Abstract
During the last decade, visual illusions have been used repeatedly to understand similarities and differences of visual perception of human and non-human animals. However, nearly all studies have focused only on illusions not related to motion perception and, to date, it is unknown whether non-human primates perceive any kind of motion illusion. In the present study we investigated whether rhesus monkeys (Macaca mulatta) perceived one of the most popular motion illusions in humans, the Rotating Snake illusion (RSI). To this purpose, we set up four experiments. In Experiment 1 subjects initially were trained to discriminate static vs. dynamic arrays. Once reaching the learning criterion, they underwent probe trials in which we presented the RSI and a control stimulus identical in overall configuration with the exception that the order of the luminance sequence was changed in a way that no apparent motion is perceived by humans. The overall performance of monkeys indicated that they spontaneously classified RSI as a dynamic array. Subsequently, we tested adult humans in the same task with the aim of directly comparing the performance of human and non-human primates (Experiment 2). In Experiment 3 we found that monkeys can be successfully trained to discriminate between the RSI and a control stimulus. Experiment 4 showed that a simple change in luminance sequence in the two arrays could not explain the performance reported in Exp. 3. These results suggest that some rhesus monkeys display a human-like perception of this motion illusion, raising the possibility that the neurocognitive systems underlying motion perception may be similar between human and non-human primates.
Keywords: Motion illusion, Rotating Snake illusion, Macaca mulatta, Comparative perception
INTRODUCTION
The visual system provides organisms with simultaneous information in their environment, including perceptions of size, shape, colour and movement of objects in a short amount of time. For humans, these perceptions are subjective, and the perception of non-human animals is likely to be subjective too. We have learned much about the physiology of photoreceptors and neural circuits supporting vision in non-human animals (e.g., Desimone and Gross 1979; Shimizu and Bowers 1999). However, physiological and neuro-anatomical studies are blind with respect to the perceptual experience of non-human animals, and behavioral studies requiring subjects to discriminate among objects differing in one physical feature are still fundamental to understand how vertebrates perceive the world.
The examination of the existence of visual illusions has been adopted as an experimental tool to compare visual perception in different species (e.g., Agrillo et al. 2013; Nakamura et al. 2008; Watanabe et al. 2013). Studies on visual illusions are also fundamental for comparisons with physiological results because they allow us to test whether neural responses correlate with the physical or perceptual features of objects. Comparative psychology has demonstrated that chimpanzees perceive the Delboeuf illusion (Parrish and Beran 2014), rhesus monkeys perceive the regular-random numerosity illusion (Beran 2006) as well as the Zöllner illusion (Agrillo et al. 2014a), and capuchin monkeys perceive the Müller-Lyer illusion (Suganuma et al. 2007). Similarly, different non-primate species also perceive the Ponzo illusion (pigeons: Fuijta et al. 1991), the Ebbinghaus illusion (domestic chicks, Rosa Salva et al. 2013) and the Kanizsa triangle (bamboo sharks, Fuss et al. 2014; redtail splitfin, Sovrano and Bisazza 2009), suggesting that the perceptual mechanisms to process size, numerosity, orientation and length are similar among vertebrates.
However, most of these studies investigated illusions not involving motion perception. Motion perception is crucial for surviving for non-human animals because it allows them to hunt and to avoid being hunted. If a similar motion processing system is evolved in human and in non-human animals it is expected that any constraints of the system will be similar. Consequently, species others than humans should perceive the same motion illusions that humans perceive.
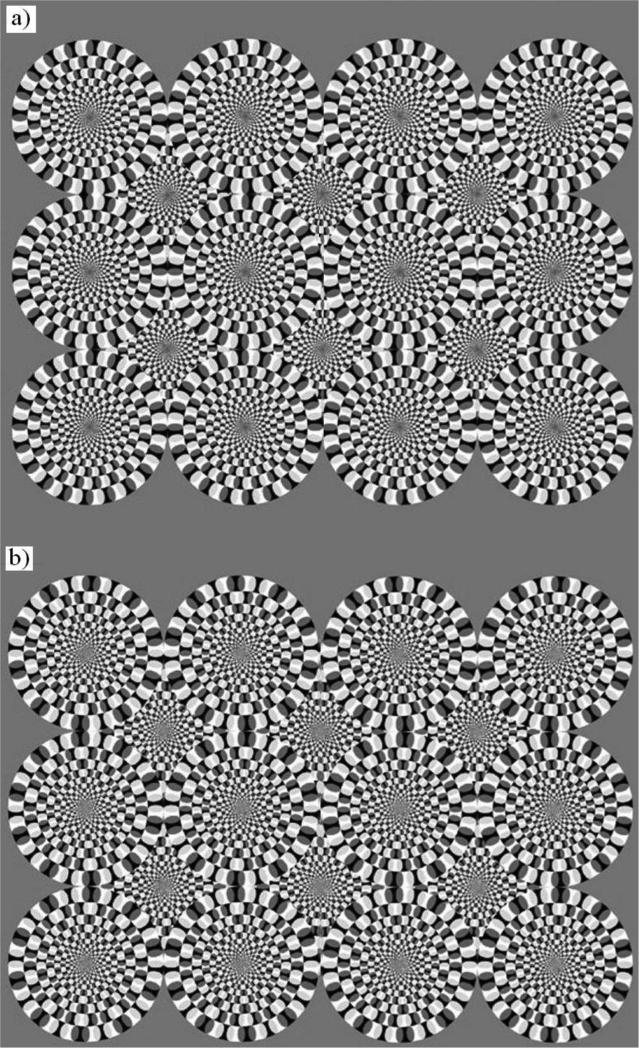
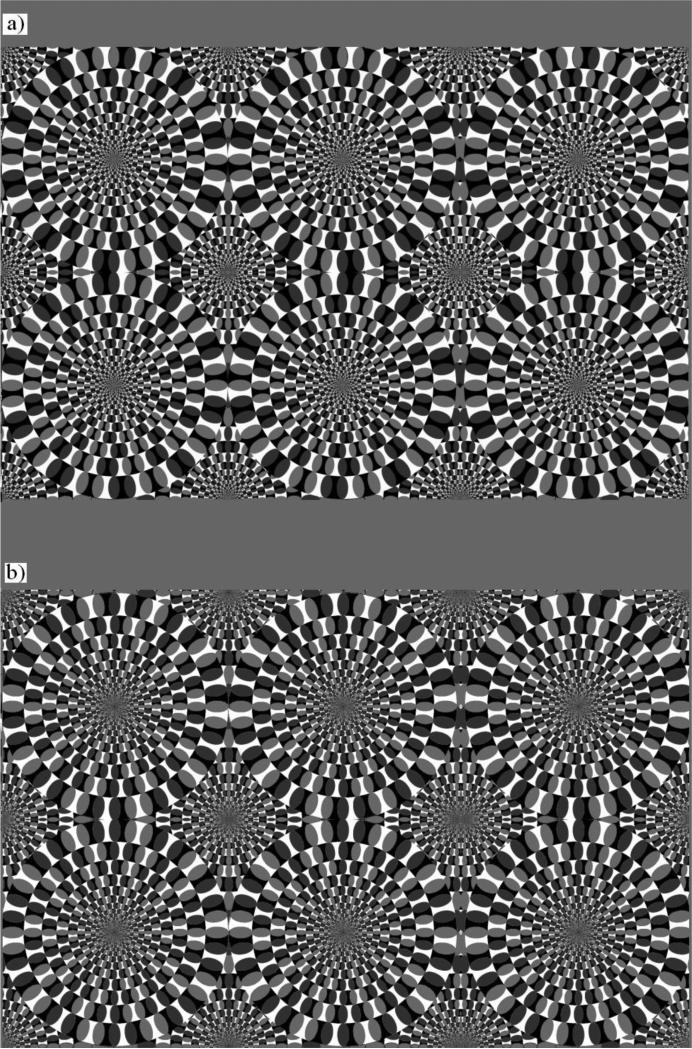
Motion illusions refer to a perception of motion that is really absent (e.g. Kitaoka and Ashida 2003; Gori et al. 2006; Spillmann 2013) and/or different (in direction, strength, etc., Gori et al. 2006, 2010, 2011) from what it is present in the physical stimulus. The illusory motion is experienced as “real motion,” and it should not be confused with the perception of dynamism evocated by specific characteristics in paintings (Gori et al. 2008) or by the speed lines often used in comics (Burr 2000). One of the most famous motion illusions that elicits global illusory motion by means of a static pattern in the human literature is called the ‘Rotating Snakes’ illusion (RSI) (Kitaoka and Ashida 2003). The illusion is a much more powerful variation of Fraser and Wilcox's (1979) pattern and consists of specific periodical arrangement of blobs having different luminance (presented according to the following luminance sequence: black - dark grey - white - light grey) along concentric circles (Fig. 1a). Only this luminance sequence is able to produce illusory motion perception in humans (e.g. Murakami et al. 2006). As opposed to other popular motion illusions, such as the Enigma illusion (Gori et al. 2006), the direction of rotation is always in the dark-to-light direction without reversals (Faubert and Herbert 1999), and it appears stronger in a stimulus presented in the peripheral visual field (Naor-Raz and Sekuler 2000). A large percentage of human observers perceive a strong global rotatory motion in the circles. The mechanisms underlying this illusion are still debated. Conway et al. (2005) reported that the differences in response latency to different contrast elements are responsible for the illusion, and they provided the first evidence that pairs of stimuli with the above mentioned luminance sequence can generate motion signals in V1 direction-selective neurons. Backus and Oruç (2005) introduced the effect of adaptation to model the smooth motion perception over several seconds. These authors hypothesized that gaze instability can improve the strength of the illusion, even though it is not necessary. They claimed that the illusion is caused directly by adaptation over time, rather than being driven by eye movements, suggesting that changes over time in the adapted states of visual neurons are sufficient to evoke a strong percept of motion (Petrov and Popple 2002). Tomimatsu et al. (2011) also confirmed the crucial role of adaptation in the Fraser-Wilcox Illusion. Other authors stressed the crucial role of the fixational eye movements in eliciting the illusory motion. While Murakami et al. (2006) and Beer et al. (2008) proposed fixational drifts as the main fixational eye movements responsible for the illusion, Otero-Millan et al. (2012) demonstrated the role of transient oculomotor events (such as microsaccades, saccades, and blinks) in initiating the illusory motion perception. The importance of cortical activity has been highlighted by other researchers. Specifically, using fMRI, Ashida et al. (2012) found an increase of neural activity in the motion network cortex during observation of the RSI pattern and concluded that local motion signals in response to asymmetric spatial patterns at the level of V1 would be integrated in the V5-MT complex, ultimately responsible for the rotatory motion perception. Ruzzoli et al. (2011) showed how illusory rotation in the Enigma illusion is based on activity in the motion sensitive cortical areas, suggesting that global illusory rotation needs the activation of a cortex characterized by large receptive fields, such as V5-MT complex.
Figure 1. Stimuli used in probe trials of Experiment 1.
(a) Rotating Snake illusion and (b) control stimulus. The former array is commonly perceived by humans as a dynamic array, the latter presents a similar overall configuration but the luminance relationship among the blobs is inverted. No illusory motion is commonly perceived by humans in this case.
To date, only two studies have investigated the RSI in non-human animals. Bååth et al. (2014) did an online survey in which participants (cats’ owners) were required to indicate whether their pet cats reacted somehow to the RSI (e.g., if they attacked one point of the illusory pattern). Results of the survey indicated that 29% of the respondents claimed that their cat reacted to the illusory pattern. This indirectly suggests that cats might be attracted by the illusory motion of the concentric blobs. An empirical investigation has been recently conducted in fish: Gori et al. (2014a) trained zebrafish and guppies to select the dynamic array from two alternative arrays composed by static and dynamic objects. In the test phase, fish were presented with the RSI and a control stimulus: results showed that both species classified the RSI as a dynamic array. However, the fact that such distantly-related species compared to humans show a human-like performance of this visual pattern does not necessarily provide information about how other primates might perceive the RSI. As fish and non-human primates evolved different perceptual mechanisms with respect of some features of objects (e.g., global-local preference: Spinozzi et al. 2003; Truppa et al. 2010; Ebbinghaus illusion: Parron and Fagot 2007; Sovrano et al. 2014), a direct investigation of this phenomenon in monkeys is needed.
In the present study we investigated whether rhesus monkeys perceived the RSI. Experiment 1 assessed whether monkeys spontaneously classified the RSI as a dynamic array. Monkeys initially were trained to discriminate between static and dynamic arrays of two-dimensional objects. After reaching the learning criterion, they were presented with the RSI and a control stimulus composed by the same local features of the RSI but flipped in their order, this stimulus does not evoke any motion perception in humans. If they perceived the illusory motion we expected a significant bias in classifying the RSI as a dynamic array. Subsequently, we set up a control experiment with human participants to compare the performance of human and non-human primates in the presence of the same experimental material (Experiment 2). In Experiment 3 we trained monkeys to discriminate between the RSI and a control stimulus. As subjects easily learned to discriminate between the two arrays, Experiment 4 investigated whether monkeys’ performance in Experiment 3 was based on the apparent motion perception of the RSI or, instead, was based on the only physical difference that existed between the two stimuli - a difference in the luminance sequence of the concentric blobs.
EXPERIMENT 1
Experiment 1 was designed to assess whether monkeys spontaneously classified the illusory pattern as a dynamic array. Subjects were trained to assess whether the arrays presented on the screen were composed of static or dynamic objects. After reaching the learning criterion, monkeys started the test phase in which they were presented with novel configurations of objects, to assess their ability to generalize the learned rule, including the RSI and a control stimulus. The assumption was that, if they perceived the illusory motion, they would classify the RSI as a dynamic array.
Subjects
Six adult male rhesus monkeys from the Language Research Center in Atlanta (Georgia, USA) were tested, including Gale (age 29), Luke (age 13), Hank (age 29), Lou (age 19), Obi (age 9) and Murph (age 19). All monkeys were captive reared and arrived at the Language Research Center in the first few years of life for training on the joystick apparatus. Subjects were singly housed during the daily test sessions with visual and auditory access to other monkeys and were paired housed for one full day per week with indoor-outdoor access to enclosures filled with enrichment items and natural substrate. When they were in their home cage, subjects had constant access to their computerized testing apparatus, thus permitting a free engagement in several cognitive/perceptual tasks to earn food pellets throughout the day. They had 24-hour access to water and were daily fed primate chow, fruits, and vegetables regardless to the experimental trials they completed. The current study complied with protocols approved by the Georgia State University IACUC and was in full accordance with the USDA Animal Welfare Act and the “Guidelines for the use of laboratory animals.”
Apparatus and stimuli
The Language Research Center's Computerized Test System, consisting of a personal computer, color monitor (Acer V173 B 17” LCD, 55-75Hz vertical refresh rate, 30-80KHz horizontal scanning frequency), digital joystick, and food pellet dispenser, was used for testing (Richardson et al. 1990). Monkeys manipulated a joystick generating isomorphic movements of a cursor on the attached screen. Normal viewing distance ranged from 30.5 to 40.6 cm, a distance commonly used to investigate visual perception in rhesus monkeys (e.g., Agrillo et al. 2014a, b). Visual angle cannot be estimated as subjects were free to move inside their home cage during the experimental session. Microsoft Visual Basic 6.0 was used to design the computer program. Food rewards were 94 mg Bio-Serv food pellets dispensed as soon as the subjects selected the correct response. All monkeys had extensive experience with computerized testing (e.g., Agrillo et al. 2014a; Beran 2006; Beran and Parrish 2013).
The stimuli employed in the training phase were previously used in the study on the RSI in fish (Gori et al. 2014a) and consisted of pairs of arrays composed by two-dimensional objects (e.g., circles, ovals, wheels, gears, squares, rectangles, diamonds; see supplemental material). Arrays had either an overall square or rectangular shape but were all inscribed within a 12 × 12 cm square during the experiment (software scaling). Within each stimulus pair, the same array could be presented in two different versions: in one version the objects rotated in either clockwise or counterclockwise direction at three different speeds (5/15/21 rpm); in the other version all figures were static. Moving and static arrays were created and presented through Adobe Flash CS4®. A total of 30 different pairs of stimuli were used and were presented randomly during the training phase.
In the test phase we presented 30 novel configurations to assess monkeys’ ability to generalize the learned rule to novel arrays. The test phase also introduced probe trials with the RSI and its control stimulus. This control stimulus – previously adopted by Murakami et al. (2006) - is identical to the illusory pattern with the exception that the luminance relationship among the blobs is inverted which completely breaks down the illusory motion perception in humans (Fig. 1b). Two different versions were created for both stimuli, each having the same size: 6-circle snakes (larger circles) or 12-circle snakes (smaller circles).
Procedure
Two different tasks were presented: a) relative judgment task and b) absolute judgment task. Three monkeys completed each task. In the relative judgment task, monkeys were required to choose which array was/appeared dynamic between two alternative arrays placed in opposite positions on the computer screen. The absolute judgment task required monkeys to assess whether the solitary array presented in the middle of the screen was/appeared static or dynamic.
Relative judgment task
Two phases were used in this task: training and test phase.
Training phase
We tested Hank, Murph and Luke. At the outset of each trial, a light grey colored rectangle appeared in the above-center part of the computer screen that was medium grey in background color. At the bottom center of the screen was a small red circle that was the cursor under control of the joystick. Subjects moved the cursor into contact with the light grey rectangle to see the next pair of arrays. These arrays were presented at left center and right center of the screen, with the red cursor directly between them. Subjects were reinforced for selecting the dynamic array. Incorrect responses led to a buzz tone and a 20 second timeout during which the screen remained blank. Correct responses led to a melodic chime sound and the delivery of a single food pellet. In either case, there was a 1 second inter-trial interval, and then the cursor and the light grey rectangle appeared to indicate the start of the next trial. As subjects worked at their own pace for different session durations, the trial counts per session varied as did the number of sessions completed. Monkeys were admitted to the test phase only after reaching the learning criterion which was 75% correct responses in the last two consecutive sessions.
Test phase
This phase was identical to the training phase with the exception that novel configurations were presented, including the RSI and its control stimulus. When pairs of static and dynamic stimuli were presented, monkeys continued to receive the reward or a 20 second timeout in case of, respectively, correct and incorrect response. However, no auditory feedback or reward/timeout was provided for any response in the presence of the RSI and the control stimulus, and the 1 second inter-trial interval began immediately after subjects’ response. The RSI and the control stimulus were presented with a random probability of 0.15 on each trial. If monkeys perceived the illusory motion we should expect a bias to choose the RSI. The experiment ended as soon as subjects performed 40 trials with the 6-circle snakes and 40 trials with the 12-circle snakes (for a total of 80 probe trials).
Absolute judgment task
Training and test
We tested Obi, Lou and Gale. In this task, monkeys were presented with a single array (the same configurations presented simultaneously in the relative judgment task) and were required to classify whether the array was static or dynamic.
Each trial presented the monkeys with a cursor that they controlled, the stimulus array and two choice icons (two different arbitrary icons used only for this experiment). The choice icons were bottom justified on the left/right side of the screen. Monkeys controlled the cursor that was centered between the choice icons and moved that cursor into contact with one of the choice icons to indicate if they perceived a static (left icon) or a dynamic (right icon) array. After reaching the learning criterion (the same used in the relative judgment task), the monkeys advanced to the test phase in which novel configurations, including the RSI and the control stimulus, were presented.
For both tasks, statistical analyses were conducted using SPSS 20.0. We first analyzed individual performance in each task. Subsequently, a group analysis was performed to assess monkeys’ overall perception of the RSI. Means and standard deviations are reported. Average reaction times were also reported in this and in the following experiments, as a general measure of the difficulty of the task. Reaction times longer than 5 seconds were excluded as subjects were not trained to respond as quickly as possible and monkeys sometimes were found to interrupt the trial before providing their response for other activities such as drinking water or observing another monkey in another part of the laboratory. We did not perform inferential statistical analysis on reaction times as longer /shorter reaction times would have not provided us useful insights about the monkeys’ perception of motion.
Results and discussion
Relative judgment task
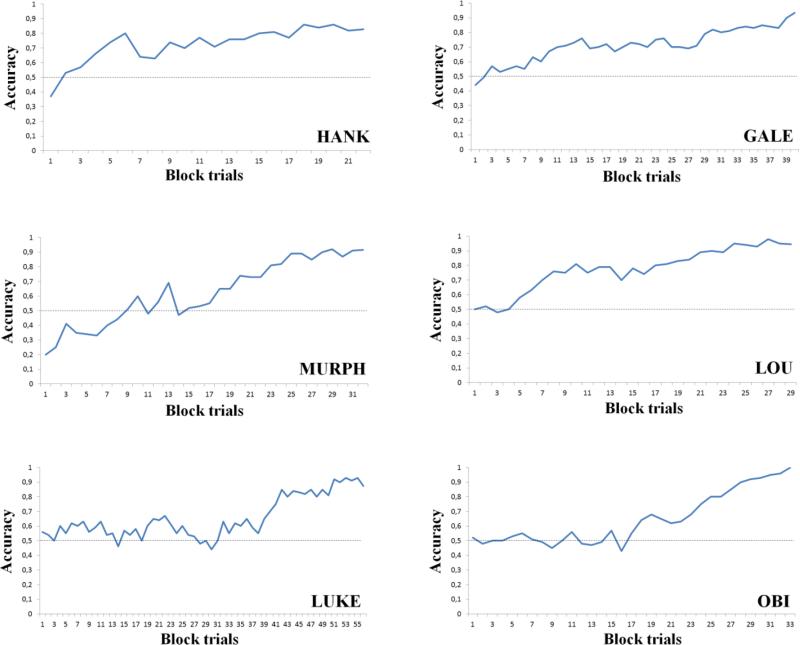
All subjects reached the learning criterion: Hank reached the criterion in 4 sessions (2,235 trials), Murph did so in 6 sessions (3,294 trials), and Luke needed 11 sessions (5,608 trials, Figure 2). The mean reaction time was 1.527 s (standard deviation, sd = 0.928 s). In the test phase, subjects generalized the learned rule to novel arrays at statistically significant levels (binomial tests, Table 1; mean reaction time: 1.409 s (sd = 0.883 s)). In probe trials showing the RSI and the control stimulus, Luke showed a significant bias to classify both the 6-circle and 12-circle RSI as a dynamic array. Murph showed no bias. Hank showed a human-like perception of the RSI only with the 12-circle snakes, whereas he showed the opposite bias for the 6-circle snakes (binomial tests, Table 1; mean reaction time: 1.734 s (sd = 0.520 s)).
Figure 2. Learning curves of training phase, Experiment 1.
Accuracy (proportion of correct choices) is plotted against block (100 trials in each block) for each monkey. As soon as monkeys reached the learning criterion (75% correct choices in the last two sessions), the training phase ended and subjects began the test phase. All subjects reached the criterion. The dashed lines represent chance level.
Absolute judgment task
All subjects reached the learning criterion: Obi reached the criterion in 6 sessions (3,320 trials), Lou in 5 sessions (2,936 trials), and Gale needed 10 sessions (4,075 trials, Figure 2). The mean reaction time was 1.670 s (sd = 0.576 s). In the test phase, monkeys generalized the learned rule to novel arrays at statistically significant levels (binomial tests, Table 1; mean reaction time: 1.587 s (sd = 0.697 s)). In probe trials showing the RSI and the control stimulus, Lou showed a significant bias in support of a human-like perception of RSI only with 6-circle snakes, while Gale showed the same bias with 12-circle snakes. Obi showed no bias (binomial tests, Table 1; mean reaction time with the RSI: 1.843 s (sd = 0.982 s)). The control stimulus was correctly classified as a static array by all subjects (mean accuracy: Gale: 0.825; Obi: 0.875; Lou: 0.900, binomial tests, all P < 0.05; mean reaction time with control stimulus: 1.543 s (sd = 0.649 s)).
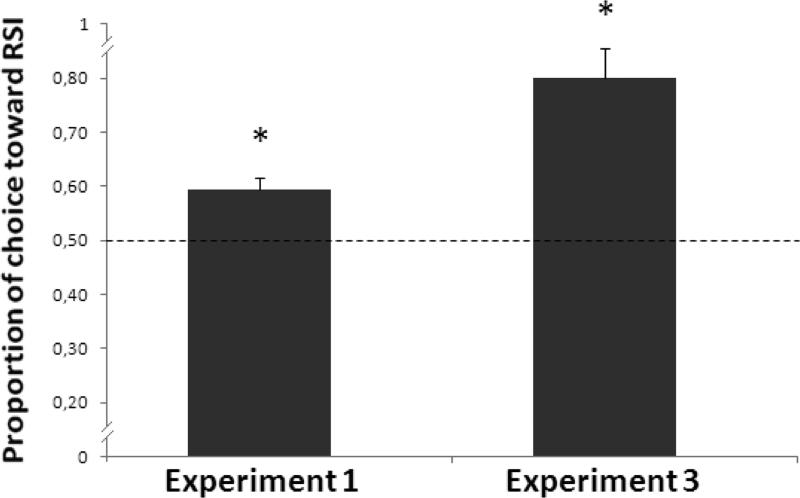
The group analysis did not show any difference in performance as a function of the type of task (relative vs. absolute, F(1,4) = 0.070, P = 0.805) or the type of stimuli (6-circle snakes vs. 12-circle snakes, F(1, 4) = 1.356, P = 0.309). No interaction was found (F(1, 4) = 0.957, P = 0.383). When we averaged monkeys’ performance (regardless to the type of task and stimuli) we found that monkeys classified the illusory pattern as a dynamic array at greater than chance levels (one sample t-test, t(5) = 4.392, P = 0.007, Figure 3).
Figure 3. Results of Experiment 1 and Experiment 3.
Monkeys spontaneously generalized the learned rule in the presence of the RSI and classified it as a dynamic array at levels significantly greater than chance (Experiment 1). When directly trained to discriminate between the RSI and the control stimulus, monkeys also solved the task (Experiment 3). Bars represent the standard error of the mean. The dashed line represents chance level. Asterisks denote a significant departure from chance (* = p < 0.05).
The results of Experiment 1 showed that monkeys classified the RSI as a dynamic array more often than not, suggesting that they might perceive the illusion. However, at the individual level, we found inconsistent evidence of a human-like perception of the RSI in this species. It is worth noting that this illusion is not universally reported in humans either. Billino et al. (2009) found that 84% of the observers perceived it while Fraser and Wilcox (1979) reported that 75% of participants perceived their original illusion. In this sense there is no reason to expect that all monkeys should perceive the illusory motion. It was possible that the heterogeneous pattern of data might be at least partially ascribed to the different experiences in computer tasks of these subjects. All of our subjects had participated in previous cognitive/perceptual tasks and were performing other experiments at the time of this study. However, those experiments investigated self-control, attention, metacognition and visual memory. No subject was involved in any other visual illusion task during this experimental period. In this sense, we do not believe that the different monkeys’ experience might primarily explain the heterogeneous pattern here reported at individual level. Apart from this, other factors may explain our results. First, we used a relatively reduced number of probe trials compared to the number of trials used for training and other test trials, and we cannot exclude that the lack of significant bias in binomial tests in most cases might be obscured by this fact. In addition, even though subjects generalized the learned rule to novel configurations, it was possible that the apparent speed of the circles included in the RSI may have substantially differed from the physical speed of the stimuli used in training and test phase. Unfortunately, the speed of the apparent motion in this visual array cannot be assessed in monkeys using these methods, and the possibility exists that monkeys did not as easily classify the RSI as a dynamic stimulus because they never encountered subtle motion discriminations in the training phase. Experiment 2 attempted to address this issue by evaluating human performance with the same stimuli.
EXPERIMENT 2
To verify if our stimuli were adequate to test the RSI in other primates, we tested adult humans. Participants were trained in comparable conditions to discriminate between the same stimuli presented to monkeys in the training phase. As soon as they reached the learning criterion, they were shown the same novel configurations presented to monkeys, including the RSI and control stimulus. If our hypothesis that monkeys see the illusion similarly to humans was correct, participants were expected to exhibit a high score in training and test phases in the presence of physically dynamic arrays.
Subjects
10 adult participants (4 males, 6 females) between the ages of 20 and 24 years (mean age 21.4 years) were tested. All had normal or corrected-to-normal vision. As the RSI is a popular illusion in psychological courses, we selected only those participants who did not study, or were not studying, psychology (e.g., engineering students). They were tested in the Department of General Psychology at the University of Padova (Italy). All participants gave their informed consent prior to participating in the experiment.
Apparatus, stimuli and procedure
The testing set-up included a personal computer, a joystick, and a 17-in LCD color monitor. The same stimuli presented to monkeys in Experiment 1 were shown in this experiment, both for training and test phase. The procedure was similar to that described with monkeys. Half of the subjects were tested with the relative judgment task, and half of the subjects were tested with the absolute judgment task. In the training phase subjects were required to select which array was dynamic (relative judgment task) or to establish whether the array presented on the screen was static or dynamic (absolute judgment task). Participants were admitted to the test phase as soon as they reached 75% correct choices in the most recent 50 trials (the same proportion used in Experiment 1 with the monkeys).
Although the two species were tested in comparable conditions, the two experiments differed with respect to some details: in the human study we used a cordless joystick ‘Logitech freedom 2.4’. No auditory feedback was given but visual feedback was provided. The word “Incorrect” or the word “Correct” appeared in the middle of the screen. Timeouts for incorrect responses were shortened to only 4 seconds. Similarly to Experiment 1, in test trials with the RSI and control stimulus no reward or timeout was provided. Each human participant completed 650 trials (training + test trials) in a single session. In the test phase, the RSI and the control stimulus were presented with a random probability of 0.15 on each trial, allowing us to collect 90 trials from each participant.
In order to reduce the methodological variability between the two experiments, human participants were not provided with specific instructions about their task, as previously done in other comparative studies (e.g., Agrillo et al. 2014b; Beran 2006). Before starting the experiment participants only read the following text: “You will perform a computer task. You will see a box that says ‘Start Trial’, and you should select it by using the joystick. Then, two arrays (or a single array, for the absolute judgment task) will appear, and you should select one of those (or select one choice icon on the bottom of the screen, for the absolute judgment task). Try to respond quickly and accurately. You may take a short break if you need to, but otherwise please try to complete as many trials as you can.” In this way, the rules for correct responding could only be inferred from the feedback, exactly as happened in Experiment 1 with the monkeys.
Results and discussion
Relative judgment task
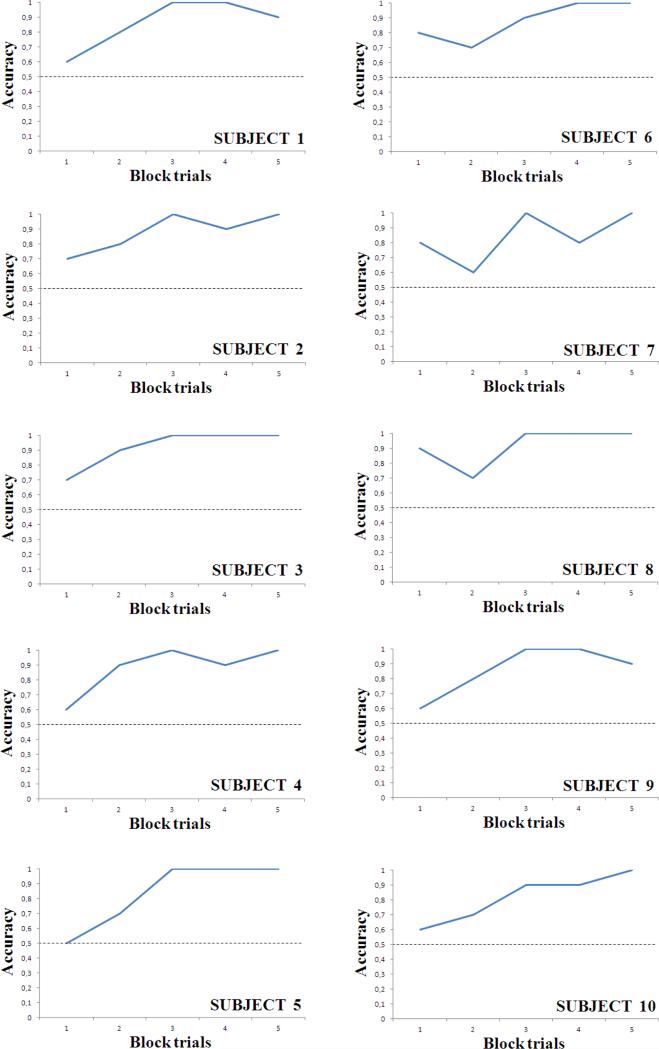
All subjects reached the learning criterion in the first 50 trials (Figure 4). The mean reaction time was 0.687 s (sd = 0.223 s). In the test phase, subjects easily generalized the learned rule to novel arrays (mean accuracy in the first 50 trials: Subject 1: 0.940, Subject 2: 0.840, Subject 3: 0.960, Subject 4: 0.980 and Subject 5: 0.860; mean reaction time: 0.634 s (sd = 0.119 s)). In probe trials showing the RSI and the control stimulus, all subjects classified the RSI as a dynamic array, both with 6-circle and with 12-circle snakes (binomial tests, all P < 0.05, Table 2; mean reaction time: 0.753 s (sd = 0.201 s)).
Figure 4. Learning curves of training phase, Experiment 2.
Data for each participant are presented as successive blocks of 10 trials. All participants reached the learning criterion in the first 50 trials. The dashed lines represent chance level.
Absolute judgment task
All subjects reached the learning criterion in the first 50 trials (Figure 4). The mean reaction time was 0.602 s (sd = 0.134 s). In the test phase, subjects easily generalized the learned rule to novel arrays (Subject 6: 0.880, Subject 7: 0.960, Subject 8: 0.980, Subject 9: 0.840 and Subject 10: 0.940, mean reaction time: 0.610 s (sd = 0.146 s)). In probe trials showing the RSI and the control stimulus, all subjects classified the RSI as a dynamic array, both with 6-circle and with 12-circle snakes (see Table 2; mean reaction time: 0.690 s (sd = 0.215 s)). The control stimulus was correctly classified as a static array (mean accuracy: Subject 6: 0.978, Subject 7: 0.844, Subject 8: 0.900, Subject 9: 0.933 and Subject 10: 0.944; mean reaction time: 0.657 s (sd = 0.211 s)).
For the group analysis, no difference was reported as a function of the type of task (overall accuracy in the relative judgment task, mean ± sd: 0.916 ± 0.062, overall accuracy in the absolute judgment task: 0.920 ± 0.058, independent t-test t(8) = 0.105, P = 0.919). Thus, we pooled the two tasks and contrasted participants’ performance in the first 50 trials of the test phase with novel configurations with the performance in the presence of the illusory pattern. Results showed a lower tendency to classify the RSI as a dynamic array compared to the physically much more dynamic arrays (accuracy with novel configurations: 0.918 ± 0.056; proportion of choices in which RSI was classified as a dynamic array: 0.788 ± 0.042, paired t-test t(9) = 5.665, P < 0.0001).
Thus, adult participants tested with the same stimuli presented to monkeys easily learned the discriminative rule based on motion cues. In the test phase they generalized this rule to novel configurations, and they classified the RSI – but not the control stimulus - as a dynamic array. However, the proportion of times in which participants classified the RSI as a dynamic array was lower compared to that reported in the presence of real motion. This suggests that the type of stimuli used in the training phase (in Experiment 2 and in Experiment 1) – and, specifically, the speed of motion – might not have been optimal for training monkeys to react to the illusory motion speed. With respect of this issue, it is worth noting that the present set of stimuli was previously used in a study of the RSI in fish. As in our study, a spontaneous classification of the RSI as dynamic array was reported at the group level for two fish species, while a lower tendency to classify the RSI as a dynamic array was reported for some individuals than the performance exhibited with real dynamic stimuli (Gori et al. 2014a).
EXPERIMENT 3
The fact that the monkeys did not easily generalize the classification rule to the RSI with the set of stimuli of Experiment 1 does not necessarily mean that they cannot perceive illusory motion. In Experiment 3 we assessed whether monkeys could discriminate between apparently slow motion and no motion in pairs of stimuli for which no true motion existed but some stimuli might be perceived to have such motion because of their luminance sequence. To do this, subjects were directly trained to discriminate between the RSI and the control stimulus.
Subjects, apparatus, stimuli and procedure
The same monkeys (N = 6) were tested as in Experiment 1.
The tasks and procedure were identical to Experiment 1 with one exception. Only two stimuli were presented: the RSI and its control. Again, Hank, Murph and Luke performed the relative judgment task, while Obi, Lou and Gale performed the absolute judgment task. In the former, monkeys were simultaneously presented with both arrays and were always reinforced for selecting the RSI. In the latter, monkeys were sequentially presented with one array at a time in random order and were required to select the correct choice icon for each array (RSI: right icon, control stimulus: left icon).
Two experimental sessions were conducted with each monkey, and all other procedural details were the same as in Experiment 1.
Results and discussion
Relative and absolute judgment task
Each monkey discriminated between the RSI and the control stimulus (binomial tests, Table 3; mean reaction time: 1.302 s (sd =0.538 s)). An one sample t-test on the overall performance confirmed that the monkeys as a group discriminated between the two arrays (t(5) = 5.486, P = 0.003, Figure 3). These data indicated that in both tasks and with both types of stimuli (6 and 12 circle snakes) monkeys discriminated between the RSI and its control stimulus. This result, however, might be due to two reasons: (a) monkeys could have discriminated on the basis of the apparent motion of the RSI or (b) monkeys could have used as a discriminative cue the subtle physical difference that existed between the two arrays: the different luminance sequence of the blobs. In Experiment 4 we assessed these possibilities.
EXPERIMENT 4
In this experiment monkeys were required to discriminate between two patterns identical to the RSI with the exception that the typical RSI adjacent luminance sequence was changed. Again, the two arrays differed in the luminance sequence of blobs (as RSI and control stimulus) but this time both luminance sequences do not generate any kind of motion illusion to humans. As shown by Murakami et al. (2006), only the exact RSI luminance sequence is, indeed, able to produce illusory motion in humans. Thus, in this experiment, monkeys were required to discriminate between the two arrays on the only basis of their luminance sequence.
To assess whether the results of Experiment 3 could be primarily ascribed to the use of a single change in the luminance sequence as discriminative cue, we compared the performance of this experiment with that reported in Experiment 3. If subjects solved the previous experiment by using the different luminance sequence, they were expected to exhibit no difference in the learning rate in the two experiments.
Subjects, apparatus, stimuli and procedure
The same rhesus monkeys (N = 6) were tested as in Experiment 3.
The tasks and procedure were identical to Experiment 3. Two stimuli were presented (Figure 5), differing only in that the black and white luminance were exchanged. As no difference was reported in Experiment 1 as a function of the type of stimuli (6/12-circle snakes), only a six-circle array was presented in all trials.
Figure 5. Stimuli used in Experiment 4.
Both arrays are static and appear to be static to human observers. The only difference between the arrays is represented by an inversion in the luminance sequence of the concentric blobs.
As in Experiment 3, Hank, Murph, and Luke performed the relative judgment task, while Obi, Lou, and Gale performed the absolute judgment task. In the relative judgment, monkeys were presented with both arrays simultaneously and were required to select the reinforced one (the “A” stimulus in Figure 5 for Murph and Luke and the “B” stimulus for Hank). In the absolute judgment, monkeys were sequentially presented with one array at a time in random order and were required to associate a different choice icon for the two arrays (the “A” stimulus was associated with the left icon and the “B” stimulus with the right icon for Gale, and the reverse was true for Obi and Lou). In order to have comparable number of trials in the two experiments, we analyzed subjects’ accuracy in the first 500 trials of each experiment.
Results and discussion
Relative judgment task
Although all subjects discriminated between the RSI and the control stimulus in Experiment 3, only Murph selected the reinforced stimulus in this experiment. Luke showed a significant preference for selecting the non-reinforced stimulus while Hank showed no difference in discrimination (binomial tests, Table 4; mean reaction time: 1.982 s (sd = 0.786 s)).
Absolute judgment task
Although all subjects discriminated between the RSI and the control stimulus in Experiment 3, only Lou discriminated between the two arrays in this experiment. Obi and Gale did not show any difference in discrimination (binomial tests, Table 4; mean reaction time: 1.734 s (sd = 0.769 s)).
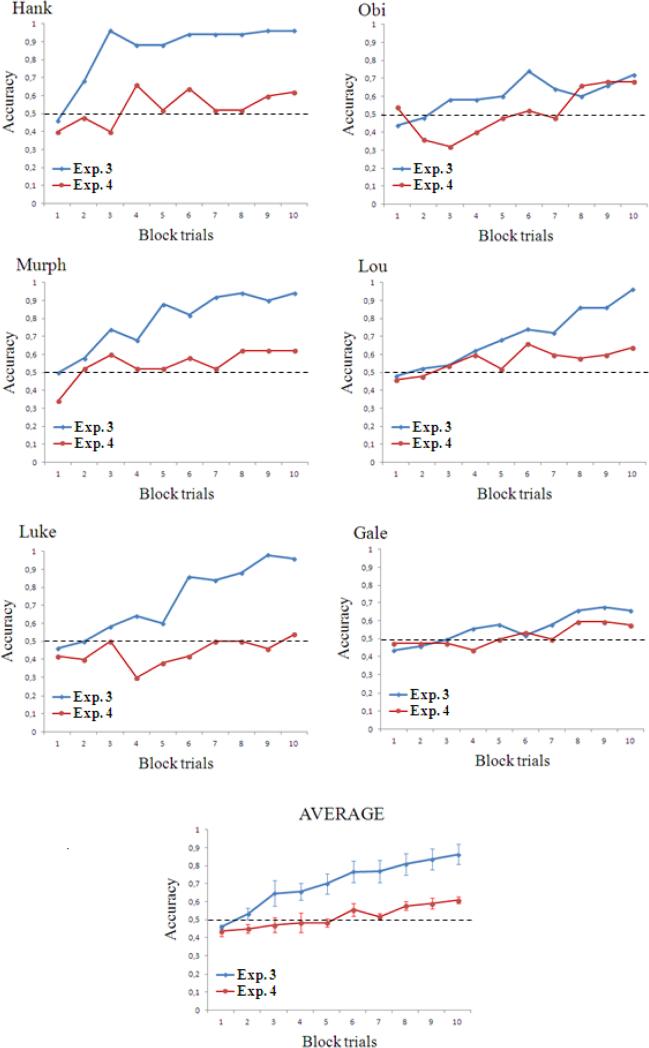
Figure 6 illustrates the learning rates in the two experiments. The data are presented as successive blocks of 50 trials, so that 10 blocks of trials are shown on the x-axis. A repeated measures ANOVA with Experiment (3 or 4) and Block (1 to 10) as within subjects factors showed main effects of Experiment (F(1,5) = 16.154, P = 0.010), Block (F(9, 45) = 24.562, P < 0.001) and a significant interaction (F(9, 45) = 3.209, P = 0.004). This indicated that monkeys showed an overall higher performance in Experiment 3, that accuracy increased with increasing the block trials and that the learning rate was different in the two experiments.
Figure 6. Learning curves of Experiment 3 (trials 1-500) and Experiment 4.
The data from each monkey are presented as successive blocks of 50 trials. Monkeys’ learning rates statistically differed in the two experiments, with subjects being quicker in the discrimination task of Experiment 3. The dashed lines represent chance level. Bars represent the standard error of the mean.
In short, monkeys proved much less able to discriminate between two visual arrays that differed from each other only with regard to a change in their luminance sequence, the exact same physical difference that distiguished the RSI and its control stimulus in the Experiment 3. As luminance sequence of the arrays represented the only physical cue that differed between the two stimuli, we concluded that the high performance exhibited in Experiment 3 was likely due to the apparent motion perception elicited by the RSI.
It is intesting to note that in this experiment one monkey, Luke, showed a significant preference for the non-reinforced pattern. We can only speculate on this unexpected result. If he could not discriminate between the two arrays, we should have expected a random performance. Our best explanation is that Luke learned to discriminate between the two stimuli but did not learn which was the positive stimulus associated with the food reward. If so, he might have chosen systematically one of the two arrays, although not the reinforced one.
GENERAL CONCLUSIONS
The perception of static illusions has been widely investigated in several non-human animals. In contrast, little attention has been devoted to motion illusions in species other than humans. In this study we asked whether rhesus monkeys perceived illusory motion. We focused on a static pattern that is known to generate a vivid motion perception, the Rotating Snake illusion (RSI). The resulting data support the idea of a human-like perception of this illusion in macaques, although the effect is not strong, and it is not shown by every monkey in every test.
In Experiment 1 monkeys were initially trained to discriminate between static and dynamic arrays; subsequently they were presented with the RSI and its control stimulus. We found an overall bias in spontaneously classifying the RSI as a dynamic array, in agreement with the idea of a similar motion illusion between humans and monkeys. We also found similar performance as a function of the type of task (relative/absolute judgment task), suggesting that monkeys’ performance was equivalent in multiple test formats. However, analyses of probe trials from individual monkeys showed that only one subject clearly perceived the illusion with both types of stimuli (6 and 12 circle snakes), while three monkeys showed a human-like perception with only one type of stimulus. We hypothesized that these results might have been partially affected by the fact that the apparent speed of circles in the RSI is poorly known and might have been more subtle compared to the speed of the objects presented in the training phase. To test this hypothesis we set up a control experiment in which human participants were tested with the same stimuli (Experiment 2). Participants exhibited a lower tendency to classify the RSI as a dynamic array than real dynamic stimuli, in line with our hypothesis, although they clearly showed the perceptual illusion itself. The choice of using this set of stimuli was motivated by the fact that a previous study using the same experimental material found an overall perception of RSI in two fish species (Gori et al. 2014a). An alternative way to select the set of stimuli could have consisted of assessing the point of subjective equality in humans first, and then using that speed to train monkeys. Even in that case, however, there would have been no guarantee that the point of subjective equality of our species would have exactly matched that of monkeys. These manipulations remain to be conducted in future research. To shed additional light on the monkeys’ ability to perceive illusory motion, in Experiment 3 we asked whether monkeys could be directly trained to discriminate between the RSI and a control stimulus. All subjects learned this discrimination after only two experimental sessions, regardless of the version of the task. As the two arrays that were discriminated have the same overall configuration but are differently perceived by humans in terms of presence/absence of motion, we hypothesized that monkeys could have used the apparent motion of the RSI as discriminative cue. However, before drawing such a conclusion we needed to establish whether monkeys could use as a discriminative cue the different luminance sequence of local features – the only physical cue that differed between RSI and control stimulus. Experiment 4 addressed this question by presenting monkeys with two arrays similar to the stimuli presented in Experiment 3 but with both now physically and perceptually static and differing only in a change in the luminance sequence, exactly the same physical difference between the RSI and its control stimulus in the Experiment 3. Only two subjects solved the task. Additionally, the monkeys’ learning rate was lower compared to that exhibited in Experiment 3. Assuming that monkeys struggled to use the luminance sequence to distinguish two visual arrays arranged exactly like the pattern of the RSI, it appears unlikely that the high performance reported in Experiment 3 could be primarily ascribed to the single change in luminance sequence of the blobs between the RSI and its control stimulus. In line with the overall analyses of Experiment 1, we believe that the strongest candidate underlying monkeys’ abilities in Experiment 3 is the apparent motion which our subjects are likely to perceive with the RSI.
The exact reason underlying the emergence of this motion illusion is not clear yet. Some authors suggested the critical role of fixational eye movements (Murakami et al. 2006; Otero-Millan et al. 2012) while others stressed that the illusory effect originated in the visual cortex (Ashida et al. 2012; Backus and Oruç 2005; Kuriki et al. 2008). Since rhesus monkeys seem to perceive the illusion in a similar way reported in humans, this species appears to be a model to better understand the neural correlates underlying this phenomenon. With respect to this issue, Conway et al. (2005) have already investigated the neural correlates of illusory motion in a macaque brain. However, their study did not show if the recorded neuronal response reaches the behavioral or perhaps even conscious level, as macaques did not perform any behavioral task of motion discrimination. Hence, no evidence of illusory perception in macaques could be provided. It is interesting to note that the ability of perceiving motion illusions is a highly heritable trait (Fraser and Wilcox, 1979; Gori et al. 2014c); it will be interesting to see if this polymorphism has a genetic basis in monkeys as well. Mild variations in visual processing (perception and attention) have been associated with developmental disorders, such as dyslexia and autism spectrum disorders in humans (e.g. Gori and Facoetti 2014, Gori et al. 2014b; Ronconi et al. 2012; Stein and Walsh 1997). Our results suggest the possibility to employ animal models in order to better understand these human disorders.
More generally, previous studies have investigated the neurophysiological mechanisms surrounding real motion perception in monkeys, finding interesting similarities with the neural circuits involved in humans’ motion perception (Orban et al. 2003; Rees et al. 2000). Our study aligns with this literature and reinforces the idea of similar perceptual mechanisms in two species that diverged more than 20 million years ago (Kumar and Hedges 1998). These mechanisms seem to be similar in the correct representation of the world but also in their constraints, which results in percepts that sometimes clearly diverge from the physical reality.
In sum, here we described four experiments suggesting that rhesus monkeys are likely to perceive the RSI as humans do. Together with recent studies on fish (Gori et al. 2014a) and cats (Bååth et al. 2014) this study represents a rare example of illusory motion perception in non-human animals. Future studies are now required to assess whether the perception of the RSI is restricted to a few species or instead occurs in several vertebrates, and those results will allow a better refinement of the phylogenetic map of experienced visual illusory motion.
Supplementary Material
Acknowledgements
This research was supported by FIRB grant 2013 (RBFR13KHFS) from ‘Ministero dell'Istruzione, Università e Ricerca’ (MIUR, Italy) to Christian Agrillo, and by funding from the National Institutes of Health to Michael J. Beran (Grant HD-060563). The authors thank the three anonymous reviewers for their useful comments, Ted Evans and Audrey E. Parrish for assistance with data collection, and the Language Research Center staff for their care of the primates.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest
REFERENCES
- Agrillo C, Miletto Petrazzini ME, Dadda M. Illusory patterns are fishy for fish, too. Front Neur Circuits. 2013;7:137. doi: 10.3389/fncir.2013.00137. doi: 10.3389/fncir.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrillo C, Parrish AE, Beran MJ. Do rhesus monkeys (Macaca mulatta) perceive the Zöllner illusion? Psych Bull Rev. 2014a;21(4):986–94. doi: 10.3758/s13423-013-0573-2. [DOI] [PubMed] [Google Scholar]
- Agrillo C, Parrish AE, Beran MJ. Do primates see the solitaire illusion differently? A comparative assessment of humans (Homo sapiens), chimpanzees (Pan troglodytes), rhesus monkeys (Macaca mulatta), and capuchin monkeys (Cebus apella). J Comp Psych. 2014b;128(4):402–413. doi: 10.1037/a0037499. [DOI] [PubMed] [Google Scholar]
- Ashida H, Kuriki I, Murakami I, Hisakata R, Kitaoka A. Direction-specific fMRI adaptation reveals the visual cortical network underlying the ‘Rotating Snakes’ illusion. NeuroImage. 2012;61:1143–1152. doi: 10.1016/j.neuroimage.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Bååth R, Seno T, Kitaoka A. Cats and Illusory Motion. Psychology. 2014 doi: 10.4236/psych.2014.59125. [Google Scholar]
- Backus BT, Oruç İ . Illusory motion from change over time in the response to contrast and luminance. J Vis. 2005;5(11) doi: 10.1167/5.11.10. article 10. [DOI] [PubMed] [Google Scholar]
- Beer AL, Heckel AH, Greenlee MW. A motion illusion reveals mechanisms of perceptual stabilization. PLoS ONE. 2008;3(7):e2741. doi: 10.1371/journal.pone.0002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ. Quantity perception by adult humans (Homo sapiens), chimpanzees (Pan troglodytes), and rhesus macaques (Macaca mulatta) as a function of stimulus organization. Int J Comp Psych. 2006;19:386–397. [Google Scholar]
- Beran MJ, Parrish AE. Visual nesting of stimuli affects rhesus monkeys’ (Macaca mulatta) quantity judgments in a bisection task. Att Percept Psychoph. 2013;75:1243–1251. doi: 10.3758/s13414-013-0474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billino J, Hamburger K, Gegenfurtner KR. Age effects on the perception of motion illusions. Perception. 2009;38:508. doi: 10.1068/p5886. [DOI] [PubMed] [Google Scholar]
- Burr D. Are ‘speed lines’ used in human visual motion? Cur Biol. 2000;10(12):R440–R443. doi: 10.1016/s0960-9822(00)00545-5. [DOI] [PubMed] [Google Scholar]
- Conway BR, Kitaoka A, Yazdanbakhsh A, Pack CC, Livingstone MS. Neural basis for a powerful static motion illusion. J Neurosci. 2005;25(23):5651–5656. doi: 10.1523/JNEUROSCI.1084-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Gross CG. Visual areas in the temporal cortex of the macaque. Brain Res. 1979;178:363–380. doi: 10.1016/0006-8993(79)90699-1. [DOI] [PubMed] [Google Scholar]
- Faubert J, Herbert AM. The peripheral drift illusion: A motion illusion in the visual periphery. Perception. 1999;28(5):617–621. doi: 10.1068/p2825. [DOI] [PubMed] [Google Scholar]
- Fraser A, Wilcox KJ. Perception of illusory movement. Nature. 1979;281:565–566. doi: 10.1038/281565a0. [DOI] [PubMed] [Google Scholar]
- Fujita K, Blough DS, Blough PM. Pigeons see the Ponzo illusion. Anim Learn Behav. 1991;19:283–293. [Google Scholar]
- Fuss T, Bleckmann H, Schluessel V. The brain creates illusions not just for us: sharks (Chiloscyllium griseum) can “see the magic” as well. Front Neur Circuits. 2014;8:24. doi: 10.3389/fncir.2014.00024. doi: 10.3389/fncir.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori S, Agrillo C, Dadda M, Bisazza A. Do fish perceive illusory motion? Scient Rep. 2014a;4:6443. doi: 10.1038/srep06443. doi:10.1038/srep06443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori S, Cecchini P, Bigoni A, Molteni M, Facoetti A. Magnocellular-dorsal pathway and sub-lexical route in developmental dyslexia. Front Hum Neurosci. 2014b;8:460. doi: 10.3389/fnhum.2014.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori S, Mascheretti S, Giora E, Ronconi L, Ruffino M, Quadrelli E, Facoetti A, Marino C. The DCDC2 intron 2 deletion impairs illusory motion perception unveiling the selective role of magnocellular-dorsal stream in reading (dis)ability. Cereb Cort. 2014c doi: 10.1093/cercor/bhu234. online first, doi:10.1093/cercor/bhu234. [DOI] [PubMed] [Google Scholar]
- Gori S, Facoetti A. Perceptual learning as a possible new approach for remediation and prevention of developmental dyslexia. Vis Res. 2014;99:78–87. doi: 10.1016/j.visres.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Gori S, Giora E, Stubbs DA. Perceptual compromise between apparent and veridical motion indices: The Unchained-Dots illusion. Perception. 2010;39:863. doi: 10.1068/p6678. [DOI] [PubMed] [Google Scholar]
- Gori S, Giora E, Yazdanbakhsh A, Mingolla E. A new motion illusion based on competition between two kinds of motion processing units: The Accordion Grating. Neur Net. 2011;24:1082–1092. doi: 10.1016/j.neunet.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Gori S, Hamburger K, Spillmann L. Reversal of apparent rotation in the Enigma-figure with and without motion adaptation and the effect of T-junctions. Vis Res. 2006;46:3267–3273. doi: 10.1016/j.visres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Gori S, Pedersini R, Giora E. How do painters represent motion in garments? Graphic invariants across centuries. Spat Vis. 2008;21:201–227. doi: 10.1163/156856808784532635. [DOI] [PubMed] [Google Scholar]
- Kitaoka A, Ashida H. Phenomenal characteristics of the peripheral drift illusion. Vision. 2003;15:261–262. [Google Scholar]
- Kumar S, Hedges SB. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- Kuriki I, Ashida H, Murakami I, Kitaoka A. Functional brain imaging of the Rotating Snakes illusion by fMRI. J Vis. 2008;8(16):11–10. doi: 10.1167/8.10.16. [DOI] [PubMed] [Google Scholar]
- Murakami I, Kitaoka A, Ashida H. A positive correlation between fixation instability and the strength of illusory motion in a static display. Vis Res. 2006;46:2421–2431. doi: 10.1016/j.visres.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Watanabe S, Fujita K. Pigeons perceive the Ebbinghaus-Titchener circles as an assimilation illusion. J Exp Psych Anim Behav Process. 2008;34:375–387. doi: 10.1037/0097-7403.34.3.375. [DOI] [PubMed] [Google Scholar]
- Naor-Raz G, Sekuler R. Perceptual dimorphism in visual motion from stationary patterns. Perception. 2000;29(3):325–335. doi: 10.1068/p3034. [DOI] [PubMed] [Google Scholar]
- Orban GA, Fize D, Peuskens H, Denys K, Nelissen K, Sunaert S, Todd J, Vanduffel W. Similarities and differences in motion processing between the human and macaque brain: evidence from fMRI. Neuropsychologia. 2003;41:1757–1768. doi: 10.1016/s0028-3932(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Otero-Millan J, Macknik SL, Martinez-Conde S. Microsaccades and blinks trigger illusory rotation in the “Rotating Snakes” illusion. J Neurosci. 2012;32:6043–6051. doi: 10.1523/JNEUROSCI.5823-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish AE, Beran MJ. When less is more: Like humans, chimpanzees (Pan troglodytes) misperceive food amounts based on plate size. Anim Cogn. 2014;17:427–434. doi: 10.1007/s10071-013-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parron C, Fagot J. Comparison of grouping abilities in humans (Homo sapiens) and baboons (Papio papio) with the Ebbinghaus illusion. J Comp Psych. 2007;121:405–411. doi: 10.1037/0735-7036.121.4.405. [DOI] [PubMed] [Google Scholar]
- Petrov YA, Popple AV. Effects of negative afterimages in visual illusions. J Opt Soc Am A. 2002;19(6):1107–1111. doi: 10.1364/josaa.19.001107. [DOI] [PubMed] [Google Scholar]
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat Neurosci. 2000;3:716–723. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- Richardson WK, Washburn DA, Hopkins WD, Savage-Rumbaugh ES, Rumbaugh DM. The NASA/LRC Computerized Test System. Behav Res Meth Instr Comput. 1990;22:127–131. doi: 10.3758/bf03203132. [DOI] [PubMed] [Google Scholar]
- Ronconi L, Gori S, Ruffino M, Franceschini S, Urbani B, Molteni M, Facoetti A. Decreased coherent motion discrimination in autism spectrum disorder: The role of attentional zoom-out deficit. Plos One. 2012;7:e49019. doi: 10.1371/journal.pone.0049019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa Salva O, Rugani R, Cavazzana A, Regolin L, Vallortigara G. Perception of the Ebbinghaus illusion in four-day-old domestic chicks (Gallus gallus). Anim Cogn. 2013;16:895–906. doi: 10.1007/s10071-013-0622-2. [DOI] [PubMed] [Google Scholar]
- Ruzzoli M, Gori S, Pavan A, Pirulli C, Marzi CA, Miniussi C. The neural basis of the Enigma illusion: A transcranial magnetic stimulation study. Neuropsychologia. 2011;49:3648–3655. doi: 10.1016/j.neuropsychologia.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Bowers AN. Visual circuits of the avian telencephalon: Evolutionary implications. Behav Brain Res. 1999;98:183–191. doi: 10.1016/s0166-4328(98)00083-7. [DOI] [PubMed] [Google Scholar]
- Sovrano VA, Bisazza A. Perception of subjective contours in fish. Perception. 2009;38:579–590. doi: 10.1068/p6121. [DOI] [PubMed] [Google Scholar]
- Sovrano VA, Albertazzi L, Rosa Salva O. The Ebbinghaus illusion in a fish (Xenotoca eiseni). Anim Cogn. 2014;18(2):533–542. doi: 10.1007/s10071-014-0821-5. [DOI] [PubMed] [Google Scholar]
- Spillmann L. The Ouchi-Spillmann illusion revisited. Perception. 2013;42(4):413–429. doi: 10.1068/p7384. [DOI] [PubMed] [Google Scholar]
- Spinozzi G, De Lillo C, Truppa V. Global and local processing of hierarchical visual stimuli in tufted capuchin monkeys (Cebus apella). J Comp Psych. 2003;117:15–23. doi: 10.1037/0735-7036.117.1.15. [DOI] [PubMed] [Google Scholar]
- Stein J, Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997;20:147–152. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Suganuma E, Pessoa V, Mongefuentes B, Castro B, Tavares M. Perception of the Müller-Lyer illusion in capuchin monkeys (Cebus apella). Behav Brain Res. 2007;182:67–72. doi: 10.1016/j.bbr.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Tomimatsu E, Ito H, Sunaga S, Remijn GB. Halt and recovery of illusory motion perception from peripherally viewed static images. Att Percept Psychoph. 2011;73(6):1823–1832. doi: 10.3758/s13414-011-0131-9. [DOI] [PubMed] [Google Scholar]
- Truppa V, Sovrano VA, Spinozzi G, Bisazza A. Processing of visual hierarchical stimuli by fish (Xenotoca eiseni). Behav Brain Res. 2010;207:51–60. doi: 10.1016/j.bbr.2009.09.039. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Nakamura N, Fujita K. Bantams (Gallus gallus domesticus) also perceive a reversed Zöllner illusion. Anim Cogn. 2013;16:109–115. doi: 10.1007/s10071-012-0556-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.