Abstract
Brain-derived neurotrophic factor (BDNF) is associated with synaptic plasticity, which is crucial for long-term learning and memory. Some studies suggest that people suffering from anxiety disorders show reduced BDNF relative to healthy controls. Lower BDNF is associated with impaired learning, cognitive deficits, and poor exposure-based treatment outcomes. A series of studies with rats showed that exercise elevates BDNF and enhances fear extinction. However, this strategy has not been tested in humans. In this pilot study, we randomized participants (N = 9, 8 females, MAge = 34) with posttraumatic stress disorder (PTSD) to (a) prolonged exposure alone (PE) or (b) prolonged exposure + exercise (PE + E). Participants randomized to the PE + E condition completed a 30-minute bout of moderate-intensity treadmill exercise (70% of age-predicted HRmax) prior to each PE session. Consistent with prediction, the PE + E group showed a greater improvement in PTSD symptoms (d = 2.65) and elevated BDNF (d = 1.08) relative to the PE only condition. This pilot study provides initial support for further investigation into exercise augmented exposure therapy.
Keywords: exercise, augmentation, exposure therapy, BDNF, anxiety disorders, pilot data, CBT
Introduction
Exposure therapy has demonstrated clear efficacy for the anxiety disorders, offering clinically meaningful advantages over psychological placebo conditions and showing improvements in symptoms comparable to established pharmacotherapies (Barlow, Gorman, Shear, & Woods, 2000; Blanco et al., 2010; Goodson et al., 2011; Hofmann & Smits, 2008; Powers & Emmelkamp, 2008; Powers, Halpern, Ferenschak, Gillihan, & Foa, 2010; Powers, Sigmarsson, & Emmelkamp, 2008; Simpson et al., 2013). However, many patients who receive exposure therapy either fail to respond or continue to experience some residual symptoms following treatment discontinuation (Barlow et al., 2000; Blanco et al., 2010; Hofmann & Smits, 2008; Simpson et al., 2013). Large clinical trials of exposure-based treatment efficacy for the various anxiety disorders have yielded non-optimal response rates of up to 49% for social anxiety disorder (Davidson et al., 2004), 38% for obsessive-compulsive disorder (Foa et al., 2005), 36% for panic disorder (Barlow et al., 2000), and 44% for PTSD (Foa, Rothbaum, Riggs, & Murdock, 1991). Accordingly, the agenda for exposure therapy research has shifted to the development of strategies to enhance the efficacy of exposure therapy. Augmentation strategies that reduce anxiety during exposure therapy (breathing retraining, anxiety reducing drugs/benzodiazepines, and use of safety behaviors) have failed to enhance outcome (Otto, Hong, & Safren, 2002; Powers, Smits, & Telch, 2004; Powers, Smits, Whitley, Bystritsky, & Telch, 2008; Schmidt et al., 2000); however, strategies that enhance the learning that takes place during exposure therapy appear more promising (Powers, Smits, Leyro, & Otto, 2007; Powers, Vervliet, Smits, & Otto, 2009). One such augmentation strategy to enhance exposure learning is physical exercise.
The primary aims of this article are to detail the rationale for use of exercise as an augmentation strategy for exposure therapy and to present findings from a small-scale randomized study that tested the efficacy of exercise as an aid to exposure therapy in adults suffering from PTSD. PTSD is most similar to the translational research on extinction research given there is a definitive conditioning event (the trauma). We have organized the article into three sections. In the first section, we present a rationale for using exercise as an augmentation strategy, focusing specifically on the capacity of exercise to manipulate a key molecular mediator of fear extinction. In the second section, we describe the methodology and findings of the pilot study. In the third and final section, we provide directions for future research in this area.
Rationale
Fear extinction as a model for exposure therapy
Emphasizing the current priority of the National Institute of Mental Health to support translational research, Anderson & Insel (2006) discussed the promise of fear extinction research, particularly that which examines fear extinction retention, for improving treatment outcome for people with anxiety disorders. As others have asserted (Bouton, 2002; Myers & Davis, 2007), Anderson & Insel (2006) draw parallels between the procedures employed in animal and human studies of extinction learning and those used in exposure therapy. Specifically, extinction training and exposure therapy involve repeated exposure to feared cues without the associated negative outcome (Pavlov, 1927). Extinction retention has been associated with greater symptom reduction following exposure therapy (Berry, Rosenfield, & Smits, 2009); hence, parameters of fear extinction training related to greater consolidation of fear extinction learning may guide the development of strategies that can augment the efficacy of exposure therapy (Berry et al., 2009; Ledgerwood, Richardson, & Cranney, 2004; Myers & Davis, 2007; Santini, Ge, Ren, Pen÷a de Ortiz, & Quirk, 2004).
Converging evidence from studies of extinction in human and non-human animals indicates that the same structures implicated in the maintenance of PTSD are involved in extinction of conditioned fear. In particular, hyperreactivity is seen in the amygdala (Nutt & Malizia, 2004) and decreased activity is exhibited in the ventromedial prefrontal cortex (vmPFC; Etkin & Wager, 2007) in individuals suffering from PTSD. Hippocampal volume and metabolic rate are also reduced in PTSD (Smith, 2005). Both human and animal studies implicate the amygdala in fear conditioning (LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; LeDoux, Sakaguchi, & Reis, 1984), indicating that the function of the amygdala is conserved across species and hyperactivity in the amygdala may contribute to enhanced recall of traumatic memories in PTSD (Pitman, Shin, & Rauch, 2001). Anatomical studies show that the vmPFC projects to the amygdala, predominantly to inhibitory neurons in the intercalated region (intercalated cells are important for inhibitory control over the amygdala), suggesting that vmPFC activity may suppress amygdala activity. Quirk and colleagues (Quirk, Russo, Barron, & Lebron, 2000) reported evidence indicating that lesions of the infralimbic region (IL) of the rodent vmPFC impaired extinction of conditioned fear. The authors proposed that the IL interacts with the amygdala to enable the extinction of conditioned fear, a finding that is now supported by further studies in rats (Mueller, Bravo-Rivera, & Quirk, 2010; Quirk, Likhtik, Pelletier, & Paré, 2003) and in humans (Linnman et al., 2012; Phelps, Delgado, Nearing, & LeDoux, 2004). One of the limitations of exposure therapy is the return of fear that occurs after the conditioned response appears to be extinguished. The hippocampus plays a role in gating of extinction to a specific conditioned stimulus, thus preventing generalization of extinction to multiple contexts (Ji & Maren, 2005). Inactivation of the hippocampus prevents the return of fear in rats exposed to the conditioned cue in a new context (Ji & Maren, 2005); however, inactivation of the hippocampus during extinction training impairs extinction recall the following day, indicating that the hippo-campus is necessary for the consolidation of extinction learning (Corcoran, Desmond, Frey, & Maren, 2005).
Fear extinction research can guide the development of strategies to enhance exposure therapy: a successful example of translational research
As described in the previous section, animal research on fear extinction has helped delineate core mechanisms and structures involved in the extinction of conditioned fear. The last decade has illustrated how this basic and mechanistic research can help identify treatment targets and corresponding interventions for enhancing exposure therapy outcomes. Specifically, in their animal research, Davis and colleagues first demonstrated that extinction learning is modulated by activity of the glutamatergic N-methyl-d-aspartate receptor (NMDA-R) in the amygdala (Davis, Ressler, Rothbaum, & Richardson, 2006). They showed that inhibitors of this activity blocked the retention of extinction learning (Falls, Miserendino, & Davis, 1992) and, likewise, partial agonists, such as d-cycloserine (DCS), enhance the consolidation of this learning (Ledgerwood, Richardson, & Cranney, 2003; Walker, Ressler, Lu, & Davis, 2002). Teaming up with clinical researchers, Davis and colleagues then translated these preclinical findings, demonstrating that the acute pre-session administration of DCS facilitated reductions in fear of heights with exposure therapy. Since then, a number of clinical trials (e.g. Hofmann et al., 2013; Smits et al., 2013; Smits et al., 2013; Tart et al., 2013) have replicated and extended these findings to other anxiety disorder samples (Rodrigues et al., 2014). This translational research success supports a continued focus on fear extinction and its core mechanisms for developing augmentation interventions for exposure therapy (Anderson & Insel, 2006).
BDNF: a key mechanism underlying fear extinction
One key target for augmentation of exposure therapy is brain-derived neurotrophic factor (BDNF). BDNF is a member of the neurotrophins—a family of structurally related proteins that promote neuronal differentiation and survival during development (Barde, 1994; Levi-Montalcini, 1987)—and has been implicated in a host of pathological conditions. BDNF is also a potent modulator of synaptic plasticity (Arancio & Chao, 2007; Bramham & Messaoudi, 2005; Schinder & Poo, 2000) and much effort has been devoted to studying its role in the hippocampus, a structure traditionally associated with learning and memory. These sets of studies suggest that BDNF plays a role in hippocampal long-term potentiation (LTP) and learning. Initial work showed deficits in early LTP (E-LTP) in BDNF knock-out mice that could be rescued by exogenous administration of BDNF (Korte et al., 1995; Patterson et al., 1996). Intrahippocampal infusions of tropomyosin receptor kinase (Trk)B Fc, which prevents BDNF from binding to TrkB receptors, was found to impair LTP induction (Figurov, Pozzo-Miller, Olafsson, Wang, & Lu, 1996; Patterson et al., 1996), E-LTP (Figurov et al., 1996; Patterson et al., 1996), and late-phase LTP (L-LTP; Kang, Welcher, Shelton, & Schuman, 1997; Patterson et al., 1996) in slice preparations. Preventing BDNF signaling also impaired hippocampus-dependent learning (Bekinschtein et al., 2007). Recent work indicates that exogenous infusions of BDNF can induce synaptic potentiation. This was first demonstrated in the slice preparation at CA3-CA1 synapses (Kang & Schuman, 1995, 1996) and, more recently, in vivo in the dentate gyrus (DG; Messaoudi, Ying, Kanhema, Croll, & Bramham, 2002). Messaoudi and colleagues’ work suggests that BDNF-induced LTP in the DG is NMDA-R independent, while high frequency stimulation (HFS)-induced LTP requires NMDA-R activation (Messaoudi et al., 2002). However, BDNF- and HFS-induced LTP share common mechanisms as evidenced by observations that BDNF-LTP is occluded when BDNF is infused post-HFS-LTP. In addition, HFS-induced LTP induces BDNF release that is dependent on NMDAR and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-R activation (Hartmann, Heumann, & Lessmann, 2001); hence, it appears that the induction of LTP by exogenous BDNF can bypass early stages of HFS-induced potentiation but that, in either case, BDNF is required for maintenance (Messaoudi et al., 2007, 2002; Santi et al., 2006). There is increasing empirical support for the role of another structure—the amygdala—in changes induced by BDNF. Indeed, temporally specific changes in BDNF gene expression were found to occur in the LA after paired stimuli that supported learning, but not after exposure to neutral or aversive stimuli alone (Rattiner, Davis, French, & Ressler, 2004).
Given the above, it is not surprising that BDNF has been shown to play an important role in the extinction of conditioned fear. Effectively, activation of the TrkB receptor by BDNF in the basolateral complex of the amygdala is necessary for the consolidation, but not acquisition, of fear extinction in rats (Chhatwal, Stanek-Rattiner, Davis, Ressler, & Amygdala, 2006). Deletion of BDNF in the adult hippocampus impairs extinction in mice (Heldt, Stanek, Chhatwal, & Ressler, 2007) and histone modifications were observed around the BDNF gene promoters in the mPFC following extinction of conditioned fear in mice (Bredy et al., 2007). These findings implicate the neurotrophin BDNF in the consolidation of fear extinction within the neurocircuitry that is affected in PTSD. Remarkably, Peters and colleagues (Peters, Dieppa-Perea, Melendez, & Quirk, 2010) showed that infralimbic BDNF infusions induced extinction of conditioned fear in rats even in the absence of extinction training. Taken together, these findings indicate that BDNF is both necessary and sufficient for the extinction of conditioned fear in rats.
Additional support for the role of BDNF in fear extinction retention comes from research linking a single nucleotide polymorphism (SNP) in the gene encoding BDNF (i.e., BDNF Val66Met genotype) to impaired extinction learning in mice and humans (Soliman et al., 2010). Extending this research, Felmingham and colleagues showed that PTSD patients with the BDNF Met66 allele were not as responsive to exposure therapy as were those with the Val/Val genotype (Felmingham, Dobson-Stone, Schofield, Quirk, & Bryant, 2013).
In summary, BDNF is crucial for synaptic plasticity in brain regions that are critically involved in the consolidation of extinction. Reduced BDNF release in humans is associated with impaired functioning of this extinction circuitry. Therefore, methods to enhance BDNF release may facilitate the consolidation of extinction learning and, consequently, improve exposure therapy outcomes.
The promise of aerobic exercise for enhancing fear extinction and exposure therapy outcomes
Basic research on fear extinction has supported the development and testing of a number of pharmacological agents, including DCS, as well as other pharmacological and somatic interventions for augmenting exposure therapy (Powers, Smits, Otto, Sanders, & Emmelkamp, 2009; Smits et al., 2013, 2014). Because patients seeking care for anxiety disorders generally prefer behavioral over pharmacological and somatic approaches (Arch, 2014; McHugh, Whitton, Peckham, Welge, & Otto, 2013; Roy-Byrne, Berliner, Russo, Zatzick, & Pitman, 2003), it is important to develop and make available behavioral interventions that can manipulate the core mechanisms involved in fear extinction/exposure therapy. Aerobic exercise emerges as a promising candidate for a number of reasons (Asmundson et al., 2013). First, patients with anxiety disorders and their providers generally already perceive exercise as a useful strategy for their recovery (Daley, 2002). Second, widely prescribed to promote health and longevity, exercise is a non-invasive intervention with low incidence of adverse events in adults with psychiatric illness (Blumenthal et al., 1999; Trivedi et al., 2011). Third, extant literature suggests that exercise significantly increases BDNF activity in regions of the brain critical to fear extinction (Cho et al., 2012; Cotman & Berchtold, 2002; Heyman et al., 2012; Stroyöhle et al., 2010). Indeed, a number of studies have shown that chronic and acute aerobic exercise increase BDNF levels in the hippocampus of rats (Chen & Russo-Neustadt, 2009; Dravid et al., 2010; Neeper, Goómez-Pinilla, Choi, & Cotman, 1996; Oliff, Berchtold, Isackson, & Cotman, 1998; Vaynman, Ying, & Goómezpinilla, 2004; Widenfalk, Olson, & Thoreón, 1999). Fourth, an acute bout of voluntary wheel running enhances extinction retention in rats (Siette, Reichelt, & Westbrook, 2014). Siette et al. (2014) showed that wheel access just before or just after extinction training resulted in reduced freezing at test. However, wheel access long before or long after extinction (6 hours) did not enhance retention. There also appeared to be a dose–response relationship with greater running distance associated with greater fear learning/extinction. Another study showed voluntary running after extinction training increased neurogenesis in the dentate gyrus and reduced contextual fear relative to controls when tested 6 weeks later (Akers et al., 2014).
An important next step in building upon the promise of using aerobic exercise as an augmentation intervention of exposure therapy is to demonstrate that exercise, when prescribed acutely, can enhance exposure therapy outcomes in humans. In addition, keeping in mind the dissemination and utilization of this augmentation strategy in clinical practice, it is important to determine whether these putative effects can be observed with a moderate-intensity acute exercise bout (which can be prescribed to most individuals without formal medical clearance (American College for Sports Medicine, 2013)). A review of 24 studies indicates that acute exercise can yield meaningful increases in serum or plasma BDNF in healthy subjects and persons with chronic disease (psychiatric or medical; Knaepen, Goekint, Heyman, & Meeusen, 2010). These studies further indicate that a meaningful increase in peripheral BDNF levels with an acute exercise bout requires at least 15 minutes of moderate-intensity exercise (Schmolesky, Webb, & Hansen, 2013; Tang, Chu, Hui, Helmeste, & Law, 2008). Limited research with psychiatric patients confirms that 30 minutes of acute moderate- to vigorous-intensity aerobic exercise confers significant BDNF increases in adults with major depressive disorder (Gustafsson et al., 2009; Laske et al., 2010) or panic disorder (Stroöhle et al., 2010).
Building upon this extant research, we conducted a small pilot study testing whether preceding exposure therapy sessions with an acute moderate-intensity exercise bout confers increases in BDNF levels and greater reductions in symptom severity. We now provide an overview of this study and its findings.
Pilot study
Method
Design
Study participants were enrolled in a 12-session prolonged exposure therapy (PE) program and randomly assigned to either complete an acute bout of exercise prior to each session (PE+E) or no exercise prior to the session (PE-Alone). Assessments of the putative mediator (BDNF) occurred prior to the first session and immediately following the last session, and assessment of outcome (symptom severity) occurred at baseline, prior to each session, and at posttreatment.
Participants
Study participants (N = 9; 8 females, 1 male; MAge = 34, SD =11.82) consisted of community individuals in the Dallas area. All participants met the following entry criteria: principal diagnosis of PTSD based on DSM-IV criteria; between the ages of 18 and 65 years; absence of physical conditions that could be exacerbated by exercise; body mass index [weight (kg)/height (m)2]<, 35; no history of bipolar or psychotic disorders; no diagnosis of an eating disorder or substance abuse or dependence (excluding nicotine) within the past three months; no history of organic brain syndrome, intellectual disability or other cognitive dysfunction that could interfere with the capacity to participate in CBT or to complete safety and efficacy assessments; for females, not currently pregnant and no plans to become pregnant during the course of the trial; no current involvement in an moderate-heavy intensity exercise program; no recent change in psychotropic medications (e.g., stabilized for at least 8 weeks); and, no concurrent trauma focused therapy. The study sample was 88.9% Caucasian (n = 8) and 11.1% (n = 1) other or mixed races, with 77.8% self identified as non-Hispanic (n = 7) and 22.2% as Hispanic (n = 2).
Procedures
Participants were recruited through a variety of local and online sources. Online advertisements contained a link to a secured eligibility prescreen survey. Individuals who appeared potentially eligible based on their survey responses were contacted via phone and given a phone screen to verify survey responses as well as information about the study. If the potential participant was still interested and appeared eligible they were invited to the site for a diagnostic screening interview. The diagnostic screen visit consisted of informed consent procedures and self-report questionnaires, followed by administration of the Structured Clinical Interview for DSM-IV-TR Diagnosis of Axis I Disorders (SCID; First, Spitzer, Gibbon, & Williams, 1996). Participants meeting all eligibility criteria were scheduled for a baseline visit one week prior to the therapy program, which consisted of an interview by an independent evaluator (IE), randomization, and a blood draw offsite (Quest Diagnostics Center). During the baseline independent evaluation, patients completed a clinician-rated assessment (PTSD Symptom Scale-Interview; PSSI) with an IE blind to study condition. Next, participants met with a trained staff member who randomized the individual (using a random number generator) and accompanied them to Quest Diagnostics, a clinical laboratory ~3 miles from the study site where 1 ml of plasma was collected via blood draw, spun, and flash frozen by Quest staff, and stored in their deep freeze until shipment. Patients then completed 12 weeks of treatment (either PE-Alone or PE+E) with an assigned protocol therapist. Immediately after the last treatment session they again met with the IE blind to condition to complete the PSSI and to Quest Diagnostics for the second and final blood draw.
Measures
Brain Derived Neurotrophic Factor (BDNF)
Plasma BDNF was analyzed by RayBiotech, Inc. (Norcross, GA) using a sandwich enzyme-linked immunosorbent assay (ELISA) kit. We sampled plasma BDNF because it correlates with central BDNF levels (Angelucci, Gelfo, De Bartolo, Caltagirone, & Petrosini, 2011; Karege, Schwald, & Cisse, 2002). PTSD Symptom Scale-Interview Version (PSSI). The PSSI (Foa, Riggs, Dancu, & Rothbaum, 1993; Powers, Gillihan, Rosenfield, Jerud, & Foa, 2012) is a psychometrically sound 17-item interview that rates each of the DSM-IV symptom criteria on a 0–3 scale of frequency and severity. The PSSI yields a total score with a possible range from 0 to 51, with higher scores indicating more severe PTSD symptoms.
Exercise intervention
Participants assigned to the PE+E condition completed a 30-minute bout of moderate-intensity treadmill exercise (70% of age-predicted HRmax) immediately prior to each PE session. During these exercise sessions, an exercise training management system was used similar to the method in previous studies (Smits et al., 2008, 2012; Smits, Meuret, Zvolensky, Rosenfield, & Seidel, 2009). The heart rate signal from a Polar transmitter (chest strap) was monitored and the clinician running the exercise session adjusted speed and incline of the treadmill accordingly. The training program consisted of a 5-minute warm-up at a progressively increasing speed until the target heart rate was reached. Participants then trained at that target heart rate for the time consistent with the training progression schedule. A 5-minute cool-down period followed, during which the speed was gradually reduced and participants then stretched.
Prolonged exposure therapy
All participants received PE. PE is a manualized treatment program consisting of 12 weekly treatment sessions that are approximately 90 minutes each (Foa, Hembree, & Rothbaum, 2007). PE includes the following procedures for treatment of PTSD: education about common reactions to trauma; breathing retraining (i.e., teaching the client how to breath in a calm way); prolonged and repeated imaginal exposure to the trauma memories to habituate to and process the traumatic experience; prolonged and repeated in vivo exposure to safe situations the client is avoiding because of trauma-related fear; assignment of homework; and monitoring compliance with homework assignments. Each participant was treated by a clinical psychology doctoral student. Therapist training included a 5-day intensive workshop and 2 closely supervised cases, developed by Dr. Edna Foa and administered for this study by Dr. Mark Powers, who also provided ongoing clinical supervision.
Results
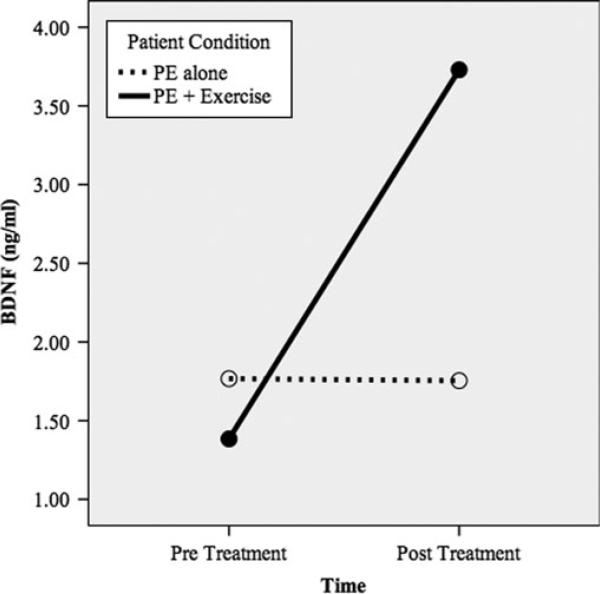
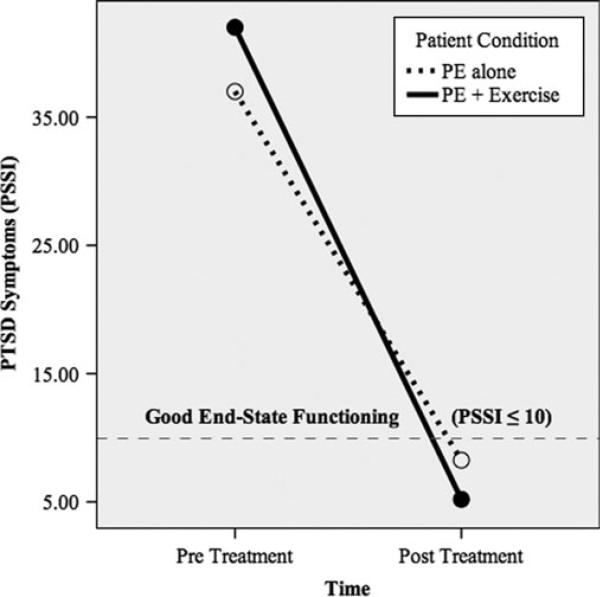
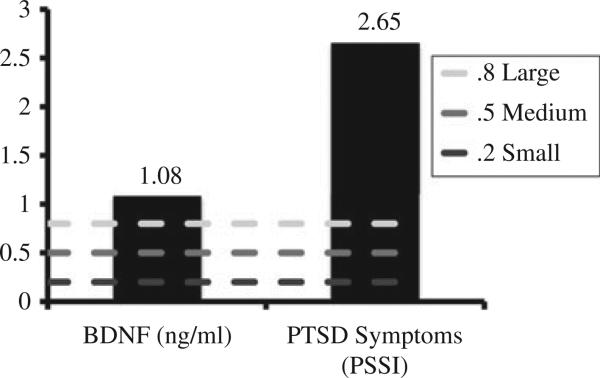
Figure 1 shows the relative increase in BDNF means and Figure 2 shows the relative declines in PTSD symptom means. More specifically, the PE pre- and post-BDNF means were 1.77 and 1.75, respectively, and the PE + E pre- and post-BDNF means were 1.38 and 3.73, respectively. The PE pre- and post-PSSI means were 37.00 and 8.25, respectively, and the PE + E pre- and post-PSSI means were 42.00 and 5.20, respectively. The dashed line in Figure 2 denotes the good end-state functioning cut-off (PSSI ≤10; Powers et al., 2012; Zandberg et al., under review). Because the sample size in this pilot trial was too small to conduct traditional significance tests, we computed between-group effect sizes (i.e., controlled Cohen's d) of pre- to post-treatment changes in the putative mediator and outcome (see Figure 3). Consistent with prediction, PE E increased BDNF to a greater degree than PE-Alone, yielding a large between group effect size (d = 1.08, SE = 0.72). Also consistent with prediction, PE + E outperformed PE-Alone on PTSD symptom reduction, yielding a very large between-group effect size (d = 2.65, SE = 0.92).
Figure 1.
BDNF as a function of treatment condition.
Figure 2.
PTSD symptoms as a function of treatment condition.
Figure 3.
Between group effect sizes (Cohen's d).
Directions for future research
Theoretical accounts and empirical data reviewed in this article support further study of exercise as an augmentation strategy for exposure therapy. As reviewed, exercise may have the potential to enhance outcome in general and aid those individuals for whom a regular exposure therapy regimen falls short in delivering the desired outcomes. More specifically, exercise acts as a cognitive enhancer, manipulating the core mechanisms of exposure therapy (e.g. synaptic plasticity). The development of this clinical application is in the early stages, and in this last section we offer a few suggestions for research that we believe can aid this effort.
Mechanisms of action
Too often treatment development has primarily focused on clinical outcomes and ignored the study of the treatment's mechanisms of action. Without investigating the putative mechanisms, it is challenging to refine an intervention, and null findings regarding clinical outcomes are especially difficult to interpret. In previous sections, we have reviewed the literature supporting the exercise–BDNF–fear extinction relation. We would like to note here that there are a number of challenges to studying this relation. First, while exercise-induced increases in peripheral BDNF concentrations are predominately a result of BDNF release in the brain regions critical to fear extinction, there are several other sources (e.g., smooth muscle, lymphocytes, skeletal muscle, endothelium; Knaepen et al., 2010; Pareja-Galeano et al., 2015) that contribute to peripheral BDNF levels. Exercise may also increase central BDNF without resulting in a change in peripheral BDNF concentrations (Knaepen et al., 2010). Accordingly, research on the exercise–BDNF–fear extinction relation in humans should be complemented by research on this relation in animals, where it may be possible to more firmly establish BDNF release in brain areas critical to fear extinction as the mediator of the effects of exercise for enhancing fear extinction. Second, our study and others examined BDNF levels in plasma. However, as discussed by Pareja-Galeano and colleagues Pareja-Galeano et al. (2015), BDNF sampling and data analytic procedures may strongly influence the magnitude of the effect of exercise on peripheral BDNF concentrations. Specifically, they found that exercise increased BDNF concentrations in serum when samples coagulated at 4°C for 24 hrs as well as in whole blood, but not in serum coagulated at 4°C for 10 minutes or in plasma. Based on their findings and review, they provided specific recommendations for accurately determining peripheral BDNF changes induced by exercise, which include adjusting for hemoconcentration as well as specific clotting temperature and time.
In addition to further studying BDNF as a mechanism of action, it is important to consider alternative and complementary mediators. For example, exercise also impacts other systems implicated in fear extinction, including NMDA receptors (Vasuta et al., 2007), the hypothalamic–pituitary–adrenal axis (De quervain et al., 2011; Roozendaal & McGaugh, 1996; Stranahan, Lee, & Mattson, 2008), and norepinephrine (Dishman, Renner, White-Welkley, Burke, & Bunnell, 2000; Dishman et al., 2000). Future investigations should seek to assess BDNF as well as indices of these other systems.
Target population
The relatively high response rates of exposure therapy suggest that applying augmentation strategies in unselected samples may lead to an underestimation of their effects and utility. If the augmentation effects of exercise are mostly explained by enhanced BDNF release, one may hypothesize that those with lower BDNF levels or expression capacity benefit more from exercise that those who do not exhibit these deficits. Initial support for this hypothesis comes from a small study by Mata and colleagues (Mata, Thompson, & Gotlib, 2010), who demonstrated that adolescent girls who are carriers of the BDNF met-allele (i.e., which is associated with lower BDNF expression) showed a stronger negative relation between exercise and depressed mood relative to girls with the val/val polymorphism. Such findings encourage further testing of this and other putative moderators of exercise augmentation, as this will enable clinicians to apply this clinical strategy more effectively. Similarly, the study we reported on only included participants with PTSD. Future trials will determine whether BDNF-mediated exercise augmentation of exposure therapy is effective in other anxiety disorders. Finally, future studies could measure physical fitness to determine whether it moderates the impact of exercise augmentation.
Treatment parameters
Another important unanswered question regarding the efficacy of exercise for mood and anxiety disorders, as well as exposure therapy augmentation, is how to best prescribe exercise. Basic research supports the application of acute bouts for enhancing BDNF release. It remains unknown, however, what the optimal intensity, duration, frequency, and timing of these bouts are for observing meaningful augmentation of exposure therapy. Here, it is interesting to note that when the aim is to enhance consolidation or retention of fear extinction, it may be important to administer the exercise bout post-session rather than pre-session, because that gives the clinician and patient the opportunity to limit the augmentation to successful sessions (see Smits et al., 2013). Likewise, it is plausible that the augmentation effects of exercise are not limited to acute bouts of aerobic activity. Studying other exercise modalities and training programs, especially in combination with patient preferences and needs, is likely to facilitate knowledge on mechanisms of action, and (thereby) aid the development of the most effective application of exercise as an augmentation strategy for exposure therapy.
Conclusions
Exercise has strong empirical support as an intervention for physical and mental health conditions. In addition to its direct effects on symptoms of anxiety and depression, exercise may be an effective aid to other established treatments for anxiety and mood disorders. In this article, we have reviewed some mechanisms by which exercise may exert these positive augmentation effects and specifically as it relates to exposure therapy. Initial data provide support and some clear direction for this research agenda.
Acknowledgments
Funding
Portions of this research were funded by grants from the National Institute on Drug Addiction: R01 DA027533 & K01 DA035930. Clinical trials registry: ClinicalTrials.gov, NCT01199107, https://clinicaltrials.gov/ct2/show/NCT01199107
Footnotes
Disclosure statement: The authors have declared that no conflict of interest exists.
References
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, Cristofaro AD, Hsiang H.-L.(Liz), Frankland PW. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. doi:10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- American College for Sports Medicine . ACSM's guidelines for exercise testing and prescription. Lippincott Williams & Wilkins; Philadelphia, PA: 2013. [Google Scholar]
- Anderson KC, Insel TR. The promise of extinction research for the prevention and treatment of anxiety disorders. Biological Psychiatry. 2006;60:319–321. doi: 10.1016/j.biopsych.2006.06.022. doi:10.1016/j.biopsych.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci F, Gelfo F, De Bartolo P, Caltagirone C, Petrosini L. BDNF concentrations are decreased in serum and parietal cortex in immunotoxin 192 IgG-Saporin rat model of cholinergic degeneration. Neurochemistry International. 2011;59(1):1–4. doi: 10.1016/j.neuint.2011.04.010. doi:10.1016/j.neuint.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Arancio O, Chao MV. Neurotrophins, synaptic plasticity and dementia. Current Opinion in Neurobiology. 2007;17:325–330. doi: 10.1016/j.conb.2007.03.013. doi:10.1016/j.conb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Arch JJ. Cognitive behavioral therapy and pharmacotherapy for anxiety: treatment preferences and credibility among pregnant and non-pregnant women. Behaviour Research and Therapy. 2014;52:53–60. doi: 10.1016/j.brat.2013.11.003. doi:10.1016/j.brat.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Asmundson GJG, Fetzner MG, DeBoer LB, Powers MB, Otto MW, Smits JAJ. Let's get physical: A contemporary review of the anxiolytic effects of exercise for anxiety and its disorders. Depression & Anxiety (1091–4269) 2013;30:362–373. doi: 10.1002/da.22043. doi:10.1002/da.22043. [DOI] [PubMed] [Google Scholar]
- Barde YA. Neurotrophic factors: An evolutionary perspective. Journal of Neurobiology. 1994;25:1329–1333. doi: 10.1002/neu.480251102. doi:10.1002/neu.480251102. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder. JAMA. 2000;283:2529–2536. doi: 10.1001/jama.283.19.2529. doi:10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LRM, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. doi:10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Berry AC, Rosenfield D, Smits JAJ. Extinction retention predicts improvement in social anxiety symptoms following exposure therapy. Depression and Anxiety. 2009;26:22–27. doi: 10.1002/da.20511. doi:10.1002/da.20511. [DOI] [PubMed] [Google Scholar]
- Blanco C, Heimberg RG, Schneier FR, Fresco DM, Chen H, Turk CL, Liebowitz MR. A placebo-controlled trial of phenelzine, cognitive behavioral group therapy, and their combination for social anxiety disorder. Archives of General Psychiatry. 2010;67:286–295. doi: 10.1001/archgenpsychiatry.2010.11. doi:10.1001/archgenpsychiatry.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Krishnan KR. Effects of exercise training on older patients with major depression. Archives of Internal Medicine. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. doi:10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. doi:10.1016/S0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Progress in Neurobiology. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. doi:10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learning & Memory. 2007;14:268–276. doi: 10.1101/lm.500907. doi:10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Running exercise-induced up-regulation of hippocampal brain-derived neurotrophic factor is CREB-dependent. Hippocampus. 2009;19:962–972. doi: 10.1002/hipo.20579. doi:10.1002/hipo.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nature Neuroscience. 2006;9:870–872. doi: 10.1038/nn1718. doi:10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H-C, Kim J, Kim S, Son YH, Lee N, Jung SH. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO2max performance in healthy college men. Neuroscience Letters. 2012;519:78–83. doi: 10.1016/j.neulet.2012.05.025. doi:10.1016/j.neulet.2012.05.025. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. Journal of Neuroscience. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. doi:10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. doi:10.1016/S0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Daley AJ. Exercise therapy and mental health in clinical populations: Is exercise therapy a worthwhile intervention? Advances in Psychiatric Treatment. 2002;8:262–270. doi:10.1192/apt.8.4.262. [Google Scholar]
- Davidson JRT, Foa EB, Huppert JD, Keefe FJ, Franklin ME, Compton JS, Gadde KM. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Archives of General Psychiatry. 2004;61:1005–1013. doi: 10.1001/archpsyc.61.10.1005. doi:10.1001/archpsyc.61.10.1005. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of d-cycloserine on extinction: Translation from preclinical to clinical work. Biological Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. doi:10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- De quervain DJ-F, Bentz D, Michael T, Bolt OC, Wiederhold BK, Margraf J, Wilhelm FH. Glucocorticoids enhance extinction-based psychotherapy. Proceedings of the National Academy of Sciences. 2011;108:6621–6625. doi: 10.1073/pnas.1018214108. doi:10.1073/pnas.1018214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, Renner KJ, White-Welkley JE, Burke KA, Bunnell BN. Treadmill exercise training augments brain norepinephrine response to familiar and novel stress. Brain Research Bulletin. 2000;52:337–342. doi: 10.1016/s0361-9230(00)00271-9. doi:10.1016/S0361-9230(00)00271-9. [DOI] [PubMed] [Google Scholar]
- Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, Le P, Traynelis SF. Structural determinants of d-cycloserine efficacy at the NR1/NR2C NMDA Receptors. Journal of Neuroscience. 2010;30:2741–2754. doi: 10.1523/JNEUROSCI.5390-09.2010. doi:10.1523/JNEUROSCI.5390-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. doi:10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. The Journal of Neuroscience. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham KL, Dobson-Stone C, Schofield PR, Quirk GJ, Bryant RA. The brain-derived neurotrophic factor Val66Met polymorphism predicts response to exposure therapy in posttraumatic stress disorder. Biological Psychiatry. 2013;73:1059–1063. doi: 10.1016/j.biopsych.2012.10.033. doi:10.1016/j.biopsych.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. doi:10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer R, Gibbon M, Williams JB. Structured clinical interview for DSM-IV Axis I disorders. Biometrics Research, New York State Psychiatric Institute; New York, NY: 1996. [Google Scholar]
- Foa EB, Hembree E, Rothbaum BO. Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences therapist guide. Oxford University Press; Oxford: 2007. [Google Scholar]
- Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, Tu X. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. American Journal of Psychiatry. 2005;162:151–161. doi: 10.1176/appi.ajp.162.1.151. doi:10.1176/appi.ajp.162.1.151. [DOI] [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress. 1993;6:459–473. doi:10.1002/jts.2490060405. [Google Scholar]
- Foa EB, Rothbaum BO, Riggs DS, Murdock TB. Treatment of posttraumatic stress disorder in rape victims: A comparison between cognitive-behavioral procedures and counseling. Journal of Consulting and Clinical Psychology. 1991;59:715–723. doi: 10.1037//0022-006x.59.5.715. doi:10.1037/0022-006X.59.5.715. [DOI] [PubMed] [Google Scholar]
- Goodson J, Helstrom A, Halpern JM, Ferenschak MP, Gillihan SJ, Powers MB. Treatment of posttraumatic stress disorder in U.S. combat veterans: A meta-analytic review. Psychological Reports. 2011;109:573–599. doi: 10.2466/02.09.15.16.PR0.109.5.573-599. doi:10.2466/02.09.15.16.PR0.109.5.573-599. [DOI] [PubMed] [Google Scholar]
- Gustafsson G, Lira CM, Johansson J, Wisén A, Wohlfart B, Ekman R, Westrin A. The acute response of plasma brain-derived neurotrophic factor as a result of exercise in major depressive disorder. Psychiatry Research. 2009;169:244–248. doi: 10.1016/j.psychres.2008.06.030. doi:10.1016/j.psychres.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. The EMBO Journal. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. doi:10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Molecular Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. doi:10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman E, Gamelin F-X, Goekint M, Piscitelli F, Roelands B, Leclair E, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans—possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–851. doi: 10.1016/j.psyneuen.2011.09.017. doi:10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders. The Journal of Clinical Psychiatry. 2008;69:621–632. doi: 10.4088/jcp.v69n0415. doi:10.4088/JCP.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Smits JAJ, Rosenfield D, Simon N, Otto MW, Meuret AE, Pollack MH. d-Cycloserine as an augmentation strategy with cognitive-behavioral therapy for social anxiety disorder. American Journal of Psychiatry. 2013;170:751–758. doi: 10.1176/appi.ajp.2013.12070974. doi:10.1176/appi.ajp.2013.12070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learning & Memory. 2005;12:270–276. doi: 10.1101/lm.91705. doi:10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. doi:10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. doi:10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: Different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. doi:10.1016/S0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neuroscience Letters. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. doi:10.1016/S0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity—exercise-induced response of peripheral brain-derived neurotrophic factor. Sports Medicine. 2010;40:765–801. doi: 10.2165/11534530-000000000-00000. doi:10.2165/11534530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proceedings of the National Academy of Sciences. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. doi:10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. doi:10.1016/S0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Laske C, Banschbach S, Stransky E, Bosch S, Straten G, Machann J, Eschweiler GW. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. The International Journal of Neuropsychopharmacology. 2010;13:595–602. doi: 10.1017/S1461145709991234. doi:10.1017/S1461145709991234. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of d-cycloserine on extinction of conditioned freezing. Behavioral Neuroscience. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. doi:10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. d-Cycloserine and the facilitation of extinction of conditioned fear: Consequences for reinstatement. Behavioral Neuroscience. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. doi:10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. The Journal of Neuroscience. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. doi:10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Linnman C, Zeidan MA, Furtak SC, Pitman RK, Quirk GJ, Milad MR. Resting amygdala and medial prefrontal metabolism predicts functional activation of the fear extinction circuit. American Journal of Psychiatry. 2012;169:415–423. doi: 10.1176/appi.ajp.2011.10121780. doi:10.1176/appi.ajp.2011.10121780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata J, Thompson RJ, Gotlib IH. BDNF genotype moderates the relation between physical activity and depressive symptoms. Health Psychology. 2010;29:130–133. doi: 10.1037/a0017261. doi:10.1037/a0017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Whitton SW, Peckham AD, Welge JA, Otto MW. Patient preference for psychological vs pharmacologic treatment of psychiatric disorders. The Journal of Clinical Psychiatry. 2013;74:595–602. doi: 10.4088/JCP.12r07757. doi:10.4088/JCP.12r07757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soulé J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. Journal of Neuroscience. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. doi:10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Ying S-W, Kanhema T, Croll SD, Bramham CR. Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. The Journal of Neuroscience. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Bravo-Rivera C, Quirk GJ. Infralimbic D2 receptors are necessary for fear extinction and extinction-related tone responses. Biological Psychiatry. 2010;68:1055–1060. doi: 10.1016/j.biopsych.2010.08.014. doi:10.1016/j.biopsych.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. doi:10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Research. 1996;726:49–56. doi:10.1016/0006-8993(96)00273-9. [PubMed] [Google Scholar]
- Nutt DJ, Malizia AL. Structural and functional brain changes in posttraumatic stress disorder. The Journal of Clinical Psychiatry. 2004;65:11–17. [PubMed] [Google Scholar]
- Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Molecular Brain Research. 1998;61:147–153. doi: 10.1016/s0169-328x(98)00222-8. doi:10.1016/S0169-328X(98)00222-8. [DOI] [PubMed] [Google Scholar]
- Otto MW, Hong JJ, Safren SA. Benzodiazepine discontinuation difficulties in panic disorder: Conceptual model and outcome for cognitive-behavior therapy. Current Pharmaceutical Design. 2002;8:75–80. doi: 10.2174/1381612023396726. doi:10.2174/1381612023396726. [DOI] [PubMed] [Google Scholar]
- Pareja-Galeano H, Alis R, Sanchis-Gomar F, Cabo H, Cortell-Ballester J, Gomez-Cabrera MC, Viña J. Methodological considerations to determine the effect of exercise on brain-derived neurotrophic factor levels. Clinical Biochemistry. 2015;48:162–166. doi: 10.1016/j.clinbiochem.2014.11.013. doi:10.1016/j.clinbiochem.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER, Recombinant BDNF. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. doi:10.1016/S0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Conditional reflex: an investigation of the psychological activity of the cerebral cortex. Oxford University Press; New York, NY: 1927. [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. doi:10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. doi:10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Shin LM, Rauch SL. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. The Journal of Clinical Psychiatry. 2001;62:47–54. [PubMed] [Google Scholar]
- Powers MB, Emmelkamp PMG. Virtual reality exposure therapy for anxiety disorders: A meta-analysis. Journal of Anxiety Disorders. 2008;22:561–569. doi: 10.1016/j.janxdis.2007.04.006. doi:10.1016/j.janxdis.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Powers MB, Gillihan SJ, Rosenfield D, Jerud AB, Foa EB. Reliability and validity of the PDS and PSS-I among participants with PTSD and alcohol dependence. Journal of Anxiety Disorders. 2012;26:617–623. doi: 10.1016/j.janxdis.2012.02.013. doi:10.1016/j.janxdis.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clinical Psychology Review. 2010;30:635–641. doi: 10.1016/j.cpr.2010.04.007. doi:10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Powers MB, Sigmarsson SR, Emmelkamp PMG. A meta-analytic review of psychological treatments for social anxiety disorder. International Journal of Cognitive Therapy. 2008;1:94–113. doi:10.1521/ijct.2008.1.2.94. [Google Scholar]
- Powers MB, Smits JAJ, Leyro TM, Otto MW. Handbook of the exposure therapies. Academic Press; New York, NY: 2007. Translational research perspectives on maximizing the effectiveness of exposure therapy. p. 126. [Google Scholar]
- Powers MB, Smits JAJ, Otto MW, Sanders C, Emmelkamp PMG. Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: A randomized placebo controlled trial of yohimbine augmentation. Journal of Anxiety Disorders. 2009;23:350–356. doi: 10.1016/j.janxdis.2009.01.001. doi:10.1016/j.janxdis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Powers MB, Smits JAJ, Telch MJ. Disentangling the effects of safety-behavior utilization and safety-behavior availability during exposure-based treatment: A placebo-controlled trial. Journal of Consulting and Clinical Psychology. 2004;72:448–454. doi: 10.1037/0022-006X.72.3.448. doi:10.1037/0022-006X.72.3.448. [DOI] [PubMed] [Google Scholar]
- Powers MB, Smits JAJ, Whitley D, Bystritsky A, Telch MJ. The effect of attributional processes concerning medication taking on return of fear. Journal of Consulting and Clinical Psychology. 2008;76:478–490. doi: 10.1037/0022-006X.76.3.478. doi:10.1037/0022-006X.76.3.478. [DOI] [PubMed] [Google Scholar]
- Powers MB, Vervliet B, Smits JAJ, Otto MW. Helping exposure succeed: Leaning theory perspectives on treatment resistance and relapse. In: Antony MM, Hofmann SG, Otto MW, editors. Resolving treatment complications in anxiety disorders. Springer; New York, NY: 2009. pp. 31–50. [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. The Journal of Neuroscience. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. The Journal of Neuroscience. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. Journal of Neuroscience. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. doi:10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues H, Figueira, IvanI, Lopes A, Gonçalves R, Mendlowicz MV, Coutinho ESF, Ventura P. Does d-cycloserine enhance exposure therapy for anxiety disorders in humans? A meta-analysis. PloS One. 2014;9:e93519. doi: 10.1371/journal.pone.0093519. doi:10.1371/journal.pone.0093519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. The memory-modulatory effects of glucocorticoids depend on an intact stria terminalis. Brain Research. 1996;709:243–250. doi: 10.1016/0006-8993(95)01305-9. doi:10.1016/0006-8993(95)01305-9. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne P, Berliner L, Russo J, Zatzick D, Pitman RK. Treatment preferences and determinants in victims of sexual and physical assault. The Journal of Nervous and Mental Disease. 2003;191:161–165. doi: 10.1097/01.NMD.0000055343.62310.73. doi:10.1097/01.NMD.0000055343.62310.73. [DOI] [PubMed] [Google Scholar]
- Santi S, Cappello S, Riccio M, Bergami M, Aicardi G, Schenk U, Canossa M. Hippocampal neurons recycle BDNF for activity-dependent secretion and LTP maintenance. The EMBO Journal. 2006;25:4372–4380. doi: 10.1038/sj.emboj.7601303. doi:10.1038/sj.emboj.7601303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. Journal of Neuroscience. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. doi:10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends in Neurosciences. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. doi:10.1016/S0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Woolaway-Bickel K, Trakowski J, Santiago H, Storey J, Koselka M, Cook J. Dismantling cognitive-behavioral treatment for panic disorder: Questioning the utility of breathing retraining. Journal of Consulting and Clinical Psychology. 2000;68:417–424. doi: 10.1037//0022-006x.68.3.417. doi:10.1037/0022-006X.68.3.417. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Webb DL, Hansen RA. The effects of aerobic exercise intensity and duration on levels of brain-derived neurotrophic factor in healthy men. Journal of Sports Science & Medicine. 2013;12:502–511. [PMC free article] [PubMed] [Google Scholar]
- Siette J, Reichelt AC, Westbrook RF. A bout of voluntary running enhances context conditioned fear, its extinction, and its reconsolidation. Learning & Memory. 2014;21:73–81. doi: 10.1101/lm.032557.113. doi:10.1101/lm.032557.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HB, Foa EB, Liebowitz MR, Huppert JD, Cahill S, Maher MJ, Campeas R. Cognitive-behavioral therapy vs risperidone for augmenting serotonin reuptake inhibitors in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:1190–1199. doi: 10.1001/jamapsychiatry.2013.1932. doi:10.1001/jamapsychiatry.2013.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: A meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. doi:10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Berry AC, Rosenfield D, Powers MB, Behar E, Otto MW. Reducing anxiety sensitivity with exercise. Depression & Anxiety 1091–4269. 2008;25:689–699. doi: 10.1002/da.20411. doi:10.1002/da.20411. [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Hofmann SG, Rosenfield D, DeBoer LB, P.T., Simon NM, Pollack MH. d-Cycloserine augmentation of cognitive behavioral group therapy of social anxiety disorder: Prognostic and prescriptive variables. Journal of Consulting and Clinical Psychology. 2013;81:1100–1112. doi: 10.1037/a0034120. doi:10.1037/a0034120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JAJ, Meuret AE, Zvolensky MJ, Rosenfield D, Seidel A. The effects of acute exercise on CO2 challenge reactivity. Journal of Psychiatric Research. 2009;43:446–454. doi: 10.1016/j.jpsychires.2008.05.009. doi:10.1016/j.jpsychires.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Rosenfield D, Davis ML, Julian K, Handelsman PR, Otto MW, Powers MB. Yohimbine enhancement of exposure therapy for social anxiety disorder: A randomized controlled trial. Biological Psychiatry. 2014;75:840–846. doi: 10.1016/j.biopsych.2013.10.008. doi:10.1016/j.biopsych.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Rosenfield D, Otto MW, Marques L, Davis ML, Meuret AE, Hofmann SG. d-Cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. Journal of Psychiatric Research. 2013;47:1455–1461. doi: 10.1016/j.jpsychires.2013.06.020. doi:10.1016/j.jpsychires.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JAJ, Rosenfield D, Otto MW, Powers MB, Hofmann SG, Telch MJ, Tart CD. d-Cycloserine enhancement of fear extinction is specific to successful exposure sessions: Evidence from the treatment of height phobia. Biological Psychiatry. 2013;73:1054–1058. doi: 10.1016/j.biopsych.2012.12.009. doi:10.1016/j.biopsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JAJ, Zvolensky MJ, Rosenfield D, Marcus BH, Church TS, Frierson GM, Briceno NF. The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with elevated anxiety sensitivity: Study protocol for a randomized controlled trial. Trials. 2012;13:207–221. doi: 10.1186/1745-6215-13-207. doi:10.1186/1745-6215-13-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Casey BJ. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. doi:10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Mattson MP. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromolecular Medicine. 2008;10:118–127. doi: 10.1007/s12017-008-8027-0. doi:10.1007/s12017-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ströhle A, Stoy M, Graetz B, Scheel M, Wittmann A, Gallinat J, Hellweg R. Acute exercise ameliorates reduced brain-derived neurotrophic factor in patients with panic disorder. Psychoneuroendocrinology. 2010;35:364–368. doi: 10.1016/j.psyneuen.2009.07.013. doi:10.1016/j.psyneuen.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neuroscience Letters. 2008;431:62–65. doi: 10.1016/j.neulet.2007.11.019. doi:10.1016/j.neulet.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tart CD, Handelsman PR, DeBoer LB, Rosenfield D, Pollack MH, Hofmann SG, Smits JAJ. Augmentation of exposure therapy with post-session administration of d-cycloserine. Journal of Psychiatric Research. 2013;47:168–174. doi: 10.1016/j.jpsychires.2012.09.024. doi:10.1016/j.jpsychires.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Church TS, Carmody TJ, Grannemann BD, Galper DI, Blair SN. Exercise as an augmentation treatment for nonremitted major depressive disorder. The Journal of Clinical Psychiatry. 2011;72:677–684. doi: 10.4088/JCP.10m06743. doi:10.4088/JCP.10m06743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasuta C, Caunt C, James R, Samadi S, Schibuk E, Kannangara T, Christie BR. Effects of exercise on NMDA receptor subunit contributions to bidirectional synaptic plasticity in the mouse dentate gyrus. Hippo-campus. 2007;17:1201–1208. doi: 10.1002/hipo.20349. doi:10.1002/hipo.20349. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gómez-pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. Journal of Neuroscience Research. 2004;76:356–362. doi: 10.1002/jnr.20077. doi:10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu K-T, Davis M. Facilitation of conditioned fear extinction by systemic administration or intraamygdala infusions of d-cycloserine as assessed with fear-potentiated startle in rats. The Journal of Neuroscience. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenfalk J, Olson L, Thorén P. Deprived of habitual running, rats downregulate BDNF and TrkB messages in the brain. Neuroscience Research. 1999;34:125–132. doi: 10.1016/s0168-0102(99)00051-6. doi:10.1016/S0168-0102(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Zandberg LJ, Rosenfield D, McLean CP, Powers MB, Asnaani A, Foa EB. Concurrent treatment for post-traumatic stress disorder and alcohol dependence: Predictors and moderators of outcome. Journal of Consulting and Clinical Psychology. doi: 10.1037/ccp0000052. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]