Abstract
Environmental stimuli that signal food availability hold powerful sway over motivated behavior and promote feeding, in part, by activating the mesolimbic system. These food-predictive cues evoke brief (phasic) changes in nucleus accumbens (NAc) dopamine concentration and in the activity of individual NAc neurons. Phasic fluctuations in mesolimbic signaling have been directly linked to goal-directed behaviors, including behaviors elicited by food-predictive cues. Food-seeking behavior is also strongly influenced by physiological state (i.e. hunger vs. satiety). Ghrelin, a stomach hormone that crosses the blood-brain barrier, is linked to the perception of hunger and drives food intake, including intake potentiated by environmental cues. Notwithstanding, whether ghrelin regulates phasic mesolimbic signaling evoked by food-predictive stimuli is unknown. Here, rats underwent Pavlovian conditioning in which one cue predicted the delivery of rewarding food (CS+) and a second cue predicted nothing (CS−). After training, we measured the effect of ghrelin infused into the lateral ventricle (LV) on sub-second fluctuations in NAc dopamine using fast-scan cyclic voltammetry and individual NAc neuron activity using in vivo electrophysiology in separate groups of rats. LV ghrelin augmented both phasic dopamine and phasic increases in the activity of NAc neurons evoked by the CS+. Importantly, ghrelin did not affect the dopamine nor NAc neuron response to the CS−, suggesting that ghrelin selectively modulated mesolimbic signaling evoked by motivationally significant stimuli. These data demonstrate that ghrelin, a hunger signal linked to physiological state, can regulate cue-evoked mesolimbic signals that underlie food-directed behaviors.
Introduction
The seminal work of Pavlov established that food-predictive stimuli engage physiological processes that prepare the body for the ingestion of calories (Pavlov 1927; Woods 1991; Woods and Ramsay 2000). Stimuli associated with food can also trigger approach and ultimately food consumption, even in sated animals (Weingarten 1983; Lovibond 1983; Roitman et al. 2001). This is especially problematic in modern cultures where humans are bombarded with promotions for unhealthy food (Mink et al. 2010), which can prime eating behavior (Harris et al. 2009). Thus, food cues and the neural circuits they engage are likely key contributors to the recent dramatic rise in overeating and incidence of obesity.
Among other circuits (Petrovich 2013), food-predictive cues activate the mesolimbic system (Tang et al. 2012), including the midbrain dopamine neurons and one of their major targets, the nucleus accumbens (NAc). Pharmacological modulation of the NAc affects cue-elicited responding for food (Wyvell and Berridge 2000; Di Ciano et al. 2001; Yun et al. 2004b; Lex and Hauber 2008; Blaiss and Janak 2009; Corbit and Balleine 2011). Food-paired cues evoke brief (phasic) increases in NAc dopamine concentration (Day et al. 2007; Stuber et al. 2008; Brown et al. 2011) and in the firing rate of NAc neurons (Nicola et al. 2004; Day et al. 2006). These cue-evoked mesolimbic signals have been directly linked to food-directed actions that culminate with food consumption (Roitman et al. 2004; Day et al. 2007; Stuber et al. 2008; Taha et al. 2007; Cacciapaglia et al. 2011; Flagel et al. 2011; McGinty et al. 2013; Roitman and Loriaux 2014; Krause et al. 2010). Taken together, phasic mesolimbic signaling evoked by food-predictive cues strongly contributes to cue-evoked food approach and food intake.
To be adaptive, the neural signals that participate in food-directed behavior should be sensitive to physiological state (i.e. hunger vs. satiety). One candidate that may link physiological state with mesolimbic signaling is the stomach hormone ghrelin; the only known peripheral peptide associated with hunger (Cummings et al. 2004). Ghrelin crosses the blood-brain barrier (Banks et al. 2002), and interacts with a distributed network of ghrelin receptor expressing nuclei (Zigman et al. 2006; Cabral et al. 2013), including the mesolimbic system (Naleid et al. 2005; Abizaid et al. 2006; Skibicka et al. 2013). Ghrelin augments the BOLD signal response to food cues in multiple limbic areas linked to appetitive behaviors (Malik et al. 2008). The ability of food-predictive cues to promote food-directed behavior is similarly regulated by both caloric state and ghrelin signaling (Corbit et al. 2007; Kanoski et al. 2012; Walker et al. 2012). These studies suggest that physiological state in general and ghrelin specifically could regulate neural responsivity to food-predictive cues. However, how ghrelin modulates mesolimbic encoding of food-predictive cues remains uninvestigated. Here, we measured dopamine signaling in the NAc using fast-scan cyclic voltammetry (FSCV) and recorded spiking activity of individual NAc neurons using electrophysiology in separate groups of rats in response to Pavlovian conditioned cues. We hypothesized that central manipulation of ghrelin would potentiate phasic mesolimbic signaling evoked by food-predictive stimuli.
Materials and Methods
Subjects
Male Sprague-Dawley rats (n=23; 350–400 g) were housed individually and maintained on a 12:12-h light-dark cycle (on at 7:00 AM). Laboratory chow (LabDiet 5012; Richmond, IN) and water were provided ad libitum. Animals were treated in accordance with the guidelines put forth by the National Institutes of Health and under the approval of the Animal Care Committee of the University of Illinois at Chicago.
Behavioral Session
A Pavlovian procedure was used in which the delivery a single 45 mg sugar pellet (3.58 kcal/g; BioServ, Frenchtown, NJ) was preceded by a distinct compound stimulus that occurred 3 s earlier (CS+). A second compound stimulus was presented but terminated after 3 s and predicted no pellet delivery (CS−). The two possible cues were a 60 dB white noise or 50 dB tone, which turned off after 1 s, and left or right cue light, which remained lit for 3 s. Cue identities (CS+ vs. CS−) were counterbalanced across rats. Trial types were randomly selected, as was the inter-trial interval (range: 30–90 s) between cue presentations. Rats were trained for 10 days prior to surgery. Training sessions consisted of 20 and 40 trials for voltammetry and electrophysiology experiments, respectively. All training and experimental sessions took place during the light phase in standard operant chambers (Med Associates, Inc.; St. Albans, VT). The same behavioral procedure was used in both voltammetry and electrophysiology experiments. All rats were fed ad libitum throughout the duration of training and testing.
FSCV Surgical Procedures
Rats (n=10) were anesthetized with ketamine hydrochloride (100 mg/kg, i.p.) and xylazine hydrochloride (10 mg/kg, i.p.). All implants were targeted relative to bregma using the rat brain atlas of Paxinos and Watson (2007). A guide cannula (Bioanalytical Systems; West Lafayette, IN) was implanted dorsal to the right NAc core (+1.3 mm AP, +1.5 mm ML, −2.5 mm DV). We targeted the NAc core because of strong evidence linking cue-evoked phasic dopamine signaling with food approach behavior (Day et al. 2007; Stuber et al. 2008; Cacciapaglia et al. 2011). A chlorinated silver reference electrode was placed in left forebrain. Additionally, a 22-gauge, 11 mm infusion cannula (GC313; Plastics One, Roanoke, VA) was implanted in the lateral ventricle (LV; −0.8 mm AP, −2.1 mm ML, −3.7 mm DV, angled 10° away from midline). Stainless steel skull screws and dental cement were used to secure the guide and infusion cannulae and reference electrode to the skull. Once dry, the obdurator was removed from the guide cannula, and a micromanipulator containing a carbon fiber electrode was attached. The electrode was then lowered into the NAc. A bipolar-stimulating electrode (0.20 mm diameter; Plastics One, Roanoke, VA) was positioned dorsal to the VTA (5.2 mm posterior to bregma, +1.0 mm lateral to midline, and 7.0 mm ventral to skull surface) and lowered in 0.2-mm increments until electrically (60 pulses, 60 Hz, 120 μA, 4 ms/phase) evoked dopamine release was detected via the carbon fiber electrode (see below for details). After optimizing evoked dopamine release, the stimulating electrode was cemented in place and the carbon fiber electrode was removed and replaced with the obdurator. After 5–7 days recovery, rats were retrained for two days. Retraining sessions were identical to earlier sessions with the exception that rats were tethered to the recording headstage for habituation. The test session occurred the day after the second re-training session.
Voltammetry recordings
FSCV in awake and behaving rats and analyte identification and quantification have been extensively described previously (Heien et al., 2004-for chemometrics; Cone et al., 2013). Briefly, a micromanipulator containing a glass-insulated carbon fiber (~75 μm; Goodfellow USA, Coraopolis, PA) (recording) electrode was inserted into the NAc guide cannula. The recording electrode was then lowered into NAc and locked into place. After the recording electrode was secured, an injector connected to a 10 μL Hamilton syringe was inserted into the LV cannula and left in place for the duration of the session. This enabled us to make mid-session LV infusions without disrupting the animal. A FSCV headstage (University of Washington EME Shop) was used to tether rats, apply voltage changes and measure resultant current changes. The electrode voltage was held at −0.4 V, relative to the Ag/AgCl reference electrode and ramped in a triangular fashion (−0.4 to +1.3 to −0.4 V; 400 V/s). This voltage ramp was repeated at 10 Hz. To verify that the NAc recording site could capture phasic dopamine release events, electrical stimulation (120 μA, 60 Hz, 24p) was delivered to the VTA/SN. If stimulation failed to evoke phasic dopamine release, the recording electrode was advanced 0.16 mm and the process was repeated.
Once detectable and stable electrically-evoked release was confirmed, electrical stimulations ceased and the experimental session, as described above, began. Another computer controlled behavioral events of the experiment (Med Associates) and sent digital outputs corresponding to each event to the FSCV interface to be time-stamped along with the electrochemical data. Electrochemical data was also synced with time-stamped video that was recorded during the entire session. After 10 CS+ and CS− presentations (mid-session) an infusion pump was activated to deliver an intra-cranial infusion. n-octanoylated ghrelin (1 μg in 1 μL 0.9% saline; American Peptide, Sunnyvale CA) or vehicle was infused at a rate of 2 μL/min through a 28 gauge injector (1 mm projection beyond the infusion cannula). When given peripherally, this dose of ghrelin generates plasma ghrelin concentrations that are below those observed following 24 h fasting (Wren et al. 2001) and we previously showed that LV delivery of 1 μg ghrelin is sufficient to increase the phasic dopamine response to food reward in the NAc and promote intake of both chow and sucrose (Cone et al. 2014). We presented an additional 10 CS+ and CS− trials following LV infusion. After the recording session, electrodes were removed; rats were then disconnected from the headstage and returned to their home cage. Following experiments, all recording electrodes were calibrated in a flow-cell as described previously (Sinkala et al. 2012) to convert detected current to concentration. The average calibration factor for all electrodes used in these experiments was 40.20 +/− 3.3 nM/nA.
Voltammetry data analysis
All rats retrieved and consumed all delivered pellets before and after pharmacological manipulations. Individual CS+ and CS− trials were background subtracted and dopamine concentration during the 5 seconds before to 10 seconds after the event of interest was extracted from voltammetric data using principal component analysis (Heien et al. 2004; Day et al. 2007). We averaged the dopamine concentration during the entire CS+ or CS− (3 second window) for each trial. These values were compared before and after LV infusions (see below).
Electrophysiology Surgical Procedures
Under ketamine (100 mg/kg i.p.) and xylazine (10 mg/kg i.p.) anesthesia, rats (n=13) were implanted with two microwire electrode arrays and an infusion cannula aimed at the LV. The electrode arrays were chronically implanted bilaterally at AP +1.5, ML ±1.1–1.3 mm relative to bregma, and −6.5 mm relative to brain surface using the rat brain atlas of Paxinos and Watson (2007). Each array was organized into two columns of four microwires (50 μm diameter, tip separation 0.25 mm; MicroProbes for Life Science, Gaithersburg, MD). The electrode arrangement and coordinates were chosen to target both NAc core and shell neurons, as cue-evoked activity in both NAc subregions have been linked to food-directed behavior (Taha et al. 2007; McGinty et al. 2013; Roitman and Loriaux 2014). Ground wires from each array were wrapped around a skull screw and implanted at a remote location ~1 mm into the brain. The 22-gauge, 11 mm LV cannula (GC313; Plastics One, Roanoke, VA) was implanted at AP −0.8, ML −2.4, and −3.75 mm relative to skull surface angled 15° away from midline. LV coordinates were adjusted from above to accommodate the left electrode array. Electrode arrays and the cannula were fixed in place with dental acrylic adhered to skull screws. All rats were given a 10-day recovery period before retraining for 2 days prior to recordings. During the two retraining sessions, rats were tethered to a headstage for habituation, but no data were collected.
Electrophysiological recordings
Before the start of each recording session, rats were connected to a flexible recording cable (Plexon, Dallas, TX, USA) attached to a commutator (Crist Instrument Company, Hagerstown, MD, USA). The headstage of each recording cable contained 16 miniature unity-gain field effect transistors. The activity of single neurons was recorded differentially between each active and an inactive (reference) electrode from the permanently implanted microwire arrays. The inactive electrode was examined before the start of the session to verify the absence of neuronal spike activity and served as the differential electrode for other electrodes with cell activity. Online isolation and discrimination of neuronal activity was accomplished using a commercially available neurophysiological system (multichannel acquisition processor; MAP System, Plexon). Another computer controlled behavioral events of the experiment (Med Associates, Inc.) and sent digital outputs corresponding to each event to the MAP system to be time-stamped along with the neural data. Principal component analysis (PCA) of continuously recorded waveforms was performed prior to each session and aided in the separation of multiple neuronal signals from the same electrode. During the recording session, waveforms that matched the templates generated by PCA were collected as the same neuron.
All rats underwent two separate recording sessions with one day in between. In the first session, rats received an LV infusion of either n-octanoylated ghrelin (1 μg in 1 μL 0.9% saline; American Peptide, Sunnyvale CA) or vehicle infused at a rate of 2 μL/min through a 28-gauge injector (1 mm projection beyond the infusion cannula). During the second session, the other infusion was made. Treatments were counterbalanced across days. The LV infusion was delivered after 20 CS+ and CS− presentations (mid-session), and we presented an additional 20 CS+ and CS− trials following infusion.
After the experimental sessions, cell recognition and sorting was finalized using an offline sorter program (Plexon), which assessed neuronal data based on PCA of the waveforms, cell firing characteristics and inter-spike intervals. Data were then exported to Neuroexplorer (Nex Technologies, Madison, AL) and Matlab (Mathworks, Natick, MA, USA) for analyses.
Electrophysiological data analysis
For electrophysiological recordings, we first identified neurons that increased their firing rate in response to the CS+ during first half of the recording session (before LV infusion). We restricted our analyses to these cells because: 1) they represented by far the most prominent type of response (67 out of a total of 88 cue-responsive cells); 2) cue-evoked increases in NAc activity is dopamine dependent (du Hoffmann and Nicola 2014); 3) cue-evoked increases participate in food-seeking actions (McGinty et al. 2013). Individual neurons were assessed for their response to the CS+ or CS− using a Wilcoxon signed-rank test. Briefly, the response window for CS trials (0.1 – 1 s following CS onset) was tested against a baseline period from −10 to −5 s before the onset of the CS. For both the CS+ and CS−, individual cells were classified as responsive cells if the average firing rate during the entire 1 s following CS onset was significantly different than baseline. Importantly, neurons were classified for their response to session events (CS+, CS−) based only on trials that occurred before LV infusions. This approach allowed us to assess how the CS populations were modulated by ghrelin or vehicle without biasing our analysis due to infusion-related changes in response properties.
We further analyzed cue-responsive neurons for the effect of LV infusions on neural responses. First, we assessed changes in cue-evoked activity resulting from LV infusion across all CS+ trials. To control for changes in baseline activity, we normalized each neuron’s firing rate to its’ average activity from −10 to −5 seconds before CS+ onset (the same baseline period used to identify responsive cells). Next, we calculated the average normalized firing rate (100 ms bins) during the 3 s CS+ for all trials before and after infusion. We then used these values to calculate the % change in average normalized firing rate during the CS+ (after vs. before LV infusion) for each neuron in 5 trial blocks (4 blocks total from 20 post-infusion trials).
To assess how discriminable CS+ neural responses were following LV infusion, we applied a Receiver Operating Characteristic (ROC) analysis (Green and Swets, 1966). For each neuron, we calculated the trial-by-trial firing rate during the 3 s CS+ for each neuron in 500 ms bins. To determine how discriminable responses were after the LV infusion compared with before, we computed the area under the ROC curve (auROC) for the CS+ firing rate distribution of each neuron during the final 10 trials of the session (after infusion) to the CS+ firing rate distribution during all 20 pre-infusion trials. A neuron with an auROC value above 0.50 indicated greater CS+ evoked activity following LV infusion, while an auROC value below 0.50 indicated reduced CS+ evoked activity after infusion. We also examined the proportions of neurons that had significant auROC values in positive (auROC>0.5) versus negative (auROC<0.5) directions. As a control, we also performed a second ROC analysis, but instead compared only trials that occurred before infusion. For this analysis, we compared the firing rate distributions during the 20 trials before infusion in 10 trial blocks (i.e firing rate on trials 11–20 vs. trials 1–10). In this case, an auROC value above 0.50 indicates greater activity in later pre-infusion trials, whereas auROC values less than 0.50 indicated greater activity in earlier trials.
Verification of cannula placements and recording sites
All LV cannula placements were verified by injecting Angiotensin II into the LV (50 ng in 2 μL saline; Sigma-Aldrich, St. Louis, MO) and measuring home cage water intake. The Angiotensin II test took place after all recording sessions were complete. Angiotensin II caused drinking (at least 7 mL in 30 min) in all rats. Following completion of experiments, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg). For the voltammetry experiments, a small electrolytic lesion was made at the voltammetry recording site by passing a 10-μA current through a polyimide-insulated stainless steel electrode (A-M Systems, Inc.) 4 times for 4 s each. For electrophysiology experiments, a 100-μA current was passed through each electrode of the microelectrode array for 4 s. In both experiments, current was administered using a lesion-making device (UGO Basile S.R.I.; Comerio, Varse, Italy). Rats were then transcardially perfused with cold 0.01 M phosphate-buffered saline followed by 10% buffered formalin solution (Sigma, Inc). Brains were removed and stored in 10% formalin (voltammetry experiments) or 10% formalin in 3% potassium ferrocyanide (electrophysiology experiments) for 24 h, after which all brains were transferred to 30% sucrose in 0.1 M phosphate buffer (PB) for 7 days. The potassium ferrocyanide reacts with deposited iron from the electrodes to reveal a blue reaction product corresponding with the location of an electrode tip. After post-fixing, 50-μm coronal brain sections were taken using a cryostat and NAc sections were mounted on glass slides. Lesion locations were verified using light microscopy in conjunction with the rat brain atlas of Paxinos and Watson (2007). All voltammetry recordings were confirmed to be from the NAc core. All electrophysiology recordings were confirmed to be in the NAc core or shell.
Statistical Analysis
CS+ selectivity was assessed within animal (voltammetry) or within neuron (electrophysiology). For voltammetry, dopamine concentration evoked by the CS+ and CS− was first separately averaged across trials prior to LV infusions and then statistically compared using a paired t-test. For electrophysiology experiments, we assessed whether the population of neurons that had significant responses to the CS+ responded preferentially to this food-predicted cue. For each neuron, the firing rate during the CS+ and CS− was separately averaged across trials prior to LV infusions and then statistically compared using a paired t-test. The effects of LV infusion were tested using two-way repeated measures ANOVA with Tukey’s HSD post-hoc tests where appropriate. In voltammetry experiments, we compared the average dopamine concentration evoked during the 3 s cue period (CS+ or CS−) with main effects of epoch (pre, post-infusion) X treatment (saline, ghrelin). In electrophysiology experiments, we compared the post-infusion change (%) in the average normalized firing rate within neuron during the 3 s CS period in 5 trial blocks. We tested for main effects of block (1, 2, 3, 4) X treatment (saline, ghrelin). In both voltammetry and electrophysiology experiments, the effects of LV infusion on retrieval latency were analyzed using a two-way repeated measures ANOVA [epoch (pre, post-infusion) and treatment (vehicle, ghrelin)]. Proportions of neurons that responded to task parameters as well as proportions of neurons with significant auROC values were compared between groups using a two-sample Z-test. Distributions of ROC values were compared using a two-sample Komolgorov-Smirnov test. Statistical analyses were performed using GraphPad 5.0 (Prism Inc.), MATLAB (Mathworks), Statistica 10 (Statsoft).
Results
Selective approach behavior evoked by the food-predictive cue (CS+)
We examined whether our rats preferentially approached the food magazine following presentation of the CS+. We determined the percentage of all CS+ and CS− trials in which rats entered the food pellet receptacle during the 3 s CS. All rats entered the food pellet receptacle before the end of the 3 s CS+ on the majority of trials before LV infusions (60.8 ± 5%). In contrast, rats entered the receptacle during the CS− on a very small percentage of trials (13.2 ± 2%). Thus, rats selectively approached the food pellet receptacle following the onset of the CS+ (t(35)=10.35, p<.00001). In addition, prior to LV infusions, there were no statistically significant differences in the latency to retrieve the sugar pellet in rats that would go on to receive saline versus ghrelin (t(17)=0.61, p>05). Next, we sought to determine whether LV infusions affected the probability or speed with which rats would approach the food receptacle following CS+ onset. Following LV infusion, there we no changes in the probability of entering the magazine during the CS+ or CS− compared to pre-infusion values (both saline and ghrelin p>.05). In addition, LV infusions had no effect on latency to enter the receptacle following CS+ onset (both saline and ghrelin p>.05). The above analyses were performed on data pooled from experiments using both recording techniques. However, the results of these analyses were identical when the individual experiments (voltmmetry, electrophysiology) were analyzed separately.
Food-predictive cues evoke greater dopamine release than neutral cues
CS+ and CS− cues elicited differential DA release in the NAc during the block of trials preceding LV infusion. Figure 1a shows robust dopamine release in response to a CS+ during a single representative trial (Figure 1a). While the CS− also evoked dopamine release, the magnitude was smaller in comparison (Figure 1b–c). For all pre-LV infusion trials, we compared the average dopamine concentration during the 1-second following the CS+ and CS− onset within rats. Consistent with a previous report (Day et al. 2007), the CS+ evoked greater dopamine release than the CS− (t(9)=5.67, p<.001; Figure 1d). The results were unaffected when groups were analyzed separately based on the identity of the LV infusion they would later receive (Saline and Ghrelin CS+ vs. CS− both p<.05). All FSCV recordings were made in the NAc core (Figure 1e).
Fig. 1.
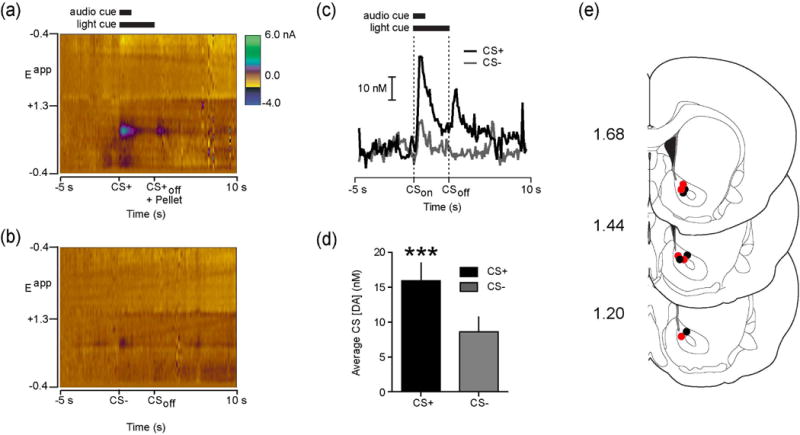
NAc dopamine release preferentially encodes food-predictive stimuli. (a) Representative single trial colorplot depicting current changes (color) as a function of electrode potential (y-axis) in the 5 s before to 10 s after (x-axis) CS+ onset. Solid Bars at top denote duration of light/tone cue. Dopamine [identified by its oxidation (~+0.6 V; green) and reduction (~−0.2 V; light yellow) features] was transiently evoked by the CS+. (b) Representative single trial colorplot from the same animal as in A but for a CS- trial. (c) Dopamine concentration extracted from the electrochemical data in A and B using chemometric analysis (Heien et al., 2004). (d) Average dopamine concentration evoked during the 1 s following CS onset for all pre-infusion CS+ and CS− trials (n=10 rats; 10 CS+ and CS− trials per rat). (e) Coronal brain sections modified from Paxinos and Watson (2007). Numbers at left indicate approximate distance from Bregma. Colored circles indicate approximate voltammetry recording locations from ad-libitum fed rats that received LV saline (n=5; black) or ghrelin (n=5; red). ***p<.001.
Food-predictive cues activate more NAc neurons and more effectively drive NAc activity than neutral cues
We recorded from a total of 199 NAc neurons from 13 rats across the two sessions (ghrelin sessions: 95 cells; vehicle sessions: 104 cells). Consistent with a previous study (Day et al. 2006), we found subpopulations of NAc neurons that increased their firing rate following the onset of the CS+ (n=67 neurons) or CS− (n=24 neurons). There was some overlap between these populations, as 19 of 24 CS− responsive cells also responded the CS+. Figure 2a shows an example perievent raster of a neuron that responded to the CS+ with a significant increase in firing rate. This same neuron had a much weaker response to the CS−. Previous studies have shown that NAc core and shell neurons preferentially encode outcome-predictive cues compared to cues not associated with any consequences (Nicola et al. 2004; Day et al. 2006; Ambroggi et al. 2011; McGinty et al. 2013). Thus, we first investigated whether the population of NAc neurons that we sampled differentially encoded the CS+ and CS−. We examined whether each neuron responded to the CS+ or CS−, relative to baseline, in the trials before pharmacological manipulations. For this analysis, cells could respond to the CS+, CS−, or both. Across all recorded cells, there were significantly more cells that responded to the CS+ (34%; 67/199) than the CS− (12%: 24/199) (Z=5.13, p<.00001). The results were unaffected when the groups were analyzed separately according to subsequent infusion (Ghrelin: Z = 4.49, p<.05; Saline: Z = 2.88, p<.05). Thus, a greater proportion of NAc neurons encoded the CS+ compared to the CS−.
Fig. 2.
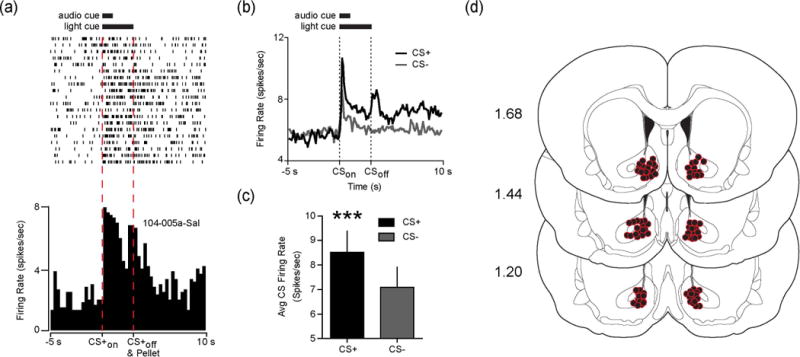
NAc neurons preferentially encode food-predictive stimuli. (a) Raster plot (above) and perievent histogram (below) aligned to the CS+ for a representative NAc neuron that responded to the CS+ with a significant increase in firing rate. (b) Average firing rate during the 5 s before to 10 s after CS onset for all pre-infusion CS+ and CS− trials across the population of neurons that responded to the CS+ (67/199 neurons from the two recording sessions; 20 CS+ and CS− trials). (c) Average firing rate of the 67 neurons plotted in B during the initial 1 s following CS+ and CS− onset. (d) Coronal brain sections modified from Paxinos and Watson (2007). Numbers at left indicate approximate distance from Bregma. Colored circles indicate confirmed electrode placements in the NAc (n=13 rats; 8 wires per hemisphere per rat). Note that some circles are obscured due to overlap. ***p<.001.
We next sought to determine whether the neurons that increased in firing rate in response to the CS+ selectively encoded the food-predictive cue. We compared the average firing rate during the 1 second following the onset of the CS+ and CS− for the 67 neurons that responded to the CS+ before LV infusions. Within this subset of neurons, we found that the firing rate during the first second following cue onset was significantly greater for the CS+ than the CS− (t(64)=7.45, p<.0001; Figure 2b–c). The results were unchanged if we analyzed the entire 3 s CS period (p<.0001). Thus, neurons that responded to the CS+ selectively encoded the motivationally relevant cue, which is consistent with previous reports (Nicola et al. 2004; Day et al. 2006; Ambroggi et al. 2011; McGinty et al. 2013). All neurons included in our analysis were confirmed to come from either the NAc core or shell (Figure 2d).
Central ghrelin selectively increases the phasic dopamine response to food-predictive cues
To determine whether central ghrelin regulates phasic dopamine signaling evoked by a food-predictive cue (CS+), we used ad libitum fed rats with cannula in the LV and infused vehicle (1 μL saline; n=5) or ghrelin (1 μg; n=5) into the LV mid-session. LV saline had no effect on CS+ evoked dopamine release (Figure 3a), whereas LV ghrelin augmented the magnitude of dopamine evoked by the CS+ (Figure 3b). An ANOVA revealed that ghrelin significantly increased the average dopamine concentration evoked by the CS+ compared to baseline values (epoch X treatment interaction (F(1,8)=11.00, p<.05); post hoc: post-ghrelin p<.01 versus pre-infusion values; Figure 3c). To determine whether ghrelin selectively augments the phasic dopamine response to motivationally significant stimuli or merely increases phasic dopamine release in a non-specific manner, we also analyzed the effects of ghrelin on the magnitude of dopamine evoked by CS− presentation. As above, we compared the average dopamine concentration during the 3 s following CS− onset before compared to after LV infusion. Consistent with the notion that ghrelin selectively modulates phasic dopamine release to motivationally significant stimuli, we found that LV ghrelin did not alter the magnitude of dopamine evoked by the CS− compared to LV saline as there was no effect of either epoch (before vs. after; F(1,8)=0.38, p=.55) nor treatment (saline vs. ghrelin; F(1,8)=1.13, p=.31) and no interaction between the main effects (F(1,8)=2.06, p=.18; Figure 3d–f).
Fig. 3.
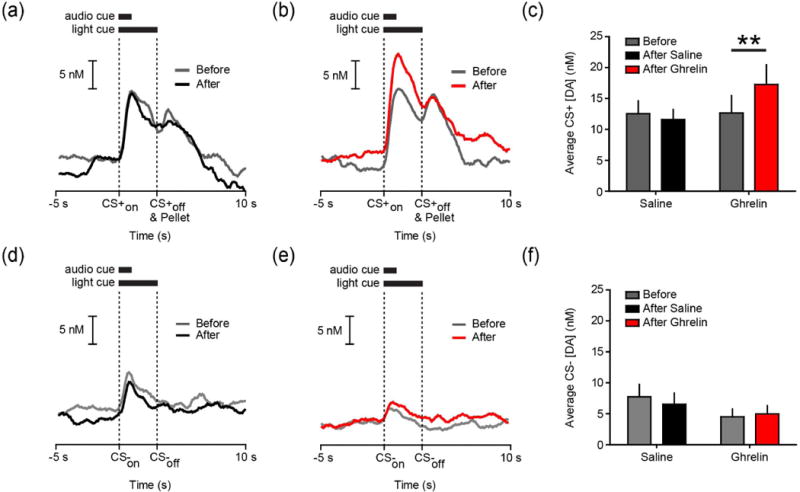
LV ghrelin increases the magnitude of dopamine evoked by the CS+. (a) Average trace of dopamine concentration during the 5 s before to 10 s after CS+ onset before (gray) and after (black) LV infusion of saline (n=5 rats; 10 trials per rat per epoch). (b) Average trace of dopamine concentration during the 5 s before to 10 s after CS+ onset before (gray) and after (red) LV infusion of ghrelin (n=5 rats; 10 trials per rat per epoch). (c) Average dopamine concentration during the 3 s CS+ before and after LV saline or ghrelin. (d) Same conventions as (a) except for the CS−. (e) Same conventions as (b) except for the CS−. (f) Average dopamine concentration during the 3 s CS− before and after LV saline or ghrelin. **p<.01.
Central ghrelin does not affect the baseline firing rate of CS+ responsive NAc neurons
Prior to examining the effects of ghrelin on neural responses to cues, we examined whether LV ghrelin affected baseline activity of NAc neurons that responded to the CS+. Indeed, an increased CS+ response could reflect an increased response to the CS+ specifically, or alternatively, having resulted from an overall increase in spiking activity for a given neuron. To partially determine this, we calculated the average firing rate for each neuron during the baseline epoch we used to identify CS responsive neurons (−10 to −5 s prior to CS+ onset). We found that LV infusion altered the baseline firing rate of the CS+ population (Saline before: 5.55±0.9; Saline after: 4.86±0.8; Ghrelin before: 5.63±0.9; Ghrelin before: 5.81±0.9 spikes/s; epoch X treatment interaction (F(1,65)=19.14, p<.001). Post hoc tests revealed that this was due to a decrement in the baseline firing rate of CS+ responsive neurons following LV saline (before vs. after: p<.05), which was absent following LV ghrelin (before vs. after: p =.22). Thus, LV ghrelin infusion did not affect the baseline activity of NAc neurons that respond to the CS+.
Central ghrelin selectively influences NAc neuron responses to a food-predictive cue
Examining the response of CS+ neurons across all pre- and post-infusion trials revealed that while there was a decrease in the magnitude of the neural response to the cue (0–3s following CS+ onset) in the saline infusion condition (Figure 4a), NAc responses to the CS+ increased following LV ghrelin infusion (Figure 4b). Thus, LV infusion appeared to have bi-directional effects depending on infusion type. To characterize the effects and time course of LV infusions on NAc activity evoked by the CS+, we compared the % change in normalized firing rate during the entire 3 s CS+ (after relative to before LV infusion) in 5 trial blocks. The enhancement of CS+ evoked activity following ghrelin administration depended on time since infusion (block X treatment ANOVA: F(3,65)=4.62, p<.01; Figure 4c). Post-hoc tests revealed that this was driven by later blocks (block 3: p=.07; block 4: p<.001). Within groups, there was no effect of saline across blocks; however, ghrelin significantly increased normalized CS+ activity during the post-infusion period (block 1 vs. block 4: p<.05; Figure 4c).
Fig. 4.
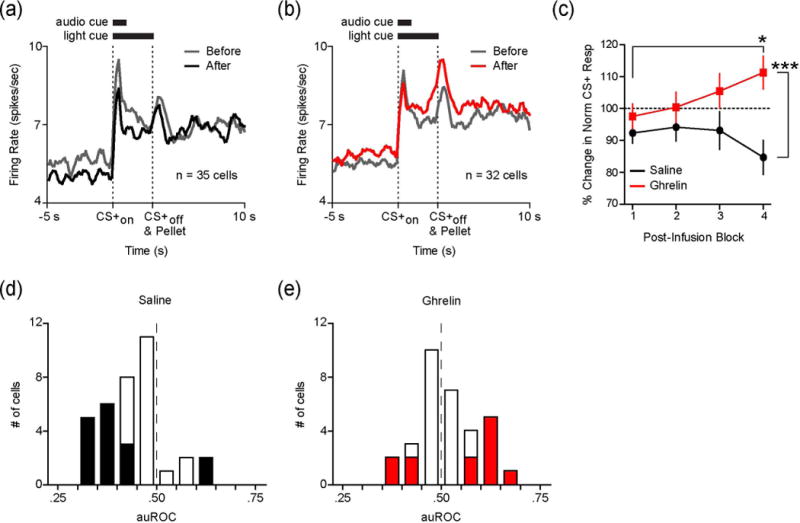
LV ghrelin increases CS+ evoked activity in NAc neurons. (a) Average firing rate of CS+ responsive cells during the 5 s before to 10 s after CS+ onset before (gray) and after (black) LV infusion of saline (n=35 neurons; 20 trials per neuron per block). (b) Average firing rate of CS+ responsive cells during the 5 s before to 10 s after CS+ onset before (gray) and after (red) LV infusion of ghrelin (n=32 neurons; 20 trials per neuron per block). (c) Average % change in normalized firing rate during the 3 s CS+ following LV saline (black) or ghrelin (red) in 5 trial blocks. Pre-infusion firing rate is indicated by the dashed line at 100%. (d) Population density histogram of differences in firing rate (plotted as auROC values) during the 3 s CS+ comparing the second block of 10 trials after LV saline to pre-infusion firing rate (auROC<0.5, after<before; 0.5, no difference; auROC>0.5, after>before). Cells with significant auROC values are filled black (p<.05). E, Population density histogram of auROC values during the 3 s CS+ comparing the second block of 10 trials after LV ghrelin to pre-infusion firing rate (auROC<0.5, after<before; auROC>0.5, after>before). Cells with significant auROC values are filled red (p<.05). *p<.05 for ghrelin block 1 compared to block 4, ***p<.001 for ghrelin block 4 compared to saline block 4.
Across all CS+ responsive neurons, ghrelin significantly augmented responses during the later trials of the recording session. This effect may reflect subtle modulation in CS+ activity across the entire population, or robust effects on a smaller group of CS+ responsive cells. Thus, we next examined how LV ghrelin influences cue-evoked activity in the NAc at the level of individual neurons. To accomplish this, we used ROC analysis to compare the firing rate of each neuron during the CS+ (0–3 s, 500 ms bins) for the last 10 trials after LV infusion compared to all pre-infusion trials. Consistent with the population level normalized firing rate changes, the distributions of auROC values were significantly different across groups. AuROC values following LV ghrelin were rightward shifted (i.e. greater activity in post-infusion trials) compared to LV saline (Komolgorov-Smirnov test; p<.01; Figure 4d–e). At the level of individual neurons, a greater proportion of cells had auROC values significantly above 0.50 following LV ghrelin (8/32) compared to LV saline (2/35; Z=2.21, p<.05). Additionally, a smaller proportion of cells had auROC values that were significantly below 0.50 following LV ghrelin (4/32) versus LV saline (14/35; Z=−2.53, p<.05; Figure 4d–e). The results of the cell-by-cell ROC analysis indicate, compared to LV saline, LV ghrelin increases the CS+ response in a greater proportion of NAc neurons while potentially preventing a decrement in the response of other cells.
Changes in firing rate during the post-infusion period contrast with those observed pre-infusion. We compared firing rates during the CS+ for the first block of 10 trials before infusion compared to second block of 10 pre-infusion trials. The average auROC value across the CS+ population was approximately 0.50 for both groups indicating the neural response to the CS+ did not change during the pre-infusion epoch (Saline auROC: 0.48 ± 0.01; Ghrelin auROC: 0.50 ± 0.01; (t(65)=1.04, p=.30), and there was no difference in the proportion of cells that had significant auROC values across groups (Saline: 6/35; Ghrelin: 6/32). This was true for neurons with significant auROC values above 0.50 (Saline: 2/35 cells; Ghrelin: 3/32), as well as neurons with significant auROC values below 0.50 (Saline: 4/35; Ghrelin: 3/32). These data suggest that the auROC effects reported above are due specifically to LV infusions rather than intra-neuronal response variability during the session.
Only 12% (24/199) of all recorded neurons responded to the CS−. Notwithstanding the small sample size, we examined whether ghrelin altered the response of NAc neurons to motivationally neutral stimuli. As above, we compared the % change in normalized firing rate following LV infusion in 5 trial blocks. LV infusion did not alter the NAc neuron response to the CS−, as we found no effect of either epoch (pre vs. post; F(3,18)=2.29, p=.09) or treatment (saline vs. ghrelin; F(1,18)=0.33, p=.57) and no interaction (F(3=3,18)=0.21, p=.88).
Central ghrelin does not recruit previously unresponsive NAc neurons
Above, we restricted our analyses to the subset of neurons that significantly responded to the CS+ prior to infusions. It is also possible that LV ghrelin recruits previously unresponsive neurons to respond to features of the behavioral paradigm. To examine this possibility, we examined the subpopulation of neurons that did not significantly respond to the CS+ or CS− during the 20 trials before infusion, to determine if any began to respond to the CS+ or CS− after LV infusion. If ghrelin infusion recruited previously unresponsive neurons, we would expect the proportion of cells that become responsive after infusion to differ between saline- and ghrelin-infused groups. While there was a small subpopulation of cells that began to respond to the CS+ after LV infusion, the proportions of these cells were not significantly different between groups (Saline: 6% (6/104); Ghrelin: 8% (8/95), p=.46). There were also no differences in the proportion of neurons that began to respond to the CS− (Saline: 7% (7/104); Ghrelin 7% (7/95); p=.85). Thus, compared to saline, LV administration of ghrelin did not augment the proportions of cells that encoded paradigm parameters after infusion.
Discussion
Food cues pervade our lives. In humans, cues associated with food powerfully enhance both ratings of appetite and food consumption (Ferriday and Brunstrom 2008). Thus, understanding the neurobiological substrates that encode such cues and how those signals are modulated is of great clinical importance especially in light of the worldwide obesity problem. Among other circuits (Petrovich 2013), food cues activate striatal circuitry. Food-cue reactivity is also influenced by physiological state (e.g. hunger, satiety; Corbit et al., 2007; D’Agostino and Small, 2012). The ‘hunger hormone’ ghrelin regulates aspects of both normal and cue-potentiated feeding (Nakazato et al. 2001; Walker et al. 2012; Kanoski et al. 2012) and modulates striatal food-cue responses as measured with fMRI (Malik et al. 2008; Kroemer et al. 2013). We found that ghrelin modulates the response of specific striatal elements to food cues – namely phasic dopamine and NAc neural activity. Critically, ghrelin did not alter encoding of a neutral stimulus, suggesting that ghrelin’s effects were specific to motivationally relevant cues. Given the extensive literature on the role of striatal signaling in cue-evoked behavior, ghrelin’s ability to regulate phasic mesolimbic signals evoked by food cues has broad implications for cue-driven behavior and may represent a novel mechanism by which ghrelin and physiological state augment food seeking.
Ghrelin affects mesolimbic circuitry (for review see (Skibicka and Dickson 2011). In animals not engaged in any feeding-related activity, both systemic and central administration of ghrelin increases dopamine concentration in the NAc. These increases were measured over many minutes using microdialysis (Jerlhag et al. 2007; Jerlhag 2008) – which might result from separate regulatory processes than phasic dopamine signaling studied here (Floresco et al. 2003). We recently showed that central ghrelin potentiates the phasic dopamine response to primary food reward (Cone et al. 2014). These responses shift from food itself to cues that predict food (Figures 1 and 3, but also Schultz, 1998; Day et al., 2007; Stuber et al., 2008). The shift and residual response at time of reward is thought to participate in associative learning (Schultz and Dickinson 2000) and incentive motivation (Berridge and Robinson 1998; Flagel et al. 2011). Indeed, the magnitude of cue-evoked phasic dopamine release has been linked to approach behaviors (Day et al. 2007; Stuber et al. 2008; Wassum et al. 2013). Moreover, genetic manipulations that attenuate phasic dopamine signaling disrupt learning about environmental cues associated with food (Zweifel et al. 2009). Recent work has provided a causal link for dopamine’s role in these phenomena. Selective phasic activation of dopamine neurons promotes learning about cues paired with food reward (Steinberg et al. 2013) and invigorates reward-seeking behaviors (Ilango et al. 2014). Together, these results suggest that ghrelin’s ability to modulate cue-evoked dopamine signaling could promote learning about environmental cues that signal food availability as well as gate the ability of food cues to drive food-seeking behavior.
Food-predictive cues also evoke phasic changes in the activity of NAc neurons (Nicola et al. 2004; Roitman et al. 2005; Day et al. 2006). Cue-evoked NAc activity has been directly linked to food-seeking in a variety of behavioral paradigms (Taha et al. 2007; McGinty et al. 2013; Roitman and Loriaux 2014). Importantly, interfering with normal phasic NAc signaling disrupts food consumption following cue presentation (Krause et al. 2010). Here, we recorded the activity of many individual NAc neurons. Given that medium spiny projection neurons (MSN) comprise approximately 95% of NAc neurons (Gerfen and Surmeier 2011), the effects reported here likely arise primarily from the activity of MSNs. Future studies should seek to parse the specific NAc cell types that are modulated by central ghrelin. That ghrelin augmented phasic NAc spiking suggests that ghrelin could gate the ability of food cues to elicit food-seeking behaviors (McGinty et al. 2013) via regulation of NAc output. In addition to driving approach behaviors, phasic activation of NAc neurons is sufficient to reinforce instrumental behavior (Britt et al. 2012). Coupled with this literature, our data suggest that modulation of NAc output by ghrelin may potentiate neural ensembles involved in food approach and consumption.
Several studies have linked the magnitude of cue-evoked signals in the NAc with aspects of approach behavior (Day et al. 2007; Stuber et al. 2008; McGinty et al. 2013; Roitman and Loriaux 2014). We quantified the proportion of trials associated with rapid approach behavior (entry into the food port during the 3 s CS+) as well as latency to approach the food port following CS+ onset. Mid-session ghrelin infusions did not affect these measures. However, there are considerable differences between our data and the studies mentioned above. Dopamine recordings that support a correlation between cue-evoked release and approach were performed in subjects that were in the early stages of acquiring a Pavlovian association (Day et al. 2007; Stuber et al. 2008). In contrast, our rats were very well trained in this relatively simple task and the rapidity with which our rats tended to enter the food receptacle may have impaired our ability to observe a change in latency following LV ghrelin. Furthermore, work that linked approach behavior and cue-evoked NAc spiking used rigorous video analysis of behavior on a trial-by-trial basis (McGinty et al. 2013) or utilized a task that featured multiple cues and behavioral response options to those cues (Roitman and Loriaux 2014). Thus, previous studies offered greater opportunities to characterize behavioral variability and link that variability with dopamine and NAc output while our study did not set out to measure this specifically. Future work will be aimed at determining whether changes in NAc dynamics by ghrelin directly affect the intensity or likelihood of food-seeking behavior evoked by food cues.
Interestingly, LV manipulations differentially affected both CS+ evoked NAc activity as well as the baseline firing rate of NAc neurons. LV saline led to a reduction in CS+ activity as well as an overall reduction in baseline activity. With respect to the CS+, the decrease in spiking activity following LV saline was not apparent until the 4th post-infusion block, suggesting that the decrement is unlikely to have resulted from the infusion. In contrast, the decrease in baseline firing rate did not occur following LV ghrelin. This suggests that in addition to augmenting CS+- evoked activity, ghrelin may help to sustain baseline levels of NAc activity during bouts of food-seeking. This notion is further supported by the fact that LV ghrelin resulted in a smaller proportion of CS+ responsive cells with significant reductions in CS+-evoked activity following ghrelin compared to LV saline. In sum, ghrelin may both augment and sustain the activity in neuronal populations that encode food-predictive stimuli.
Ghrelin is secreted from the stomach, and plasma levels increase in proportion to the time from the last meal (Cummings et al. 2004). Peripheral ghrelin crosses the blood-brain barrier (Banks et al. 2002) and once in the brain can be found in virtually all ghrelin receptor expressing nuclei (Zigman et al. 2006; Cabral et al. 2013). Thus, ghrelin could modulate cue-evoked mesolimbic activity by acting on a distributed network of structures that innervate the VTA and the NAc. Indeed, ghrelin increases the BOLD response to food cues in multiple brain regions linked to appetitive behavior, including the striatum, amygdala, hippocampus, and orbitofrontal cortex (Malik et al. 2008). In contrast, the change in cue-evoked NAc spiking following LV ghrelin could have resulted from augmented cue-evoked dopamine release. Indeed, coincident changes in NAc cell firing and phasic dopamine release have been observed at the same recording site (Cheer et al. 2007; Cacciapaglia et al. 2011) and pharmacological attentuation of VTA activity reduce cue-evoked NAc spiking (Yun et al. 2004a; Cacciapaglia et al. 2011). Moreover, a recent study demonstrated that blocking either D1- or D2-type dopamine receptors in the NAc attenuates cue-evoked increases in the firing rate of NAc neurons (du Hoffman and Nicola 2014). Taken together, this suggests that NAc dopamine facilitates cue-evoked increases in NAc activity. Phasic dopamine release is thought to preferentially activate D1 receptors (Dreyer et al. 2010; but see Marcott et al. 2014), which alter the excitability of striatal neurons through calcium channels (Surmeier et al. 1995) as well as AMPA and NMDA receptor trafficking (Snyder et al. 2000; Hallett et al. 2006). Our data show that ghrelin increased cue-evoked dopamine within the first 10 post-infusion trials, while the effects on phasic NAc cue responses were not observed until later trials. This is consistent with the time course of dopamine-dependent GluR1 trafficking in the NAc (Mangiavacchi and Wolf 2004; Chao et al. 2002; Sun et al. 2008). These data raise the intriguing possibility that ghrelin’s enhancement of cue-evoked dopamine release renders subsets of NAc neurons more sensitive to food-cue related input.
In summary, ghrelin – a peripheral hormone associated with hunger – augments cue-evoked activity in striatal elements that jointly participate in cue-related learning and cue-evoked behavior. Our results, therefore, implicate physiological state in general, and ghrelin specifically, as strong regulators of mesolimbic activity. In addition, these data highlight novel mechanisms through which ghrelin influences neural signals that are known to contribute to food-directed behaviors that culminate in food consumption.
Acknowledgments
The authors would like to thank Dr. James E. McCutcheon for valuable discussions and Dr. Matthew McMurray for technical assistance. This work was supported by NIH grant R01-DA025634 (MFR) and the National Center for Advancing Translational Sciences grant TL1TR000049 (JJC). JJC also received support from the Chicago Biomedical Consortium and the University of Illinois at Chicago Dean’s Scholar Fellowship.
Abbreviations
- BLA
Basolateral Amygdala
- CS+
Food-predictive cue
- CS−
Neutral cue
- FSCV
Fast-scan cyclic voltammetry
- LV
Lateral Ventricle
- NAc
Nucleus Accumbens
- ROC
Receiver Operating Characteristic
- VTA
Ventral tegmental area
Footnotes
The authors have no conflicts of interest to declare.
References
- Abizaid A, Liu ZWW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggi F, Ghazizadeh A, Nicola SM, Fields HL. Roles of nucleus accumbens core and shell in incentive-cue responding and behavioral inhibition. J Neurosci. 2011;31:6820–6830. doi: 10.1523/JNEUROSCI.6491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Tschöp M, Robinson SM, Heiman ML, Op MT. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Janak PH. The nucleus accumbens core and shell are critical for the expression, but not the consolidation, of Pavlovian conditioned approach. Behav Brain Res. 2009;200:22–32. doi: 10.1016/j.bbr.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci. 2011;34:1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral A, Fernandez G, Perello M. Analysis of brain nuclei accessible to ghrelin present in the cerebrospinal fluid. Neuroscience. 2013;253:406–15. doi: 10.1016/j.neuroscience.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciapaglia F, Wightman RM, Carelli RM. Rapid Dopamine Signaling Differentially Modulates Distinct Microcircuits within the Nucleus Accumbens during Sucrose-Directed Behavior. J Neurosci. 2011;31:13860–13869. doi: 10.1523/JNEUROSCI.1340-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao SZ, Ariano MA, Peterson DA, Wolf ME. D1 dopamine receptor stimulation increases GluR1 surface expression in nucleus accumbens neurons. J Neurochem. 2002;83:704–712. doi: 10.1046/j.1471-4159.2002.01164.x. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Aragona BJ, Heien MLAV, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007;54:237–44. doi: 10.1016/j.neuron.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Ciano P Di, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone JJ, Chartoff EH, Potter DN, Ebner SR, Roitman MF. Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS One. 2013;8:e58251. doi: 10.1371/journal.pone.0058251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 2014;34:4905–13. doi: 10.1523/JNEUROSCI.4404-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The general and outcome-specific forms of pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J Neurosci. 2011;31:11786–11794. doi: 10.1523/JNEUROSCI.2711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH, Balleine BW. General and outcome-specific forms of Pavlovian-instrumental transfer: The effect of shifts in motivational state and inactivation of the ventral tegmental area. Eur J Neurosci. 2007;26:3141–3149. doi: 10.1111/j.1460-9568.2007.05934.x. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab. 2004;287:E297–304. doi: 10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- D’Agostino AE, Small DM. Neuroimaging the interaction of mind and metabolism in humans. Mol Metab. 2012;1:10–20. doi: 10.1016/j.molmet.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–8. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Day JJ, Wheeler RA, Roitman MF, Carelli RM. Nucleus accumbens neurons encode Pavlovian approach behaviors: evidence from an autoshaping paradigm. Eur J Neurosci. 2006;23:1341–1351. doi: 10.1111/j.1460-9568.2006.04654.x. [DOI] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J Neurosci. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriday D, Brunstrom JM. How does food-cue exposure lead to larger meal sizes? Br J Nutr. 2008;100:1325–1332. doi: 10.1017/S0007114508978296. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. Wiley; New York: 1966. [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmision. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW. Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. J Neurosci. 2006;26:4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JL, Bargh JA, Brownell KD. Priming effects of television food advertising on eating behavior. Health Psychol. 2009;28:404–413. doi: 10.1037/a0014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien MLAV, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697–704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- du Hoffman, Nicola Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J Neurosci. 2014;34:14349–14364. doi: 10.1523/JNEUROSCI.3492-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Broker CJ, Wang DV, Ikemoto S. Phasic excitation of ventral tegmental dopamine neurons potentiates the initiation of conditioned approach behavior: parametric and reinforcement-schedule analyses. Front Behav Neurosci. 2014;8:155. doi: 10.3389/fnbeh.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol. 2008;13:358–363. doi: 10.1111/j.1369-1600.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JAJA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry. 2012;73:915–923. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci. 2010;30:4746–4756. doi: 10.1523/JNEUROSCI.0197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer NB, Krebs L, Kobiella A, Grimm O, Pilhatsch M, Bidlingmaier M, Zimmermann US, Smolka MN. Fasting levels of ghrelin covary with the brain response to food pictures. Addict Biol. 2013;18:855–862. doi: 10.1111/j.1369-1600.2012.00489.x. [DOI] [PubMed] [Google Scholar]
- Lex A, Hauber W. Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn Mem. 2008;15:483–491. doi: 10.1101/lm.978708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF. Facilitation of instrumental behavior by a Pavlovian appetitive conditioned stimulus. J Exp Psychol Anim Behav Process. 1983;9:225–247. [PubMed] [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin Modulates Brain Activity in Areas that Control Appetitive Behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- Marcott PF, Mamaligas AA, Ford CP. Phasic dopamine release drives rapid activation of striatal d2-receptors. Neuron. 2014;84:164–76. doi: 10.1016/j.neuron.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty VB, Lardeux S, Taha SA, Kim JJ, Nicola SM. Invigoration of reward seeking by cue and proximity encoding in the nucleus accumbens. Neuron. 2013;78:910–922. doi: 10.1016/j.neuron.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink M, Evans A, Moore CG, Calderon KS, Deger S. Nutritional imbalance endorsed by televised food advertisements. J Am Diet Assoc. 2010;110:904–910. doi: 10.1016/j.jada.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J Neurophysiol. 2004;91:1840–1865. doi: 10.1152/jn.00657.2003. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Oxford University Press; London: 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic; London: 2007. [Google Scholar]
- Petrovich GD. Forebrain networks and the control of feeding by environmental learned cues. Physiol Behav. 2013;121:10–18. doi: 10.1016/j.physbeh.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman JD, Loriaux AL. Nucleus accumbens responses differentiate execution and restraint in reward-directed behavior. J Neurophysiol. 2014;111:350–60. doi: 10.1152/jn.00350.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Dijk G Van, Thiele TE, Bernstein IL. Dopamine mediation of the feeding response to violations of spatial and temporal expectancies. Behav Brain Res. 2001;122:193–199. doi: 10.1016/s0166-4328(01)00189-9. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–71. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–97. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Sinkala E, McCutcheon JE, Schuck MJ, Schmidt E, Roitman MF, Eddington DT. Electrode calibration with a microfluidic flow cell for fast-scan cyclic voltammetry. Lab Chip. 2012;12:2403–2408. doi: 10.1039/c2lc40168a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP, Dickson SL. Ghrelin and food reward: the story of potential underlying substrates. Peptides. 2011;32:2265–73. doi: 10.1016/j.peptides.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Shirazi RH, Rabasa-Papio C, Alvarez-Crespo M, Neuber C, Vogel H, Dickson SL. Divergent circuitry underlying food reward and intake effects of ghrelin: Dopaminergic VTA-accumbens projection mediates ghrelin’s effect on food reward but not food intake. Neuropharmacology. 2013;73C:274–283. doi: 10.1016/j.neuropharm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16:966–73. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, Ridder B de, Bowers MS, Joosten RN, Feenstra MG, Bonci A. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–2. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Taha SA, Nicola SM, Fields HL. Cue-evoked encoding of movement planning and execution in the rat nucleus accumbens. J Physiol. 2007;584:801–818. doi: 10.1113/jphysiol.2007.140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav. 2012;106:317–24. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Walker AK, Ibia IE, Zigman JM. Disruption of cue-potentiated feeding in mice with blocked ghrelin signaling. Physiol Behav. 2012;25:34–43. doi: 10.1016/j.physbeh.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Loewinger GC, Maidment NT. Phasic mesolimbic dopamine release tracks reward seeking during expression of pavlovian-to-instrumental transfer. Biol Psychiatry. 2013;73:747–55. doi: 10.1016/j.biopsych.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- Woods SC. The eating paradox: how we tolerate food. Psychol Rev. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- Woods SC, Ramsay DS. Pavlovian influences over food and drug intake, in. Behav Brain Res. 2000;110:175–182. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2549–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004a;24:2923–33. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA, Nicola SM, Fields HL. Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci. 2004b;20:249–263. doi: 10.1111/j.1460-9568.2004.03476.x. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]


