Abstract
Vaccine-induced mucosal antibodies are often evaluated using small volumes of secretory fluids. However, fecal matter containing mucosal IgA is abundant. We purified fecal IgA from five SIV-vaccinated and five SIV-infected rhesus macaques by sequential affinity chromatography. The purified IgA was dimeric by native PAGE, contained secretory component, and was analogous to IgA in colostrum and vaginal fluid by western blot. IgA from one infected and four vaccinated animals neutralized H9-derived SIVmac251 with IC50s as low as 1µg/mL. Purified IgAs inhibited transcytosis and exhibited phagocytic activity, the latter significantly correlated with SIVmac251 Env-specific IgA in the purified samples. Among different affinity resins, peptide M was optimal compared to jacalin, anti-monkey IgA and SSL7 for IgA purification, as confirmed using tandem peptide M/anti-monkey IgA columns. Fecal IgA provided material sufficient for several assays relevant to protective efficacy, and was shown to be multifunctional. Our approach is potentially applicable to human clinical studies.
Keywords: Non-human primate mucosal IgA, Feces, Peptide M, Neutralization, Phagocytosis, Transcytosis inhibition
1. INTRODUCTION
Mucosal immunity is an important facet of many vaccine strategies aimed at preventing infection and/or disease progression by infectious agents. Our group has been pursuing development of an HIV vaccine that elicits strong mucosal responses. This is an important goal, as the majority of HIV transmission events occur mucosally, and it is in tissue near mucosal sites that infection is established [1, 2]. One difficulty in designing a mucosal vaccine lies in adequately evaluating mucosal immunity elicited by various vaccine candidates. As assessment of mucosal cellular immunity is limited by the need for tissue biopsies, routine monitoring of mucosal humoral responses in mucosal secretions is often conducted. In this regard, vaccine induction of HIV/SIV-specific IgA, the antibody isotype predominant at gastrointestinal surfaces, has been demonstrated. The majority of such studies, conducted in mice using vaccine approaches including recombinant DNA or viral vectors and HIV protein immunizations with mucosal adjuvants, have simply reported the induction of HIV-specific mucosal IgA [3-6]. Other murine studies have shown elicitation of functional mucosal IgA in secretions able to neutralize HIV or inhibit transcytosis [7-9]. Pastori et al. [10] used Peyers patch supernatants to demonstrate induction of anti-CCR5 IgA with HIV blocking activity. Similarly, studies conducted in non-human primates (NHPs) showed HIV− or SIV-specific mucosal IgA induced by vaccination with replication-competent adenovirus, modified vaccinia Ankara, or DNA recombinant vectors with or without subsequent DNA or protein boosts [11-15]. A phase I clinical trial in humans of a novel gp41-P1 peptide approach [16] was also shown to elicit mucosal IgA. Overall, however, few pre-clinical vaccine studies in macaques have correlated mucosal IgA with protective efficacy. Xiao et al. [17] demonstrated an association of vaccine- induced anti-SIV Env IgA able to inhibit transcytosis with reduced viremia. Bomsel et al. [18] showed protection against SHIV infection with mucosal IgA that possessed transcytosis inhibiting activity. Xiao et al. [19] correlated delayed SIVmac251 acquisition with rectal IgA. In natural infection, mucosal IgA with HIV neutralizing activity has been implicated in maintaining the negative serologic status of highly exposed female sex workers (20]. In view of the multiple activities of mucosal IgA, including its role in immune exclusion by providing a protective physical barrier in mucus against pathogens [21], an in depth investigation of vaccine elicited mucosal IgA responses is needed to clearly identify functions correlated with protective efficacy.
As rhesus macaques are the current model of choice for pre-clinical vaccine development, it is important to take into account what is currently known about macaque IgA. Immunoglobulins bearing an alpha heavy chain are the major antibody isotype found in external mucosal secretions, as well as being present in serum [22]. In humans, there are two subclasses of IgA, IgA1 and IgA2, encoded by two distinct Ig heavy α constant genes. IgA1 has an elongated proline-rich hinge region [23]. Old world monkeys have only one Ig heavy α constant gene [24], which exhibits significant sequence variability, with frequent mutations in the hinge region [25, 26]. This variability may affect isolation strategies utilizing specific binding epitopes, thereby decreasing the effectiveness of such approaches. Furthermore, the same variability can affect antigen binding, as evidenced by the studies of Tudor et al. where functionality and binding of an HIV-specific mAb were altered by using different heavy chains, and human IgA2 and IgG1 were demonstrated to exhibit different functions despite having the same variable regions [27].
Numerous methods have been used to purify immunoglobulins, including phage display technology, ammonium sulfate precipitation, size exclusion chromatography, anion exchange chromatography, and high-performance liquid chromatography [28-30]. Affinity purification is also used to purify Igs, and commercial methods of IgG purification have been well characterized and utilized, including Protein G, Protein A, and Protein L [31, 32]. Several commercially available methods for affinity purification of IgA have been developed. These include peptide M, SSL7, and jacalin. Peptide M, or Sap, is a 50-residue synthetic peptide derived from streptococcal M protein that has been shown to bind human monomeric and dimeric IgA1 and IgA2 with high affinity [33]. SSL7, Staphylococcus aureus superantigen-like protein 7, similarly binds the Fc of monomeric human IgA1 and IgA2, as well as IgA from cow and sheep’s milk, although it does not bind bovine, rabbit, mouse or goat serum IgA [34, 35]. Jacalin is an alpha D-galactose binding lectin that binds human IgA and IgD [36, 37]. It specifically binds O-linked glycans found in the hinge region of IgA1[38], although it also binds IgA2 in some assays [37]. Jacalin, Peptide M, and SSL7 have not yet been shown to bind monkey IgA.
Macaque mucosal IgA is often studied using small volumes of secretions collected by swabs or lavages containing limited amounts of IgA. This in turn limits the number and type of functional assays that can be performed. A further complication is the potential for blood contamination, particularly in rectal secretions, which can confuse assay results by introducing non-mucosal systemic antibodies into samples. Additionally, macaques must be anesthetized in order to collect secretions. While alternative methods may not be available for collection of vaginal and nasal secretions, the rectal and gastrointestinal mucosae may be better sampled using IgA from fecal matter.
In healthy human subjects, there are around 65mg of total IgA per 100g of feces [39]. This represents a large amount of available mucosal IgA that could potentially be utilized for characterizing IgA specificity and functional activity. Feces have been successfully used as a source for IgA isolation from humans, dogs, and mice [40]. It has been demonstrated in mice that IgA from feces is representative of its mucosal immunoglobulin [41]. With respect to the non-human primate model, feces also represent an easily collectible sample, not requiring anesthesia, and available as often as the animal defecates, without interfering with the rectal mucosa, unlike a swab. Feces certainly contain functional IgA, but this IgA must be purified if it is to be used to evaluate and characterize mucosal immune responses. Here we show that the commercially available resins: jacalin, peptide M, and SSL7, can be used for purification of macaque mucosal IgA. Additionally, we demonstrate that feces represent an inexpensive and readily obtainable source of mucosal IgA exhibiting multiple functions, and providing ample amounts of mucosal IgA for extensive characterization.
2. MATERIALS AND METHODS
2.1 Cell lines
HeLa TZM-BL cells were maintained in DMEM with 5% FBS. THP-1 cells, a monocytic cell line, were maintained in RPMI 1640 with 10% FBS and 0.05mM β-mercaptoethanol. HT-29 and H9 cells were maintained in RPMI 1640 with 10% FBS.
2.2 Animals
Indian rhesus macaques were maintained at Advanced BioScience Laboratories, Inc. (ABL) and the NCI animal facility, according to the standards of the Association for Assessment and Accreditation of Laboratory Animal Care International and the Guide for the Care and Use of Laboratory Animals of the NIH. Animal protocols were approved by the ABL Animal Care and Use Committee (ACUC) and the NCI ACUC prior to implementation. Animals P275 and ZC10 were naïve, untreated animals. Vaccinated animals P878, P879, P888, P889, and P897 received ALVAC SIVgag/pro/env vaccinations at 0 and 1 month, and twice more at 3 and 6 months together with Env protein formulated in alum. Animals P888 & P897 received SIVm766 gD-gp120 & SIVCG7V gD-gp120 while P878, P879 and P889 received two full length single chain (FLSC) proteins [42] composed of SIVm766 gp120 or SIVCG7V gp120 attached to rhesus CD4 by a flexible amino acid linker. The vaccinated macaques were challenged intrarectally 4 weeks after their last vaccination with repeated weekly doses (125 TCID50) of SIVmac251, a stock prepared by Ronald Desrosiers and obtained from Nancy Miller, DAIDS, NIAID. Vaccinated animals received 10 weekly challenges, and remained uninfected (Franchini et al., unpublished data). Four months after their last challenge, they received an ALVAC-SIV/gp120 boost and thereafter were boosted monthly with ALVAC-SIV/gp120. SIVmac251 infected animals, P436, P437, P644, P735, and P876, were enrolled in a pathogenesis study. All had detectable viral loads 5 months prior to sampling, ranging from 5.4×104 to 5.1×106 SIV RNA copies/mL plasma. Animals P436, P437, and P735 had viral loads of 1.5×106, 6.5×104, and 4.2×105 respectively around the time of sampling. SIVmac251-infected animals 897, DELP, and DFDM were chronically infected at the time of sample collection. Feces were collected from the animal cages within several hours of expulsion, and stored at -20°C until thawed for homogenization.
2.3 Purification of IgA
Feces were homogenized in RPMI 1640 supplemented with antimycotic and antibiotic (Anti-Anti, Invitrogen) and incubated at 4°C for 4-8 hours. Homogenate was clarified by centrifugation at 2500rpm for 20 minutes, followed by 13000 rpm for 20 minutes. The clarified homogenate was filtered through successively smaller filters (0.8μm down to 0.2μm), and run through a protein G-agarose column (ThermoScientific), then a jacalin-agarose column (ThermoScientific), and lastly a custom-conjugated anti-monkey IgA column (α-Mon IgA, KPL) in series (5mL of agarose/column). Jacalin, α-Mon IgA, peptide M and SSL7 (Invivogen) agarose columns were also tested separately for their ability to purify mucosal IgA, as was a peptide M/α-Mon IgA tandem combination. Columns were washed with 3-5 column-volumes of DPBS. The jacalin column was eluted with 2 column-volumes of 0.1M D-galactose; Protein G, α-Mon IgA, peptide M, and SSL7 columns were eluted with 2 column-volumes of 0.1M glycine pH 2.5 and immediately neutralized with pH 8.0 Tris-HCl. Column eluates were dialyzed overnight in DPBS at 4°C (12000 MWCO Puralyzer, Sigma). The Jacalin and α-Mon IgA column eluates were combined and concentrated (20000 MWCO Pierce concentrators, Thermo- Pierce) as were eluates from the peptide M/α-Mon IgA tandem procedure.
2.4 Western Blotting
For SDS-PAGE, samples were added to laemmli sample buffer (Bio-rad) with β-mercaptoethanol, and heated at 90°C for 10 minutes. Samples and molecular weight markers (Novex Sharp Protein Standard for denaturing gels, Invitrogen) were run on 4-20% Tris-Glycine gels (Invitrogen), and transferred using the iBlot system (Invitrogen) onto a nitrocellulose membrane. The membrane was blocked with 3% BSA in Tris-buffered saline containing 0.05% Tween-20 (TBS-T), and incubated overnight at 4°C with 1:1000 α-Mon IgA-peroxidase, 1:1500 α-Mon IgG-peroxidase, or 1:500 α-Hu SC-peroxidase (all from Nordic Immunology) diluted in blocking buffer. Chemiluminescent substrate (Supersignal West Pico, Thermo Scientific) was used to visualize bands.
For blue native PAGE, samples were added to native sample buffer (Invitrogen), and the recommended amount of 5% G-250 (Invitrogen). Samples and molecular weight markers (Native MARK unstained protein standard for Native gels, Invitrogen) were run at 4°C on 4-16% Bis-Tris gels (Invitrogen) at 150V for 60 minutes, followed by 250V for 90 minutes. Gels were transferred onto PVDF membranes (Invitrogen) using the iBlot system (Invitrogen), incubated with 8% acetic acid for 5 minutes, then washed with ultrapure water. Membranes were dried, washed three times with methanol, then blocked with 3% BSA in TBS-T, before incubating overnight at 4°C with 1:1000 α-Mon IgA-peroxidase in blocking buffer. They were visualized in the manner described above.
2.5 ELISAs
96-well ½ area plates (Greiner) were incubated overnight at 4°C with α-Mon IgA or IgG (100ng/well, KPL) or SIVmac251 gp120 (200ng/well, ABL) in carbonate-bicarbonate buffer (Sigma), then blocked for two hours at RT with 1% BSA blocking solution (KPL). Plates were loaded with samples, total Ig standards, or dilutions of Env-specific IgA and IgG standards derived from IgG-depleted pooled serum or purified serum IgG, respectively, obtained from SIVmac251- infected macaques and quantified as previously described [43]. Following incubation at 37°C for one hour, the plates were washed 5 times with wash buffer, and α-Mon IgA- peroxidase or α-Mon IgG-peroxidase (Nordic Immunology) diluted in blocking buffer was added and incubated for 1 hour at RT. After washing 5 times, peroxidase substrate was added (KPL), and after 15 minutes Phosphoric acid (1M) was added before reading absorbance at 450 nm. Results are expressed in the concentrations listed based upon the standard curves generated. The ELISA for secretory component followed the same procedure as above, using 96-well ½ area plates coated with unconjugated α-Mon IgA. Following incubation with purified fecal IgA samples, secretory component was detected using α-monkey SC-peroxidase (Nordic Immunology).
2.6 Neutralization
HeLa TZM-bl cells (0.1 ml) were seeded into 96-well trays (8 x104 cells/ml) one day prior to infection. 200 focus forming units (FFU) of SIVmac251 were mixed with twofold serial dilutions of isolated IgA. After 1 h of incubation at 37°C, these mixtures were added to target cells and incubated for 8 hrs at 37°C. Then, the virus-antibody mixture was removed, DMEM with 5% FBS was added, and infected cells were incubated at 37°C for 48 h. Medium was removed, and 100 μl of medium without phenol red was added. Cells were then fixed and solubilized by adding 100 μl of BriteLite (Company). Luminescence was read with a Vector luminometer.
2.7 Antibody-dependent cellular phagocytosis (ADCP)
SIVmac251 gp120 was biotinylated (Biotin-XX microscale protein labeling kit, Invitrogen), and 3-5 µg were incubated for 25 minutes at room temperature in the dark with streptavidin- fluorescent beads (FluoroSpheres® NeutrAvidin® Labeled Microspheres, 1.0 µm, Yellow-Green Fluorescent (505/515), 1% solids) that were diluted 100-fold. THP-1 cells were plated in a 96- well U-bottom plate (250,000-300,000 per well), and samples were added. The bead-gp120 mix was further diluted in media 5-fold, and 50µL was added to cells, incubated for 3 hours at 37°C, washed at low speed, and fixed in 2% PFA. Cells were assayed for fluorescent bead uptake by flow cytometry using a BD Biosciences LSR II. Results are reported as the phagocytic score as previously described [44], calculated as (% phagocytosis x MFI)/106.
2.8 Transcytosis Inhibition
SIV transcytosis across epithelial cells was performed as previously described (17) with some modifications. Briefly, the intestinal epithelial HT-29 cell line was grown as a tight, polarized monolayer in 24-Transwell plates (6.5-mm diameter, 0.4-µm pore size; Costar, Corning) for 20 days in RPMI 1640 containing 10% fetal calf serum. The tightness of the monolayer was monitored by transepithelial resistance (>250 Ω/cm2) with a Millicell ohmmeter (Millipore) and confirmed by a fluorescein (Sigma) leakage test. SIVmac251-infected H9 cells (1×106 cells/well) were washed three times and added to the apical chamber with 5ug purified IgA. Transcytosis was assessed after 4 h by measuring p27 antigen in the basal chamber by antigen capture ELISA (ABL). Inhibition of SIVmac251 transcytosis was calculated as follows: (1- [p27 in the basal chamber of the test sample]/[average p27 in the basal chamber of the naïve samples]) x 100.
3. Results
3.1 Quantity of mucosal IgA purified from feces of vaccinated and infected macaques
It was not known if affinity chromatography designed for human IgA purification could be used to isolate IgA from rhesus macaque mucosal samples, including fecal matter. To address this, we utilized conjugated agarose columns in series to purify IgA from various fecal samples. A protein G column, which does not bind IgA, was first used to deplete the mucosal sample of IgG. The sample was then run through a jacalin column to bind IgA, followed by a custom conjugated α-Mon IgA column in series to catch IgA not bound by jacalin. After washing these columns, the IgA was eluted. The eluates were combined, dialyzed, and concentrated for further characterization. This method was applied to fecal samples from five SIV-hyper-vaccinated rhesus macaques, and five SIV-infected macaques, as well as naïve controls.
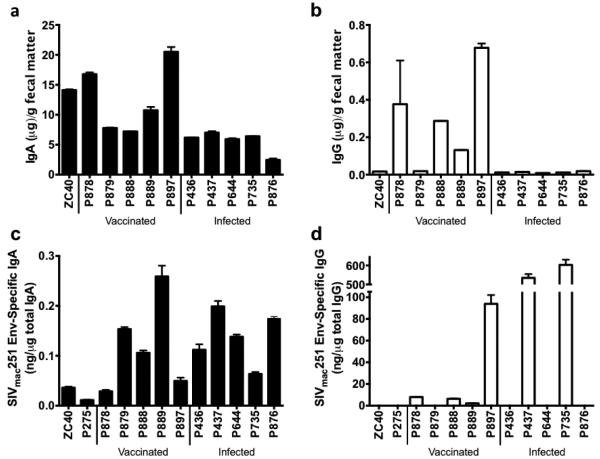
Purified IgA and IgG samples were quantified by ELISA (Fig. 1). Roughly 10 μg of total IgA per gram feces could be obtained, with higher amounts obtained from the naïve and vaccinated macaques and lower amounts from the infected macaques (Fig. 1a). Vaccinated animals had a mean of 12.6±2.6 μg total IgA per g of feces, and the infected animals had a mean of 5.6±0.8. The highest yield was 700 μg total IgA from a 56g fecal sample. For comparison, we obtained a mean of 2.8±0.4 μg total IgA per swab in a separate study using rectal swabs obtained from 22 naïve rhesus macaques [45]. We also quantified fecal IgG purified on the Protein G column. As with the mucosal IgA, feces from vaccinated animals yielded significantly more total IgG than feces from infected animals. Minimal amounts of total IgG were obtained, roughly 0.17 μg per gram of feces (Fig. 1b). Vaccinated animals had a mean of 0.33±0.12 μg total IgG per g of feces, and the infected animals had a mean of 0.012±0.0017. A mean of 1.97±0.6 μg total IgG per swab was obtained using rectal swabs from 22 naïve macaques [45].
Fig. 1. Quantity of total and SIVmac251 Env-specific IgA purified from fecal matter.
IgA was purified from the feces of 5 vaccinated and 5 infected macaques, along with 2 naïve animals. (a) Total IgA purified from fecal matter quantified by ELISA. (b) Total IgG purified from fecal matter quantified by ELISA. (c) SIVmac251 Env-specific IgA purified from fecal matter quantified by ELISA. (d) SIVmac251 Env-specific IgG purified from fecal matter quantified by ELISA. P275 is not included in total Ig measurements as fecal matter was not weighed prior to Ig purification.
IgA specific for SIVmac251 Env was detected in all samples, although only a background level, defined by samples from the naïve macaques, was present in vaccinated macaque P878 (Fig. 1c). Vaccinated animals had a mean of 0.12±0.04 ng specific IgA per μg total IgA, and the infected animals had a mean of 0.14±0.05. Aside from vaccinated macaque, P889, with a very high level of SIV Env-specific IgA, levels of specific IgA/μg total IgA in the vaccinated and infected animals were very similar. SIVmac251 Env-specfic IgG was also detected, but in cases where significant amounts of total IgG were purified, surprisingly, most of the total IgG was specific for SIVmac251 Env, although significant variability was observed (Fig. 1d). Vaccinated animals had a mean of 28±44 ng specific IgG/μg total IgG for 4 of 5 positive macaques, and the infected animals had a mean of 568±48ng specific IgG/μg total IgG for 2 of 5 positive macaques. A preponderance of HIV-specific IgG antibodies compared to IgA has been reported in supernatants of duodenal biopsies of HIV infected individuals [46], although the mechanism responsible is not clear. Further investigation of this finding is warranted.
3.2 Characterization of Purified IgA
The purified fecal IgA was then characterized to determine if it was representative of mucosal IgA found in macaque mucosal secretions. IgA samples were run under denaturing and non-denaturing conditions. Under denaturing conditions, western blotting for monkey IgA showed that IgA isolated from two different fecal samples exhibited a clear α heavy chain band comparable to those found in unpurified vaginal swab and rhesus colostrum samples (Fig. 2a). A second band of smaller molecular weight was observed in both purified fecal samples, possibly resulting from degradation of IgA within the gastrointestinal tract. The amount of IgA in the unpurified rectal swab sample (Fig. 2a) was too little to be detected under the conditions used.
Fig. 2. Characterization of IgA affinity purified from rhesus fecal matter.
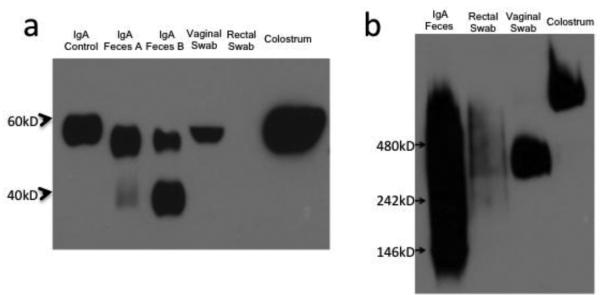
(a) IgA control is rhesus IgA (NIH Non-Human Primate Reagent Resource), IgA from feces A and B (from two different naïve animals), vaginal swab (pooled from several animals), and rhesus colostrum western blotted with α-monkey IgA-peroxidase. (b) Native-PAGE western blotted with α- monkey IgA-peroxidase. 4-20% Tris-glycine gels used for SDS-PAGE in (a), and 4-16% Bis- Tris gel used for blue native-PAGE in (b).
Using blue native PAGE, the purified fecal IgA appeared largely dimeric (MW ~320,000), similar to that present in rectal and vaginal swabs (Fig. 2b) and consistent with SIgA, known to be dimeric [47]. Nevertheless, some polymeric IgA as well as degradation products in the fecal IgA were observed. The IgA in rhesus colostrum was essentially all polymeric, as evidenced by a higher molecular weight band, which has been reported previously, and is most likely functionally similar to dimeric IgA [48]. There may be distinct differences in mucosal IgA found in different tissue compartments, but this remains to be investigated further.
The purified fecal IgA was tested in a semi-quanititative ELISA for the presence of secretory component (SC), the portion of the poly-Ig receptor that is cleaved and remains attached to the transcytosed mucosal dimeric IgA [49]. SC was present in each IgA sample isolated from feces (Table I). However, only a small amount of SC was detectable in the fecal IgA samples, which required roughly 1μg of purified IgA for SC detection, possibly due to degradation in the intestinal track or loss during purification. We were unable to test for the presence of J-chain due to the lack of a reagent for rhesus macaques. Purified IgA was free of IgG, as verified by western blot (data not shown).
Table I.
Secretory component (SC) and endotoxin in purified fecal IgA.
| SC detected in ng of IgAa |
Endotoxin EU/mL |
||
|---|---|---|---|
| Naïve | ZC40 | 485 | 2188 |
|
| |||
| P275 | 457 | 1632 | |
| Vaccinated | P878 | 104 | NT |
| P879 | 2841 | NT | |
| P888 | 1386 | 1603 | |
| P889 | 1295 | 2503 | |
| P897 | 1724 | NT | |
|
| |||
| Infected | P436 | 934 | 2143 |
| P437 | 753 | 9266 | |
| P644 | 845 | 3975 | |
| P735 | 900 | 10640 | |
| P876 | 182 | 2268 | |
|
| |||
| Average | 992 | 4024 | |
|
| |||
| Vaginal Swabb | 152 | ||
| Rectal Swab | 220 | ||
Purified IgA samples were serially diluted and evaluated by ELISA for secretory component. The IgA concentration of the highest dilution for each sample giving a positive signal is listed.
Endotoxin levels present in unpurified representative vaginal and rectal swabs are listed for comparison.
NT = Not Tested
3.3 Endotoxin in fecal IgA
The purified fecal IgA samples from the vaccinated and infected animals were also tested for endotoxin (Table I). As expected, they contained a relatively high amount of endotoxin (an average of 4000 EU/mL, or roughly 400ng/mL). In comparison, the IgA in vaginal swabs contained 150 EU/mL, while the IgA in rectal swabs contained 220 EU/mL. The amount of endotoxin present in the fecal IgAs precludes their in vivo usage outside of the rectal compartment without further endotoxin removal.
3.4 Neutralization of SIVmac251 by purified IgA
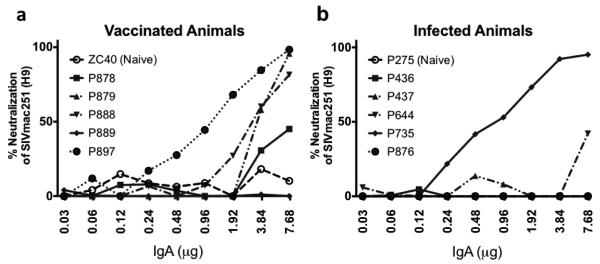
To determine if the purified fecal IgA mediated functional activities, we first assayed for neutralization capacity. SIVmac251 derived from H9-infected cells was used in a TZM-BL neutralization assay using up to 8μg of purified IgA (Fig. 3). This assay is unaffected by endotoxin present in the samples [50]. In the vaccinated cohort, 4 of 5 fecal IgA samples exhibited neutralizing activity, with the exception being animal P889 (Fig. 3a). IgA from macaque P878 did not reach 50% neutralization, but IC50 values for IgA from the other three vaccinated macaques ranged from 1 to 4 μg. Infected animal, P735, exhibited neutralizing activity with an IC50 less than 1μg of total mucosal IgA. Fecal IgA from the other infected animals did not exhibit significant neutralization of the autologous virus (Fig. 3b). Overall, purified fecal IgA from vaccinated animals more frequently exhibited neutralizing activity in comparison to the infected animals. The IgA from naïve animals exhibited no neutralization of the virus.
Fig. 3. Neutralization of H9-derived SIVmac251 by IgA purified from the feces of vaccinated and infected animals.
TZM-BL neutralization assay was conducted using H9-derived SIVmac251 and purified IgA from (a) 5 vaccinated animals and one naïve animal and (b) 5 infected animals and one naïve animal. IgA purified using sequential ProteinG/jacalin/anti-monkey IgA agarose columns.
3.5 Phagocytic Activity of IgA
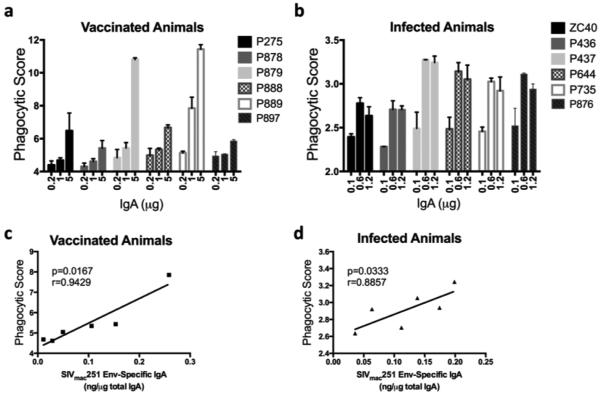
We next determined the ability of the purified fecal IgA to mediate ADCP using a target antigen, SIVmac251 gp120. Among the vaccinated animals, P879 and P889 exhibited elevated phagocytic activity (Fig. 4a), and in addition, macaque P888 showed phagocytic activity greater than the naïve animal (P275). With regard to IgA purified from feces of infected macaques, all but one (P436) displayed phagocytic activity greater than that of the naïve animal, ZC40 (Fig. 4b). The phagocytic activity of IgA from both the vaccinated and infected animals correlated significantly with the amount of SIVmac251 Env-specific IgA present in each sample (p=0.0167 and p=0.0333 for vaccinated and infected animals, respectively; Fig. 4c and d), indicating that recognition by fecal IgA of specific SIV gp120 epitopes was responsible for the phagocytic activity. The p value for the vaccinated animals was better than for the infected animals, and the spearman correlation coefficient (r value) was closer to 1 for the vaccinated animals. Further, the slope of the linear regression fit line for the vaccinated animals was steeper than for the infected animals (5.66 vs. 3.14), indicating overall that the phagocytic capacity of the IgA from the vaccinated animals was better.
Fig. 4. ADCP activity of IgA purified from feces of vaccinated and infected animals.
THP-1 cells were incubated with SIVmac251 gp120-coated fluorescent beads and IgA from feces. After 3 hours, phagocytosis was quantified by flow cytometry of samples from (a) 5 vaccinated animals and one naïve animal (P275) and (b) 5 infected animals and one naïve animal (ZC40). The phagocytic activities of the vaccinated group (c) and the infected group (d) were then correlated to the respective animals’ SIVmac251 Env-specific IgA isolated from feces using Spearman correlation. IgA purified using sequential ProteinG/jacalin/anti-monkey IgA agarose columns.
3.6 Transcytosis Inhibition
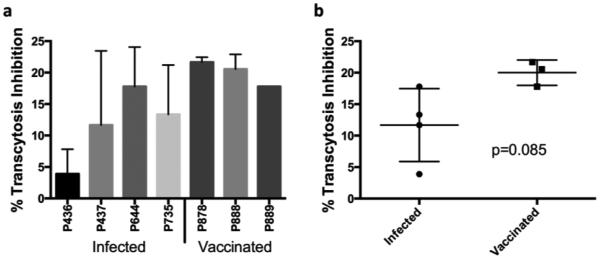
To further examine the functional capacity of the purified fecal IgA, we assessed its ability to inhibit transcytosis of cell-associated SIVmac251 across a tight epithelial cell barrier in vitro. After subtracting the averaged background of two naïve animals, purified fecal IgA from all vaccinated and infected macaques that were tested exhibited transcytosis inhibition (Fig. 5a). Consistent with the results for other functional activities, the mean levels of transcytosis inhibition by purified fecal IgA of the infected animals were lower than the mean levels for the vaccinated animals tested, but not significantly so (Fig. 5b).
Fig. 5. Transcytosis inhibition of cell-associated SIVmac251 by purified IgA.
SIV-infected H9 cells and IgA were added to the apical side of an HT-29 epithelial monolayer. After 4 hours, p27 levels were measured in the basolateral medium. (a) 5μg of purified IgA from 4 infected and 3 vaccinated animals. (b) Comparison between infected and vaccinated groups. p=0.085, value determined using Mann-Whitney test. All percentages calculated as % inhibited beyond the mean value from two naïve animals. IgA purified using sequential ProteinG/jacalin/anti-monkey IgA agarose columns.
3.7 Alternative affinity purification methods for mucosal IgA
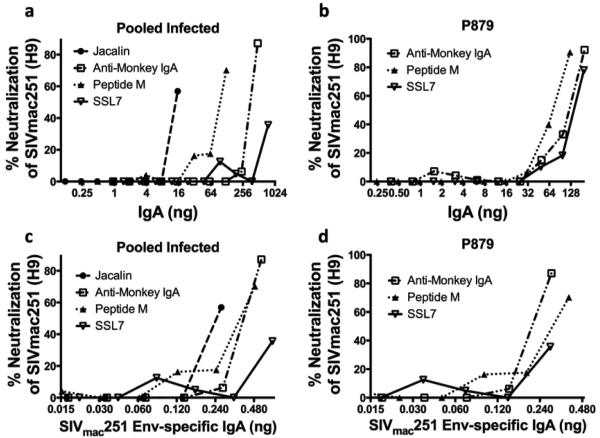
After demonstrating that a combination of Jacalin and anti-monkey IgA resins successfully purified fecal IgA with several functional activities, we investigated other affinity purification methods. We first used jacalin, α-Mon IgA, peptide M, and SSL7 separately for affinity purification of macaque mucosal IgA using 14.2g of fecal matter from the same sample from a naïve animal for each purification. Their efficiency of binding mucosal IgA was first determined by comparing the amount obtained from the column eluates to the total amount in the flowthrough plus eluate fractions (Table II). SSL7 bound IgA most efficiently, α-Mon IgA and Peptide M exhibited moderate efficiencies, while jacalin was least efficient. However, a comparison by western blot under denaturing conditions revealed that although all the purified IgAs contained the IgA heavy chain, all but that purified using jacalin contained a second smaller molecular weight band representing apparently degraded heavy chain (Fig. 6a) suggesting affinity purification by jacalin might yield better quality, non-degraded mucosal IgA. Under native conditions, all methods appeared to purify polymeric IgA from this particular macaque sample (Fig. 6b). Thus jacalin, α-Mon IgA, peptide M, and SSL7 all appeared useful for mucosal IgA purification from macaque feces, although multiple forms of IgA were obtained with SSL7.
Table II.
Relative efficiency of IgA affinity purification.
| IgA (ug/ml) | |||
|---|---|---|---|
| Column Eluate |
Column Flowthrough |
% IgA bound by column |
|
| Jacalin | 0.2 | 103.6 | 0.2 |
| Anti-Monkey IgA | 18.8 | 32.4 | 36.7 |
| SSL7 | 125.7 | 4.2 | 96.7 |
| Peptide M | 32.2 | 6.3 | 83.7 |
Columns were previously used and regenerated. Results are averaged from 3 separate samples.
Fig. 6. Jacalin, Peptide M, α-Mon IgA, and SSL7 can be used for affinity purification of rhesus IgA from feces.
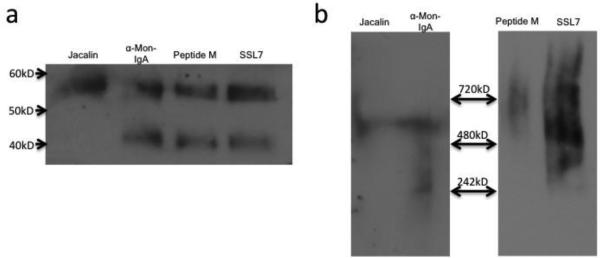
(a) 60ng of IgA affinity purified respectively left to right on jacalin, α- Mon IgA, peptide M, and SSL-7 columns were western blotted with α-monkey IgA-peroxidase. (b) 60ng of IgA affinity purified on Jacalin and α-Mon IgA columns, and 450ng of IgA affinity purified on peptide M and SSL7 columns were run on the same blue native-PAGE, exposed for different times using chemiluminescence, then western blotted with α-monkey IgA-peroxidase. 4-20% Tris-glycine gel used for SDS-PAGE in (a), 4-16% Bis-Tris gel used for blue native- PAGE in (b).
3.8 Functional comparison of mucosal IgA purification methods
Having demonstrated the ability to purify IgA using jacalin, α-Mon IgA, peptide M, and SSL7 columns, we wished to determine if IgAs obtained using these different methods were equally functional. Fecal matter from infected macaques (pooled in order to obtain enough material for purification by the 4 different methods and then evaluation across several functional assays) and fecal matter from a hyper-vaccinated animal, P879, were purified using each of the four resins, and the IgAs obtained were compared for the ability to neutralize H9-derived SIVmac251 virus (Fig. 7). The IgA obtained from the pooled feces of SIV-infected macaques indicated that jacalin yielded the most functional IgA, followed by peptide M, α-Mon IgA, and SSL7 (Fig. 7a). P879 IgA (Fig. 7b) showed similar results, although IgA from jacalin was not tested due to the minimal amount obtained. Similar results were obtained when neutralization was plotted versus the amount of SIVmac251 Env-specific IgA present in the total IgA used (Fig. 7c,d). In this case, IgA from the α-Mon IgA column exhibited slightly more neutralizing activity than that from the peptide M column. All methods yielded functional IgA with respect to neutralization, although jacalin and α-Mon IgA purification resulted in slightly better functionality.
Fig. 7. Neutralization of H9-derived SIVmac251 by IgA purified by different methods from feces.
TZM-BL neutralization assay was conducted using H9-derived SIVmac251 and (a) purified IgA from pooled fecal matter from SIV-infected macaques using jacalin, α-Mon IgA, Peptide M, and SSL7 columns, respectively, with the total IgA used on the X-axis. (b) Purified IgA from fecal matter from vaccinated macaque P879 using α-Mon IgA, Peptide M, and SSL7 columns with the total IgA used on the X-axis. (c)&(d) same as (a)&(b) respectively, only with amount of SIVmac251 Env-specific IgA used on the X-axis.
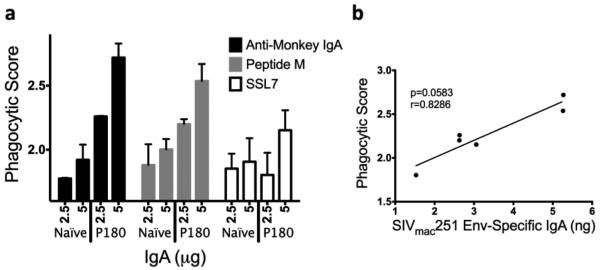
Fecal IgA from a naïve animal and an infected animal (P180) were similarly purified by the various affinity methods and compared for ADCP activity. Phagocytosis is important in clearing infectious agents, as well as in the initial immune response to an antigen that is recognized, phagocytosed, and degraded. IgA is known to play a role in mucosal immune responses through modulation of phagocytosis [51]. Fig. 8a shows similar functionality between IgA from peptide M and α-Mon IgA columns, both of which exhibited better phagocytic activity than IgA isolated using SSL7. IgA from jacalin was not tested due to the small amount obtained. The phagocytic score of the isolated total IgA plotted against the amount of SIVmac251 Env- specific IgA present in the sample (Fig. 8b), trended towards a positive correlation, although it was marginally non-significant. When the overall functionality of the mucosal IgA isolated by these various methods was compared, IgA purified using SSL7 did not perform as well as the other methods, despite demonstrating the best binding efficiency (Table II).
Fig. 8. ADCP activity of IgA purified by several methods from feces.
THP-1 cells were incubated with SIVmac251 gp120-coated fluorescent beads and IgA from feces. After 3 hours, phagocytosis was quantified by flow cytometry. (a) Fecal IgAfrom a naïve monkey and an infected monkey (P180) were purified using α-Mon IgA, Peptide M, and SSL7 columns, respectively, and IgA was used in the ADCP assay. (b) The phagocytic score was plotted against the amount of SIVmac251 Env-specific IgA present in each P180 sample used.
As IgA purified by both peptide M and α-Mon IgA exhibited good functional activity and also bound IgA more efficiently than jacalin (Table II), we considered that sequential peptide M/ α-Mon IgA affinity purification might be a preferred method. IgA was purified using this tandem method from fecal homogenates from three SIV-infected animals, 897, DELP, and DFDM, run using denaturing and non-denaturing conditions, and immunoblotted for IgA, IgG, and secretory component (Fig. 9). Under denaturing conditions, IgA heavy chain was detected in all three samples, along with some degradation products, and no IgG was detected (Fig. 9a). Under non- denaturing conditions, monomeric IgA was detected in the purified IgA from animals DELP and DFDM, and dimeric IgA was detected in the DELP sample (Fig. 9b). IgA was not detected in the sample from macaque 897 on the native gel, consistent with greater degradation in this purified IgA (Fig. 9a). The dimeric IgA purified from DELP was also positive for secretory component, consistent with mucosal IgA. Overall the peptide M/α-Mon IgA tandem procedure is a suitable method for the affinity purification of mucosal IgA from rhesus macaque fecal matter. It provides adequate amounts of mucosal IgA, allowing for multiple immunological assays to be conducted on pre-clinical samples. Potentially, similar methodology could be applied to purification of mucosal IgA from feces of clinical samples.
Fig. 9. Characterization of IgA affinity purified from rhesus fecal matter using a tandem peptide M/α-Mon IgA procedure.
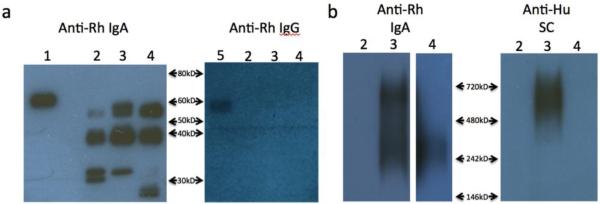
(a) SDS-PAGE western blotted with α-mon IgA-PO and α- mon IgG-PO. 1)rhesus IgA standard 2)Animal 897 3)Animal DELP 4)Animal DFDM 5)rhesus IgG standard (b) Native-PAGE of Rh IgA western blotted with α-mon IgA-PO and α-human secretory component-PO(Nordic). Lane 4 was exposed longer than lanes 2 and 3. 4-20% Tris- glycine gels used for SDS-PAGE in (a), 4-16% Bis-Tris gel used for blue native-PAGE in (b).
4. Discussion
Identification of immune mechanisms correlated with protection against HIV/SIV viral transmission is important for the rational design of prophylactic vaccine strategies. As most HIV transmissions occur mucosally, investigation of immune responses at mucosal sites is critical. To date, the study of mucosal immunity has been largely limited to monitoring mucosal immunoglobulins by ELISA techniques. Amounts of mucosal antibody available for functional studies have been limited. Here we report the purification of macaque mucosal IgA from fecal material, a source readily obtainable in large quantity. Feces represent a virtually unlimited amount of material that can be easily collected and stored frozen until IgA is purified. The only limitation is the amount of feces produced by the animal. One can easily obtain 30 grams per day, which based on our results here, could provide 300 ug of purified IgA per day. Up to 70 times more IgA can be purified from feces compared to secretions collected by rectal swabs. The use of fecal extracts for monitoring mucosal IgA in the gastrointestinal tract has been criticized as unrepresentative of amounts present in whole gut lavages [52]. However, this previous study did not purify IgA. Further, the amount of viral-specific IgA can be easily normalized to the total amount of IgA obtained. The amount of endotoxin present in purified fecal IgA might be of concern, but can be addressed using a commercially available endotoxin removal kit. Overall, utilization of fecal matter as a source of mucosal IgA could be applied to human clinical trials as readily as macaque studies. Samples obtained need only be frozen, and do not require a visit to a physician.
Purified fecal IgA was shown to be largely dimeric and contained SC (Fig. 2b, Table 1). The quantity of purified IgA obtained from individual fecal samples provided sufficient material for investigation of several functional activities. Fecal matter collected from two cohorts of macaques, one hyper-vaccinated and the other SIV-infected, along with naïve controls, was purified and the IgA obtained was shown to be multifunctional, exhibiting phagocytic, neutralizing, and transcytosis inhibiting activities. As expected, functionality of the vaccinated animals was greater than that of the infected macaques.
With regard to phagocytic activity, several studies have identified a role for IgA in phagocytosis involving interaction with FcαR1 [51, 53, 54]. THP-1 cells used as phagocytes in this study have been shown to express FcαRI [55]. The phagocytic activity exhibited by the purified IgA samples correlated with the amount of Env-specific IgA detected, for both the vaccinated and the infected groups, suggesting that the Env-specific IgA was responsible for the phagocytosis (Fig. 4c,d). It is unclear whether this IgA activity in the vaccinated rhesus macaques contributed to their observed protection from 10 sequential low-dose intrarectal SIV challenges (Franchini et al., unpublished data), as the fecal samples studied were obtained post- SIV challenge.
In addition to phagocytosis, the ability of the purified IgAs to neutralize H9-derived SIVmac251 was tested (Fig. 3). Overall, only two animals, one vaccinated and one infected, exhibited strong neutralization capacity with IC50 values of approximately 1μg/mL, but the vaccinated animals as a group exhibited greater neutralizing activity than the infected animals. The vaccinated animals’ neutralization capacity of mucosal IgA might be better than infected animals due to their hyper-vaccination against the autologous virus used. Moreover, the replicating virus in the infected animals could have undergone clonal change, resulting in altered antibody reactivity and poor neutralization. While the relative potency of the fecal IgA was less than that of many well characterized broadly neutralizing antibodies, the neutralizing activity exhibited contributes overall to their function. Additionally, the mucosal IgA from vaccinated animals tended to inhibit transcytosis better than that from infected animals (Fig 5). The neutralizing activity of the fecal IgA reflects functionality of the Fab, which when combined with the phagocytic activity attributed to the Fc portion of the antibody and the ability to inhibit transcytosis across epithelial membranes, may cumulatively contribute to protective efficacy. Overall, these studies clearly demonstrated that the purified fecal IgA was multifunctional.
Affinity chromatography purification of human IgA has been well characterized, but until now has not been applied to non-human primate samples. Previous methods of IgA purification were very cumbersome, costly, quite time consuming, and yielded very limited quantities of IgA. The affinity methods described here for purification of fecal IgA can also be applied to other mucosal samples, such as milk and vaginal and rectal swabs. They require no special equipment and are less complicated than HPLC methodology. Additionally, affinity purification is preferable over other methods that largely rely on protein size, which could encompass other undesired immunoglobulin isotypes or other proteins. Affinity purification methods should prove to be valuable for studying mucosal immunity in the context of the non-human primate vaccine model, and potentially applicable in human clinical trials.
With regard to the various affinity resins tested, jacalin has been used for years to isolate IgA, but had not been applied to isolation of monkey IgA. As shown here, the degraded product seen under denaturing conditions of IgA purified on α-Mon IgA, peptide M, and SSL7 columns as a smaller band below the 60kD α heavy chain band, did not appear in the sample purified on Jacalin (Fig. 6a). This may be due to the fact that jacalin binds glycans in the hinge region of IgA (38), and may require an intact molecule with appropriate conformation for epitope accessibility. In addition, monkey IgA is inherently variable [26, 27], perhaps also affecting binding. That the jacalin-purified IgA was of high quality was also seen in its better neutralization capability (Fig. 7a,c). However, the overall efficiency of jacalin binding to IgA was poor (Table II). Its use in tandem with a custom-conjugated α-Mon IgA column made up for any lack of specificity and efficiency. However, comparative results on the efficiency of binding and functional immune assays suggested peptide M might be a better choice for the tandem protocol as IgA isolated on peptide M was comparable functionally to that obtained using jacalin. Further, peptide M bound IgA efficiently (Table II). SSL7, despite exhibiting the most efficient capture of mucosal IgA from feces (Table II), was not preferable as SSL7-purified IgA exhibited the poorest functionality of the four resins tested (Fig. 7&8). We subsequently validated the peptide M/α-Mon IgA method by purification of mucosal IgA from fecal samples (Fig. 9).
5. Conclusion
We have demonstrated in a non-human primate model that mucosal IgA possessing multifunctional activities and present in great quantities in feces can be efficiently purified. Affinity purification of fecal IgA should prove useful not only in characterizing mucosal immune responses to various vaccine candidates, but also in further elucidating the host immune response to a spectrum of mucosal pathogens. Importantly, our approach has the potential to contribute significantly to the study of mucosal immunity in human clinical studies.
Highlights.
Mucosal IgA was affinity purified from rhesus macaque fecal homogenates
Large quantities of fecal IgA could be purified enabling multiple functional assays
Purified fecal IgA mediated neutralization, phagocytosis, and transcytosis inhibition
Mucosal IgA from SIV-vaccinated vs infected macaques exhibited functional differences
Evaluation of fecal mucosal IgA is potentially applicable to human clinical studies
Acknowledgements
We gratefully acknowledge the animal caretakers at ABL and the NIH Animal Center for their excellent care of the animals and collection of samples. Rhesus IgA and IgG standards were obtained through the NIH Nonhuman Primate Reagent Resource. This work was supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract No. HHSN26100800001E, and in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MS, Shaw GM, McMichael AJ, Haynes. BF. Acute HIV-1 Infection. The New England journal of medicine. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaneko H, Bednarek I, Wierzbicki A, Kiszka I, Dmochowski M, Wasik TJ, Kaneko Y, Kozbor. D. Oral DNA vaccination promotes mucosal and systemic immune responses to HIV envelope glycoprotein. Virology. 2000;267:8–16. doi: 10.1006/viro.1999.0093. [DOI] [PubMed] [Google Scholar]
- 4.Cristillo AD, Ferrari MG, Hudacik L, Lewis B, Galmin L, Bowen B, Thompson D, Petrovsky N, Markham P, Pal. R. Induction of mucosal and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations. The Journal of general virology. 2011;92:128–140. doi: 10.1099/vir.0.023242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domm W, Brooks L, Chung HL, Feng C, Bowers WJ, Watson G, McGrath JL, Dewhurst. and S. Robust antigen-specific humoral immune responses to sublingually delivered adenoviral vectors encoding HIV-1 Env: association with mucoadhesion and efficient penetration of the sublingual barrier. Vaccine. 2011;29:7080–7089. doi: 10.1016/j.vaccine.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marinaro M, Riccomi A, Rappuoli R, Pizza M, Fiorelli V, Tripiciano A, Cafaro A, Ensoli B, De Magistris. MT. Mucosal delivery of the human immunodeficiency virus-1 Tat protein in mice elicits systemic neutralizing antibodies, cytotoxic T lymphocytes and mucosal IgA. Vaccine. 2003;21:3972–3981. doi: 10.1016/s0264-410x(03)00295-0. [DOI] [PubMed] [Google Scholar]
- 7.Rowcliffe E, Bolmstedt A, Biller M, Wahren B, Olofsson S, Hinkula. J. Demonstration of neutralizing mucosal IgA response to intranasal HIV-1 env DNA vaccines with or without the V3 glycosylation site. Scandinavian journal of infectious diseases. 2004;36:360–364. doi: 10.1080/00365540410020208. [DOI] [PubMed] [Google Scholar]
- 8.Kato H, Bukawa H, Hagiwara E, Xin KQ, Hamajima K, Kawamoto S, Sugiyama M, Sugiyama M, Noda E, Nishizaki M, Okuda. K. Rectal and vaginal immunization with a macromolecular multicomponent peptide vaccine candidate for HIV-1 infection induces HIV-specific protective immune responses. Vaccine 18: 1151-1160. 2000 doi: 10.1016/s0264-410x(99)00385-0. [DOI] [PubMed] [Google Scholar]
- 9.Jain S, Rosenthal. KL. The gp41 epitope, QARVLAVERY, is highly conserved and a potent inducer of IgA that neutralizes HIV-1 and inhibits viral transcytosis. Mucosal immunology. 2011;4:539–553. doi: 10.1038/mi.2011.21. [DOI] [PubMed] [Google Scholar]
- 10.Pastori C, Diomede L, Venuti A, Fisher G, Jarvik J, Bomsel M, Sanvito F, Lopalco. L. Induction of HIV-blocking anti-CCR5 IgA in Peyers's patches without histopathological alterations. Journal of virology. 2014;88:3623–3635. doi: 10.1128/JVI.03663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hidajat R, Xiao P, Zhou Q, Venzon D, Summers LE, Kalyanaraman VS, Montefiori DC, Robert-Guroff. M. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. Journal of virology. 2009;83:791–801. doi: 10.1128/JVI.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson LJ, Kuate S, Daltabuit-Test M, Li Q, Xiao P, McKinnon K, DiPasquale J, Cristillo A, Venzon D, Haase A, Robert-Guroff. M. Replicating adenovirussimian immunodeficiency virus (SIV) vectors efficiently prime SIV-specific systemic and mucosal immune responses by targeting myeloid dendritic cells and persisting in rectal macrophages, regardless of immunization route. Clinical and vaccine immunology : CVI. 2012;19:629–637. doi: 10.1128/CVI.00010-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai L, Kwa SF, Kozlowski PA, Montefiori DC, Nolen TL, Hudgens MG, Johnson WE, Ferrari G, Hirsch VM, Felber BK, Pavlakis GN, Earl PL, Moss B, Amara RR, Robinson. HL. SIVmac239 MVA vaccine with and without a DNA prime, similar prevention of infection by a repeated dose SIVsmE660 challenge despite different immune responses. Vaccine. 2012;30:1737–1745. doi: 10.1016/j.vaccine.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fouda GG, Amos JD, Wilks AB, Pollara J, Ray CA, Chand A, Kunz EL, Liebl BE, Whitaker K, Carville A, Smith S, Colvin L, Pickup DJ, Staats HF, Overman G, Eutsey-Lloyd K, Parks R, Chen H, Labranche C, Barnett S, Tomaras GD, Ferrari G, Montefiori DC, Liao HX, Letvin NL, Haynes BF, Permar. SR. Mucosal immunization of lactating female rhesus monkeys with a transmitted/founder HIV-1 envelope induces strong Env-specific IgA antibody responses in breast milk. Journal of virology. 2013;87:6986–6999. doi: 10.1128/JVI.00528-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vajdy M, Singh M, Kazzaz J, Soenawan E, Ugozzoli M, Zhou F, Srivastava I, Bin Q, Barnett S, Donnelly J, Luciw P, Adamson L, Montefiori D, O'Hagan. DT. Mucosal and systemic anti-HIV responses in rhesus macaques following combinations of intranasal and parenteral immunizations. AIDS research and human retroviruses. 2004;20:1269–1281. doi: 10.1089/aid.2004.20.1269. [DOI] [PubMed] [Google Scholar]
- 16.Leroux-Roels G, Maes C, Clement F, van Engelenburg F, van den Dobbelsteen M, Adler M, Amacker M, Lopalco L, Bomsel M, Chalifour A, Fleury. S. Randomized Phase I: Safety, Immunogenicity and Mucosal Antiviral Activity in Young Healthy Women Vaccinated with HIV-1 Gp41 P1 Peptide on Virosomes. PloS one. 2013;8:e55438. doi: 10.1371/journal.pone.0055438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, Hidajat R, Demberg T, Robert-Guroff. M. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. Journal of virology. 2010;84:7161–7173. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, Devillier G, Cong Z, Wei Q, Gao H, Qin C, Yang GB, Zurbriggen R, Lopalco L, Fleury. S. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, Zhao J, DiPasquale J, Fenizia C, Lee EM, Kalisz I, Kalyanaraman VS, Pal R, Montefiori D, Keele BF, Robert-Guroff. M. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. Journal of virology. 2012;86:4644–4657. doi: 10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broliden K, Hinkula J, Devito C, Kiama P, Kimani J, Trabbatoni D, Bwayo JJ, Clerici M, Plummer F, Kaul. R. Functional HIV-1 specific IgA antibodies in HIV-1 exposed, persistently IgG seronegative female sex workers. Immunology letters. 2001;79:29–36. doi: 10.1016/s0165-2478(01)00263-2. [DOI] [PubMed] [Google Scholar]
- 21.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthesy. B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 22.Kerr MA. The structure and function of human IgA. The Biochemical journal. 1990;271:285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flanagan JG, Lefranc MP, Rabbitts. TH. Mechanisms of divergence and convergence of the human immunoglobulin alpha 1 and alpha 2 constant region gene sequences. Cell. 1984;36:681–688. doi: 10.1016/0092-8674(84)90348-9. [DOI] [PubMed] [Google Scholar]
- 24.Kawamura S, Tanabe H, Watanabe Y, Kurosaki K, Saitou N, Ueda. S. Evolutionary rate of immunoglobulin alpha noncoding region is greater in hominoids than in Old World monkeys. Molecular biology and evolution. 1991;8:743–752. doi: 10.1093/oxfordjournals.molbev.a040687. [DOI] [PubMed] [Google Scholar]
- 25.Sumiyama K, Saitou N, Ueda. S. Adaptive evolution of the IgA hinge region in primates. Molecular biology and evolution. 2002;19:1093–1099. doi: 10.1093/oxfordjournals.molbev.a004167. [DOI] [PubMed] [Google Scholar]
- 26.Scinicariello F, Attanasio. R. Intraspecies heterogeneity of immunoglobulin alpha-chain constant region genes in rhesus macaques. Immunology. 2001;103:441–448. doi: 10.1046/j.1365-2567.2001.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tudor D, Yu H, Maupetit J, Drillet AS, Bouceba T, Schwartz-Cornil I, Lopalco L, Tuffery P, Bomsel. M. Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12680–12685. doi: 10.1073/pnas.1200024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinholdt J, Kilian. M. Lack of cleavage of immunoglobulin A (IgA) from rhesus monkeys by bacterial IgA1 proteases. Infection and immunity. 1991;59:2219–2221. doi: 10.1128/iai.59.6.2219-2221.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoyama K, Chiba. J. Separation of different molecular forms of mouse IgA and IgM monoclonal antibodies by high-performance liquid chromatography on spherical hydroxyapatite beads. Journal of immunological methods. 1993;162:201–210. doi: 10.1016/0022-1759(93)90385-k. [DOI] [PubMed] [Google Scholar]
- 30.Avril A, Froude JW, Mathieu J, Pelat T, Thullier. P. Isolation of antibodies from non-human primates for clinical use. Current drug discovery technologies. 2013 doi: 10.2174/15701638113109990030. [DOI] [PubMed] [Google Scholar]
- 31.Eliasson M, Olsson A, Palmcrantz E, Wiberg K, Inganas M, Guss B, Lindberg M, Uhlen. M. Chimeric IgG-binding receptors engineered from staphylococcal protein A and streptococcal protein G. The Journal of biological chemistry. 1988;263:4323–4327. [PubMed] [Google Scholar]
- 32.Nilson BH, Logdberg L, Kastern W, Bjorck L, Akerstrom. B. Purification of antibodies using protein L-binding framework structures in the light chain variable domain. Journal of immunological methods. 1993;164:33–40. doi: 10.1016/0022-1759(93)90273-a. [DOI] [PubMed] [Google Scholar]
- 33.Sandin C, Linse S, Areschoug T, Woof JM, Reinholdt J, Lindahl. G. Isolation and detection of human IgA using a streptococcal IgA-binding peptide. J Immunol. 2002;169:1357–1364. doi: 10.4049/jimmunol.169.3.1357. [DOI] [PubMed] [Google Scholar]
- 34.Langley R, Wines B, Willoughby N, Basu I, Proft T, Fraser. JD. The staphylococcal superantigen-like protein 7 binds IgA and complement C5 and inhibits IgA-Fc alpha RI binding and serum killing of bacteria. J Immunol. 2005;174:2926–2933. doi: 10.4049/jimmunol.174.5.2926. [DOI] [PubMed] [Google Scholar]
- 35.Ramsland PA, Willoughby N, Trist HM, Farrugia W, Hogarth PM, Fraser JD, Wines. BD. Structural basis for evasion of IgA immunity by Staphylococcus aureus revealed in the complex of SSL7 with Fc of human IgA1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15051–15056. doi: 10.1073/pnas.0706028104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregory RL, Rundegren J, Arnold. RR. Separation of human IgA1 and IgA2 using jacalin-agarose chromatography. Journal of immunological methods. 1987;99:101–106. doi: 10.1016/0022-1759(87)90037-8. [DOI] [PubMed] [Google Scholar]
- 37.Aucouturier P, Duarte F, Mihaesco E, Pineau N, Preud'homme. JL. Jacalin, the human IgA1 and IgD precipitating lectin, also binds IgA2 of both allotypes. Journal of immunological methods. 1988;113:185–191. doi: 10.1016/0022-1759(88)90331-6. [DOI] [PubMed] [Google Scholar]
- 38.Kondoh H, Kobayashi K, Hagiwara. K. A simple procedure for the isolation of human secretory IgA of IgA1 and IgA2 subclass by a jackfruit lectin, jacalin, affinity chromatography. Molecular immunology. 1987;24:1219–1222. doi: 10.1016/0161-5890(87)90169-6. [DOI] [PubMed] [Google Scholar]
- 39.Meillet D, Raichvarg D, Tallet F, Savel J, Yonger J, Gobert. JG. Measurement of total, monomeric and polymeric IgA in human faeces by electroimmunodiffusion. Clinical and experimental immunology. 1987;69:142–147. [PMC free article] [PubMed] [Google Scholar]
- 40.Tress U, Suchodolski JS, Williams DA, Steiner. JM. Development of a fecal sample collection strategy for extraction and quantification of fecal immunoglobulin A in dogs. American journal of veterinary research. 2006;67:1756–1759. doi: 10.2460/ajvr.67.10.1756. [DOI] [PubMed] [Google Scholar]
- 41.Grewal HM, Karlsen TH, Vetvik H, Ahren C, Gjessing HK, Sommerfelt H, Haneberg. B. Measurement of specific IgA in faecal extracts and intestinal lavage fluid for monitoring of mucosal immune responses. Journal of immunological methods. 2000;239:53–62. doi: 10.1016/s0022-1759(00)00171-x. [DOI] [PubMed] [Google Scholar]
- 42.DeVico A, Fouts T, Lewis GK, Gallo RC, Godfrey K, Charurat M, Harris I, Galmin L, Pal. R. Antibodies to CD4-induced sites in HIV gp120 correlate with the control of SHIV challenge in macaques vaccinated with subunit immunogens. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17477–17482. doi: 10.1073/pnas.0707399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manrique M, Kozlowski PA, Wang SW, Wilson RL, Micewicz E, Montefiori DC, Mansfield KG, Carville A, Aldovini. A. Nasal DNA-MVA SIV vaccination provides more significant protection from progression to AIDS than a similar intramuscular vaccination. Mucosal immunology. 2009;2:536–550. doi: 10.1038/mi.2009.103. [DOI] [PubMed] [Google Scholar]
- 44.Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, Irvine DJ, Burton DR, Alter. G. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. Journal of immunological methods. 2011;366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vargas-Inchaustegui DA, Tuero I, Mohanram V, Musich T, Pegu P, Valentin A, Sui Y, Rosati M, Bear J, Venzon DJ, Kulkarni V, Alicea C, Pilkington GR, Liyanage NP, Demberg T, Gordon SN, Wang Y, Hogg AE, Frey B, Patterson LJ, DiPasquale J, Montefiori DC, Sardesai NY, Reed SG, Berzofsky JA, Franchini G, Felber BK, Pavlakis GN, Robert-Guroff. M. Humoral immunity induced by mucosal and/or systemic SIV-specific vaccine platforms suggests novel combinatorial approaches for enhancing responses. Clin Immunol. 2014;153:308–322. doi: 10.1016/j.clim.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider T, Zippel T, Schmidt W, Pauli G, Heise W, Wahnschaffe U, Riecken EO, Zeitz M, Ullrich. R. Abnormal predominance of IgG in HIV-specific antibodies produced by short-term cultured duodenal biopsy specimens from HIV-infected patients. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1997;16:333–339. doi: 10.1097/00042560-199712150-00004. [DOI] [PubMed] [Google Scholar]
- 47.Strugnell RA, Wijburg. OL. The role of secretory antibodies in infection immunity. Nature reviews. Microbiology. 2010;8:656–667. doi: 10.1038/nrmicro2384. [DOI] [PubMed] [Google Scholar]
- 48.Brandtzaeg P, Farstad IN, Johansen FE, Morton HC, Norderhaug IN, Yamanaka. T. The B-cell system of human mucosae and exocrine glands. Immunological reviews. 1999;171:45–87. doi: 10.1111/j.1600-065X.1999.tb01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mostov KE. Transepithelial transport of immunoglobulins. Annual review of immunology. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- 50.Geonnotti AR, Bilska M, Yuan X, Ochsenbauer C, Edmonds TG, Kappes JC, Liao HX, Haynes BF, Montefiori. DC. Differential inhibition of human immunodeficiency virus type 1 in peripheral blood mononuclear cells and TZM-bl cells by endotoxin-mediated chemokine and gamma interferon production. AIDS research and human retroviruses. 2010;26:279–291. doi: 10.1089/aid.2009.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franca EL, Morceli G, Fagundes DL, Rudge MV, Calderon Ide M, Honorio-Franca. AC. Secretory IgA-Fcalpha receptor interaction modulating phagocytosis and microbicidal activity by phagocytes in human colostrum of diabetics. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2011;119:710–719. doi: 10.1111/j.1600-0463.2011.02789.x. [DOI] [PubMed] [Google Scholar]
- 52.Ferguson A, Humphreys KA, Croft. NM. Technical report: results of immunological tests on faecal extracts are likely to be extremely misleading. Clinical and experimental immunology. 1995;99:70–75. doi: 10.1111/j.1365-2249.1995.tb03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herr AB, White CL, Milburn C, Wu C, Bjorkman. PJ. Bivalent binding of IgA1 to FcalphaRI suggests a mechanism for cytokine activation of IgA phagocytosis. Journal of molecular biology. 2003;327:645–657. doi: 10.1016/s0022-2836(03)00149-9. [DOI] [PubMed] [Google Scholar]
- 54.Weisbart RH, Kacena A, Schuh A, Golde. DW. GM-CSF induces human neutrophil IgA-mediated phagocytosis by an IgA Fc receptor activation mechanism. Nature. 1988;332:647–648. doi: 10.1038/332647a0. [DOI] [PubMed] [Google Scholar]
- 55.Boltz-Nitulescu G, Willheim M, Spittler A, Leutmezer F, Tempfer C, Winkler. S. Modulation of IgA, IgE, and IgG Fc receptor expression on human mononuclear phagocytes by 1 alpha,25-dihydroxyvitamin D3 and cytokines. Journal of leukocyte biology. 1995;58:256–262. doi: 10.1002/jlb.58.2.256. [DOI] [PubMed] [Google Scholar]