Abstract
Increasing evidence supports the critical role of AMPA glutamate receptors in psychostimulant action. These receptors are regulated via a phosphorylation-dependent mechanism in their trafficking, distribution and function. The hippocampus is a brain structure important for learning and memory and is emerging as a critical site for processing psychostimulant effects. To determine whether the hippocampal pool of AMPA receptors is regulated by stimulants, we investigated and characterized the impact of amphetamine (AMPH) on phosphorylation of AMPA receptors in the adult rat hippocampus in vivo. We found that AMPH markedly increased phosphorylation of AMPA receptor GluA1 subunits at serine 845 (S845) in the hippocampus. The effect of AMPH was dose-dependent. A single dose of AMPH induced a rapid and transient increase in S845 phosphorylation. Among different hippocampal subfields, AMPH primarily elevated S845 phosphorylation in the Cornu Ammonis area 1 and dentate gyrus. In contrast to S845, serine 831 phosphorylation of GluA1 and serine 880 phosphorylation of GluA2 were not altered by AMPH. In addition, surface expression of hippocampal GluA1 was upregulated, while the amount of intracellular GluA1 fraction was concurrently reduced in response to AMPH. GluA2 protein levels in either the surface or intracellular pool were insensitive to AMPH. These data demonstrate that the AMPA receptor in the hippocampus is sensitive to dopamine stimulation. Acute AMPH administration induces dose-, time-, site-, and subunit-dependent phosphorylation of AMPA receptors and facilitates surface trafficking of GluA1 AMPA receptors in hippocampal neurons in vivo.
Keywords: CA1, dentate gyrus, GluA1, GluA2, glutamate, stimulant, S831, S845, S880
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor is expressed throughout the mammalian brain and is essential for excitatory synaptic transmission. This receptor functions as a homo- or heterotetrameric complex composed from multiple subunits (GluA1-4 or formerly GluR1-4) (Hollmann and Heinemann, 1994). Typical transmembrane topology of an AMPA receptor subunit contains an intracellular C-terminal (CT) tail, which provides a key region for protein-protein interactions and posttranslational modifications. An important posttranslational modification is phosphorylation (Wang et al., 2006; Mao et al., 2011; Lu and Roche, 2012; Huganir and Nicoll, 2013). By altering phosphorylation levels at a specific amino acid in the CT domain, various protein kinases regulate trafficking, distribution and function of modified receptors. Early studies identified two major phosphorylation sites in GluA1 CT, i.e., serine 831 (S831) and serine 845 (S845) (Roche et al., 1996; Barria et al., 1997; Mammen et al., 1997; Serulle et al., 2007). While S831 is phosphorylated by protein kinase C (PKC) and Ca2+/calmodulin-dependent protein kinase II, S845 is phosphorylated by protein kinase A (PKA) and cyclic guanosine monophosphate-dependent protein kinase II. Another phosphorylation site is serine 880 (S880) in GluA2 CT which is phosphorylated by PKC (Matsuda et al., 1999; Chung et al., 2000; McDonald et al., 2001). It is clear that phosphorylation at these sites modifies the number of AMPA receptors at a distinct subcellular site and determines the efficacy of AMPA receptors and excitatory synapses (Chung et al., 2000; Xia et al., 2000; Kim et al., 2001).
Phosphorylation of AMPA receptors is subject to the modulation by psychostimulants (Wolf and Ferrario, 2010; Mao et al., 2011). Early studies focused on the striatum, a key target of stimulants and an area enriched with GluA1/GluA2 AMPA receptors (Bernard et al., 1997; Kondo et al., 2000; Hu et al., 2004; Reimers et al., 2011). Dopamine D1 receptor agonists elevated GluA1 S845 phosphorylation in the striatum (Price et al., 1999; Snyder et al., 2000; Chao et al., 2002b; Swayze et al., 2004). Dopamine stimulants, such as cocaine and amphetamine (AMPH), were also effective in stimulating striatal S845 phosphorylation (Snyder et al., 2000; Li et al., 2011; Mao et al., 2013; Xue et al., 2014). Thus, AMPA receptors in the striatum are sensitive to stimulants and may play a role in the remodeling of synaptic plasticity related to addictive properties of drugs of abuse. In addition to the striatum, the hippocampus draws increasing attention as a responsive brain region in drug action. This area shows high levels of dopamine D1 and D2 receptors (Fremeau et al., 1991; Gangarossa et al., 2012) and AMPA receptors (Wenthold et al., 1996). As a major structure underlying learning and memory, the hippocampal formation is believed to play a critical role in the experience-based and learning-dependent drug addiction (Fuchs et al., 2005; Robbins et al., 2008; Koob and Volkow, 2010). However, the hippocampal importance has been less studied and the responsivity of the AMPA receptor population in the hippocampus to stimulants has not been fully investigated.
In this study, we investigated the impact of AMPH on AMPA receptor phosphorylation in the hippocampus of adult rat brains in vivo. Specifically, we monitored changes in GluA1 phosphorylation at S831 and S845 and GluA2 phosphorylation at S880 in the hippocampus after AMPH administration. We first conducted two studies to characterize dose- and time-dependent effects of AMPH on AMPA receptor phosphorylation. We then mapped the phosphorylation response of AMPA receptors in hippocampal subfields. Finally, we monitored correlated changes in surface GluA1 and GluA2 expression in hippocampal neurons in response to AMPH.
Materials and methods
Animals
Adult male Wistar rats weighing 220–275 g (Charles River, New York, NY) were used in this study. Animals were individually housed in a controlled environment at a constant temperature of 23°C and humidity of 50 ± 10% with food and water available ad libitum. The animal room was on a 12-h/12-h light/dark cycle. Rats were allowed 6–7 days of habituation to the animal colony. All animal use and procedures were in strict accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. The Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines have been followed.
AMPH administration
The stimulant d-amphetamine sulfate (Sigma-Aldrich, St. Louis, MO) was dissolved in saline. A single intraperitoneal (i.p.) injection of saline or AMPH was given to individual rats. In a dose-response study, rats were treated with saline or AMPH at one of three different doses (0.2, 1, or 5 mg/kg) and were sacrificed 15 min after drug injection. The dose of the drug was calculated as the salt. In a time-course study, rats were treated with saline or AMPH (5 mg/kg, i.p.). Rats were then sacrificed at different time points (15 min, 1 h, or 6 h after drug injection) for brain tissue harvest for Western blot analysis.
Synaptosomal preparation
After drug administration, rats were anesthetized with equithesin (5 ml/kg, i.p.) and were decapitated. Rat brains were removed and cut into coronal slices (600–800 μm). The entire striatum containing the caudate putamen and nucleus accumbens and hippocampus were removed at 4°C. To isolate hippocampal subfields, Cornu Ammonis area 1 (CA1), the bend region of CA3, and the dentate gyrus (DG) region centered by the dentate hilus were separated from the slices by microdissection (Zhao et al., 2001). The dissected hippocampal and striatal tissues were homogenized in isotonic sucrose homogenization buffer (0.32 M sucrose, 10 mM HEPES, pH 7.4, and 2 mM EDTA) containing a protease inhibitor cocktail and a phosphatase inhibitor cocktail (Thermo Scientific, Rochester, NY). The homogenate was centrifuged at 800 g (10 min). The supernatant was centrifuged at 10,000 g (30 min). The pellet 2 (P2) containing crude synaptosomal plasma membranes was washed and centrifuged again at 10,000 g (30 min). The supernatant was removed and the pellet (synaptosomal membranes) was resuspended and solubilized in the sucrose homogenization buffer with 0.5% Triton X-100, 1% sodium dodecyl sulfate (SDS), a protease inhibitor cocktail (Thermo Scientific), and a phosphatase inhibitor cocktail (Thermo Scientific). Protein concentrations were determined. Samples were stored at −80°C until use.
Western blot analysis
The equal amount of proteins was loaded and separated on SDS NuPAGE Novex 4–12% gels (Invitrogen, Carlsbad, CA). Proteins on gels were transferred to the polyvinylidene fluoride membrane (Millipore, Bedford, MA). The membrane was blocked in a blocking buffer (5% nonfat dry milk in phosphate-buffered saline and 0.1% Tween 20) for 1 h. The membrane was washed and incubated in the blocking buffer containing a primary antibody usually at 1:1000 overnight at 4°C. This was followed by 1 h incubation in a horseradish peroxidase-linked secondary antibody against rabbit or mouse IgG (Jackson Immunoresearch Laboratory, West Grove, PA) at 1:5000. Immunoblots were developed with the enhanced chemiluminescence reagents (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). MagicMark XP Western protein standards (Invitrogen) were used for protein size determination. Optical density of immunoblots was measured using NIH ImageJ gel analysis software. Values of optical density were normalized to actin. Primary antibodies used in this study include rabbit polyclonal antibodies against GluA1 with phosphorylated S831 (pS831) (PhosphoSolutions, Aurora, CO), GluA1 with phosphorylated S845 (pS845) (PhosphoSolutions), GluA2 with phosphorylated S880 (pS880) (PhosphoSolutions), GluA1 (Millipore, Billerica, MA), GluA2 (PhosphoSolutions), or actin (Sigma), and mouse antibodies against α-actinin (Millipore).
Surface receptor cross-linking assays
To detect surface versus intracellular receptor expression in hippocampal neurons of adult rat brains, we performed surface receptor cross-linking assays as described previously (Boudreau and Wolf, 2005; LacKamp et al., 2009; Mao et al., 2009). Briefly, after rats were anesthetized and decapitated, brains were removed and cut into coronal sections (300–400 μm) with a vibratome (Leica VT1200 S). The hippocampi were rapidly dissected and added into Eppendorf tubes containing ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM) 10 glucose, 124 NaCl, 3 KCl, 1.25 KH2PO4, 26 NaHCO3, 2 MgSO4, and 2 CaCl2, bubbled with 95% O2-5% CO2, pH 7.4. To cross-link surface receptors, we added a bis(sulfosuccinimidyl)suberate (BS3) reagent (Pierce, Rockford, IL) to a final concentration of 2 mM. Slices were incubated for 45 min with gentle agitation at 4°C. The cross-linking reaction was terminated by quenching with 20 mM of glycine (10 min, 4°C). The sections were then washed four times (5 min each) and sonicated in ice cold radioimmunoprecipitation assay (RIPA) buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.25% deoxycholic acid, and 1% NP-40. Protein concentrations were measured and proteins were analyzed by SDS-PAGE with 4–12% Trisglycine gels (Invitrogen).
Statistics
The results are presented as means ± SEM. These results were statistically analyzed using a one-way analysis of variance followed by a Bonferroni (Dunn) comparison of groups using least squares-adjusted means or two-tailed unpaired Student's t-test. Probability levels of < 0.05 were considered statistically significant.
Results
Effects of AMPH on AMPA receptor phosphorylation: a dose-response study
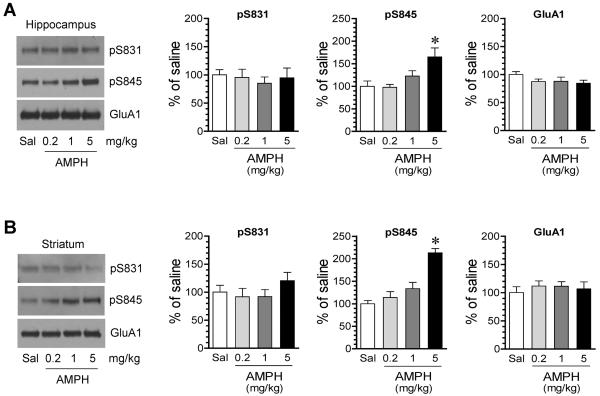
We first investigated the effect of AMPH in a dose-response study. In this experiment, we subjected rats to a single i.p. injection of saline or AMPH at different doses (0.2, 1, or 5 mg/kg). We then sacrificed rats 15 min after drug administration to analyze the impact of AMPH on AMPA receptor phosphorylation. We focused on the hippocampus. The striatum, an area in which the effect of AMPH on AMPA receptor phosphorylation has been well documented (Mao et al., 2013), served as a regional control. In immunoblot analysis of GluA1 S831 phosphorylation, we found that AMPH did not alter pS831 protein levels in the hippocampus at all three doses surveyed (Fig. 1A). In the striatum, the stimulant induced an insignificant change in pS831 expression (Fig. 1B), similar to early observations in this region (Mao et al., 2013). Total cellular levels of GluA1 proteins in both regions remained stable following AMPH administration. These data indicate that GluA1 S831 phosphorylation in the hippocampus is insensitive to AMPH.
Figure 1. Effects of AMPH at different doses on GluA1 phosphorylation in the rat hippocampus.
(A) Effects of AMPH on S831 and S845 phosphorylation in the hippocampus. (B) Effects of AMPH on S831 and S845 phosphorylation in the striatum. Representative immunoblots are shown to the left of the quantified data. Note that AMPH induced a site-specific and dose-dependent increase in S845 phosphorylation in both regions. Rats were treated with a single i.p. injection of saline (Sal) or AMPH at different doses (0.2, 1, or 5 mg/kg) and were sacrificed 15 min after drug injection for preparing synaptosomal proteins for immunoblot analysis. Data are presented as means ± SEM (n = 5 per group). *P < 0.05 versus saline.
We next assayed GluA1 phosphorylation at S845 in the same samples. Unlike S831, S845 phosphorylation in the hippocampus was significantly altered by AMPH. As shown in Fig. 1A, pS845 protein levels were dose-dependently elevated following AMPH administration. At two lower doses (0.2 and 1 mg/kg), AMPH did not significantly alter pS845 expression. At a higher dose (5 mg/kg), AMPH induced a marked increase in pS845 proteins. AMPH also dose-dependently increased S845 phosphorylation in the striatum (Fig. 1B) as expected based on our previous study (Mao et al., 2013). These data suggest that S845 in hippocampal neurons is sensitive to AMPH. Since total GluA1 proteins remained unchanged, a higher level of pS845 signals reflects an increase in the portion of phosphorylated proteins.
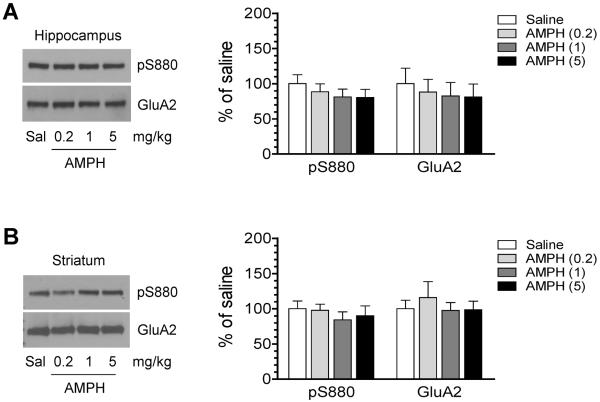
In addition to GluA1, GluA2 is phosphorylated at S880 (Matsuda et al., 1999; Chung et al., 2000; McDonald et al., 2001). We then assessed changes in this site phosphorylation. In the hippocampus, pS880 protein levels were not altered by AMPH at three doses (Fig. 2A). Similarly, pS880 protein levels in the striatum of AMPH-treated rats did not differ from those in saline-treated rats (Fig. 2B). Total GluA2 protein levels in both regions were not altered after AMPH administration. These results indicate that GluA2 S880 phosphorylation is not regulated by AMPH in both hippocampal and striatal neurons.
Figure 2. Effects of AMPH at different doses on GluA2 phosphorylation in the rat hippocampus.
(A) Effects of AMPH on S880 phosphorylation in the hippocampus. (B) Effects of AMPH on S880 phosphorylation in the striatum. Representative immunoblots are shown to the left of the quantified data. Rats were treated with a single i.p. injection of saline (Sal) or AMPH at different doses (0.2, 1, or 5 mg/kg) and were sacrificed 15 min after drug injection for preparing synaptosomal proteins for immunoblot analysis. Data are presented as means ± SEM (n = 5 per group).
Effects of AMPH on AMPA receptor phosphorylation: a time course study
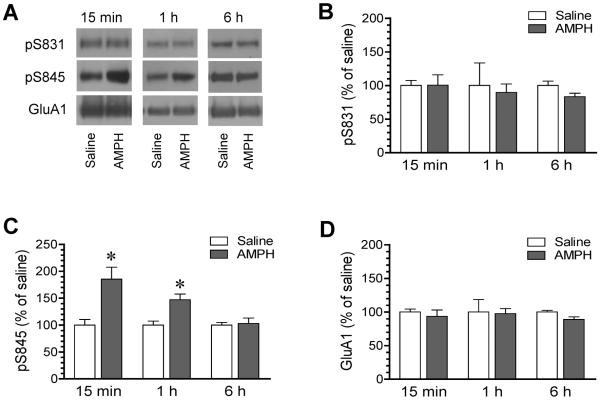
To determine temporal properties of the AMPH effect, we carried out a separate time course study. In this experiment, we administered AMPH to rats at a single effective dose (5 mg/kg). We then sacrificed rats at different time points (15 min, 1 h, or 6 h after drug injection) to monitor changes in AMPA receptor phosphorylation in the hippocampus. At each time point, saline injection served as a control. We first assayed S831 phosphorylation. We found that AMPH was still ineffective in affecting S831 phosphorylation. At 15 min, pS831 levels in the hippocampus were not changed in AMPH-treated rats relative to saline-treated rats (Fig. 3A and 3B), consistent with the above finding. At the two later time points (1 and 6 h), pS831 levels in AMPH-treated rats were not different from those in saline-treated rats. At all three time points, total GluA1 showed a minimal change (Fig. 3A and 3D). These data support that S831 is an insensitive site to AMPH.
Figure 3. Time course analysis of effects of AMPH on GluA1 phosphorylation in the rat hippocampus.
(A) Representative immunoblots illustrating effects of AMPH on S831 and S845 phosphorylation surveyed at different time points after drug injection. (B–D) The quantified data showing effects of AMPH on S831 (B) and S845 (C) phosphorylation and GluA1 (D) expression at different time points. Note that AMPH increased pS845 protein levels at 15 min and 1 h and did not alter pS845 levels at 6 h. Rats were treated with a single i.p. injection of saline or AMPH (5 mg/kg) and were sacrificed at different time points (15 min, 1 h, or 6 h after drug injection) for preparing synaptosomal proteins for immunoblot analysis. Data are presented as means ± SEM (n = 4–6 per group). *P < 0.05 versus saline at the same time point.
Unlike S831, S845 phosphorylation was clearly regulated in a time-dependent fashion. A robust increase in pS845 protein abundance was seen in the hippocampus at 15 min after AMPH administration (Fig. 3A and 3C). A significant increase persisted at 1 h. At 6 h, the amount of pS845 proteins measured in AMPH-treated rats returned to a level insignificantly different from that detected in the saline group. These results reveal a dynamic and reversible phosphorylation of S845 in hippocampal neurons following AMPH stimulation.
Finally, GluA2 S880 phosphorylation was analyzed in the time course study. No significant difference in hippocampal pS880 levels was found between AMPH- and saline-treated rats at 15 min (Fig. 4). Similar results were observed at 1 and 6 h. Throughout the time course surveyed, total GluA2 levels did not show a significant change. Thus, GluA2 S880 phosphorylation in hippocampal neurons is resistant to AMPH.
Figure 4. Time course analysis of effects of AMPH on GluA2 S880 phosphorylation in the rat hippocampus.
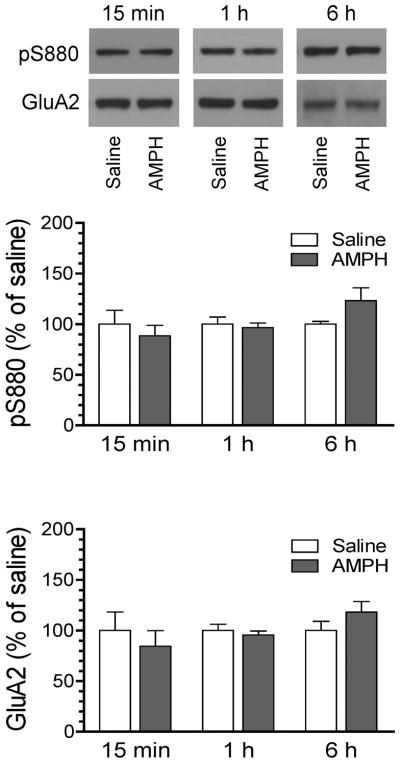
Representative immunoblots are shown above the quantified data. Rats were treated with a single i.p. injection of saline or AMPH (5 mg/kg) and were sacrificed at different time points (15 min, 1 h, or 6 h after drug injection) for preparing synaptosomal proteins for immunoblot analysis. Data are presented as means ± SEM (n = 4–6 per group).
Effects of AMPH on AMPA receptor phosphorylation in hippocampal subfields
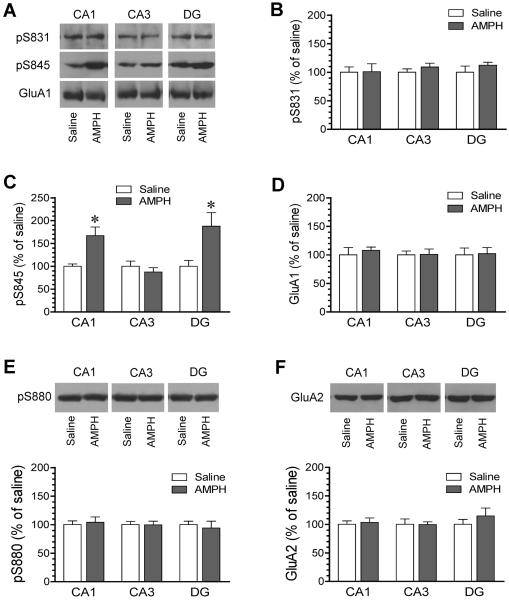
To determine the effect of AMPH in hippocampal subfields, we microdissected CA1, CA3 and DG structures from rats treated with saline or AMPH. Following AMPH administration (5 mg/kg, i.p.; 15 min prior to tissue collection), changes in AMPA receptor phosphorylation in all three types of principal hippocampal neurons (i.e., the giant pyramidal neurons in the CA3, the smaller pyramidal neurons in the CA1, and the granule neurons in the DG) were measured by immunoblots. AMPH had no significant effect on pS831 protein levels in CA1, CA3, and DG subregions (Fig. 5A and 5B). AMPH however induced a significant increase in pS845 levels in the CA1 and DG, while the stimulant did not alter pS845 levels in the CA3 (Fig. 5A and 5C). Changes in GluA2 phosphorylation were also measured in the same samples. No significant change in GluA2 S880 phosphorylation was observed in AMPH-treated rats compared to saline-treated rats (Fig. 5E). AMPH did not alter total amounts of GluA1 and GluA2 in the three subregions (Fig. 5D and 5F). These data demonstrate that the AMPH effect was subfield-specific as manifested by induction of S845 phosphorylation primarily in CA1 and DG neurons.
Figure 5. Effects of AMPH on AMPA receptor phosphorylation in the hippocampal subfields.
(A) Representative immunoblots illustrating effects of AMPH on S831 and S845 phosphorylation in CA1, CA3, and DG subregions. (B–D) The quantified data showing effects of AMPH on S831 (B) and S845 (C) phosphorylation and GluA1 (D) expression in CA1, CA3, and DG subregions. Note that AMPH induced an increase in S845 protein levels in CA1 and DG but not CA3 areas. (E and F) Effects of AMPH on S880 phosphorylation (E) and GluA2 expression (F) in CA1, CA3, and DG subregions. Representative immunoblots are shown above the quantified data. Rats were treated with a single i.p. injection of saline or AMPH (5 mg/kg) and were sacrificed 15 min after drug injection for microdissection of hippocampal subfields. Synaptosomal proteins were prepared for immunoblot analysis. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus saline.
Effects of AMPH on surface and intracellular expression of GluA1 and GluA2
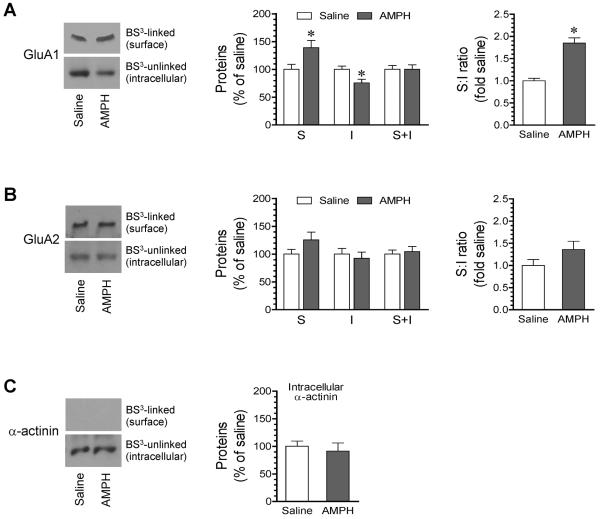
To determine possible subcellular redistribution of the receptors, we used a crosslinking assay to isolate receptors from specific surface and intracellular pools in intact hippocampal neurons in vivo. In these assays, a membrane-impermeable reagent BS3 was used to selectively crosslinks surface membrane-bound receptors to form high-molecular weight aggregates. These aggregates can be readily separated from unlinked intracellular receptors bound on light membranes of the intracellular endoplasmic reticulum, Golgi, and vesicular structures on gel electrophoresis. In experiments testing the effect of AMPH, we found that AMPH (5 mg/kg, i.p., 1 h) induced a slight but significant increase in BS3-linked surface GluA1 protein levels (Fig. 6A). The BS3-unlinked intracellular GluA1 was concurrently reduced. As a result, the surface to intracellular ratio was markedly enhanced. There was no significant change in GluA2 levels in either the surface or the intracellular pool or in the surface to intracellular ratio (Fig. 6B). Expression of α-actinin, an intracellular protein which was inaccessible to BS3 and served as a control, remained unchanged in response to AMPH (Fig. 6C). These results indicate that AMPH selectively modulates GluA1 trafficking in hippocampal neurons by redistributing GluA1 from the intracellular compartment to the surface membrane.
Figure 6. Effects of AMPH on surface and intracellular expression of AMPA receptor subunits in the rat hippocampus.
(A–B) Effects of AMPH on surface (S) and intracellular (I) expression of GluA1 (A) and GluA2 (B) and on the S:I ratio of GluA1 (A) and GluA2 (B) in hippocampal neurons. (C) Effects of AMPH on α-actinin expression in hippocampal neurons. Representative immunoblots are shown to the left of the quantified data. Rats were treated with a single i.p. injection of saline or AMPH (5 mg/kg) and were sacrificed 1 h after drug injection for BS3 crosslinking assays of changes in surface versus intracellular expression of GluA1 and GluA2 in the hippocampus. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus saline.
Discussion
Effects of AMPH on phosphorylation and surface expression of AMPA receptors were investigated in adult rat brains in vivo with a focus on the hippocampus. A single dose of AMPH increased GluA1 S845 phosphorylation in this brain region. The effect of AMPH was dose-dependent. The rapidly-induced S845 phosphorylation was reversible. In contrast to S845, S831 phosphorylation was not altered by AMPH. S880 phosphorylation in hippocampal GluA2 was also resistant to AMPH. In surface receptor crosslinking assays, we found that AMPH slightly elevated surface levels of GluA1 although not GluA2 proteins. These results indicate that S845 phosphorylation of AMPA receptors is selectively regulated by AMPH in hippocampal neurons. AMPH was able to induce a dose- and time-dependent rise in S845 phosphorylation, accompanied by a parallel increase in surface-expressed GluA1.
S845 phosphorylation and surface trafficking of receptors
The site selective nature of AMPH action is noteworthy. Early studies observed that AMPH at 5 mg/kg increased S845 phosphorylation in the striatum and prefrontal cortex, while AMPH did not alter S831 and S880 phosphorylation in these regions (Mao et al., 2013; Xue et al., 2014). Similarly, in the hippocampus, we found that AMPH stimulated the phosphorylation at S845, while sparing S831 and S880. Apparently, S845 is a preferred site by AMPH in all brain regions surveyed so far. This reflects the ability of AMPH to enhance dopamine levels and subsequently activate dopamine D1 receptors and associated PKA, leading to changes in GluA1 phosphorylation at the PKA site (S845). Regarding functional outcome, the phosphorylation at S845 has been found to drive surface trafficking of GluA1-containing receptors onto extrasynaptic sites (Chao et al., 2002a; Mangiavacchi et al., 2004; Man et al., 2007), which facilitates their insertion into synapses (Esteban et al., 2003; Sun et al., 2005; Oh et al., 2006; Gao et al., 2006; Sun et al., 2008) and potentiates the peak current of AMPA receptor channels (Roche et al., 1996). It is likely that AMPH enhances S845 phosphorylation to accelerate surface delivery of GluA1. This likelihood is supported by our finding that AMPH induced a correlated increase in surface expression of GluA1. Of note, the increase in surface expression primarily occurred to GluA1, indicating an increase in surface expression of homomeric GluA1 receptors. In other studies, increased GluA1 surface expression in the nucleus accumbens was associated with an increase in pS845 protein levels in surface and extrasynaptic pools in rats self-administering cocaine (Ferrario et al., 2011), although surface GluA1 expression in the nucleus accumbens was not elevated following behavioral sensitization to AMPH (Nelson et al., 2009). Parallel increases in both GluA1 and pS845 proteins occurred in the rat ventral tegmental area (VTA) following cocaine self-administration (Choi et al., 2011).
Hippocampal roles in stimulant action
The hippocampal formation is another important region for stimulant action (Robbins et al., 2008; Ricoy and Martinez, 2009; Koob and Volkow, 2010). Although not traditionally considered part of the reward circuitry, the hippocampus is intimately connected to the reward system with the primary dopaminergic input from the VTA and a major glutamatergic output to the nucleus accumbens (Gasbarri et al., 1997; Luo et al., 2011; Britt et al., 2012). A growing appreciation is that the hippocampus as a major central site for learning and memory is significantly implicated in experience-dependent drug seeking behavior. This appreciation is supported by increasing evidence. For instance, AMPH increased hippocampal glucose utilization (Engber et al., 1998). Acute AMPH or cocaine increased dopamine levels in the hippocampus (Borgkvist et al., 2012). Since the hippocampus expresses few, if any, dopamine transporters (Lorang et al., 1994), stimulants are reasoned to block the norepinephrine transporter that is suggested to clear dopamine in the hippocampus to enhance dopamine in the region (Borgkvist et al., 2012). Cocaine self-administration also activated hippocampal neurons as detected by Fos expression (Neisewander et al., 2000). Behaviorally, lidocaine inactivation of hippocampal neurons blocked behavioral sensitization to AMPH (Degoulet et al., 2008) and attenuated drug seeking behavior (Sun and Rebec, 2003). Electrical stimulation of the hippocampus or intra-hippocampal injection of methamphetamine elicited drug seeking behavior (Vorel et al., 2001; Taepavarapruk and Phillips, 2003; Ricoy and Martinez, 2009). A number of other studies further support the role of the hippocampus in environmental context- or drug-primed seeking behavior (Black et al., 2004; Fuchs et al., 2005; 2007; Rogers and See, 2007; Lansink et al., 2009; Hearing et al., 2010; Lasseter et al., 2010; Xie et al., 2010; 2014) and in AMPH or cocaine conditioned place preference (Meyers et al., 2003; 2005; Rademacher et al., 2006). In electrophysiological experiments, cocaine and methamphetamine altered synaptic plasticity in the form of long-term potentiation, a cellular substrate of learning, recorded in the hippocampus (Swant et al., 2010; Keralapurath et al., 2014). These results collectively support that the hippocampus is critical for learning and memory diseases such as stimulant addiction (Kelley, 2004; Hyman et al., 2006).
Hippocampal AMPA receptors and drug effects
The AMPA receptor population in the hippocampus is believed to contribute to local synaptic plasticity and drug-associated learning. In addition to dopamine D1 and D2 receptors expressed in hippocampal neurons (Bruinink et al., 1986; Fremeau et al., 1991; Gangarossa et al., 2012), AMPA receptors are enriched in this area with two major populations of GluA1/GluA2 and GluA2/GluA3 heteromers and a smaller amount of GluA1 homomers (Wenthold et al., 1996). The D1 agonist SKF81297 induced a sustained enhancement of AMPA receptor-mediated currents in rat hippocampal CA1 neurons (Yang, 2000). Re-exposing mice to a cocaine-associated environment increased GluA1 S845 phosphorylation in the dorsal hippocampus (Tropea et al., 2008). Cocaine self-administration also increased S831 and S845 phosphorylation in the CA1 hippocampus (Edwards et al., 2007). Morphine administration enhanced hippocampal GluA1 phosphorylation and expression at synaptic and extrasynaptic sites (Billa et al., 2009; 2010). Intra-hippocampal infusion of a cell permeable TAT-S845 peptide that selectively inhibited S845 phosphorylation suppressed acute behavioral responses to AMPH (Du et al., 2008). In this study, we found that AMPH enhanced S845 phosphorylation and surface GluA1 expression in hippocampal neurons. This adds additional evidence for the critical role of hippocampal AMPA receptors in stimulant action. Future studies need to elucidate accurate underlying mechanisms linking phosphorylation changes to receptor plasticity in relation to drug seeking behavior. In addition, it is unclear why AMPH did not alter S845 phosphorylation in the CA3 subregion. Given a set of anatomical and physiological differences among the hippocampal subfields (Gaarskjaer, 1986; Zhao et al., 2001), future studies will also need to explore subfield-specific responses of AMPA receptors to stimulants and contributions of the subfield receptor to drug action.
Acknowledgements
This work was supported by NIH grants R01DA10355 (JQW) and R01MH61469 (JQW).
Abbreviations used
- ACSF
artificial cerebrospinal fluid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPH
amphetamine
- BS3
bis(sulfosuccinimidyl)suberate
- CA
Cornu Ammonis area
- CT
C-terminus
- DG
dentate gyrus
- ECL
enhanced chemiluminescence
- EDTA
ethylenediaminetetraacetic acid
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- PKA
protein kinase A
- PKC
protein kinase C
- RIPA
radioimmunoprecipitation assay
- SDS
Sodium dodecyl sulfate
- VTA
ventral tegmental area.
Footnotes
Conflict of interest: The authors declare that there are no potential conflicts of interest.
References
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J. Biol. Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rats. J. Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billa SK, Liu J, Bjorklund NL, Sinha N, Fu Y, Shinnick-Gallagher P, Moron JA. Increased insertion of glutamate receptor 2-lacking alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors at hippocampal synapses upon repeated morphine administration. Mol. Pharmacol. 2010;77:874–883. doi: 10.1124/mol.109.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billa SK, Sinha N, Rudrabhatla SR, Moron JA. Extinction of morphine-dependent conditioned behavior is associated with increased phosphorylation of the GluR1 subunit of AMPA receptors at hippocampal synapses. 2009;29:55–64. doi: 10.1111/j.1460-9568.2008.06560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Hippocampal memory system function and the regulation of cocaine self-administration behavior in rasts. Behav. Brain Res. 2004;151:225–238. doi: 10.1016/j.bbr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Malmlof T, Feltmann K, Lindskog M, Schilstrom B. Dopamine in the hippocampus is cleared by the norepinephrine transporter. Int. J. Neuropsychopharmacol. 2012;15:531–540. doi: 10.1017/S1461145711000812. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J. Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinink A, Bischoff A. Detection of dopamine receptors in homogenates of rat hippocampus and other brain areas. Brain Res. 1986;386:78–83. doi: 10.1016/0006-8993(86)90143-5. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Ariano MA, Peterson DA, Wolf ME. D1 dopamine receptor stimulation increases GluR1 phosphorylation in nucleus accumbens neurons. J. Neurochem. 2002a;83:704–712. doi: 10.1046/j.1471-4159.2002.01164.x. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME. D(1) dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J. Neurochem. 2002b;81:984–992. doi: 10.1046/j.1471-4159.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- Choi KH, Edwards S, Graham DL, Larson EB, Whisler KN, Simmons D, Friedman AK, Walsh JJ, Rahman Z, Monteggia LM, Eisch AJ, Neve RL, Nestler EJ, Han MH, Self DW. Reinforcement-treated regulation of AMPA glutamate receptor subunits in the ventral tegmental area enhances motivation for cocaine. J. Neurosci. 2011;31:7927–7937. doi: 10.1523/JNEUROSCI.6014-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J. Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degoulet M, Rouillon C, Rostain JC, David HN, Abraini JH. Modulation by the dorsal, but not the ventral, hippocampus of the expression of behavioral sensitization to amphetamine. Int. J. Neuropsychopharmacol. 2008;11:497–508. doi: 10.1017/S146114570700822X. [DOI] [PubMed] [Google Scholar]
- Du J, Greson TK, Wu LJ, Ren M, Gray NA, Falke C, Wei Y, Wang Y, Blumenthal R, Machado-Vieira R, Yuan P, Chen G, Zhou M, Manji HK. The role of hippocampal GluR1 and GluR2 receptors in manic-like behavior. J. Neurosci. 2008;28:68–79. doi: 10.1523/JNEUROSCI.3080-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Graham DL, Bachtell RK, Self DW. Region-specific tolerance to cocaine-regulated cAMP-dependent protein phosphorylation following chronic self-administration. Eur. J. Neurosci. 2007;25:2201–2213. doi: 10.1111/j.1460-9568.2007.05473.x. [DOI] [PubMed] [Google Scholar]
- Engber TM, Dennis SA, Jones BE, Miller MS, Contreras PC. Brain regional substrates for the actions of the novel wake-promoting agent modafinil in the rat: comparison with amphetamine. Neuroscience. 1998;87:905–911. doi: 10.1016/s0306-4522(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Estaban JA, Shi H, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Duncan GE, Fornaretto MG, Dearry A, Gingrich JA, Breese GR, Caron MG. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc. Natl. Acad. Sci. USA. 1991;88:3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su Z, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur. J. Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Gaarskjaer FB. The organization and development of the hippocampal mossy fiber system. Brain Res. 1986;396:335–357. doi: 10.1016/0165-0173(86)90004-4. [DOI] [PubMed] [Google Scholar]
- Gangarossa G, Longueville S, Bundel DD, Perroy J, Herve D, Girault JA, Valjent E. Characterization of dopamine D1 and D2 receptor-expressing neurons in the mouse hippocampus. Hippocampus. 2012;22:2199–2207. doi: 10.1002/hipo.22044. [DOI] [PubMed] [Google Scholar]
- Gao C, Sun X, Wolf ME. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J. Neurochem. 2006;98:1664–1677. doi: 10.1111/j.1471-4159.2006.03999.x. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1997;21:1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Hearing MC, Schochet TL, See RE, McGinty JF. Context-driven cocaine-seeking in abstinent rats increases activity-regulated gene expression in the basolateral amygdala and dorsal hippocampus differentially following short and long periods of abstinence. Neuroscience. 2010;170:570–579. doi: 10.1016/j.neuroscience.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Chen LW, Yung KK, Chan YS. Differential expression of AMPA receptor subunits in substance P receptor-containing neurons of the caudate-putamen of rats. Neurosci. Res. 2004;49:281–288. doi: 10.1016/j.neures.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Keralapurath MM, Clark JK, Hammond S, Wagner JJ. Cocaine- or stress-induced metaplasticity of LTP in the dorsal and ventral hippocampus. Hippocampus. 2014;24:577–590. doi: 10.1002/hipo.22250. [DOI] [PubMed] [Google Scholar]
- Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc. Natl. Acad. Sci. USA. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Okabe S, Sumino R, Okado H. A high GluR1:GluR2 expression ratio is correlated with expression of Ca2+-binding proteins in rat forebrain neurons. Eur. J. Neurosci. 2000;12:2812–2822. doi: 10.1046/j.1460-9568.2000.00167.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LacKamp A, Zhang GC, Mao LM, Fibuch EE, Wang JQ. Loss of surface N-methyl-D-aspartate receptor proteins in mouse cortical neurones during anaesthesia induced by chloral hydrate in vivo. Br. J. Anaesth. 2009;102:515–522. doi: 10.1093/bja/aep009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 2009;7:e1000173. doi: 10.1371/journal.pbio.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience. 2010;171:830–839. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Herrera S, Bubula N, Nikitina E, Palmer AA, Hanck DA, Loweth JA, Vezina P. Casein kinase 1 enables nucleus accumbens amphetamine-induced locomotion by regulating AMPA receptor phosphorylation. J. Neurochem. 2011;118:237–247. doi: 10.1111/j.1471-4159.2011.07308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorang D, Amara SG, Simerly RB. Cell-type-specific expression of catecholamine transporters in the rat brain. J. Neurosci. 1994;14:4903–4914. doi: 10.1523/JNEUROSCI.14-08-04903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Roche KW. Posttranslational regulation of AMPA receptor trafficking and function. Curr. Opin. Neurobiol. 2012;22:470–479. doi: 10.1016/j.conb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: A functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J. Biol. Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc. Natl. Acad. Sci. USA. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiawacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J. Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- Mao LM, Diaz JA, Fibuch EE, Wang JQ. Regulation of phosphorylation of synaptic and extrasynaptic GluA1 AMPA receptors in the rat forebrain by amphetamine. Eur. J. Pharmacol. 2013;715:164–171. doi: 10.1016/j.ejphar.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Guo ML, Jin DZ, Fibuch EE, Choe ES, Wang JQ. Posttranslational modification biology of glutamate receptors and drug addiction. Front. Neuroanat. 2011;5:19. doi: 10.3389/fnana.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat. Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Mikawa S, Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J. Neurochem. 1999;73:1765–1768. doi: 10.1046/j.1471-4159.1999.731765.x. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Chung HJ, Huganir RL. Identification of protein kinase C phosphorylation sites within the AMPA receptor GluR2 subunit. Neuropharmacology. 2001;41:672–679. doi: 10.1016/s0028-3908(01)00129-0. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Neisewander JL. Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. NeuroReport. 2003;14:2127–2131. doi: 10.1097/00001756-200311140-00023. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Speer CM, Neisewander JL. Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav. Neurosci. 2006;120:401–412. doi: 10.1037/0735-7044.120.2.401. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J. Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CL, Milovanovic M, Wetter JB, Ford KA, Wolf ME. Behavioral sensitization to amphetamine is not accompanied by changes in glutamate receptor surface expression in the rat nucleus accumbens. J. Neurochem. 2009;109:35–51. doi: 10.1111/j.1471-4159.2009.05911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Price CJ, Kim P, Raymond LA. D1 dopamine receptor-induced cyclic AMP-dependent protein kinase phosphorylation and potentiation of striatal glutamate receptors. J. Neurochem. 1999;73:2441–2446. doi: 10.1046/j.1471-4159.1999.0732441.x. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Kovacs B, Shen F, Napier TC, Meredith GE. The neural substrates of amphetamine conditioned place preference: implications for the formation of conditioned stimulus-reward associations. Eur. J. Neurosci. 2006;24:2089–2097. doi: 10.1111/j.1460-9568.2006.05066.x. [DOI] [PubMed] [Google Scholar]
- Reimers JM, Milovanovic M, Wolf ME. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011;1367:223–233. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricoy UM, Martinez JL., Jr. Local hippocampal methamphetamine-induced reinforcement. Front. Behav. Neurosci. 2009;3:47. doi: 10.3389/neuro.08.047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann. NY Acad. Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol. Learn Mem. 2007;87:688–692. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:670–688. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J. Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subicullum attenuates cocaine-seeking behavior in rats. J. Neurosci. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J. Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J. Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swant J, Chirwa S, Stanwood G, Khoshbouei H. Methamphetamine reduces LTP and increases baseline synaptic transmission in the CA1 region of mouse hippocampus. PLoS One. 2010;5:e11382. doi: 10.1371/journal.pone.0011382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayze RD, Lise MF, Levinson JN, Phillips A, El-Husseini A. Modulation of dopamine mediated phosphorylation of AMPA receptors by PSD-95 and AKAP79/150. Neuropharmacology. 2004;47:764–778. doi: 10.1016/j.neuropharm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Phillips AG. Neurochemical correlates of relapse to d-amphetamine self-administration by rats induced by stimulation of the ventral subiculum. Psychopharmacology. 2003;168:99–108. doi: 10.1007/s00213-002-1337-2. [DOI] [PubMed] [Google Scholar]
- Tropea TF, Kosofsky BE, Rajadhyaksha AM. Enhanced CREB and DARPP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. J. Neurochem. 2008;106:1780–1790. doi: 10.1111/j.1471-4159.2008.05518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Liu X, Zhang G, Parelkar NK, Arora A, Haines M, Fibuch EE, Mao L. Phosphorylation of glutamate receptors: a potential mechanism for the regulation of receptor function and psychostimulant action. J. Neurosci. Res. 2006;84:1621–1629. doi: 10.1002/jnr.21050. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J. Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci. Biobehav. Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2010;208:1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wells AM, Fuchs RA. Cocaine seeking and taking: role of hippocampal dopamine D1-like receptors. Int. J. Neuropsychopharmacol. 2014;17:1533–1538. doi: 10.1017/S1461145714000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Edwards MC, Mao LM, Guo ML, Jin DZ, Fibuch EE, Wang JQ. Rapid and sustained GluA1 S845 phosphorylation in synaptic and extrasynaptic locations in the rat forebrain following amphetamine administration. Neurochem. Int. 2014;64:48–54. doi: 10.1016/j.neuint.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN. Sustained enhancement of AMPA receptors- and NMDA receptor-mediated currents induced by dopamine D1/D5 receptor activation in the hippocampus: an essential role of postsynaptic Ca2+ Hippocampus. 2000;10:57–63. doi: 10.1002/(SICI)1098-1063(2000)10:1<57::AID-HIPO6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lein ES, He A, Smith SC, Aston C, Gage FH. Transcriptional profiling reveals strict boundaries between hippocampal subregions. J. Comp. Neurol. 2001;441:187–196. doi: 10.1002/cne.1406. [DOI] [PubMed] [Google Scholar]