Abstract
Perfluorooctanoic acid (PFOA) is a known developmental toxicant in mice, with varied strain outcomes depending on dose and period of exposure. The impact of PFOA on female mouse pubertal development at low doses (≤1 mg/kg), however, has yet to be determined. Therefore, female offspring from CD-1 and C57Bl/6 dams exposed to PFOA, creating serum concentrations similar to humans, were examined for pubertal onset, including mammary gland development. Mouse pups demonstrated a shorter PFOA elimination half-life than that reported for adult mice. Prenatal exposure to PFOA caused significant mammary developmental delays in exposed female offspring in both strains. Delays started during puberty and persisted into young adulthood; severity was dose-dependent. In contrast, an evaluation of serum hormone levels and pubertal timing onset in the same offspring revealed no effects of PFOA compared to controls in either strain. Therefore, our data suggest that the mammary gland is more sensitive to the effects of early low level PFOA exposures compared to other pubertal endpoints, regardless of strain.
Keywords: Perfluorooctanoic acid, mammary gland, puberty, CD-1, C57Bl/6, vaginal opening, estrous cycle
1. Introduction
Perfluorooctanoic acid (PFOA) is an eight carbon member of the perfluoroalkyl acid family. Its use as a surfactant in various industrial and consumer products, as well as its ability to resist further degradation under extreme temperatures, has made it both persistent and ubiquitous within the environment resulting in the inevitable exposure to both humans and wildlife.
Serum PFOA levels in the general U.S. population have declined from 5.21 ng/mL (1999-2000) to 3.07 ng/mL over a decade (2009-2010) [1], potentially due to consumer awareness and a gradual phase out of PFOA production in the U.S. However, residents living in areas of elevated PFOA exposure (sites near manufacturing facilities) have serum levels 10-100 times higher than the national average, and children aged 2-5 years demonstrated a geometric mean serum concentration of 600 ng/ml (as of 2006) [2]. Pubertal aged children from this area also experienced higher total geometric mean serum levels (<12 years, 77.6 ng/ml and 12-19 years, 59.9 ng/ml) [3]. Exposure at these ages is mostly related to drinking water sources but may also be contributed to by the ingestion of contaminated dust, food sources and hand to mouth contact. PFOA exposure may also occur during gestation or shortly after birth, as PFOA was detected in human and rodent milk, rodent serum, urine, amniotic fluid and at relatively low levels in human cord blood [4, 5 and 6]. These findings suggest that PFOA is not only capable of being transferred from mother to offspring due to its long half-life (3.8-4.4 years in humans [7]), but that the longer the exposure, the higher the potential is for increased serum PFOA levels in offspring. Parental exposures prior to conception may compromise embryonic development and continued placental and lactational [8] exposures may have deleterious effects on the developing fetus, newborn and infant. Early life exposures to PFOA may induce alterations in the epigenome, such as altered DNA methylation [9, 10], that have the potential to alter normal development and lead to adverse health outcomes in later life.
High dose exposures in adult rodents (≥10 mg/kg PFOA) have resulted in hepatotoxicity, tumors of the liver and pancreas and in pregnant adult mice, neonatal toxicity and mortality of their offspring [11, 12 and 13]. Lower perinatal doses, however, have resulted in an array of reproductive and developmental alterations in mouse offspring that have included advanced preputial separation in males, sex specific neurobehavioral differences [14] and impaired mammary gland development [15]. The mammary gland is a unique organ in that the majority of its development occurs postnatally. Although differentiation is not complete until late pregnancy, puberty demarks an exponential growth phase that is facilitated by various paracrine and endocrine factors including estrogen and progesterone and includes an increased mammary stem cell population [16, 17, and 18]. Additionally, the highly proliferative terminal end buds (TEBs) lead the migration of the mammary epithelium into the fat pad resulting in the extensive ductal branching patterns of the gland. Exposures to endocrine disrupting chemicals (EDCs) during gestation or early life are especially of concern because they may affect the normal progression of mammary development that may not become apparent until puberty or later life. EDCs can disrupt normal functions by mimicking naturally occurring hormones, inhibiting hormone receptor binding or causing an abnormal inhibitory or stimulatory endocrine response [19]. High dose PFOA exposure has demonstrated these properties in the mammary gland by stimulating growth in the glands of peripubertal exposed C57Bl/6 female mice [20]. These effects were only apparent when the ovaries were present and also resulted in the upregulation of estrogen receptor alpha (ERα) expression within the gland. However, the mechanism for prenatal PFOA exposure on mammary gland development has yet to be determined, but it is evident that early exposures set in motion key alterations that may be exacerbated by circulating endogenous hormones. This could potentially further target the proliferating TEBs resulting in the increased potential for cellular transformations that may make the gland more susceptible to developing later life diseases, such as breast cancer [21, 22].
Full and late gestational exposure to low dose PFOA has been shown to induce stunted mammary epithelial growth in CD-1 female offspring, an abnormality that persisted into adulthood, which can begin as early as postnatal day (PND) 56 in some mouse strains. Macon et al. [15] reported adverse changes in female CD-1 mouse mammary gland development from 0.01 to 1 mg/kg PFOA following a late gestation exposure, which is defined as a one-time daily oral gavage exposure between gestational days 10-17. Multigenerational effects have also been observed in CD-1 mice administered separate and combined oral gavage and drinking water exposure to 5 μg/L PFOA. All PFOA treated F1 females demonstrated reduced mammary development that continued until 63 days of age (young adulthood) [23]. Mammary development was also attenuated at weaning in F2 females that were never exposed to PFOA via placental transfer. Much higher oral exposures appear to be required for mammary effects in other strains, using peripubertal exposures. Yang et al. [24] reported striking differences among C57Bl/6 and Balb/c mice peripubertally exposed to ≥5 mg/kg PFOA. Peripubertal PFOA exposure significantly inhibited mammary growth at 5 and 10 mg/kg in Balb/c animals, whereas in C57Bl/6 mice stimulatory effects were reported at 5 mg/kg, but inhibitory effects predominated at 10 mg/kg. Mammary gland development was not altered in either strain following peripubertal exposures at 1.0 mg/kg suggesting that some mouse strains are more sensitive than others to PFOA effects and/or the effects in the mammary gland are highly dependent upon the timing of exposure (peripubertal vs. in utero).
PFOA has also been associated with changes in other pubertal developmental landmarks. Girls living in close proximity to PFOA-polluted areas have self-reported delays in menarche that were associated with increased serum PFOA levels [25]. Rodent models have also been assessed for similar hallmarks that include timing of first estrus, vaginal opening (VO) and estrous cyclity, as the onset of cyclity occurs after VO and first estrus; VO and first estrus in prenatally exposed pups were slightly delayed at dose levels outside those that humans would be exposed to (20 mg/kg) and unchanged at the lowest dose tested (1.0 mg/kg) [13]. EDCs have been reported to elicit non-monotonic responses, wherein lower doses may produce changes not observed at higher doses [26]. To date, no study has examined the low-dose effects of PFOA on all pubertal events in prenatally exposed female mice nor determined how the mouse strain may influence any potential effects. Our goal was to provide a thorough evaluation of the low dose effects of PFOA exposure on the timing of critical pubertal events in the CD-1 and C57Bl/6 female mouse including mammary gland development, VO and first estrus, in addition to the evaluation of serum steroid hormone and PFOA levels. These assessments may provide valuable information as to the most sensitive pubertal targets influenced by prenatal PFOA exposure, as well as indicate pathways that may be perturbed by other related toxicants during pubertal onset.
2. Materials and Methods
2.1 PFOA
Perfluorooctanoic acid (PFOA, ammonium salt; >98% pure) was obtained from Fluka Chemical (Steinheim, Switzerland) and 1, 2-13C2-perfluoroctanoic acid (13C2-PFOA) was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). PFOA dosing solutions were dissolved in deionized water (DI water) and freshly prepared daily.
2.2 Animals
Timed pregnant CD-1 and C57Bl/6 mice were obtained from Charles River Laboratories (Raleigh, NC) on gestational day 0 (GD 0) when they were confirmed sperm positive. Upon arrival, each dam was weighed and randomly assigned to one of five treatment groups, equalizing the mean starting body weight in each group. Pregnant dams were individually housed in polypropylene cages with microbarrier lids and received chow (LabDiet 5001, PMI Nutrition International LLC, Brentwood, MO) and tap water ad libitum. Animal facilities were maintained on 12:12h light-dark cycle, at a controlled temperature (20-24 °C), with 40-60% relative humidity. All animals were treated humanely and in accordance with the National Institute of Environmental Health Sciences Animal Care and Use Committee (ACUC).
2.3 Experimental Design
For space reasons, the studies in CD-1 mice were completed in three blocks (block 1 n=97, block 2 n= 40, and block 3 n=26). Studies with the C57Bl/6 animals were completed in one block (n=41). For each experimental block, timed pregnant dams were orally gavaged daily with vehicle (DI water), 0.01, 0.1, 0.3 or 1.0 mg PFOA/kg/body weight (bw) between GD 1-17. A full gestation exposure design was chosen to recapitulate murine serum levels that have been established in previous experiments and were found to overlap with PFOA serum levels in residents living in heavily contaminated areas in the U.S. Dams were weighed daily to administer a dose volume of 10 μl/g bw. Parturition normally occurred on the eve of GD 18 and PND 1 was defined as the first day following parturition (usually > 0.5 days old). On PND 3, pup sex was determined and CD-1 litters were equalized to 10 pups per dam. If numbers permitted, each dam received 6-7 females and 3-4 males. Pregnancy rates in CD-1 females were >60% (block 1 =78% block 2 =67% and block 3 = 88%); however, the C57Bl/6 block yielded a much lower rate of approximately 27% (41/152). Regardless of treatment, C57Bl/6 dams produced litter sizes of between 5-8 pups. Any litters with an n<5 pups were excluded from these studies. On PND 21, pups of both strains were weaned and all female pups were retained and housed 3-5 mice per cage in an effort to keep litter mates together. Male weanlings from controls were retained in order to circulate pheromones into the room that are known to assist in normalizing female cycling [27, 28]. Additionally, every 3-4 days (at cage changes) a small sample of bedding from the males was placed into each female cage.
2.4 Necropsy
With the exception of PND 21 (sexually immature), vaginal lavage was performed on all females prior to necropsy to determine their stage of the estrous cycle. We attempted to necropsy females during the first day of estrus in order to minimize morphological and hormonal fluctuations caused by normal changes in the estrous cycle. Vaginal smears were acquired between 6 and 8 AM on the day of sacrifice and necropsied shortly thereafter (9 AM-12 PM) [29]. On PND 21, 35, and 56 for CD-1 mice (n=4-7 litters/treatment), and PND 21(n=2-6 litters/treatment) and 61 (n= 3-9litters/treatment) for C57Bl/6 mice (fewer time points due to this strain historically having fewer pups per live birth and low pregnancy rates following plug identification) female pups were first weighed and then sacrificed using swift decapitation to obtain trunk blood for PFOA and hormone analysis. Mammary glands were collected from the fourth and fifth inguinal glands and prepared as carmine stained whole mounts. Body and liver weights were also recorded.
2.5 Pubertal Endpoints
Pubertal maturation in females was assessed using VO and vaginal cytology to determine timing to first estrus in the estrous cycle. Visual assessment for VO started on PND 18 in CD-1 mice and on PND 23 in C57Bl/6 mice, as VO has been reported to begin as late as PND 30 in that strain [30]. Once VO occurred, daily vaginal lavage was performed using 1X phosphate buffered saline and a sterile eyedropper to obtain cells to determine the presence of cornified epithelial cells, the first indication of estrus [13, 31]. All samples were evaluated fresh daily on a Leica DM2000 light-microscope (Leica Microsystems). Body weights were taken daily and recorded on the day of VO and first estrus. Pubertal endpoints were only measured in CD-1 block 1 (n= 7-8/treatment) and 2 (n=4-5/treatment) litters. Three to seven C57Bl/6 pups were evaluated per litter.
2.6 Mammary Whole Mount Preparation and Analysis
The entire fourth and fifth mammary gland from each mouse was placed on a charged slide and flattened to the natural surface area. Each whole mount was fixed in Carnoy’s solution (EtOH, acetic acid and chloroform), stained in carmine alum and de-fatted in xylene [32]. For each collection, all samples were compared to age-matched animals within the same group and across other treatment groups [32]. Each gland was assessed and assigned a qualitative score on a scale of 1-4 (1=poor development and 4=best development) using a Leica Z16APO and DFC295 light microscope and camera (Leica Microsystems Inc., Buffalo Grove, IL), respectively. Qualitative scoring criteria is based on, but not limited to, lateral and longitudinal epithelial growth, branching density, changes in epithelial growth, appearance of budding from ductal tree, number of differentiating duct ends and the presence or absence of terminal end buds. Each gland was scored by two individuals without knowledge of treatment and averaged to obtain a final score [15]. Approximately, 4-7 CD-1 females/treatment/block and 2-10 C57Bl/6 offspring/treatment were scored.
2.7 PFOA Serum
Serum concentrations of PFOA were obtained using the methods reported by Reiner et al. [33]. Briefly, 25-50 μL of serum from each individual was transferred to its own 15 ml propylene tube. A predetermined amount of internal standard (13C2-PFOA) was added to approximate the midpoint of the calibration curve for the anticipated sample range. 0.1 M Formic acid was added to the sample to denature the proteins and it was vortexed. Cold acetonitrile (-20C) was added to precipitate the proteins followed by vortexing and centrifugation at 2000 x g for 3 min. Supernatant from the acetonitrile mixture were placed in liquid chromatography vials containing ammonium acetate buffer (pH 6.5) (1:1). All samples were analyzed using a Waters Acquity Ultra Performance Liquid Chromatograph interfaced with a Waters Quattro Premier XE triple quadropole mass spectrometer (Waters Corporation, Milford, MA). Blank matrices were obtained using Pel-Freeze Biologicals CD-1 control mouse serum (Rogers, AR). At least six standards were used to generate the calibration curve with the coefficients of determination at 0.99 or higher. Calibration curves were obtained by plotting the ratio of PFOA peak area to 13C2-PFOA peak area vs. concentration and were fitted to a linear regression equation with 1/x weighting. The limit of quantitation (LOQ) was defined as the lowest point on the standard curve at which analysis may be reported +/− 30% confidence. Quality control (QC) samples were interspersed throughout the analytical run and were run in duplicates for accuracy determination. Method accuracy and precision were determined by analyzing the QC samples repeatedly. Accuracy was calculated as the percentage of the concentration found compared with the theoretical concentration and the precision was calculated using the average relative standard deviation of the replicate analysis of the QC materials. Additionally, approximately 10% of all unknown samples were analyzed in duplicate for precision measurements. Each sample batch run contained a calibration standard and a matrix and methanol blank that were prepared under the same conditions as the unknown samples. The LOQ for CD-1 serum samples ranged from 5-100 ng/ml (Control=5 ng/ml, 0.01mg/kg=10 ng/ml, 0.1, 0.3 and 1.0 mg/kg=100 ng/ml). The LOQ for C57Bl/6 animals was 10 ng/ml. Samples that were below the LOQ were reported as the LOQ /√2 for statistical purposes. Serum PFOA was measured from at least one pup from each litter per treatment group in the CD-1 (n=5-10) and C57Bl/6 (n= 2-6) studies.
2.8 Serum Estradiol and Progesterone Analysis
Serum estradiol and progesterone levels were measured using a commercially available assay kit (Meso Scale Discovery, Gaithersburg, MD). Coefficients of variation ≤ 20% were considered acceptable. Assays were performed in a multiplex format in 96-well, 4 spot plates that were pre-coated with estradiol and progesterone capture antibodies and 50 μl serum samples. Sandwich immunoassays were conducted by adding test serum followed by conjugated detection antibodies (anti-estradiol and anti-progesterone) containing an electrochemiluminescent compound. Electrochemiluminescence was detected using a SECTOR Imager 2400. Quantitation of estradiol or progesterone was based on the intensity of the emitted light. A rat serum sample of known concentration was also tested for QC purposes. Data from the Sector Imager was transferred to Excel worksheets for further analysis. The limit of detection (LOD) for estradiol was 5 pg/ml and 0.07 ng/ml for progesterone.
All unknown serum samples were analyzed in duplicate and standard curves for estradiol and progesterone were generated using calibrators supplied with the kit. Estradiol concentrations between 0 and 4 ng/ml and progesterone concentrations between 0 and 50 ng/ml were fitted to a sigmoid dose response curve and used to quantify the level of each hormone in test samples. Serum samples from each treatment group were stratified across plates so that not all of one dose group was assayed on the same plate.
2.9 Statistical Analysis
For all studies, dams or litters were considered the unit of measurement. Therefore, if a dam was represented by more than one pup, their values were averaged. Outlier values were determined by bodyweight and were calculated using GraphPad’s QuickCalc Grubb’s test (http://graphpad.com/quickcalcs/grubbs1/). Outliers were only removed when the animal presented as being moribund. When an individual animal was found to be an outlier within a specific end point, the data for that individual was removed from all data sets that it was contained in. Replicated experiments were treated as blocks and were analyzed using two-way analysis of variance (ANOVA) to determine block and treatment effects using Tukey’s post hoc test (p<0.05). A linear regression test was performed on the mammary gland scores to determine dose related effects. Serum PFOA, estradiol and progesterone levels were assessed using ANOVA followed by a Dunnett’s post hoc test. Graphs and tables were created using GraphPad Prism 6 (La Jolla, CA) and Microsoft Excel. All data are represented as the mean ± SEM.
3. Results
3.1 Internal PFOA burdens and liver enlargement
Serum PFOA concentrations in exposed female offspring were measured to determine the internal concentrations at which pubertal effects were being observed. PFOA levels were detectable in all exposed female offspring from both strains at PND 21 (Table 1). PFOA was undetectable in control mice of both strains. Serum PFOA concentrations increased in a dose dependent manner and were significantly different at the two highest doses in the CD-1 females at PND 21 and 35 compared with control females. Detectable levels were measured in all treated animals at PND 35, however, by PND 56 all animals experienced a 4-5 fold decrease in serum levels compared to littermates collected at PND 35, a decrease that was larger than expected, given the reported 15.6-21.7 d half-life of PFOA in adult CD-1 mice [34] following a single oral adulthood exposure [34]. By PND 56, serum PFOA levels were below LOQ in the 0.01mg/kg group and only statistically increased from control levels at 1.0 mg/kg. The same dose dependent trend observed at PND 21 in CD-1 mice was also shown in the C57Bl/6 pups; however, differences were not significant, more than likely reflecting the fair amount of variability between mice in a small litter size, rather than treatment. Additionally, treatment with the same PFOA exposures in C57Bl/6 mice resulted in serum levels that were lower on average compared to those observed in the CD-1 strain of the same age. By PND 61 the C57Bl/6 animals in the three highest dose groups had measurable levels that were still above the LOQ threshold, however, these levels were ~10-100 fold reduced from those observed in their PND 21 litter mates. Taken together, these data suggest a faster rate of clearance of PFOA from the C57Bl/6 offspring and potentially a faster clearance in rapidly growing offspring than the 15.6-21.7 d half-life previously reported in adult CD-1 mice [34]. Based on our 1.0 mg PFOA/kg data and serum data collected previously [15], we estimated half-life clearance in the CD-1 animals between PND 21 to PND 56 to be 7.3-8.9 days and C57Bl/6 to be 6.0 days between PND 21 and PND 61.
Table 1.
PFOA Serum Concentrations in CD-1 and C57Bl/6 Mice
| Control (n) | 0.01 mg/kg (n) | 0.1 mg/kg (n) | 0.3 mg/kg (n) | 1.0 mg/kg (n) | |
|---|---|---|---|---|---|
| CD-1 | |||||
| PND 21 | <LOQ (6) | 74.8 ± 16.9 (10) | 457.3± 91.0 (9) | 904.8 ± 131.5 (10)* | 3119.0 ± 396.4 (5)** |
| PND 35 | <LOQ (11) | 14.3 ± 2.3 (12) | 61.7 ± 6.5 (9) | 200.9 ± 32.2 (11)* | 889.8 ± 117.5 (11)** |
| PND 56 | < LOQ (5) | < LOQ (5) | 15.0 ± 5.0 (4) | 46.2 ± 7.5 (6) | 200.2 ± 21.1 (7)** |
| C57Bl/6 | |||||
| PND 21 | <LOQ (2) | 26.1 ± 19.0 (2) | 247.1 ± 11.4 (2) | 891.3 ± 528.7 (3) | 2141.67 ± 666.8 (2) |
| PND 61 | <LOQ (6) | <LOQ (2) | 27.7 ± 10.4 (3)* | 9.3± 2.2 (5) | 22.0 ± 7.6 (5) |
Data presented as the mean ± SEM. Significance observed in comparison to control;
p≤0.05.
The LOQ (limit of quantitation) for C57Bl/6 animals was <10 ng/ml. CD-1 LOQ: Control 5 ng/ml, 0.01mg/kg=10 ng/ml, 0.1, 0.3 and 1.0 mg/kg=100 ng/ml. All controls at PND 21 and 61 and 0.01, 0.1 and 0.3 mg/kg at PND 61 were <LOQ.
(n) = # of animals per dose group; CD-1 n= 4-12; C57Bl/6 n= 2-6
Full gestation PFOA exposure (0.01 to 1 mg/kg) did not affect absolute body weight measurements in either CD-1 or C57Bl/6 female offspring (Tables 2 and 3, respectively). Net body weight was defined as the liver weight subtracted from the body weight and was found to be significantly reduced in the highest dose group in CD-1 females (1.0 mg/kg) at PND 21 and 35. This effect had little biological significance, as it resolved and net body weights were comparable to the control levels by PND 56. These finding are in agreement with the body weights observed at post weaning ages following a full gestation exposure to PFOA levels ≤ 1.0 mg/kg [13, 15]. No significant effects for net body weight were observed in the C57Bl/6 females.
Table 2.
Body and Liver Weights of Female CD-1 Mice
| Control (n) | 0.01 mg/kg (n) | 0.1 mg/kg (n) | 0.3 mg/kg (n) | 1.0 mg/kg (n) | |
|---|---|---|---|---|---|
| Body Weight (g) | |||||
| PND 21 | 11.9 ± 0.2 (20) | 12.1 ± 0.2 (22) | 12.5 ± 0.3 (22) | 11.6 ± 0.3 (22) | 10.9 ± 0.2 (21) |
| PND 35 | 23.1 ± 0.3 (17) | 22.9 ± 0.5 (16) | 23.0 ± 0.4 (14) | 22.2 ± 0.3 (17) | 21.8 ± 0.4 (16) |
| PND 56 | 26.6 ± 0.8 (9) | 27.7 ± 0.7 (14) | 27.6 ± 0.2 (8) | 25.69 ± 0.83 (4) | 28.5 ± 0.7 (9) |
| Net Body Weight (g) | |||||
| PND 21 | 11.3 ± 0.2 (19) | 11.5 ± 0.2 (22) | 11.9 ± 0.3 (22) | 11.1 ± 0.3 (22) | 10.3 ± 0.2 (21)* |
| PND 35 | 22.0 ± 0.3 (17) | 21.7 ± 0.4 (16) | 21.9 ± 0.4 (14) | 21.1 ± 0.3 (17) | 20.7 ± 0.3 (16)* |
| PND 56 | 25.2 ± 0.8 (9) | 26.3 ± 0.7 (13) | 26.3 ± 0.2 (8) | 25.6 ± 0.5 (10) | 27.1 ± 0.6 (9) |
| Absolute Liver Weight (g) | |||||
| PND 21 | 0.60 ± 0.02 (19) | 0.62 ± 0.02 (22) | 0.61 ± 0.02 (22) | 0.59 ± 0.02 (22) | 0.62 ± 0.02 (21) |
| PND 35 | 1.16 ± 0.03 (17) | 1.14 ± 0.04 (16) | 1.13 ± 0.04 (14) | 1.13 ± 0.02 (17) | 1.13 ± 0.04 (16) |
| PND 56 | 1.36 ± 0.05 (9) | 1.35 ± 0.04 (13) | 1.29 ± 0.03 (8) | 1.22 ± 0.02 (10) | 1.36 ± 0.07 (9) |
| Relative Liver | |||||
| PND 21 | 0.051 ± 0.002 (19) | 0.051 ± 0.001 (22) | 0.049 ± 0.001 (22) | 0.051 ± 0.001 (22) | 0.057 ± 0.001 (21)* |
| PND 35 | 0.050 ± 0.001 (17) | 0.050 ± 0.002 (16) | 0.049 ± 0.002 (14) | 0.051 ± 0.001 (17) | 0.051 ± 0.001 (16) |
| PND 56 | 0.052 ± 0.002 (9) | 0.048 ± 0.003 (13) | 0.047 ± 0.001 (8) | 0.046 ± 0.001 (10)* | 0.048 ± 0.001 (9) |
Data are represented as mean ± SEM. Significance observed in comparison to control;
p≤0.05
Net Body Weight= Body weight (g) – Liver Weight (g); Relative Liver weight = Body weight (g)/Liver weight (g)
(n) = # of animals per dose group; n= 8-22
Table 3.
Body and Liver Weights of Female C57Bl/6 Mice
| Control (n) | 0.01 mg/kg (n) | 0.1 mg/kg (n) | 0.3 mg/kg (n) | 1.0 mg/kg (n) | |
|---|---|---|---|---|---|
| Body Weight (g) | |||||
| PND 21 | 8.4 ± 0.4 (6) | 8.6 ± 0.1 (4) | 9.5 ± 0.9 (2) | 8.2 ± 0.7 (5) | 7.5 ± 0.3 (5) |
| PND 61 | 19.1 ± 0.3 (9) | 19.8 ± 0.3 (5) | 20.1 ± 0.5 (3) | 20.1 ± 0.4 (9) | 19.9 ± 0.5 (8) |
| Net Body Weight (g) | |||||
| PND 21 | 8.0 ± 0.4 (6) | 8.3 ± 0.03 (4) | 9.1 ± 0.9 (2) | 7.9 ± 0.6 (5) | 7.1 ± 0.3 (5) |
| PND 61 | 18.2 ± 0.2 (9) | 18.8 ± 0.3 (5) | 19.2 ± 0.5 (3) | 19.2 ± 0.3 (9) | 19.0 ± 0.4 (8) |
| Absolute Liver Weight (g) | |||||
| PND 21 | 0.37 ± 0.03 (6) | 0.43 ± 0.04 (4) | 0.45 ± 0.03 (3) | 0.38 ± 0.03 (6) | 0.39 ± 0.01 (5) |
| PND 61 | 0.93 ± 0.02 (9) | 0.97 ± 0.03 (5) | 0.90 ± 0.05 (3) | 0.95 ± 0.05 (9) | 0.89 ± 0.03 (8) |
| Relative Liver | |||||
| PND 21 | 0.044 ± 0.002 (6) | 0.049 ± 0.004 (4) | 0.048 ± 0.001 (2) | 0.045 ± 0.001 (5) | 0.052 ± 0.001 (5) |
| PND 61 | 0.048 ± 0.001 (9) | 0.049 ± 0.001 (5) | 0.045 ± 0.002 (3) | 0.047 ± 0.002 (9) | 0.045 ± 0.002 (8) |
Data are represented as mean ± SEM
Net Body Weight= Body weight (g) – Liver Weight (g); Relative Liver weight = Body weight (g)/Liver weight (g)
(n) = # of animals per dose group; N= 2-9
Absolute liver weight differences in both strains were not significantly altered in comparison to their respective controls (Tables 2 and 3). CD-1 females in the 1.0 mg/kg dose group exhibited significantly elevated relative liver weights at PND 21 that recovered to normal levels by PND 35. As relative liver weights had returned to values similar to controls by PND 28 following a full gestational exposure in a previous study, our transient finding is thought to be biologically irrelevant [15]. C57Bl/6 mice did not demonstrate any significant relative liver weight changes at PND 21 or PND 61. Together these data confirm that liver effects resulting from early and low exposures are transient and may begin to dissipate during early puberty. This appears to correlate well with the higher internal body burdens in CD-1 mice that showed increased liver hypertrophy (relative liver weight) in 1 mg PFOA/kg bw weanlings compared to no effects seen in C57Bl/6 pups (Tables 2 and 3).
3.2 Pubertal Assessment
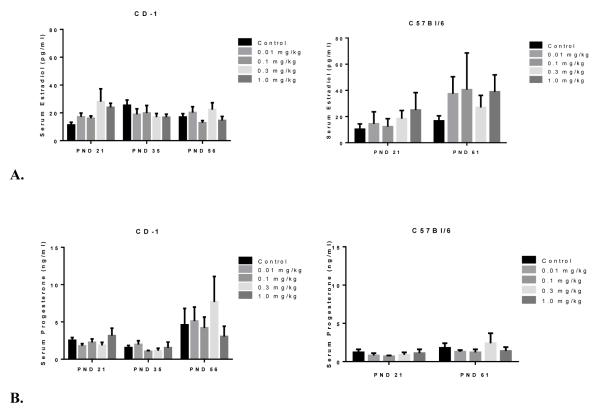
Pubertal onset in the female mouse is associated with an increased production of the steroid hormones estradiol and progesterone as a result of increased signaling from the hypothalamus and pituitary gland to the ovaries. Both hormones are critical for ovulation and uterine changes, as well as normal mammary gland development and sexual maturation [35, 36]. To understand the effects of prenatal PFOA on circulating steroids, serum was obtained from females in the stage of estrus, except at PND 21 where all hormone analysis samples were from females that had not undergone VO. Figure 1A illustrates that estradiol levels in both strains were similar between PFOA treated and control mice. A similar lack of statistical difference for treatment effect was shown for progesterone (Figure 1B), however; progesterone levels were overall elevated in CD-1 animals at PND 56, in all exposure groups, compared to levels seen at PND 21 and 35. Progesterone levels in PFOA-exposed C57Bl/6 females were comparable to control at both PND 21 and 61.
Figure 1.
Effects of PFOA treatment on serum hormone levels A) estradiol B) progesterone levels. All animals were sacrificed during estrus, with the exception of PND 21 when vaginal opening had not occurred. CD-1 litter/treatment group n=12-15 (PND 21), n=9-10 (PND 35) and n=8-10 (PND 56). C57Bl/6 litter n= 3-4 (PND 21) and n=3-9 (PND 61). Data presented as the mean ± SEM.
An assessment of the effects of PFOA on VO and first estrus are summarized in Table 4. Females from only two of the three CD-1 blocks were measured. Although all assessments were completed in an identical manner, it was necessary to assess each block separately due to block effect differences. VO occurred ~4 days later in block 2 compared to block 1, however, comparisons between the control and treatment groups within each block revealed no significant differences due to treatment. The reason for the block difference could not be determined. Timing to first estrus was also unchanged within each block. However, block 1 females appeared to undergo their average first estrus 2-3 days following VO whereas it almost immediately followed VO in block 2 females, with the exception of the 1.0 mg/kg group (~2.5 days timing to first estrus). Neither body weight at VO nor first estrus timing was changed by PFOA treatment in either block. The age of VO, day of first estrus and body weights at these times was also unchanged in the C57Bl/6 animals. The fact that no treatment related effects were observed in either strain implies that the differences in the normal timing of these events in each strain are likely a result of strain differences rather than PFOA exposure.
Table 4.
Assessment of Female Pubertal Events Following Prenatal PFOA Exposures in CD-1 and C57Bl/6 Mice
| Control | 0.01 mg/kg (n) | 0.1 mg/kg (n) | 0.3 mg/kg (n) | 1.0 mg/kg (n) | |
|---|---|---|---|---|---|
| CD-1 (Block 1) | |||||
| Vaginal Opening | 22.8 ± 0.7 (8) | 24.1 ± 0.7 (11) | 23.8 ± 0.9 (7) | 25.7 ± 0.6 (7) | 23.9 ± 0.7 (10) |
| Vaginal Opening BW | 13.8 ± 0.5 (8) | 14.2 ± 0.6 (11) | 15.6 ± 0.9 (7) | 16.6 ± 1.8 (7) | 14.6 ± 0.8 (10) |
| First Estrus | 25.1 ± 0.6 (8) | 26.7 ± 0.7 (11) | 26.1 ± 0.9 (7) | 28.2 ± 0.6 (7) | 26.3 ± 0.6 (10) |
| CD-1 (Block 2) | |||||
| Vaginal Opening | 28.2 ± 1.4 (5) | 27.5 ± 1.2 (6) | 28.8 ± 0.4 (5) | 28.1 ± 1.4 (5) | 26.6 ± 0.9 (4) |
| Vaginal Opening BW | 18.5 ± 1.2 (5) | 18.5 ± 1.5 (6) | 19.5 ± 0.4 (5) | 18.5 ± 0.9 (5) | 17.5 ± 1.0 (4) |
| First Estrus | 28.8 ± 1.2 (5) | 29.8 ± 1.2 (6) | 28.9 ± 0.4 (5) | 29.2 ± 1.4 (5) | 29.1 ± 0.4 (4) |
| C57Bl/6 | |||||
| Vaginal Opening | 31.7 ± 0.8 (7) | 31.2 ± 0.4 (3) | 29.9 ± 0.3 (3) | 31.3 ± 0.7 (5) | 31.6 ± 0.6 (5) |
| Vaginal Opening BW | 14.8 ± 0.2 (7) | 14.9 ± 0.3 (3) | 14.8 ± 0.7 (3) | 15.4 ± 0.2 (5) | 14.5 ± 0.4 (5) |
| First Estrus | 33.6 ± 0.9 (7) | 32.9 ± 0.1 (3) | 31.9 ± 1.0 (3) | 31.7 ± 0.7 (5) | 32.4 ± 0.8 (5) |
Data are represented as mean ± SEM. All animals evaluated within each block were assessed as close as possible to the same time of day to minimalize variability.
(n) = # of animals per dose group; CD-1 n= 4-11; C57Bl/6 n=3-7
Units for these measures are as follows: Vaginal opening and first estrus are postnatal age in days and body weight (BW) is reported in grams.
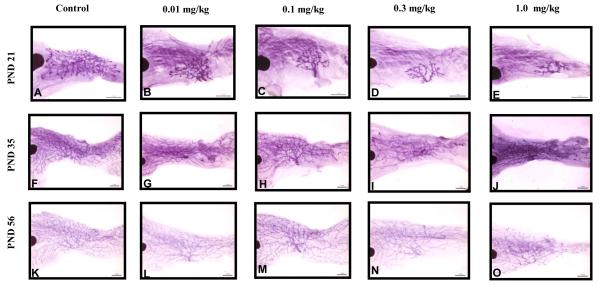
The effects of PFOA on mammary gland development were assessed by whole mount using factors that include lateral and longitudinal branching, the presence or absence of terminal end buds and branching density. However, these criteria were dependent upon the age of development. Both CD-1 and C57Bl/6 strains exhibited developmental delays that were apparent at the lowest dose (0.01 mg/kg) and were significantly different from controls in CD-1 mice. As early as PND 21 we observed a dose dependent decrease in developmental scores in the CD-1 animals with the highest dose group scoring ~1.2 points lower than control (Table 5). Score reductions were significant at PND 21 in the three highest dose groups (0.1-1.0 mg/kg); however, by PND 35 and 56 growth delays were obvious in all CD-1 treatment groups resulting in significantly lower scores. When a trend analysis was performed on the mammary developmental scores of CD-1 mice, an inverse relationship was noted between scores and PFOA dose (p<0.001), indicating that increased dose induced the developmental delays observed. Representative morphological evaluations of the CD-1 glands are illustrated in Figure 2. TEBs, lateral and longitudinal branching and secondary branching were all decreased with increased PFOA dose, resulting in a much smaller gland (Figure 2). By PND 35, in addition to the growth defects already described, PFOA caused a delay in the fourth and fifth glands growing together. Although, our evaluation of pubertal timing in both strains has established that C57Bl/6 animals reach sexual maturation at a later point than CD-1 mice, mammary glands were still evaluated on PND 21 to remain consistent throughout each block. We did note that control scores from both strains were very similar; it should be noted that scoring is based on the level of development compared to controls and may be based on entirely different criteria that can still result in similar scores across strains.
Table 5.
Mammary Gland Developmental Scores
| Control (n) | 0.01 mg/kg (n) | 0.1 mg/kg (n) | 0.3 mg/kg (n) | 1.0 mg/kg (n) | |
|---|---|---|---|---|---|
| CD-1 | |||||
| PND 21 | 2.9 ± 0.1 (19) | 2.4 ± 0.1 (22) | 2.3 ± 0.1 (22)** | 2.0 ± 0.1 (21)*** | 1.7 ± 0.1 (21)**** |
| PND 35 | 3.1 ± 0.1 (16) | 2.3 ± 0.2 (17)** | 2.2 ± 0.2 (14)** | 2.3 ± 0.1 (16)** | 1.9 ± 0.2 (14)**** |
| PND 56 | 3.3 ± 0.1 (9) | 2.3 ± 0.2 (13)** | 2.5 ± 0.2 (8)* | 2.2 ± 0.1 (10)** | 1.9 ± 0.2 (9)**** |
| C57Bl/6 | |||||
| PND 21 | 2.9 ± 0.2 (7) | 2.5 ± 0.4 (5) | 2.1 ± 0.7 (2) | 1.8 ± 0.3 (6)* | 1.8 ± 0.2 (5)* |
| PND 61 | 2.8 ± 0.2 (10) | 2.2 ± 0.2 (5) | 2.6 ± 0.1 (3) | 2.1 ± 0.1 (10)* | 1.7 ± 0.1 (8)*** |
Data are represented as mean ± SEM. Mammary glands scored between 1 (poor development) and 4(best development). Individual pup scores were averaged and are represented by the mean values for each treatment group.
(n) = # of animals per dose group; CD-1 n= 8-19 and C57Bl/6 n=2-10.
Significance observed in comparison to control;
p≤0.05,
p≤0.01,
p≤0.001 and
p≤0.0001.
Figure 2.
Mammary whole mount assessment of early and late pubertal glands in CD-1 offspring. Representative image of control A) PND 21, F) PND 35, and K) PND 56; 0.01 mg/kg B) PND 21, G) PND 35 and L) PND 56, 0.1 mg/kg C) PND 21, H)PND 35 and M)PND 56; 0.3 mg/kg D) PND 21, I) PND 35 and N) PND 56 and 1.0 mg/kg E) PND 21, J) PND 35 and O)PND 56. CD-1 n= 4-11 litters/treatment group. Significant inverse trends were noted between developmental scores and PFOA dose, indicating higher PFOA exposure was related to lower (more severe) developmental scores (p<0.05).
In the C57Bl/6 strain, only the 0.3 and 1.0 mg PFOA/kg offspring demonstrated mammary developmental scores that were significantly reduced from that of controls (Table 5). A trend analysis confirmed a similar trend as those seen in the CD-1 mice, in which higher PFOA doses induced mammary developmental delays. The presence of TEBs was minimal at PND 21, considering that estradiol levels in both strains at this time point were within the same range. However, significant defects in branching density due to PFOA exposure were visible in the 0.3 and 1.0 mg/kg PFOA groups at both PND 21 and 61 (Table 5). C57Bl/6 PFOA treated animals exhibited, at most, minimal budding and secondary branching with little to no lateral and longitudinal branching (Figure 3). At PND 61, the C57Bl/6 glands at the highest dose still had TEBs and internal budding throughout the fat pad; whereas at the same time point control glands were almost fully matured. This demonstrates that there are significant differences in the natural rate of mammary gland development in two different strains. Also, the impact of PFOA treatment on C57Bl/6 females was not as severe as that seen in CD-1 females and is likely due to differences in body burden, as serum PFOA levels were lower in C57Bl/6 than CD-1 across dose groups at the same ages. Regardless of those strain differences, the effects of PFOA on mammary gland development persisted in both strains through early adulthood.
Figure 3.
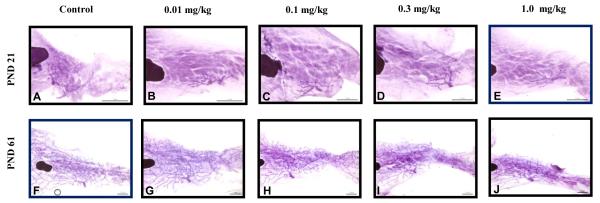
Mammary whole mount assessment of early and late pubertal glands in C57Bl/6 offspring. Representative image of control A) PND 21 and F) PND 61, 0.01 mg/kg B)PND 21 and G) PND 61, 0.1 mg/kg C) PND 21 and H)PND 61, 0.3 mg/kg D) PND 21 I) PND 61 and 1.0 mg/kg E) PND 21 and J) PND 61. C57Bl/6 n=2-10 litters/treatment group. Significant inverse trends were noted between developmental scores and PFOA dose, indicating higher PFOA exposure was related to lower (more severe) developmental scores (p<0.05).
4. Discussion
The objective of these studies was to determine the pubertal development effects of early life PFOA exposures, which produce serum concentrations in the CD-1 and C57Bl/6 female mice that coincide with reported human blood PFOA concentrations in contaminated areas, specifically evaluating for dose and strain-related effects. Perinatal low dose exposures to PFOA resulted in greater alterations in the pubertal mammary gland for both strains compared to all other measured pubertal endpoints. Mammary gland developmental delays were visible as early as PND 21 in both strains and were significantly different from controls at concentrations as low as 0.01 mg/kg in the CD-1 mouse. Our study suggests that at these low doses the strain differences, in response to PFOA, are dose dependent. Given identical oral doses during the exact same window of exposure, both strains experienced delayed mammary development at 0.3 and 1.0 mg/kg PFOA and lacked changes in the timing of other pubertal events. The CD-1 mouse responded to lower doses of PFOA, demonstrating effects on mammary development at 0.01 and 0.1 mg/kg, along with the higher doses. This is likely a reflection of the higher and longer circulating PFOA levels within the blood of the CD-1 mice. Although an increase in relative liver weight was evident in CD-1 mice and not the C57Bl/6 at 1.0 mg/kg PFOA exposure, we are unclear whether this observation was biologically relevant without having measurements from earlier time-points. From these data, we suggest that it is the peak serum PFOA concentration that regulates these effects (may have occurred between birth and within the first two weeks of life), rather than the serum PFOA level at the time of evaluation. This is further supported by earlier findings from Macon et al. [15] in which serum collected from a full gestation 1.0 mg/kg PFOA revealed that peak concentrations occurred at PND 14, rather than PND 7. To date, serum levels between PND 0 and PND 6 have yet to be reported for a full gestation study. In one study, where PFOA was administered once during late gestation (GD 17), pup serum concentrations peaked at PND 1 for all concentrations [5]. Therefore, the observed effects are likely a result of the in utero exposure, followed by exacerbation of effect from the exposure during lactation.
In these studies, PFOA induced abnormal mammary gland development in both strains. This suggests that either the other pubertal end points measured have a much different dose response threshold or that the hypothalamic ovarian axes that govern the various processes differ in their response to PFOA. Full gestation (GD1-17) PFOA exposure studies have previously reported delayed mammary development at concentrations ≤ 3.0 mg/kg in the CD-1 mouse [15]. Our current findings fully support and extend those previous observations with reduced developmental scores at the lowest dose of 0.3 mg/kg, and with an n that is 3-4 times that in the previous experiments. Previous changes were evident beginning on PND 7 and persisted until the last collection on PND 84. We confirm here the persistent mammary effects at lower doses and also determined through trend analysis that the severity of the effects increased with higher doses in both strains (p <0.001). Macon and colleagues [15]] also observed reduced mammary gland scores at PND 21 following a late gestation exposure (GD10-17) to PFOA in all concentrations including the 0.01 mg/kg dose. While they noted reduced mammary developmental scores following PFOA exposure, longitudinal growth measurements were only changed in the 1.0 mg/kg group and the number of TEBs were significantly reduced in the 0.1 and 1.0 mg/kg groups. All other quantitative measurements, however, were similar to age matched controls. Studies comparing the effects of peripubertal PFOA exposure in C57Bl/6 and Balb/c have also shown differences by strain in mammary gland development [24, 37]. Although they found no significant effects at 1.0 mg/kg in either strain compared to control for ductal length, number of TEBs or stimulated terminal ducts, C57Bl/6 animals had an increased number of TEBs compared to the Balb/c at PND 56. Many of the glands from our C57Bl/6 highest dose group (1.0 mg/kg) also exhibited poor branching and differentiation; TEBs were very apparent at PND 61, in addition to the substantial number of long ducts with little to no branching. Zhao et al. [37] also evaluated serum PFOA in C57Bl/6 and Balb/c pups, and as concentrations increased, C57Bl/6 mice were shown to have much lower serum PFOA levels compared to Balb/c females (≥5 mg/kg). While we are aware of the fact that concentration plays a major role in the depth of effects seen, our data further supports that the excretion rate for PFOA varies amongst mouse strain, and plays a significant role in whether or not PFOA affects the mammary gland at the lowest doses tested. The fact that we saw adverse mammary changes in the CD-1 mice at 0.01 and 0.1 mg/kg suggests that this strain is the most sensitive to prenatal PFOA exposures for mammary gland developmental delays.
Puberty demarks a pivotal window of susceptibility for environmental exposures and the development of endocrine regulated tissues in both humans and rodents. During this period of development, upstream pulsatile increases from gonadotropin releasing hormone trigger sex hormones, such as estrogen and progesterone, to circulate at higher concentrations. In turn, other downstream cues are prompted that result in the occurrence of VO (rodents) or first menarche (humans), sexual maturation and rapid mammary gland development. Early exposure to EDCs, however, can alter the timing at which these processes occur or alter the morphological development altogether resulting in the increased risk of development of adulthood diseases, such as breast cancer [21, 22]. Since the onset of puberty is highly influenced by sex hormones, we were also interested in measuring circulating estradiol and progesterone to determine whether PFOA contributed to altered levels. Progesterone is involved in developmental branching [15, 16, 20] and estradiol has both paracrine and endocrine roles in mammary development [15, 16, 20]. We collected all post-pubertal animals on the same stage of the estrous cycle (first day of estrus) to minimize variability and found no difference in serum estradiol or progesterone in either strain in response to PFOA exposure. These findings are both supported and contrasted by the work of Zhao et al. [20]. C57Bl/6 mice exposed to 5 mg/kg during adolescence/puberty had no change in serum estradiol, yet were reported to have increased levels of progesterone. In a follow up study using PFOA doses of 2.5 mg/kg in Balb/c or 7.5 mg/kg in C57Bl/6 the same group of researchers were unable to see significant changes in progesterone due to an inadequate number of controls in each stage of the estrous cycle; however, they reported a significant decrease in ovarian protein levels of StAR, CYP11A1, HSD3β1, HSD17β1, aromatase and PPARα, which are all involved in the biosynthesis pathway. Taken together, it appears that circulating levels of hormones have little, if any, effect on PFOA-induced decreases in mammary branching density. Thus, while other endocrine disruptors have been shown to alter estradiol and progesterone levels at much lower doses, we postulate that PFOA may alter mammary branching density through other endocrine related mechanisms that are specific to the mammary gland.
Evaluation of vaginal cytology is also a valid tool to identify the effects of toxicants, toxins or xenobiotics on the hypothalamic-pituitary-ovarian axis in rodents. In mice, VO is thought to denote the onset of puberty and is heavily influenced by increases in serum estradiol; however, it doesn’t necessarily reflect the onset of sexual maturity. In the untreated CD-1 mouse VO typically occurs around PND 23, although normal timing to this event can vary in other mouse strains. Neither strain in our study exhibited changes in timing of either VO or first estrus following prenatal exposure to PFOA. Body weights were unchanged at the time of these events, as well (Table 4). Lau et al. [13] have shown a 3 day delay for VO and first estrus at 10 mg/kg following prenatal exposure, and consistent with our findings, the 1.0 mg/kg group showed no effect. Serum levels were not reported in their study but it was implied that increased PFOA serum levels in rodents result in greater pubertal delays. Although no differences were observed between treated and control animals, there were marked differences between the timing of VO and first estrus in CD-1 controls compared to C57Bl/6 controls. This observation has been previously noted [29]. Inbred strain comparisons at puberty showed that C3H and DBA animals began VO at approximately PND 22-24, whereas C57Bl/6 mice were around PND 27 [29]. C57Bl/6 mice were also the last to exhibit cornification in that 3-strain comparison. Our data supports these findings and reinforces the idea that even in non-treated animals there appears to be differences in genetic profiles between strains that govern the timing of these occurrences. This innate difference may also influence windows of sensitivity to chemicals. Variability within the outbred CD-1 strain was also apparent within our vaginal cytology data and thus the reason for representing it in two separate blocks. The different seasons during which these blocks were tested could have contributed to these differences, as it has been noted that VO tends to occur earlier in rodents born during the summer months compared to those born during winter [38]. Our animals in block 1 were born in mid-summer whereas block 2 females were born during early winter. Body weights at VO in both blocks were similar indicating that our second block required additional time to achieve the body weight necessary to begin these events.
Serum PFOA levels were measured in our studies to determine the internal doses required for pubertal changes, such as mammary gland development, and also confirmed our lab’s previous findings that internal exposures were within the range of serum PFOA levels reported in humans living in contaminated areas of the U.S. Children in the Ohio River Valley have been reported to have geometric mean (GM) serum PFOA levels as high as 600 ng/ml [2] and more recently, reports demonstrated levels of PFOA in children aged 12-19 even within the general population (GM: 1.81 ng/ml) that are comparable to those found in adults aged ≥20 (GM 2.12) ([39]). Most noteworthy from these findings are the fact that our PND 21 serum concentrations from the 0.01 mg/kg treated groups in both strains fell within the serum range reported in male and female participants aged <12 years old from the C8 Science panel (GM 34.8 ng/ml) [3]. PFOA levels were elevated in a dose dependent manner and were within the same range at PND 21 in both strains, and were detectable in the three highest treatment groups on the final collection date in each strain.
Interesting correlations between potential rodent and adolescent health impact may be realized if the half-life of PFOA following exposure during gestation is compared. With a half-life of about 3 years in humans, by the time prenatal exposure had gone through three half-lives, a child would be around 9 or 10 years old (average age for Tanner Stage II; [40]). Similarly, in the prenatally exposed mouse pup with a PFOA half-life of 6-7 days (as calculated from our data), three half-lives would equate to PND 18-21 days old, which is also the time that the mammary tissue begins to develop. While there haven’t been any reports that have directly linked PFOA exposure to breast developmental timing in girls, new evidence suggests that there may be a correlation. Higher serum PFOA levels were associated with the length of time that 6-8 year old girls had been breastfed [41] and their source of water and serum PFOA concentrations were highly correlated. In another study [42], breastfed girl’s experienced delayed onset of breast development in comparison to formula fed girls and was even more exacerbated by the length of time that they were breastfed.
Girls living in the Ohio River Valley area reported increased delays (self-reported) to menarche within the measured concentration ranges that were observed in our 0.01 and 0.1 mg/kg group. This may indicate a lack of concordance between rodents and humans for other pubertal indicator endpoints following PFOA exposure. It has been well documented that the mode of action for these pubertal endpoints [43] are fairly different between rodents and girls and that VO and time to first estrus in rodents may not be translatable indicators for puberty timing in girls.
While most of organogenesis occurs during embryonic development, the mammary gland undergoes most of its growth during puberty. Still, exposure to excessive amounts of hormones or EDCs too early in life can have a permanent effect on the developing gland. Because mammary alterations were documented following early low dose PFOA exposures in our study and by others [23], without altering other pubertal endpoints or liver: body weight ratios, implies that the mammary gland may be the most sensitive tissue to the prenatal effects of PFOA. This can also be said for other chemicals in which the mammary gland has been studied. Early exposure to human relevant levels of endocrine disruptors in rodents has led to both accelerated (Bisphenol A) and delayed mammary gland growth (TCDD and atrazine) that have presented in the form of an extended presence of TEBs [44], increased stromal and epithelial tissue and sensitization to estradiol [45, 46, 47]. Some of those chemicals, as well as PFOA [23, 48], have adversely affected the lactational capacity of rodents to nourish their litters. Dams [48] and their F1 offspring [23] demonstrate abnormal lactating gland morphology, decreased pup weight, and/or altered milk protein gene expression following exposure to PFOA during pregnancy. Studies using slightly higher exposures of PFOA [49] have also shown that the developmental effects of prenatal exposure had long-lasting effects; increased stromal hyperplasia and disorganized, scant mammary epithelium were reported in 18 month old PFOA-exposed mice.
The hypothesized mode of action for some of these chemicals involves either direct or indirect alterations to key receptors, such as ERα, or their corresponding signaling pathways. At least within the mammary gland PFOA has been shown to not directly bind to the ERα, nor cause a stimulatory effect in ovariectomized animals [20]. PPARα knock-out mice exhibit mammary effects following a peripubertal exposure indicating that PPARα may not play a predominate role in the mammary gland changes observed after PFOA exposure as those seen in rodent livers [20]. Work by Macon and colleagues (in press) suggest that post transcriptional modifications may play a role in the observed mammary changes [50]. Pparα and γ expression were decreased in microarray validation studies, however, only Pparγ protein levels were increased. Additionally, Pparγ molecular weight shifts were also found in Westerns, leading the authors to propose phosphorylating modifications. The stroma may play an important role, as lipid metabolism genes were also found to be altered. Therefore, we conclude that during early PFOA exposures post- transcriptional modifications in the gland itself may be responsible for the altered epithelium and microenvironment. Also, since PFOA was still present in the serum 5 weeks following weaning, as a result of a lactational exposure, the effects may have been compounded and the peripubertal mechanism suggested by Zhao et al. [20] may have also taken effect. Regardless of the mechanism behind the outcome, each of these chemicals, including PFOA, has the potential to significantly alter the glands normal development, thereby increasing the gland’s risk to further environmental insults.
The few studies that have tried to correlate breast cancer risks to perfluorinated chemicals (PFCs) found that in a Greenland Inuit cohort the PFC levels were linear to the cancer risks [51], while a Danish cohort reported weak significance [52]. Whether PFOA directly causes cancer still requires additional evaluation, however, the delayed phenotype that it creates in the rodent ultimately leaves the gland more susceptible to other harmful exposures. Because of the potential for increased lifetime susceptibility to disease or dysfunction, pubertal assessments of chemical effect performed in rodent models should include an evaluation of the mammary gland. This suggestion has recently been echoed by the scientific community [53, 54, 55]. It will also be necessary to evaluate for changes in breast development based on early life or cord blood PFOA levels in future epidemiological studies.
Although PFOA production and use in the USA is scheduled to be phased out by 2015 [55], its lasting effects may continue to be seen well after this time due to its persistence within the environment and long half-life in humans. Because PFOA exposure occurs throughout the lifetime in humans, it may be necessary to continue monitoring heavily exposed populations to determine if later life diseases arise. These studies confirm that the mammary gland is highly sensitive to low dose prenatal PFOA exposure. Our findings suggest that measures should be put in place to limit PFOA exposures in sensitive populations, especially in pregnant women and prepubescent females. Decreased exposure could potentially minimize early life effects, as well as those that may manifest throughout life, to reduce mammary gland susceptibility for later life disease and dysfunction.
HIGHLIGHTS.
Given an identical oral exposure, C57Bl/6 offspring achieve lower serum PFOA than CD-1
In utero exposure to PFOA stunts the developing mammary gland of CD-1 and C57Bl/6 mice
Mouse pups demonstrate a potential for a shorter PFOA half-life than adult mice
Early PFOA exposure alters the mammary gland without changing other pubertal endpoints
Acknowledgements
The authors would like to thank Dr. Grace Kissling, NIEHS, for helping with statistical assessments and the ILS, Inc. team for contracted assistance with serum hormone analysis.
Funding This work was supported by the National Toxicology Program, National Institute of Environmental Health Sciences, NIH. Deirdre Katrese Tucker was funded by an Intramural Research Training Award, NIEHS, NIH and the Curriculum of Toxicology at UNC Chapel Hill, Chapel Hill, NC, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest The authors declare that there are no competing financial or other conflicts of interest.
References
- [1]. [Accessed February 20, 2014];CDC Fourth National Report on Human Exposure to Environmental Chemicals: Updated tables March 2013. http://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Mar2013.pdf.
- [2].Emmett E, Zhang H, Shofer F, Freeman D, Rodway N, Desai C, Shaw L. Community exposure to perfluorooctanoate: relationships between serum levels and certain health parameters. J Occup Environ Med. 2006;48(8):771–779. doi: 10.1097/01.jom.0000233380.13087.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Frisbee S, Brooks A, Maher A, Flensborg P, Arnold S, Flethcher T, Steenland K, Shankar A, Knox S, Pollard C, Halverson J, Vieira V, Jin C, Leyden L, Ducatman A. The C8 health project: design, methods, and participants. Environ Health Perspect. 2009;117(12):1873–1882. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Apelberg B, Witter F, Herbstman J, Calafat A, Halden R, Needham L, Goldman L. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect. 2007;115(11):1670–1676. doi: 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fenton S, Reiner J, Nakayama S, Delinsky A, Stanko J, Hines E, White S, Lindstrom A, Strynar M, Petropoulou S. Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod Toxicol. 2009;27(3-4):365–372. doi: 10.1016/j.reprotox.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fromme H, Midasch O, Twardella D, Angerer J, Boehmer S, Liebl B. Occurrence of perfluorinated substances in an adult German population in southern Bavaria. Int Arch Occup Environ Health. 2007;80(4):313–319. doi: 10.1007/s00420-006-0136-1. [DOI] [PubMed] [Google Scholar]
- [7].Olsen G. Evaluation of the half-life (t1/2) of elimination of perfluorooctanesulfonate (PFOS), perfluorohexanesulfonate (PFHS), and perfluorooctanoate (PFOA) from human serum; International Syposium on Fluorinated Alkyl Organics in the Environment, TOX017; FLUOROS. 2005. [Google Scholar]
- [8].Mondal D, Weldon R, Armstrong B, Gibson L, Lopez-Espinosa M, Shin H, Fletcher T. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ Health Perspect. 2014;122(2):187–192. doi: 10.1289/ehp.1306613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Guerrero-Preston R, Goldman L, Brebi-Mieville P, Ili-Gangas C, Lebron C, Witter F, Apelberg B, Hernandez-Roystacher M, Jaffe A, Halden R, Sidransky D. Global DNA hypomethylation is associated with in utero exposure to cotinine and perfluorinated alkyl compounds. Epigenetics. 2010;5(6):539–546. doi: 10.4161/epi.5.6.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Knower K, To S, Leung Y, Ho S, Clyne C. Endocrine disruption of the epigenome: a breast cancer link. Endocrine Related Cancer. 2014;21(2):T33–T-55. doi: 10.1530/ERC-13-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Biegel L, Hurtt M, Frame S, O’Connor J, Cook J. Mechanisms of extrahepatic tumor induction by peroxisomes proliferators in male CD rats. Toxicol Sci. 2001;60(1):44–55. doi: 10.1093/toxsci/60.1.44. [DOI] [PubMed] [Google Scholar]
- [12].Kennedy G, Butenhoff J, Olsen G, O’Connor J, Seacat A, Perkins R, Biegel L, Murphy S, Farrar D. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34(4):351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- [13].Lau C, Thibodeaux J, Hanson R, Narotsky M, Rogers J, Lindstrom A, Stryar M. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci. 2006;90(2):510–518. doi: 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- [14].Onishchenko N, Fischer C, Wan Ibrahim W, Negri S, Spulber S, Cottica D, Ceccatelli S. Prenatal exposure to PFOS or PFOA alters motor function in mice in a sex-related manner. Neurotox Res. 2011;19(3):452–461. doi: 10.1007/s12640-010-9200-4. [DOI] [PubMed] [Google Scholar]
- [15].Macon M, Villanueva L, Tatum-Gibbs K, Zehr R, Strynar M, Stanko J, White S, Helfant L, Fenton S. Prenatal perfluorooctanoic acid exposure in CD-1 mice: low-dose developmental effects and internal dosimetry. Toxicol Sci. 2011;122(1):134–145. doi: 10.1093/toxsci/kfr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gilberstein G. Postnatal mammary gland morphogenesis. Microscopy Research and Technique. 2001;52:155–162. doi: 10.1002/1097-0029(20010115)52:2<155::AID-JEMT1001>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- [17].Gjorevski N, Nelson C. Integrated morphodynamic signaling of the mammary gland. Nature Reviews Molec Cell Biol. 2011;12:581–593. doi: 10.1038/nrm3168. [DOI] [PubMed] [Google Scholar]
- [18].Tiede B, Kang Y. From milk to malignancy; the role of mammary stem cells in development, pregnancy and breast cancer. Cell Research. 2011;21:245–257. doi: 10.1038/cr.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Endocrine Disruptors: Basic Information. US EPA Office of Science and Coordination and Policy; [Accessed on July 1, 2014]. http://www.epa.gov/endo/pubs/edspoverview/whatare.htm. [Google Scholar]
- [20].Zhao Y, Tan Y, Haslam S, Yang C. Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in C57BL/6 mice. Toxicol Sci. 2010;115(1):214–224. doi: 10.1093/toxsci/kfq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fenton S. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147(6 Suppl):S18–24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- [22].Macon M, Fenton S. Endocrine disruptors and the breast: early life effects and later life disease. J Mammary Gland Biol Neoplasia. 2013;18(1):43–61. doi: 10.1007/s10911-013-9275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].White S, Fenton S, Hines E. Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol. 2011;127(1-2):16–26. doi: 10.1016/j.jsbmb.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang C, Tan Y, Harkema J, Haslam S. Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains. Reprod Toxicol. 2009;27(3-4):299–306. doi: 10.1016/j.reprotox.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lopez-Espinosa J, Fletcher T, Armstrong B, Genser B, Dhatariya K, Mondal D, Ducatman A, Leonardi G. Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ Sci Technol. 2011;45(19):8160–8166. doi: 10.1021/es1038694. [DOI] [PubMed] [Google Scholar]
- [26].Vandenberg L, Colborn T, Hayes T, Heindel J, Jacobs D, Lee D, Shioda T, Soto A, vom Saal F, Welshons W, Zoeller R, Myers J. Hormones and endocrine disrupting chemicals: low dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Whitten W. Modification of the oestrous cycle of the mouse by external stimuli associated with the male. J Endocrinol. 1956;13(4):399–404. doi: 10.1677/joe.0.0130399. [DOI] [PubMed] [Google Scholar]
- [28].Whitten W, Bronson F, Greenstein J. Estrus-inducing pheromone of male mice: transport by movement of air. Science. 1968;161(3841):584–585. doi: 10.1126/science.161.3841.584. [DOI] [PubMed] [Google Scholar]
- [29].Silver L. [Accessed September 13, 2014];Mouse Genetics: Concepts and applications. http://www.informatics.jax.org/silver/chapters/4-3.shtml.
- [30].Nelson J, Karelus K, Felicio L, Johnson T. Genetic Influences on the timing of Puberty in Mice. Biol Reprod. 1990;42:649–655. doi: 10.1095/biolreprod42.4.649. [DOI] [PubMed] [Google Scholar]
- [31].Caligioni C. Assessing Reproductive Status/Stages in Mice. Curr Prot Neurosci. 2009;48:A.4I.1–A.4I.8. doi: 10.1002/0471142301.nsa04is48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Davis B, Fenton S. The mammary gland. In: Haschek WM, Rousseaux CG, Wallig MA, editors. Haschek and Rousseaux’s Handbook of Toxicologic Pathology. 3rd Edition. Elsevier, Inc., Academic Press (USA); 2013. pp. 2665–2694. Chapter 61. [Google Scholar]
- [33].Reiner J, Nakayam S, Delinsky A, Stanko J, Fenton S, Lindstrom A, Strynar M. Analysis of PFOA in dosed CD1 mice. Part 1. Methods development for the analysis of tissues and fluids from pregnant and lactating mice and their pups. Reprod Toxicol. 2009;27(3-4):360–364. doi: 10.1016/j.reprotox.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lou I, Wambaugh J, Lau C, Hanson R, Lindstrom A, Strynar M, Zehr R, Setzer R, Barton H. Modeling single and repeated dose pharmacokinetics of PFOA in mice. Toxicol Sci. 2009;107:331–341. doi: 10.1093/toxsci/kfn234. [DOI] [PubMed] [Google Scholar]
- [35].Brisken C, O’Malley B. Hormone action in the mammary gland. Cold Spring Harbor Perspect Biol. 2010;2(a003178):1–17. doi: 10.1101/cshperspect.a003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bordini B, Rosenfield R. Normal pubertal development: Part I: The endocrine basis of puberty. Pediatrics in Review. 2011;32(6):223–229. doi: 10.1542/pir.32-6-223. [DOI] [PubMed] [Google Scholar]
- [37].Zhao Y, Tan Y, Strynar M, Perez G, Haslam S, Yang C. Perfluorooctanoic acid effects on ovaries mediate its inhibition of peripubertal mammary gland development in Balb/c and C57Bl/6 mice. Reprod Toxicol. 2012;33(4):563–576. doi: 10.1016/j.reprotox.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yoon C. Homeostasis Associated with Heterozygosity in the Genetics of Timing of Vaginal opening in the house mouse. Genetics. 1955;40(3):297–309. doi: 10.1093/genetics/40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. [Accessed July 21, 2014];CDC Fourth National Report on Human Exposure to Environmental Chemicals: Updated tables July 2014. http://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Jul2014.pdf.
- [40].Biro F, Greenspan L, Galvez M, Pinney S, Teitelbaum S, Windham G, Deardorff J, Herrick R, Succop P, Hiatt R, Kushi L, Wolff M. Onset of breast development in a longitudinal cohort. Pediatrics. 2013 doi: 10.1542/peds.2012-3773. doi: 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pinney S, Biro F, Windham G, Herrick R, Yaghjyan L, Calafat A, Succop P, Sucharew H, Ball K, Kato K, Kushi L, Bornschein R. Serum biomarkers of polyfluoroalkyl compound exposure in young girls in Greater Cincinnati and the San Francisco Bay Area, USA. Environ Poll. 2014;184:327–334. doi: 10.1016/j.envpol.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kale A, Deardorff J, Lahiff M, Laurent C, Greenspan L, Hiatt R, Windham G, Galvez M, Biro F, Pinney S, Teitelbaum S, Wolff M, Barlow J, Mirabedi A, Lasater M, Kushi L. Breastfeeding versus formula-feeding and girls’ pubertal development. Maternal and Child Health Journal. 2014 doi: 10.1007/s10995-014-1533-9. DOI 10.1007/s10995-014-1533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Buck G, Gray L, Marcus M, Ojeda S, Pescovitz O, Witchel S, Sippell W, Abbott D, Soto A, Tyl R, Bourguignon J, Skakkebaek N, Swan S, Golub M, Toppari J, Euling S. Environmental factors and puberty timing: expert panel research needs. Pediatrics. 2008;121(Sup 3):S192–S207. doi: 10.1542/peds.1813E. [DOI] [PubMed] [Google Scholar]
- [44].Fenton S, Hamm J, Birnbaum L, Youngblood G. Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Sci. 2002;67(1):63–74. doi: 10.1093/toxsci/67.1.63. [DOI] [PubMed] [Google Scholar]
- [45].Markey C, Luqui E, Munoz-de Toro M, Sonnenschein C, Soto A. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Repro. 2001;65(4):12515–1223. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- [46].Munoz-de Toro M, Markey C, Wadia P, Luque E, Rubin B, Sonnenschein C, Soto A. Perinatal exposure to bisphenol A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146(9):4138–4147. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wadia P, Vandenberg L, Schaeberle C, Rubin B, Sonnenschein C, Soto A. Perinatal bisphenol A exposure increases estrogen sensitivity of the mammary gland in diverse mouse strains. Environ Health Perspect. 2007;115(4):592–598. doi: 10.1289/ehp.9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].White S, Calafat A, Kuklenyik Z, Villanueva L, Zehr R, Helfant L, Strynar M, Lindstrom A, Thibodeaux J, Wood C, Fenton S. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci. 2007;96(1):133–144. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- [49].White S, Kato K, Jia L, Basden B, Calafat A, Hines E, Stanko J, Wolf C, Abbott B, Fenton S. Effects of Perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross foster and restricted gestational exposure. Reprod Toxicol. 2007;27:289–298. doi: 10.1016/j.reprotox.2008.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Macon M, Ren H, Fenton S. Prenatal PFOA exposure alters gene expression pathways in murine mammary gland. Toxicol Sci. doi: 10.1093/toxsci/kfu253. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bonefeld-Jorgensen C, Long M, Bossi R, Ayotte P, Asmund G, Kruger T, Ghisari M, Mulvad G, Kern P, Nzulumiki P, Dewailly E. Perfluorinated compounds are related to breast cancer risk in Greenlandic Inuit: a case control study. Environ Health. 2011;10:88. doi: 10.1186/1476-069X-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bonefeld-Jørgensen E, Long M, Fredslund S, Bossi R, Olsen J. Breast cancer risk after exposure to perfluorinated compounds in Danish women: a case-control study nested in the Danish National Birth Cohort. Cancer Causes Control. 2014 doi: 10.1007/s10552-014-0446-7. PMID: 25148915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].IBCERCC. Interagency Breast Cancer and the Environment Research Coordinating Committee. Department of Health and Human Services [February, 2013];Breast Cancer and the Environment: Prioritizing Prevention. Available at: http://www.niehs.nih.gov/about/assets/docs/ibcercc_full_508.pdf.
- [54].Osborne G, Rudel R, Schwarzmann M. Evaluating chemical effects on mammary gland development: A critical need in disease prevention. Reprod Toxicol. 2014 Aug 1; doi: 10.1016/j.reprotox.2014.07.077. available online. Doi: 10.1016/j.reprotox.2014.07.077. [DOI] [PubMed] [Google Scholar]
- [55]. [Accessed July 21, 2014];US EPA Chemical Safety and Pollution Prevention-Pollution Prevention and Toxics: Perfluorooctanoic Acid (PFOA) and Fluorinated Telomers. http://www.epa.gov/oppt/pfoa/