Abstract
Yersinia pestis, the causative agent of bubonic, septicemic and pneumonic plague, encodes a multitude of Fe transport systems. Some of these are defective due to frameshift or IS element insertions, while others are functional in vitro but have no established role in causing infections. Indeed only 3 Fe transporters (Ybt, Yfe and Feo) have been shown to be important in at least one form of plague. The yersiniabactin (Ybt) system is essential in the early dermal/lymphatic stages of bubonic plague, irrelevant in the septicemic stage, and critical in pneumonic plague. Two Mn transporters have been characterized (Yfe and MntH). These two systems play a role in bubonic plague but the double yfe mntH mutant is fully virulent in a mouse model of pneumonic plague. The same in vivo phenotype occurs with a mutant lacking two (Yfe and Feo) of four ferrous transporters. A role for the Ybt siderophore in Zn acquisition has been revealed. Ybt-dependent Zn acquisition uses a transport system completely independent of the Fe-Ybt uptake system. Together Ybt components and ZnuABC play a critical role in Zn acquisition in vivo. Single mutants in either system retain high virulence in a mouse model of septicemic plague while the double mutant is completely avirulent.
Introduction
The transition metals iron, manganese and zinc play key roles in the metabolism of eukaryotes as well as bacteria. Hosts restrict access to these metals in an attempt to prevent bacterial pathogens from proliferating.1-6 While copper serves as a cofactor in some proteins, bacteria also possess transporters and periplasmic proteins designed to avoid copper toxicity.7 Yersinia pestis has multiple, proven ferric and ferrous transport systems (Fig. 1) but only two transport systems each for manganese and zinc8-13 (Fig. 2) and no Cu resistance mechanism investigated. This article reviews these transport mechanisms and their roles in the virulence of Y. pestis.
Fig. 1.
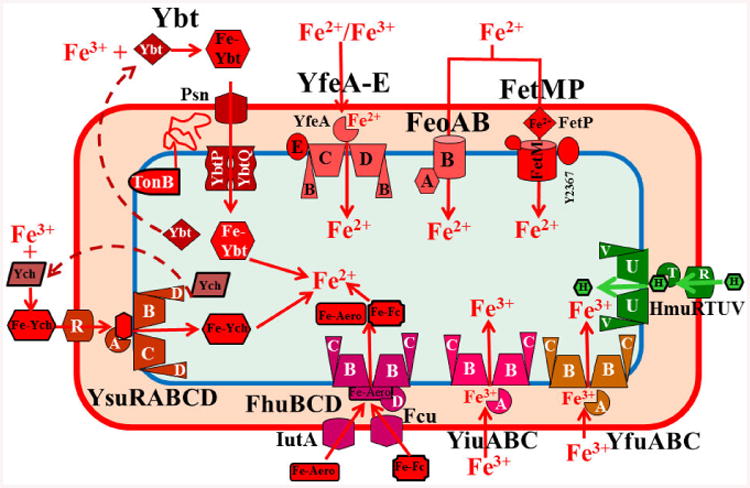
Y. pestis iron transport systems that are proven functional in vitro. These include a heme transporter (Hmu), three ferrous transporters (Yfe, Feo, and, Fet), three “ferric” transporters (Yfe, Yfu, and Yiu), one siderophore-dependent system (Ybt) fully functional in all Y. pestis biotypes, one system (Fhu/IutA/Fcu) capable of transporting, but not synthesizing aerobactin and ferrichrome bound to Fe (FeAero and FeFc). Finally, the Ysu system of Y. pestis CO92 (orientalis biotype) synthesizes and transports the Yersiniachelin (Ych) siderophore while Y. pestis KIM and Antiqua (medaevalis and antiqua biotypes) have a frameshift mutation that disrupts Ych synthesis. Although the fitABC and efeUOB loci are intact, their functionality remains to be tested. All OM receptors shown are TonB-dependent. FetMP requires one or more of the flp gene products (Y2363-Y2367) to be functional; for simplicity only Y2367 is shown. The Yfe ABC transporter can utilize ferric iron as a substrate, but its oxidation state during transport is undetermined. Dashed arrows in all figures indicate unknown components or unconfirmed steps.
Fig. 2.
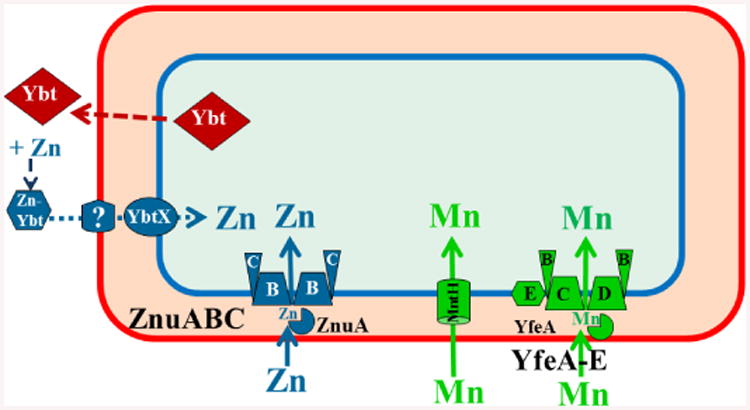
Y. pestis manganese and zinc transporters functional in vitro. Two widespread Mn transporters (MntH and Yfe/Sit) are functional in Y. pestis. The Znu and Ybt systems are also widespread with the Ybt siderophore and YbtX recently implicated in Zn uptake. Our model shows Zn bound to Ybt. However, this has not been demonstrated and the role of Ybt in Zn acquisition may be indirect.
Y. pestis causes primarily three forms of plague: bubonic, septicemic and pneumonic. Bubonic plague, caused by the bite of an infected flea, has an early lymphatic stage prior to development of systemic disease while septicemic plague bypasses the lymphatic stage. In humans, these forms of plague occasionally develop into secondary pneumonic plague. Infectious respiratory droplets transmitted from these individuals are the cause of the highly fatal primary pneumonic plague.
Two unique aspects of Y. pestis metal homeostasis are that each type of plague infection depends upon different metal transporters for virulence and the in vitro and in vivo phenotypes of metal transport mutants often differ.
In vitro and in silico analysis of transition metal transporters in Yersinia pestis
Iron
Iron is required for the growth of nearly all bacterial species and is found in heme proteins such as catalase and cytochromes, iron-sulfur cluster (Fe/S)-containing electron carriers and enzymes, and non-heme Fe enzymes. In most cells, Fe has multiple essential roles.14
Jackson and Burrows discovered that Y. pestis required iron for in vivo growth in the 1950's. Genomic and experimental analysis has demonstrated that Y. pestis encodes a multitude of proven (Fig. 1), putative, and non-functional iron transport systems9, 12 which can be divided into the four categories below. TonB/ExbB/ExbD requirements and Fur regulation of the promoters of these systems, are typical of other Gram-negative bacteria and will not be addressed here.
Heme uptake systems
The Y. pestis HmuP'RSTUV ABC transporter for heme and a variety of hemoproteins (Fig. 1) is typical for Gram-negatives and is nearly identical to Yersinia enterocolitica HemPRSTUV. HmuR is the TonB-dependent outer membrane (OM) receptor.15-17 The substrate-binding protein (SBP) HmuT binds two stacked hemes with nanomolar dissociation constants suggesting transport of two heme molecules in one reaction cycle.18 HmuU and HmuV are the inner membrane (IM) permease and the ATPase, respectively,17 and have a 2:2 stoichiometry. Mutational analysis of HmuU identified key amino acids for interaction with HmuT or heme transport. Analysis of the crystal structure of HmuUV showed an outward-facing cavity in the nucleotide-free complex. This suggests that this and other type II ABC transporters, which include siderophore and cobalamin transporters, may have coupling mechanisms that differ from those of other ABC transporters.19 HmuS is a homologue of the cytoplasmic heme-binding protein ShuS of Shigella dysenterieae.16, 17
A Y. pestis hmuR mutant was unable to use heme or any of the tested hemoproteins (haemoglobin, myglobin, haemoglobin-haptoglobin, heme-albumin or hemopexin). In contrast an hmuS mutant was fully competent in heme/hemoprotein use.17 In Y. enterocolitica an hemS mutation is lethal.16 A Y. pestis hmuT∷cat mutation prevented the use of heme and all hemoproteins except haemoglobin and hemopexin.17
Although Y. pestis encodes an intact hemophore system (hasRADEB and hasF) reminiscent of those found in other Gram-negative bacteria, in vitro studies were unable to demonstrate transport activity for the Has hemophore system in Y. pestis.20, 21
Ferric ABC transporters
Y. pestis has several ABC transporters that share similarity to ones with proven roles in Fe acquisition in other organisms. In this section only the non-siderophore-associated systems for ferric iron are discussed. Three ABC transporters (YfeABCDE, YfuABC and YiuABC) have proven iron uptake activity during aerobic growth (Fig. 1). Their efficacy has a definite hierarchy with Yfe>Yfu>Yiu, as determined by growth studies with mutants in a chelex-100 extracted, defined medium (cPMH2).22-25 Genome analysis identified fitABC as a potential Fe ABC transporter; however no experimental analysis of the system has been performed.9, 12 Finally, a frameshift mutation has disrupted y2840 of the fiuABC locus (y2842-y2837) making it unlikely that this gene expresses a functional IM permease.9
Growth studies of a yfe mutant demonstrated an aerobic and microaerboic growth defect under iron-depleted conditions, implicating it in both ferric and ferrous uptake. A reduction in ferric uptake by this mutant was directly demonstrated. Thus Yfe appears to function aerobically and microaerobically (Fig. 1). Whether ferric uptake by Yfe is due to reduction to ferrous prior to transport through this system remains to be determined.
Ferrous transporters
Three (Yfe, Feo and Fet) of the four ferrous transporters of Y. pestis have demonstrated iron acquisition activity under microaerobic conditions.26, 27 The Feo, Efe and Yfe/Sit systems are widespread while the more recently discovered Fet system appears to be more narrowly distributed (Fig. 1 and 3).26, 28-31 The transport activities of Yfe for Fe and Mn under aerobic conditions are described elsewhere in this article.
Fig. 3.
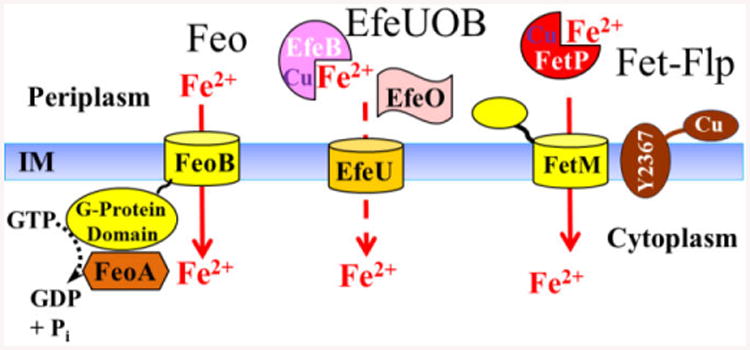
Ferrous transporters of Y. pestis. The Yfe ABC transporter is depicted in Fig. 1 and 5. Y. pestis FeoAB is typical of Gram-negative Feo systems. Both FeoA and FeoB are required with FeoB proposed to serve as the permease with its G-protein domain and FeoA acting to help hydrolyze GTP to energize transport. FeoC has been proposed or shown to affect levels of FeoB,28, 34 however, a Y. pestis feoC mutation has no affect on feoABC transcription or growth under iron-defidient conditions.26, 28 Bacterial Efe systems are members of the OFeT family that are related to the yeast FTR family but lack the Fet3p component. EfeU is the permease while EfeO and EfeB are periplasmic proteins, EfeO has putative metal binding sites (Cu and Fe), an N-terminal cupridoxin domain and a C-terminal peptidase-M75 domain. EfeB is predicted to have heme peroxidase-like activity 30, 35-37. FetMP is distantly related to the yeast FTR system with mutations in E. coli and Y. pestis having different phenotypes (see text). In addition, an insertion in Y. pestis y2367 (flpD; the first of 6 linked genes) causes loss of function. Although only Y2367 is shown in the model, the identity and number of flp genes (fet-linked phenotype) required for FetMP function is undetermined 10, 26, 29
Under microaerobic, iron-restricted conditions, the yersiniabactin (Ybt) siderophore-dependent iron transport system still plays a role in growth. This might be due to residual ferric iron from aerobic medium preparation; to avoid this complication a Δpgm or irp2 (iron-regulated protein whose gene product is required for Ybt synthesis) background was used in microaerobic and reducing conditions.32, 33 The pgm locus is a 102-kb chromosomal region that is spontaneously deleted in Y. pestis during in vitro growth and encodes the Ybt and Fet systems as well as numerous other proteins.9, 26
Single yfe or feo mutants exhibit a significant growth defect; however a double yfe feo mutant has a more severe growth restriction. Complementation with either locus, supplementation with Fe, or aerobic growth alleviate this growth inhibition.26, 27 Further experiments demonstrated that the Fet-Flp transporter (Fig. 1 and 3) also contributes to microaerobic iron acquisition and that a yfe feo fet mutant is defective in short-term iron uptake under reducing conditions where the oxidation state would be primarily ferrous.26
Studies in E. coli demonstrated that FetM is an essential IM component while FetP is an accessory periplasmic component.29 In contrast, a Y. pestis fetP mutant has a more severe growth defect than a fetM mutant. The FetMP system in Y. pestis (Fig. 3) appears to be linked to a downstream locus (y2367-y2362) which we have designated flpDABCTE (fet-linked phenotype). A polar mutation in flpD has a phenotype similar to a fetP mutation. FlpD (Y2367) has a partial YHS domain found in copper-transporting ATPases but lacks two cys residues that may be required for its function.26, 38 FlpA and B (Y2366 and Y2365, respectively)likely are ABC permeases with similarities to those predicted to export antimicrobial peptides while FlpC is the associated ATPase. FlpT has a periplasmic thioredoxin-like domain implicated in disulphide reductase activity and FlpE is a member of the cytochrome c superfamily.26 The number and identity of genes in the flp locus required for iron acquisition via FetMP remains to be determined.
In other bacteria, feo is transcriptionally regulated by ArcA, Fnr, and RstA as well as Fur.39-42 In Salmonella FeoC binding to FeoB resulted in higher levels of FeoB possibly by preventing degradation by FtsH.34 FeoC has also been proposed as a transcriptional regulator.28, 43 However, our studies with an feo transcriptional reporter have failed to identify a role for any of these regulators in the expression of the feoABC operon in Y. pestis.26
Another unique aspect of feo regulation in Y. pestis is our finding that Fe-Fur represses transcription of an feo reporter under microaerobic but not aerobic conditions. In contrast, all other tested iron-regulated promoters of Y. pestis are repressed aerobically by iron and Fur.12, 26, 44 The mechanism by which Fe-Fur fails to repress expression of feo under aerobic growth conditions is being explored.
Siderophore systems
In Y. pestis strains we analysed, the ynp locus (y3404-y3423) is disrupted by an IS100 element and/or a frameshift mutation in the second gene of the locus encoding a putative siderophore biosynthetic gene or is absent entirely. In the two Y. pseudotuberculosis strains examined, the locus appears intact, encoding genes for siderophore biosynthesis and transport with significant similarities to the Ybt system. However, no studies on the functionality of this locus have been performed in either organism.9
The aerobactin system of Y. pestis has a frameshift mutation in iucA that abolishes synthesis of the siderophore. However, Y. pestis cells use exogenously supplied aerobactin and ferrichrome for iron acquisition via the IutA/FhuBCD and likely Fcu/FhuBCD transport systems, respectively (Fig. 1).9, 45
Y. pestis strains are divided into epidemic strains (biotypes/biovars antiqua, mediaevalis and orientalis) that have caused epidemic/pandemics and more ancient endemic strains (Microtus or Pestoides) which cause bubonic plague in some animals and are occasionally isolated from humans.46-49 In Y. pestis KIM (Kurdistan Iran Man; mediaevalis) and Antiqua (antiqua) the first gene of the ysu locus has a frameshift mutation that should prevent synthesis of a putative hydroxamate siderophore. In Y. pestis CO92 (orientalis), 91001 (Microtus) and Pestoides F (Pestoides) as well as two Y. pseudotuberculosis strains, the ysu locus is intact (ypo1528-ypo1538 in CO92) (Fig. 1). Analysis of the biosynthetic proteins encoded by the intact locus suggested two alternative biosynthetic pathways with two possible products for each pathway. The four predicted, alternative siderophore structures are all cyclic hydroxamates with estimated molecular masses ranging from ∼370 to ∼600 Da.9 Recently Rakin et al13 have demonstrated that the ysu locus is responsible for the synthesis of a siderophore they named yersiniachelin (Ych) in a Y. pestis strain with an intact ysu locus.
The Ybt system is encoded on a pathogenicity island and is functional in a number of different Gram-negative pathogens.50 Early research on the Ybt system focused on Y. enterocoltiica and Y. pestis. Indeed, Y. enterocolitica was first used to purify and determine the Ybt structure (Fig. 4) and identify synthesis of Ybt diastereomers.51, 52 The Yersiniae Ybt system has been reviewed recently,53 consequently, biosynthesis of the Ybt siderophore will not be discussed here and regulation will only be briefly summarized.
Fig. 4.
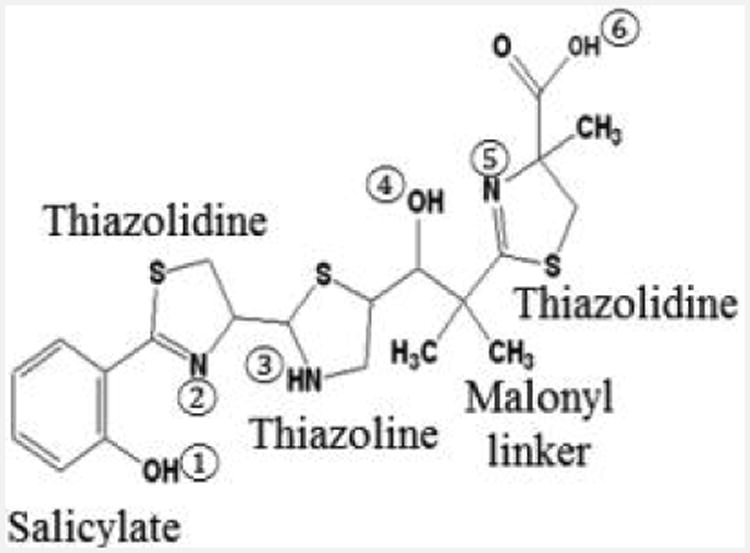
Structure of the Ybt siderophore. Salicylate, thiazolidine, and thiazoline rings as well as the malonyl linker are labelled. The six coordinate binding sites for Fe3+ are shown.
The mechanism for secretion of Ybt has not been elucidated. Once in the environment, Ybt can remove iron from transferrin and lactoferrin under in vitro conditions and has a calculated proton-independent stability constant for Fe3+ of 4 × 1036 M-1 with three nitrogen and three hydroxyl coordination sites (Fig. 4).54, 55 The crystal structure of Fe3+-Ybt has been characterized.56 Although uptake of Fe from Ybt has been demonstrated, translocation of the siderophore into the bacterial cytoplasm has not.53 Uptake requires the TonB-dependent OM receptor Psn (also required for sensitivity to the bacteriocin pesticin and termed FyuA in other bacteria) and two fused-function IM permease-ATPases, YbtP and YbtQ (Fig. 1). Although this type of fused-function ABC transporter is usually associated with Type I secretion systems, ybtP and ybtQ mutants exhibit reduced iron uptake and iron-deficient growth but normal levels of secreted Ybt.57 No SBP for this transport system has been identified and it may be that none is required. Compared to the parent strain and an irp2 (biosynthetic) mutant, psn, ybtP and ybtQ mutants all show reduced iron-deficient growth. It is likely the secreted Ybt in the transport mutants sequesters residual iron making it unavailable for use by other iron transport systems. Indeed, mutations in other iron transport systems have no in vitro phenotype unless the Ybt system is also mutated. Where or how Fe is removed from the Ybt siderophore also remains unresolved.53, 55
The function of YbtX, an IM protein with similarities to pyochelin and rhizobactin siderophore uptake permeases but also with modest similarities to alcaligin and enterobactin siderophore exporters, remained unknown until recently (see Zinc section below). Although encoded within the ybtPQXS operon, a ybtX mutant shows normal Ybt secretion and iron uptake. In addition, iron-deficient growth of this mutant is modestly better than the parent strain indicating that YbtX has no significant role in Fe uptake by the Ybt system.57
The ynp locus (see above) has genes which encode proteins with similarities to some Ybt biosynthetic enzymes and to Psn, YbtP, YbtQ, and YbtX. However, none of these proteins correct defects in Ybt synthesis or transport due to ybt mutations.9, 53
In addition to typical Fe-Fur repression of the four ybt operons encoded within the pathogenicity island, transcription is regulated by YbtA, a member of the AraC family of transcriptional regulators, and the Ybt siderophore. YbtA in the presence of Ybt activates transcription of the irp2irp1ybtUTE, ybtPQXS, and psn operons and represses its own transcription. All mutations that prevent Ybt synthesis show loss of transcriptional activation by YbtA with one exception. A ybtS mutant which cannot synthesize salicylate and had no detectable Ybt still exhibits normal YbtA-dependent transcriptional activation. The mechanism behind this anomaly is under investigation.33, 57-59
Zinc
Zinc is an essential element with no known examples of organisms that have completely dispensed with a Zn requirement. Zn is not redox active under normal physiological conditions, but serves as an electrophilic catalyst (Lewis acid) in numerous enzymes and as a scaffold for organizing protein domains 14. Our studies have shown a Zn requirement for in vitro growth of Y. pestis.60
Bioinformatic analysis of the Y. pestis KIM10+ genome identified the typical ZnuABC transporter as the only Zn uptake system (Fig. 2) and 4 Zn efflux systems (ZntA, ZitB, ZntB, and FieF).60 No experimental work has been published on Zn efflux in Y. pestis and Zn-Zur regulation is typical of other Gram-negative bacteria and will not be discussed here.60, 61
Compared to the parent strain, a Y. pestis znu mutant had a significant growth defect in cPMH2 (residual Zn content of ∼0.5 μM) which is corrected by supplementation with Zn but not Fe and by complementation with the cloned znuABC locus. However, the znu mutant responded to the addition of Zn to 0.4 μM suggesting the presence of an additional high-affinity Zn uptake system.60
In our search for this putative Zn transporter, we mutated or overexpressed other divalent cation transporters, transporters whose expression is Zn-regulated and the homolog of ZevAB, a more recently identified Zn transporter of Haemophilus influenzae.62 We also tested the growth of a septuplet mutant (znu yfe mntH efe fetMP feo y2842) in case there are three or more semi-redundant Zn transporters in Y. pestis. None of these studies indicated a role in Zn acquisition for any of these systems, at least under our in vitro Zn-deficient growth conditions. Thus the ferrous transporters (Yfe, Feo, Efe, Fet), manganese uptake systems (MntH and Yfe), ZevAB, Y2842 (a Zn-responsive SBP named FiuA), Zn-responsive putative K-channel Y1247, and FieF appear to play no role in Zn acquisition in Y. pestis (unpublished observations).8, 10, 60,62 Although FieF is a Zn and Fe effluxer in bacteria, human and plant homologues of FieF appear to import Zn.63-65
Since Ybt binds another divalent cation (Cu2+),66 we constructed an irp2 znu mutant and found that this double mutant, unable to synthesize Ybt, was unable to grow in cPMH2 supplemented with 1 μM Fe (to compensate for loss of the Ybt system) and 0.6 μM Zn. Growth was restored by complementation with the cloned irp2 gene, supplementation with 2.5 μM Zn or addition of purified Ybt siderophore. These results suggest that the Ybt siderophore is involved in high-affinity Zn acquisition and may serve as a zincophore. However, we have not demonstrated Zn binding by Ybt.8 Pyoverdine and pyochelin bind a variety of divalent and trivalent cations but these non-Fe complexes appear to be involved in preventing metal toxicities.67-69 Both pyochelin and micacocidin (an anti-mycoplasma compound produced by a Pseudomonad and Ralstonia solanacearum) bind Zn and have structures similar to Ybt.67, 70,71 In R. solanacearum, Kreutzer et al used gallium as a surrogate for Fe to demonstrate potential siderophore activity by micacocidin.71 Coelibactin, produced by Streptomyces coelicolor has been proposed as a zincophore based on Zn and Zur regulation of the coelibactin locus.72, 73 However, neither Zn binding nor uptake via coelibactin has been demonstrated. The only secondary metabolite that has been shown to both bind Zn and deliver it to the bacterial cell is pyridine-2,6-bis(thiocarboxylic acid) (PDTC) produced by some Pseudomonads. Although PDTC is classified as a siderophore and delivers Fe more effectively than Zn to the cell, it may serve to reduce Fe to the ferrous state.74, 75
To identify possible Ybt transport components involved in Zn acquisition, we assessed the Zn-deficient growth of psn, tonB, ybtP, ybtQ and ybtX mutants in a znu background. These studies demonstrated that Psn, TonB, YbtP and YbtQ, required for Fe uptake via Ybt, are not involved in Zn acquisition. Instead, the IM permease YbtX is required for Zn uptake via Ybt (Fig. 2).8 This is the first siderophore system with demonstrated involvement in Fe and Zn acquisition by completely independent uptake systems.
Manganese
Y. pestis has two identified Mn transporters that are widespread in bacteria (Fig. 5). MntH, an Nramp1 family member, was first characterized in Salmonella and E. coli as a proton-dependent Mn transporter. The Y. pestis MntH is highly similar to other characterized MntH transporters involved in Mn uptake.11, 64, 65
Fig. 5.
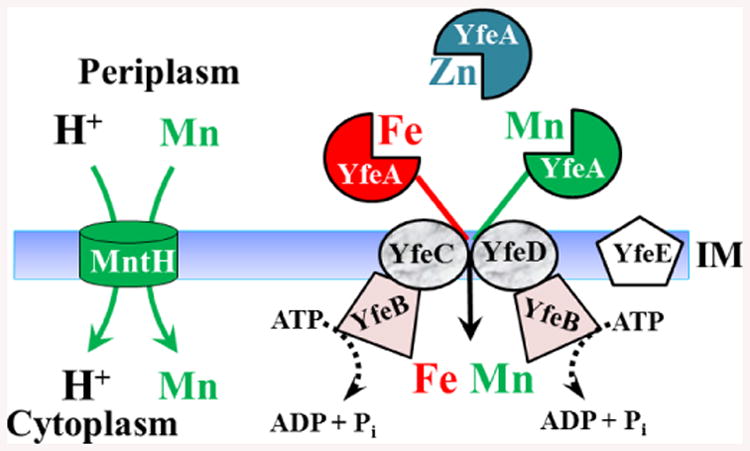
Models of proven Y. pestis Mn transporters YfeABCDE and MntH. The Yfe ABC transporter also accumulated Fe under aerobic and microaerobic conditions. In addition, the YfeA SBP binds Zn but does not transport it into the cytoplasm 22, 23, 26, 60
ABC transporters for Mn were first described in the cyanobacterium Synechocystis, Streptococci and then Y. pestis.22, 23, 76-78 In Y. pestis, it was cloned in a screen for iron transport systems and named Yfe; however, Yfe transports both Fe and Mn.22, 23 Subsequently Gram-negative orthologues were identified in Samonella and Shigella and termed Sit.79-81 While YfeA-D is a typical ABC Mn transporter,6, 22, 23 the SBP YfeA binds Mn and Zn with similar high affinities (18 nM and 7 nM, respectively) yet transports only Fe and Mn.60 The PsaA SBP component of the Mn ABC transporter of Streptococcus pneumonica (PsaABC) also binds both Mn and Zn but transports only Mn. Couñago et al have shown that Zn binding by PsaA locks it in a closed state that prevents release of the cation;82 perhaps a similar mechanism occurs with the related YfeA of Y. pestis. In Y. pestis, there is an additional IM component, YfeE (Fig. 5). In cPMH supplemented with conalbumin or 2,2′-dipyridyl (DIP), a Y. pestis ΔyfeE mutant had a moderate delay in growth while ΔyfeA, ΔyfeAB and ΔyfeBCDE mutants all showed significant growth inhibition.22, 23 The role of YfeE in Fe or Mn transport remains to be determined. In unchelated cPMH2 (containing ∼0.5 μM residual Mn), single mntH and yfe Y. pestis mutants have no significant growth defect, while the mntH yfeAB double mutant has a modest growth defect. This double mutant also shows complete loss of Mn uptake in short-term (30 min) transport assays.11
The modest growth defect of this double mutant and a growth response to micromolar Mn suggests that a third, unrecognized high-affinity Mn transporter may provide Mn. Our initial tests of a quintuplet mutant (yfe mntH feo fet efe) and septuplet mutant (with additional znu y2842 mutations) indicate that none of these systems are the putative 3rd Mn transporter. Indeed, the septuplet mutant responds to Mn supplementation to 1 μM. 11 While this supports a putative 3rd Mn transporter, the modest in vitro growth defect may simply reflect little or no requirement for Mn by Y. pestis. Indeed there is significant evidence that E. coli does not require Mn but can use Fe to replace Mn in some mononuclear enzymes and has Fe-dependent isozymes.83, 84 One example is ribonucleotide reductase (RNR); Y. pestis, E. coli and other enterics have two Fe-dependent RNRs and one Mn-dependent RNR. In contrast, Bacillus subtilis has a single Mn-dependent RNR and appears to have an absolute requirement for Mn.14, 85, 86 The ability of Fe supplementation to 1 μM to stimulate growth of our yfe mntH mutant supports the view that Y. pestis does not have an essential requirement for Mn, at least under our in vitro growth conditions. The modest in vitro growth defect of the double mutant under Fe- and Mn-deficient growth conditions may result from diversion of scarce Fe into some normally Mn-dependent enzymes and mismetallation of some mononuclear enzymes by Zn, preventing enzymatic activity.84, 87, 88
Nothing is known about the translocation of Mn through the Y. pestis OM. Although an OM porin (MnoP) for Mn uptake has been described for Bradyrhizobium japonicum,89 no protein with significant similarity to MnoP is encoded by the Y. pestis genome.
A unique aspect of Mn-responsive regulation in Y. pestis is the lack of MntR or Mur transcriptional regulators. Instead Fe-Fur and Mn-Fur repress transcription of the mntH and yfeABCD promoters (∼7-10-fold and 2-fold repression by Fe and Mn, respectively). All other tested Y. pestis Fe-Fur repressible promoters are not repressed by Mn supplementation. In addition, Mn repression of these two promoters is not a unique feature of the Y. pestis Fur protein – E. coli Fur causes similar Mn repression of these Y. pestis promoters. A fifteen nucleotide segment that partially overlaps the Fur box of the yfeABCD promoter displays significant similarity to a region located upstream of the Fur box in the mntH promoter. When these nucleotides (catcgctaatggtat) replace similarly positioned nucleotides of the hmuP'R promoter, the hybrid promoter is converted from repression by Fe only to repression by Fe and Mn.10, 11, 23 While all other tested Fe-Fur responsive promoters do not respond to Mn,10 it is likely that any additional Y. pestis promoters that respond to Mn will also be repressed by Fe.
In other bacteria, mntH is also regulated by OxyR while yfe is regulated by a LysR-type transcriptional regulator (YPTB0333) in Yersinia pseudotuberculosis.81, 90, 91 In Y. pestis, regulation of yfe by the LysR-type homologue and mntH by OxyR has not been tested.
Copper
While copper is required for growth in a number of bacteria and serves as a cofactor in cellular processes;14 excess Cu is toxic. Relatively few Cu import systems have been identified – with P-type ATPases in Pseudomonas aeruginosa and Listeria monocytogenes being two exceptions.92, 93 Cu resistance systems have been characterized in a number of bacteria.7 In Y. pestis no experimental work on these systems has been performed. Our search of the Y. pestis KIM10+ genome did not identify a P-type ATPase Cu importer. An in silico analysis of 14 copper homeostasis proteins identified 7 (CopA, CueO, CueP, CusC, CutF [NlpE], PcoC and YebZ [PcoD]) which are prevalent in Yersinia species.7 Our search of the Y. pestis KIM10+ genome identified an additional 3 genes encoding potential copper homeostasis proteins. CueR is a transcriptional regulator controlling expression of cueO and copA in other enterics, while Y2522 and Y2523 have similarities to CopD/PcoD (35% identity/42% similarity) and CopC/PcoC (28% identity/48% similarity) in Pseudomonas syringae, respectively. PcoC and PcoD may import copper into the cytoplasm. Periplasmic PcoC binds Cu+ and Cu2+ and may interact with membrane protein PcoD (YebZ) (Fig. 6).7 Based on bioinformatics and functions characterized in other bacteria, Fig. 6 shows our proposed model of copper homeostatis in Y. pestis. CopA may export Cu+ from the cytoplasm to the periplasm. In the periplasm CueP could be a Cu-binding protein involved in copper resistance in Salmonella 84 while CueO appears to be a multi-copper oxidase that converts Cu+ to Cu2+.7 In E. coli, CutF is an OM protein involved in copper tolerance while CusC is the OM component of the CusCBAF complex that exports Cu+ from the cytoplasm to the extracellular environment.7 Whether CusC is capable of Cu+ export in the absence of the IM and periplasmic components of the system is unknown.
Fig. 6.
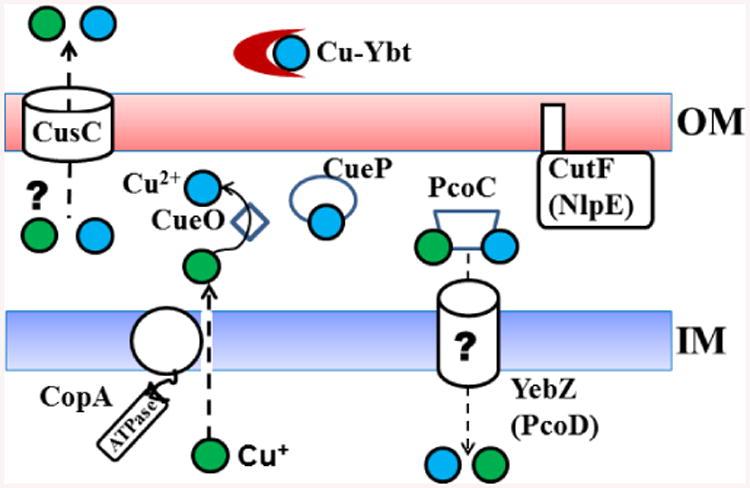
Proposed Y. pestis copper transport and resistance mechanisms. Proposed functions for Y. pestis proteins are based solely on similarities to those with established functions in other bacteria. Cu2+ binding by the Ybt siderophore has been experimentally demonstrated.66 Cu+, green spheres; Cu2+, blue spheres.
In E. coli, the Ybt siderophore has been shown to bind Cu2+, enhancing copper tolerance. In addition, Cu-Ybt acts as a superoxide dismutase.66, 94
Ybt enhances intracellular survival of E. coli in copper-replete RAW264.7 cells suggesting that Cu2+ binding and/or superoxide dismutase activity may play a role in virulence.94 Copper resistance genes have been associated with higher expression and/or increased survival of bacterial pathogens in vivo.84, 94-97 Microarrays analysis found that Y. pestis cueO was expressed in the rat bubo but not in the flea while cueR was upregulated 4.5-fold in the bubo compared to the flea. However, no differential expression of any other copper resistance genes was noted.98
In vivo roles of transition metal transporters in Y. pestis
Y. pestis is an obligate pathogen with a life cycle alternating between growth in its vector (fleas) and mammalian hosts, primarily rodents. This section will describe our current knowledge of the transition metal transporters required for growth in the flea and for the progression of the three primary forms of plague – bubonic, septicemic and pneumonic as well as expression of these transporters in the diverse environments of the flea and different mammalian organs.
Fleas
Upon ingestion of an infected blood meal, Y. pestis migrates from the foregut, consisting of an unbranched esophagus and proventriculus (a valve separating the esophagus and the midgut). into the midgut. In the midgut, blood is incompletely digested by various degradative enzymes and moved to the hindgut for excretion. Y. pestis is restricted to this simple alimentary tract. In the classic plague vector, the oriental rat flea (Xenopsylla cheopis), Y. pestis produces the Hms biofilm in the flea gut and proventriculus that is essential for blockage-dependent transmission of Y. pestis from an individual flea to mammals. Biofilm formation is thought to be important for spread and long-term maintenance in natural reservoirs.98-102 Early biofilm-dependent blockage of some fleas and enhanced transmission by laboratory-reared fleas exposed to natural environments suggests that blockage-dependent transmission may also play a large role in epidemics.103, 104 A second proven transmission mechanisms, early-phase transmission, which occurs within 24-72 h of an infectious blood meal, is proposed to play an important role in transmission from multiple flea bites and during fast-progressing plague epizootics in prairie dogs.100, 104, 105 One study suggests that early-phase transmission is biofilm-independent.101
Although no experimental studies on requirements for transition metal transporters in fleas have been published, a microarray study has yielded insights into their expression. A comparison of the transcriptomes of Y. pestis cells from the flea midgut to those from rat lymphnodes (buboes) revealed that no iron transport systems are highly expressed in the flea.98, 106 This presumed iron-rich environment might result from the lysis of red blood cells releasing haemoglobin and the degradation of various serum proteins that chelate Fe and hemin. Although yfe levels were higher in the bubo compared to the flea, mntH levels were similar in both environments.98, 106 Since both yfe and mntH are similarly regulated by Fe and Mn availability, this difference might be due to additional regulation of yfe and/or mntH or to technical difficulties. The flea mid-gut may be somewhat Zn-restrictive since expression levels of rpmJ (ypo3135; L36 ribosomal subunit protein) and ypo1343 (y2842;FiuA), both highly Zn-repressed in vitro, 61 were similar in the bubo and flea (see Bubonic plague below). Finally, in the flea, there was evidence for expression of genes encoding proteins predicted to be involved in copper resistance mechanisms.98, 106
Bubonic Plague
Relatively small numbers of Y. pestis cells (∼100) are inoculated into the dermis from the infected flea bite.107 The first stage of infection is growth at the bite site and dissemination to the draining lymph node. This early stage is proposed to have an intracellular phase since the Y. pestis antiphagocytic mechanisms may not be fully induced. Growth in the lymph node is largely extracellular, at least at the later stages, and is characterized by a swollen lymph node or bubo. Finally bacteria disseminate via the bloodstream to internal organs (liver, spleen, lungs, etc.) where they grow to high populations and eventually cause septic shock. Symptoms of bubonic plague are a sudden onset of fever, headache, chills, malaise, regional lymphadenopathy (swollen lymph node) and sepsis. Untreated, the fatality rate is 50-60% in humans. In rodents, a high level of bacteria in the bloodstream is a requirement for transmission to a naïve flea, which completes the natural life cycle of Y. pestis. Humans are dead end hosts and do not contribute to the maintenance of Y. pestis in natural reservoirs.61, 98-100, 108, 109
Sebbane et al. did a microarray analysis of strain 195P cells isolated 48-72 h post-infection from lymph nodes compared to cells grown in a Luria Bertani/MOPS medium (LB/MOPS) at 37°C.110 Fur-regulated genes were generally derepressed with 120-fold increases in some ybt operons/genes. However, neither iutA (needed for aerobactin use) nor feoAB were differentially regulated (Table 1).111 Our finding that feoABC is not repressed by Fe under aerobic conditions in vitro26 likely explains the lack of increased feoABC expression in vivo. Both Mn- and Fe-regulated mntH and yfeA were more highly expressed in the bubo compared to in vitro as were Zn-regulated znuA and rpmJ (Table 1).110 These results suggest that the infected bubo is an environment low in Fe, Mn, and Zn.
Table 1. Microarray analysis of Y. pestis gene expression under in vivo conditions.
| Genea | Bubonic plagueb | “Septicemic plague”b | Pneumonic plagueb |
|---|---|---|---|
| Fe-responsive genes | |||
| irp2 (ypo1911) | 197.3 | 7.1 | 2.1 |
| ybtP (ypo1913) | B | 4 | 3.3 |
| ybtX (ypo1915) | 97.8 | 2.9 | 3.1 |
| psn (ypo1906) | 179.7 | 2.5 | 2.3 |
| ybtA (ypo1912) | 24.2 | 1.4 | - |
| iucA (ypo0989) | B | 2.1 | - |
| ysuI (ypo1530) | 4.5 | 1.3 | - |
| ysuF (ypo1528) | 11 | 3.1 | - |
| ysuH (ypo1531) | 5 | 1.2 | - |
| ysuR (ypo1537) | - | 3.3 | - |
| ysuE (ypo1538) | - | 6.1 | - |
| yfuA (ypo2958) | 18.7 | 3 | - |
| yiuA (ypo1310) | 12.6 | 1.7 | - |
| yiuR (ypo1313) | 11.9 | 1.5 | - |
| feoB (ypo0132) | - | 1.1 | -2.8 |
| feoA (ypo0133) | - | 1.1 | -3.2 |
| efeU (ypo1854) | 5.5 | 1.9 | - |
| fetM (ypo1941) | 11.8 | - | 2.2 |
| flpD (y2367/ypo1943) | - | - | 2.2 |
| flpA (y2366/ypo1944) | 7.1 | - | - |
| hmuR/YPO0283 | 10.7 | 2 | - |
| tonB (ypo2193) | 5.4 | 2.6 | - |
| exbD (ypo0683) | 12.1 | 2.5 | - |
| Mn- and Fe-responsive genes | |||
| mntH (ypo2982) | 8.5 | 3 | - |
| yfeA (ypo2439) | 9.6 | 10.2 | 2.8 |
| Zn-responsive genes | |||
| znuA (ypo2061) | 3.2 | 2.2 | 2.1 |
| rpmJ (ypo3135) | 22.7 | - | 23.5 |
| fiuA (ypo1343) | - | 1.2 | 28.1 |
| zntA (ypo3820) | - | - | -5.4 |
| Cu-responsive genes | |||
| pcoC (y2523/ypo1784) | - | 3.6 | - |
Some are the first gene of an operon. The ypo numbers are locus tags for Y. pestis CO92, referenced in the microarray studies; y numbers are locus tags for Y. pestis KIM10+. Positive numbers indicated fold increases in expression in vivo vs in vitro. Dashes indicate that the gene was not listed in the table of differentially regulated genes which could indicate no detected expression or no significant difference between the two environments. B – detected only in cells from the bubo.
Bubonic plague – cells from rat bubo vs log-phase cells grown in Luria Bertani/MOPS broth at 37°C111; “septicemic plague” – cells grown in heat-inactivated human serum vs cells grown in Luria Bertani broth at 37°C86, 115; pneumonic plague – cells from bronchoalveolar lavage 48h post infection vs cells grown in Brain Heart Infusion broth + 2.5 mM CaCl2 at 37°C116
Long before technology allowed researchers to determine that Y. pestis Pgm- mutants (unable to adsorb hemin, a characteristic of the Hms biofilm) result from a 102-kb chromosomal deletion, Jackson and Burrows112 determined that Pgm- mutants were avirulent in mice by an intraperitoneal route unless hemin or inorganic iron was co-injected. A KIM Δpgm mutant is also avirulent in mice via subcutaneous injection (sc).113 Our mouse studies, using KIM strains, have shown that specific ybt mutations cause a similar loss of virulence by an sc route. Note that mutations in biosynthetic- and transport-encoding genes cause a similar degree of virulence loss (Table 2).8, 55 In vivo studies with strains that possess intact ysu and ych loci (e.g., CO92 and endemic strains), show a similar virulence loss due to ybt mutations. This suggests that the Ysu/Ych siderophore-dependent transport system does not compensate for the loss of Ybt in vivo.46, 114 Mutations in the Hmu and Has transporters or Yfu and Yiu do not affect virulence (Table 2).20, 25
Table 2.
Virulence of Y. pestis with Fe, Mn, or Zn mutations in mouse models of bubonic, septicemic, and pneumonic plague.
| Strain or mutation (Function) | Bubonic plague LD50sa | Septicemic plague LD50sa | Pneumonic plague LD50sa | Reference |
|---|---|---|---|---|
| Parent strain | 25 | <14 | 329 | 8, 55 |
| Fe transport mutants | ||||
| Psn (Fe3+-Ybt uptake) | >2.6 × 107 (>1.13 × 106-fold) | <14 | 1.1 × 104 (33-fold) | 8, 55 |
| irp2 (Ybt synthesis) | >1.3 × 107 (>5.2 × 105-fold) | ∼48 | 2.6 × 105 (790-fold) | 8, 55 |
| ΔfeoB (Fe2+ uptake) | 55 (NS) | Not tested | 211 (NS) | 26 |
| ΔyfeAB (Fe & Mn uptake) | 205 (9-fold; NS) 74.3 (8-fold) | Not tested | 139 (NS) | 24, 26 |
| ΔyfeAB ΔfeoB (Fe2+ & Mn uptake) | 2,035 (89-fold) | Not tested | 407 (NS) | 26 |
| Δpgm (Ybt-Fet-Flp-; Fe3+-Ybt & Fe2+uptake) | > 107 | < 6 (NS)b | >3.9 × 106 (>1.2 × 104-fold) | 8, 22, 55 |
| Δpgm ΔyfeAB (Ybt- Fet-Flp-; Fe3+ & Fe2+uptake) | Not tested | > 1.7 × 107b (>1.2 × 106-fold) | Not tested | 22 |
| ΔhmuP'RSTUV Delta;hasRADE (heme uptake) | <4.2 (NS) | Not tested | 273 (NS)c | 20 (unpublished) |
| Mn transport mutants | ||||
| ΔmntH (Mn uptake) | 36 (delayed TTD) | Not tested | Not tested | 11 |
| ΔyfeAB ΔmntH (Mn & Feuptake) | 3,068 (133-fold) | Not tested | 142 (NS) | 11 |
| Zn transport mutants | ||||
| znu (Zn uptake) | 266 ± 149 (NS)d | ∼11 ± 5 | 1,113 ± 1,376 (NS)d | 8, 60 |
| psn ΔznuBC (Fe3+-Ybt & Zn uptake) | Not tested | ∼40 (delayed TTD) | Not tested | 8 |
| irp2 znu (Ybt synthesis & Zn uptake) | Not tested | ∼6.0 × 106 (>4.3 × 105-fold) | Not tested | 8 |
| Δpgm znu (Ybt- Fet-Flp-; Fe3+, Fe2+ & Zn uptake) | Not tested | 5.7 × 106 (>9.5 × 105-fold) | Not tested | 8 |
All studies used outbred Swiss Webster mice. Bubonic plague – subcutaneous injection; Septicemic plague – retro-orbital injection; Pneumonic plague – intranasal instillation; delayed TTD – delayed time-to-death at low doses compared to parent/wild-type strain except for the psn ΔznuBC mutant which was compared to the znu mutant. NS – not significantly different from the wild-type strain.
The Δpgm and Δpgm ΔyfeAB mutants have a jopJ∷Mud Δpsa background. A combination of ΔyfeAB and genes encoded within the pgm locus could contribute to the large virulence loss in the Δpgm ΔyfeAB mutant.
Unpublished data – Swiss Webster mice were infected by intranasal instillation with Y. pestis cells grown as described in other studies on pneumonic plague. All mouse experiments in Table 2 were conducted in accordance with the Animal Welfare Act and approved by the University of Kentucky Institutional Animal Care and Use Committee.
Standard deviations are shown only for the znu mutant because of high variations. For clarity, standard deviations are not show for all other mutants tested.
A single feoB mutant is fully virulent while three trials of the single yfeAB mutant show an 8-9-fold loss of virulence compared to the parent strain. While statistics currently indicate this difference is not significant, further mouse studies could change this calculation. However, a feoB yfeAB double mutant has an 89-fold loss of virulence compared to the parent strain (Table 2).26 Thus the Ybt-siderophore dependent system is essential for the progression of bubonic plague while the Feo and Yfe transporters play a lesser role. An open question is whether a strain mutated in all 4 ferrous transporters would have reduced virulence compared to the ΔfeoB ΔyfeAB double mutant.
Using Y. pestis CO92 in a Brown-Norway rat bubonic plague model, Pradel et al. found that single mutations in most Fe transport systems did not affect virulence. These Fe uptake systems included Efe, FcuA, Fit, Fiu, Hmu, Iuc, IutA, Yiu, Yfu, Ynp and Ysu.117 However, with potentially redundant siderophore, ferric and ferrous transporters, multiple mutations are often required to identify the virulence roles of these systems.26
Our znu mutants retain high virulence (Table 2) and Pradel et al. obtained a similar result in Brown-Norway rats.60, 117 Because an irp2 mutantis completely avirulent in the bubonic plague model, we did not test the irp2 znu mutant that is more severely defective in Zn acquisition in vitro. Since a ybtX mutation does not affect Fe uptake via Ybt, we are testing the virulence of a znu ybtX mutant to assess the role of Zn acquisition in the progression of bubonic plague.
Mn acquisition also plays a role in bubonic plague. While an mntH mutant remains highly virulent in both mouse and rat models, our yfeAB mntH double mutant has a 133-fold virulence loss compared to the parent strain in the mouse model (Table 2).11, 117 We propose that the decreased virulence of the double mutant, compared to its modest in vitro growth defect, may be due to impaired oxidative stress defences. Although Mn is not essential for growth of E. coli, MntH is required during peroxide stress and Mn has been implicated in oxidative defences.4, 83
Thus full virulence in a mouse model of bubonic plague requires Ybt and Yfe or Feo for Fe acquisition and Yfe or MntH for Mn acquisition with only a mutation in the Ybt system causing a potentially complete loss of virulence (Fig. 7A; Table 2).
Fig. 7.
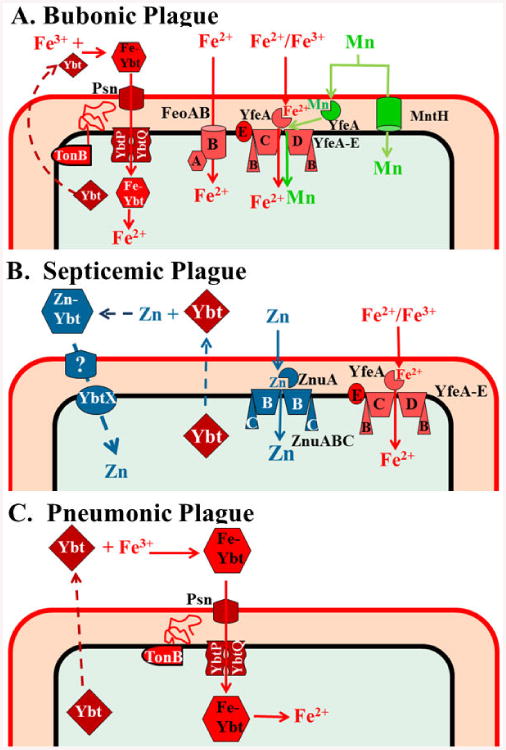
Fe, Zn, and Mn transporters with proven roles in mouse models of bubonic (A), septicemic (B), and pneumonic (C) plague. The Yfe system is shown as “transporting” both Fe3+ and Fe2+ since there is evidence it functions under aerobic conditions where Fe3+ would predominate and under microaerobic or reducing conditions where Fe2+ would be prevalent. However, the oxidation state transported is undetermined. Zn binding by Ybt (B) has not been shown; thus Ybt could play an indirect role in Zn uptake.
Septicemic Plague
Septicemic plague, which is less common, has been attributed to cutaneous cuts that allow direct entry to the blood stream.100, 109 Recently, Sebbane et al. 118 demonstrated that bites from multiple infected oriental rat fleas (Xenopsylla cheopis, a classical plague vector) could, at low incidence, cause primary septicemic plague. Septicemic plague is essentially bubonic plague without the early dermal and lymphatic stages and is characterized by fever and sepsis without regional lymphadenopathy (buboes) 100, 109.
Gene expression studies on Y. pestis cells isolated from the liver and spleen have not been performed. A microarray study using cells cultured ex vivo in heat-inactivated human plasma is the only genome-wide gene expression study relevant to septicemic plague and the latter stages of bubonic plague. Although most Fe transporters were more highly expressed in plasma compared to growth in LB at 37°C, iutA, fhuC, feoAB and fet-flp were not differentially expressed (Table 1).86, 115 Both Mn- and Fe-regulated systems (MntH and Yfe) had a 3-10.2-fold increase in expression in plasma compared to LB. Expression of genes involved in Zn and Cu transport or resistance was variable. Expression of znuA was moderately increased in plasma but other highly Zn-repressed genes (rpmJ and fiuA) were not. Finally, pcoC showed moderately higher expression in plasma compared to LB while all other Cu-related genes did not (Table 1).86, 115 Perhaps additional non-metal regulatory elements affected expression of some of these systems. Finally heat inactivation may have denatured some plasma metal-chelating proteins, affecting expression of some of these systems.
While Jackson and Burrows 112 showed that a component of the Pgm+ phenotype was involved in Fe acquisition in vivo, Une and Brubaker 113 demonstrated that a Pgm- KIM strain was highly virulent via intravenous (iv) injection,113 indicating that the then unidentified Ybt system was not needed in septicemic plague or the latter stages of bubonic plague. We replicated this finding by retro-orbital (similar to iv) injection in the now genetically defined Δpgm mutation in strain KIM and demonstrated that strains with an irp2 or a psn mutation are also highly virulent by this route of infection (Table 2).8, 55 However, the majority of mice survived doses between 10-20 cells of the irp2 mutant while all mice given similar doses of the Ybt+ parent succumbed to disease.8 Thus Ybt may play a modest, but not essential, role in septicemic plague and in the systemic stages of infections from low infectious doses. Using infection by oriental rat fleas, Sebbane et al. 118 showed that an irp2 mutant was able to cause septicemic plague in mice at low incidence. Recently, Tn-Seq analysis by Palace et al. found that mutants with insertions in psn, ybtP or ybtA were selected against in a mouse model of septicaemic plague.119 This suggests that in mixed infections, Ybt+ strains out compete mutants unable to use Ybt (e.g., psn, ybtP or ybtA mutants). However,in the absence of Ybt+ competitors, the Ybt system is not required for septicemic plague or the latter stages of bubonic plague but is essential in the early dermal/lymphatic stages of bubonic plague (Table 2).
Relatively few other metal transporters have been tested in the mouse model of septicemic plague. A Δpgm ΔyfeAB yopJ psa mutant was avirulent in our mouse model of septicemic plague (Table 2).22 This degree of virulence loss may be due to other components within the pgm locus (Fet-Flp or non-metal related factors) in combination with reduced Mn and/or Fe acquisition due to the ΔyfeAB mutation. In addition, background mutations in yopJ and psa in combination with the ΔyfeAB mutation may have increased virulence loss. However, the yopJ and psa mutations, designed to slightly attenuate this strain, did not cause a substantial loss of virulence in the absence of the ΔyfeAB mutation (Table 2).22
As expected, a single znu mutant was fully virulent in this model. However the irp2 znu mutant had over a 4 × 105-fold loss of virulence. The high virulence of a psn znu mutant demonstrates that the loss of virulence by the irp2 znu mutant was due to the defect in Zn acquisition and not a combination of defects in Zn and Fe acquisition (Table 2).8 While a large number of transition metal systems remain to be tested, the Yfe system for Fe and Mn transport as well as Znu and Ybt for Zn transport play a role in septicemic plague (Fig. 7B).
Pneumonic Plague
Secondary pneumonic plague is an occasional complication of bubonic or septicemic infection that occurs from hematogenous spread to the lungs. In humans and non-human primates (but not rodents) with secondary pneumonic plague transmission can occur by infected respiratory droplets causing primary pneumonic plague. Symptoms of fever, cough, and chest pain develop rapidly and radiology reveals a patchy bronchopneumonia or confluent consolidation of the lungs. While bacteria spread hematogenously to other internal organs, pneumonia is typically the cause of death in untreated cases. Untreated, fatality rates approach 100% and treatment with antibiotics delayed more than 24 h after symptoms develop is often unsuccessful 100, 108, 109, 120, 121.
A microarray analysis of Y. pestis cells isolated by bronchioalveolar lavage from the lungs of mice 48 h post intranasal infection was compared to cells grown in Brain Heart Infusion broth at 37°C. At this point in the infection mice are showing signs of illness, some lung necrosis, and bacteria appear to be extracellular in alveoli and the small bronchioles.116, 121 Compared to in vitro grown cells, Y. pestis cells from the lungs showed moderately increased expression of ybt, yfe and fet-flp but not other Fe transporters (Table 1).116 Moderately lower expression of feoAB in the lungs is curious, although in vitro these genes are not Fe repressed during aerobic growth. These results suggest a moderately iron-deficient environment with necrosis and immune responses possibly affecting iron-chelation mechanisms. Although yfeA exhibited moderate differential expression (2.8-fold), mntH levels were similar in vivo and in vitro. All three genes used as indicators for Zn levels show increased expression in the lung compared to in vitro. Thus the lung appears to be a Fe-and Zn-deficient environment with Mn levels less clear.
Analysis of Ybt system mutants in the mouse intranasal infection model yielded the intriguing result that mutations in irp2 were more highly attenuated (790-fold loss) than those in psn (33-fold loss). Thus mutants able to synthesize but not transport Ybt are 24-fold more virulent than mutants unable to synthesize Ybt. Both mutants exhibited significantly increased time-to-death (TTD) compared to much lower doses of the parent Ybt+ strain. The LD50 and time-to-death results are the opposite of the in vitro phenotype in which transport mutants have a more severe growth defect than biosynthesis mutants (Table 2).55 There are a number of different possible mechanisms for this difference, some with supporting in vitro evidence: 1) an unidentified,Ybt-YbtA-regulated gene might be required for virulence via the respiratory route – this gene would be transcriptionally active in the psn mutant but not in an irp2 mutant; 2) Ybt may have direct toxic effects in the lungs; 3) Ybt may affect host immune cell recruitment and/or cytokine signalling; 4) Cu-Ybt complexes may reduce Cu toxicity; 5) the SOD activity of Cu-Ybt may play a role in the lung; and 6) in the lungs, Ybt plays a greater role in Zn acquisition. 8, 55, 66, 94 Note that Zn uptake via Ybt would not be affected by a psn mutation8. Clearly, the Ybt system is a critical component in inhalation disease (Fig. 7C) with differential effects due to biosynthetic versus transport mutants.
We also found that a Δpgm mutant had an increased TTD and an LD50 of >4 × 106 in this model; that is a 15-fold greater loss of virulence than the irp2 mutant (Table 2).55 Lee-Lewis and Anderson122 found that the Δpgm KIM strain had a delay in TTD and did not cause a typical pneumonic infection. While bacteria survived in the lungs, they did not proliferate but spread hematogenously to other internal organs with septicaemia, not pneumonia, as the likely cause of death. Thus, the increased TTD of our irp2 and psn mutants55 might indicate death due to septicaemia not pneumonia. Supplementation with iron administered intraperitoneally reduced survival time but did not restore pneumonic disease, suggesting that an additional virulence factor(s), unrelated to Fe-acquisition, is encoded within the pgm locus.122 It should be noted that other researchers have determined LD50s ranging from 104 to 106 with Δpgm strains of KIM and CO92 in mice.114, 122,123 In some cases, BALB/c mice, which are more sensitive to plague were used; however, the reason(s) for the large differences in LD50s remains to be fully examined and explained.
Our analysis of yfe feo (Fe transport), yfe mntH (Mn transport) and znu mutants also demonstrated full virulence in the mouse model of pneumonic plague (Table 2). Analysis of a yfe feo efe fet-flp mutant is needed to determine whether or not ferrous transport processes are needed in lung infections. Analysis of a znu ybtX mutant should determine whether this more severely Zn-transport-deficient mutant will be attenuated. Nevertheless, the differences in virulence of the yfe feo and yfe mntH mutants in bubonic versus pneumonic infection models are intriguing.
Conclusions and future directions
Under in vitro aerobic conditions, the Fe-Ybt transport system is the most important of the multitude of Fe transporters. Under microaerobic conditions, the Yfe, Feo, and Fet-Flp systems each contribute to Fe-deficient growth. The Yfe and MntH Mn tansporters have apparently redundant roles in vitro while the Znu system is primarily responsible for Zn aqcuisition. However, a second Zn transporter involving the Ybt siderophore and YbtX also functions in vitro.
Virulence studies with Y. pestis indicate that in vitro and in vivo phenotypes of metal transport mutants may differ significantly.10, 53 The Mn transport double mutant has a modest in vitro growth defect but a more severe virulence defect in the bubonic plague model.11 In contrast, the znu mutant has the opposite phenotype. A znu mutant has a severe in vitro growth defect while a second irp mutation is required for virulence loss in the septicemic plague model.8, 60
Our in vivo studies have also clearly demonstrated that different metal transporters are required in specific organs or disease stages. The most striking example is Ybt: the three forms of plague show differing requirements for the Ybt system with pulmonary infections also showing an intriguing difference between Ybt biosynthetic mutants versus transport mutants.55 In addition, the combination of Yfe/Feo Fe transporters and Yfe/MntH Mn transporters are important for bubonic plague progression but not pneumonic plague.11, 26
Except for Ybt, the Fe, Mn and Zn transporters that may be important in pneumonic disease have not been identified. For Y. pestis strains with functional Ysu and/or Ych systems, studies are clearly needed to determine the in vivo importance of these siderophore-dependent transport systems. In CO92 and two endemic strains, the functional Ysu-Ych system does not compensate for loss of Ybt in vivo. Further studies with the septicemic model are required to identify whether transporters are important in the early lympathic stages of bubonic disease, the later septicemic stage, or both.
The demonstration that Ybt is involved in Zn acquisition in vitro and in vivo is an exciting new development. However, direct binding of Zn by Ybt has not been demonstrated and it is possible that the siderophore plays an indirect role. In addition, an OM receptor (if required) has not been identified for uptake via YbtX. Since YbtX is not involved in Fe-Ybt uptake, we can use the znu ybtX mutant to examine the role of Zn acquisition in bubonic and pneumonic plague.
While great progress has been made in our understanding of transition metal uptake systems in Y. pestis and their roles in causing disease, much remains to be elucidated.
Supplementary Material
Acknowledgments
The authors and our metal-related research studies cited here are supported by Public Health Services grant AI33481 from the US National Institutes of Health.
Biography

Dr. Alexander Bobrov (left) earned his M.D. in Pediatrics at the Saratov State Medical University in 1991 and a Ph.D. in Microbiology at the Microbe Anti-Plague Institute in 1995. He worked there for four years and was promoted to Senior Scientist. Alex started as a postdoctoral researcher at the University of Kentucky (UK) in 1999 and has continued as a Research Associate since 2002.
Dr. Jacqueline Fetherston earned a B.S. in medical technology at Michigan State University (MSU) in 1974 and obtained her Ph.D. in cell biology in 1981 at Washington University. She performed postdoctoral research at MSU and the National Institutes of Health, U.S.A. She was a Research Associate at UK and a Senior Research Associate at the Louisiana State University School of Medicine in Shreveport (LSUMC-S). In 1991 she started as a Research Assistant Professor and has been promoted to Research Professor at UK
Dr. Robert Perry (right) obtained his B.A. in biology at Indiana University in 1972 and earned his M.S. (1975) and Ph.D. (1978) degrees in microbiology at MSU. He performed postdoctoral research at Washington University, MSU, and UK. He was an Assistant Professor of microbiology at LSUMCS from 1986-1991. In 1991 he joined the Department of Microbiology and Immunology at the University of Kentucky where he was promoted to Professor.
References
- 1.Haley KP, Skaar EP. Microbes Infect. 2012;14:217–227. doi: 10.1016/j.micinf.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hood MI, Skaar EP. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kehl-Fie TE, Skaar EP. Curr Opin Chem Biol. 2010;14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lisher JP, Giedroc DP. Front Cell Infect Microbiol. 2013;3:91. doi: 10.3389/fcimb.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skaar EP. PLoS Pathog. 2010;6:e1000949. doi: 10.1371/journal.ppat.1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papp-Wallace KM, Maguire ME. Annu Rev Microbiol. 2006;60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez-Montes G, Arguello JM, Valderrama B. BMC Microbiol. 2012;12:249. doi: 10.1186/1471-2180-12-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobrov AG, Kirillina O, Fetherston JD, Miller MC, Burlison JA, Perry RD. Mol Microbiol. 2014;93:759–775. doi: 10.1111/mmi.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forman S, Paulley JT, Fetherston JD, Cheng YQ, Perry RD. BioMetals. 2010;23:275–294. doi: 10.1007/s10534-009-9286-4. [DOI] [PubMed] [Google Scholar]
- 10.Perry RD, Bobrov AG, Kirillina O, Rhodes ER, Actis LA, Fetherston JD. Adv Exp Med Biol. 2012;954:267–279. doi: 10.1007/978-1-4614-3561-7_34. [DOI] [PubMed] [Google Scholar]
- 11.Perry RD, Craig SK, Abney J, Bobrov AG, Kirillina O, Mier I, Jr, Truszczynska H, Fetherston JD. Microbiology. 2012;158:804–815. doi: 10.1099/mic.0.053710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry RD, Fetherston JD. In: Yersinia Molecular and Cellular Biology. Carniel E, Hinnebusch BJ, editors. Horizon Bioscience; Norfolk, U.K.: 2004. pp. 257–283. [Google Scholar]
- 13.Rakin A, Schneider L, Podladchikova O. Front Cell Infect Microbiol. 2012;2:151. doi: 10.3389/fcimb.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merchant SS, Helmann JD. Adv Microb Physiol. 2012;60:91–210. doi: 10.1016/B978-0-12-398264-3.00002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornung JM, Jones HA, Perry RD. Mol Microbiol. 1996;20:725–739. doi: 10.1111/j.1365-2958.1996.tb02512.x. [DOI] [PubMed] [Google Scholar]
- 16.Perkins-Balding D, Rasmussen A, Stojiljkovic I. In: Iron Transport in Bacteria. Crosa JH, Mey AR, Payne SM, editors. ASM Press; Washington, D.C.: 2004. pp. 66–85. [Google Scholar]
- 17.Thompson JM, Jones HA, Perry RD. Infect Immun. 1999;67:3879–3892. doi: 10.1128/iai.67.8.3879-3892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattle D, Zeltina A, Woo JS, Goetz BA, Locher KP. J Mol Biol. 2010;404:220–231. doi: 10.1016/j.jmb.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Woo JS, Zeltina A, Goetz BA, Locher KP. Nat Struct Mol Biol. 2012;19:1310–1315. doi: 10.1038/nsmb.2417. [DOI] [PubMed] [Google Scholar]
- 20.Rossi MS, Fetherston JD, Létoffé S, Carniel E, Perry RD, Ghigo JM. Infect Immun. 2001;69:6707–6717. doi: 10.1128/IAI.69.11.6707-6717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wandersman C, Delepelaire P. Mol Microbiol. 2012;85:618–631. doi: 10.1111/j.1365-2958.2012.08136.x. [DOI] [PubMed] [Google Scholar]
- 22.Bearden SW, Perry RD. Mol Microbiol. 1999;32:403–414. doi: 10.1046/j.1365-2958.1999.01360.x. [DOI] [PubMed] [Google Scholar]
- 23.Bearden SW, Staggs TM, Perry RD. J Bacteriol. 1998;180:1135–1147. doi: 10.1128/jb.180.5.1135-1147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong S, Bearden SW, Geoffroy VA, Fetherston JD, Perry RD. Infect Immun. 2001;67:2829–2837. doi: 10.1128/IAI.67.5.2829-2837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirillina O, Bobrov AG, Fetherston JD, Perry RD. Infect Immun. 2006;74:6171–6178. doi: 10.1128/IAI.00874-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fetherston JD, Mier I, Jr, Truszczynska H, Perry RD. Infect Immun. 2012;80:3880–3891. doi: 10.1128/IAI.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry RD, Mier I, Jr, Fetherston JD. BioMetals. 2007;20:699–703. doi: 10.1007/s10534-006-9051-x. [DOI] [PubMed] [Google Scholar]
- 28.Cartron ML, Maddocks S, Gillingham P, Craven CJ, Andrews SC. BioMetals. 2006;19:143–157. doi: 10.1007/s10534-006-0003-2. [DOI] [PubMed] [Google Scholar]
- 29.Koch D, Chan ACK, Murphy MEP, Lilie H, Grass G, Nies DH. J Biol Chem. 2011;286:25317–25330. doi: 10.1074/jbc.M111.222745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajasekaran M, Nilapwar S, Andrews S, Watson K. Biometals. 2010;23:1–17. doi: 10.1007/s10534-009-9262-z. [DOI] [PubMed] [Google Scholar]
- 31.Nies DH, Grass G. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Böck A, Curtiss R 3d, Kaper JB, Neidhardt FC, Nyström T, Rudd KE, Squires CI, editors. ASM Press; Washington, D.C.: 2009. ch. 5.4.4.3. [Google Scholar]
- 32.Bearden SW, Fetherston JD, Perry RD. Infect Immun. 1997;65:1659–1668. doi: 10.1128/iai.65.5.1659-1668.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller MC, Fetherston JD, Pickett CL, Bobrov AG, Weaver RH, DeMoll E, Perry RD. Microbiology. 2010;156:2226–2238. doi: 10.1099/mic.0.037945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim H, Lee H, Shin D. J Bacteriol. 2013;195:3364–3370. doi: 10.1128/JB.00343-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao J, Woodhall MR, Alvarez J, Cartron ML, Andrews SC. Mol Microbiol. 2007;65:857–875. doi: 10.1111/j.1365-2958.2007.05802.x. [DOI] [PubMed] [Google Scholar]
- 36.Große C, Scherer J, Koch D, Otto M, Taudte N, Grass G. Mol Microbiol. 2006;62:120–131. doi: 10.1111/j.1365-2958.2006.05326.x. [DOI] [PubMed] [Google Scholar]
- 37.Debut AJ, Dumay QC, Barabote RD, Saier MH., Jr J Mol Microbiol Technol. 2006;11:1–9. doi: 10.1159/000092814. [DOI] [PubMed] [Google Scholar]
- 38.Inesi G, Pilankatta R, Tadini-Buoninsegni F. Biochem J. 2014;463:167–176. doi: 10.1042/BJ20140741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boulette ML, Payne SM. J Bacteriol. 2007;189:6957–6967. doi: 10.1128/JB.00621-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi E, Groisman EA, Shin D. J Bacteriol. 2009;191:7174–7181. doi: 10.1128/JB.00958-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeon J, Kim H, Yun J, Ryu S, Groisman EA, Shin D. J Bacteriol. 2008;190:7326–7334. doi: 10.1128/JB.00903-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kammler M, Schön C, Hantke K. J Bacteriol. 1993;175:6212–6219. doi: 10.1128/jb.175.19.6212-6219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo J, Nair MK, Galvan EM, Liu SL, Schifferli DM. Microb Pathog. 2011;51:121–132. doi: 10.1016/j.micpath.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao H, Zhou D, Li Y, Guo Z, Han Y, Song Y, Zhai J, Du Z, Wang X, Lu J, Yang R. J Bacteriol. 2008;190:3063–3075. doi: 10.1128/JB.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forman S, Nagiec MJ, Abney J, Perry RD, Fetherston JD. Microbiology. 2007;153:2332–2341. doi: 10.1099/mic.0.2006/004275-0. [DOI] [PubMed] [Google Scholar]
- 46.Anisimov AP, Lindler LE, Pier GB. Clin Microbiol Rev. 2004;17:434–464. doi: 10.1128/CMR.17.2.434-464.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou D, Tong Z, Song Y, Han Y, Pei D, Pang X, Zhai J, Li M, Cui B, Qi Z, Jin L, Dai R, Du Z, Wang J, Guo Z, Wang J, Huang P, Yang R. J Bacteriol. 2004;186:5147–5152. doi: 10.1128/JB.186.15.5147-5152.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui Y, Li Y, Gorgé O, Platonov ME, Yan Y, Guo Z, Pourcel C, Dentovskaya SV, Balakhonov SV, Wang X, Song Y, Anisimov AP, Vergnaud G, Yang R., 3 Public Library of Science. 2008 doi: 10.1371/journal.pone.0002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cui Y, Yu C, Yan Y, Li D, Li Y, Jombart T, Weinert LA, Wang Z, Guo Z, Xu L, Zhang Y, Zheng H, Qin N, Xiao X, Wu M, Wang X, Zhou D, Qi Z, Du Z, Wu H, Yang X, Cao H, Wang H, Wang J, Yao S, Rakin A, Li Y, Falush D, Balloux F, Achtman M, Song Y, Wang J, Yang R. Proc Natl Acad Sci USA. 2013;110:577–582. doi: 10.1073/pnas.1205750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesic B, Carniel E. In: Yersinia Molecular and Cellular Biology. Carniel E, Hinnebusch BJ, editors. Horizon Bioscience; Norfolk, UK: 2004. pp. 285–306. [Google Scholar]
- 51.Chambers CE, McIntyre DD, Mouck M, Sokol PA. BioMetals. 1996;9:157–167. doi: 10.1007/BF00144621. [DOI] [PubMed] [Google Scholar]
- 52.Drechsel H, Stephan H, Lotz R, Haag H, Zähner H, Hantke K, Jung G. Liebigs Ann. 1995;1995:1727–1733. [Google Scholar]
- 53.Perry RD, Fetherston JD. Microbes Infect. 2011;13:808–817. doi: 10.1016/j.micinf.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perry RD, Balbo PB, Jones HA, Fetherston JD, DeMoll E. Microbiology. 1999;145:1181–1190. doi: 10.1099/13500872-145-5-1181. [DOI] [PubMed] [Google Scholar]
- 55.Fetherston JD, Kirillina O, Bobrov AG, Paulley JT, Perry RD. Infect Immun. 2010;78:2045–2052. doi: 10.1128/IAI.01236-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller MC, Parkin S, Fetherston JD, Perry RD, DeMoll E. J Inorg Biochem. 2006;100:1495–1500. doi: 10.1016/j.jinorgbio.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Fetherston JD, Bertolino VJ, Perry RD. Mol Microbiol. 1999;32:289–299. doi: 10.1046/j.1365-2958.1999.01348.x. [DOI] [PubMed] [Google Scholar]
- 58.Fetherston JD, Bearden SW, Perry RD. Mol Microbiol. 1996;22:315–325. doi: 10.1046/j.1365-2958.1996.00118.x. [DOI] [PubMed] [Google Scholar]
- 59.Perry RD, Abney J, Mier I, Jr, Lee Y, Bearden SW, Fetherston JD. Adv Exp Med Biol. 2003;529:275–283. doi: 10.1007/0-306-48416-1_53. [DOI] [PubMed] [Google Scholar]
- 60.Desrosiers DC, Bearden SW, Mier I, Jr, Abney J, Paulley JT, Fetherston JD, Salazar JC, Radolf JD, Perry RD. Infect Immun. 2010;78:5163–5177. doi: 10.1128/IAI.00732-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Qiu Y, Gao H, Guo Z, Han Y, Song Y, Du Z, Wang X, Zhou D, Yang R. BMC Microbiol. 2009;9:128. doi: 10.1186/1471-2180-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosadini CV, Gawronski JD, Raimunda D, Argüello JM, Akerley BJ. Infect Immun. 2011;79:3366–3376. doi: 10.1128/IAI.05135-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valentine RA, Jackson KA, Christie GR, Mathers JC, Taylor PM, Ford D. J Biol Chem. 2007;282:14389–14393. doi: 10.1074/jbc.M701752200. [DOI] [PubMed] [Google Scholar]
- 64.Bloß T, Clemens S, Nies DH. Planta. 2002;214:783–791. doi: 10.1007/s00425-001-0677-1. [DOI] [PubMed] [Google Scholar]
- 65.Grass G, Otto M, Fricke B, Haney CJ, Rensing C, Nies DH, Munkelt D. Arch Microbiol. 2005;183:9–18. doi: 10.1007/s00203-004-0739-4. [DOI] [PubMed] [Google Scholar]
- 66.Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE, Henderson JP. Nat Chem Biol. 2012;8:731–736. doi: 10.1038/nchembio.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brandel J, Humbert N, Elhabiri M, Schalk IJ, Mislin GLA, Albrecht-Gary AM. Dalton Trans. 2012;41:2820–2834. doi: 10.1039/c1dt11804h. [DOI] [PubMed] [Google Scholar]
- 68.Schalk IJ, Guillon L. Environ Microbiol. 2013;15:1661–1673. doi: 10.1111/1462-2920.12013. [DOI] [PubMed] [Google Scholar]
- 69.Schalk IJ, Hannauer M, Braud A. Environ Microbiol. 2011;13:2844–2854. doi: 10.1111/j.1462-2920.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- 70.Kobayashi S, Nakai H, Ikenishi Y, Sun WY, Ozaki M, Hayase Y, Takeda R. J Antibiot (Tokyo) 1998;51:328–332. doi: 10.7164/antibiotics.51.328. [DOI] [PubMed] [Google Scholar]
- 71.Kreutzer MF, Kage H, Gebhardt P, Wackler B, Saluz HP, Hoffmeister D, Nett M. Appl Environ Microbiol. 2011;77:6117–6124. doi: 10.1128/AEM.05198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hesketh A, Kock H, Mootien S, Bibb M. Mol Microbiol. 2009;74:1427–1444. doi: 10.1111/j.1365-2958.2009.06941.x. [DOI] [PubMed] [Google Scholar]
- 73.Zhao B, Moody SC, Hider RC, Lei L, Kelly SL, Waterman MR, Lamb DC. Int J Mol Sci. 2012;13:8500–8513. doi: 10.3390/ijms13078500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cortese MS, Paszczynski A, Lewis TA, Sebat JL, Borek V, Crawford RL. BioMetals. 2002;15:103–120. doi: 10.1023/a:1015241925322. [DOI] [PubMed] [Google Scholar]
- 75.Leach LH, Morris JC, Lewis TA. BioMetals. 2007;20:717–726. doi: 10.1007/s10534-006-9035-x. [DOI] [PubMed] [Google Scholar]
- 76.Bartsevich VV, Pakrasi HB. J Biol Chem. 1996;271:26057–26061. doi: 10.1074/jbc.271.42.26057. [DOI] [PubMed] [Google Scholar]
- 77.Dintilhac A, Alloing G, Granadel C, Claverys JP. Mol Microbiol. 1997;25:727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 78.Kolenbrander PE, Andersen RN, Baker RA, Jenkinson HF. J Bacteriol. 1998;180:290–295. doi: 10.1128/jb.180.2.290-295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Janakiraman A, Slauch JM. Mol Microbiol. 2000;35:1146–1155. doi: 10.1046/j.1365-2958.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- 80.Kehres DG, Janakiraman A, Slauch JM, Maguire ME. J Bacteriol. 2002;184:3159–3166. doi: 10.1128/JB.184.12.3159-3166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Runyen-Janecky L, Dazenski E, Hawkins S, Warner L. Infect Immun. 2006;74:4666–4672. doi: 10.1128/IAI.00562-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Couñago RM, Ween MP, Begg SL, Bajaj M, Zuegg J, O'Mara ML, Cooper MA, McEwan AG, Paton JC, Kobe B, McDevitt CA. Nat Chem Biol. 2014;10:35–41. doi: 10.1038/nchembio.1382. [DOI] [PubMed] [Google Scholar]
- 83.Anjem A, Varghese S, Imlay JA. Mol Microbiol. 2009;72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Imlay JA. J Biol Chem. 2014;289:28121–28128. doi: 10.1074/jbc.R114.588814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y, Stubbe J. Biochemistry. 2011;50:5615–5623. doi: 10.1021/bi200348q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marceau M, Simonet M. In: Yersinia Systems Biology and Control. Carniel E, Hinnebusch BJ, editors. Caister Academic Press; Norfolk, UK: 2012. pp. 19–42. ch. 2. [Google Scholar]
- 87.Anjem A, Imlay JA. J Biol Chem. 2012;287:15544–15556. doi: 10.1074/jbc.M111.330365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin JE, Imlay JA. Mol Microbiol. 2011;80:319–334. doi: 10.1111/j.1365-2958.2011.07593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hohle TH, Franck WL, Stacey G, O'Brian MR. Proc Natl Acad Sci USA. 2011;108:15390–15395. doi: 10.1073/pnas.1110137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kehres DG, Janakiraman A, Slauch JM, Maguire ME. J Bacteriol. 2002;184:3151–3158. doi: 10.1128/JB.184.12.3151-3158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arafah S, Rosso ML, Rehaume L, Hancock REW, Simonet M, Marceau M. Microbiology. 2009;155:2168–2181. doi: 10.1099/mic.0.026690-0. [DOI] [PubMed] [Google Scholar]
- 92.Francis MS, Thomas CJ. Molec Gen Genet. 1997;253:484–491. doi: 10.1007/s004380050347. [DOI] [PubMed] [Google Scholar]
- 93.Lewinson O, Lee AT, Rees DC. Proc Natl Acad Sci USA. 2009;106:4677–4682. doi: 10.1073/pnas.0900666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chaturvedi KS, Hung CS, Giblin DE, Urushidani S, Austin AM, Dinauer MC, Henderson JP. ACS Chem Biol. 2013;9:551–561. doi: 10.1021/cb400658k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Livny J, Zhou X, Mandlik A, Hubbard T, Davis BM, Waldor MK. Nucleic Acids Res. 2014;42:12212–12223. doi: 10.1093/nar/gku891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hunt JB, Neece SH, Ginsburg A. Anal Biochem. 1985;146:150–157. doi: 10.1016/0003-2697(85)90409-9. [DOI] [PubMed] [Google Scholar]
- 97.Kraemer SM, Duckworth OW, Harrington JM, Schenkeveld WDC. Aquat Geochem. Springer Netherlands: 2014. pp. 1–37. [DOI] [Google Scholar]
- 98.Hinnebusch BJ, Sebbane F, Vadyvaloo V. In: Yersinia: systems biology and control. Carniel E, Hinnebusch BJ, editors. Caister Academic Press; Norfolk, UK: 2012. pp. 1–18. [Google Scholar]
- 99.Gage KL, Kosoy MY. Annu Rev Entomol. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- 100.Eisen RJ, Gage KL. Annu Rev Entomol. 2012;57:61–82. doi: 10.1146/annurev-ento-120710-100717. [DOI] [PubMed] [Google Scholar]
- 101.Vetter SM, Eisen RJ, Schotthoefer AM, Montenieri JA, Holmes JL, Bobrov AG, Bearden SW, Perry RD, Gage KL. Microbiology. 2010;156:2216–2225. doi: 10.1099/mic.0.037952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hinnebusch BJ. Adv Exp Med Biol. 2012;954:237–243. doi: 10.1007/978-1-4614-3561-7_30. [DOI] [PubMed] [Google Scholar]
- 103.Jones RT, Vetter SM, Gage KL. The American Journal of Tropical Medicine and Hygiene. 2013;89:784–787. doi: 10.4269/ajtmh.13-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Webb CT, Brooks CP, Gage KL, Antolin MF. PNAS. 2006;103:6236–6241. doi: 10.1073/pnas.0510090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Eisen R, Borchert J, Holmes J, Amatre G, Van Wyk K, Enscore R, Babi N, Atiku LA, Wilder A, Vetter S, Bearden S, Montenieri J, Gage K. Am J Trop Med Hyg. 2008;78:949–956. [PubMed] [Google Scholar]
- 106.Vadyvaloo V, Jarrett C, Sturdevant DE, Sebbane F, Hinnebusch BJ. PLoS Pathog. 2010;6:e1000783. doi: 10.1371/journal.ppat.1000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lorange EA, Race BL, Sebbane F, Hinnebusch BJ. J Infect Dis. 2002;191:1907–1912. doi: 10.1086/429931. [DOI] [PubMed] [Google Scholar]
- 108.Perry RD, Fetherston JD. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Butler T. Plague and otherYersinia Infections. Pleunum Press; New York: 1983. [Google Scholar]
- 110.Sebbane F, Lemaître N, Sturdevant DE, Rebeil R, Virtaneva K, Porcella SF, Hinnebusch BJ. Proc Natl Acad Sci U S A. 2006;103:11766–11771. doi: 10.1073/pnas.0601182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Comer JE, Sturdevant DE, Carmody AB, Virtaneva K, Gardner D, Long D, Rosenke R, Porcella SF, Hinnebusch BJ. Infect Immun. 2010;78:5086–5098. doi: 10.1128/IAI.00256-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jackson S, Burrows TW. Br J Exp Pathol. 1956;37:577–583. [PMC free article] [PubMed] [Google Scholar]
- 113.Une T, Brubaker RR. Infect Immun. 1984;43:895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Welkos S, Pitt MLM, Martinez M, Friedlander A, Vogel P, Tammariello R. Vaccine. 2002;20:2206–2214. doi: 10.1016/s0264-410x(02)00119-6. [DOI] [PubMed] [Google Scholar]
- 115.Chauvaux S, Rosso ML, Frangeul L, Lacroix C, Labarre L, Schiavo A, Marceau M, Dillies MA, Foulon J, Coppée JY, Médigue C, Simonet M, Carniel E. Microbiology. 2007;153:3112–3124. doi: 10.1099/mic.0.2007/006213-0. [DOI] [PubMed] [Google Scholar]
- 116.Lathem WW, Crosby SD, Miller VL, Goldman WE. Proc Natl Acad Sci U S A. 2005;102:17786–17791. doi: 10.1073/pnas.0506840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pradel E, Lemaître N, Merchez M, Ricard I, Reboul A, Dewitte A, Sebbane F. PLoS Pathog. 2014;10:e1004029. doi: 10.1371/journal.ppat.1004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sebbane F, Jarrett C, Gardner D, Long D, Hinnebusch BJ. PLoS ONE. 2010;5:e14379. doi: 10.1371/journal.pone.0014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Palace SG, Proulx MK, Lu S, Baker RE, Goguen JD. mBio. 2014;5:e01385–01314. doi: 10.1128/mBio.01385-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Agar SL, Sha J, Foltz SM, Erova TE, Walberg KG, Baze WB, Suarez G, Peterson JW, Chopra AK. Microbes Infect. 2009;11:205–214. doi: 10.1016/j.micinf.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 121.Fellows P, Lin W, Detrisac C, Hu SC, Rajendran N, Gingras B, Holland L, Price J, Bolanowski M, House RV. Clin Vaccine Immunol. 2012;19:468–476. doi: 10.1128/CVI.05591-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee-Lewis H, Anderson DM. Infect Immun. 2010;78:220–230. doi: 10.1128/IAI.00559-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Parent MA, Berggren KN, Kummer LW, Wilhelm LB, Szaba FM, Mullarky IK, Smiley ST. Infect Immun. 2005;73:7304–7310. doi: 10.1128/IAI.73.11.7304-7310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


