Abstract
Background
Three costimulation-blockade-based regimens have been explored after transplantation of hearts from pigs of varying genetic backgrounds to determine whether CTLA4-Ig (abatacept) or anti-CD40mAb+CTLA4-Ig (belatacept) can successfully replace anti-CD154mAb.
Methods
All pigs were on an α1,3-galactosyltransferase gene-knockout/CD46 transgenic (GTKO.CD46) background. Hearts transplanted into Group A baboons (n=4) expressed additional CD55, and those into Group B (n=3) expressed human thrombomodulin (TBM). Immunosuppression included anti-thymocyte globulin with anti-CD154mAb (Regimen 1: n=2) or abatacept (Regimen 2: n=2) or anti-CD40mAb+belatacept (Regimen 3: n=2). Regimens1/2 included induction anti-CD20mAb and continuous heparin. One further baboon in Group B (B16311) received a modified Regimen 1. Baboons were followed by clinical/laboratory monitoring of immune/coagulation parameters. At biopsy, graft failure, or euthanasia, the graft was examined by microscopy.
Results
Group A baboons survived 15–33 days, whereas Group B survived 52, 99 and 130 days, respectively. Thrombocytopenia and reduction in fibrinogen occurred within 21 days in Group A, suggesting thrombotic microangiopathy (TM), confirmed by histopathology. In Group B, with follow-up for >4m, areas of myofiber degeneration and scarring were seen in 2 hearts at necropsy. A T cell response was documented only in baboons receiving Regimen 2.
Conclusions
The combination of anti-CD40mAb+belatacept proved effective in preventing a T cell response. Expression of TBM prevented thrombocytopenia, and may possibly delay the development of TM and/or consumptive coagulopathy.
Keywords: α1,3-galactosyltransferase gene-knockout; baboon; complement-regulatory proteins; costimulation blockade; pig, heart; thrombomodulin; thrombotic microangiopathy; xenotransplantation
INTRODUCTION
The availability of α1,3-galactosyltransferase gene-knockout (GTKO) pigs (1, 2) enabled pig hearts to function heterotopically in baboons for <6m (3–5). Using costimulation blockade, graft failure was from thrombotic microangiopathy (TM) in the longer-term survivors (6, 7). With added anti-CD20mAb, Mohiuddin et al. reported up to 8m survival of hearts from GTKO pigs transgenic for the human complement-regulatory protein CD46 (membrane cofactor protein) (8). Recently, based on early work by others (9–11), hearts expressing human thrombomodulin (TBM) have functioned for >1y, but required a continuous heparin infusion (12, 13).
We report the transplantation of hearts from GTKO.CD46 pigs that expressed either a second human complement-regulatory protein, CD55 (14), or TBM (15–18). We report results from three immunosuppressive regimens, based on (i) an anti-CD154mAb, (ii) CTLA4-Ig (abatacept), or (iii) a combination of an anti-CD40mAb and a high-affinity variant of CTLA4-Ig (belatacept), respectively. The aims were to determine whether (i) expression of TBM delayed/prevented TM, and (ii) a costimulation-based regimen without anti-CD154mAb successfully prevented an adaptive immune response.
The omission of anti-CD154mAb (with its thrombogenic effect [19–21]) and the expression of TBM on the pig organ enabled us to manage these recipients without continuous heparin, thus enabling removal of intravascular catheters, reducing the incidence of catheter-related complications. Anti-CD40mAb+belatacept prevented a T cell response as effectively as anti-CD154mAb. Our results support the premise that TBM expression delayed features of TM.
METHODS
Animals
Pigs
Homozygous GTKO pigs transgenic for CD46 and either CD55 (n=4; Group A) or TBM (n=3; Group B) (17, 22), all of blood group O (nonA), 10–20 kg, were sources of hearts (Table 1). All pigs were provided by Revivicor (Blacksburg, VA), although two of the TBM pigs were cloned from cells provided by LMU (Munich, Germany) where the TBM transgene was on Revivicor’s GTKO.CD46 background (17, 23). Tissues from all major organs were negative for Galα1, 3Gal expression and positive for CD46 and CD55 (>85%, by flow cytometry).
Table 1.
Details of Group A and B experiments
| Baboon # | Pig | Immuno- suppressive Regimen* |
Graft/Recipient Survival (days) |
Complications | Cause of Termination (graft score) |
|---|---|---|---|---|---|
| Group A (all received GTKO.CD46.CD55 pig hearts) | |||||
| B 19110 | 1 | 33 | Hemorrhage (a) Left hemiplegia (b) |
Euthanized (1/3) | |
| B 19010 | 1 | 18 | Massive ascites (c) | Euthanized (1/3) | |
| B 19510 | 2 | 15 | Ascites Septic peritonitis (d) |
Died (2/3) | |
| B 18910 | 2 | 23 | Septicemia/ascites (e) | Euthanized (1.5/3) | |
| Group B (all received GTKO.CD46.TBM pig hearts)** | |||||
| B 5512 | TBM (8%) (pig TBM promoter) | 3 | 99 | CMV pneumonia | Euthanized (2.5/3) |
| B 5712 | TBM (26%) (pig TBM promoter) | 3 | 130 | None | Graft failure (0.5/3) |
| B 16311 | TBM (96%) (ICAM-2 promoter/enhancer) | 1 | 52 | Pneumonia (f) | Euthanized (3/3) |
For details, see Table 2.
Percentage refers to percentage of donor pig AECs expressing human TBM.
Related to high heparin dosage; treated successfully.
Intra-arterial catheter thromboembolic complication.
Ascites drained on two occasions (day 14 = 500 mL and day 16 = 750 mL); reaccumulated rapidly; ascitic fluid sterile.
Blood - enterococcus faecalis; ascites drained at necropsy (day 15 = 600 mL); ascitic fluid also grew enterococcus faecalis.
Blood - enterococcus faecalis; ascites drained on day 14 (<50 mL) and at necropsy (day 23 = 700 mL); ascitic fluid sterile.
This baboon did not receive anti-CD20mAb. Pneumonia related to Enterococcus faecalis and Escherischia coli.
TBM transgenesis used two different techniques (Table 1). Two TBM expression vectors were constructed. Endothelium-specific expression of the human TBM coding DNA sequence (CDS) was driven by a 0.9 kb porcine ICAM-2 promoter fragment, preceded by a 1.4 kb porcine ICAM-2 enhancer originating from intron 1 of the pig ICAM-2 gene. The expression cassette was flanked by multiple copies (two copies at the 5’ end and 4 copies at the 3’ end) of chicken beta-globin insulator. An additional TBM expression vector was built at LMU using an 8.9 kb region upstream of the porcine TBM gene as promoter for expression of the human TBM CDS. This vector also contained a neomycin resistance cassette located downstream of the bovine growth hormone polyadenylation cassette inserted behind the TBM CDS.
Linear plasmid fragments were prepared and used to transfect GTKO.CD46 porcine fibroblast cell lines, in which human CD46 is expressed as a minigene under control of the endogenous promoter (24). Transfected pig fibroblasts were selected by antibiotic resistance and either screened for the presence of the transgene by polymerase chain reaction (PCR) before nuclear transfer, or used directly for nuclear transfer. Derived fetuses or live pigs were screened by Southern analysis for presence of the transgenes. Southern-positive pigs or fetuses were screened for transgene expression by RT-PCR, immunofluorescence, and/or flow cytometry. One high-expressing ICAM2-TBM line and one moderate-expressing TBM-TBM line were used to produce the pigs used in these studies. TBM expression in the 3 donor pigs was 96%, 26%, and 8%, respectively.
Baboons
Male baboons (n=7, Papio anubis, University of Oklahoma Health Sciences Center, Oklahoma City, OK), weighing 5–9 kg, of blood groups A, B and AB, were recipients of pig hearts (Table 1).
All animal care was in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1985). Protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Surgical procedures
Anesthesia, intravascular catheter placement in baboons, heart excision in pigs, and heterotopic intra-abdominal pig heart transplantation in baboons have been described previously (3–5, 25). In 2 baboons (Group B), an open needle biopsy was obtained of the graft left ventricular myocardium approximately 3m after transplantation.
Immunosuppressive and supportive therapy
Baboons received one of three immunosuppressive/supportive regimens (Table 2). Regimens 2/3 were aimed at replacing anti-CD154mAb (19, 26). Regimen 2 (n=2) was directed towards blockade of the CD28:B7 pathway with abatacept. To eliminate other variability between Regimens 1/2, all baboons in both regimens received a heparin infusion and the anti-platelet agent, ketorolac (Table 2).
Table 2.
| A: Comparison of Regimens 1, 2, and 3 |
|---|
| Regimen 1 (n=3): |
| Induction: ATG, CVF (a), anti-CD20mAb, methylprednisolone |
| Maintenance: Anti-CD154mAb, MMF, methylprednisolone, heparin, ketorolac, ganciclovir (i.v. daily) |
| Regimen 2 (n=2): |
| Induction: ATG, CVF*, anti-CD20mAb, methylprednisolone |
| Maintenance: CTLA4-Ig (abatacept), MMF, methylprednisolone, heparin, ketorolac, ganciclovir (i.v. daily) |
| Regimen 3 (n=2): |
| Induction: ATG, methylprednisolone |
| Maintenance: Anti-CD40mAb + CTLA4-Ig (belatacept), rapamycin (B5512) or tacrolimus (B5712), methylprednisolone, low molecular weight heparin (c), ganciclovir (i.v. ×2 weekly), valganciclovir (p.o. daily) |
| B: Details of immunosuppressive and supportive therapy | ||
|---|---|---|
| Dose | Duration | |
| Induction therapy | ||
| Thymoglobulin (ATG) (a) | 1–10 mg/kg i.v. | days -3, -1 a |
| Cobra venom factor | 100 units/day i.v. | days -1, 0, 1 |
| Methylprednisolone | 5 mg/kg i.v. | before each dose of ATG, and on days -1, 0, 1 |
| Anti-CD20mAb (b) | 20 mg/kg i.v. | Single dose on day -2 |
| Maintenance costimulation blockade | ||
| Anti-human CD154mAb (c) | 25 mg/kg i.v | days -1, 0, 4, 7, 10, 14, 19 and then every 7 days (Regimen 1 only) |
| OR | ||
| Abatacept (d) | 25 mg/kg i.v | days -1, 0, 2, 4, 7, 10, 14, 21 and then every 7 days (Regimen 2 only) |
| OR | ||
| Anti-CD40mAb (e) Belatacept (f) |
25 mg/kg i.v. 20 mg/kg i.v. |
days -1, 0, 4, 7, 10, 14 and then every 7 days. days -1, 0, 4, 7, 14 and then every 14 days (Regimen 3 only) |
| Maintenance pharmacologic immunosuppressive and supportive therapy | ||
| MMF | 25–110 mg/kg/day | continuous i.v. infusion from day -3 (to maintain a constant blood level of 3–5 µg/mL) |
| Rapamycin | 0.01 mg/kg i.m. ×2/day | to maintain a blood trough level of 10–15 ng/mL |
| Tacrolimus | 0.05-0.03 mg/kg i.m. ×2/day | to maintain a blood trough level of 10–15 ng/mL |
| Methylprednisolone | 5 mg/kg i.m. daily | tapering to 0.25 mg/kg/day i.m. at 6 weeks |
| Heparin (g) | 5–50 U/kg/h i.v. | from day 0 (to maintain ACT at 200–250sec from day 3) (Regimens 1 and 2 only) |
| Low molecular weight heparin | 700 IU daily i.m. | from day 1 (Regimen 3 only) |
| Ketorolac (h) | 0.5 mg/kg i.v. | before each dose of anti-CD154mAb or CTLA4-Ig, beginning on day -1 (Regimens 1 and 2 only) |
| Ganciclovir | 5 mg/kg/day i.v. | in Regimen 3, it was administered daily for 14 days and then ×2 weekly |
| Valganciclovir | 15 mg/kg/day ×2 daily p.o. | in Regimen 3 only |
Cobra venom factor was administered to one Group A baboon that had a particularly high level of anti-nonGal IgM, and so for consistency was administered to all 4 baboons in Group A and 1 in Group B. We subsequently concluded that cobra venom factor is unnecessary when adequate complement-regulation is provided by the graft, and it was omitted in the remaining 2 baboons in Group B.)
Rapamycin and tacrolimus were tested to determine whether there was a major difference in outcome.)
Although low molecular weight heparin alone is unlikely to prevent TM, we felt it might augment the effect of the expression of TBM.
Day-1 dose of ATG (Rabbit derived, Genzyme, Cambridge, MA) was given only if needed to reduce lymphocyte count <500×103/mm3.
Anti-CD20mAb (Rituxan, Biogen Idec/Genentech, South San Francisco, CA)
Anti-CD154mAb (Hu5c8 in a mouse/human IgG1 chimeric; NIH NHP Reagent Resource, Boston, MA) kindly provided by Dr. Keith Reimann.
Abatacept (Orencia; BMS, Princeton, NJ)
Anti-CD40mAb (2C10R4; NIH NHP Reagent Resource) kindly provided by Dr. Keith Reimann.
Belatacept (Nulojix; BMS, Princeton, NJ)
Heparin and ketorolac were given in Regimens 1 and 2 only.
One further Group B baboon (B16311) is considered separately as it received a modification of Regimen 1 which did not include anti-CD20mAb (Table 1); in addition, the TBM promoter was different from that in the other two Group B pigs.
Regimen 3 (n=2) was directed towards blockade of both pathways by administering a mouse-rhesus chimeric anti-CD40 IgG4 mAb (2C10R4) (27) in combination with belatacept. (Belatacept was not available to us when Regimen 2 was investigated [28].) In Regimen 3, rapamycin replaced mycophenolate mofetil (MMF) in one case and tacrolimus in the other, simply because we administer MMF by a continuous i.v. infusion, whereas rapamycin or tacrolimus can be administered i.m.. In Regimen 3 the baboons did not receive i.v. heparin or ketorolac, but did receive low molecular weight heparin s.c. (Table 2).
Monitoring of recipient baboons
Graft contractions were monitored by palpation by two independent observers twice weekly and scored on a scale from 0–3 (with 3 being strong contractions). In baboons receiving heparin, activated clotting time was monitored. Blood cell counts, chemistry, and coagulation parameters were measured initially daily and then less frequently (29). Blood cultures were performed whenever indicated. Anti-CD154mAb, anti-CD40mAb, abatacept, and belatacept levels were not measured, but the dosages were based on previous studies by us and others (3–5, 19, 28, 30–37).
Immunological monitoring
These methods have been described previously (38–40), and included:-
(i) Flow cytometry to monitor T and B cell numbers (to determine the effect of anti-thymocyte globulin). (ii) Flow cytometry to monitor binding of xenoreactive anti-nonGal antibodies (IgM and IgG) to aortic endothelial cells (AECs) from GTKO pigs (pAECs) (38, 40). (iii) The CFSE-MLR (carboxyfluorescein succinimidyl ester-mixed lymphocyte reaction) was carried out with stored peripheral blood mononuclear cells (PBMCs) pre-transplantation and at the time of euthanasia. (iv) Total complement activity.
Histopathology and Immunohistopathology of pig heart grafts
Biopsies of xenografts were obtained at the time of euthanasia in all cases, and 3m after transplantation in 2 cases in Group B (41).
Statistical analysis
In view of the small number of experiments and the several variables, no statistical analyses were carried out.
RESULTS
Gal epitopes and transgene products on pAECs and PBMCs
GTKO.CD46.CD55 and GTKO.CD46.TBM pigs did not express Gal on either pAECs or PBMCs. High expression of CD46 in all pigs and of CD55 (in Group A) on pAECs was documented, with variable expression of TBM (in Group B) from 8% to 96% (Table 1).
Pig heart graft survival and complications
Recipient baboons survived from 15–130d (Table 1). All baboons were euthanized or died with functioning hearts, although function had deteriorated in most cases (Table 1). Mean survival in Group A was limited to 22d (median 21d), but was extended to 94d (median 99d) in Group B. Graft function deteriorated more quickly in Group A than Group B (Table 1). In Group A, there appeared no difference in survival between Regimens 1/2.
When compared with our previous studies (3–5, 19), the addition of anti-CD20mAb to Regimens 1/2 appeared to be associated with a particularly early incidence of infectious complications, but with no increase in graft survival. In addition, three baboons developed massive (n=2) or modest (n=1) ascites (Table 1). Graft function, determined by the strength of contractions by palpation, had weakened in all Group A hearts to a palpation score of 1 or 2 (out of 3) within two weeks, whereas all Group B hearts retained a score of 3/3 at this time interval.
Immunologic Monitoring
T and B cell counts
In all baboons, after anti-thymocyte globulin on day -3, a profound depletion of T cells was observed for approximately one week, after which there was some recovery, which was quicker in baboons that received abatacept (Regimen 2) (suggesting that abatacept is not as T cell-depleting as the other costimulation-blockade agents). However, CD3+T cell counts were generally maintained <500/mm3, with CD4+ and CD8+ cell numbers frequently less than half of this, throughout the periods of follow-up, even beyond 4m. When anti-CD20mAb was added to the regimen (n=4), B cell subsets were also depleted dramatically, and in those receiving Regimen 2 remained almost undetectable throughout the period of follow-up.
CFSE-MLR
CFSE-MLR was carried out only in baboons receiving Regimen 3. [Previous studies had indicated that regimens based on anti-CD154mAb completely suppressed T cell proliferation (5, 19)]. Post-transplant, when stimulation of PBMCs, CD3+, CD4+, or CD8+ cells was against donor-specific cells, cell proliferation was greatly reduced (compared to pre-transplantation), irrespective of the nature of the heart graft or immunosuppressive regimen (not shown).
Anti-pig antibodies and complement
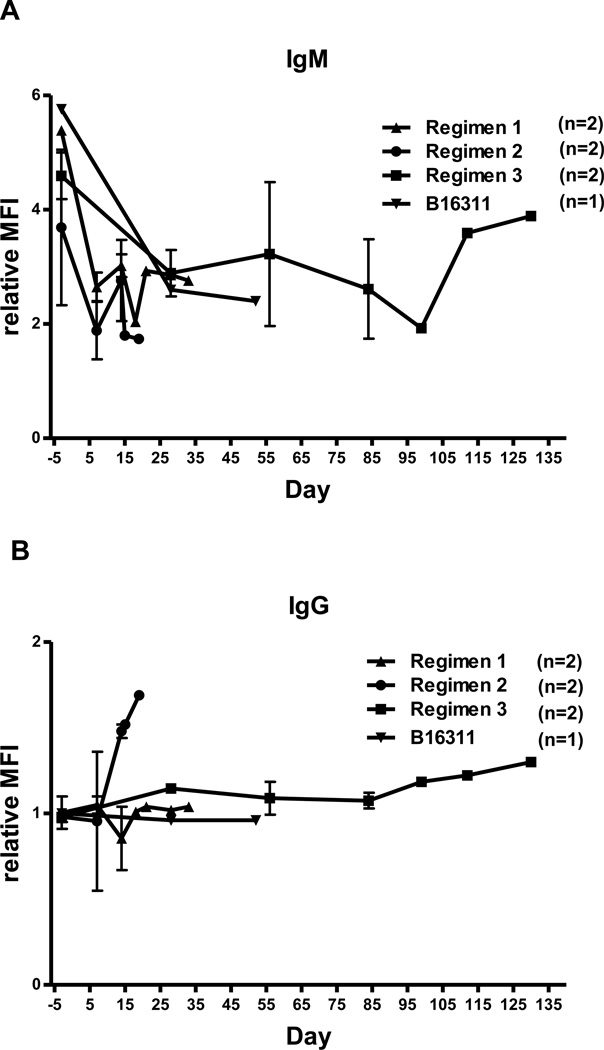
There was no increase in xenoreactive IgM in any baboon (Figure 1A). IgG sensitization to nonGal antigens expressed on the pAECs did not occur in baboons receiving Regimens 1 or 3, but was seen in baboons receiving Regimen 2 (abatacept-based) (Figure 1B). There were no differences related to the genetic manipulation of the pig, where all were on a GTKO.CD46 background.
Figure 1. Xenoreactive IgM and IgG binding to GTKO pAECs in baboons receiving Regimens 1, 2, and 3, and modified Regimen 1 (B16311).
No increase in IgM was observed in any of the baboons treated with any of the regimens (A). The baboons treated with Regimen 2 (abatacept-based) developed an elicited anti-pig IgG antibody response (B). An elicited IgG antibody response was not seen in baboons receiving Regimens 1 or 3 or modified Regimen 1 (B).
Relative mean fluorescence intensity (MFI) = MFI of anti-nonGal antibody / MFI of isotype control.
Coagulation dysfunction
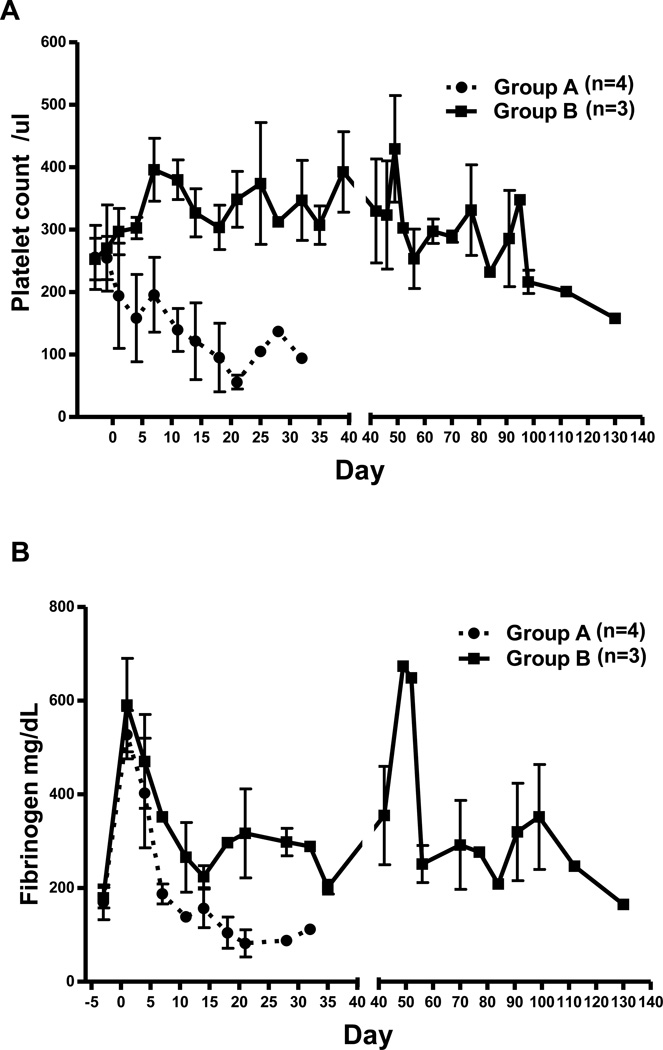
Platelet counts, fibrinogen levels, D-dimer
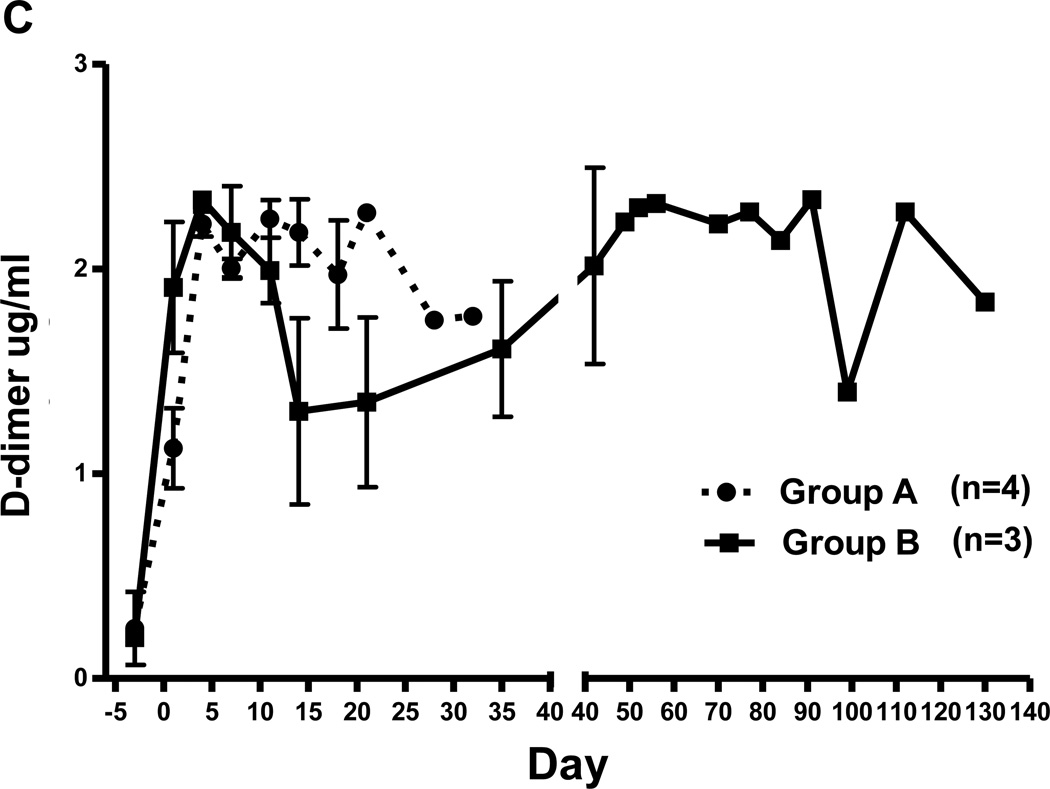
After heart xenotransplantation, in our experience the development of thrombocytopenia and decreased fibrinogen are systemic indicators of TM and/or consumptive coagulopathy. There was a decrease in platelet count to <50,000/µL or <20% baseline within 14d in all recipients in Group A, but not in Group B (Figure 2A). After an initial increase due to the surgical procedure, fibrinogen levels decreased to <100 mg/dL or <50% baseline level within 21d in Group A, and were subsequently maintained at low levels (Figure 2B), but did not decrease in Group B. In both Groups A/B, D-dimer levels initially rose, and remained above the normal range (Figure 2C).
Figure 2. Mean (+/− SE) platelet counts (A), fibrinogen (B), and D-dimer (C) in baboons of Groups A and B.
(A) In Group A, thrombocytopenia developed within 21 days (95×103 /µl) post-transplant. In contrast, there was no decrease in platelet count in Group B.
(B) Fibrinogen level was dramatically decreased in Group A by day 21 (105 mg/dL), whereas no reduction of fibrinogen was observed in Group B.
(C) In both groups, there was an immediate rise in the D-dimer levels by day 3 (>2 ug/ml). This increased D-dimer level was sustained in Group A, although it slightly recovered in day 14 (1.3 ug/ml), and then gradually increased throughout the post-transplant course in Group B.
Of note, no thrombocytopenia or reduction in fibrinogen was seen in the single baboon in Group B (B16311) that received an anti-CD154mAb-based regimen (Regimen 1), indicating that this agent is not the cause of these changes.
Two of the four Group A baboons developed sepsis which could possibly have been a factor in the loss of platelets and fibrinogen. However, one baboon in Group B developed pneumonia (for which it required euthanasia), and yet did not develop thrombocytopenia or a reduction in fibrinogen, suggesting that sepsis may not have been a dominant factor in the Group A baboons.
Histopathology and immunohistopathology of pig hearts
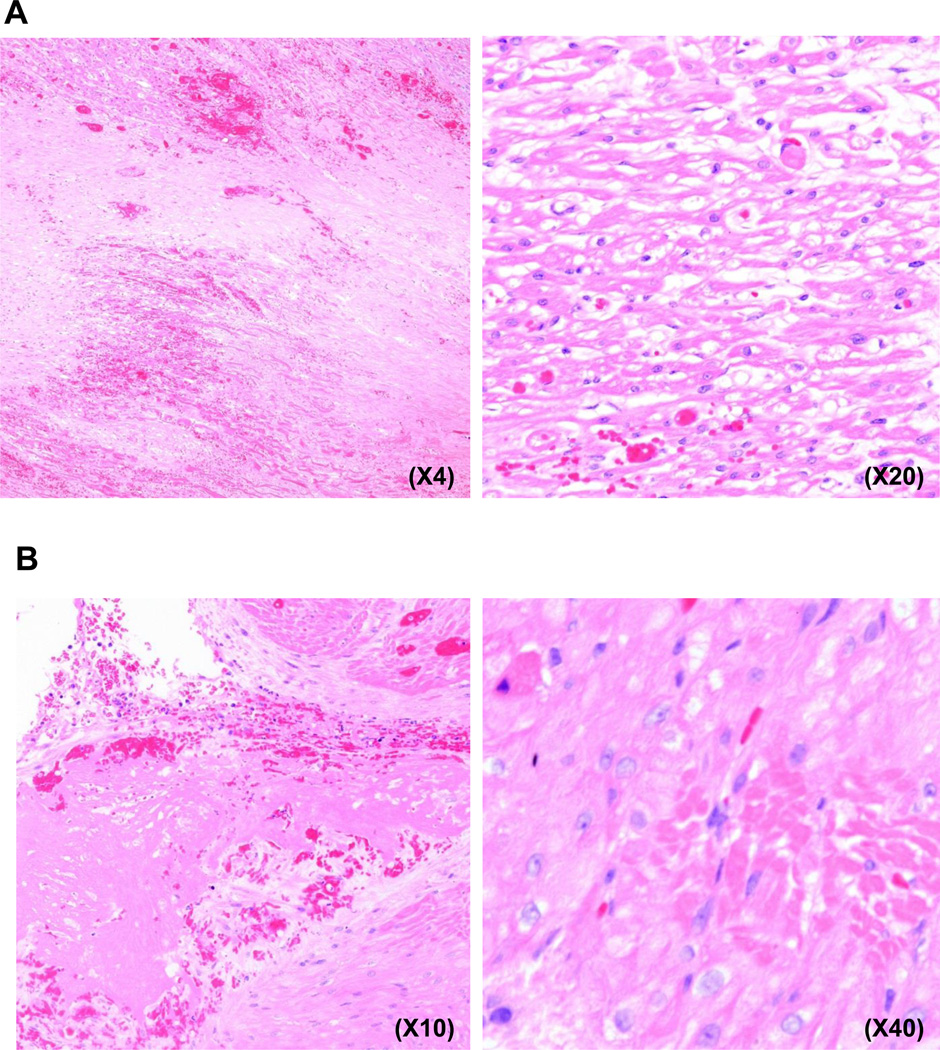
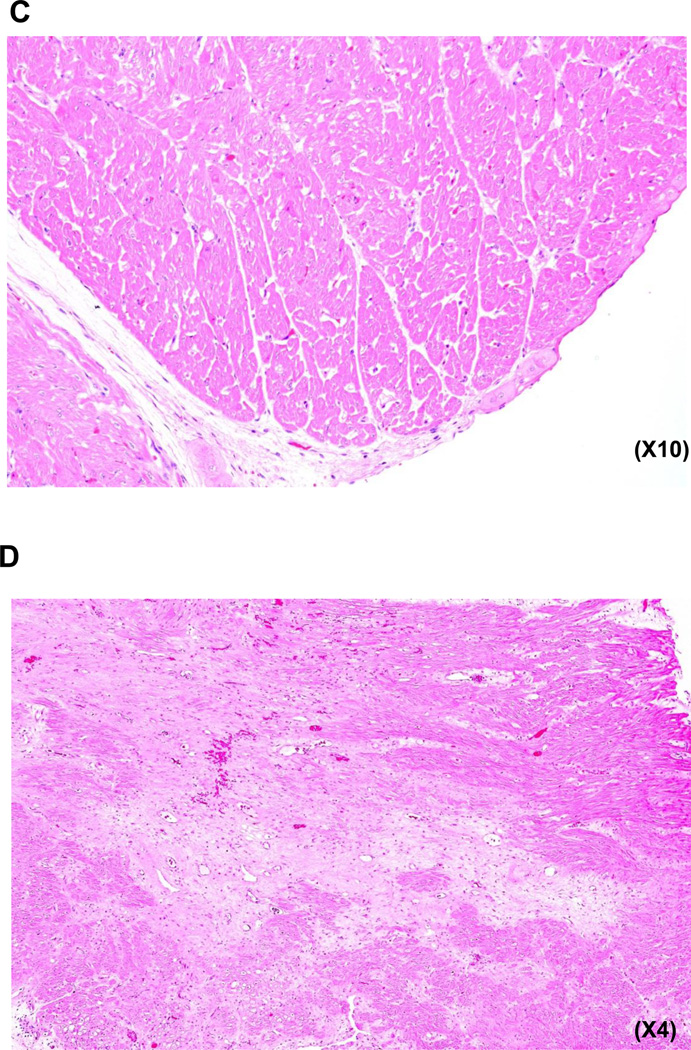
There were no features suggestive of acute cellular rejection (graft infiltrating cells), although eosinophils (but not neutrophils) were seen in three of the four hearts in Group A, but not in Group B, possibly indicating an inflammatory response. Despite their short survival (15–33d), all Group A hearts exhibited focal areas of thrombosis, myofiber loss, myocardial ischemia (necrosis) and infarction, vacuolization, and early fibrosis, suggesting ischemic injury (Figures 3A, B). In Group B, virtually normal histology was seen in one heart at 52d (in B16311) (Figure 3C), though patchy, focally extensive areas of fibrosis (scarring) were seen in the other two Group B baboons on biopsy at 3m and at the time of euthanasia at 14w and 18w, respectively (Figure 3D). However, thrombosed or recanalizing vessels were not apparent, casting doubt on the cause of the observed changes. Immunofluorescence indicated weak IgM, IgG, and complement (C3) deposition in all grafts in both groups (not shown). No attempt was made to determine expression of the transgenes in the explanted hearts.
Figure 3. Histopathology and immunohistopathology in baboons of Groups A and B.
(A) GTKO.CD46.CD55 pig heart in a baboon that received Regimen 1 (B19110) 33 days after transplantation. (Left) Areas of fibrosis/scar adjacent to infarcted myocardium. (Right) Vacuolated and attenuated myofibers adjacent to areas of frank infarction and focal small thrombus.
(B) GTKO.CD46.CD55 pig heart in a baboon that received Regimen 1 (B19010) 18 days after transplantation. (Left) large thrombus in ventricular lumen with evidence of re-endothelialization. (Right) Focal area of eosinophilic myofiber degeneration.
(C) Minimal features of myocardial ischemia or injury, with no cellular infiltrate in a GTKO.CD46.TBM pig heart in a baboon that received Regimen 1 (B16311) 52 days after transplantation. (TBM expression on the pig aortic endothelial cells was 96%).
(D) Patchy focally extensive areas of fibrosis (scarring) in a GTKO.CD46.TBM pig heart in a baboon that received Regimen 3 (B5512) 99 days after transplantation. (TBM expression on the pig aortic endothelial cells was 8%).
DISCUSSION
Unless immunosuppressive therapy is adequate, a T cell response develops after pig organ (or artery patch) transplantation in nonhuman primates, even if the graft is from a genetically-modified pig (19, 42). The adaptive immune response is prevented by anti-CD154mAb-based therapy (3–5, 8, 19, 30). This present study explored (i) the effect of the replacement of anti-CD154mAb by either abatacept or anti-CD40mAb+belatacept, and (ii) the effect of TBM expression on the pig vascular endothelium.
The number of experiments is small, and there are several variables that make comparisons difficult, e.g., (i) replacement of anti-CD154mAb by abatacept or anti-CD40mAb+belatacept, (ii) administration or not of an anti-CD20mAb, (iii) slight variation in adjunctive immunosuppressive therapy (e.g., MMF, tacrolimus, or rapamycin), and (iv) expression of either CD55 or TBM. However, we suggest that the following conclusions can reasonably be drawn.
Hearts from GTKO.CD46.TBM pigs are protected from hyperacute rejection in the absence of an anti-complement agent.
Although others have reported prolonged graft survival when an anti-CD20mAb was added to a costimulation blockade-based regimen (8), it was possibly associated with a higher incidence of (i) early infectious complications and (ii) ascites than we have seen previously (3–5). (Ascites does not appear to have been reported by others.) Although most infections were associated with positive blood cultures, no change had been made to our previous regimen of care of intravascular catheters or antibiotic prophylaxis. The ascites were not associated with hypoalbuminemia, and had not developed in previous baboons administered cobra venom factor.
-
Blockade of the CD28:B7 pathway alone (with abatacept) did not prevent the adaptive response, whereas blockade of both CD40:CD154 and CD28:B7 pathways with anti-CD40mAb+belatacept successfully prevented this response, and, in future studies, can replace an anti-CD154mAb-based regimen (4, 5, 8, 19, 26, 30). These observations correlate with our studies in pig artery patch transplantation, which have also indicated that CD28:B7 pathway blockade alone (with abatacept or belatacept) is inadequate to inhibit the adaptive response even when combined with MMF or rapamycin or corticosteroids (26). (We did not test anti-CD40mAb alone as our in vitro studies indicated a stronger suppressive effect of CTLA4-Ig. However, we [Iwase H, et al, unpublished] and others (12, 13) have subsequently obtained prolonged xenograft survival with high-dose anti-CD40mAb alone.)
Endothelial cells from GTKO pigs or from pigs expressing a human complement-regulatory protein induce a weaker T cell response than do wild-type pig endothelial cells (43, 44), and this may be a factor in the successful inhibition of the adaptive response in the current study. Furthermore, thrombin induces a human T cell response that is not associated with SLA class II expression (45), and the expression of TBM in the pig hearts in the two baboons that received Regimen 3 may also have been beneficial in this respect.
The two Group B baboons that received Regimen 3 are the first we have been able to maintain for a relatively prolonged period of time (14–18w) without an indwelling intravascular catheter (which were removed within the first 30 days), which is a step towards clinical application.
TBM-expressing hearts (Group B) are possibly associated with delayed onset of features of TM, though this complication may slowly develop. This study supports, but does not definitively confirm, that TBM-expressing pig organs may have advantages. The number of experiments in Group B was too small for us to draw any conclusions in regard to outcome relating to the TBM promoter.
Importantly, in two cases, the delay in TM/graft injury occurred in the absence of heparinization, which is the first time we have achieved this (and was not the case in the recently-reported graft survival for >1y [12, 13]). The absence of thrombocytopenia or reduction in fibrinogen (with follow-up for >4m) suggests an absence of development of consumptive coagulopathy, which is in contrast to Group A and several previous studies (3–5). This delay was observed whether anti-CD154mAb or anti-CD40mAb+belatacept had been administered, and so was not simply related to the absence of anti-CD154mAb in the regimen.
Furthermore, although TM was delayed after GTKO heart transplantation in baboons in Kuwaki’s series (3,4), all baboons in that series received continuous infusions of high-dose heparin (+/− aspirin), whereas in two of the present Group B baboons only low molecular weight heparin (+ aspirin) was administered. In addition to simple anticoagulation, heparin may have immunomodulatory actions beneficial to xenograft survival (46–49). In our platelet aggregation assay and following pig artery patch transplantation, heparin proved to be a strong inhibitor of thrombin, which is a key factor in the development of TM.
Graft survival was surprisingly poor in Group A, in which expression of CD46 and CD55 would have been anticipated to protect the graft from early immune-mediated injury. As cobra venom factor was also administered to some of these baboons, complement-mediated injury was almost certainly prevented. We subsequently concluded that cobra venom factor is unnecessary when adequate complement-regulation is provided by the graft.
In two cases in Group A, the early positive blood cultures could possibly have been a factor in the development of features of consumptive coagulopathy, but these features were not seen in a Group B baboon that required euthanasia for sepsis/pneumonia.
In summary, anti-CD40mAb+belatacept proved as effective in preventing a T cell response as anti-CD154mAb, but allowed easier management of the baboon with fewer complications. In contrast to another report (8), anti-CD20mAb in this series was associated with a high incidence of early infection. TBM expression on the graft may possibly delay TM and/or consumptive coagulopathy.
ACKNOWLEDGEMENTS
Burcin Ekser, MD, was a recipient of NIH NIAID T32 AI 074490 training grant. Mohamed Ezzelarab, MD, was supported in part by the Joseph A. Patrick Fellowship at the Thomas E. Starzl Transplantation Institute. Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is, or has been, supported in part by NIH grants #U19 AI090959, #U01 AI068642, and # R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA. The baboons used in the study were from the Oklahoma University Health Sciences Center, Division of Animal Resources, which is supported by NIH P40 sponsored grant RR012317-09. We would like to express our gratitude to Dr. Keith Reimann for providing us anti-CD40 and anti-CD154 mAb by the NHP Reagent Resource and contract HHSN272200900037C.
ABBREVIATIONS
- AECs
aortic endothelial cells
- CFSE-MLR
carboxyfluorescein succinimidyl ester-mixed lymphocyte reaction
- GTKO
α1,3-galactosyltransferase gene-knockout
- MFI
mean fluorescence intensity
- MMF
mycophenolate mofetil
- P
pig
- PBMCs
peripheral blood mononuclear cells
- TBM
thrombomodulin
- TM
thrombotic microangiopathy
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
Carol Phelps and David Ayares are employees of Revivicor, Inc. Keith Reimann is an employee of Mass Biologics. No other author has a conflict of interest.
REFERENCES
- 1.Kolber-Simonds D, Lai L, Watt SR, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 4.Tseng YL, Kuwaki K, Dor FJ, et al. alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80:1493–1500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 5.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houser SL, Kuwaki K, Knosalla C, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11:416–425. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 2008;172:1471–1481. doi: 10.2353/ajpath.2008.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohiuddin MM, Corcoran PC, Singh AK, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012;12:763–771. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawson JH, Daniels LJ, Platt JL. The evaluation of thrombomodulin activity in porcine to human xenotransplantation. Transplant Proc. 1997;29:884–885. doi: 10.1016/s0041-1345(96)00192-3. [DOI] [PubMed] [Google Scholar]
- 10.Siegel JB, Grey ST, Lesnikoski BA, et al. Xenogeneic endothelial cells activate human prothrombin. Transplantation. 1997;64:888–896. doi: 10.1097/00007890-199709270-00017. [DOI] [PubMed] [Google Scholar]
- 11.Kopp CW, Grey ST, Siegel JB, et al. Expression of human thrombomodulin cofactor activity in porcine endothelial cells. Transplantation. 1998;66:244–251. doi: 10.1097/00007890-199807270-00019. [DOI] [PubMed] [Google Scholar]
- 12.Mohiuddin MM, Singh AK, Corcoran PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14:488–489. doi: 10.1111/ajt.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohiuddin M, Singh AK, Corcoran PC, et al. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. JTCS. 2014;148:1106–1113. doi: 10.1016/j.jtcvs.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1:964–966. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 15.Petersen B, Ramackers W, Tiede A, et al. Pigs transgenic for human thrombomodulin have elevated production of activated protein C. Xenotransplantation. 2009;16:486–495. doi: 10.1111/j.1399-3089.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- 16.Miwa Y, Yamamoto K, Onishi A, et al. Potential value of human thrombomodulin and DAF expression for coagulation control in pig-to-human xenotransplantation. Xenotransplantation. 2010;17:26–37. doi: 10.1111/j.1399-3089.2009.00555.x. [DOI] [PubMed] [Google Scholar]
- 17.Wuensch A, Baehr A, Bongoni AK, et al. Regulatory sequences of the porcine THBD gene facilitate endothelial-specific expression of bioactive human thrombomodulin in single- and multitransgenic pigs. Transplantation. 2014;97:138–147. doi: 10.1097/TP.0b013e3182a95cbc. [DOI] [PubMed] [Google Scholar]
- 18.Yazaki S, Iwamoto M, Onishi A, et al. Production of cloned pigs expressing human thrombomodulin in endothelial cells. Xenotransplantation. 2012;19:82–91. doi: 10.1111/j.1399-3089.2012.00696.x. [DOI] [PubMed] [Google Scholar]
- 19.Ezzelarab MB, Ekser B, Echeverri G, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012;19:221–232. doi: 10.1111/j.1399-3089.2012.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 21.Knosalla C, Gollackner B, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism revisted. Transplantation. 2002;74:416–417. doi: 10.1097/00007890-200208150-00024. [DOI] [PubMed] [Google Scholar]
- 22.Ayares D, Phelps C, Vaught TD, et al. Multi-transgenic pigs for vascularized pig organ xenografts. Xenotransplantation. 2011;18:269. (abstract #119). [Google Scholar]
- 23.Klymiuk N, Wuensch A, Kurome M, et al. GalT-KO/CD46/hTM triple-transgenic donor animals for pig-to-baboon heart transplantation. Xenotransplantation. 2011;18:271. (Abstract #126). [Google Scholar]
- 24.Loveland BE, Milland J, Kyriakou P, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004;11:171–183. doi: 10.1046/j.1399-3089.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 25.Cooper DKC, Ye Y, Niekrasz M. Heart transplantation in primates. In: Cramer DVPL, Makowka L, editors. Handbook of Animal Models in Transplantation Research. Boca Raton: CRC Press; 1994. pp. 173–200. [Google Scholar]
- 26.Iwase H, Ekser B, Satyananda V, et al. Pig artery patch transplants in baboons using costimulation blockade and mutant MHC (CIITA-DN) pigs. Transplant Immunol. 2015 doi: 10.1016/j.trim.2015.02.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe M, Badell IR, Thompson P, et al. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am J Transplant. 2012;12:2079–2087. doi: 10.1111/j.1600-6143.2012.04054.x. 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 29.Ekser B, Bianchi J, Ball S, et al. Comparison of hematologic, biochemical, and coagulation parameters in alpha1,3-galactosyltransferase gene-knockout pigs, wild-type pigs, and four primate species. Xenotransplantation. 2012;19:342–354. doi: 10.1111/xen.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buhler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69:2296–2304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 31.Adams AB, Shirasugi N, Jones TR, et al. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J Immunol. 2005;174:542–550. doi: 10.4049/jimmunol.174.1.542. [DOI] [PubMed] [Google Scholar]
- 32.Haanstra KG, Ringers J, Sick EA, et al. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation. 2003;75:637–643. doi: 10.1097/01.TP.0000054835.58014.C2. [DOI] [PubMed] [Google Scholar]
- 33.Kenyon NS, Chatzipetrou M, Masetti M, et al. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci U S A. 1999;96:8132–8137. doi: 10.1073/pnas.96.14.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson TC, Trambley J, Odom K, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation. 2002;74:933–940. doi: 10.1097/00007890-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 36.Tai HC, Campanile N, Ezzelarab M, Cooper DK, Phelps C. Measurement of anti-CD154 monoclonal antibody in primate sera by competitive inhibition ELISA. Xenotransplantation. 2006;13:566–570. doi: 10.1111/j.1399-3089.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 37.Thompson P, Cardona K, Russell M, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant. 2011;11:947–957. doi: 10.1111/j.1600-6143.2011.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ezzelarab M, Hara H, Busch J, et al. Antibodies directed to pig non-Gal antigens in naive and sensitized baboons. Xenotransplantation. 2006;13:400–407. doi: 10.1111/j.1399-3089.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- 39.Hara H, Ezzelarab M, Rood PP, et al. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation. 2006;13:357–365. doi: 10.1111/j.1399-3089.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 40.Hara H, Long C, Lin YJ, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–1174. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 41.Ekser B, Klein E, He J, et al. Genetically-engineered pig-to-baboon liver xenotransplantation: histopathology of xenografts and native organs. PLoS One. 2012;7:e29720. doi: 10.1371/journal.pone.0029720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11:1295–1298. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ezzelarab M, Ezzelarab C, Wilhite T, et al. Genetically-modified pig mesenchymal stromal cells: xenoantigenicity and effect on human T-cell xenoresponses. Xenotransplantation. 2011;18:183–195. doi: 10.1111/j.1399-3089.2011.00635.x. [DOI] [PubMed] [Google Scholar]
- 44.Wilhite T, Ezzelarab C, Hara H, et al. The effect of Gal expression on pig cells on the human T-cell xenoresponse. Xenotransplantation. 2012;19:56–63. doi: 10.1111/j.1399-3089.2011.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ezzelarab C, Ayares D, Cooper DK, Ezzelarab MB. Human T-cell proliferation in response to thrombin-activated GTKO pig endothelial cells. Xenotransplantation. 2012;19:311–316. doi: 10.1111/j.1399-3089.2012.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorski A, Wasik M, Nowaczyk M, Korczak-Kowalska G. Immunomodulating activity of heparin. FASEB J. 1991;5:2287–2291. doi: 10.1096/fasebj.5.9.1860620. [DOI] [PubMed] [Google Scholar]
- 47.Heinzelmann M, Bosshart H. Fondaparinux sodium lacks immunomodulatory effects of heparin. Am J Surg. 2004;187:111–113. doi: 10.1016/j.amjsurg.2003.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Hochart H, Jenkins PV, Preston RJ, Smith OP, White B, et al. Concentration-dependent roles for heparin in modifying lipopolysaccharide-induced activation of mononuclear cells in whole blood. Thromb Haemost. 2008;99:570–575. doi: 10.1160/TH07-06-0424. [DOI] [PubMed] [Google Scholar]
- 49.Ranjbaran H, Wang Y, Manes TD, et al. Heparin displaces interferon-gamma-inducible chemokines (IP-10, I-TAC, and Mig) sequestered in the vasculature and inhibits the transendothelial migration and arterial recruitment of T cells. Circulation. 2006;114:1293–1300. doi: 10.1161/CIRCULATIONAHA.106.631457. [DOI] [PubMed] [Google Scholar]