiccating environment on the severity of irritation symptoms and ocular surface epithelial disease. The study was conducted in accordance with the Declaration of Helsinki, and the Baylor College of Medicine Institutional Review Board approved the protocol and informed consent form prior to study initiation. The trial was also registered with clinicaltrials.org (# NCT01797822).
Twenty subjects were enrolled in the study after providing written informed consent. Subjects were selected based on the following inclusion criteria: inferior tear meniscus height of ≤ 230 um as a measure of low tear volume, corneal and conjunctival dye staining score of ≥ 3 in at least one eye, normal corneal sensitivity (score ≥ 5 measured with the Cochet Bonnet esthesiometer), absence of other ocular surface disease, including anterior blepharitis, meibomian gland disease, conjunctivochalasis and conjunctival scarring, tear break of time (TBUT) < 7 seconds, and ocular surface disease index (OSDI) symptom score > 20. Patients were excluded if they had a corneal transplant, retinal detachment or glaucoma filtering surgery in the past, cataract or LASIK surgery within 12 months of study enrollment, diabetes mellitus, uncontrolled systemic infection or inflammation or used prescription eye drops within 4 weeks of study enrollment.
Experimental Low Humidity Environment
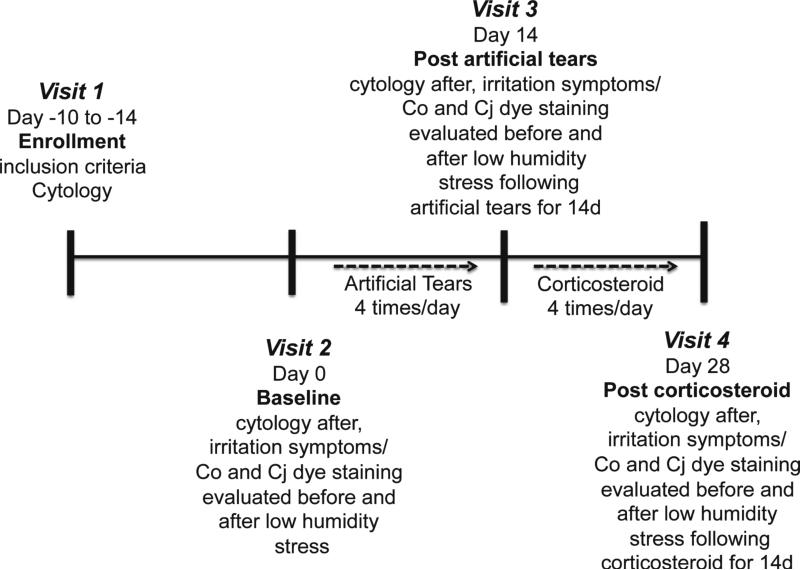
The study design is summarized in Figure 1. Subjects who met inclusion criteria were enrolled at visit 1 and returned 10 to 14 days later to be subjected to a baseline controlled low humidity environment using a previously reported goggles system (visit 2), and repeat low humidity exposures after using artificial tears for 14 days (visit 3) followed by dexamethasone drops for 14 days (visit 4), for a total of three low humidity challenges during the study period. At each visit, subjects wore the controlled environmental goggles for 90 minutes while watching a movie on a video display placed on a stand, elevated slightly above eye level, 4.5 feet away.7 Ambient air was pumped continuously at a flow rate of 2 to 5 L/min through a conditioning system into the goggles such that the air surrounding the eyes and eyelids maintained a relative humidity of 18% to 25% throughout the test period. Relative humidity, temperature and airflow was measured every 10 seconds by sensors in the goggles and was recorded in an electronic database.
Figure 1.
Summary of study design to investigate the effects of dry eye therapies on environmentally induced ocular surface disease. The study consisted of 4 visits. Study visit 1 (enrollment) established that subjects met inclusion criteria for signs and symptoms and conjunctival cytology was performed; at study visit 2 (baseline), irritation symptoms and corneal and conjunctival dye staining were measured before and after experimental low humidity stress and cytology was performed after the stress; at study visit 3 (post artificial tears) - irritation symptoms and corneal and conjunctival dye staining were measured before and after experimental low humidity stress in subjects treated with preservative-free artificial tear drops 4 times daily for 14 days, cytology was performed after the stress; at study visit 4 (post corticosteroid) - irritation symptoms and corneal and conjunctival dye staining were measured before and after experimental low humidity stress in subjects treated with corticosteroid (dexamethasone 0.1%) drops 4 times daily for 14 days, cytology was performed after the stress. Co = corneal, Cj = conjunctival
Blink Rate Measurement
Blink rate was measured using electromyography (EMG) signals detected by the NeuroSky(TM) MindBand Bluetooth device (NeuroSky, Silicon Valley, CA). The MindBand was placed on the forehead directly above the goggles, for the duration of the 90 minute test period. The dry electrodes on the MindBand measured the changing electrical potential of muscles during blinks. The signal was sent by Bluetooth to a computer, translated into real-time waveforms by NeuroSky software, and displayed in the ClimaTears Test Suite (Biocentric Developments LLC, Austin, TX). To determine the blink threshold specific to each subject, the size of the waveform was observed and the threshold was set to the point at which the waveform consistently crossed the threshold during a blink. The blink count readings were verified with manual blink counting for one minute by the investigator at 5 minutes, 45 minutes, and 75 minutes into the study.
Assessments
The same examiner performed all of the objective clinical tests. An ocular surface evaluation was performed before and after the 90-minute low humidity exposure at visits 2, 3 and 4 (Figure 1). Tear and ocular surface evaluations included fluorescein tear break up time (TBUT) as a measure of tear stability, and corneal fluorescein and conjunctival lissamine green staining as measures of epithelial cell health. Tear breakup time (TBUT) was performed by a previously reported method.17 Corneal fluorescein staining viewed through a yellow filter was graded in 5 zones in the cornea and 6 zones in the exposed bulbar conjunctiva (3 nasal and 3 temporal) using a previously reported grading scheme that was corrected for the percent area of staining in each zone. 17,20 In addition to biomicroscopic grading of the fluorescein staining at the time of each visit, the corneal fluorescein staining was also imaged using the camera of an iPod Touch (Apple, Cupertino, CA) with the ProCamera application (Cocologics, Mannheim, Germany) at each visit before and after the low humidity challenge. The same examiner viewed the videos and graded the staining, while blinded to subjects’ identity, at the conclusion of the study.
Severity of eye irritation symptoms was measured using a validated questionnaire adapted from the Ocular Surface Disease Index (OSDI).4 The questionnaire contained five questions regarding the frequency of photophobia, gritty/sandy sensation, burning/stinging, blurred vision and fluctuating vision. We also assessed severity of ocular symptoms using a Visual Analog Scale (VAS) measuring the severity and frequency of dry eye symptoms, severity of blurred vision related to dry eye and frequency of blinking.
Treatment
After the baseline low humidity exposure (visit 2), subjects used artificial tears (preservative- free Refresh Optive Sensitive, Allergan, Inc. Irvine, CA) four times daily for two weeks and returned to our clinic for a second 90-minute exposure to the low humidity environment (visit 3). After the third visit, subjects used preservative free 0.1% dexamethasone solution (Greenpark pharmacy, Houston, TX) four times daily for two weeks and returned to our clinic for a third and final 90-minute exposure to the low humidity environment at visit 4.
Conjunctival Gene Expression
Cells obtained by impression cytology of the nasal bulbar conjunctiva of each eye using the EyePrim device (OpiaTech, Paris France) that applies a Supor 450 membrane (Pall, Port Washington, NY) with uniform pressure to the conjunctival surface, were placed in 0.5mL of RNA lysis buffer (Qiagen, Valencia, CA, USA) and stored at −80°C. Total RNA was isolated from the membranes using an RNeasy Mini Plus Kit (Qiagen, Valencia, CA) following the manufacturer protocol. Cytology was performed at the enrollment visit and following the low humidity exposure at visits 2-4. The RNA concentration was measured by its absorption at 260 nm using a spectrophotometer (NanoDrop 2000, Thermo Scientific, Wilmington, DE) and first strand cDNA was synthesized using random hexamers and M-MuLV reverse transcriptase (Ready-To-Go You-Prime First-Strand Beads; Amersham Pharmacia Biotech, Inc., Piscataway, NJ) and the DNA concentration was measured with Qubit spectrophotometer (Life Technologies, Grand Island, NY). Digital PCR to detect copy number of HLA-DR transcripts was performed with a QuantStudio™ 3D Digital PCR system (Life Technologies, Grand Island, NY) according to the manufacturer's instructions and normalized by concentration of cDNA. The fold changes in HLA-DR expression relative to the baseline visit were calculated for each patient and the fold changes among all patients per visit were averaged.
Statistical Analyses
We used GraphPad (Prism 6, La Jolla, CA) to perform sample size calculations using the results from a prior study of 25 subjects exposed to a low humidity environmental challenge.7 A sample size of 20 subjects was calculated to have 90% power of detecting a statistical difference (p=0.05) in total corneal and conjunctival staining before and after the environmental challenge. Data was compared using paired t-test or repeated measures analysis of variance (one-way ANOVA) with GraphPad Prism (La Jolla, CA).
RESULTS
Twenty subjects meeting the inclusion criteria were enrolled in the study. All subjects had aqueous tear deficiency and were classified as either non-Sjögren syndrome aqueous tear deficiency (non-SS ATD) (n=14) or Sjögren syndrome aqueous tear deficiency (SS ATD) (n=6) using the proposed American College of Rheumatology criteria.21 We chose subjects with aqueous tear deficient dry eye based on prior findings of a significant ocular surface response to a controlled low humidity environment in this patient population.7 One subject had LASIK surgery 13 years ago and none had systemic diseases except for Sjögren syndrome.
Corneal Staining and tear break-up time
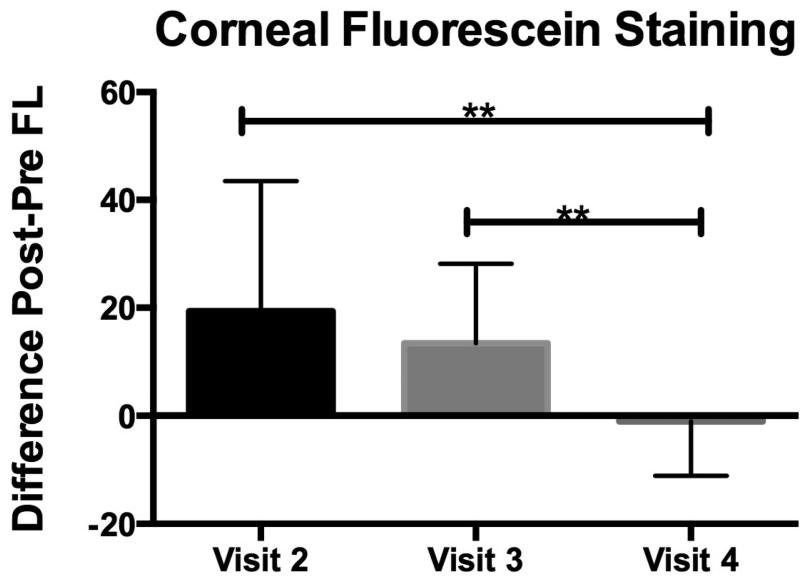
Fluorescein staining is an indicator of corneal epithelial barrier disruption from epithelial cell damage that is attributed to dry eye induced stress and inflammation on the ocular surface.3 We calculated the difference (deltas, n=20 eyes) in fluorescein corneal staining before (pre) and after (post) the low humidity challenge at visits 2-4. There was no statistical difference in the deltas between visits 2 (baseline with no treatment) and 3 (post artificial tears). However, there was a statistically significance difference (p=0.002) between visits 2 and 4 (post steroid), and visits 3 and 4 (p=0.003)(Figure 2). Subjects with SS ATD had a similar magnitude of response as those with non-SS ATD. Heterogeneity in subject responses consisted of varying magnitudes of worsening in corneal fluorescein staining after low humidity exposure. There was no statistical difference in tear break-up time before and after the low humidity challenge at any of the visits.
Figure 2.
Post to pre difference in corneal fluorescein staining after exposure to low humidity goggles environment at study visit 2 (baseline), visit 3 (post artificial tears) and 4 (post dexamethasone). Mean differences in corneal fluorescein staining scores graded after goggles (post) and before goggles (pre) at visits 2-4 where the 90-minute low humidity exposure was performed (n=20). **Denotes significance between groups indicated (p<0.005).
Conjunctival Staining
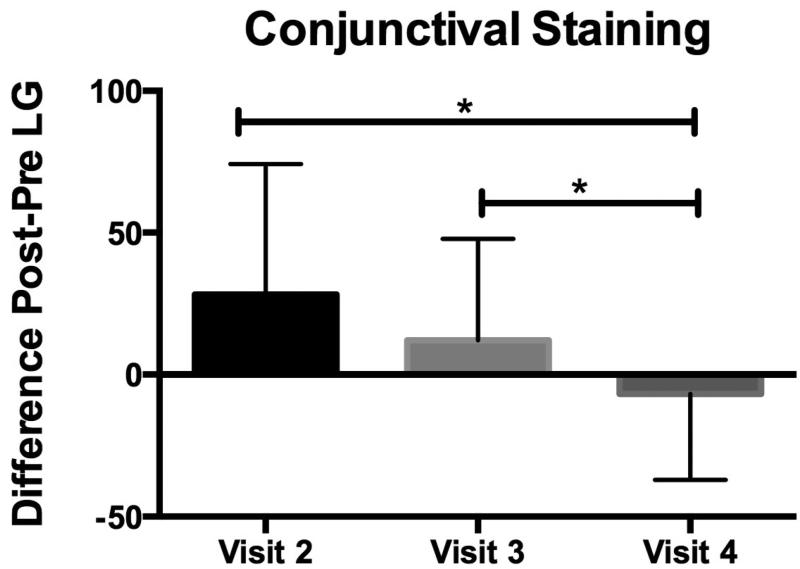
Lissamine green staining is routinely used to evaluate conjunctival cell membrane abnormalities in dry eye.3 We calculated the difference (delta, n=20 eyes) in conjunctival lissamine green staining before (pre) and after (post) the low humidity challenge at visits 2-4. There was no statistical difference in the deltas between visits 2 (baseline with no treatment) and 3 (post artificial tears). There was a statistically significance difference (p=0.01) between visit 2 and visit 4 (post steroid), and between visits 3 and 4 (p=0.03) (Figure 3).
Figure 3.
Post to pre difference in conjunctival lissamine green staining after exposure to low humidity goggles environment at study visit 2 (baseline), visit 3 (post artificial tears) and 4 (post dexamethasone). Mean differences in conjunctival lissamine green staining scores graded after goggles (post) and before goggles (pre) at visits 2-4 where the 90-minute low humidity exposure was performed (n=20). *Denotes significance between groups indicated (p<0.05).
Eye irritation symptoms
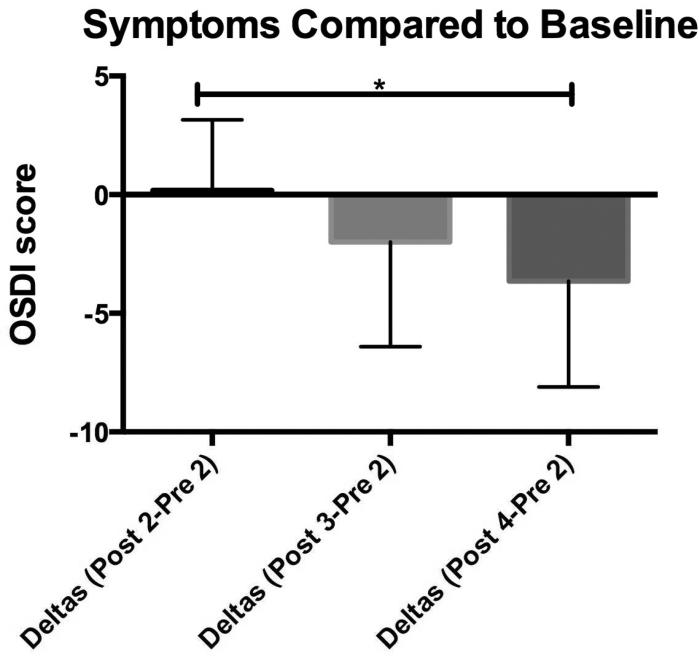
Dry eye patients often complain of eye irritation, blurry vision and photophobia. Therefore, we measured the difference (delta, n= 20 subjects) in symptom severity scores between baseline at visit 2 (pre-exposure) and after the low humidity challenges at visits 2-4. We compared symptoms after low humidity exposure at each visit (post-2, post-3, post-4) to the initial symptoms at visit 2 (pre-2) as a measure of the effectiveness of treatments despite several low humidity exposures. There was no statistical difference in the deltas between visits 2 and 3, and visits 3 and 4, however a statistical difference was present in the deltas between visits 2 and 4 (p=0.01) (Figure 4). There were no seasonal variations in symptom severity noted, nor statistical difference between the pre and post OSDI scores at each visit, which we attribute to the difficulty subjects had in responding to the Likert scale questions of the OSDI after only a 90 minute low humidity exposure. There were also no differences in the individual questions of the OSDI between visits nor any statistical difference in eye irritation symptoms between visits for the visual analog scale (VAS).
Figure 4.
Change in ocular irritation symptoms after exposure to low humidity environment at study visit 2 (baseline), visit 3 (post artificial tears) and 4 (post dexamethasone). Mean difference between ocular surface disease index questionnaire (OSDI) scores after 90-minute low humidity exposure was performed at visits 2-4 and OSDI score obtained before the 90-minute low humidity exposure at visit 2 (n=20). *Denotes significance between groups indicated (p=0.01).
Blink rate has been reported as an indicator of sensory irritation of the ocular surface.22 Therefore, we also compared the mean blink rates during the environmental challenge at visits 2-4 and the change in the blink rate between 15, 45 and 85 minutes during the low humidity challenge at each visit. No statistical change in blink rate was found during the challenge at each visit, nor were the mean differences between visits statistically different.
Conjunctival Gene Expression
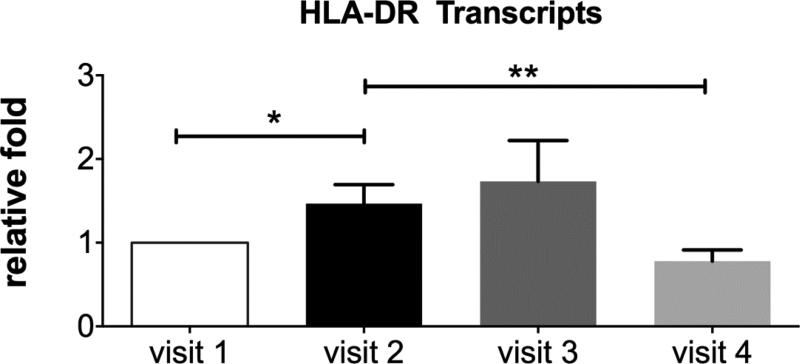
Expression of HLA-DR (human leukocyte antigen) gene was evaluated in conjunctival impression cytology samples using digital PCR. HLA-DR is a major histocompatibility complex (MHC) class II cell surface receptor expressed by antigen presenting dendritic cells that can be induced in conjunctival epithelial cells by inflammatory cytokines.23,24 This marker was chosen because it was found to be elevated in the conjunctiva of dry eye patients and a significant decrease was observed in subjects treated with either topical cyclosporin A or oral polyunsaturated fatty acids.25-26 Impression cytology was performed to obtain cells from the nasal bulbar conjunctiva at the enrollment (visit 1) and after each of the 90-minute low humidity challenges (visits 2-4). Levels of HLA-DR RNA transcripts significantly increased after the initial low humidity challenge at visit 2 (P = 0.04). There was a non-significant increase in transcripts after the low humidity exposure performed at visit 3 (post-artificial tears) and a significant decrease after the environmental challenge at visit 4 (post corticosteroid), compared to visit 2. These findings indicate that corticosteroids are capable of suppressing expression of an ocular surface inflammatory marker that is increased in dry eye and acutely stimulated by desiccating stress.
There were no adverse events during the study. Two subjects experienced a slight steroid induced intraocular pressure increase (< 5mm Hg) at the conclusion of the study.
DISCUSSION
Relative low humidity and constant air flow in modern air conditioned office environments are major contributing factors to the dry eye symptoms and visual disturbances experienced by office workers, which have been reported to impact work productivity and may have an economic impact on society as a whole.27-29 Adverse environmental conditions not only contribute to the progression of dry eye disease, they also are a risk factor for developing dry eye disease in those with predisposing characteristics, such as low tear volume.7 Therapies to blunt the ocular surface response to these adverse conditions are important in improving comfort, quality of life, and productivity in those with dry eye disease. In this study, we found that dry eye signs and symptoms worsened after exposure to a low humidity environment when subjects were untreated and also when subjects were treated with two weeks of artificial tears. However, after two weeks of treatment with dexamethasone, subjects reported decreased eye irritation symptoms compared to their initial evaluation, and they had a significantly lower increase in HLA-DR expression and reduced corneal and conjunctival staining after the low humidity exposure compared to prior exposures. This indicates that topically applied corticosteroids can suppress acute environmentally induced ocular surface inflammation.
Subjects were initially treated with artificial tears because they are frequently utilized to alleviate eye irritation symptoms in dry eye patients. Various types of artificial tears have been reported to reduce eye irritation symptoms and improve some clinical signs of dry eye disease.30,31 Artificial tears primarily function to hydrate and lubricate the ocular surface, but there are no studies demonstrating artificial tears inhibit activation of innate inflammatory pathways in response to a desiccating environment. We found no significant difference in the magnitude of discomfort or ocular surface disease that developed following the low humidity environmental challenge between visit 2 when subjects received no treatment, and visit 3 after two weeks of artificial tears.
The mechanism(s) by which corticosteroids prevented worsening of cornea and conjunctival epithelial disease in response to the low humidity challenge was not evaluated in this study, but animal and culture models suggest the effects of corticosteroids may be due to suppression of MAPK or NFκβ stress signaling pathways that are activated by desiccation or they may result from stabilizing cell membranes that are damaged by hyperosmolar tears.32,33 For example, fluorometholone treatment decreased IL-1β and TNF-α immunostaining in the corneal epithelium of a Botulinum toxin B induced mouse dry eye model, while methylprednisolone significantly improved dry eye signs and symptoms under non-stressed environmental conditions and decreased osmolarity and concentrations of IL-1β, IL-8 and MCP-1 in tears of moderate to severe dry eye patients who had not responded to artificial tears doses 4 times per day. 34,35 Because expression of HLA-DR is increased following MAPK activation and/or exposure to inflammatory cytokines (IFN-γ and TNF-α), it is likely that the observed effect of corticosteroid was due to suppression of one or more these inflammatory mediators/pathways.36
It is possible that prior artificial tear use may have potentiated the effects of dexamethasone; however, the majority of dry eye sufferers use artificial tears, yet many still experience exacerbations of irritation and ocular surface disease in low humidity environments. Further, it is a common practice for patients to use preservative-free artificial tears up until enrollment and treatment randomization in clinical trials of dry medications seeking FDA approval.37
An interesting finding in this study is that the severity of corneal fluorescein and conjunctival lissamine green staining was lower after the 90-minute low humidity stress than before the environmental challenge at visit 4 after subjects had been treated with a topical corticosteroid for 2 weeks. Fluorescein stains intercellular spaces in areas of tight junction disruption and will permeate into cells with damaged cell membranes.38 Sloughing of the superficial dead or damaged cells after the low humidity stress at visit 4 may have caused a restriction of fluorescein movement into or around the underlying healthy cells, resulting in less staining.
Sample size is the biggest limitation in this study. The sample size was based on anticipated changes in corneal fluorescein staining that was observed in our initial study using this low humidity goggles system.7 We believe that a larger sample size may have been required to observe greater between group differences in eye irritation symptoms measured with the VAS and the individual questions of the OSDI. Another limitation of this study is that we didn't evaluate how long it takes for the ocular surface changes induced by short-term exposure to a desiccating environment to return to normal levels. It is possible that the ocular surface epithelial disease may persist for days and that repeat exposure to low humidity stress may exacerbate the severity of dry eye. Mice exposed to a low humidity environment for 14 days continued to have corneal fluorescein staining greater than baseline for 4 months and were found to develop memory T helper 17 (Th17) cells that were capable of inducing severe corneal fluorescein staining when adoptively transferred to naïve recipients 120 days after the low humidity exposure.39 Further studies, are also needed to determine the duration of protection against the adverse effects low humidity exposure that two weeks of dexamethasone treatment confers and whether this treatment prevents development of memory T cells capable of causing dry eye disease.
In conclusion, our study shows that corticosteroids can mitigate the adverse effects of low humidity environmental stress on the ocular surface in individuals with dry eye disease. This suggests that the increased irritation and ocular surface epithelial disease that develops following a desiccating environmental challenge is due to inflammation that can be modulated by a corticosteroid. While extended use of corticosteroids is not indicated due to potential side effects (e.g. cataract and elevated intraocular pressure), other anti-inflammatory therapies with activity against stress activated pathways may prove to be effective in blunting the impact of desiccating stress on the ocular surface. The development of anti-inflammatory therapies for the ocular surface will become increasingly important to reduce the impact of dry eye disease as the use of digital technology rises and more people work in office environments.
Figure 5.
Levels of inflammatory biomarker HLA-DR in the conjunctiva at visit 1 (enrollment) and after exposure to low humidity goggles environment at study visit 2 (baseline), visit 3 (post artificial tears) and 4 (post dexamethasone). Digital polymerase chain reaction (PCR) was used to measure HLA-DR RNA transcripts in conjunctival cells obtained by impression cytology taken from the nasal bulbar conjunctiva. Transcript levels at each visit are expressed as the fold difference relative to the baseline level measured in samples taken at the enrollment visit (visit 1). *P<0.05, ** P=0.01
ACKNOWLEDGMENTS
a. Funding/Support (including none): Bausch and Lomb (Bridgewater, NJ), NIH Grant EY11915, NEI/NIH Core Grant EY-002520 (Bethesda, MD), Research to Prevent Blindness (New York, NY), Oshman Foundation (Houston, TX), William Stamps Farish Fund (Houston, TX), Hamill Foundation (Houston, TX)
d. Other Acknowledgments: There are no individuals who contributed to the work reported in this manuscript other than those listed as authors.
Biography
 Quianta L. Moore, M.D., J.D., received her medical degree from Baylor College of Medicine, Houston, Texas and her law degree from the University of Houston Law Center, Houston, Texas. She was the first person to complete the dual-degree program between both colleges, and post-graduation spent two years in a research fellowship studying ocular surface disease and public health implications. Her research interests are public health, policy and dry eye disease.
Quianta L. Moore, M.D., J.D., received her medical degree from Baylor College of Medicine, Houston, Texas and her law degree from the University of Houston Law Center, Houston, Texas. She was the first person to complete the dual-degree program between both colleges, and post-graduation spent two years in a research fellowship studying ocular surface disease and public health implications. Her research interests are public health, policy and dry eye disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
b. Financial Disclosures (including none): Quianta L Moore has no financial disclosures. Cintia S De Paiva has no financial disclosures. Stephen C Pflugfelder has the following financial disclosures: Consulting and research funding from Allergan, and research funding from Bausch and Lomb.
c. Contributions to Authors in each of these areas: design and conduct of the study (QLM, CDP, SCP); collection, management, analysis, and interpretation of the data (QLM, CDP, SCP); and preparation, review, or approval of the manuscript (QLM,CDP,SCP). Each author meets the four criteria set by the ICMJE required to claim authorship.
REFERENCES
- 1.Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17(6):584–9. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bron AJ, Tomlinson A, Foulks GN, et al. Rethinking dry eye disease: A perspective on clinical implications. The Ocular Surface. 2014;12(2S):S1–S31. doi: 10.1016/j.jtos.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Pflugfelder SC, Stern ME, Beuerman R. Dysfunction of the integrated functional unit: impact on tear film stability and composition. In: Pflugfelder SC, Stern ME, Beuerman R, editors. Dry eye and the Ocular Surface. Marcel Dekker; New York, NY: 2004. pp. 63–88. [Google Scholar]
- 4.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 5.Shimazaki-Den S, Dogru M, Higa K, Shimazaki J. Symptoms, visual function, and mucin expression of eyes with tear film instability. Cornea. 2013;32(9):1211–8. doi: 10.1097/ICO.0b013e318295a2a5. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Miguel A, Teson M, Martin-Montanez V, et al. Dry eye exacerbation in patients exposed to desiccating stress under controlled environmental conditions. Am J Ophthalmol. 2014;157(4):788–798. doi: 10.1016/j.ajo.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Alex A, Edwards A, Hays JD, et al. Factors predicting the ocular surface response to desiccating environmental stress. Invest Ophthalmol Vis Sci. 2013;54(5):3325–3332. doi: 10.1167/iovs.12-11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barabino S, Shen L, Chen L, Rashid S, Rolando M, Dana MR. The controlled environment-chamber: A new mouse model of dry eye. Invest Ophthalmol Vis Sci. 2005;46(8):2766–2771. doi: 10.1167/iovs.04-1326. [DOI] [PubMed] [Google Scholar]
- 9.Abusharha AA, Pearce EI. The effect of low humidity on the human tear film. Cornea. 2013;32(4):429–434. doi: 10.1097/ICO.0b013e31826671ab. [DOI] [PubMed] [Google Scholar]
- 10.Smith JA, Albietz J, Begley C, et al. The epidemiology of dry eye disease: Report of the epidemiology subcommittee of the International Dry Eye Workshop. Ocul Surf. 2007;5(2):93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 11.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 12.Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men. Arch Ophthalmol. 2009;127(6):763–768. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45(12):4293–301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 14.Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32(1):19–41. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beuerman R, Stern ME, Mircheff A, Pflugfelder SC. In: The Lacrimal Functional Unit in Dry Eye and the Ocular Surface. Pflugfelder SC, Stern ME, Beuerman R, editors. Marcel Dekker; New York: 2004. pp. 11–40. [Google Scholar]
- 16.Corrales RM, de Paiva CS, Li DQ, et al. Entrapment of conjunctival goblet cells by desiccation-induced cornification. Invest Ophthalmol Vis Sci. 2011;52(6):3492–3499. doi: 10.1167/iovs.10-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tung CI, Perin AF, Gumus K, Pflugfelder SC. Tear meniscus dimensions in tear dysfunction and their correlation with clinical parameters. Am J Ophthalmol. 2014;157(2):301–310. e1. doi: 10.1016/j.ajo.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47(7):2847–56. doi: 10.1167/iovs.05-1281. [DOI] [PubMed] [Google Scholar]
- 19.Patane MA, Cohen A, From S, Torkildsen G, Welch D, Ousler GW. Ocular iontophoresis of EGP-437 (dexamethasone phosphate) in dry eye patients: results of a randomized clinical trial. Clin Ophthalmol. 2011;5(5):633–643. doi: 10.2147/OPTH.S19349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Situ P, Simpson T, Jones LW, Fonn D. Effects of Silicone Hydrogel Contact Lens Wear on Ocular Surface Sensitivity to Tactile, Pneumatic Mechanical and Chemical Stimulation. Invest Ophthalmol Vis Sci. 2010;51(12):6111–6117. doi: 10.1167/iovs.09-4807. [DOI] [PubMed] [Google Scholar]
- 21.Shiboski SC, Shiboski CH, Criswell L, et al. American College of Rheumatology classification criteria for Sjogren's syndrome: a data-driven, expert consensus approach in the Sjogren's International Collaborative Clinical Alliance cohort. Arthritis Care Res. 2012;64(4):475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiesswetter E, van Thriel C, Schaper M, Blaszkewicz, Seeber A. Eye blinks as indicator for sensory irritation during constant and peak exposures to 2-ethylhexanol. Environ Toxicol Pharmacol. 2005;19(3):531–41. doi: 10.1016/j.etap.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 23.Baudouin C, Brignole F, Pisella PJ, Becquet F, Philip PJ. Immunophenotyping of human dendriform cells from the conjunctival epithelium. Curr Eye Res. May. 1997;16(5):475–81. doi: 10.1076/ceyr.16.5.475.7053. [DOI] [PubMed] [Google Scholar]
- 24.Tsubota K, Fukagawa K, Fujihara T, et al. Regulation of human leukocyte antigen expression in human conjunctival epithelium. Invest Ophthalmol Vis Sci. 1999;40(1):28–34. [PubMed] [Google Scholar]
- 25.Brignole F, Pisella PJ, De Saint Jean M, Goldschild M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest Ophthalmol Vis Sci. 2001;42(1):90–5. [PubMed] [Google Scholar]
- 26.Epstein SP, Gadaria-Rathod N, Wei Y, Maguire MG, Asbell PA. HLA-DR expression as a biomarker of inflammation for multicenter clinical trials of ocular surface disease. Exp Eye Res. 2013;111(6):95–104. doi: 10.1016/j.exer.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada M, Mizuno Y, Shigeyasu C. Impact of dry eye on work productivity. Clinicoecon Outcomes Res. 2012;4(10):307–312. doi: 10.2147/CEOR.S36352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchino M, Uchino Y, Dogru M, et al. Dry eye disease and work productivity loss in visual display users: The Osaka Study. Am J Ophthalmol. 2014;157(2):294–300. doi: 10.1016/j.ajo.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Abetz L, Rajagopalan K, Mertzanis P, Begley C, Barnes R, Chalmers R. Development and validation of the Impact of Dry Eye on Everyday Life (IDEEL) questionnaire, a patient-reported outcomes (PRO) measure for the assessment of the burden of dry eye on patients. Health Quality Life Outcomes. 2011;9(12):111. doi: 10.1186/1477-7525-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang TJ, Wang IJ, Ho JD, Chou HC, Lin SY, Huang MC. Comparison of the clinical effects of carbomer-based lipid containing gel and hydroxypropyl-guar gel artificial tear formulations in patients with dry eye syndrome: A 4-week prospective, open-label, randomized, parallel-group, non-inferiority study. Clinical Therapeutics. 2010;32(1):44–52. doi: 10.1016/j.clinthera.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 31.Kaercher T, Thelen U, Brief G, Morgan-Warren RJ, Leaback R. A prospective, multicenter, non-interventional study of Optive Plus in the treatment of patients with dry eye: the prolipid study. Clin Ophthalmol. 2014;8(6):1147–1155. doi: 10.2147/OPTH.S58464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83(3):526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Li DQ, Luo L, Chen Z, Kim HS, Song XJ, Pflugfelder SC. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82(4):588–96. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu L, Zhang C, Chuck RS. Topical steroid and non-steroidal anti-inflammatory drugs inhibit inflammatory cytokine expression on the ocular surface in the botulinum toxin B-induced murine dry eye model. Mol Vis. 2012;18(7):1803–12. [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Min K, Kim SK, Kim EK, Kim TI. Inflammatory cytokine and osmolarity changes in the tears of dry eye patients treated with topical 1% methylprednisolone. Yonsei Med J. 2014;55(1):203–208. doi: 10.3349/ymj.2014.55.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martins I, Deshayes F, Baton F, Forget A, Ciechomska I, Sylla K, Aoudjit F, Charron D, Al-Daccak R, Alcaide-Loridan C. Pathologic expression of MHC class II is driven by mitogen-activated protein kinases. Eur J Immunol. 2007;37(3):788–97. doi: 10.1002/eji.200636620. [DOI] [PubMed] [Google Scholar]
- 37.Sheppard JD, Torkildsen GL, Lonsdale JD, D'Ambrosio FA, Jr, McLaurin EB, Eiferman RA, Kennedy KS, Semba CP. OPUS-1 Study Group. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014;121(2):475–83. doi: 10.1016/j.ophtha.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Xu M, Sivak JG, McCanna DJ. Comparison of the effects of ophthalmic solutions on human corneal epithelial cells using fluorescent dyes. J Ocul Pharmacol Ther. 2013;29(9):794–802. doi: 10.1089/jop.2013.0002. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Chauhan SK, Lee HS, Saban DR, Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2014;7(1):38–45. doi: 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]