Abstract
Anti-phospholipid antibodies (APLA) represent a diagnostic criterion of systemic lupus erythematosus (SLE) and cause morbidity, termed anti-phospholipid syndrome (APS). Activation of the mechanistic target of rapamycin (mTOR) has been recently associated with APS. mTOR is a sensor of oxidative stress. Therefore, we examined mitochondrial mass, superoxide production, mTOR and FoxP3 expression in 72 SLE patients, twelve of whom also had APS, and 54 healthy controls by flow cytometry. Mitochondrial mass was increased in CD4−CD8− double-negative (DN) T cells of SLE patients with APS (2.7-fold) in comparison to those without APS (1.7-fold; p=0.014). Superoxide production was increased in all lymphocyte subsets of APS patients. FoxP3+ cells were depleted within CD4+CD25+ Tregs in patients with APS (28.4%) relative to those without APS (46.3%, p=0.008). mTOR activity was similar between SLE patients with and without APS. Thus, oxidative stress and Treg depletion rather than mTOR activation underlie APS in patients with SLE.
Keywords: Systemic lupus erythematosus, Anti-phospholipid syndrome, Oxidative stress, Mechanistic target of rapamycin, mTOR, Treg
1. INTRODUCTION
The pathogenesis of systemic lupus erythematosus (SLE) is incompletely understood which limits the development of effective treatments (1). As recently recognized, SLE patients exhibit activation of the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) in T cells that can be reversed with clinical efficacy upon treatment with rapamycin (2–5). The activation of mTORC1 has been attributed to oxidative stress both outside (6;7) and within the immune system (3;5;8). Oxidative stress originates in mitochondria of lupus T cells (8–10) which accumulate both in patients and mice with SLE (11). In turn, oxidative stress is associated with depletion of the intracellular antioxidant, glutathione (9). Importantly, the reversal of glutathione depletion with N-acetylcysteine (NAC) also blocked mTORC1 with promising therapeutic efficacy in a randomized double-blind placebo controlled pilot study of SLE patients (5). mTORC1 has been also identified as a metabolic sensor of oxidative stress in SLE (3;6;8) and principal driver of pro-inflammatory expansion of CD4−CD8− double-negative (DN) T cells (1;4;12–14). These findings are in line with recent discoveries that the metabolic pathways which control mTOR activation are key regulators of T-cell lineage specification both under physiological conditions and in autoimmunity (15–18).
Anti-phospholipid antibodies (APLA) represent a component of the diagnostic criteria for SLE (19–21). They also contribute to significant pathologies, termed anti-phospholipid syndrome (APS), both in patients with or without SLE (22). Oxidative stress has been recently implicated in driving the immunogenicity of phospholipid antigens (23–25). In a retrospective study of 10 patients with APS nephropathy, who required renal transplantation and received treatment with rapamycin, also known as sirolimus, 7 of 10 patients (70%) had a functioning allograft 144 months after transplantation versus 3 of 27 patients treated without rapamycin (11%) (26). Efficacy of rapamycin was attributed to mTOR activation in renal vascular endothelial cells. Interestingly, the majority of APS patients in this study also had SLE (16/28 = 57%) (26). However, it has not been disclosed how many of the 7 patients who actually benefited from rapamycin (26) satisfied the diagnosis of SLE (19;20) or APS (22). mTOR activity has not been measured within the immune system itself (26), which is considered to be the principal mediator of autoimmunity both in APS (24) and SLE (1). Therefore, critical gaps exist in our knowledge about the role of mTOR activation and its relationship to oxidative stress in APS. In the present study, we examined mTORC1 activity and metabolic biomarkers of oxidative stress (4;5) in 72 SLE patients, 12 of whom also had APS, as well as in 54 healthy controls matched for age, gender, and ethnicity for each blood donation. The results indicate that oxidative stress and Treg depletion rather than mTOR activation underlie APS in patients with SLE.
2. MATERIALS AND METHODS
2.1. Human subjects
Peripheral blood lymphocytes (PBL) were isolated from 72 SLE patients. Each patient satisfied the criteria for a definitive diagnosis of SLE (19;20). 12 SLE patients also satisfied the diagnostic criteria for APS (22). Nine APS patients were Caucasian females, one was a Caucasian male, and one was an African American female. Among the SLE patients without APS, two were Caucasian males, one was African American female, and the remaining were Caucasian females. The mean (±SEM) age of patients was 43.3±1.5 years, ranging between 21–62 years. 54 healthy subjects were individually matched for each patient blood donation for age within ten years, gender, and ethnic background and their freshly isolated cells were studied in parallel as controls for flow cytometry studies. The mean (±SEM) age of controls was 45.6±1.4 years, ranging between 20–62 years. 47 controls were females including 40 Caucasians, five African-Americans, and two Hispanic. 7 controls were Caucasian males.
2.2. Flow cytometry of oxidative stress, mTOR activity, and FoxP3 expression
T-cell subsets were analyzed by staining with antibodies to CD4, CD8, and CD25. B cell subsets were identified by CD19 and CD25 staining (4). Mitochondrial transmembrane potential (ΔΨm) was assessed with tetra-methyl-rhodamine methyl ester (TMRM, 100 nM, excitation: 543 nm, emission: 567 nm recorded in FL-2). Mitochondrial mass was evaluated with MitoTracker Green-FM (MTG, 100 nM; excitation: 490 nm, emission: 516 nm recorded in FL-1). Reactive oxygen intermediates (ROI) were assessed with superoxide-sensing hydroethidine (HE, 1 μM). All metabolic and mitochondrial sensor dyes were obtained from Invitrogen (Carlsbad, CA) and used as earlier described (4;27). For detection of mTOR activity and FoxP3 expression, cells were permeabilized with Cytofix/CytopermPlus (eBiosciences) and stained with AlexaFluor-488-conjugated antibody to pS6RP and AlexaFluor-647-conjugated antibody to FoxP3, as earlier described (5). Each patient’s cells were freshly isolated, stained and analyzed in parallel with a matched control. Mean channel fluorescence (MFI) values of patient samples were normalized to controls set at 1.0 for each analysis and expressed as fold changes. Frequencies of cell populations were compared as absolute values.
2.3. Statistics
Data were analyzed with t-test using the Prism software (GraphPad, San Diego, CA).
3. RESULTS
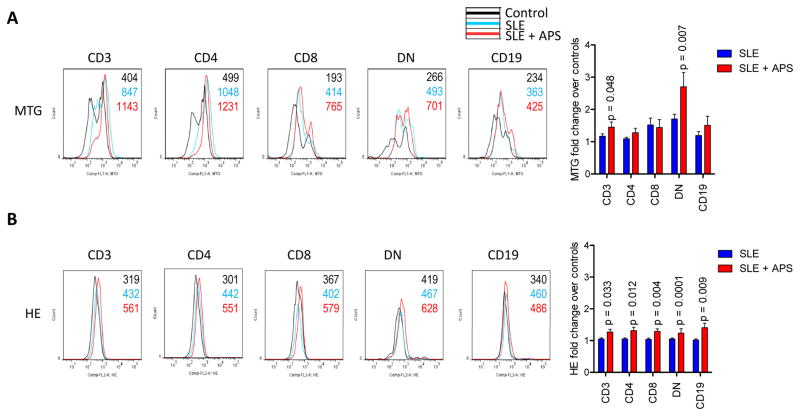
Twelve of the 72 patients (17%) satisfied the diagnostic criteria for APS (22). The accumulation of oxidative stress-generating mitochondria was significantly greater in DN T cells of SLE patients with APS (2.7-fold) in comparison to those without APS (1.7-fold; two-tailed p=0.014; Fig. 1A). Elevation of Δψm or mitochondrial hyperpolarization, which drives the production of reactive oxygen intermediates (ROI) (8), i.e., oxidative stress, was as also increased in DN T cells of patients with APS (TMRMhi : 25.3±4.4%) relative to those without APS (TMRMhi : 16.8±1.5%; p=0.029). While mitochondrial mass was only elevated in DN T cells (Fig. 1A), the intracellular production of superoxide, i.e., oxidative stress, was increased in all lymphocyte subsets of patients with APS in comparison to those without APS (Fig. 1B). In accordance with earlier findings (4;5), mTORC1 was markedly elevated in DN T cells relative to CD4 (8.5-fold, p=2.8×10−9) or CD8 T cells of all SLE patients combined (9.4-fold, p=3.1×10−10). However, mTORC1 activity was similar between SLE patients with and without APS (Fig. 2). No discrete population with elevated mTORC1 activation was detectable in CD19+ B cells (data not shown). Given that Tregs are depleted in SLE patients (28), which, in turn contribute to autoantibody production (29), we examined their prevalence in patients with and without APS. The frequency of CD4+CD25+FoxP3+ Tregs was reduced in patients with APS (Fig. 3). In particular, FoxP3+ expression was diminished within CD4+CD25+ Tregs in SLE patients with APS (28.4%) relative to those without APS (46.3%, p=0.008; Fig. 3).
Figure 1.
Increased oxidative stress in SLE patients with APS. Peripheral blood lymphocytes (PBL) of 72 lupus patients were freshly isolated and studied in parallel with 54 healthy control subjects matched for age within 10 years, gender, and ethnicity for each blood donation, as earlier described (4;5). A, Mitochondrial mass was measured by MitoTracker Green (MTG) fluorescence in CD3+, CD4+, CD8+, and CD3+CD4−CD8− double-negative (DN) T cells and CD19+ B cells. Left panels show histogram overlays of healthy (black) and SLE subjects with (red) and without APS (blue). Bar chart represents mean ± SEM of fold changes relative to paired controls normalized at 1.0. B, Oxidative stress, that is, the production of reactive oxygen intermediates (ROI) was measured by hydroethidine (HE) fluorescence. Left panels show representative histogram overlays, while bar chart depicts the mean ± SEM of fold changes relative to paired controls normalized at 1.0. p values reflect comparison between SLE patients with and without APS using unpaired t-test.
Figure 2.
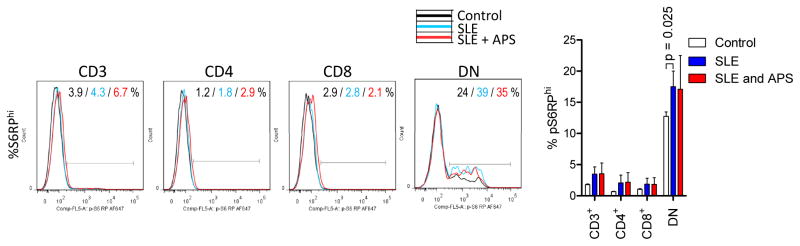
Comparable mTOR pathway activity in lupus T cells of patients with or without APS. mTORC1 activity was measured by the frequency of cells with elevated intracellular phosphorylated S6 ribosomal protein levels (%pS6RPhi). Left panels show representative histogram overlays, while bar chart depicts the mean ± SEM of fold changes relative to paired controls normalized at 1.0. p values reflect comparison between SLE patients with and without APS using unpaired t-test.
Figure 3.
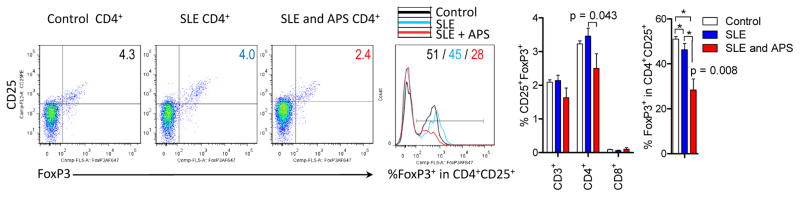
Treg depletion in SLE patients with APS. CD25+FoxP3+ Tregs were enumerated within CD4+ T cells and FoxP3 expression was also assessed by intracellular staining of permeabilized CD4+CD25+ T cells (4;5). * reflect p values < 0.05 comparing groups of subjects marked by brackets. Exact p values of 0.043 and 0.008 reflect comparisons between SLE patients with and without APS using unpaired t-test.
4. DISCUSSION
The present study reveals the accumulation of mitochondria in DN T cells and enhanced superoxide production in all lymphocytes subsets of SLE patients with APS. The prominent changes in DN T cells are consistent with earlier findings of their pro-inflammatory metabolic state (4;30) that generates oxidative stress in SLE (8). However, mTORC1 activity, which is responsive to oxidative stress (6;7), was similar within the immune system of SLE patients irrespective of APS status. On the one hand, these findings suggest that activation of the mTOR pathway does not differentiate lupus patients with APS from those without APS. On the other hand, mTOR blockade may be equally beneficial for both groups of patients due to the underlying SLE. Indeed, rapamycin showed clinical efficacy both in mouse models (11;31) and patients with SLE (2;4;11). Therefore, the benefit from rapamycin treatment in APS nephropathy may be attributed to underlying lupus, given that the majority of patients in that study had SLE as well (16/28 = 57%) (26). Moreover, in accordance with mTOR being a sensor of mitochondrial dysfunction (32) and oxidative stress (3;6), its activation is responsive to antioxidant treatment in vitro (6;7) and in vivo (5). The enhanced production of superoxide in SLE patients with APS is consistent with earlier observations that oxidative stress spreads from DN T cells to other cells and through the bloodstream (8). Along these lines, oxidative modification triggers the immunogenicity of phospholipid antigens (23–25). Furthermore, these studies reveal a profound depletion of Tregs in SLE patients having concurrent APS. Given that antioxidant treatment with NAC blocked mTOR and reversed the depletion of Tregs in SLE patients (5), such intervention may be particularly effective in patients with APS. Therefore, follow-up clinical studies with NAC, which are clearly warranted to confirm its promising therapeutic efficacy in SLE, should include patients with APS.
HIGHLIGHTS.
Anti-phospholipid antibodies (APLA) is a diagnostic criterion of SLE
APLA cause anti-phospholipid syndrome (APS).
Mitochondrial mass is increased in T cells of SLE patients with APS
Oxidative stress is increased in SLE patients with APS
Tregs are depleted in SLE patients with APS
Acknowledgments
This work was supported in part by grants RO1 AI 072648 and RO1 AT 04332 from the National Institutes of Health and the Central New York Community Foundation.
References
- 1.Tsokos GC. Systemic Lupus Erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez D, Bonilla E, Mirza N, Niland B, Perl A. Rapamycin reduces disease activity and normalizes T-cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2983–2988. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez DR, Telarico T, Bonilla E, Li Q, Banerjee S, Middleton FA, Phillips PE, Crow MK, Oess S, Muller-Esterl W, Perl A. Activation of mTOR controls the loss of TCR. in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol. 2009;182:2063–2073. doi: 10.4049/jimmunol.0803600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai Z-W, Borsuk R, Shadakshari A, Yu J, Dawood M, Garcia R, Francis L, Tily H, Bartos A, Faraone SV, Phillips PE, Perl A. mTOR activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus eryhthematosus. J Immunol. 2013;191:2236–2246. doi: 10.4049/jimmunol.1301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, Miklossy G, Jimah J, Doherty E, Tily H, Francis L, Garcia R, Dawood M, Yu J, Ramos I, Coman I, Faraone SV, Phillips PE, Perl A. N-acetylcysteine reduces disease activity by blocking mTOR in T cells of lupus patients. Arthritis Rheum. 2012;64:2937–2946. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarbassov dD, Sabatini DM. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J Biol Chem. 2005;280:39505–39509. doi: 10.1074/jbc.M506096200. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida S, Hong S, Suzuki T, Nada S, Mannan AM, Wang J, Okada M, Guan KL, Inoki K. Redox Regulates Mammalian Target of Rapamycin Complex 1 (mTORC1) Activity by Modulating the TSC1/TSC2-Rheb GTPase Pathway. J Biol Chem. 2011;286:32651–32660. doi: 10.1074/jbc.M111.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perl A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat Rev Rheumatol. 2013;9:674–686. doi: 10.1038/nrrheum.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gergely PJ, Grossman C, Niland B, Puskas F, Neupane H, Allam F, Banki K, Phillips PE, Perl A. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arth Rheum. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty E, Oaks Z, Perl A. Increased Mitochondrial Electron Transport Chain Activity at Complex I is Regulated by N-acetylcysteine in Lymphocytes of Patients with Systemic Lupus Erythematosus. Antiox Redox Signal. 2014;21:56–65. doi: 10.1089/ars.2013.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caza TN, Fernandez D, Talaber G, Oaks Z, Haas M, Madaio MP, Lai ZW, Miklossy G, Singh RR, Chudakov DM, Malorni W, Middleton FA, Banki K, Perl A. HRES-1/RAB4-Mediated Depletion of DRP1 Impairs Mitochondrial Homeostasis and Represents a Target for Treatment in SLE. Ann Rheum Dis. 2014;73:1887–1897. doi: 10.1136/annrheumdis-2013-203794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shivakumar S, Tsokos GC, Datta SK. T cell receptor alpha/beta expressing double-negative (CD4−/CD8−) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol. 1989;143:103–112. [PubMed] [Google Scholar]
- 13.Kato H, Perl A. MTORC1 expands Th17 and IL-4+ DN T cells and contracts Tregs in SLE. J Immunol. 2014;192:4134–4144. doi: 10.4049/jimmunol.1301859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koga T, Hedrich CM, Mizui M, Yoshida N, Otomo K, Lieberman LA, Rauen T, Crispín JC, Tsokos GC. CaMK4-dependent activation of AKT/mTOR and CREM-α underlies autoimmunity-associated Th17 imbalance. J Clin Invest. 2014;124:2234–2245. doi: 10.1172/JCI73411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez D, Perl A. Metabolic control of T cell activation and death in SLE. Autoimmun Rev. 2009;8:184–189. doi: 10.1016/j.autrev.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray JP, Craft J. PTENtiating autoimmunity through Treg cell deregulation. Nat Immunol. 2015;16:139–140. doi: 10.1038/ni.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, Sobel ES, Brusko TM, Morel L. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med. 2015;7:274ra18. doi: 10.1126/scitranslmed.aaa0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arth Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arth Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 21.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sánchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G, Magder LS. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arth Rheum. 2012;64:2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) [see comment] [136 refs] J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 23.Ioannou Y, Zhang JY, Qi M, Gao L, Qi JC, Yu DM, Lau H, Sturgess AD, Vlachoyiannopoulos PG, Moutsopoulos HM, Rahman A, Pericleous C, Atsumi T, Koike T, Heritier S, Giannakopoulos B, Krilis SA. Novel assays of thrombogenic pathogenicity in the antiphospholipid syndrome based on the detection of molecular oxidative modification of the major autoantigen b2-glycoprotein I. Arth Rheum. 2011;63:2774–2782. doi: 10.1002/art.30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannakopoulos B, Krilis SA. The Pathogenesis of the Antiphospholipid Syndrome. N Engl J Med. 2013;368:1033–1044. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Sanchez C, Barbarroja N, Messineo S, Ruiz-Limon P, Rodriguez-Ariza A, Jimenez-Gomez Y, Khamashta MA, Collantes-Estevez E, Cuadrado MJ, Aguirre MA, Lopez-Pedrera C. Gene profiling reveals specific molecular pathways in the pathogenesis of atherosclerosis and cardiovascular disease in antiphospholipid syndrome, systemic lupus erythematosus and antiphospholipid syndrome with lupus. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204600. In press. [DOI] [PubMed] [Google Scholar]
- 26.Canaud G, Bienaimé F, Tabarin F, Bataillon G, Seilhean D, Noël LH, Dragon-Durey MA, Snanoudj R, Friedlander G, Halbwachs-Mecarelli L, Legendre C, Terzi F. Inhibition of the mTORC Pathway in the Antiphospholipid Syndrome. N Engl J Med. 2014;371:303–312. doi: 10.1056/NEJMoa1312890. [DOI] [PubMed] [Google Scholar]
- 27.Perl A, Hanczko R, Doherty E. Assessment of mitochondrial dysfunction in lymphocytes of patients with systemic lupus erythematosus. In: Perl A, editor. Meth Mol Med Autoimmunity: Methods and Protocols. Springer; Clifton, N J: 2012. pp. 61–89. [DOI] [PubMed] [Google Scholar]
- 28.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T Regulatory Cell Function in Patients with Active Systemic Lupus Erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 29.Fujio K, Okamura T, Sumitomo S, Yamamoto K. Regulatory cell subsets in the control of autoantibody production related to systemic autoimmunity. Ann Rheum Dis. 2013;72:ii85–ii89. doi: 10.1136/annrheumdis-2012-202341. [DOI] [PubMed] [Google Scholar]
- 30.Perl A, Hanczko R, Lai Z-W, Oaks Z, Kelly R, Borsuk R, Asara JM, Phillips PE. Comprehensive metabolome analyses reveal N-acetylcysteine-responsive accumulation of kynurenine in systemic lupus erythematosus: implications for activation of the mechanistic target of rapamycin. Metabolomics. 2015 doi: 10.1007/s11306-015-0772-0. In press, http://link.springer.com/article/10.1007/s11306-015-0772-0. [DOI] [PMC free article] [PubMed]
- 31.Warner LM, Adams LM, Sehgal SN. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arth Rheum. 1994;37:289–297. doi: 10.1002/art.1780370219. [DOI] [PubMed] [Google Scholar]
- 32.Desai BN, Myers BR, Schreiber SL. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc Natl Acad Sci USA. 2002;99:4319–4324. doi: 10.1073/pnas.261702698. [DOI] [PMC free article] [PubMed] [Google Scholar]