Abstract
Objective
The requirement for toll-like receptors in lung ischemia reperfusion injury (LIRI) has been demonstrated but not fully characterized. We have previously reported that toll-like receptor-4 is required by alveolar macrophages but not pulmonary endothelial or epithelial cells for the development of LIRI. Additionally, we have demonstrated differential patterns of mitogen-activated protein kinase activation and cytokine release in these cell types during LIRI. We sought to determine whether the differences in their activation responses related to cell specific toll-like receptor activation requirements.
Methods
Primary cultures of alveolar macrophages, pulmonary endothelial, and immortalized epithelial cells were pretreated with toll-like receptor-2 or -4 short interference (si)RNA prior to hypoxia and reoxygenation. Cell lysates and media were analyzed for receptor knockdown, mitogen-activated protein kinase activation, and cytokine production. Rats were pretreated with toll-like receptor-2 or -4 siRNA prior to lung ischemia reperfusion and changes in lung vascular permeability were assessed.
Results
Toll-like receptor-2 knockdown in alveolar macrophages did not affect mitogen-activated protein kinase phosphorylation or cytokine secretion. Conversely, toll-like receptor-2 knockdown in pulmonary endothelial and epithelial cells demonstrated significant reductions in ERK 1/2 activation and cytokine secretion. Toll-like receptor-4, but not toll-like receptor-2, decreased lung permeability index in LIRI.
Conclusions
Differential toll-like receptor signaling and mitogen-activated protein kinase activation in response to LIRI appear to be cell specific. siRNA provides an outstanding tool for examination of the underlying mechanism.
Keywords: Ischemia/reperfusion (Lung), reoxygenation, TLR-2, TLR-4, proinflammatory mediators
INTRODUCTION
Despite improvements in organ preservation and donor and recipient management, lung ischemia reperfusion injury (LIRI) remains a significant clinical problem following lung transplantation.1,2 Up to 25% of lung transplant recipients develop significant reperfusion injury in the hours following implantation.3 LIRI increases the risk of acute rejection, adversely affects early post-operative mortality, and is the strongest acute risk factor for obliterative bronchiolitis.4,5 Characterization of the cell specific inflammatory signaling events in LIRI is a crucial step toward identifying strategies and treatments aimed at ameliorating LIRI and improving outcomes.
Work in vitro has demonstrated that in response to oxidative stress, activation of the mitogen-activated protein kinases (MAPK), p38, and c-Jun N-terminal kinase (JNK), is discretely associated with the alveolar macrophage.6 Additionally, we have demonstrated the importance of early activation of the alveolar macrophage in the development of LIRI.7 Conversely, activation of extracellular signal-regulated kinase (ERK) 1/2, but not p38 or JNK, occurs in pulmonary artery endothelial cells and type 2 pneumocytes (non-alveolar macrophage).6,7 These discrete cell specific patterns of MAPK activation in vitro have also been demonstrated in an in situ model of left LIRI using immunohistochemistry.6 This differential pattern of MAPK activation in alveolar macrophages versus other cell types suggests alternative requirements for initiation of the proinflammatory signaling cascade in response to oxidative stress. While MAPK activation is centrally important in LIRI, it is likely the upstream signaling where differentiated cellular responses are rendered.
Toll-like receptors (TLR) are an evolutionarily conserved family of pattern recognition receptors critical to innate immunity. This ability to sense alarm and initiate inflammation makes them excellent candidates for early signaling in LIRI. TLR-4 is activated by a wide array of signals including stressed, necrotic or injured tissue but it’s response to lipopolysaccharide is most well characterized.11,12 TLR-4 activation in response to lipopolysaccharide stimulation initiates downstream recruitment and activation of specific adaptor proteins, signaling kinases, and transcription factors, ultimately resulting in the transcription and secretion of proinflammatory cytokines and chemokines.9,10 TLR-4 has also been implicated as a key modulator in a number of models of ischemia and reperfusion. TLR-4 deletion or pharmacologic antagonism has been shown to reduce injury severity in cardiac, hepatic, renal, and cerebral models of ischemia reperfusion.13–16 Shimamoto et al recently demonstrated the requirement of TLR-4 for LIRI using knockout mice which supports our work using siRNA for target molecular deletion of TLR-4.17–19 We demonstrated that TLR-4 knockdown in the alveolar macrophage protects against LIRI through decreased MAPK phosphorylation, proinflammatory cytokine production, and a corresponding reduction in permeability index. However, cytokine production remained elevated in pulmonary artery endothelial cells and type 2 pneumocytes in response to oxidative stress despite TLR-4 knockdown.18,19 This lack of response to TLR-4 knockdown in these cell types combined with the differential MAPK phosphorylation in these cell types suggests a different initial site or sequence of inflammatory activation.
TLR-2 exhibits a similar array of ligands including both bacterial products such as lipotheichoic acid as well as host molecules. Furthermore, it is known to have an important role in the initiation of inflammatory responses and the development of ischemic injury in the heart and kidney.20,21 In the kidney, TLR-2 is strongly expressed within glomerular endothelial cells and epithelial cells of Bowman’s capsule. TLR-2 knockout mice demonstrate an attenuated injury response to renal ischemia and reperfusion.20 There are currently no reports examining the role of TLR-2 in the mediation of LIRI. However, TLR-2 are expressed on bronchial epithelial cells, and has been shown to be involved in various organ-specific models of ischemia reperfusion.13–16,22,23 Therefore, TLR-2 is a candidate for the initial site of inflammatory signaling activation in pulmonary artery endothelial cells and type 2 pneumocytes in response to oxidative stress.
The purpose of this study was to determine whether the differential responses to oxidative stress among alveolar macrophages, pulmonary artery endothelial cells, and type 2 pneumocytes relate to cell specific toll-like receptor activation patterns leading to differential downstream signaling responses. Previous in vivo studies demonstrated that intravenous administration of siRNA transfects alveolar macrophages but not pulmonary artery endothelial cells or type 2 pneumocytes, necessitating the use of in vitro studies to investigate the effects of TLR-2 deletion on these non-alveolar macrophage cell types in response to oxidative stress.18 Using siRNA to achieve targeted molecular deletion of TLR-2 and TLR-4 in cultured alveolar macrophages, pulmonary artery endothelial cells and type 2 pneumocytes, we examined the cell-specific requirements for TLR activation in LIRI. Additionally, a well-developed model of LIRI in the rat was utilized for further characterization.
MATERIALS AND METHODS
Short Interference Ribonucleic Acid
The siRNAs used in this study were designed (Invitrogen, Carlsbad, California) and implemented as previously described.18,19 A single siRNA duplex targeting TLR-4 was utilized based on prior work demonstrating its efficacy of uptake and TLR-4 knockdown in the cell types of interest as well as the animal model.18,19 Three unique, non-overlapping siRNA duplexes were designed to target TLR-2. A non-coding, scrambled siRNA sequence was utilized as a control. For our in vitro experiments, 100pmol of oligonucleotide was diluted in 50µl of growth media per well in a 12-well culture plate, incubated for 15 minutes and then combined with either 3µl of lipofectamine (Lipofectamine 2000, Invitrogen Corporation, Carlsbad, CA) diluted in 50µl of growth media for alveolar macrophages or 1µl of lipofectamine diluted in 50µl of growth media for type 2 pneumocytes and pulmonary artery endothelial cells. This siRNA-lipid mixture replaced the cell culture media and cells were incubated with this mixture for a minimum of 6 hours. For our in vivo experiment, 10nM of siRNA was diluted in 250µl of sterile saline and combined with 100µl/kg of lipid vector diluted in 250µl of sterile saline and administered twice, 24 and 48 hours prior to undergoing our IR protocol. The siRNA-lipid complex was incubated for 15 minutes and then rapidly injected into the penile vein. TLR-2 sequence-1 was utilized for TLR-2 knockdown given it yielded the greatest knockdown efficacy. The antisense sequences used for the siRNA were as follows:
| TLR-4 seq2 | 5’-UCU GCU CCA CCA UUG GGC UUA ACC A-3’ |
| TLR-2 seq1 | 5’-GAC AAC AUC AUU GAU UCC AUC GAA A-3’ |
| TLR-2 seq2 | 5’-GGA GCU AGG UAA AGU AGA AAC GGU A-3’ |
| TLR-2 seq3 | 5’-CCU CGG CUC CAA GAG CUG UAU AUU U-3’ |
| Control | Non-coding siRNA, Medium GC content |
Ischemia Reperfusion (IR) Protocol
Pathogen free adult male Long-Evans rats (Harlan Sprague Dawley, Indianapolis, Indiana), weighing 275–300 grams, were used for all in vivo experiments. All procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee at the University of Washington.
A well established, in situ warm IR model was utilized as previously described.18,24 Briefly, rodents were anesthetized with 30mg of intraperitoneal pentobarbital and a 14-gauge angiocatheter was placed under direct vision into the trachea through a midline neck incision. The catheter was secured and the animal was placed on a Harvard Rodent Ventilator (Harvard Apparatus, Boston, Massachusetts). Ventilator settings were standardized with an inspired oxygen content of 60%, 2 cm water of positive end-expiratory pressure, and a respiratory rate of 80. A left thoracotomy was performed and the left lung was mobilized. Fifty units of heparin (500µL) was administered via the penile vein and allowed to circulate for five minutes. A non-crushing clamp was placed across the left lung hilum, occluding the pulmonary artery, vein and mainstem bronchus. After 90 minutes, the clamp was removed and the left lung allowed to reventilate and reperfuse for 4 hours, during which 500µL of warm saline was injected subcutaneously every hour to maintain hydration. At the end of reperfusion, a midline laparotomy and sternotomy were performed. Blood samples were taken from the inferior vena cava and the animals were euthanized. The heart-lung block was excised and the pulmonary circulation flushed with 20 mL of saline. The left lung was then separated from other mediastinal structures.
In Vivo Experimental Groups
Four experimental groups were studied. Negative control animals (non-coding siRNA and no IR protocol) and positive control animals (non-coding siRNA and IR protocol). The two remaining experimental groups received either TLR-2 or TLR-4 siRNA prior to undergoing the IR protocol.
Alveolar Macrophage Harvest
Alveolar macrophages were isolated and cultured as described previously.7, 25 Briefly, the heart lung block was subsequently excised via a median sternotomy. Intratracheal lavage was performed with 50mL of cold phosphate-buffered saline (PBS) as previously described. The effluent was centrifuged at 1,500g for 10 minutes and the cell pellet was resuspended in serum free RPMI (Gibco BRL). Cell counts and viability were assessed by standard trypan blue exclusion methods. Cells were then plated at a density of 1,000,000 cells per well.
Type 2 Pneumocytes
A rat T2P cell line, RLE-6TN (American Tissue Cell Company, Manassas, VA) was maintained in Hams F-12 culture media containing 10% heat inactivated FBS. The T2P were cultured in 12 well plates at a density of 200,000/mL. Cell counts and viability were assessed by a standard trypan blue exclusion technique and media was replenished every 48 hours until confluence was achieved.
Rat Pulmonary Artery Endothelial Cell Culture
The heart-lung block was harvested and bronchoalveolar lavage performed as described above except 21-day-old rats were utilized. Pulmonary artery endothelial cells were isolated, cultured, and purified as described previously by our group.27 Endotracheal lavage was performed in a similar fashion as described above for the alveolar macrophage harvest except PBS containing 0.25 mM Ethylenediaminetetraacetic Acid was used for the lavage fluid to deplete alveolar macrophages. Two millimeter strips of peripheral lung were removed from all lung lobes. Tissue was minced, rinsed in RPMI media, transferred to a dispase (10mg/mL) solution, and then incubated for 60 minutes at 37°C. The cell suspension was homogenized and incubated for 5 minutes at 37°C. Ten milliliters of complete media containing 10% fetal bovine serum was added to terminate the reaction, and the cellular suspension was filtered through a 100µm mesh. The filtrate was centrifuged at 800g for 8 minutes, resuspended in supplemented RPMI media, and plated on gelatin-coated culture dishes. The media was changed every 48 hours during incubation until confluent. Cells were labeled for 8 hours with 4µg/mL of acetylated low-density lipoproteins, which bind selectively to endothelial cells. Cells were separated using flow cytometry (FACSTAR Plus, San Jose, CA) to maintain a pure culture of endothelial cells. All cells used were from passages 4 to 8 to obtain optimal results. Cells were plated in 12-well plates at a concentration of 500,000 cells/mL, and used for experiments when confluent.
Hypoxia and Reoxygenation (HR) Protocol
Hypoxia and reoxygenation was performed as previously described.25,27 Briefly, once type 2 pneumocytes or pulmonary artery endothelial cells reached confluence, or alveolar macrophages have quiesced for at least 6 hours, the cells were placed in a hypoxic chamber (FiO2 0.5%) for 90 minutes, and then placed in a normoxic (FiO2 21%) incubator for up to 4 hours. Negative controls remained in the normoxic incubator for up to 6 hours. After 15 min of reoxygenation, total protein was harvested for p38, JNK activation and after 1 hr of reoxygenation, total protein was harvested for ERK1/2 activation. Following 4 hours of reoxygenation, media was harvested and analyzed for cytokine-induced neutrophil chemoattractant (CINC) secretion by enzyme-linked immunosorbent assay (ELISA).
In Vitro Experimental Groups
To determine sequence-specific knockdown efficacy, alveolar macrophages were pretreated with either TLR-2 sequence-1, sequence-2, or sequence-3 siRNA and compared to positive controls that received pretreatment with non-coding siRNA prior to undergoing HR. Negative control cells (non-coding siRNA and no HR protocol) and positive control (non-coding siRNA and HR protocol). The two remaining experimental groups received either TLR-2 or TLR-4 siRNA prior to undergoing the HR protocol.
Western Blot Analysis
Total protein was extracted from whole left lung homogenates and cultured cells.33,34 40µg of protein was electrophoresed on 10% sodium dodecylsulfate-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Amersham Biosciences, NJ). The membrane was blocked for 1 hour at room temperature with 5% blotto and then incubated with either a phospho-specific ERK 1/2 (Thr202/Tyr204) antibody, a phospho-specific p38 (Thr180/Tyr182) antibody, a phospho-specific JNK (Thr183/Tyr 185) polyclonal antibody, or a TLR-2-specific antibody (Cell Signaling, Beverly, MA) overnight at 4°C. The membrane was then incubated with a horseradish-peroxidase conjugated secondary antibody for 1 hour at room temperature. The blot was developed using the SuperSignal chemiluminescent substrate (Pierce, Rockford, IL), and exposed on Kodak film. Densitometry was performed using Image J (version 1.2; Cybernetics Corp, Silver Springs, Maryland).
Protein analysis for Cytokine Content by ELISA
Following four hours of in vitro reoxygenation, media was collected and processed as previously described.24,25 Media was then analyzed for CINC content via sandwich ELISA as previously described.24,25
Lung Permeability Index
To quantitate lung injury, permeability index of the lung was determined as previously described.24 I125-radiolabeled bovine serum albumin (125I-BSA) (NEN Life Sciences, Boston, MA), titrated to an activity of 800,000 counts per minute (cpm) per dose, was intravenously injected in a final volume of 500 µl of 1% BSA-PBS solution 5 minutes prior to removal of the hilar clamp. Following 4 hours of reperfusion, the radioactivity counts were quantitated in the left lung and in 1mL of inferior vena cava blood using a scintillation counter and calculated as follows:
Permeability index = left lung (cpm)/1mL blood (cpm)
This ratio corrects for any variation in systemic blood levels of radioactivity and provides a reproducible measure of lung microvascular permeability.
Statistical Analysis
All data is presented as mean values + the standard error of the mean. Comparisons among groups were made using one-way analysis of variance. Bonferroni’s method was used to adjust for multiple comparisons. A post-hoc two-tailed Student’s t-test was performed to assess statistical differences between individual groups, which is defined for all tests as a p< 0.05.
RESULTS
Sequence-Specific TLR-2 Knockdown in vitro
Primary cultures of alveolar macrophages pretreated with TLR-2 siRNA sequence-1, sequence-2, and sequence-3 demonstrated a reduction in TLR-2 expression by 87% (p<0.001), 78% (p<0.01), and 84% (p<0.001), respectively, compared to alveolar macrophages pretreated with non-coding siRNA, as assessed by relative optical densitometry of Western blot analysis (Figure 1). TLR-2 sequence-1 was used for all subsequent experiments as it was the most efficacious at achieving TLR-2 knockdown.
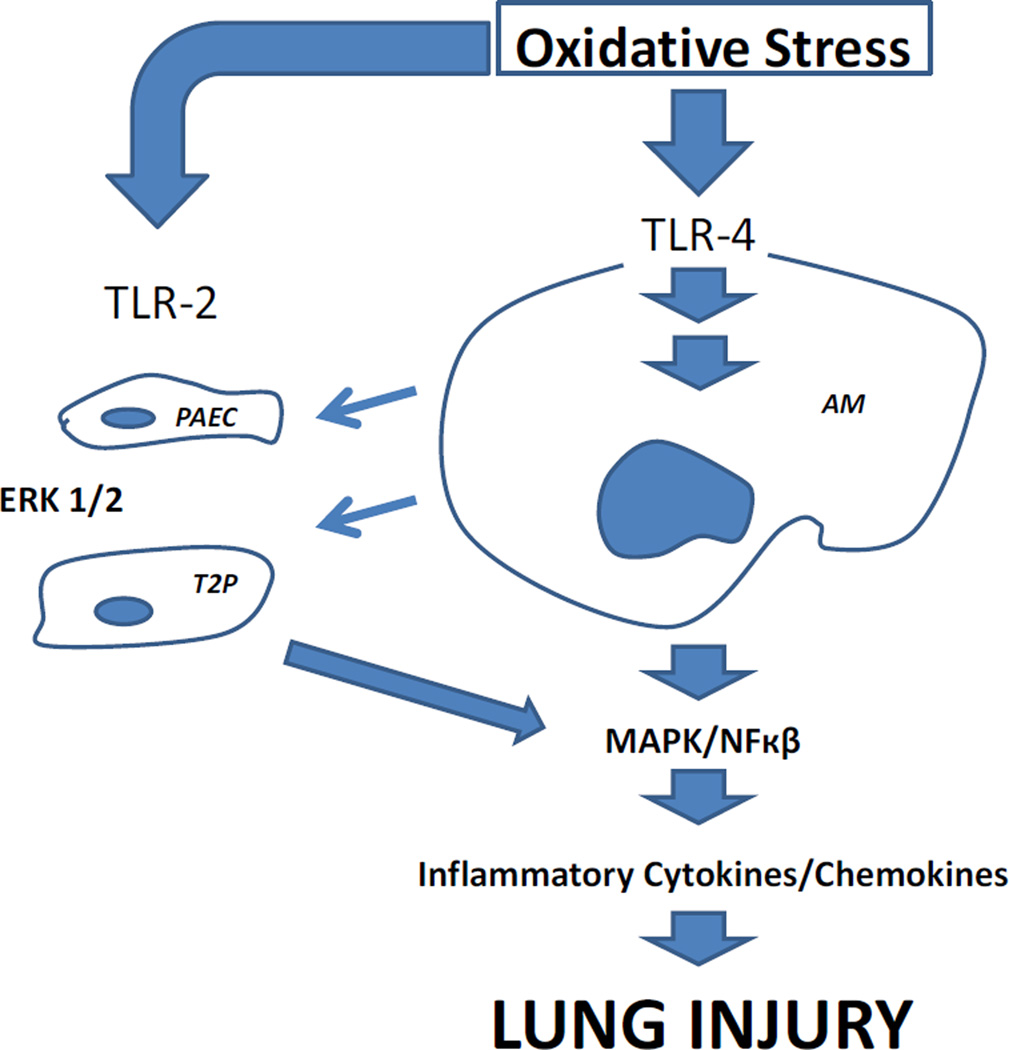
Figure 1. Overview of Toll-like Receptor Activation in Lung Ischemia Reperfusion Injury.
Oxidative stress is initially transduced through TLR-4 activation in alveolar macrophages (AM), triggering an inflammatory signaling cascade leading to the development of lung injury. AM activation products augment and amplify the inflammatory responses via TLR-2 pathways from both pulmonary artery endothelial cells (PAEC) and type 2 pneumocytes (T2P).
Efficacy of TLR-2 Knockdown in vitro
TLR-2 knockdown in siRNA pretreated alveolar macrophages, pulmonary artery endothelial cells and type 2 pneumocytes was confirmed by Western Blot analysis with densitometry. Preteatment with TLR-2 siRNA sequence-1 resulted in a reduction in TLR-2 expression by 86% (p<0.01) in alveolar macrophages, 77% (p<0.01) in pulmonary artery endothelial cells, and 82% (p<0.01) in type 2 pneumocytes compared to non-coding siRNA pretreated cells as assessed by relative optical densitometry of Western blot analysis.
MAPK Phosphorylation Following TLR-2 Knockdown in vitro
MAPK phosphorylation persisted (p>0.02 and p>0.02, respectively for phosphorylated p38 and JNK) in alveolar macrophages pretreated with TLR-2 siRNA compared to non-coding siRNA pretreated control alveolar macrophages (Figure 3). Conversely, ERK phosphorylation was significantly diminished in TLR-2 siRNA pretreated pulmonary artery endothelial cells (p<0.01) and type 2 pneumocytes (p<0.01) compared to non-coding siRNA pretreated control cells (Figure 4). Additionally, there was no alteration (p>0.02) in ERK phosphorylation in pulmonary artery endothelial cells pretreated with TLR-4 siRNA compared to non-coding siRNA pretreated control pulmonary artery endothelial cells (Figure 5).
Figure 3. MAPK Phosphorylation Following TLR-2 Knockdown in Alveolar Macrophages.
Phosphorylation of p38 (pp38) and JNK (pJNK) persisted (p>0.02 and p>0.02, respectively) for in alveolar macrophages pretreated with TLR-2 siRNA compared to non-coding siRNA pretreated control alveolar macrophages after undergoing hypoxia and reoxygenation (HR15) as assessed by relative optical densitometry of Western blot analysis (n=12 wells per group).
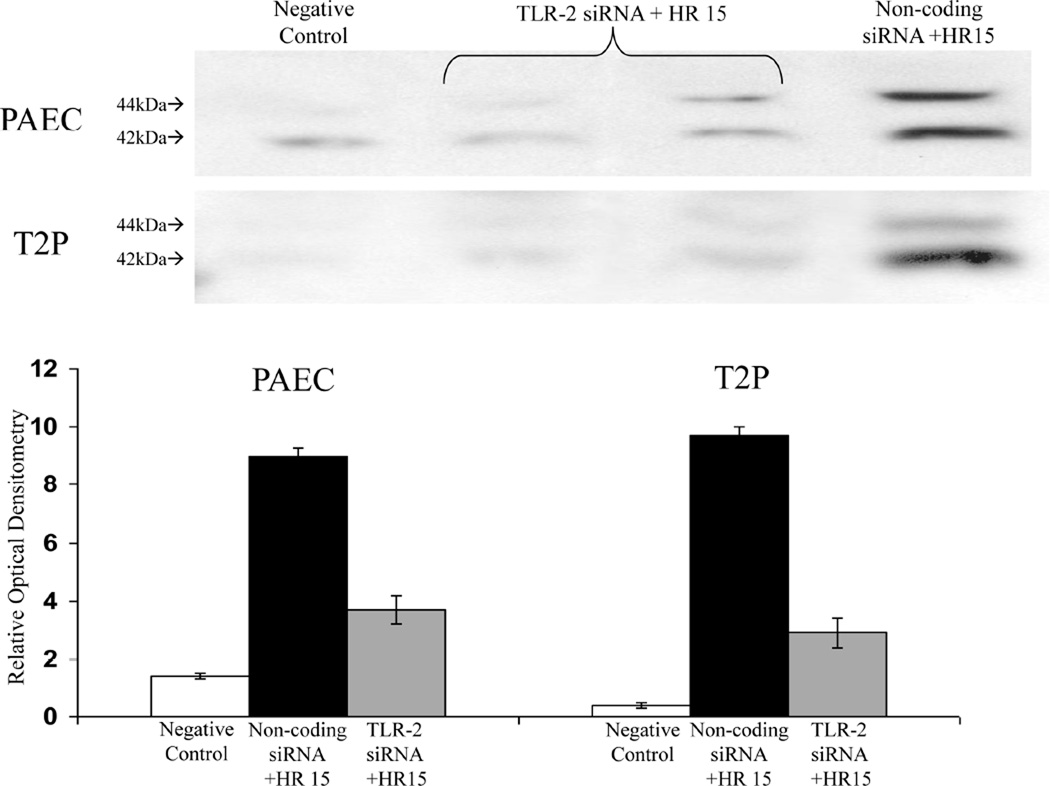
Figure 4. ERK Phosphorylation Following TLR-2 Knockdown in vitro.
Pulmonary artery endothelial cells (PAEC) and type 2 pneumocytes (T2P) pretreated with TLR-2 siRNA demonstrated significant reduction in ERK phosphorylation (p-ERK; p<0.01 and p<0.01, respectively) compared to non-coding siRNA pretreated control cells after undergoing hypoxia and reoxygenation (HR15) as assessed by relative optical densitometry of Western blot analysis (n=12 wells per group).
Figure 5. ERK Phosphorylation Following TLR-2 Knockdown Pulmonary Artery Endothelial Cells.
There was no alteration (p>0.02) in ERK phosphorylation in pulmonary artery endothelial cells pretreated with TLR-4 siRNA compared to non-coding siRNA pretreated control pulmonary artery endothelial cells after undergoing hypoxia and reoxygenation (HR15) as assessed by relative optical densitometry of Western blot analysis (n=12 wells per group).
Cytokine Production following TLR-2 and TLR-4 Knockdown in vitro
TLR-2 knockdown did not alter (p>0.02) secretion of the inflammatory cytokine CINC in alveolar macrophages compared to non-coding siRNA pretreated control alveolar macrophages (Figure 6). Conversely, TLR-2 knockdown decreased CINC secretion in pulmonary artery endothelial cells and type 2 pneumocytes by 76% (p<0.001) and 78% (p<0.001), respectively, compared to non-coding siRNA pretreated control cells. In cultured alveolar macrophages pretreated with TLR-4 siRNA there was a significant reduction in CINC secretion (p<0.0001) compared to non-coding siRNA pretreated control alveolar macrophages. However, TLR-4 knockdown did not affect CINC secretion in pulmonary artery endothelial cells or type 2 pneumocytes (p>0.02 and p>0.02, respectively) compared to non-coding siRNA pretreated control cells.
Figure 6. Cytokine Production following TLR-2 and TLR-4 Knockdown in vitro.
CINC production is not affected (p>0.02 and p>0.02, respectively) by TLR-4 knockdown in pulmonary artery endothelial cells (PAEC) or type 2 pneumocytes (T2P) but is reduced by 76% (p<0.001) and 78% (p<0.001), respectively with TLR-2 knockdown in these cell types compared to non-coding siRNA pretreated control cells after undergoing hypoxia and reoxygenation (HR4). Conversely, CINC production is not affected (p>0.02) by TLR-2 knockdown in alveolar macrophages (AM) but is reduced by 97% (p<0.001) with TLR-4 knockdown in alveolar macrophages compared to non-coding siRNA pretreated control cells after undergoing hypoxia and reoxygenation (HR4).
Permeability Index following TLR-2 and TLR-4 Knockdown in vivo
With TLR-4 siRNA pretreatment, lung vascular permeability was reduced by 85% (p<0.001) compared to non-coding siRNA pretreated control animals after undergoing 90 minutes of ischemia followed by 4 hours of reperfusion. In fact, there was no statistical difference (p>0.02) in the vascular permeability from animals pretreated with TLR-4 siRNA compared to negative control. In contrast, pretreatment with TLR-2 siRNA did not confer protection (p>0.02) from subsequent ischemia reperfusion injury compared to non-coding siRNA pretreated animals after undergoing 90 minutes of ischemia followed by 4 hours of reperfusion (Figure 7).
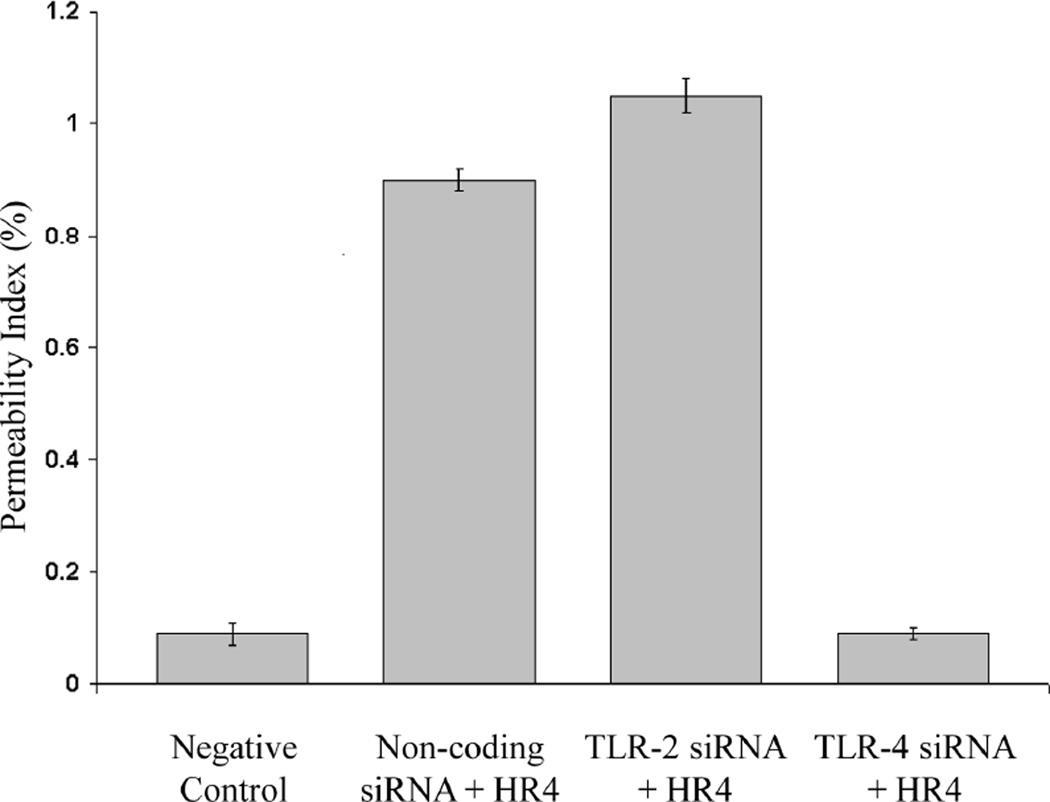
Figure 7. Permeability Index following TLR-2 and TLR-4 Knockdown in vivo.
With TLR-4 siRNA pretreatment, the vascular permeability was reduced by 85% (p<0.001) compared to non-coding siRNA pretreated control animals after undergoing ischemia and reperfusion (IR4). There was no statistical difference (p>0.02) in the vascular permeability between animals pretreated with TLR-4 siRNA prior to undergoing ischemia and reperfusion compared to negative control animals which did not receive pretreatment or undergo ischemia reperfusion. In contrast, pretreatment with TLR-2 siRNA did not confer protection (p>0.02) from subsequent ischemia reperfusion injury compared to non-coding siRNA pretreated animals after undergoing ischemia and reperfusion (n=4 animals per group).
DISCUSSION
These experiments establish differential roles for TLR-2 and TLR-4 dependent proinflammatory signaling in response to oxidative stress in the lung. While we have previously shown that TLR-4 activation in the alveolar macrophage is centrally important, these data suggests a novel role for TLR-2 in the proinflammatory response of pulmonary artery endothelial cells and type 2 pneumocytes to oxidative stress in the lung. Knockdown of TLR-4, but not TLR-2, in the alveolar macrophage in vivo led to marked protection from lung injury. The protection seen with TLR-4 knockdown in vivo was likely secondary diminished TLR-4 mediated MAPK phosphorylation in alveolar macrophages as our prior work has shown.18,19
The lack of protective response to TLR-2 siRNA administration in vivo may be due to several factors. Prior in vivo studies have shown that intravenous administration of TLR-4 siRNA resulted in preferential uptake by alveolar macrophages, not pulmonary artery endothelial cells or type 2 pneumocytes. Therefore, when TLR-2 siRNA is administered intravenously, it may not be taken up by pulmonary artery endothelial cells or type 2 pneumocytes and instead be taken up by the alveolar macrophages. Alternatively, though TLR-2 knockdown diminished ERK phosphorylation and resultant downstream proinflammatory signaling in response to oxidative stress in cultured type 2 cells and PAEC, TLR-4 dependent proinflammatory signaling in the alveolar macrophage may be the predominant signaling response to oxidative stress. As such, protection from LIRI may not be achieved without TLR-4 knockdown in alveolar macrophages.
It is interesting to note that TLR-4 knockdown in the pulmonary artery endothelial cells and type 2 pneumocytes did not diminish inflammatory cytokine production or ERK 1/2 phosphorylation. These results support the hypothesis that TLR-2 regulates the response to oxidative stress in these cell types, while TLR-4 does so in the alveolar macrophage. Further studies evaluating the effects of simultaneously TLR-2 and TLR-4 knockdown in vivo may be warranted to confirm a greater protection form LIRI with simultaneous knockdown and inhibition of both proinflammatory signaling pathways in these relevant lung types. Such studies are limited however by the selective inability to transfect type 2 cells and PAEC in vivo.
Investigating ischemia reperfusion injury requires not only pertinent, appropriate animal models, but cell culture models as well in order to discretely map critical cell signaling events. Acknowledging that in vitro studies fall short of reconstituting complex in vivo interactions, they serve as an important complement to the animal studies in order to characterize critical signaling events. Our warm hilar occlusion model is specific and reliable for the study of mechanisms of injury related to warm atelectatic ischemia and reperfusion inevitable circumstances in clinical lung transplantation. Our model is not only relevant, but addresses an important clinical problem that has a very predictable time of onset and allows for practical pretreatment and intervention unlike most types of acute inflammatory lung injury (i.e. Acute Respiratory Distress Syndrome).
To address the upstream, cell surface receptor initiated signaling events in LIRI in vivo and in vitro, we developed a method of specific molecular knockdown utilizing siRNA targeting TLR-2 and TLR-4. While in vitro transfection of multiple different cell types using siRNA has been widely utilized, in vivo intravenous delivery and uptake in the lung in a model of LIRI has not yet been reported.28,29 This novel approach, while not without limitations, provides a reliable, reproducible and specific means for targeting candidate molecules involved in LIRI. Furthermore, our method of TLR knockdown using siRNA is consistent between in vivo and in vitro models, eliminating a methodological difference between cell culture and animal models. Finally, widely utilized pharmacologic inhibitors have drawbacks with regards to loss of specificity at higher doses, incomplete blockade, and availability. Such limitations are not relevant concerns with our approach.
Concerns regarding the use of siRNA relate broadly to the sometimes overlapping off-target effects, experimental controls, and specificity. To address these concerns, specificity and methodological controls must be employed. In our study, a number of approaches were engaged; preliminary dose response experiments provided the minimal effective dose for TLR knockdown (20nM in vivo) thereby minimizing dose-related off-target effects; toxicity control experiments utilized lipofectamine 2000 vector alone in all experiments, which did not induce injury or an inflammatory response alone; negative controls received non-coding siRNA; and 3 non-overlapping, independent siRNA sequences were tested for TLR-2 and TLR-4, all of which demonstrated effective target protein knockdown and the desired phenotype, increasing our confidence that the observed phenotype can be attributed to target gene knockdown. Additionally, Stealth siRNA (Invitrogen) are designed to ensure that only the antisense strand of the siRNA duplex is processed by the endogenous RNAi machinery, limiting the potential off-target effects from the sense strand. Finally, we have demonstrated effective target protein knockdown in the context of the above controls.
Though we have previously demonstrated that MAPK activation and inflammatory signaling in the alveolar macrophage in response to oxidative stress is TLR-4 dependent, a similar requirement for TLR-2 activation in the alveolar macrophage does not appear to exist. Moreover, TLR-2 appears to regulate pulmonary artery endothelial cell and type 2 pneumocyte responses to hypoxia and reoxygenation while a similar role for TLR-4 was not demonstrated. Thus we have identified specific cellular sites of TLR-2 and TLR-4 activation and the downstream effects on signaling. Our novel in vivo and in vitro use of siRNA targeting the lung or resident pulmonary cells provide us with an effective tool for studying the mechanisms, potential ligands and relevant adaptor proteins responsible for TLR signaling.
These studies are a fundamental step toward identifying potential treatments aimed at improving clinical outcomes following lung transplantation. Therapies aimed at blocking a single inflammatory cytokine or signaling molecule such as the MAPK have failed in both treatments and prophylaxis of LIRI. The current study provides evidence that TLR signaling pathways play a significant role in the development of LIRI and intervention at the early stages of these pathways pose potentially attractive novel targets for therapeutic strategies. Additional studies will be required to assess the efficacy of TLR targeted therapies in LIRI prior to moving to clinical trials.
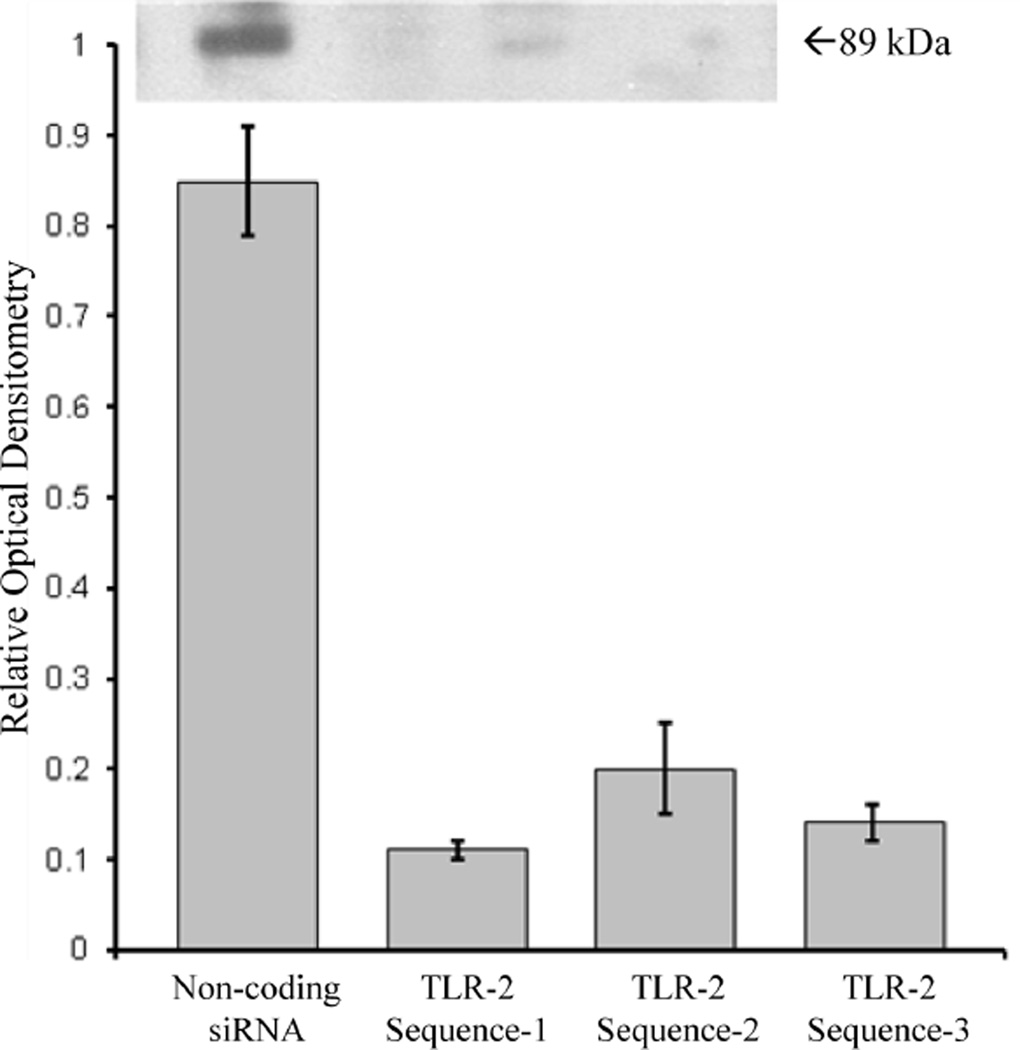
Figure 2. Sequence-Specific TLR-2 Knockdown in vitro.
Primary cultures of alveolar macrophages pretreated with TLR-2 siRNA sequence-1, sequence-2, and sequence-3 demonstrated a reduction in TLR-2 expression by 87% (p<0.001), 78% (p<0.01), and 84% (p<0.001), respectively, compared to alveolar macrophages pretreated with non-coding siRNA, as assessed by relative optical densitometry of Western blot analysis (n=12 wells per group).
Acknowledgments
Sources of Funding: NIH RO1 Role of TLR-4 in Lung Reperfusion Injury
Grant Support: The authors are grateful for research funding from the National Institutes of Health, Bethesda, MD grant R01HL093097. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Patrick Phelan drafted and revised the manuscript, co-supervised the study, analyzed and interpreted the data.
- Heather E Merry revised the manuscript, co-supervised the study, analyzed and interpreted the data.
- Billanna Hwang revised the manuscript, interpreted the data.
- Michael S Mulligan designed and supervised the study, and revised the manuscript.
Patrick Phelan, No conflicts of interest.
Heather E Merry, No conflicts of interest.
Billanna Hwang, No conflicts of interest.
Michael S Mulligan, No conflicts of interest.
REFERENCES
- 1.Angel LF, Levine DJ, Restrepo MI, Johnson S, Sako E, Carpenter A, et al. Impact of a lung transplantation donor-management protocol on lung donation and recipient outcomes. Am J Respir Crit Care Med. 2006;174(6):710–716. doi: 10.1164/rccm.200603-432OC. [DOI] [PubMed] [Google Scholar]
- 2.Pierre AF, Keshavjee S. Lung transplantation: donor and recipient critical care aspects. Curr Opin Crit Care. 2005;11(4):339–344. doi: 10.1097/01.ccx.0000170507.55443.90. [DOI] [PubMed] [Google Scholar]
- 3.King RC, Binns OA, Rodriguez F, Kanithanon RC, Daniel TM, Spotnitz WD, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg. 2000;69:1681–1685. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 4.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73:1041–1048. doi: 10.1016/s0003-4975(01)03606-2. [DOI] [PubMed] [Google Scholar]
- 5.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 6.Wolf PS, Merry HE, Farivar AS, McCourtie AS, Mulligan MS. Stress-activated protein kinase inhibition to ameliorate lung ischemia reperfusion injury. J Thorac Cardiovasc Surg. 2008;135:656–665. doi: 10.1016/j.jtcvs.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Naidu BV, Krishnadasan B, Farivar AS, Woolley SM, Thomas R, Van Rooijen N, et al. Early activation of the alveolar macrophage is critical to the development of lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2003;126:200–207. doi: 10.1016/s0022-5223(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 8.Akira S, Kawai T. TLR signaling. Seminars in Immunology. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Takeda K, Akira A. TLR signaling pathways. Semin Immunol. 2004 Feb;16(1):3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Toll-IL-1 receptor domain-containing adaptor protein is critical for early lung immune responses against Escherichia coli lipopolysaccharide and viable Escherichia coli. J Immunol. 2005 Dec 1;175(11):7484–7495. doi: 10.4049/jimmunol.175.11.7484. [DOI] [PubMed] [Google Scholar]
- 11.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005 Nov;11(11):1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 12.Oppenheim JJ, De Y. Alarmins: chemotactic activators f immune responses. Curr Opinion in Immunology. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, Yang CW. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation. 2005 May 27;79(10):1370–1377. doi: 10.1097/01.tp.0000158355.83327.62. [DOI] [PubMed] [Google Scholar]
- 14.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, Pohlman TH, Shimpo H, Verrier ED. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006 Jul 4;114(1 Suppl):I270–I274. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 15.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, Yada M, Pohlman TH, Verrier ED. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg. 2004 Aug;128(2):170–179. doi: 10.1016/j.jtcvs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, Geller DA, Billiar TR. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005 Dec 1;175(11):7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 17.Shimamoto A, Pohlman TH, Shomura S, Tarukawa T, Takao M, Shimpo H. Toll-like receptor 4 mediates lung ischemia–reperfusion injury. Ann. Thorac. Surg. 2006;82:2017–2023. doi: 10.1016/j.athoracsur.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 18.Merry HE, FitzSullivan E, Wolf PS, Keech JC, McCourtie AS, Mulligan MS. The Role of Innate Immunity in the Development of Lung Ischemia Reperfusion Injury. Submitted to American Journal of Transplantation for consideration for publication 4/2010. [Google Scholar]
- 19.Merry HE, FitzSullivan E, Keech JC, Wolf PS, Mulligan MS. Alveolar Macrophage Proinflammatory Response in Oxidative Stress is Toll-Like Receptor 4 Dependent. Submitted to Annals of Thoracic Surgery for consideration for publication 4/2010. [Google Scholar]
- 20.Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, Mackman N, McKay DB. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-depedent and independent pathways. J of Immunology. 2007;178:6252–6258. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 21.Favre J, Musette P, Douin-Echinard V, Laude K, Henry JP, Arnal JF, Thuillez C, Richard V. Toll-Like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2007;27:1064–1071. doi: 10.1161/ATVBAHA.107.140723. [DOI] [PubMed] [Google Scholar]
- 22.Bachar O, Adner M, Uddman R, Cardell LO. Toll-like receptor stimulation induces airway hyper-responsiveness to bradykinin, and effect mediated by JNK and NF-kappa B signaling pathways. Eur J Immunol. 2004;34:1196–1207. doi: 10.1002/eji.200324569. [DOI] [PubMed] [Google Scholar]
- 23.Droemann D, Goldmann T, Branscheid D, Clark R, Dalhoff K, Zabel P, Vollmer E. Toll-like receptor 2 is expressed by alveolar epithelial cells type II and macrophages in the human lung. Histochem Cell Biol. 2003;119:103–108. doi: 10.1007/s00418-003-0497-4. [DOI] [PubMed] [Google Scholar]
- 24.Krishnadasan B, Naidu BV, Byrne K, Fraga C, Verrier ED, Mulligan MS. The role of proinflammatory cytokines in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2003 Feb;125(2):261–272. doi: 10.1067/mtc.2003.16. [DOI] [PubMed] [Google Scholar]
- 25.Naidu BV, Krishnadasan B, Byrne K, Farr AL, Rosengart M, Verrier ED, Mulligan MS. Regulation of chemokine expression by cyclosporine A in alveolar macrophages exposed to hypoxia and reoxygenation. Ann Thorac Surg. 2002 Sep;74(3):899–905. doi: 10.1016/s0003-4975(02)03746-3. discussion 905. [DOI] [PubMed] [Google Scholar]
- 26.Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol. 1990 258 Apr;(4 Pt 1):L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- 27.Naidu BV, Farivar AS, Woolley SM, Byrne K, Mulligan MS. Chemokine response of pulmonary artery endothelial cells to hypoxia and reoxygenation. J Surg Res. 2003 Oct;114(2):163–171. doi: 10.1016/s0022-4804(03)00330-5. [DOI] [PubMed] [Google Scholar]
- 28.Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 29.McManus MT, et al. Small interfering RNA-mediated gene silencing in T lymphocytes. J. Immunol. 2002;169:5754–5760. doi: 10.4049/jimmunol.169.10.5754. [DOI] [PubMed] [Google Scholar]
- 30.Fedorov Y, et al. Off-target effects by siRNA can induce toxic phenotype. RNA. 2006;12:1188–1196. doi: 10.1261/rna.28106. [DOI] [PMC free article] [PubMed] [Google Scholar]