Abstract
Purpose
To evaluate the relationship between rates of change on frequency doubling technology (FDT) perimetry and longitudinal changes in quality of life (QoL) of glaucoma patients.
Design
Prospective observational cohort study.
Methods
One hundred fifty-two subjects (127 glaucoma and 25 healthy) followed for an average of 3.2 ± 1.1 years. All subjects were evaluated with National Eye Institute Visual Function Questionnaire (NEI VFQ-25), FDT and standard automated perimetry (SAP). Glaucoma patients had a median of 3 NEI VFQ-25, 8 FDT and 8 SAP tests during follow up. Mean sensitivities of the integrated binocular visual fields were estimated for FDT and SAP and used to calculate rates of change. A joint longitudinal multivariable mixed model was used to investigate the association between change in binocular mean sensitivities and change in NEI VFQ-25 Rasch-calibrated scores.
Results
There was a statistically significant correlation between change in binocular mean sensitivity for FDT and change in NEI VFQ-25 scores during follow-up in the glaucoma group. In multivariable analysis with the confounding factors, each 1dB/year change in binocular FDT mean sensitivity corresponded to a change of 0.8 units per year in the NEI VFQ-25 scores (P = 0.001). For binocular SAP mean sensitivity, each 1 dB/year change was associated with 2.4 units per year change in NEI VFQ-25 scores (P < 0.001). The multivariable model containing baseline and rate of change information from SAP had stronger ability to predict change in NEI VFQ-25 scores compared to the equivalent model for FDT (R2 of 50% and 30%, respectively; P = 0.001).
Conclusion
SAP performed significantly better than FDT in predicting change in NEI VFQ-25 scores in our population, suggesting that it may still be the preferable perimetric technique for predicting risk of disability from the disease.
INTRODUCTION
Glaucoma is an optic neuropathy characterized by progressive degeneration of retinal ganglion cells, leading to characteristic changes to the optic nerve and loss of visual function.1 The main goal of glaucoma treatment is to prevent patients from developing significant functional loss that could lead to disability and decrease in quality of life (QoL).2,3 The understanding of how visual function loss in glaucoma affects QoL is important in guiding therapeutic decisions. Such assessment has frequently been done by evaluating the relationship between visual field loss, as measured by standard automated perimetry (SAP), and QoL outcomes, as measured by standardized questionnaires such as the National Eye Institute Visual Function Questionnaire (NEI VFQ-25).2
In a recent longitudinal study, we demonstrated that rates of progressive visual field loss on SAP were significantly associated with decline in QoL over time.4 However, although SAP remains the gold standard test for assessing functional loss in glaucoma, other tests have been proposed, such as frequency doubling technology (FDT) perimetry.5 FDT is based on the hypothesis that contrast sensitivity to frequency doubling stimuli (low spatial frequency gratings flickering at high temporal frequency) is reduced in glaucoma.5,6 The test has been shown to perform as well as, if not better than, SAP for detection of glaucomatous visual field defects with high sensitivity and specificity and less variability in areas of low sensitivity.7–9 Recent studies have also suggested that FDT perimetry may detect progressive visual field defects not apparent on SAP.10
FDT has been proposed as a selective perimetric test that attempts to target the parasol ganglion cells (M cells).8 These cells project to the magnocellular layers of the lateral geniculate nucleus and are sensitive to low contrast and to high temporal and low spatial frequency stimuli linked to motion perception.11,12 In contrast, SAP is not selective for a particular ganglion cell type and any of the primary ganglion cell subtypes can respond to an achromatic stimulus presented on an achromatic background.8 Although the selectivity of FDT testing has been a matter of controversy,13 it is conceivable that due to their different stimulus properties, these tests may have different relationships with measures of QoL.
The purpose of the present study was to evaluate and compare the relationship between progressive visual field loss in FDT and SAP with changes in QoL as measured by the NEI VFQ-25 questionnaire in a cohort of glaucoma patients followed over time.
METHODS
This was an observational cohort study. Participants from this study were included in a prospective longitudinal study designed to evaluate functional impairment in glaucoma, the Diagnostic Innovations in Glaucoma Study: Functional Impairment, conducted at the Visual Performance Laboratory, Department of Ophthalmology, University of California San Diego.14 Written informed consent was obtained from all participants. This study received institutional review board approval and methodology adhered to the tenets of the Declaration of Helsinki and to the Health Insurance Portability and Accountability Act.
At each visit during follow-up, subjects underwent a comprehensive ophthalmic examination, including review of medical history, best corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure measurement using Goldmann applanation tonometry, gonioscopy, dilated ophthalmoscopic examination using a 78-diopter lens and stereoscopic photographs of the optic nerves. Only patients with open angles on gonioscopy were included. Subjects with coexisting retinal disease, uveitis or non-glaucomatous optic disc neuropathy were excluded from the study.
This study enrolled glaucoma patients diagnosed based on the presence of glaucomatous optic neuropathy and/or repeatable visual field defects. As SAP and FDT were being compared, we avoided a classification of glaucoma based solely on the presence of visual field defects by one or the other perimetric technique. Glaucomatous appearance of the optic disc was determined by the presence of neuroretinal rim thinning, excavation, or retinal nerve fiber layer defects as evaluated by masked assessment of stereoscopic photographs. Visual field defects were determined by the presence of pattern standard deviation with P < 0.05, and/or glaucoma hemifield test results outside normal limits for both perimetric techniques.
We also enrolled a group of 25 healthy subjects with normal optic disc evaluation and normal SAP and FDT results (defined as a pattern standard deviation within 95% confidence limits and a glaucoma hemifield test result within normal limits). The NEI VFQ-25 questionnaires were obtained annually, and both SAP and FDT tests were obtained at 6-month intervals. For inclusion, all subjects were required to have had a minimum of 2 NEI VFQ-25 questionnaires and at least 5 SAP and 5 FDT tests during follow-up.
Perimetric testing
All patients underwent SAP testing with the Swedish interactive threshold algorithm (SITA) standard 24-2 strategy using the Humphrey Field Analyzer II (Carl Zeiss Meditec, Inc, Dublin, CA). FDT was performed using the Humphrey Matrix (Carl-Zeiss Meditec, Inc, Dublin, CA) with 24-2 threshold strategy. Only reliable tests were included (less than 33% fixation losses and false negatives, and less than 15% false positives). An integrated binocular field was obtained using the monocular fields for the right and left eyes according to the binocular summation technique described by Nelson-Quigg et al.15 After binocular summation thresholds were obtained, an average of all thresholds was calculated to obtain the mean sensitivity for FDT and SAP.
Rasch Analysis of the NEI-VFQ-25 questionnaire
QoL was assessed by the NEI VFQ-25 questionnaire.16 This questionnaire consists of 25 questions measuring overall vision, difficulty with near-vision and distance activities, ocular pain, driving difficulties, limitations with peripheral vision and color vision, social functioning, role limitations, dependency and mental health symptoms related to vision plus an additional single-item general health rating question. Rasch analysis was performed to obtain final estimates of “person measures” or Rasch scores, summarizing the NEI-VFQ responses.
We have previously published the details of the Rasch modeling procedure in this population.4 In brief, Rasch scores can be used to express where each respondent falls on a linear scale representing the degree of impairment as measured by the NEI VFQ-25 and can be used for subsequent parametric statistical analyses.17,18 Rasch analysis was performed using Andrich rating-scale models to obtain the estimates of the required ability of each item, perceived ability of each subject, and the category thresholds for each response categories.19 Rasch analysis locates item difficulty and person ability on a logit (log odds) scale. Person ability scores were rescaled linearly to range from 0 to 100. Person and item measures were examined for fit to the Rasch model using infit and outfit item fit statistics and model fitting has been previously described.4
In order to account for lack of unidimensionality, the final Rasch model used only the items belonging to the general vision, near vision, distance vision, peripheral vision, color vision and social functioning subscales. Items belonging to the dependency (3 items), mental health (3 items) and role limitations (2 items) were excluded as previous work has found these items belong to a separate socioemotional dimension not directly related to visual functioning.20 In addition, the 2 items belonging to the subscale of ocular pain were also excluded as they could not fit into the Rasch model.20 This is also an expected result as ocular pain would likely produce changes in QoL that are not directly related to those produced by loss of vision from glaucoma.
Clinical, demographic and socioeconomic variables
Visual acuity was measured using the Early Treatment Diabetic Retinopathy Study chart21 and change in visual acuity during follow-up was calculated as the difference between the logMAR visual acuity at the last followup visit and the baseline visit. We also collected information about marital status [married (yes/no)], presence of health insurance (yes/no), degree of education [at least high school degree (yes/no)] and income [less than $25,000/year (yes/no)].
Presence or history of systemic medical conditions was also investigated, including diabetes mellitus, arthritis, high blood pressure, heart disease, stroke, depression, asthma and cancer. A simple summation score was used to create a co-morbidity index ranging from 1 to 4.22 In addition, we collected information on whether the patient had undergone glaucoma filtering surgery and/or cataract surgery during follow-up. As these variables could potentially affect patient perceptions about QoL, they were included as potentially confounding factors in the analysis of relationship between FDT and SAP with NEI VFQ-25 results.
Statistical Analyses
The investigation of the association between NEI VFQ-25 scores, FDT and SAP data was performed with a joint multivariable longitudinal linear mixed model.23 Details about this model have been presented elsewhere.23–28 In this type of analysis the average evolution of a specific response is described using a linear function of time, and subject-specific deviations from this average evolution are introduced by random intercepts and random slopes, allowing the study of effects of different baseline values and different rates of change for each patient. In a joint-modeling approach using mixed models, the random-effects are determined for each response process and different processes are associated by imposing a joint multivariable distribution on the random effects.29
Multivariable linear regression models were also performed to evaluate the association between rates of change on FDT, SAP and NEI VFQ-25 scores after adjusting for potentially confounding socioeconomic and clinical variables. Statistical analysis was performed using commercially available software Winsteps version 3.81.0 (Chicago, Illinois, USA) and Stata version 13 (StataCorp LP, College Station, Texas, USA). The alpha level (type I error) was set at 0.05.
RESULTS
The study included 254 eyes of 127 glaucoma patients and 50 eyes of 25 healthy participants. Table 1 summarizes clinical and demographic characteristics of included subjects. In the glaucoma group mean age at baseline was 65.8 ± 12.3 years. Glaucoma patients were followed for an average of 3.2 years ± 1.1 years and had a median of 3 NEI VFQ-25 questionnaires, 8 FDT tests and 8 SAP tests. Mean ± standard deviation (SD) of the binocular mean sensitivity at baseline was 24.5 ± 4.5 dB and 29.5 ± 3.2 dB (for FDT and SAP respectively).
Table 1.
Baseline demographic and clinical characteristics of glaucoma patients and healthy subjects who had longitudinal visual field testing and quality of life assessment.
| Variables | Glaucoma group | Control group |
|---|---|---|
| (n = 127) | (n = 25) | |
| Age, years | 65.8 ± 12.3 | 48.0 ± 10.9 |
| Gender, % female | 48% | 72% |
| Race, % black | 24% | 32% |
| LogMAR visual acuity (better eye) | −0.06 ± 0.11 | −0.10 ± 0.10 |
| LogMAR visual acuity (worse eye) | 0.03 ± 0.17 | −0.07 ± 0.11 |
| FDT (median number of tests and IQR) | 8 (5–9) | 7 (6–8) |
| FDT baseline MD (better eye), dB | −2.5 ± 4.1 | −0.3 ± 1.8 |
| FDT baseline MD (worse eye), dB | −5.7 ± 5.6 | −1.3 ± 1.9 |
| SAP (median number of tests and IQR) | 8 (6–10) | 7 (6–8) |
| SAP baseline MD (better eye), dB | −1.4 ± 3.4 | 0.4 ± 0.9 |
| SAP baseline MD (worse eye), dB | −4.0 ± 5.7 | −0.1 ± 0.8 |
| FDT baseline binocular mean sensitivity, dB | 24.5 ± 4.5 | 28.5 ± 2.0 |
| SAP baseline binocular mean sensitivity, dB | 29.5 ± 3.2 | 32.5 ± 0.8 |
| NEI VFQ-25 (median number of questionnaires and IRQ) | 3 (2–3) | 2 (2–3) |
| Baseline NEI VFQ-25 score | 67.8 ± 21.6 | 82.8 ± 20.5 |
| Filtering surgery during follow-up, % yes | 6% | 0% |
| Cataract surgery during follow-up, % yes | 9% | 0% |
| Education, % with at least high school degree | 96% | 92% |
| Income, lower than $25,000 | 7% | 0% |
| Marital status, % married | 70% | 64% |
| Comorbidity index | 1.2 ± 1.1 | 0.4 ± 0.6 |
| Health Insurance, % yes | 93% | 96% |
FDT = frequency doubling technology; dB = decibels; MD = mean deviation SAP = standard automated perimetry; IQR = interquartile range NEI VFQ-25 = National Eye Institute Visual Function Questionnaire 25
There was a significant correlation between change in NEI VFQ-25 scores and change in binocular FDT mean sensitivity during follow-up (R2 = 14%; P < 0.001) (Figure 1 Top). There was also a relationship between severity of the visual field defect on FDT perimetry at baseline and change in NEI VFQ-25 scores. Subjects with more severe disease at baseline were more likely to have a decrease in NEI VFQ-25 scores during follow-up (R2 = 23%; P < 0.001) (Figure 1 Bottom). In a multivariable model including both baseline and rate of change in FDT mean sensitivity, each 1dB/year change in binocular FDT mean sensitivity corresponded to a change of 0.8 units/year in NEI VFQ-25 scores.
FIGURE 1.
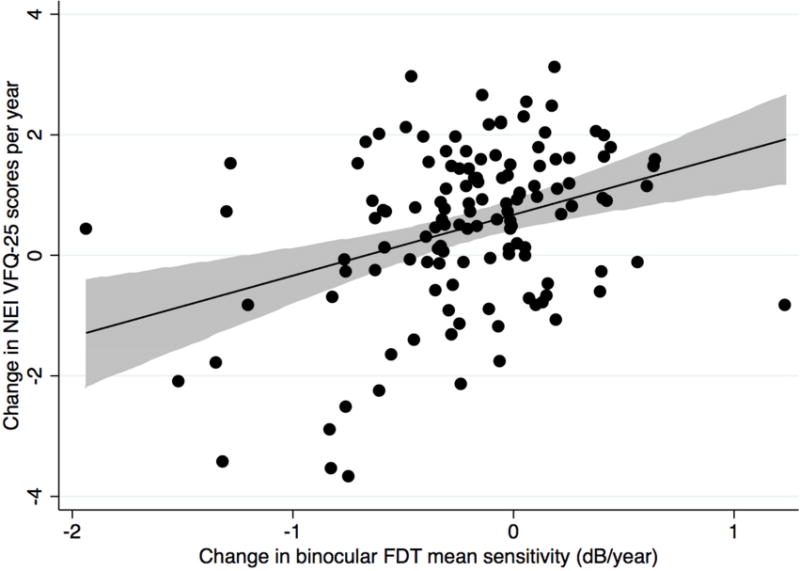
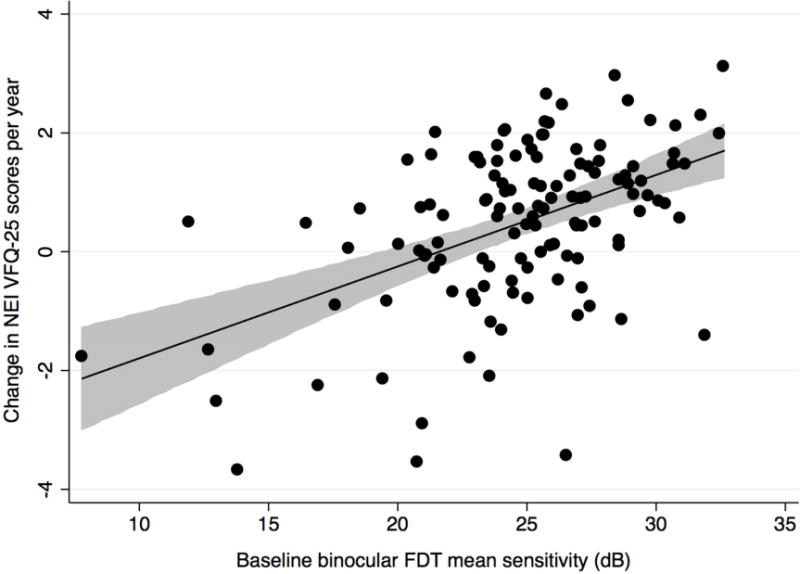
Scatterplots showing the relationship between change in binocular Frequency Doubling Technology mean sensitivity (decibels/year) and change in National Eye Institute Visual Function Questionnaire-25 scores (Top) and the relationship between baseline binocular Frequency Doubling Technology mean sensitivity (decibels) and change in National Eye Institute Visual Function Questionnaire-25 scores in the glaucoma group (Bottom) (shaded area represents 95% confidence interval of the regression). dB= decibel.
There was also a correlation between change in binocular SAP mean sensitivity and NEI VFQ-25 scores during follow-up (R2 = 44%, P < 0.001) (Figure 2 Top). Eyes with more severe disease measured by SAP at baseline were also more likely to have a decrease in NEI VFQ-25 scores during follow-up (R2 = 27%, P < 0.001) (Figure 2 Bottom). In a multivariable model including both baseline and rates of SAP mean sensitivity change, each 1dB/year change in binocular SAP mean sensitivity was associated with a change of 2.2 units per year in NEI VFQ-25 scores. The predictive ability of the multivariable model containing baseline and longitudinal SAP information (R2 = 50%) was superior to that of the equivalent model for FDT (R2 = 30%; P = 0.001).
FIGURE 2.

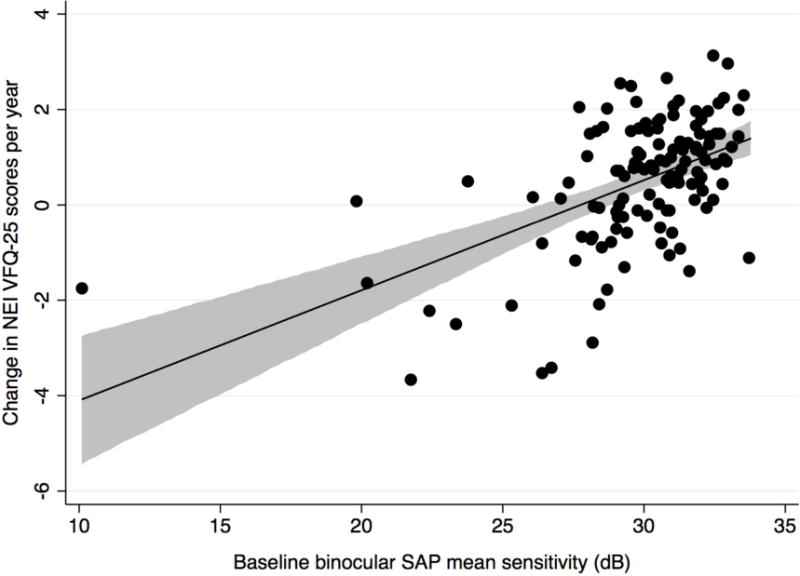
Scatterplots showing the relationship between change in binocular Standard Automated Perimetry mean sensitivity (decibels/year) and change in National Eye Institute Visual Function Questionnaire-25 scores (Top) and the relationship between baseline binocular Standard Automated Perimetry mean sensitivity (decibels) and change in National Eye Institute Visual Function Questionnaire-25 scores in the glaucoma group (Bottom) (shaded area represents 95% confidence interval of the regression). dB= decibel.
Table 2 shows multivariable models for predicting change in NEI VFQ-25 scores while adjusting for potentially confounding variables. Both FDT and SAP rates of change were still predictors of change in NEI VFQ-25 scores (P = 0.001, P<0.001, respectively) after adjustment for confounding variables. Due to the strong correlation between rates of change for FDT and SAP (R2 = 62%; P < 0.001), it was not possible to include both metrics as predictors of change in NEI VFQ-25 scores in the same model due to multicollinearity30.
Table 2.
Results of the multivariable regression models evaluating the association between longitudinal assessment in binocular frequency doubling technology and binocular standard automated perimetry with changes in National Eye Institute Visual Function Questionnaire-25 Scores in the glaucoma group, with adjustment for potentially confounding clinical and socio-economic variables.
|
|
||||
|---|---|---|---|---|
| FDT | SAP | |||
|
| ||||
| Variables | Coefficient (95% CI) | P – value | Coefficient (95% CI) | P – value |
| Change in binocular mean sensitivity (dB/year) | 0.86 (0.35 – 1.37) | 0.001 | 2.47 (1.84 – 3.10) | <0.001 |
| Baseline binocular mean sensitivity, dB | 0.11 (0.06 – 0.17) | <0.001 | 0.10 (−0.03 – 0.72) | 0.001 |
| Change in visual acuity, per 0.1 LogMAR | 0.08 (−1.44 – 1.57) | 0.938 | 0.46 (−0.46 – 1.39) | 0.322 |
| Age, per decade older | −0.05 (-0.04 – 0.25) | 0.593 | −0.03 (−0.14 – 0.20) | 0.942 |
| Gender, female | 0.62 (0.21 – 1.30) | 0.003 | 0.46 (0.11 – 0.82) | 0.011 |
| Race, black | 0.38 (−0.04 – 0.80) | 0.107 | 0.23 (−0.12 – 0.5) | 0.279 |
| Filtering surgery during follow-up, yes | 0.08 (−0.81 – 0.98) | 0.741 | 0.03 (−0.61 – 0.62) | 0.074 |
| Cataract surgery during follow-up, yes | 0.35 (−0.37 – 1.07) | 0.524 | 0.05 (−0.22 – 0.62) | 0.519 |
| Comorbidity index | −0.04 (−0.22 – 0.13) | 0.575 | −0.06 (−0.22 – 0.08) | 0.431 |
| Education, with at least high school degree | −0.02 (−1.20 – 1.15) | 0.993 | −0.69 (−1.75 – 0.36) | 0.083 |
| Income, lower than $25,000 | 0.23 (−0.04 – 0.51) | 0.211 | 0.14 (−0.09 – 0.38) | 0.311 |
| Marital status, married | 0.25 (−0.23 – 0.74) | 0.267 | 0.22 (−0.19 – 0.63) | 0.209 |
| Insurance, yes | −0.03 (−0.93 – 0.94) | 0.955 | −0.23 (−1.01 –0.54) | 0.772 |
FDT = frequency doubling technology; MD = mean deviation; SAP = standard automated perimetry. CI = confidence interval
We also examined the association between change in NEI VFQ-25 scores and change in binocular mean sensitivity for FDT (Figure 3 Top) and SAP (Figure 3 Bottom) in the group of 25 healthy subjects followed over time. No statistically significant associations were found for either test.
FIGURE 3.

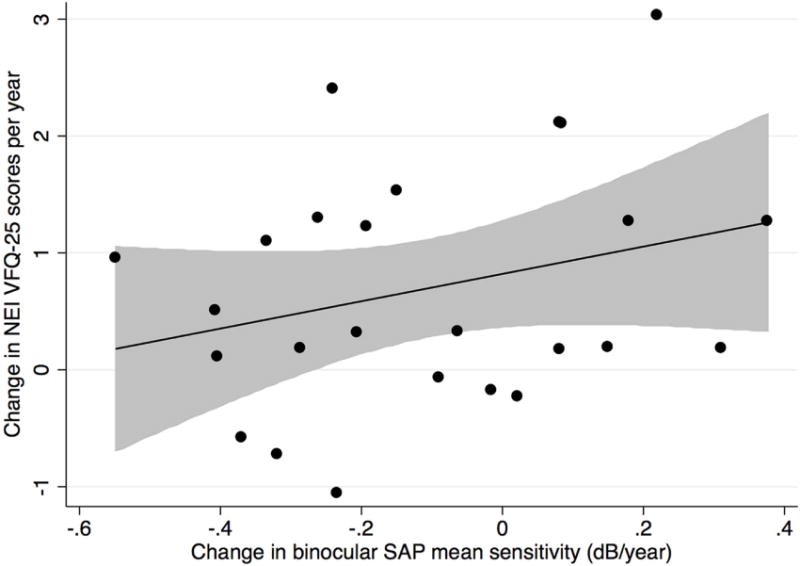
Scatterplot showing the relationship between change in binocular Frequency Doubling Technology (Top) and binocular Standard Automated Perimetry (Bottom) mean sensitivity (decibels/year) and change in National Eye Institute Visual Function Questionnaire-25 scores for the control group (shaded area represents 95% confidence interval of the regression). dB= decibel.
Figure 4 shows an example of a patient from the study illustrating the relationship between progressive visual field loss on FDT and SAP and change in NEI VFQ-25 scores.
FIGURE 4.
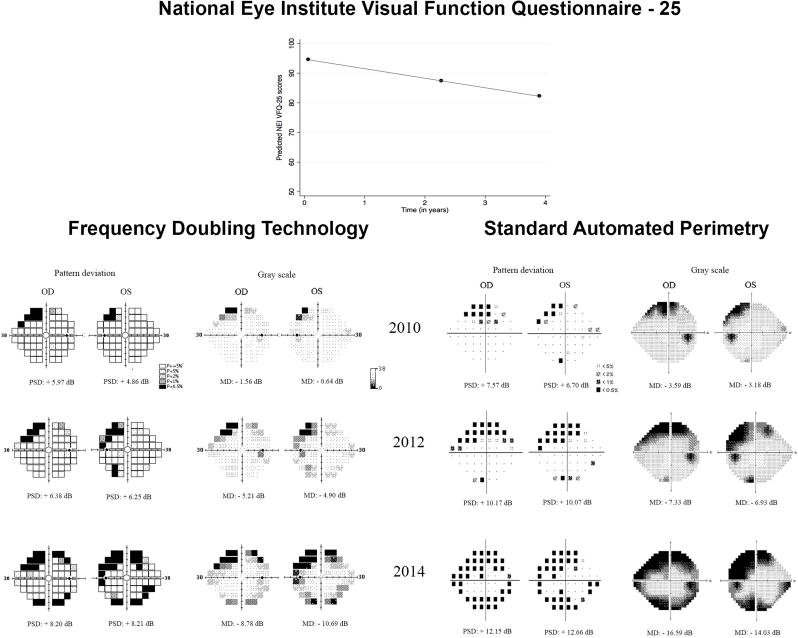
Representative tests from a 70-year-old glaucoma patient enrolled in the study and followed for an average of 3.8 years. The patient was pseudophakic at baseline in both eyes. Best-corrected visual acuities were 20/20 in both eyes, which remained essentially unchanged during follow-up. The patient had a decline of approximately 3.2 points per year in the National Eye Institute Visual Function Questionnaire-25 scores. Patient presented progressive superior and inferior visual field defects in both Frequency Doubling Technology and Standard Automated Perimetry tests, but the defects appear more pronounced for Standard Automated Perimetry on the pattern deviation plots.
DISCUSSION
In the current study, we evaluated the relationship between two types of perimetric tests (SAP and FDT) and change in QoL as measured by the NEI VFQ-25 questionnaire. Our results showed that there were statistically significant associations between longitudinal changes in NEI VFQ-25 scores and rates of change in both perimetric tests. However, compared to FDT, longitudinal changes in SAP showed stronger association with changes in QoL. These results suggest that SAP may be a better predictor of overall patient-reported disability in glaucoma than FDT.
In the multivariable model, each 1dB/year change in binocular FDT mean sensitivity was associated with a change of 0.8 units per year in NEI VFQ-25 scores. In addition, the amount of baseline visual field damage was also an important factor influencing the impact of visual field change on QoL. Considerably larger declines in QoL were seen for those subjects who had worse disease severity at baseline. For example, a patient with baseline binocular FDT mean sensitivity of 30 dB with a sustained loss of 5 dB in binocular mean sensitivity over a period of 5 years (a rate of 1dB/year) would experience a drop of approximately 4 units in NEI VFQ-25 scores. However, the same amount of visual field loss over time would result in approximately 11 units of decrease in NEI VFQ scores for a patient with a baseline FDT mean sensitivity of 20 dB. As Rasch scores were scaled to range from 0 to 100, a 11-unit change in NEI VFQ-25 scores corresponded to approximately 11% change in a scale going from the worst reported QoL in our sample to the best reported one. For SAP, both baseline and rates of change in mean sensitivity were also associated with change in NEI VFQ-25 scores. However, the multivariable model containing baseline and longitudinal SAP information performed better than a similar model for FDT in predicting change in NEI VFQ-25 scores. A control group was also included in our study in order to investigate whether ageing effects could explain a significant relationship between changes in mean sensitivity and changes in NEI VFQ-25 scores over time. No significant associations were seen in the control group, suggesting that the relationships seen in glaucoma patients were actually related to progression of the disease and its impact on QoL. It is important to note, however, that controls were significantly younger than glaucoma patients in our study, which may limit this comparison.
Only a few cross-sectional studies have investigated the relationship between FDT measurements and information about QoL.31,32 Van Landingham et al investigated 2934 adults from the National Health and Nutrition Examination Survey, aged 40 years or older examining relationship between FDT (with 19-point supra-threshold screening test) and physical activity.31 Their results concluded that individuals with bilateral visual field loss on FDT took 17% fewer steps per day (P < 0.01) and were engaged in 30% less minutes of physical activity (P = 0.02) than individuals without visual field loss.31 In another study, Qiu et al found that a greater severity of visual field abnormality on FDT was associated with significantly greater odds of physical function disability in everyday tasks, including daytime driving in familiar places and noticing objects off to the side while walking.32 Although these two cross-sectional investigations support the use of FDT in predicting risk of disability, it is important to note that they did not provide any comparison against SAP, as performed in our study. In addition, the use of cross-sectional data may be limited by the wide interindividual variability in subjective perceptions about QoL and by the possible development of compensatory mechanisms.33 Patients with slowly progressive glaucomatous damage may adapt to reduce the impact of visual loss on activities of daily living.34 If this were to be the case, one would expect those with faster rates of progression to be more likely to have reductions in QoL. In fact, our current study and our previous investigations support a significant relationship between rates of glaucomatous change and patient-reported disability.4,34,35
FDT was developed to specifically target the magnocellular pathway.5 This pathway plays a prominent role in motion perception, being responsive to low-contrast stimuli with low spatial- and high temporal-frequency.36 Although there is no consensus with regard to how effectively FDT is able to target the magnocellular pathway,37 several studies have suggested that FDT may be able to detect field losses not apparent on standard perimetry.10,38 In the current investigation, we found that SAP was superior to FDT in predicting overall patient-reported disability as assessed by the NEI VFQ-25 questionnaire. However, the disability measured by the NEI VFQ-25 represents an overall assessment of several vision-dependent tasks.16 It is possible that FDT may have a superior ability compared to SAP for predicting performance on tasks that are more dependent on characteristics related to the magnocellular pathway, such as reading or driving.32 Although the NEI VFQ-25 provides different subscales related to specific tasks, a separate analysis of the impact of FDT on the different subscales was not possible due to the limited sample size of our study. In addition, the validity of these subscales has been questioned in the literature.39
Our study has limitations. The average follow-up time was relatively short. Despite that, we found significant associations between longitudinal changes in perimetric tests and QoL scores. As subjects were followed for the same period of time with both FDT and SAP, we do not expect this to have influenced the comparisons presented in our study. It should be noted that although we demonstrated a significant association between baseline glaucoma severity and change in NEI VFQ-25 scores, our sample contained relatively few patients with severe disease in both eyes. Therefore, caution should be exercised when extrapolating our results to different patient populations. Another limitation of our study is that we assumed linear changes over time both in perimetric sensitivity as well as in NEI VFQ-25 scores. However evidence suggests that changes in visual function may not follow a linear course over the natural history of the disease.40,41 Although the assumption of linearity seems to be a sensible one for the follow-up period available in our study, future investigations with longer follow-up times are required to assess this assumption. A limitation of our multivariable model is that it included only baseline values for the confounding socioeconomic and comorbidity variables. Although changes in these variables could potentially impact NEI VFQ-25 scores over time, investigating change in these variables is difficult, as we did not obtain data quantifying the severity of comorbidities such as heart disease or other systemic conditions. However, we believe that it is unlikely that significant changes in these variables would have occurred in the timeframe of the study.
It is important to note that even though the longitudinal model including SAP information performed better than the equivalent FDT model, it was able to explain only approximately 50% of the variability in change in NEI VFQ-25 scores. QoL was assessed based on patient-reported outcomes (NEI VFQ-25), which fundamentally depend on subjective patient perceptions about the impact of vision loss on disability42 which perimetric tests may fail to capture. Future investigations should also evaluate the role of SAP and FDT in predicting objective measures of patient performance on daily tasks.
In conclusion, the results of this study demonstrated that rates of progressive visual field loss on FDT perimetry were significantly associated with decline in patient-reported QoL in glaucoma. However, SAP performed significantly better than FDT in predicting NEI VFQ-25 scores in our population, suggesting that it may still be the preferable perimetric technique for predicting risk of disability from the disease.
Acknowledgments
A. Funding/Support: Supported in part by National Institutes of Health/National Eye Institute grants EY021818 (FAM), EY11008 (LMZ), EY14267 (LMZ), EY019869 (LMZ) and core grant P30EY022589; an unrestricted grant from Research to Prevent Blindness, New York; Brazilian National Research Council-CAPES grant 12309-13-3 (CPBG); grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck and Santen.
B. Financial Disclosure(s): RYA: none; CPBG: none; ADF: none; LMZ: research support from National Eye Institute, Carl Zeiss Meditec, Optovue, Heidelberg Engineering, Topcon and Nidek; RNW: research support from Carl Zeiss Meditec, Optovue; consultant for Alcon, Allergan, Bausch & Lomb; FAM: research support from Alcon Laboratories, Bausch & Lomb, Carl Zeiss Meditec, Heidelberg Engineering, Merck, Allergan, Sensimed, Topcon, Reichert, National Eye Institute; Consultant for Allergan, Carl Zeiss Meditec, Novartis. No companies were involved in planning or executing the project.
C. Other acknowledgments: None
Biography

Ricardo Yuji Abe, MD, is a glaucoma specialist and a research fellow at the Hamilton Glaucoma Center, University of California, San Diego. Dr. Abe has completed his residency in ophthalmology at the University of Campinas, Brazil. He is a member of the Brazilian Council of Ophthalmology and Pan-American Association of Ophthalmology. He has received the Tim & Judith Sear Scholarship from the Pan-American Ophthalmological Foundation. His research interests include the study of glaucoma, quality of life and blindness prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spaeth G, Walt J, Keener J. Evaluation of quality of life for patients with glaucoma. Am J Ophthalmol. 2006;141(1 Suppl):S3–14. doi: 10.1016/j.ajo.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 3.Medeiros FA. Biomarkers and surrogate endpoints in glaucoma clinical trials. Br J Ophthalmol. 2014 Jul 17; doi: 10.1136/bjophthalmol-2014-305550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medeiros FA, Gracitelli CP, Weinreb RN, Zangwill LM, Boer ER, Rosen PN. Rates of Progressive Visual Field Loss and Longitudinal Changes in Quality of Life of Glaucoma Patients. Ophthalmology. 2015;122(2):293–301. doi: 10.1016/j.ophtha.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson AJ, Johnson CA. Frequency-doubling technology perimetry. Ophthalmol Clin North Am. 2003;16(2):213–225. doi: 10.1016/s0896-1549(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 6.Kelly DH. Frequency doubling in visual responses. J Opt Soc Am. 1966;56(11):1628–1633. [Google Scholar]
- 7.Cello KE, Nelson-Quigg JM, Johnson CA. Frequency doubling technology perimetry for detection of glaucomatous visual field loss. Am J Ophthalmol. 2000;129(3):314–322. doi: 10.1016/s0002-9394(99)00414-6. [DOI] [PubMed] [Google Scholar]
- 8.Sample PA, Medeiros FA, Racette L, et al. Identifying glaucomatous vision loss with visual-function-specific perimetry in the diagnostic innovations in glaucoma study. Invest Ophthalmol Vis Sci. 2006;47(8):3381–3389. doi: 10.1167/iovs.05-1546. [DOI] [PubMed] [Google Scholar]
- 9.Medeiros FA, Sample PA, Zangwill LM, Liebmann JM, Girkin CA, Weinreb RN. A statistical approach to the evaluation of covariate effects on the receiver operating characteristic curves of diagnostic tests in glaucoma. Invest Ophthalmol Vis Sci. 2006;47(6):2520–2527. doi: 10.1167/iovs.05-1441. [DOI] [PubMed] [Google Scholar]
- 10.Meira-Freitas D, Tatham AJ, Lisboa R, et al. Predicting progression of glaucoma from rates of frequency doubling technology perimetry change. Ophthalmology. 2014;121(2):498–507. doi: 10.1016/j.ophtha.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sample PA, Bosworth CF, Blumenthal EZ, Girkin C, Weinreb RN. Visual function-specific perimetry for indirect comparison of different ganglion cell populations in glaucoma. Invest Ophthalmol Vis Sci. 2000;41(7):1783–1790. [PubMed] [Google Scholar]
- 12.Sun H, Swanson WH, Arvidson B, Dul MW. Assessment of contrast gain signature in inferred magnocellular and parvocellular pathways in patients with glaucoma. Vision Res. 2008;48(26):2633–2641. doi: 10.1016/j.visres.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin L, Wanger P, Vancea L, Göthlin B. Concordance of high-pass resolution perimetry and frequency-doubling technology perimetry results in glaucoma: no support for selective ganglion cell damage. J Glaucoma. 2003;12(1):40–44. doi: 10.1097/00061198-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Racette L, Liebmann JM, Girkin CA, et al. African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol. 2010;128(5):551–559. doi: 10.1001/archophthalmol.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson-Quigg JM, Cello K, Johnson CA. Predicting binocular visual field sensitivity from monocular visual field results. Invest Ophthalmol Vis Sci. 2000;41(8):2212–2221. [PubMed] [Google Scholar]
- 16.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 17.Bond TG, Fox CM. Applying the Rasch Model: Fundamental Measurement in the Human Sciences. New York: Taylor & Francis; 2007. pp. 29–48. [Google Scholar]
- 18.Boone WJ. Understanding Person Measures. In: Boone WJ, Staver JR, Yeale MS, editors. Rasch Analysis in the Human Sciences. New York: Springer; 2014. pp. 69–92. [Google Scholar]
- 19.Andrich D. Rating scales and Rasch measurement. Expert Rev Pharmacoecon Outcomes Res. 2011;11(5):571–585. doi: 10.1586/erp.11.59. [DOI] [PubMed] [Google Scholar]
- 20.Marella M, Pesudovs K, Keeffe JE, O’Connor PM, Rees G, Lamoureux EL. The psychometric validity of the NEI VFQ-25 for use in a low-vision population. Invest Ophthalmol Vis Sci. 2010;51(6):2878–2884. doi: 10.1167/iovs.09-4494. [DOI] [PubMed] [Google Scholar]
- 21.Ferris FL, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–96. [PubMed] [Google Scholar]
- 22.Globe DR, Varma R, Torres M, Wu J, Klein R, Azen SP. Self-reported comorbidities and visual function in a population-based study: the Los Angeles Latino Eye Study. Arch Ophthalmol. 2005;123(6):815–821. doi: 10.1001/archopht.123.6.815. [DOI] [PubMed] [Google Scholar]
- 23.Crowther MJ, Abrams KR, Lambert PC. Joint modeling of longitudinal and survival data. The Stata Journal. 2013;13(1):165–184. [Google Scholar]
- 24.Medeiros FA, Zangwill LM, Alencar LM, Sample PA, Weinreb RN. Rates of progressive retinal nerve fiber layer loss in glaucoma measured by scanning laser polarimetry. Am J Ophthalmol. 2010;149(6):908–915. doi: 10.1016/j.ajo.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Alencar LM, Zangwill LM, Weinreb RN, et al. A comparison of rates of change in neuroretinal rim area and retinal nerve fiber layer thickness in progressive glaucoma. Invest Ophthalmol Vis Sci. 2010;51(7):3531–3539. doi: 10.1167/iovs.09-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medeiros FA, Leite MT, Zangwill LM, Weinreb RN. Combining structural and functional measurements to improve detection of glaucoma progression using Bayesian hierarchical models. Invest Ophthalmol Vis Sci. 2011;52(8):5794–5803. doi: 10.1167/iovs.10-7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medeiros FA, Zangwill LM, Girkin CA, Liebmann JM, Weinreb RN. Combining structural and functional measurements to improve estimates of rates of glaucomatous progression. Am J Ophthalmol. 2012;153(6):1197–1205. doi: 10.1016/j.ajo.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miki A, Medeiros FA, Weinreb RN, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014;121(7):1350–1358. doi: 10.1016/j.ophtha.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beckett LA, Tancredi DJ, Wilson RS. Multivariate longitudinal models for complex change processes. Stat Med. 2004;23(2):231–239. doi: 10.1002/sim.1712. [DOI] [PubMed] [Google Scholar]
- 30.Kraha A, Turner H, Nimon K, Zientek LR, Henson RK. Tools to support interpreting multiple regression in the face of multicollinearity. Front Psychol. 2012;3:44. doi: 10.3389/fpsyg.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Landingham SW, Willis JR, Vitale S, Ramulu PY. Visual field loss and accelerometer-measured physical activity in the United States. Ophthalmology. 2012;119(12):2486–2492. doi: 10.1016/j.ophtha.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 32.Qiu M, Wang SY, Singh K, Lin SC. Association between visual field defects and quality of life in the United States. Ophthalmology. 2014;121(3):733–740. doi: 10.1016/j.ophtha.2013.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huxlin KR. Perceptual plasticity in damaged adult visual systems. Vision Res. 2008;48(20):2154–2166. doi: 10.1016/j.visres.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Lisboa R, Chun YS, Zangwill LM, et al. Association between rates of binocular visual field loss and vision-related quality of life in patients with glaucoma. JAMA Ophthalmol. 2013;131(4):486–494. doi: 10.1001/jamaophthalmol.2013.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gracitelli CP, Abe RY, Tatham AJ, et al. Association between Progressive retinal nerve fiber layer loss and Longitudinal Change in Quality of Life in Glaucoma. JAMA Ophthalmol. 2015 Jan 08; doi: 10.1001/jamaophthalmol.2014.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hebart MN, Hesselmann G. What visual information is processed in the human dorsal stream? J Neurosci. 2012;32(24):8107–8109. doi: 10.1523/JNEUROSCI.1462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White AJ, Sun H, Swanson WH, Lee BB. An examination of physiological mechanisms underlying the frequency-doubling illusion. Invest Ophthalmol Vis Sci. 2002;43(11):3590–3599. [PubMed] [Google Scholar]
- 38.Medeiros FA, Sample PA, Weinreb RN. Frequency doubling technology perimetry abnormalities as predictors of glaucomatous visual field loss. Am J Ophthalmol. 2004;137(5):863–871. doi: 10.1016/j.ajo.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Pesudovs K, Gothwal VK, Wright T, Lamoureux EL. Remediating serious flaws in the National Eye Institute Visual Function Questionnaire. J Cataract Refract Surg. 2010;36(5):718–732. doi: 10.1016/j.jcrs.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26(6):688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medeiros FA, Zangwill LM, Bowd C, Mansouri K, Weinreb RN. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci. 2012;53(11):6939–6946. doi: 10.1167/iovs.12-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubenstein LZ, Schairer C, Wieland GD, Kane R. Systematic biases in functional status assessment of elderly adults: effects of different data sources. J Gerontol. 1984;39(6):686–691. doi: 10.1093/geronj/39.6.686. [DOI] [PubMed] [Google Scholar]


