Abstract
Early life exposure to estrogens and estrogen like contaminants in the environment are thought to contribute to the early onset of puberty and consequently increase the risk of developing breast cancer in the exposed female. The results of this study show that in utero exposure to the metalloestrogen arsenite altered mammary gland development prior to its effect on puberty onset. In the prepubertal gland, in utero exposure resulted in an increase in the number of mammosphere-forming cells and an increase in branching, epithelial cells, and density. In the postpubertal gland, in utero exposure resulted in the overexpression of estrogen receptor-alpha (ERα) that was due to the increased and altered response of the ERα transcripts derived from exons O and OT to estradiol. These results suggest that, in addition to advancing puberty onset, in utero exposure to arsenite alters the pre- and postpubertal development of the mammary gland and possibly, the risk of developing breast cancer.
Keywords: arsenite, in utero, mammary stem/pogenitor cells, estrogen receptor-alpha
Introduction
It has been suggested that the high incidence of hormone related diseases in children and adults, such as early onset of puberty and breast cancer, may be due to exposure to environmental estrogens. Since the beginning of the 20th century, there has been a trend towards an earlier onset of puberty. Although the trend has ceased during the last several decades in many European countries (1), it continued in the United States, especially in African American girls (2). Early puberty onset is associated with adverse social and clinical outcomes. Early thelarche (breast development) (3) and menarche (first menstrual period) (4) are risk factors or earlier development of breast cancer and early adrenarche (increase production of androgens) is associated with an increased risk of developing obesity, type 2 diabetes, and cardiovascular disease (5). Thelarche is triggered by the activation of the hypothalamic-pituitary-gonadal axis and the increase in circulating ovarian and pituitary hormones that initiate and promote the growth and morphogenesis of the breast (6).
The mammary gland is unique in that it grows and develops throughout the lifetime of a female (7). Development begins in fetal life and ends following the first full term pregnancy with the greatest growth occurring at puberty. In the immature gland, mammary stem cells are responsible for growth and development. Located in the terminal end buds (8) and along the ducts (9), these cells have the capacity to self renew and to generate progenitor cells that proliferate and differentiate into luminal and basal cells resulting in the elongation of the ducts and the development of branches. With the onset of puberty, elongation and branching occur in response to estrogens, progestins, and growth hormone. As estrogens play a central role in puberty onset and breast development, it has been suggested that exposure to environmental estrogens in early life, when the fetus and infant are susceptible to small hormonal changes (10;11), contributes to early puberty onset. In support of this hypothesis, epidemiological studies have linked exposure to the phythoestrogens in soy based formulas (12) and the xenoestrogen polybrominated biphenol (13) to early puberty onset and animal studies have shown that early life exposure to genistein (14) or estradiol (15) advances the onset of puberty.
Breast cancer is also associated with exposure to estrogens and the mammary stem and/or progenitor cells are thought to be the targets of malignant transformation because of their ability to self renew and proliferate. The most prominent risk factors for developing breast cancer are associated with increased lifetime exposure to estrogens, either to endogenous estrogens (e.g., early age at thelarche and menarche and late age at menopause) or exogenous estrogens (e.g., combined oral contraceptives or hormone replacement therapy (16;17)). In addition to lifetime exposure, the timing of exposure to estrogens appears to be a critical determinant of risk. Increased exposure to estrogens during pregnancy is associated with an increased risk of developing breast cancer in both the mother and the daughter; while decreased exposure to estrogens in daughters, whose mothers suffered from preeclampsia, is associated with a lower risk of developing the disease (18–23). Animal studies show that female offspring exposed in utero to estradiol, diethylstilbestrol, or genistein have an earlier vaginal opening and an increased risk of developing mammary tumors (14;24–26) suggesting that early life exposure to estrogens and estrogen like substances is also a risk factor of developing breast cancer.
In contrast to the naturally occurring phytoestrogens and synthetic xenoestrogens, less is known about the contribution of the metalloestrogens, such as the metalloid arsenite (27), to the high incidence of early puberty onset and breast cancer. Arsenic is a semi-metal having both metal and nonmetal properties. It is rarely found as a native element but occurs as an organic compound with carbon and hydrogen or as an inorganic compound with oxygen, chlorine, and sulfur. Arsenic is a prevalent environmental contaminant that has no known physiological function, is present in the body as a result of occupational and non-occupational exposures (28) and crosses the placental (29–31) and blood-brain barriers (32). Although the Environmental Protection Agency set the Reference Dose for inorganic arsenic as 0.3 µg/kg body weight (bw)/day (33) and the Joint FAO/WHO Expert Committee on Food Additives set the health concern level as 2.0 to 7.0 ug/kg bw/day (34), some populations may exceed these exposures. In the general population, the estimated daily intake of arsenic from food ranges from 0.01 to 5.6 µg/kg bw/day and from drinking water ranges from 0.2 to 0.539 ug/kg bw/day (35) but may be as high as 12.5 ug/kg bw/day in some areas (36). The estimated daily exposure from inhaled arsenic is also significant and ranges from 0.02 to 0.2 ug/kg bw/day in populations living in rural areas and from 0.4 to 0.6 ug/kg bw/day in populations living in cities (37) and may be higher in populations living near nonferrous smelters and power plants that burn coal high in arsenic. Cigarette smokers also inhale arsenic (0.7–6.0 ug/day) (38) and prior to the ban of arsenic in pesticides, smokers in the 1950s inhaled more than 100 µg of arsenic per day.
We have previously shown that arsenite, an oxyanionic form of arsenic, but not arsenate, has potent estrogen like activity in vitro due to a high affinity interaction with the ligand binding domain of estrogen receptor-alpha (ERα) (27). Similar to estradiol, arsenite induces the growth and expression of estrogen regulated genes in breast cancer cells and activates ERα in transient transfection experiments (27). Others have shown in a spontaneous mouse model that arsenite in drinking water increase the growth of mammary tumors (39) and that in utero exposure to arsenite influences the estrogen response pathways in both male and female offspring (29;30;40) suggesting that arsenite also has estrogen like activity in vivo. In this study, we show that, in female offspring, in utero exposure to an environmentally relevant dose of arsenite advances the timing of vaginal opening and alters the development of the mammary gland prior to its effect on the hypothalamic-pituitary-gonadal axis. In utero exposure to the metalloid also results in an expansion of the mammosphere-forming cell population in the prepubertal mammary gland and alters the expression and regulation of ERα in the postpubertal gland suggesting that in utero exposure to arsenite causes changes in the mammary gland which result in abnormal growth and potentially, increases the susceptibility of the gland to neoplasia later in life.
Materials and Methods
Animals
All animal studies were conducted in accordance with the Georgetown University Animal Care and Use Committee. Pregnant Sprague-Dawley rats were delivered on day 7 of gestation from Harlan Breeding Facilities (Frederick, MD) and maintained on a purified phytoestrogen-free diet that contained no detectable arsenic and was not supplemented with copper, chromium, and selenium (Tekland Lab Animal Diets TD02373). The animals were provided with purified water. The amount of arsenic in the water was not measured but was expected to be negligible. Pregnant female rats were treated with 5 µg/kgbw of arsenite dissolved in water by intraperitoneal (i.p.) injection on days 12 and 17 of gestation or with 50 µg/kgbw of ethinyl estradiol dissolved in corn oil by daily oral gavage starting on day 12 of gestation until birth. To avoid metabolism in the liver, ethinyl estradiol was given instead of estradiol. Arsenite is methylated primarily in the liver (28;41). When given orally, arsenite is absorbed from the gastrointestinal tract and transported to the liver where it is methylated (28;42;43). When arsenite is inhaled or given by intratracheal instillation, it is absorbed through the naso-pharynx, tracheobronchial, and pulmonary compartments and transported in the plasma to multiple organs in the body (28;42;43). Similar to inhalation, when arsenite is given by intravenous or intraperitoneal injection, it is transported to multiple organs (28;43;44). As arsenite is the form of arsenic that activates ERα (27), arsenite was administered by i.p. injection to increase its bioavailability. The control animals were treated with water by i.p. injection. In this study, there were ten control dams, nine arsenite exposed dams, and four ethinyl estradiol exposed dams. At 21 days of age, the pups were weaned and continued on the purified diet. For the vaginal opening and postpubertal studies, pups were randomly fostered with dams from the same treatment group and all of the pups were included in the analyses. For the time course study, the pups were randomly fostered with dams from the same treatment group. For the time points, three to four pups were then randomly selected from different dams. To monitor normal development, eye lid opening and weekly weights were determined. Vaginal opening was monitored daily from postnatal day 25 to 40.
Morphological analysis of the mammary gland
For morphological analysis, the mammary glands were excised and processed as whole mounts. The glands were fixed in Carnoy’s fixative for 4 hrs to overnight, defatted overnight in xylene, rehydrated, stained overnight with carmine alum (Sigma), dehydrated in a series of graded alcohols, and cleared in xylene. Digital images were obtained and analyzed using MetaMorph Microscopy Automation & Image Analysis Software (Sunnyvale, CA). To determine mammary gland density, the images were binarized and the density was calculated as the percentage of the epithelium compared to the fat pad. The mammary gland branching was calculated as the number of branching points per unit length of two branches from the nipple to the terminal end.
Mammosphere culture
Whole mammary glands from 5 day old animals were minced, suspended in DMEM/F12 medium containing collagenase and hyaluronidase (Stemcell Technologies), and incubated for 7 hrs in a 5% CO2 incubator (45). Epithelial organoids were isolated by centrifuged at 80×g for 30 seconds. Five ml of pre-warmed trypsin-EDTA (Stemcell Technologies) was added to the pellet and the pellet resuspended by pipetting. Ten ml of cold Hanks’ Balanced Salt Solution Modified (Stemcell Technologies) supplemented with 2% FBS (HF) was added and the solution centrifuged at 350×g for 5 min. Two ml of pre-warmed 5 mg/ml Dispase and 200 ul of 1 mg/ml DNase I was added and the pellet was resuspended by pipetting. To obtain a single cell suspension, the cell sample was diluted with 10 ml of cold HF and filtered through a 40-µm cell strainer. The sample was then centrifuged at 350×g for 5 min and resuspended in 1–2 ml of serum-free mammary epithelial growth medium (MEGM, Lonza) supplemented with B-27 supplement minus vitamin A (2×; Invitrogen), 20 ng/ml recombinant rat EGF (PeproTech), 20 ng/ml recombinant rat bFGF (PeproTech), and 4 µg/ml heparin (Sigma). The cells were counted and visualized using a hemocytometer. In all experiments, the number of single cells was greater than 99%. Cells were plated in ultralow attachment 6 well plates (Corning) at a density of 40,000 cells in 2 ml of the supplemented MEGM per well and incubated in 5% CO2 incubator at 37°C for 7–10 days. Although cell aggregates were present, only mammospheres larger than 60 µm in size (may have solid or hollow morphology) were counted and photo-documented. For mammosphere subcultures, the mammospheres were collected by centrifugation at 350×g for 5 min and dissociated with 0.5–1 ml of pre-warmed trypsin-EDTA. Mammospheres were triturated using a P-1000 pipette for 1.5–2 min. Five ml of cold HF was added and the suspension was centrifuged at 350×g for 5 min. Supernatant was aspirated and the pellet resuspended in 2 ml of supplemented MEGM. Single cells were plated at a density of 10,000 cells in 2 ml in ultralow attachment 6 well plates and incubated for 7–10 days as described above.
Real time reverse transcriptase-polymerase chain reaction
For RNA extraction, frozen tissue was pulverized in liquid nitrogen using Spectrum Bessman Tissue Pulverizers. One ml of Trizol reagent (Invitrogen) was added and the tissue was then homogenized using Omni Tip Soft Tissue Homogenizer. The homogenate was centrifuged for 10 min at 12,000rpm at 4°C, the lipid layer was removed, and the supernatant was collected. After a 5 min incubation at room temperature, chloroform (0.2 ml) was added for each 1 ml of sample. The sample was vortexed and incubated at room temperature for 3 min. The sample was centrifuged for 15 min at 12,000rpm at 4°C. The upper phase was collected and an equal volume of isopropanol was added. The sample was incubated at −20°C for 2 hrs to overnight and centrifuged for 15 min at 12,000rpm at 4°C. Following centrifugation, the pellet washed with 1 ml of 75% ethanol, centrifuged for 5 min at 7,000rpm at 4°C, dried, and resuspended in 50 µl of DEPC treated water. The 260:280 ratio and concentration were determined. Samples were aliquoted and stored at −80°C.
For the reverse transcriptase reaction, deoxyribonuclease I (2 µl, Invitrogen) and 2.5 µl of 10× buffer (200mM Tris-HCl pH 8.4, 20mM MgCl2, 500mM KCl; Invitrogen) was added to 2 µg of RNA and incubated at room temperature for 15 min. Then 2 µl of EDTA (25mM; Invitrogen) was added and the sample incubated for 15 min at 65°C. Each 70 µl RT reaction contained 7µl of 10× Taqman RT Buffer (500mM KCl, 100mM Tris-HCl pH 8.3), 15.4 µl of 25mM MgCl2, 14 µl of dNTPs, 3.5 µl of random hexamers, 1.4 µl of RNase inhibitor, 1.75 µl of MultiScribe reverse transcriptase (Applied Biosystems), 2 µg of RNA, and DEPC treated water to 70 µl. The mixture was incubated in the thermal cycler for 10 min at 25°C, 30 min at 48°C, and 5 min at 95°C.
For the real-time polymerase chain reaction, each 10 µl reaction contained 5 µl of Sensimix II Probe Mastermix (Bioline), 0.5 µl of 20× Assay on Demand (Applied Biosystems) and 4.5 µl of cDNA; or 5 µl of Sensimix SYBR (Bioline), 0.25 µl of 20 µM forward or reverse primer, and 4.5 µl of cDNA. Samples were then run on the 7900HT (Applied Biosystems) and the data analyzed by the 2−ΔΔCt method using the SDS 2.1 software (Applied Biosystems).
Nuclear run-on assay
For the preparation of nuclei, mammary glands were frozen, pulverized in liquid nitrogen, and resuspended in nuclei isolation buffer (15mM HEPES, 1.5M sucrose, 60mM KCl, 15mM NaCl, 0.15mM spermine, 0.5mM spermidine, 2mM EDTA, 0.5mM EGTA, 0.1mM PMSF*, 2mM DTT*; *added fresh) plus 0.1% Triton-X (Promega). Twelve ml ice-cold nuclei isolation buffer were added to approximately 500 mg of frozen pulverized tissue and the tissue was homogenized in a Dounce homogenizer. The homogenate was filtered through four-six layers of Miracloth (EMD Chemicals). The nuclei were collected by centrifugation at 250×g for 10 min at 4°C, washed with nuclear isolation buffer, resuspended in 100 µl of freezing buffer (50mM HEPES, 0.1mM EDTA, 5mM MgCl2, 40% glycerol), and stored at −80°C.
For the run-on assay, nuclei were thawed on ice in one volume of transcription buffer 2× (20mM HEPES, 200mM KCl, 5mM MgCl2, 200mM sucrose, 4mM ATP, 4mM CTP, 4mM GTP, 20% glycerol, 4mM DTT-added fresh). Eight ul of biotin-16-UTP (Roche Diagnostics) were added and the nuclei were incubated for 1 hr at 26°C. The reaction was stopped by adding 6 µl of 250mM CaCl2. The nuclei were collected by centrifugation for 20 min at 4°C at 12,000rpm. The RNA was isolated using Trizol and resuspended in 50 µl of DEPC treated water. Dynabeads, M-280 Streptavidin (Invitrogen), were used to purify the nascent mRNA following the manufacturer’s protocol. Briefly, the Dynabeads were added to the mRNA samples and incubated for 20 min at 42°C and then 2 hrs at room temperature. The mRNA-bead complex was separated using a Dynal magnet, washed, resuspended in 30 µl of DEPC water, and used to synthesize cDNA for a qRT-PCR assay.
Statistical Analysis
Vaginal opening was determined by an investigator that was blinded to treatment and the data were analyzed by one-way ANOVA. All other data are presented as means ± SEM. Statistical analyses were performed only on groups that contained three or more animals using a two-tailed unpaired Student's test with unequal variance. Statistical significance was defined as P < 0.05.
Results
Effects of early life exposure to arsenite on vaginal opening in female offspring
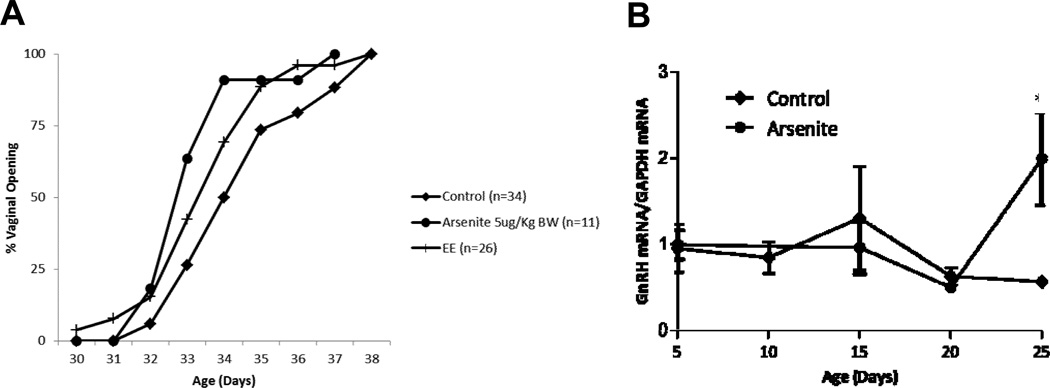
To determine the effects of early life exposure to an environmentally relevant dose of arsenite on vaginal opening, pregnant animals were treated with 5 µg/kgbw of arsenite by i.p. injection on day 12 and on day 17 of gestation or with ethinyl estradiol (50 µg/kgbw; positive control) by daily oral gavage starting on day 12 of gestation until birth (Figure 1a). Vaginal opening was monitored in female offspring beginning on postnatal day 25. There was a significant difference in the time of vaginal opening between control animals (postnatal day 34.76 ± 0.32) and animals exposed in utero to arsenite or ethinyl estradiol (postnatal day 33.45 ± 0.41 and 33.81 ± 0.32, respectively, p <0.05) that was not due to an increase in body weight (data not shown). As vaginal opening is controlled by the reactivation of the GnRH pulse generator (46), the amount of GnRH mRNA in the hypothalamus was measured on postnatal days 5, 10, 15, 20 and 25 (Figure 1b) to rule out a nonspecific effect of arsenite. In non-treated Sprague-Dawley rats, GnRH mRNA levels generally increase between postnatal day 28 and 34 (47;48). In the animals exposed in utero to arsenite, GnRH mRNA levels began to increase between postnatal days 20 and 25. As expected, there was no increase in the levels of GnRH mRNA in control animals. The earlier increase in GnRH mRNA in the exposed animals suggests that in utero exposure to arsenite alters GnRH expression and consequently the time of vaginal opening.
Figure 1. Effects of early life exposure to arsenite on puberty onset in female offspring.
Pregnant female rats were treated with arsenite (5 µg/kg bw) by i.p. injection on days 12 and 17 of gestation or ethinyl estradiol (EE, positive control, 50 µg/kg bw) by daily oral gavage beginning on day 12 until birth and the female offspring were examined.
(a) Effect of early life exposure to arsenite on the time of vaginal opening. Pregnant female rats were treated with arsenite or ethinyl estradiol and the female offspring were monitored for vaginal opening. Data are plotted as percent animals with vaginal opening (n=11–34/group).
(b) Effect of early life exposure to arsenite on the expression of GnRH in the hypothalamus. Pregnant rats were treated with arsenite and the expression of GnRH in the female offspring was determined. The amount of GnRH mRNA was measured on postnatal days 0, 5, 10, 15, 20 and 25 by a qRT-PCR assay and normalized to the amount of GAPDH mRNA (mean ±SEM; n=2–3/group; * p<0.05).
Effects of early life exposure to arsenite on the morphology of the mammary gland of female offspring
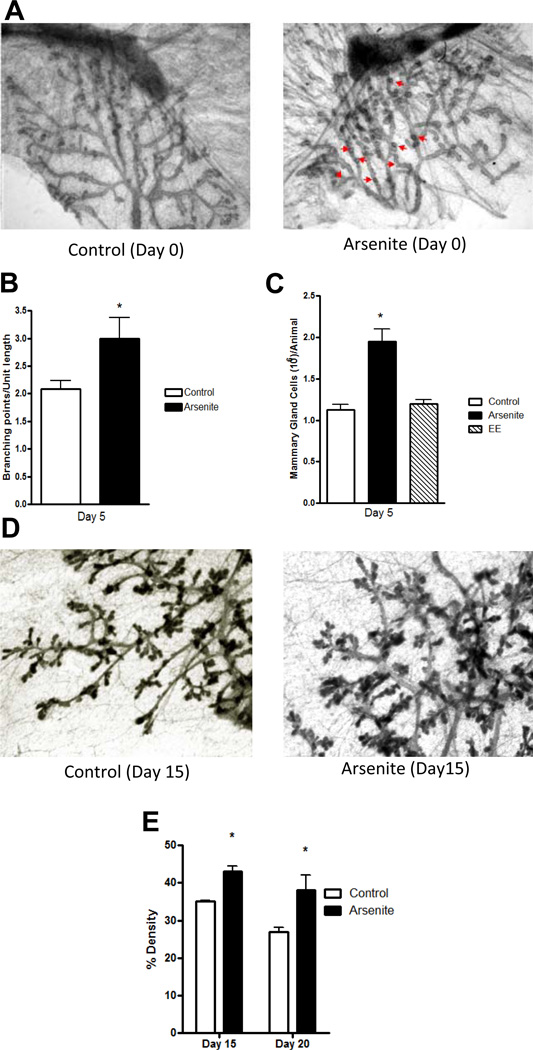
Early puberty onset may alter the development and/or morphology of the mammary gland due to an earlier increase in the synthesis of estrogens in the ovaries. To ask whether in utero exposure to arsenite alters mammary gland morphology and development prior to the onset of puberty, pregnant animals were again treated with arsenite and the female offspring examined. The fourth abdominal mammary glands were dissected and processed as whole mounts, and examined on postnatal days 0, 5, 15, and 20. On postnatal day 0, an abnormal pattern of lateral budding (49) was observed along the ducts of the mammary glands of animals exposed while in utero to arsenite, suggesting that the in utero exposure to the metalloid alters branching in the mammary gland (Figure 2a). To determine whether exposure to the metalloid alters branching in the mammary gland, the number of branch points along the ducts was counted on postnatal day 5 (Figure 2b). There was a significant increase in the number of branch points in the mammary glands of animals exposed to arsenite (2.99 ± 0.65 branch points/unit length) compared to the number of branch points in the mammary gland of control animals (2.08 ± 0.26 branch points/unit length) suggesting that in utero exposure to arsenite increases the branching of the mammary gland. To ask whether the increase in branching is associated with an increase in epithelial cells, the cells were isolated and counted (Figure 2c). There was a significant 1.7-fold increase (p < 0.05) in the number of cells in the mammary glands of animals exposed to arsenite compared to control animals. To determine whether the increase in epithelial cells continues throughout the prepubertal growth of the gland, mammary gland density was measured on postnatal days 15 and 20 (Figures 2d–e). In animals exposed to arsenite, there was a significant increase (p<0.05) in mammary gland density (43% and 38%, respectively) compared to controls (35% and 27%, respectively). Taken together, the results demonstrate a consistent increase in the number of branch points, number of epithelial cells, and density in the mammary glands of animals exposed to arsenite while in utero. Since the morphological and developmental effects of arsenite were observed in animals exposed while in utero and occurred prior to the onset of puberty, the results suggest a direct effect of early life exposure to arsenite on the mammary gland.
Figure 2. Effects of early life exposure to arsenite on the morphology of the mammary gland of female offspring.
Pregnant female rats were treated with arsenite or ethinyl estradiol as described in Figure 1. For morphological analyses, the fourth abdominal mammary glands were excised.
(a) In utero effects on mammary gland morphology. Digital image on postnatal day 0 from a control animal (representative image, n=2) or an animal exposed to arsenite (representative image, n=3). Arrows indicate abnormal lateral budding.
(b) In utero effects on mammary gland branching. The number of branch points was quantified on postnatal day 5 as branches per unit length (mean±SEM; n=3/group; * p<0.05).
(c) In utero effects on the number of epithelial cells in the mammary gland. Both fourth abdominal mammary glands were excised on postnatal day 5. Epithelial cells were isolated and counted (mean±SEM; n=4/control group, n=3/arsenite group, n=2/ethinyl estradiol group; *p<0.05 for control vs arsenite).
(d–e) In utero effects on mammary gland density on postnatal days 15, and 20. (d) Digital image from 15 day old control animal (representative image, n=3) or animal exposed to arsenite (representative image, n=3). (e) Mammary gland density is expressed as the percent of epithelium in the fat pad (mean±SEM; n=3/group; * p<0.05).
Effects of early life exposure to arsenite on mammosphere-forming cells in the glands of female offspring
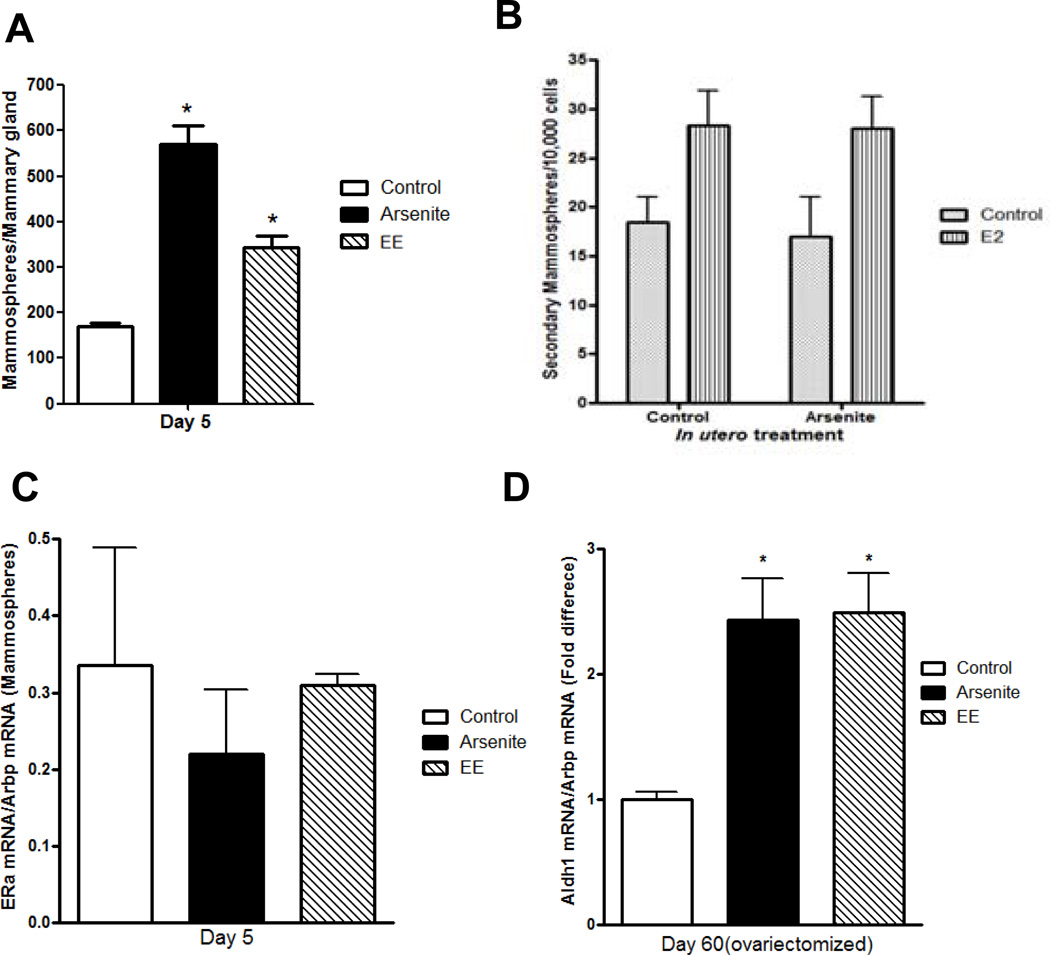
The increase in the number of branch points suggests that in utero exposure to arsenite increases the number of mammary stem and/or progenitor cells (9). Mammary stem cells form mammospheres when grown in nonadherent cell culture conditions. To ask whether there was an increase in the number of mammosphere-forming cells, mammospheres (50;51) were grown from cells isolated from the glands of 5 day old offspring (Figure 3a). Compared to control animals, there was a 3.1-fold increase in the number of mammospheres derived from the mammary glands of animals exposed in utero to arsenite and a 2-fold increase in animals exposed in utero to ethinyl estradiol. To ask whether the mammospheres were derived from a stem-like cell, the potential for self-renewal was tested. When subcultured every 10 days, the primary generation mammospheres produced secondary and tertiary generation mammospheres with an efficiency of approximately 2/1000 cells (Figure 3b). The mammospheres expressed aldehyde dehydrogenase 1 (Aldh1) (52), CD24 (53), epithelial cell adhesion molecule (EpCAM) (53) (data not shown), and estrogen receptor-alpha (ERα) (54–56) (Figure 3c). There was no significant difference in the expression of ERα mRNA in mammospheres isolated from control or exposed animals (Figure 3c). When the second generation mammospheres were treated with 17β-estradiol, there was an increase in the number of mammospheres but no difference in the size (data not shown) or fold increase in the number of mammospheres derived from control or arsenite exposed animals (1.53- and 1.64-fold increase, respectively; Figure 3b) consistent with the expression of ERα. To ask whether in utero exposure to the metalloid alters the stem/progenitor like cells in the adult gland, pregnant animals were treated with arsenite or ethinyl estradiol and the expression of Aldh1 was measured in virgin animals (Figure 3d). To avoid the effects of estrogens on gene expression, the animals were ovariectomized on postnatal day 45 and examined on postnatal day 60. There was a 2.43- and 2.49-fold increase in Aldh1 mRNA in animals exposed in utero to arsenite or estradiol, respectively. The increase in the number of mammospheres together with the increase in mammary gland Aldh1 suggests that in utero exposure to arsenite results in the expansion of the mammary stem/progenitor cell population that persists through development.
Figure 3. Effects of early life exposure to arsenite on mammosphere-forming cells in the mammary gland of female offspring.
Pregnant female rats were treated with arsenite or ethinyl estradiol as described in Figure 1.
(a) In utero effects on the number of mammospheres obtained from female offspring on postnatal day 5 (mean±SEM; n=4 animals/group; * p<0.05).
(b) In utero effects on estradiol induced proliferation of mammospheres. First generation mammospheres from control animals and arsenite exposed animals were digested with trypsin, stem/progenitor cells were selected in serum-free media under non-adherent conditions in the presence or absence of estradiol (1 nM), and the second generation mammospheres were counted (mean±SEM; n= 2/group).
(c) In utero effects on ERα expression in mammospheres obtained from female offspring on postnatal day 5. ERα mRNA was determined by a qRT-PCR assay and normalized to Arbp mRNA (mean±SEM; n=2/group).
(d) In utero effects on Aldh1 expression in the adult gland. Female offspring were ovariectomized on postnatal day 45, and on postnatal day 60, the amount of Aldh1 mRNA was measured by a qRT-PCR assay, normalized to Arbp mRNA, and presented as fold difference (mean±SEM; n=4–11/group; * p<0.05).
Effects of early life exposure to arsenite on gene expression in the mammary glands of female offspring
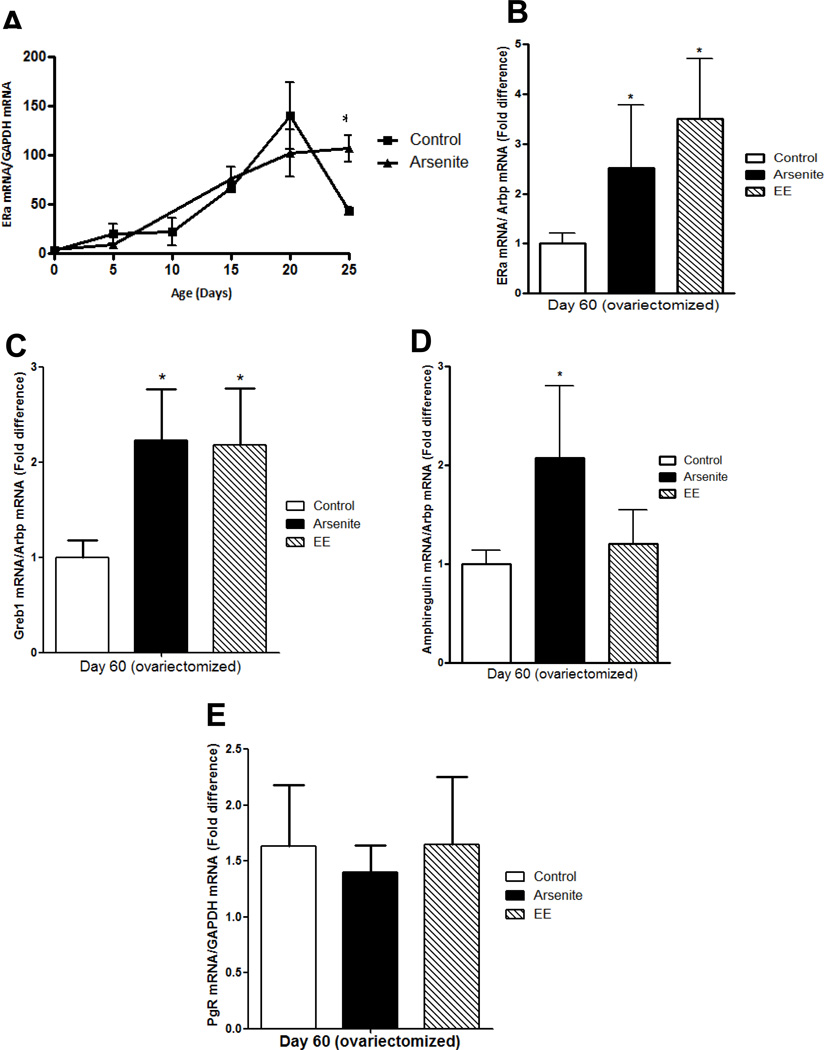
In utero exposure to endocrine disrupting chemicals has been shown to alter expression of ERα in the exposed offspring, e.g., in utero exposure to genistein or TCDD increases the expression of ERα in the mammary gland and in utero exposure to dioxin increase expression in the testis while in utero exposure to BPA decreases expression of ERα in the vagina of exposed offspring (14;57–59). To determine whether in utero exposure to arsenite alters ERα expression in the mammary gland, ERα mRNA was measured on postnatal days 0, 5, 10, 15, 20, and 25 and on postnatal day 60. To avoid the effects of estradiol on gene expression, the latter animals were ovariectomized on postnatal day 45 (Figures 4a–b). In control animals, the expression of ERα mRNA increased from postnatal day 0 to day 20 and decreased on postnatal day 25. In animals exposed in utero to arsenite, the expression of ERα mRNA increased from postnatal day 0 to day 20 but remained at the same level on postnatal day 25. In the ovariectomized animals (postnatal day 60), basal expression of ERα mRNA remained elevated with a 2.5- and 3.5-fold increase (p<0.05) in ERα mRNA in animals exposed in utero to arsenite or ethinyl estradiol, respectively (Figure 4b) suggesting that in utero exposure to arsenite leads to the overexpression of ERα that persists into adulthood.
Figure 4. Effects of early life exposure to arsenite on gene expression in the mammary gland of female offspring.
Pregnant female rats were treated with arsenite or ethinyl estradiol as described in Figure 1 and the female offspring were examined.
(a) In utero effects on ERα expression in the prepubertal gland. The amount of ERα mRNA was measured on postnatal days 0, 5, 10, 15, 20 and 25 by qRT-PCR assay and normalized to Arbp mRNA (mean±SEM; n=3–11/group; * p<0.05).
(b) In utero effects on ERα expression in the adult gland. Female offspring were ovariectomized on postnatal day 45. On postnatal day 60, the amount of ERα mRNA was measured by a qRT-PCR assay and normalized to Arbp mRNA. Data are presented as fold difference (mean±SEM; n=4–11/group; * p<0.05).
(c–e) In utero effects on gene expression in the adult gland. Female offspring were ovariectomized on postnatal day 45. On postnatal day 60, the amount of Greb1 (c), amphiregulin (d), and PgR (e) mRNA was measured by a qRT-PCR assay, normalized to Arbp mRNA, and presented as fold difference (mean±SEM; n=4–11/group; * p<0.05).
To ask whether in utero exposure to arsenite alters the expression of genes that are regulated by estradiol, candidate genes were measured in the mammary gland of the ovariectomized animals (Figure 4c–e). In utero exposure to arsenite increased the expression of GREB1 (60) and amphiregulin (61) mRNA but had no effect on progesterone receptor (PgR) mRNA. Although in utero exposure to arsenite increased the expression of amphiregulin mRNA, in utero exposure to ethinyl estradiol had no effect on its expression.
Effects of early life exposure to arsenite on the expression of ERα transcripts in the mammary gland of female offspring
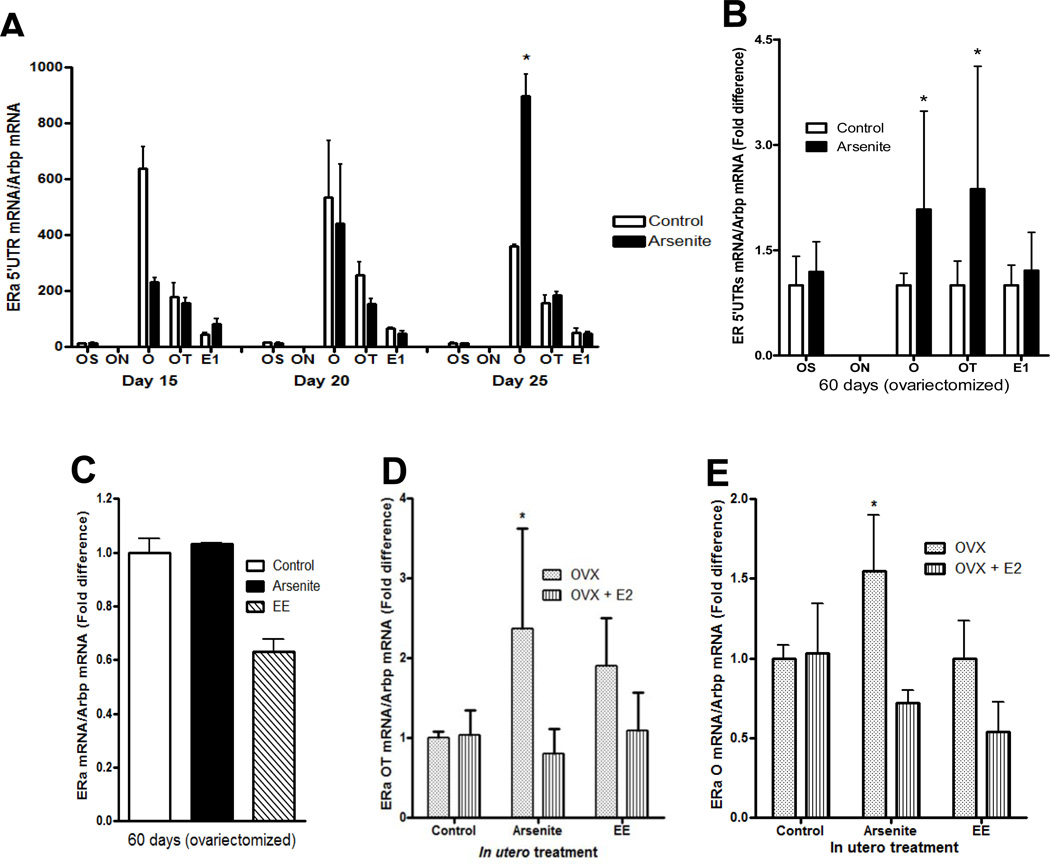
The rat ERα gene has five promoters (OS, ON, O, OT, and E1) (62–65) that confer tissue specific expression (63;65) and result in ERα transcripts with different 5’ untranslated regions (5’ UTR),. To determine which promoter is utilized in the mammary gland, the 5’UTRs of the ERα transcript were measured using primers for exons OS, ON, O, OT, and E1 (Figure 5). In control animals (postnatal day 15 to 25), the 5’UTR of ERα mRNA was derived predominantly from exons O and OT with expression of exon O approximately 2- to 4.5-fold greater than exon OT (Figure 5a). In the ovariectomized animals (postnatal day 60), the 5’UTR was derived from exon O followed by exons E1, OT and OS suggesting that throughout development, the ERα transcript derived from exon O is the predominant transcript in the mammary gland. To determine whether in utero exposure to arsenite alters expression of the ERα transcripts, the 5’UTRs of ERα mRNA were again measured on postnatal days 15 to day 25 (Figure 5a), and in the ovariectomized adult animals (Figure 5b). Compared to control animals, the transcript derived from exon O increased from postnatal day 5 to 20 but there appeared to a delay in the increase in expression. In the exposed animals, the exon O transcript was significantly less on postnatal day 15 (2.75-fold, p < 0.05), similar on postnatal day 20, and significantly higher on postnatal day 25 (2.28-fold; p < 0.05). In contrast to the transcript derived from exon O, the transcript derived from exon OT did not significantly change from postnatal day 5 to 25. In the exposed adult gland, expression of the transcripts derived from exons O and OT was increased (2.08- and 2.37-fold; p<0.05, respectively). The increase in ERα mRNA may be due to an increase in basal transcription of the ERα gene, however, a nuclear run on assay showed that there was no difference in transcription of the ERα gene between control animals and animals exposed in utero arsenite or ethinyl estradiol (Figure 5c). To ask whether in utero exposure alters the regulation of the O and OT transcripts, the ovariectomized animals were treated with 17β-estradiol (Figures 5d–e). Treatment with estradiol had no effect on the expression of the O and OT transcripts in control animals but decreased the expression of the O and OT transcripts in animals exposed to arsenite while in utero. Taken together, the data show that in utero exposure to arsenite increases the expression of the O and OT transcripts and alters their regulation by estradiol. The data also suggest that overexpression of ERα mRNA may be due, in part, to alternate promoter usage and/or posttranscriptional regulation.
Figure 5. Effects of early life exposure to arsenite on the expression of ERa transcripts in the mammary gland of female offspring.
Pregnant female rats were treated with arsenite (5 µg/kg bw) or ethinyl estradiol (50 µg/kg bw) as described in Figure 1 and the female offspring were examined.
(a) In utero effects on ERα transcripts in the prepubertal gland. The amount of 5’UTR ERα mRNA was measured on postnatal days 15, 20 and 25 by qRT-PCR and normalized to Arbp mRNA (mean±SEM; n=2–3/group; * p<0.05).
(b) In utero effects on ERα transcripts in the adult gland. Female offspring were ovariectomized on postnatal day 45. On postnatal day 60, the amount of ERα 5’UTR transcript was measured by a qRT-PCR assay and normalized to Arbp mRNA (mean±SEM; n=4–12/group; * p<0.05).
(c) In utero effects on ERα gene transcription in the adult gland. Female offspring were ovariectomized on postnatal day 45, nuclei were isolated on postnatal day 60, and a nuclear run on assay was performed. The amount of the nascent ERα transcript was measured by qRT-PCR, normalized to Arbp transcript, and presented as fold difference (n=4–10).
(d–e) In utero effects on the regulation of ERα transcripts in the adult gland. Female offspring were ovariectomized on postnatal day 45 and treated for four days with estradiol beginning on postnatal day 56. On postnatal day 60, the amount of O and OT transcripts was measured by a qRT-PCR assay, normalized to Arbp mRNA, and presented as fold difference (mean±SEM; n=4–11/group; * p<0.05).
Discussion
Previous studies have shown that the metalloid arsenite has estrogen like activity in vitro that is due to a high affinity interaction with the ligand binding domain of ERα (27) suggesting that arsenite may be a potent endocrine disruptor. The results of this study show that in utero exposure to an environmentally relevant dose of the metalloid mimics many of the effects of in utero exposure to ethinyl estradiol in female offspring. Similar to ethinyl estradiol, in utero exposure to arsenite advanced vaginal opening, increased the number of mammosphere-forming cells and epithelial density in the prepubertal mammary gland, and increased the expression of genes associated with stem- and progenitor-like cells as well as the expression of ERα and estrogen regulated genes in the postpubertal gland. In contrast to ethinyl estradiol, in utero exposure to arsenite also increase the number of epithelial cells and the expression of specific ERα transcripts suggesting that not all of the effects of arsenite are mediated by ERα. However, as many of the developmental changes in the mammary gland mimic the effects of ethinyl estradiol and occurred prior to the increase in GnRH, the results suggest a direct estrogen like effect of arsenite on the mammary gland that altered its pre- and postpubertal development. In contrast to these results, a recently published study showed that prepubertal exposure to arsenic delayed vaginal opening and suppressed the development of the mammary gland (66). The reason for the differences between the two studies is not clear but may be due to differences in the dose and timing of exposure to the metalloid. There is increasing evidence that the dose-response curves of some endocrine disruptors are nonlinear (67) and that the timing of exposure, i.e., exposure during sensitive stages of development, alters later development (68). In the mammary gland, the periods of development that are most sensitive to endocrine disruptors are the prenatal development of the epithelial sprout, ductal elongation during puberty, and lobuloalveolar expansion during pregnancy (68). In the present study, animals were exposed to a low dose of arsenite while in utero (5 ug/ kg bw on gestation days 12 and 17) whereas in the previously published study, animals were exposed to a higher dose prior to the onset of puberty (10 mg/kg bw by daily gavage beginning on postnatal day 12) which may affect the response of the mammary gland to the metalloid. Although the reasons for the different results between the studies remain to be determined, the results from the present study further support for the hypothesis that, during early development, the mammary gland is particularly susceptible to exogenous hormone like substances which affect its development (69;70).
During development, mammary stem cells generate progenitor cells that proliferate and differentiate into luminal and basal cells resulting in the elongation and branching of the ducts. The increase in the number of mammosphere-forming cells in the neonatal gland followed by the increase in the number of branches, epithelial cells, and epithelial density in the prepubertal gland and the increase in the expression of genes associated with stem- and progenitor-like cells in the nonpregnant adult gland suggests that in utero exposure to arsenite altered mammary gland development through an expansion of the stem/progenitor cell population in the neonatal gland that persisted in the adult gland.
In addition to altering the morphological development of the mammary gland, in utero exposure to arsenite increased the expression of ERα and estrogen regulated genes in the adult gland. In the pubertal and adult gland, there was an increase in the expression of ERα transcripts derived from exons O and OT and a change in their regulation by estradiol. Interestingly, the overexpression and altered regulation of exons O and OT transcripts are similar to the changes in the expression of the ERα transcripts in human breast cancer. The human ERα gene has eight promoters resulting in transcripts with 5’ UTRs derived from exons A, B, C(2), D, T1/T2, E1/E2, and F (71). In normal human breast tissue, the ERα transcripts are derived predominantly from exons A and C with the expression of the transcript derived from exon A greater than the transcript derived from exon C. In human breast tumors and breast cancer cells, ERα is overexpressed due to an increase in the transcripts derived from exons A, B, and C with the expression of the transcript derived from exon C greater than the transcript derived from exon A (72–74). Exons O and OT in the rat are homologous to exons C and B in the human, respectively, suggesting that in utero exposure to arsenite alters ERα expression similar to the alterations observed in breast cancer. In addition to the overexpression of specific transcripts, in utero exposure altered the regulation of ERα by estradiol similar to the regulation of ERα by estradiol in breast cancer cells (75). As stem/progenitor cells are thought to be the targets of malignant transformation in the breast and overexpression of ERα is thought to be the initial event in the development of ER positive tumors, the results of this study suggest that early life exposure to arsenite may increase the risk of developing breast cancer.
Taken together, the results of this study support the growing evidence that the developing organism is particularly susceptible to small changes in its environment that can induce phenotypic changes which effect tissue function and response in later life (69;70). Although several laboratories have studied the impact of phytoestrogens, pesticides, fungicides, plasticizers, and industrial chemicals on puberty onset, mammary gland development, and breast cancer (11), there is limited research on metals and metalloids with estrogen like activity. Thus, it is important to understand the impact of environmental exposure to metalloestrogens on puberty onset, mammary gland development, and the risk of developing breast cancer.
Highlights.
in utero exposure to arsenite mimics many of the effects of estradiol
in utero exposure alters the pre- and postpubertal development of the mammary gland
exposure alters the expression and regulation of estrogen receptor-alpha
Acknowledgements
We thank Drs. G. Chepko, N. Kenney, and P. Furth for helpful discussions and critical reading of the manuscript and A. Foxworth, S. Abdulla, V. Vasqez, C. Beitez, and M. Cruz for help with the animals.
Financial support: This work was supported, in part, by grants from EPA-R83213601 (MBM), NIH-R21-ES015160 (MBM), DOD-BC083291 (DAP), NIH-P30-CA51008, HHMI Undergraduate Program (MG, AC, and AA), NIH T32-CA009686, and Gewirz Foundation (MBM).
Abbreviations
- DTT
dithiothreitol
- DEPC
diethylpyrocarbonate
- EDTA
ethylenediamine tetraacetic acid
- EGF
epidermal growth factor
- ERα
estrogen receptor-alpha
- FGF
fibroblast growth factor
- HEPES
N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid
- i.p.
intraperitoneal
- kgbw
kilogram per body weight
- PgR
progesterone receptor
- qRT-PCR
quantitative real time polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors report no conflicts of interest.
References
- 1.DeMuinck Keizer-Schrama SMPF, Mul D. Trends in pubertal development in Europe. Human Reproduction Update. 2001;7:287–291. doi: 10.1093/humupd/7.3.287. [DOI] [PubMed] [Google Scholar]
- 2.Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99:505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton AS, Mack TM. Puberty and genetic susceptibility to breast cancer in a case-control study in twins. N Engl J Med. 2003;348:2313–2322. doi: 10.1056/NEJMoa021293. [DOI] [PubMed] [Google Scholar]
- 4.Feigelson HS, Henderson BE. Estrogens and breast cancer. Carcinogenesis. 1996;17:2279–2284. doi: 10.1093/carcin/17.11.2279. [DOI] [PubMed] [Google Scholar]
- 5.Ibanez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche--normal variant or forerunner of adult disease? Endocr Rev. 2000;21:671–696. doi: 10.1210/edrv.21.6.0416. [DOI] [PubMed] [Google Scholar]
- 6.Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1:467–475. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- 7.Russo IH, Russo J. Mammary gland neoplasia in long-term rodent studies. Environ Health Perspect. 1996;104:938–967. doi: 10.1289/ehp.96104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel CW, Silberstein GB. Postnatal development of the rodent mammary gland. In: Neville MC, Daniel CW, editors. The mammary gland. New York: Plenum Press; 1987. pp. 3–36. [Google Scholar]
- 9.Kenney NJ, Smith GH, Lawrence E, Barrett JC, Salomon DS. Identification of Stem Cell Units in the Terminal End Bud and Duct of the Mouse Mammary Gland. J Biomed Biotechnol. 2001;1:133–143. doi: 10.1155/S1110724301000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colborn T, von Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenton SE, Reed C, Newbold RR. Perinatal environmental exposures affect mammary development, function, and cancer risk in adulthood. Annu Rev Pharmacol Toxicol. 2012;52:455–479. doi: 10.1146/annurev-pharmtox-010611-134659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freni-Titulaer LW, Cordero JF, Haddock L, Lebron G, Martinez R, Mills JL. Premature thelarche in Puerto Rico. A search for environmental factors. Am J Dis Child. 1986;140:1263–1267. doi: 10.1001/archpedi.1986.02140260065028. [DOI] [PubMed] [Google Scholar]
- 13.Blanck HM, Marcus M, Tolbert PE, Rubin C, Henderson AK, Hertzberg VS, Zhang RH, Cameron L. Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology. 2000;11:641–647. doi: 10.1097/00001648-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Hilakivi-Clarke L, Cho E, Onojafe I, Raygada M, Clarke R. Maternal exposure to genistein during pregnancy increases carcinogen- induced mammary tumorigenesis in female rat offspring. Oncol Rep. 1999;6:1089–1095. doi: 10.3892/or.6.5.1089. [DOI] [PubMed] [Google Scholar]
- 15.Hilakivi-Clarke L, Cho E, Clarke R. Maternal genistein exposure mimics the effects of estrogen on mammary gland development in female mouse offspring. Oncol Rep. 1998;5:609–616. doi: 10.3892/or.5.3.609. [DOI] [PubMed] [Google Scholar]
- 16.Kampert JB, Whittemore AS, Paffenbarger RS., Jr Combined effect of child-bearing, menstrual events, and body size on age-specific breast cancer risk. Am J Epidemiol. 1988;128:962–979. doi: 10.1093/oxfordjournals.aje.a115070. [DOI] [PubMed] [Google Scholar]
- 17.Butler LM, Potischman NA, Newman B, Millikan RC, Brogan D, Gammon MD, Swanson CA, Brinton LA. Menstrual risk factors and early-onset breast cancer. Cancer Causes Control. 2000;11:451–458. doi: 10.1023/a:1008956524669. [DOI] [PubMed] [Google Scholar]
- 18.Hankinson SE, Willett WC, Manson JE, Colditz GA, Hunter DJ, Spiegelman D, Barbieri RL, Speizer FE. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 19.Gerhard I, Vollmar B, Runnebaum B, Kubli F. Weight percentile at birth. I. Clinical data of pregnancy and relevance for early childhood development. Eur J Obstet Gynecol Reprod Biol. 1987;26:303–311. doi: 10.1016/0028-2243(87)90128-6. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh C, Pavia M, Lambe M, Lan SJ, Colditz GA, Ekbom A, Adami HO, Trichopoulos D, Willett WC. Dual effect of parity on breast cancer risk. Eur J Cancer. 1994;30A:969–973. doi: 10.1016/0959-8049(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 21.Ekbom A, Trichopoulos D, Adami HO, Hsieh CC, Lan SJ. Evidence of prenatal influences on breast cancer risk. Lancet. 1992;340:1015–1018. doi: 10.1016/0140-6736(92)93019-j. [DOI] [PubMed] [Google Scholar]
- 22.Michels KB, Trichopoulos D, Robins JM, Rosner BA, Manson JE, Hunter DJ, Colditz GA, Hankinson SE, Speizer FE, Willett WC. Birthweight as a risk factor for breast cancer. Lancet. 1996;348:1542–1546. doi: 10.1016/S0140-6736(96)03102-9. [DOI] [PubMed] [Google Scholar]
- 23.Sanderson M, Williams MA, Malone KE, Stanford JL, Emanuel I, White E, Daling JR. Perinatal factors and risk of breast cancer. Epidemiology. 1996;7:34–37. doi: 10.1097/00001648-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Hilakivi-Clarke L, Cho E, Cabanes A, DeAssis S, Olivo S, Helferich W, Lippman ME, Clarke R. Dietary Modulation of Pregnancy Estrogen Levels and Breast Cancer Risk among Female Rat Offspring. Clin Cancer Res. 2002;8:3601–3610. [PubMed] [Google Scholar]
- 25.Boylan ES, Calhoon RE. Mammary tumorigenesis in the rat following prenatal exposure to diethylstilbestrol and postnatal treatment with 7,12-dimethylbenz[a]anthracene. J Toxicol Environ Health. 1979;5:1059–1071. doi: 10.1080/15287397909529814. [DOI] [PubMed] [Google Scholar]
- 26.Nikaido Y, Yoshizawa K, Danbara N, Tsujita-Kyutoku M, Yuri T, Uehara N, Tsubura A. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod Toxicol. 2004;18:803–811. doi: 10.1016/j.reprotox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Stoica A, Pentecost E, Martin MB. Effect of arsenite on estrogen receptor-a expression and activity in MCF-7 breast cancer cells. Endocrinol. 2000;141:3595–3602. doi: 10.1210/endo.141.10.7704. [DOI] [PubMed] [Google Scholar]
- 28.Agency for Toxic Substances and Disease Registry. Toxicological profile of arsenic. Atlanta, GA: U.S. Department of Health and Human Services; 2007. [PubMed] [Google Scholar]
- 29.Waalkes MP, Ward JM, Diwan BA. Induction of tumors of the liver, lung, ovary and adrenal in adult mice after brief maternal gestational exposure to inorganic arsenic: promotional effects of postnatal phorbol ester exposure on hepatic and pulmonary, but not dermal cancers. Carcinogenesis. 2004;25:133–141. doi: 10.1093/carcin/bgg181. [DOI] [PubMed] [Google Scholar]
- 30.Waalkes MP, Ward JM, Liu J, Diwan BA. Transplacental carcinogenicity of inorganic arsenic in the drinking water: induction of hepatic, ovarian, pulmonary, and adrenal tumors in mice. Toxicol Appl Pharmacol. 2003;186:7–17. doi: 10.1016/s0041-008x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 31.Devesa V, Adair BM, Liu J, Waalkes MP, Diwan BA, Styblo M, Thomas DJ. Arsenicals in maternal and fetal mouse tissues after gestational exposure to arsenite. Toxicology. 2006;224:147–155. doi: 10.1016/j.tox.2006.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin Y, Wang G, Zhao F, Liao Y, Sun D, Zhong Y, Yu X, Lv X, Li G, Sun G. Distribution of speciated arsenicals in mice exposed to arsenite at the early life. Ecotoxicol Environ Saf. 2010;73:1323–1326. doi: 10.1016/j.ecoenv.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Environmental Protection Agency. Integrated Risk Information System: Arsenic, inorganic. 2012 http://wwwepagov/iris/subst/0278htm.
- 34.JECFA; 2010. Joint FAO/WHO Expert Committee on Food Additives Seventy-second Meeting. [Google Scholar]
- 35.Xue J, Zartarian V, Wang SW, Liu SV, Georgopoulos P. Probabilistic Modeling of Dietary Arsenic Exposure and Dose and Evaluation with 2003–2004 NHANES Data. Environ Health Perspect. 2010;118:345–350. doi: 10.1289/ehp.0901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evaluation of certain food additives and contaminants. Fifty-fifth report of the joint FAO/WHO expert committee on food additives; 6–15 June 2000; Geneva. Geneva: World Health Organization; 2001. [Google Scholar]
- 37.Agency for Toxic Substances and Disease Registry. Toxicological profile of arsenic. Atlanta, GA: U.S. Department of Health and Human Services; 1991. [Google Scholar]
- 38.Agency for Toxic Substances and Disease Registry. Toxicological profile for arsenic. Atlanta, GA: U.S. Department of Health and Human Services; 1993. [PubMed] [Google Scholar]
- 39.Schrauzer GN, White DA, McGinness JE, Schneider CJ, Bell LJ. Arsenic and cancer: effects of joint administration of arsenite and selenite on the genesis of mammary adenocarcinoma in inbred female C3H/St mice. Bioinorg Chem. 1978;9:245–253. doi: 10.1016/s0006-3061(78)80005-2. [DOI] [PubMed] [Google Scholar]
- 40.Waalkes MP, Liu J, Chen H, Xie Y, Achanzar WE, Zhou YS, Cheng ML, Diwan BA. Estrogen signaling in livers of male mice with hepatocellular carcinoma induced by exposure to arsenic in utero. J Natl Cancer Inst. 2004;96:466–474. doi: 10.1093/jnci/djh070. [DOI] [PubMed] [Google Scholar]
- 41.Styblo M, Delnomdedieu M, Thomas DJ. Mono- and dimethylation of arsenic in rat liver cytosol in vitro. Chem Biol Interact. 1996;99:147–164. doi: 10.1016/0009-2797(95)03666-0. [DOI] [PubMed] [Google Scholar]
- 42.Mann S, Droz PO, Vahter M. A physiologically based pharmacokinetic model for arsenic exposure. II. Validation and application in humans. Toxicol Appl Pharmacol. 1996;140:471–486. doi: 10.1006/taap.1996.0244. [DOI] [PubMed] [Google Scholar]
- 43.Mann S, Droz PO, Vahter M. A physiologically based pharmacokinetic model for arsenic exposure. I. Development in hamsters and rabbits. Toxicol Appl Pharmacol. 1996;137:8–22. doi: 10.1006/taap.1996.0052. [DOI] [PubMed] [Google Scholar]
- 44.Hughes MF, Kenyon EM, Edwards BC, Mitchell CT, Thomas DJ. Strain-dependent disposition of inorganic arsenic in the mouse. Toxicology. 1999;137:95–108. doi: 10.1016/s0300-483x(99)00068-2. [DOI] [PubMed] [Google Scholar]
- 45.Prater M, Shehata M, Watson CJ, Stingl J. Enzymatic dissociation, flow cytometric analysis, and culture of normal mouse mammary tissue. Methods Mol Biol. 2013;946:395–409. doi: 10.1007/978-1-62703-128-8_25. [DOI] [PubMed] [Google Scholar]
- 46.Banerjee I, Clayton P. The genetic basis for the timing of human puberty. J Neuroendocrinol. 2007;19:831–838. doi: 10.1111/j.1365-2826.2007.01598.x. [DOI] [PubMed] [Google Scholar]
- 47.Gore AC, Wu TJ, Rosenberg JJ, Roberts JL. Gonadotropin-releasing hormone and NMDA receptor gene expression and colocalization change during puberty in female rats. J Neurosci. 1996;16:5281–5289. doi: 10.1523/JNEUROSCI.16-17-05281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwasa T, Matsuzaki T, Murakami M, Kinouchi R, Gereltsetseg G, Yamamoto S, Kuwahara A, Yasui T, Irahara M. Delayed puberty in prenatally glucocorticoid administered female rats occurs independently of the hypothalamic Kiss1-Kiss1r-GnRH system. Int J Dev Neurosci. 2011;29:183–188. doi: 10.1016/j.ijdevneu.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Welm BE, Freeman KW, Chen M, Contreras A, Spencer DM, Rosen JM. Inducible dimerization of FGFR1: development of a mouse model to analyze progressive transformation of the mammary gland. J Cell Biol. 2002;157:703–714. doi: 10.1083/jcb.200107119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Dev Biol. 2005;277:443–456. doi: 10.1016/j.ydbio.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 51.Stingl J. Detection and analysis of mammary gland stem cells. J Pathol. 2009;217:229–241. doi: 10.1002/path.2457. [DOI] [PubMed] [Google Scholar]
- 52.Ma I, Allan AL. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev. 2011;7:292–306. doi: 10.1007/s12015-010-9208-4. [DOI] [PubMed] [Google Scholar]
- 53.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clarke RB. Isolation and characterization of human mammary stem cells. Cell Prolif. 2005;38:375–386. doi: 10.1111/j.1365-2184.2005.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Booth BW, Smith GH. Estrogen receptor-alpha and progesterone receptor are expressed in label-retaining mammary epithelial cells that divide asymmetrically and retain their template DNA strands. Breast Cancer Res. 2006;8:R49. doi: 10.1186/bcr1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stingl J. Estrogen and progesterone in normal mammary gland development and in cancer. Horm Cancer. 2011;2:85–90. doi: 10.1007/s12672-010-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barthold JS, Kryger JV, Derusha AM, Duel BP, Jednak R, Skafar DF. Effects of an environmental endocrine disruptor on fetal development, estrogen receptor(alpha) and epidermal growth factor receptor expression in the porcine male genital tract. J Urol. 1999;162:864–871. doi: 10.1097/00005392-199909010-00079. [DOI] [PubMed] [Google Scholar]
- 58.Lewis BC, Hudgins S, Lewis A, Schorr K, Sommer R, Peterson RE, Flaws JA, Furth PA. In utero and lactational treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs mammary gland differentiation but does not block the response to exogenous estrogen in the postpubertal female rat. Toxicol Sci. 2001;62:46–53. doi: 10.1093/toxsci/62.1.46. [DOI] [PubMed] [Google Scholar]
- 59.Schonfelder G, Flick B, Mayr E, Talsness C, Paul M, Chahoud I. In utero exposure to low doses of bisphenol A lead to long-term deleterious effects in the vagina. Neoplasia. 2002;4:98–102. doi: 10.1038/sj.neo.7900212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosh MG, Thompson DA, Weigel RJ. PDZK1 and GREB1 are estrogen-regulated genes expressed in hormone-responsive breast cancer. Cancer Res. 2000;60:6367–6375. [PubMed] [Google Scholar]
- 61.Kariagina A, Xie J, Leipprandt JR, Haslam SZ. Amphiregulin mediates estrogen, progesterone, and EGFR signaling in the normal rat mammary gland and in hormone-dependent rat mammary cancers. Horm Cancer. 2010;1:229–244. doi: 10.1007/s12672-010-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monje L, Varayoud J, Luque EH, Ramos JG. Neonatal exposure to bisphenol A modifies the abundance of estrogen receptor alpha transcripts with alternative 5'-untranslated regions in the female rat preoptic area. J Endocrinol. 2007;194:201–212. doi: 10.1677/JOE-07-0014. [DOI] [PubMed] [Google Scholar]
- 63.Hirata S, Koh T, Yamada-Mouri N, Hoshi K, Kato J. The untranslated first exon 'exon 0S' of the rat estrogen receptor (ER) gene. FEBS Lett. 1996;394:371–373. doi: 10.1016/0014-5793(96)00987-8. [DOI] [PubMed] [Google Scholar]
- 64.Hirata S, Koh T, Yamada-Mouri N, Kato J. The novel untranslated first exon "exon 0N" of the rat estrogen receptor gene. Biochem Biophys Res Commun. 1996;225:849–854. doi: 10.1006/bbrc.1996.1262. [DOI] [PubMed] [Google Scholar]
- 65.Osada N, Hirata S, Shoda T, Hoshi K. The novel untranslated exon "exon 0T" encoded between the exon 0 and exon 1 of the rat estrogen receptor alpha (ER alpha) gene. Endocr J. 2001;48:465–472. doi: 10.1507/endocrj.48.465. [DOI] [PubMed] [Google Scholar]
- 66.Reilly MP, Saca JC, Hamilton A, Solano RF, Rivera JR, Whitehouse-Innis W, Parsons JG, Dearth RK. Prepubertal exposure to arsenic(III) suppresses circulating insulin-like growth factor-1 (IGF-1) delaying sexual maturation in female rats. Reprod Toxicol. 2014;44:41–49. doi: 10.1016/j.reprotox.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, Vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147:S18–S24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 69.Bern HA. The development of the role of hormones in development--a double remembrance. Endocrinology. 1992;131:2037–2038. doi: 10.1210/endo.131.5.1425407. [DOI] [PubMed] [Google Scholar]
- 70.Soto AM, Brisken C, Schaeberle C, Sonnenschein C. Does cancer start in the womb? altered mammary gland development and predisposition to breast cancer due to in utero exposure to endocrine disruptors. J Mammary Gland Biol Neoplasia. 2013;18:199–208. doi: 10.1007/s10911-013-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kos M, Reid G, Denger S, Gannon F. Minireview: Genomic organization of the human ERa gene promoter region. Mol Endocrinol. 2001;15:2057–2063. doi: 10.1210/mend.15.12.0731. [DOI] [PubMed] [Google Scholar]
- 72.Weigel RJ, Crooks DL, Iglehart JD, deConinck EC. Quantitative analysis of the transcriptional start sites of estrogen receptor in breast cancer. Cell Growth Differ. 1995;6:707–711. [PubMed] [Google Scholar]
- 73.Tanimoto K, Eguchi H, Yoshida T, Hajiro-Nakanishi K, Hayashi S. Regulation of estrogen receptor alpha gene mediated by promoter B responsible for its enhanced expression in human breast cancer. Nucleic Acids Res. 1999;27:903–909. doi: 10.1093/nar/27.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayashi S, Imai K, Suga K, Kurihara T, Higashi Y, Nakachi K. Two promoters in expression of estrogen receptor messenger RNA in human breast cancer. Carcinogenesis. 1997;18:459–464. doi: 10.1093/carcin/18.3.459. [DOI] [PubMed] [Google Scholar]
- 75.Saceda M, Lippman ME, Chambon P, Lindsey RL, Ponglikiturongkol M, Puente M, Martin MB. Regulation of the estrogen receptor in MCF-7 cells by estradiol. Mol Endocrinol. 1988;2:1157–1162. doi: 10.1210/mend-2-12-1157. [DOI] [PubMed] [Google Scholar]