Abstract
Purpose
To determine the effects of keratocyte loss on optical properties and vision after laser in situ keratomileusis (LASIK) with the flap created with a femtosecond laser or a mechanical microkeratome.
Design
Randomized clinical paired-eye study.
Methods
Both eyes of 21 patients received LASIK for myopia or myopic astigmatism. One eye of each patient was randomized by ocular dominance to flap creation with a femtosecond laser and the other eye to flap creation with a mechanical microkeratome. Before LASIK and at 1, 3, 6 months and 1, 3, and 5 years after LASIK, keratocyte density was measured by using confocal microscopy, and high-contrast visual acuity and anterior corneal wavefront aberrations were measured by standard methods. At each visit, all variables were compared between methods of creating the flap and to the same variable before treatment by using paired tests with Bonferroni correction for multiple comparisons.
Results
Keratocyte density in the flap decreased by 20% during the first year after LASIK and remained low through 5 years (p<0.001). High-order wavefront aberrations increased and uncorrected visual acuity improved immediately after surgery but these variables did not change further to five years. There were no differences in any variables between treatments.
Conclusions
A sustained reduction in keratocyte density does not affect vision or optical properties of the cornea through 5 years after LASIK. The method of creating a LASIK flap does not influence the changes in keratocyte density in the flap.
Introduction
In the first year after laser in situ keratomileusis (LASIK), cell density in the stromal portion of the flap decreases considerably1–4 and does not return to pre-surgical densities after at least 5 years.4, 5 Most of these stromal cells are keratocytes, and normally, the density of these cells is highest in the anterior-most layer of the sub-basal stroma.6, 7 Keratocytes maintain the health of the stroma, and although the purpose of their high concentration in the anterior region is not fully understood, their demise could lead to an eventual degradation of stromal tissue that might increase corneal haze or progressively increase anterior corneal aberrations.8 In spite of the known chronic changes in cell density, in clinical practice there appears to be little impact of ketatocyte loss on structural or visual function of corneas after LASIK, although delayed manifestations of these changes are unknown.
If anterior stromal cell loss is a consequence of cutting the corneal stroma, then it is also possible that cell loss could vary with the method of creating the LASIK flap. Netto found that after cutting a flap with the microkeratome, cells were primarily degraded by apoptosis, while after creating incisions with a 30-kHz femtosecond laser cells were lost by necrosis, which has more expanded effects on surrounding tissue.9 If the hypothesis that decreased keratocyte density restricts the ability of the cornea to maintain ideal structural and optical properties is true, then optical properties of the cornea, such as surface irregularities or light scattered from the stroma, should change as cell density decreases. If methods of cutting the flap differentially affect the function of keratocytes, then these properties should also depend on the method used to cut the flap. Differences could appear sooner or later, depending on how these treatments affect the function of the keratocytes and the role that keratocytes play in maintaining these features of the cornea.
In this randomized paired-eye study, we compared cell loss between the methods of flap creation and investigated the effect of decreased cell density in the stromal flap on visual and optical properties after LASIK with the flap created either by using a femtosecond laser or by using a mechanical microkeratome. We previously reported comparisons of visual acuity and aberrations between flap creation methods through 3 years,10–12 and here we extend these measurements through 5 years. In addition, we include measurements of forward scatter and backscatter from 1 year through 5 years, and examine possible relationships with stromal cell density.
Methods
Participants and Randomization
This study explored the relationships between stromal cell loss and visual and optical properties for 5 years after LASIK with the flap cut by femtosecond laser or mechanical microkeratome, in a randomized paired-eye design. Twenty-one patients eligible for LASIK to treat myopia or myopic astigmatism were recruited from the refractive surgery service at Mayo Clinic, Rochester, MN, between 2004 and 2005. This group has been described in detail previously.13 Patients were excluded if they had corneal abnormalities, a history of ocular disease, trauma, surgery, diabetes mellitus, or other systemic diseases that affect the eye, or used any ocular or systemic medications that affect the cornea or anterior segment. The study was reviewed and approved prospectively by the Mayo Clinic Institutional Review Board and conformed to the Declaration of Helsinki. All patients provided written informed consent to participate after review and discussion of the nature and possible consequences of the study. The trial was registered at www.clinicaltrials.gov, # NCT00350246.
Each patient was randomized to treatment with the flap created by using a femtosecond laser in either the dominant eye or the non-dominant eye and with the flap created by using a mechanical microkeratome in the fellow eye. An equal number of patients had the femtosecond laser incision in the dominant eye as had it in the non-dominant eye. Ocular dominance was determined by observing the eye used by the patient to view a distant object through a frame made with the forefinger and thumb of both hands. Patients were examined before LASIK and at 1, 3, 6, months and 1, 3, and 5 years after LASIK.
Twenty participants with normal eyes were also prospectively examined as a control group. These subjects met the same inclusion criteria as the LASIK patients except that they did not have LASIK. One eye of each participant was randomly selected to compare with the eye treated with the femtosecond laser in the LASIK group (femtosecond laser control eye) and the other eye was compared with the mechanical microkeratome eye (mechanical microkeratome control eye). Participants were examined at an initial visit and 1 month and 1, 3, and 5 years after the initial visit. At each visit, the same variables were measured in both eyes of the control group as were measured in eyes in the LASIK group.
Surgical Treatment
The surgical procedures for LASIK have been described.11–13 Briefly, bladeless flaps were created by using a 15 kHz femtosecond laser (Intralase FS, Intralase Corp., Irvine, CA). All flaps had an intended thickness of 120 µm and superior hinge. The energy of each laser pulse was 2.3 µJ in the raster cut and 2.5 µJ in the side cuts. Raster line and spot separation were 9 and 11 µm respectively. In the contralateral eye, the flap was created by a mechanical microkeratome (Hansatome, Bausch & Lomb) with an intended thickness of 180 µm and superior hinge. All eyes received a non-wavefront- guided stromal ablation with an excimer laser (Star S4, Visx, Inc.) to a depth required by the refractive correction.
Emmetropia was attempted in all eyes by using an ablation zone from 6.5 mm×6.5 mm for spherical correction to 6.5 mm×5.0 mm for spherocylindrical correction. Both eyes of all patients had the same postoperative treatment, ciprofloxacin ophthalmic solution four times per day for 5 days and fluorometholone 0.1% four to eight times per day with a taper over three weeks.
Keratocyte Density
At each visit, both corneas of all participants were examined by clinical confocal microscopy with a Tandem Scanning confocal microscope (Tandem Scanning, Reston, VA), as described previously.7, 14 From images of the full-thickness cornea, 2 frames were selected from each of 6 layers of stroma, the anterior and posterior halves of the flap, the anterior and posterior halves of the stroma between the interface and 100 µm deep to the interface (retroablation zone), 66% to 90% of stromal depth, and the posterior 10% of stroma.3, 15 Cell nuclei were identified and counted in each selected frame by using an automated program14 and cell density was expressed as volumetric density (cells/mm3).
Assessment of Visual Acuity and High-order Aberrations
High-order aberrations and visual acuity had been measured through 36 months after surgery as reported earlier.11 In this study, corneal topography in all subjects was re-examined by using a Humphrey Atlas corneal topography system (Humphrey Systems, Pleasanton, CA) at 60 months. Wavefront aberrations were expressed as Zernike Polynomials (VOLCT; Sarver and Associates) and the root-mean-square (RMS) wavefront error from Zernike orders 3 – 6, as well as spherical aberration, coma, and trefoil, were calculated as described by Calvo et al.11 High-contrast best spectaclecorrected visual acuity (BCVA) and uncorrected visual acuity (UCVA) were determined by the electronic Early Treatment of Diabetic Retinopathy Study (ETDRS) protocol.16 Visual acuity was recorded as letter scores and converted to logarithm of the minimum angle of resolution (LogMAR).
Backscatter and Straylight
The original study design did not include estimates of backscatter or straylight (forward scatter) because we did not have the instruments to make these measurements at inception. During the first year of the study we obtained a ConfoScan 4 confocal microscope (Nidek Technologies, Inc., Fremont, CA), which allowed us to record confocal image brightness as a measure of corneal backscatter. We also obtained a C-Quant straylight meter (Oculus, Lynnwood, WA), which allowed us to estimate forward scatter. We report these data at 12, 36, and 60 months.
The cornea was scanned through its full depth by using the ConfoScan 4 clinical confocal microscope equipped with a z-ring adapter to stabilize the eye and report depth in the cornea of each frame. Backscatter was the mean brightness in the center of the confocal images standardized to a scatter standard (Amco Clear, GSF Chemicals, Columbus, OH) as described previously.17 Depth in the cornea was expressed as distance from the stromal surface scaled as a percentage of stromal thickness. After LASIK, depth was also scaled so the surgical interface appeared at the mean interface depth expressed as a percent of stromal thickness.
Retinal straylight (forward scatter) was estimated by using the compensation comparison method implemented with the straylight meter as described in detail elsewhere.18 Forward light scatter was expressed as the log of the stray-light parameter determined after each subject completed a 2-alternative forced-choice protocol that was under computer control.
Statistical Analysis
Differences between treatments and differences between preoperative and postoperative variables within treatments at each visit were examined by using two-tailed paired t-tests if the data were distributed normally and Wilcoxon signed-rank tests if they were not. Variables in the LASIK patients were compared to those in the control subjects by using non-paired tests. P-values were adjusted for multiple comparisons by using the Bonferroni method; p<0.05 was considered significant.
Results
Keratocyte Density, LASIK Patients
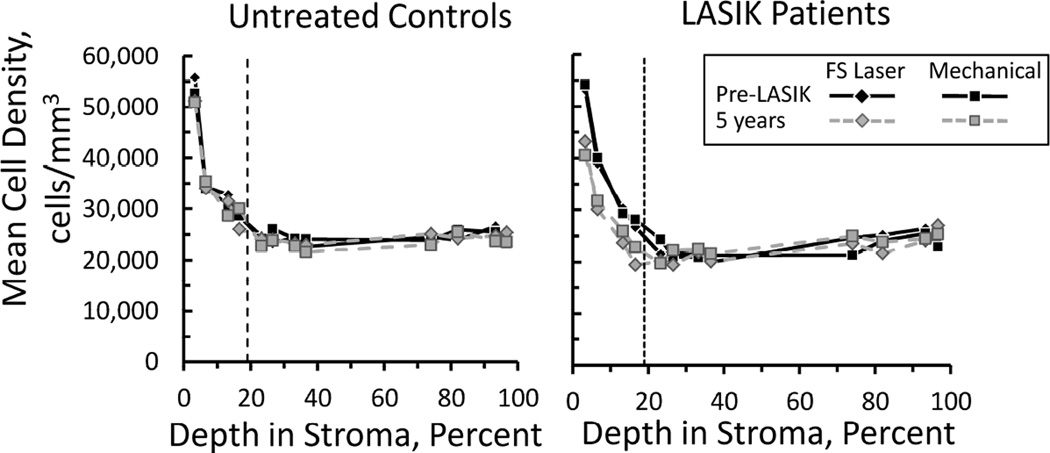
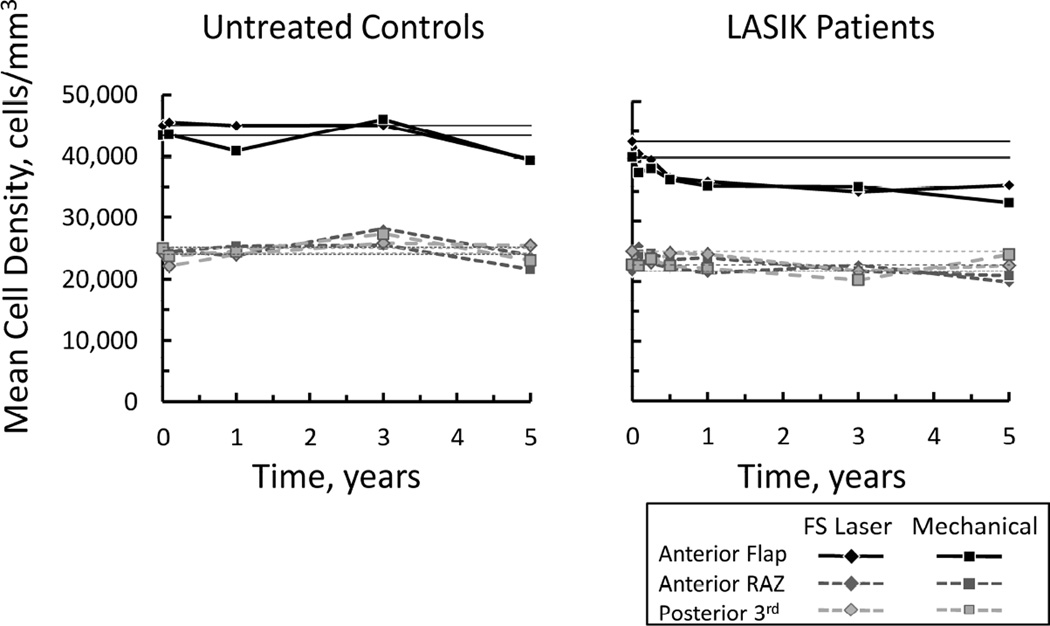
Before LASIK, cell densities were not different between eyes destined for flap creation by the femtosecond laser or by the mechanical microkeratome (p>0.9; Bonferroni-adjusted for 7 comparisons, Figures 1 and 2, Table 1). During the first year after LASIK, cell density in the flap gradually decreased after both procedures (p<0.032, Bonferroni-adjusted for 6 comparisons). There were no other consistent significant changes in cell density in layers deep to the surgical interface. Cell density in the anterior stroma did not recover, but remained low after both treatments to 5 years (p<0.001). Cell densities in the flap were generally lower than cell densities in analogous regions of the anterior stroma in the control subjects (Table 1).
Figure 1.
Mean cell density through the corneal stroma in untreated control subjects (left) and LASIK patients (right), before LASIK and at 5 years. The vertical broken line indicates the average depth of the surgical interface. Depth of each frame was scaled as a percentage of the total stromal depth, based on landmarks of the stromal boundaries in each scan. In control participants, fellow eyes were randomized for comparison to eyes in LASIK patients with flaps created by the femtosecond laser (FS Laser) or mechanical microkeratome (Mechanical). Cell density decreased in the flap after surgery and remained low at 5 years. There were no differences between treatments.
Figure 2.
Change in mean cell density in untreated control participants (left) and LASIK patients (right). After LASIK cell density in the anterior flap decreased during the first year and remained low through 5 years. In the anterior retro-ablation zone (RAZ, region 100 µm deep to the surgical interfaced) and posterior third of the stroma, cell density did not change. In control participants, fellow eyes were randomized for comparison to eyes in LASIK patients with flaps created by the femtosecond laser (FS Laser) or mechanical microkeratome (Mechanical). There were no differences between treatments. Horizontal lines represent pre-LASIK densities.
Table 1.
Keratocyte density in the anterior flap before and after LASIK.
| LASIK Patients | Control | |||
|---|---|---|---|---|
| Treatment: | Mechanical | Femtosecond | Randomized to Mechanical |
Randomized to Femtosecond |
| Visit | ||||
| Pre-LASIK | 47,147 ± 6,071 | 46,093 ± 6,038 | 43,348 ± 7,124 | 45,054 ± 9,332 |
| 1 month | 42,557 ± 7,277 | 44,709 ± 5,488 | 43,463 ± 8,271 | 45,515 ± 7,935 |
| 3 months | 41,108 ± 6,823b | 41,349 ± 7,092 | - | - |
| 6 months | 41,349 ± 7,286a | 41,305 ± 7,026a | - | - |
| 1 year | 41,393 ± 5,970b | 40,295 ± 4,905bd | 40,848 ± 8,334 | 44,998 ± 5,507d |
| 3 years | 37,407 ± 5,022ce | 35,406 ± 7,457ce | 46,022 ± 9,473e | 45,054 ± 8,192e |
| 5 years | 36,105 ± 7,897c | 36,635 ± 5,017ce | 43,194 ± 7,667 | 42,694 ± 5,811e |
LASIK - laser in situ keratomileusis
Mean cell density ± standard deviation, cells/mm3.
a p<0.05, b p<0.04, c p<0.001, compared to pre-LASIK
d p<0.04, e p<0.03, compared to control
Control corneas from each participant were randomized for purposes of comparison to the treated corneas in LASIK patients. p-values were Bonferroni-adjusted adjusted for 6 and 5 comparisons with pre-LASIK and control corneas respectively.
Cell density was not different between treatments at any time in any region (p> 0.059). The average minimum detectable difference in the flap was 6,400 cells/mm3 and in layers deep to the interface was 4,540 cells/mm3 (α=0.05, β=0.20, Bonferroni adjusted for 7 comparisons).
Keratocyte Density, Control Subjects
In control subjects, there were no consistent changes in cell densities in all 6 layers through 5 years (Figures 1 and 2). There were no differences in keratocyte density between corneas randomized for comparison to the femtosecond laser-created flaps and the fellow eyes (p>0.19).
Visual Acuity and Refractive Error
Before LASIK and at 1 month and 5 years, mean UCVA was not different between treatments (p>0.34, Bonferroni-adjusted for 3 comparisons); the minimum detectable difference was 0.10 LogMAR, or 5 ETDRS letters (α=0.05, β=0.20). At 5 years, mean uncorrected visual acuity was 0.01 ± 0.13 LogMAR and 0.04 ± 0.15 LogMAR in the eyes treated with the femtosecond laser and mechanical microkeratome respectively (p=0.30). These were not different from the visual acuity at 1 month of 0.01 ± 0.14 LogMAR and 0.00 ± 0.13 LogMAR after these treatments respectively (p>0.43).
Mean spherical equivalent before and at 1 month and 5 years after LASIK were not different between treatments (p>0.6, Bonferroni-adjusted for 3 comparisons); the minimum detectable difference was 0.50 D before LASIK and 0.25 D after LASIK (α=0.05, β=0.20). At 5 years mean spherical equivalent was −0.18 ± 0.37 D and −0.15 ± 0.29 D in eyes treated with the femtosecond laser and mechanical microkeratome respectively (p=0.65). These had not changed from −0.09 ± 0.28 D and −0.17 ± 0.27 D at 1 month after these treatments respectively (p>0.30). Best corrected visual acuity before and at one month and 5 years was not different between treatments (p>0.13), and the minimum detectable difference was 0.06 LogMAR, or 3 ETDRS letters. At 5 years, BCVA was −0.08 ± 0.06 LogMAR and −0.04 ± 0.08 LogMAR in the eyes treated with the femtosecond laser and mechanical microkeratome respectively, and was not different from BCVA of −0.05 ± 0.08 LogMAR and −0.05 ± 0.09 LogMAR after these treatments respectively at 1 month (p>0.09). All measurements other than at 5 years were from Calvo et al.11
High-order aberrations
Before LASIK and at 1 month and 5 years, mean total high-order aberrations, spherical aberrations, coma, and trefoil were not different between treatments (p>0.12) at any pupil diameter, except coma was slightly higher at one month after the femtosecond laser treatment at a 5 mm pupil (p=0.035; Bonferroni adjusted for 3 comparisons, Table 2). At 5 years these aberrations were not different from high-order aberrations at 1 month at any pupil diameter (p>0.08), except in the mechanical microkeratome group spherical aberration increased slightly across a 5 mm pupil (p=0.044) and 6 mm pupil (p=0.008, Table 2). These changes were not considered to be clinically important.
Table 2.
High-order aberrations before and after LASIK with flaps cut by using the mechanical microkeratome or femtosecond laser.
| All High | Sphere | Coma | Trefoil | |||||
|---|---|---|---|---|---|---|---|---|
| Mechanical | Femtosecond | Mechanical | Femtosecond | Mechanical | Femtosecond | Mechanical | Femtosecond | |
| 4 mm Pupil | ||||||||
| Pre-LASIK | 0.13 ± 0.04 |
0.14 ± 0.07 |
0.04 ± 0.02 |
0.04 ± 0.02 |
0.06 ± 0.03 |
0.07 ± 0.06 |
0.06 ± 0.03 |
0.06 ± 0.05 |
| 1 month | 0.17c ± 0.04 |
0.19a ± 0.06 |
0.06 ± 0.03 |
0.07a ± 0.04 |
0.08a ± 0.04 |
0.11 ± 0.06 |
0.08 ± 0.04 |
0.07 ± 0.04 |
| 5 years | 0.19c ± 0.05 |
0.18 ± 0.05 |
0.06a ± 0.04 |
0.08c ± 0.03 |
0.10c ± 0.04 |
0.11 ± 0.06 |
0.09a ± 0.05 |
0.07 ± 0.04 |
| 5 mm Pupil | ||||||||
| Pre-LASIK | 0.26 ± 0.09 |
0.26 ± 0.09 |
0.12 ± 0.05 |
0.12 ± 0.04 |
0.14 ± 0.10 |
0.14 ± 0.11 |
0.11 ± 0.06 |
0.10 ± 0.06 |
| 1 month | 0.33b ± 0.09 |
0.38c ± 0.11 |
0.16b ± 0.06 |
0.19c ± 0.06 |
0.18fa ± 0.11 |
0.23fa ± 0.12 |
0.13 ± 0.06 |
0.12 ± 0.10 |
| 5 years | 0.37b ± 0.13 |
0.37b ± 0.09 |
0.19db ± 0.08 |
0.20b ± 0.07 |
0.20a ± 0.11 |
0.23a ± 0.12 |
0.16 ± 0.11 |
0.12 ± 0.08 |
| 6 mm Pupil | ||||||||
| Pre-LASIK | 0.47 ± 0.22 |
0.46 ± 0.18 |
0.26 ± 0.09 |
0.26 ± 0.09 |
0.27 ± 0.23 |
0.26 ± 0.20 |
0.18 ± 0.10 |
0.16 ± 0.09 |
| 1 month | 0.66c ± 0.21 |
0.76c ± 0.25 |
0.40c ± 0.15 |
0.43c ± 0.16 |
0.37 ± 0.26 |
0.46b ± 0.26 |
0.20 ± 0.10 |
0.22 ± 0.20 |
| 5 years | 0.70c ± 0.19 |
0.75c ± 0.22 |
0.44ec ± 0.15 |
0.46c ± 0.13 |
0.35 ± 0.22 |
0.44a ± 0.26 |
0.23 ± 0.16 |
0.21 ± 0.17 |
LASIK - laser in situ keratomileusis
Aberrations expressed as root-mean-squared wavefront error, µm, mean ± standard deviation.
Different from pre-LASIK: ap≤0.045, bp≤0.01, cp≤0.001 (Bonferroni-adjusted for 2 comparisons)
Different from 1 month: dp=0.044, ep=0.008
Different between treatments: fp=0.035 (Bonferroni-adjusted for 3 comparisons)
Backscatter after LASIK
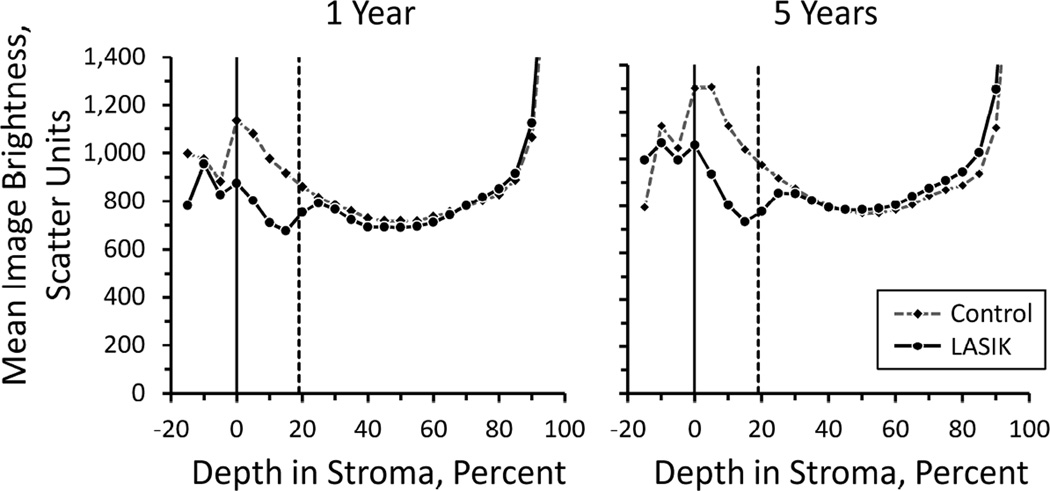
After LASIK, backscatter decreased in the flap as compared to backscatter in control corneas regardless of the method of flap creation (Figure 3). Mean image brightness from the 5% stromal layers (layers with a thickness of 5% of stromal thickness) centered on 0, 5, 10, and 15 percent of stromal thickness were approximately 26% lower in the LASIK group than in the control group (p<0.001, Bonferroni-adjusted for comparisons of 20 regions; at 5 years, adjusted p=0.023 at 0% and p<0.001 at 20% stromal thickness). Backscatter in regions deep to the surgical interface (25 – 85% of stromal depth) were not different from those in control corneas at similar stromal depths (p>0.07, unadjusted for multiple comparisons). Backscatter was not different between treatments (p>0.07, unadjusted for multiple comparisons) at any depth in the stroma and at any time after LASIK. The typical minimum detectable difference between treatments was 115 scatter units (α=0.05, β=0.20, n=17).
Figure 3.
Mean backscatter (haze) in control subjects and after LASIK at 1 year (left) and 5 years (right). Backscatter was significantly lower in the flap than in the equivalent region of un-operated control corneas at 1 year, and this reduction was consistent through 5 years (p<0.001, Bonferroni-adjusted for 20 layers).
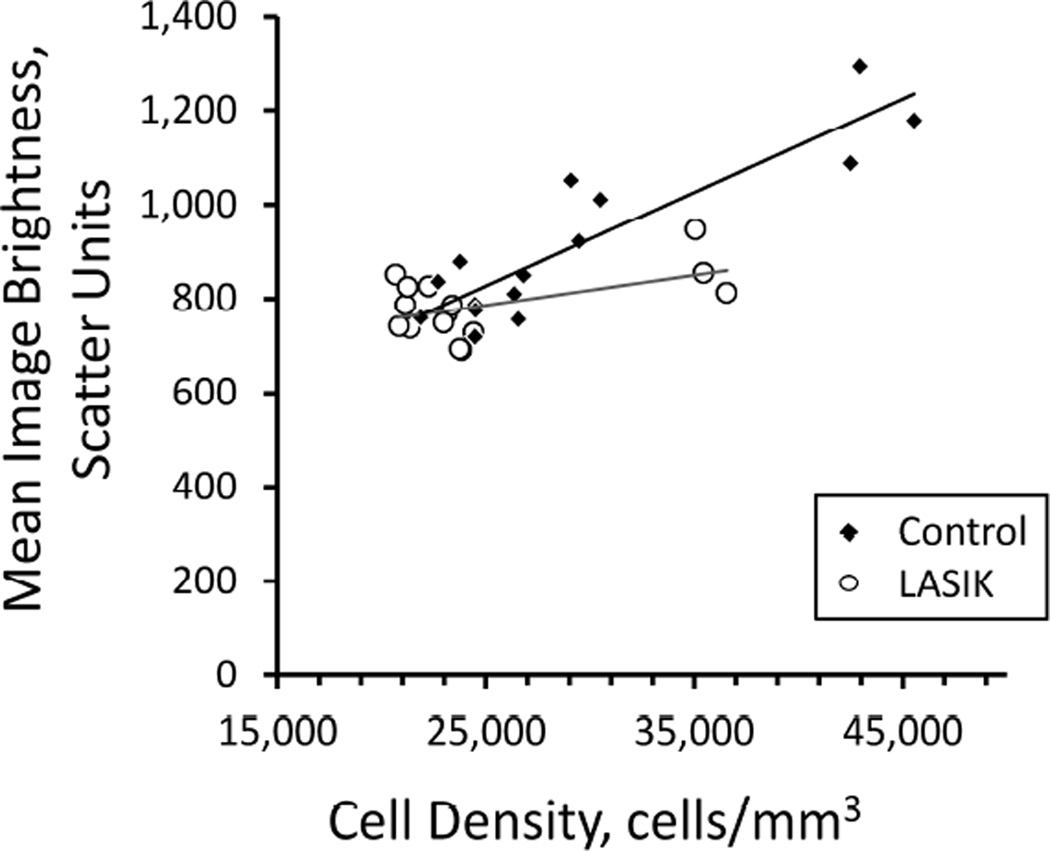
The relationship between mean image brightness and cell density was somewhat different between corneas after LASIK and normal corneas. In both groups the mean image brightness increased as mean cell density increased in the anterior stroma (Figure 4), although in LASIK patients with the reduced anterior cell densities, mean image brightness did not increase by as much as expected from their cell density as compared to control corneas.
Figure 4.
Relationship between mean backscatter (confocal image brightness) and cell density in control subjects and LASIK patients. Each point represents the mean image brightness and cell density in one layer of stroma from all subjects at one visit. After LASIK, backscatter was lower in the flap (three right-most open circles) than would be expected from the decrease in cell density based on the relationship between image brightness and cell density in the control group. The frames closest to the endothelium were excluded because the image brightness was dominated by reflectance from the endothelial surface. Data were from visits at 1, 3, and 5 years.
Straylight after LASIK
In LASIK patients, straylight at 1, 3, and 5 years was not different between treatments (p>0.9; Bonferroni-adjusted for 3 comparisons, Table 3). Straylight at 3 and 5 years was not different from straylight at 1 year (p>0.11, Bonferroni-adjusted for 2 comparisons), except in patients with flaps cut by the femtosecond laser, straylight was slightly higher at 5 years (p=.044, Bonferroni adjusted for 2 comparisons); this difference of 0.07 straylight units was not considered to be clinically important. At all visits, straylight was not different in each group of the LASIK eyes from straylight in the corresponding group of control eyes (p>0.10, Bonferroni-adjusted for 3 comparisons).
Table 3.
Straylight in LASIK patients with flap creation by the mechanical microkeratome and femtosecond laser.
| LASIK Patients | Control Subjects | |||
|---|---|---|---|---|
| Treatment: | Mechanical | Femtosecond | Randomized to Mechanical |
Randomized to Femtosecond |
| Visit | ||||
| 1 year | 1.10 ± 0.12 | 1.07 ± 0.09 | 1.04 ± 0.17 | 1.01 ± 0.09 |
| (21) | (21) | (19) | (19) | |
| 3 years | 1.17 ± 0.36 | 1.15 ± 0.18 | 1.11 ± 0.08c | 1.06 ± 0.07bc |
| (21) | (21) | (18) | (18) | |
| 5 years | 1.16 ± 0.12 | 1.14 ± 0.14a | 1.13 ± 0.17 | 1.15 ± 0.26 |
| (21) | (21) | (13) | (13) | |
LASIK - laser in situ keratomileusis
Mean ± standard deviation (number of observations).
a p=0.044, b p=0.027, compared to measurement at 1 year (Bonferroni-adjusted for 2 measurements)
cp=0.012, Mechanical vs FS (Bonferroni-adjusted for 3 measurements)
In the control group, straylight was not different between randomizations at 1 and 5 years (p>0.9) although there was a small difference between randomizations at 3 years (p=0.012, Bonferroni-adjusted for 3 comparisons). Straylight in both randomizations was not different at 5 years from straylight at one year (p>0.07) although it was somewhat elevated in the eyes randomized for comparison to the femtosecond laser group at 3 years compared to 1 year (p=0.03, Bonferroni-adjusted for 2 comparisons, Table 3).
Discussion
In study, we confirmed our previous observation in a separate cohort that keratocyte density in the stromal flap decreases after LASIK.1 While other investigators found similar changes in keratocyte density after LASIK, by using confocal microscopy4 and by histologic methods,19 the present study further explored possible differences in the corneal response between eyes treated with flap creation with a mechanical microkeratome or a femtosecond laser and whether the cell loss induced by either of these methods was associated with degradation of visual performance, high-order aberrations, or scattered light through five years. The randomized paired-eye design of this study provided the most sensitive method to compare cellular response differences between treatments in the fellow eyes. This prospective design enabled each subject to serve as his or her own control to reduce the sensitivity of the study to variations in the population. Even with this sensitive study design we were not able to detect any relationships between visual performance and keratocyte density changes after LASIK, nor could we detect differences in visual performance or cell status between methods of flap creation. The contemporaneous group of normal untreated subjects served as a control for possible changes in the instrumentation over the 5-year observation period that might have affected our ability to determine cell density, visual function, or optical properties of the cornea, changes that could suggest a drift in sensitivity of the measurements.
If cells are lost primarily by necrosis after cutting the stroma with a femtosecond laser and by apoptosis after cutting the flap with a microkeratome, then it is reasonable to hypothesize that there might be differences in the short or long-term responses between these methods.9 However, our data show that this was not the case despite the use of a 15 kHz laser with a 2.3 µJ pulse, a relatively high-energy pulse. Since our patients were enrolled in this study, femtosecond lasers have continued to evolve, and most current lasers use higher pulse frequencies and lower energy.9, 20 If anything, these features should improve the effect of the laser on optical properties of the eye by decreasing the spacing between pulses9, 20, 21; the advantages of higher frequency and lower power femtosecond lasers have been reviewed.22 Newer femtosecond lasers would likely have a lesser effect on keratocyte density or visual responses as compared to the femtosecond laser used here.
LASIK induces immediate changes in the visual and optical properties of the eye, the most pronounced change being a reduction of refractive error and improvement of uncorrected visual acuity, the goal of the procedure. Similarly, anterior corneal wavefront aberrations are known to increase immediately after LASIK, and from our measurements, this cannot be attributed to keratocyte loss. Because a chronic decrease in anterior keratocyte density might reduce the keratocytes’ maintenance of the stroma and increased anterior surface irregularity, we compared anterior corneal aberrations at 5 years to those at 1 year after LASIK and found no clinically significant changes. Keratocytes still may play a role in shaping the stroma and inducing high-order aberrations after a change in their activity across the stroma, but the relationship may not be manifest until substantially more keratocytes are lost than the 25% decrease typical after LASIK, or with a follow-up longer than 5 years.
The reason for reduced backscatter from the flap after LASIK is not known. This reduction was pronounced, consistent through 5 years, and was independent of the method used to create the flap. It did not correspond to a detectable reduction in forward scatter (straylight), which manifests as disability glare. One explanation for reduced backscatter is the decreased number of cells available to scatter and reflect light; Figure 4 demonstrates a clear relationship between cell density and backscatter in the control corneas. In the LASIK flap, cell density was lower than the maximum density in analogous regions of the control corneas but still higher than density in the mid stroma. Backscatter, however, was not as high as expected from the linear relationship between these extremes in the control corneas (Figure 4), suggesting that the properties of the tissue in this region had also somehow changed to reduce its scattering and reflectivity rather than simply decreasing the number of cells that scatter light. These differences could be related to mechanical changes in the lamellae after cutting and repositioning the flap in addition to the reduction of cell density, or to changes in the refractive index difference between the keratocytes and background substance that would change reflectance at their surfaces. Confocal microscopy does not provide the resolution to determine the exact source or nature of the reduced backscatter.
In summary, this study confirms our earlier work that demonstrated a decrease in keratocyte density in the anterior stroma through 5 years after LASIK and shows that cell density decreases regardless of the method used to create the flap. The method of creating the flap also does not affect high-order aberrations from the cornea, corneal haze, or visual function. Although the reasons for keratocyte loss after LASIK are poorly understood, it is reassuring that there are no subtle changes in optical and visual function through 5 years as a result of the sustained cell loss.
Acknowledgments
Funding and Support:
Research to Prevent Blindness, Inc., New York, NY (an unrestricted departmental grant, and SVP as Olga Keith Wiess Special Scholar)
Mayo Foundation, Rochester, MN.
NIH Grant EY 02037 (WMB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
All Authors: none
References
- 1.Erie JC, Nau CB, McLaren JW, Hodge DO, Bourne WM. Long-term keratocyte deficits in the corneal stroma after LASIK. Ophthalmology. 2004;111(7):1356–1361. doi: 10.1016/j.ophtha.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Pisella PJ, Auzerie O, Bokobza Y, Debbasch C, Baudouin C. Evaluation of corneal stromal changes in vivo after laser in situ keratomileusis with confocal microscopy. Ophthalmology. 2001;108(10):1744–1750. doi: 10.1016/s0161-6420(01)00771-0. [DOI] [PubMed] [Google Scholar]
- 3.Mitooka K, Ramirez M, Maguire LJ, et al. Keratocyte density of central human cornea after laser in situ keratomileusis. Am J Ophthalmol. 2002;133(3):307–314. doi: 10.1016/s0002-9394(01)01421-0. [DOI] [PubMed] [Google Scholar]
- 4.Canadas P, de Benito-Llopis L, Hernandez-Verdejo JL, Teus MA. Comparison of keratocyte density after femtosecond laser vs mechanical microkeratome from 3 months up to 5 years after LASIK. Graefes Arch Clin Exp Ophthalmol. 2013;251(9):2171–2179. doi: 10.1007/s00417-013-2357-9. [DOI] [PubMed] [Google Scholar]
- 5.Erie JC, McLaren JW, Hodge DO, Bourne WM. Long-term corneal keratoctye deficits after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2005;103:56–66. discussion 67-68. [PMC free article] [PubMed] [Google Scholar]
- 6.Hatou S, Shimmura S, Shimazaki J, et al. Mathematical projection model of visual loss due to fuchs corneal dystrophy. Invest Ophthalmol Vis Sci. 2011;52(11):7888–7893. doi: 10.1167/iovs.11-8040. [DOI] [PubMed] [Google Scholar]
- 7.Patel SV, McLaren JW, Hodge DO, Bourne WM. Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 2001;42(2):333–339. [PubMed] [Google Scholar]
- 8.Muller LJ, Pels E, Vrensen GF. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85(4):437–443. doi: 10.1136/bjo.85.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netto MV, Mohan RR, Medeiros FW, et al. Femtosecond laser and microkeratome corneal flaps: comparison of stromal wound healing and inflammation. J Refract Surg. 2007;23(7):667–676. doi: 10.3928/1081-597x-20070901-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel SV, McLaren JW, Kittleson KM, Bourne WM. Subbasal nerve density and corneal sensitivity after LASIK: Femtosecond laser versus mechanical microkeratome. Arch Ophthalmol. 2010;128(11):1413–1419. doi: 10.1001/archophthalmol.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvo R, McLaren JW, Hodge DO, Bourne WM, Patel SV. Corneal aberrations and visual acuity after laser in situ keratomileusis: femtosecond laser versus mechanical microkeratome. Am J Ophthalmol. 2010;149(5):785–793. doi: 10.1016/j.ajo.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klingler KN, McLaren JW, Bourne WM, Patel SV. Corneal endothelial cell changes 5 years after laser in situ keratomileusis: femtosecond laser versus mechanical microkeratome. J Cataract Refract Surg. 2012;38(12):2125–2130. doi: 10.1016/j.jcrs.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SV, Maguire LJ, McLaren JW, Hodge DO, Bourne WM. Femtosecond laser versus mechanical microkeratome: A randomized controlled study. Ophthalmology. 2007;114(8):1482–1490. doi: 10.1016/j.ophtha.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 14.McLaren JW, Patel SV, Nau CB, Bourne WM. Automated assessment of keratocyte density in clinical confocal microscopy of the corneal stroma. J Microsc. 2008;229(Pt 1):21–31. doi: 10.1111/j.1365-2818.2007.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erie JC, Patel SV, McLaren JW, Hodge DO, Bourne WM. Corneal keratocyte deficits after photorefractive keratectomy and laser in situ keratomileusis.[see comment] Am J Ophthalmol. 2006;141(5):799–809. doi: 10.1016/j.ajo.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 17.McLaren JW, Bourne WM, Patel SV. Standardization of Corneal Haze Measurement in Confocal Microscopy. Invest Ophthalmol Vis Sci. 2010;51(11):5610–5616. doi: 10.1167/iovs.10-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franssen L, Coppens JE, van den Berg TJTP. Compensation comparison method for assessment of retinal straylight. Invest Ophthalmol Vis Sci. 2006;47(2):768–776. doi: 10.1167/iovs.05-0690. [DOI] [PubMed] [Google Scholar]
- 19.Dawson DG, Kramer TR, Grossniklaus HE, Waring GO, 3rd, Edelhauser HF. Histologic, ultrastructural, and immunofluorescent evaluation of human laser-assisted in situ keratomileusis corneal wounds. Arch Ophthalmol. 2005;123(6):741–756. doi: 10.1001/archopht.123.6.741. [DOI] [PubMed] [Google Scholar]
- 20.Sarayba MA, Ignacio TS, Binder PS, Tran DB. Comparative study of stromal bed quality by using mechanical, IntraLase femtosecond laser 15- and 30-kHz microkeratomes. Cornea. 2007;26(4):446–451. doi: 10.1097/ICO.0b013e318033e7cc. [DOI] [PubMed] [Google Scholar]
- 21.de Medeiros FW, Kaur H, Agrawal V, et al. Effect of femtosecond laser energy level on corneal stromal cell death and inflammation. J Refract Surg. 2009;25(10):869–874. doi: 10.3928/1081597X-20090917-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santhiago MR, Wilson SE. Cellular effects after laser in situ keratomileusis flap formation with femtosecond lasers: a review. Cornea. 2012;31(2):198–205. doi: 10.1097/ICO.0b013e3182068c42. [DOI] [PubMed] [Google Scholar]