Abstract
We review herein the basis for using dietary components to treat and/or prevent Helicobacter pylori infection, with emphasis on: (a) work reported in the last decade, (b) dietary components for which there is mechanism-based plausibility, and (c) components for which clinical results on H. pylori amelioration are available. There is evidence that a diet-based treatment may reduce the levels and/or the virulence of H. pylori colonization without completely eradicating the organism in treated individuals. This concept was endorsed a decade ago by the participants in a small international consensus conference held in Honolulu, Hawaii, USA, and interest in such a diet-based approach has increased dramatically since then. This approach is attractive in terms of cost, treatment, tolerability and cultural acceptability. This review therefore highlights specific foods, food components, and food products, grouped as follows: bee products (e.g. honey and propolis), probiotics, dairy products, vegetables, fruits, oils, essential oils, and herbs, spices and other plants. A discussion of the small number of clinical studies that are available is supplemented by supportive in vitro and animal studies. This very large body of in vitro and pre-clinical evidence must now be followed up with rationally designed, unambiguous human trials.
Keywords: anti-inflammatory, broccoli sprouts, cancer, colonization, diet, dietary, food, garlic, gastric, Helicobacter, H. pylori, infection, stomach, ulcer
1. Introduction
Major cancer burdens in humans, especially cancers of the liver, uterine cervix, and stomach, are caused by infectious agents. Infections of the human gut with the bacterium Helicobacter pylori have only been recognized for about three decades and have achieved widespread acceptance only over the past two decades [1]. Clinical studies and basic research on the organism and its close relatives [2] have now so thoroughly validated its discovery and the public health importance of that discovery, for which a Nobel Prize was awarded, that it put the word “Helicobacter” on the tips of tongues worldwide [3]. Alongside a dramatically increased awareness of this infectious agent, there has been a proliferation of strategies for cures, some real, and many imagined, to eradicate H. pylori infection.
1.1. Approach, and Scope of Literature Reviewed
We have reviewed herein, the basis for using dietary components or ingredients (food) to treat and/or prevent H. pylori infection, with emphasis on work reported since the comprehensive review of Mahady ten years ago [4], and with emphasis on components for which there is mechanism-based plausibility, and there have been published clinical results. For this purpose, the PubMed, Scopus, and ClinicalTrials.gov databases were searched for relevant studies using keywords related to Helicobacter through February 2015, without restrictions, and by reviewing the reference lists from retrieved papers. Focusing upon the components illuminated by this strategy resulted in an examination of bee products (eg. honey and propolis), probiotics and dairy products, vegetables, fruits, oils, essential oils, herbs, and spices. We have highlighted the work done with these dietary compounds, following a critical examination of the assumption that the only good H. pylori is a dead H. pylori (e.g. that complete eradication is necessary) (Section 2), and that foods present an alternative to pharmaceuticals for a variety of sound scientific reasons (Section 3).
1.2 Helicobacter Infection
Helicobacter pylori is recognized by the World Health Organization as a Class I human carcinogen. Infection with H. pylori is implicated causally in development of chronic gastritis and in peptic ulcer disease (PUD). The pathophysiology of infection has been exhaustively reviewed by others, notably by Kusters and colleagues [5]. Briefly, this gram-negative, flagellated, spirilliform (rapidly motile) bacterium (order: Campylobacterales), utilizes the enzyme urease, (not present in mammalian tissues), to convert urea in the stomach to carbon dioxide and ammonia, thus elevating the highly acidic pH of the gastric lumen and allowing it to survive an otherwise exceedingly hostile environment. H. pylori “tunnels” into the mucus layer covering the gastric epithelium and may persist for decades where it can deliver a highly immunogenic protein dubbed “CagA” and/or a vacuolization inducing protein dubbed “VacA” to epithelial cells (these are strain-dependent) thus activating both immune and inflammatory responses.
H. pylori infection is an important factor leading to a progression through acute or chronic inflammation of the gastric mucosa and peptic ulcer disease (PUD). This gastritis, if persistent, can lead to duodenal ulcers and to mucosa-associated lymphoid tissue (MALT) lymphoma. If atrophic, it can lead to gastric ulcers and to metaplasia, dysplasia, and gastric cancer. H. pylori infection results in a 3- to 6-fold increase in the relative risk for developing gastric adenocarcinoma and MALT lymphoma. Although more than half of the world’s population is infected with H. pylori (usually in childhood), the vast majority of infected individuals never develop gastric cancer. For those individuals who are infected, attributable risk estimates range from 50 to 73%, such that about half a million new cases of gastric cancer yearly (about 55% of the total number of cases), are directly attributable to infection with H. pylori [6]. Societal costs, not only of these cancers, but of gastric and duodenal ulcer, are enormous.
1.3 Gastric cancer
Stomach cancer, as well as gastritis, gastric ulcers, and duodenal ulcers, are diseases of both the industrialized and the developing world. In many developing countries, over 90% of the population is infected, but not all developing countries have a high incidence of gastric cancer. Many African countries were originally reported to have an extremely low incidence of gastric cancer and very high rates of H. pylori infection [7], leading to examination of factors such as bacterial virulence genotype, dietary factors, and host (human) genetic polymorphism, to help explain the gastric cancer incidence in this region [8–11]. Although infection with H. pylori is rapidly declining in Western nations, 50–80% of adults in Asia and 70–90% of adults in South and Central America are colonized [12]. Globally, gastric cancer is the third leading cause of cancer mortality of both sexes, with more than 951,000 cases worldwide and approximately 723,000 deaths [13], and it is still a leading cause of cancer death in many countries.
1.4 Treatment of Helicobacter infection
The development or identification of ways in which to lower the prevalence of H. pylori infection and the consequent risk of cancer is of compelling importance because infection can result in gastritis, gastric and duodenal ulcers and perhaps other sequelae [14,15]. There are currently no vaccines against this infection and expectations for their future development are generally negative [16,17]. Combinations consisting of twice daily treatment for 7 to 14 days, with: (1) a proton pump inhibitor (PPI) such as omeprazole or lansoprazole, and the antibiotics (2) amoxicillin, and (3) clarithromycin or metronidazole (dubbed “triple therapy”) [18,19], are generally effective therapies for those who can afford them (e.g. residents of industrialized countries). However, antibiotic therapy for infected individuals in most of the developing world is impractical due to complex economic, social and logistic considerations. There are other problems with antibiotic treatment in that the development of antibiotic resistance is of considerable concern (discussed in more detail later in this review), and eradication rates in many studies are as low as 70%. This bodes poorly for a strategy of treating entire populations with antibiotics.
However, complete eradication of H. pylori in symptom-free people might not be prudent due to the intriguing, but not yet proven possibilities of adverse side-effects. These may include such things as an increased risk of lower esophageal adenocarcinoma and an exaggeration of gastroesophageal reflux symptoms [20,21]. The concept that a diet-based treatment could reduce levels of H. pylori colonization or virulence, mitigate gastritis, inhibit progression of corpus atrophy, and perhaps eventually delay or prevent development of gastric cancer -- without completely eradicating the organism in treated individuals -- presents alternatives from a number of perspectives including those of cost, treatment tolerability, and cultural acceptability. Evaluation of a number of potential diet-based treatments (e.g. Lactobacillus spp., Saccharomyces boulardii, broccoli sprouts, honey, cranberries and garlic) have been reported. Scores of other indigenous plants and other foods have been used for many centuries by native and traditional healers as cures for syndromes that are likely to involve H. pylori infection [22–31]. The proposed mechanisms of action for these natural products, although beyond the scope of this review, include a direct antimicrobial effect, as well as anti-inflammatory, antioxidant, anti-adhesive, and immune stimulatory effects, and the inhibition of the enzyme urease, one of the bacterial pathogenesis factors (Fig. 1).
Figure 1.
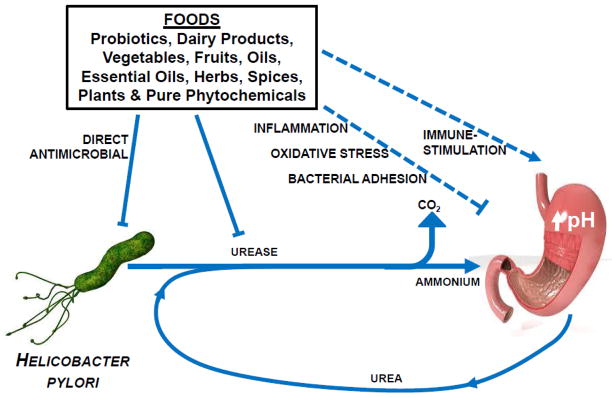
A large number of foods have been evaluated for their ability to inhibit Helicobacter pylori, in vitro. Some of these, and other food components reduce colonization of the stomach and the sequelae of colonization, in animal models and small human trials. Primary mechanisms of action are not in all cases focused on the direct antimicrobial effects, but include inhibition of the urease produced by H. pylori as a pathogenic factor, anti-inflammatory antioxidant, anti-adhesive, and immune-stimulatory properties of these foods.
Clinical trials to conclusively support or refute the efficacy of such treatments are fraught with difficulties. Numerous endpoints or biomarkers are available – many involve endoscopy and/or gastric biopsy and thus place medical, economic and logistical limits upon the numbers of subjects that can be enrolled. They also place ethical limitations on such studies, which serve to focus scientific attention on individuals with symptoms when, in fact, asymptomatic but infected individuals may be a much more useful and/or appropriate population for study, as well as being the most logical target for preventive strategies [32].
2. Reduction of the Impact, or Elimination of Infection?
A question that underlies much of the dietary strategy to reduce the incidence of gastritis, ulcers, or stomach cancer, can be very simply stated as follows:
Is it necessary to completely prevent or eradicate infection with H. pylori, or will reducing the level and/or virulence of colonization (of the gastric mucosae), result in a reduced risk of disease or reduced disease severity? Martin Blaser provides cogent analyses of this issue [20,21,33–36]. Almost two decades ago he concluded that:
“Although further research may show that human beings are better off without their long-time companions H. pylori, I maintain that we are at present too ignorant of the diversity of H pylori strains and their interactions with human beings to advocate their total elimination” [20].
Blaser’s earlier predictions (based upon his understanding of H. pylori ecology), have provoked much discussion, (see for example: [4,22,37,38]), however the tools of modern microbiology are reinforcing his early insights regarding H. pylori and what he now calls the “disappearing microbiota hypothesis” [39]. Further, and addressed by Blaser and many others, is the fact that there are deep, long-lasting, and severe effects of antibiotic therapy on the gut microbiota. It has only been in the past decade or so that the gravity of these effects and their impact on host immunity, metabolism, and even neuropsychiatry have been understood and clinically demonstrated [39,40], but it is the collective effect that mitigates against the frivolous use of antibiotics for treatment of H. pylori infection [19]. Suitable endpoints for interventions designed to reduce damage to the human stomach resulting from infection with H. pylori, without necessarily eradicating the bacterium, must thus be identified. Several primary non-invasive endpoints for detecting eradication of infection have been used in recent years: (A) the urea breath test (UBT), (B) fecal antigen detection, (C) serum pepsinogen (PG) I and PGII levels and ratios, (D) culture from a “string test”, and (E) markers of inflammation. [N.B. The UBT, (for which patients consume labelled urea and then provide a breath sample that exploits H. pylori’s high urease activity to create labelled CO2 that is measured in exhaled breath), is simple to conduct, is inexpensive, and has high sensitivity and specificity for detection of active H. pylori infection]. However, the adaptability of such tests to the accurate measurement of a reduction of bacterial levels or bacterial virulence-related genotypes is problematic. Determination of the most appropriate biomarkers to use in these human clinical trials is still far from complete [41,42].
3. Why A Food-Based Approach to H. pylori Treatment?
Graham and colleagues have shown that many compounds have anti-H. pylori activity in vitro, but do not eradicate infection in vivo in human beings [43,44]. Likewise reductions in H. pylori colonization following treatment, without achieving its eradication, have been shown following treatment of animals with dietary/phytochemical agents [45,46]. We have approached the question of diet-based treatment from a slightly different perspective, however: If by following a dietary regimen one can achieve a reduction in indicators of inflammation and of colonization, but not complete resolution of that infection, then is this dietary strategy not worthy of further development, either by itself or in a combinative approach, since complete resolution may be undesirable? We suggest that a number of foods may each have a small but measurable effect on the severity of infection and/or the risk of gastritis, ulcers, or stomach cancer, and that it is therefore worthwhile to utilize the available biomarkers of H. pylori colonization in order to evaluate the efficacy of this incremental effect in vivo. These questions must of course be considered in the context of rapid development of H. pylori strains resistant to synthetic antibiotics, oppressive poverty in many of the areas with very high prevalence of H. pylori infection, and the huge monetary cost of any long-term pharmaceutical preventive strategy [19,47].
Compared with the use of synthetic pharmaceuticals, a dietary approach to prevention can be very inexpensive, and may be the only practical or affordable approach to take in areas underserved by healthcare systems [32]. If indigenous plants and/or foods can be identified which are effective in preventing or reducing H. pylori infection, these could be introduced in such areas [48]. For example, potent anti-inflammatory activity [49] and rapid uptake by cells and organs [50] has been demonstrated for certain phytochemicals (e.g. sulforaphane from broccoli sprouts). There is also considerable precedent for treating and ameliorating gastritis and digestive disorders with foods (e.g. garlic, honey, Lactobacillus spp.). The literature on phytochemical mechanisms and their employment to treat gastric disorders is extensive, and we cite some key reviews, but do not cover the subject exhaustively herein [51–57].
4. Foods and dietary ingredients with activity against H. pylori
Phytochemical components of vegetables (and fruits) have been examined in many laboratories for their effects on H. pylori infection (Fig. 1). Additionally, many epidemiologists have evaluated the effects of fruit and vegetable consumption on cancer incidence. They have attempted to draw conclusions that relate to specific phytochemical ingredients of those dietary components.
Clinical studies lag behind both popular and scientific interest in this area: Of the 230 Helicobacter studies catalogued in the USA government’s ClinicalTrials.gov database, only 6 of them deal with dietary/food ingredients (NCT01028690, Lactobacillus reuterii; NCT01593592, L. reuterii; NCT01456728, L. reuterii; NCT01115296, probiotics; NCT02018328, curcumin; NCT01045408, berries) -- most of the balance are drug or epidemiologic investigations [58]. The 236 clinical trials posted come from the following regions (number of studies in each, in parentheses): China & Korea (102), Europe (44), USA (29), Middle East (21), Japan (10), S.E. Asia (8), Africa (6), India (5), South America (5), Canada (3), Mexico (2), Russian Federation (1).
In Table 1 we highlight primary references, reviews, and meta-analyses of clinical studies. Key recent examples of in vitro and animal studies for the best-studied foods are summarized in Table 2, which, along with the remainder of this section, focus on research published since the topic was comprehensively reviewed ten years ago [4], and studies of foods for which no robust clinical trial results have been published.
Table 1.
Clinical studies evaluating effects of selected foods and food components on H. pylori and gastric symptoms.
| FOOD PRODUCTS1 | PRIMARY REFERENCE
|
REVIEW3 | META-ANALYSIS4 | |
|---|---|---|---|---|
| EFFECT2 | ||||
| POSITIVE | NEGATIVE | |||
| Bee (Apis mellifera) Products | ||||
| Manuka honey | 59 | |||
| Probiotics | ||||
| (general) | 60 | 61 | 62 | 61 |
| Lactobacilli | 63 | 64 | ||
| Bifidobacterium bifidum | 65 | |||
| Saccharomyces boulardii | 66 | 66 | ||
| Fermented milk based- | 67 | 68 | 69 | |
| Dairy Products | ||||
| Lactoferrin | 70,71 | 72,73 | ||
| Vegetables | ||||
| Broccoli sprouts | 46,75,120 | |||
| Broccoli | 76 | 77 | ||
| Garlic | 78 | |||
| Pepper (red; capsaicin) | 78,79 | |||
| Fruits | ||||
| Cranberry | 80 | 81 | ||
| Oils | ||||
| Blackcurrant Seed- | 82 | |||
| Fish- | 82 | 83 | ||
| Essential Oils | ||||
| Hericium erinaceus (fungus) | 84 | |||
| Herbs, Spices, and Other Plants | ||||
| Mastic Gum & Resin | 85 | 86 | ||
| Cinnamon | 87 | 4,56 | ||
Foods, food products, or extracts of foods or plants used for food;
Outcome of clinical trial, as reported;
Reviews;
Meta-analyses of multiple clinical trials.
Table 2.
Effects of selected foods and food components on H. pylori and gastric symptoms.
| FOOD PRODUCTS1 | BIOLOGICAL ACTIVITY MEASURED2 | ||||
|---|---|---|---|---|---|
| ADH | AOX | IMM | INF | MIC | |
| Bee (Apis mellifera) Products | |||||
| Honey (general) | 88 | 31,51,89–94 | |||
| Manuka Honey | 88 | 59,94,95 | |||
| Propolis | 46 | 96 | 91,96,97 | ||
| Probiotics | |||||
| (general) | 64,98,99 | 64,99,100 | 98,100–104 | ||
| Dairy Products | |||||
| Lactoferrin | 105 | 105–109 | |||
| Colostrum | 110,111 | ||||
| Cow’s Milk | 110,111 | ||||
| Vegetables | |||||
| Broccoli sprouts | 112,113 | 45,46,88,114–116 | 75,94,114,117–121 | ||
| Garlic | 44,121 | ||||
| Cabbage-radish (hybrid) | 114 | 114 | |||
| Okra | 94 | ||||
| Fruits | |||||
| Pomegranate | 52 | ||||
| Cranberry | 122–124 | ||||
| Berries (blue-, bil-, rasp-, straw-, elder-) | 94,125 | ||||
| Apple | 126 | 126 | |||
| Grapes (incl. skin/seed extracts, wine, winery byproducts & resveratrol) | 127 | 127 | 127 | 127 | 127,128 |
| Oils | |||||
| Blackcurrant Seed- | 94 | ||||
| Fish- | 129 | 88 | 94,129 | ||
| Essential Oils | |||||
| Many, including: cinnamon, lemon verbena, manuka, carrotseed, N. African thyme, rosegum, and fool’s watercress | 53,54 | ||||
| Wild oregano | 130 | ||||
| Peppermint | 55 | 53,54 | |||
| Caraway | 55 | ||||
| Ginger, turmeric, licorice | 131,132 | ||||
| Nutmeg | 133 | ||||
| Chili pepper | 79 | ||||
| Herbs, Spices, and Other Plants | |||||
| Mastic Gum & Resin | 134 | ||||
| Cinnamon | 56,87 | ||||
| Licorice | 135 | 23 | |||
| Oregano | 130 | 130 | |||
| Lotus | 136 | ||||
| Many, including: catmint, Chinese goldenthread, fingerroot, forsythia, ginger, goldenseal, great burdock, green tea, hops, mango ginger, meadowsweet, monkey-face tree, nutmeg, red ginseng, sage, sticklewort, tea, turmeric, wormwood | 26 | 137 | 26,137–140 | ||
| Algae: Cladosiphon fucoida (seaweed) & sulphated polysachharides | 141 | 136 | |||
Foods, food products, or extracts of foods or plants used for food;
Primary reports or reviews of anti-H.pylori activity. Column headings indicate general types of activities measured and descriptions in parentheses outline general approaches used for these determinations: ADH - antiadhesion (enumeration of H. pylori cells in culture, adhered to cultured gastric epithelial cells); AOX - antioxidant (determination of antioxidant capacity or reactive oxygen species, ROS, neutralization capacity of the food [e.g. ORAC] and/or total antioxidant capacity of plasma before and after treatment [TEAC]); IMM -immuno-stimulatory (measurement of stimulation of macrophage recruitment and release of cytokines [IL-2] and interferon-γ); INFL - anti-inflammatory (measurement of inflammatory cytokines, of nitric oxide release [e.g. indicative of an induction of the NfkB pro-inflammatory pathway, and inducible nitric oxide synthase, iNOS]; MICRO – direct antimicrobial (plate counts or other enumeration of H. pylori cells to determine minimum inhibitory concentration, MIC; or zone of inhibition).
4.1. Bee products
Honey in general, and a specific honey harvested from the flowers of the manuka bush, (Leptospermum scoparium) have activity against H. pylori and other bacteria in vitro [51,89–92]. However, in vivo studies have not been able to demonstrate eradication of the bacterium [59]. A large body of work over the years by Peter Molan and colleagues in New Zealand has demonstrated effects of manuka honey on wound healing and other bacteria-related pathologies [142]. In the case of Helicobacter infection, Molan and others have invoked both peroxide and non-peroxide mediated mechanisms [93,95]. Of the non-peroxide effects, (phytochemical content and simple osmotic effects), the osmotic effects appear to best explain the in vitro evidence [89] and this may be why in vivo activity of honey(s) against H. pylori has not been demonstrated [59]. It is impractical to maintain a solution of, for example, 15% honey at the gastric epithelium for sufficient time for it to have direct osmotic effects.
Propolis (a flavonoid-rich by-product of bees) also manifests anti-H. pylori activity in vitro [96,97], that has not been confirmed in vivo. Propolis also has anti-inflammatory and immune stimulatory activity [97] – both mechanisms clearly being important in the pathophysiology of H. pylori infection.
4.2. Probiotics and Dairy Products
Probiotics are introduced-, and frequently transient members of the gastrointestinal flora. They have been defined by the FAO/WHO as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (143). Prebiotics are ingested fiber that serves as food for our gastrointestinal microflora, but is not directly utilized by our [mammalian] cells. As defined by Gibson and colleagues in 2010 they are “a selectively fermented ingredient that results in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) on host health” [29]. Probiotics, along with prebiotics, saliva, and gastrointestinal secretions, are all considered important for optimal digestive function. Approximately two-thirds of the immune system is localized in the gastrointestinal tract, and the literature abounds with reports and with controversy over whether prebiotics or probiotics offer protection against- and cure of a variety of endemic and acute diseases, influencing the immune system through several molecular mechanisms.
Many probiotics are composed of mixtures of bacteria that produce lactic acid, and they are thus resistant to acidic conditions found in the stomach. According to Johnson-Henry and colleagues, they may also be able to colonize the stomach at least in a transient fashion, can competitively exclude some pathogens such as Escherichia coli 0157:H7 from gastrointestinal niches, may enhance mucin secretion, may act as bactericides, and may enhance epithelial barrier integrity [101]. Thirty-three clinical studies of the effects of probiotics supplements on H. pylori colonization were evaluated in a meta-analysis that concluded there was a significantly higher pooled H. pylori eradicating effect of the supplemented groups compared to control groups [102].
Various species of Lactobacillus (a common constituent of yogurt, and a component of a variety of probiotic commercial products) have in vitro activity against H. pylori [98,100,103,104], and in vivo activity in animals [98,100,103,104] and in humans [62–65,67,98,99]. Lactobacillus species also possess anti-inflammatory activity [100] and immune-stimulant activity [98]. A meta analysis concluded that Lactobacillus supplementation could be effective in increasing eradication rates of anti-H. pylori therapy for first-treated patients, and had a positive impact on some H. pylori therapy related side effects [60]. Saccharomyces boulardii has also been the subject of intense clinical scrutiny and a recent meta analysis of its use as an adjuvant to triple therapy gave a strong odds ratio for efficacy (OR=0.46)[66].
Colostrum and cow’s milk show activity against H. pylori in vitro [110,111], as do fermented milk products [68]. A component of cow’s milk, as well as human milk, is the iron-binding glycoprotein (and natural antibiotic), lactoferrin. Lactoferrin, which is also found in neutrophils, has an inhibitory effect on the growth of a number of bacteria including H. pylori, perhaps due to the high affinity of lactoferrin for iron [105–107]. Dial and colleagues have evaluated the effects of oral lactoferrin on Helicobacter in a mouse model, and demonstrated significant effects on Helicobacter-induced gastritis [108,109]. The results of clinical trials of the effects on humans of oral lactoferrin have been both negative [72,73] and positive [70,71]. A recent meta analyses of the effects of fermented milk-based probiotic preparations on H. pylori eradication shows that they improved eradication rates by 5–15% [69]. Interestingly, this has led to the European gastroenterology community’s recent inclusion of a tempered and qualified recommendation in their Consensus Report, that “Certain probiotics show promising results as an adjuvant treatment in reducing side effects” [144].
4.3. Vegetables
4.3.1. Broccoli sprouts
Anecdotal evidence that the consumption of broccoli sprouts may relieve peptic ulcer symptoms has led to the discovery that the isothiocyanate sulforaphane, most abundant in broccoli sprouts and currently under intense investigation for its protective effects against a variety of chronic diseases, is a very potent and selective antibiotic against H. pylori [117]. The same team later showed that a few other plant-derived isothiocyanates (e.g. 8-methylthiooctyl- and 4-rhamnopyranosyloxybenzyl-isothiocyanate) had similar activity against H. pylori, but many other isothiocyanates were not active [118]. Using a gastric xenograft model in nude mice and both histological and bacteriological evaluation criteria, Lozniewski and colleagues showed that H. pylori infections were effectively eliminated in 8 of 11 transplants following in situ infusion of 7.5 μmol sulforaphane on five successive days [119]. Other in vitro studies have underscored the efficacy of both the phytochemical and the broccoli sprouts from which they come [88,94,112,114], and have demonstrated that in addition to its direct bactericidal activity sulforaphane inactivates H. pylori urease, whereas numerous other isothiocyanates do not inactivate it [74].
In vivo studies in a Helicobacter-infected mouse model have shown a dramatic protective effect of broccoli sprouts against salt-induced gastritis. The bactericidal effectiveness of sulforaphane against H. pylori appears to be at least in part systemic, since it inhibited colonization, inflammation, and gastric mucosal atrophy in an H. pylori-infected, high salt diet mouse model [45]. The protective effects were much less pronounced in nrf2 gene knockout mice [113]. An initial study with H. pylori-colonized human subjects in Japan was unable to demonstrate an effect of broccoli sprouts owing to the use of a single biomarker (UBT; the 13C Urea Breath Test) and an insufficient sample size [145].
A pilot study in H. pylori-infected subjects also reported loss of H. pylori colonization following broccoli sprout treatment in four of nine subjects [120]. A small study of five subjects, in which very low levels of market stage broccoli were mixed with Tibetan yogurt, failed to show an effect of the treatment on UBT [76]. This is not surprising given the presumed very low levels of active ingredient (actual levels not reported), and the fact that for technical reasons, the homogenization and food processing used would be expected to have destroyed most of any sulforaphane released, prior to ingestion. Yanaka and colleagues in Japan reported a significant reduction in markers of inflammation, H. pylori levels, and UBT scores in colonized adults following daily consumption of broccoli sprouts containing ca. 420 μmol of the sulforaphane precursor, glucoraphanin, for 2 months, and these effects disappeared at the end of the intervention [45,115,116]. More recently, using similar metrics and outcomes to the Yanaka study, a group in Iran showed substantial effects of a 4 week dietary supplementation (86 Type 2 diabetes patients) with 6 g/d of high sulforaphane broccoli sprouts (about 135 μmol/d)[75].
4.3.2. Crucifers and other Vegetables
Numerous epidemiological studies have examined the association between cruciferous vegetable consumption and gastric cancer (see, for example [77,146,147]). These studies point to an inverse relationship, but whether this might be due to an anti-H. pylori effect, or a more general, systemic induction of cancer protective defenses is not clear at this time. Cabbage juice has historically been used to treat stomach ulcers [148], and has significant inhibitory effects on H. pylori-induced gastritis in a Mongolian gerbil model [114]. Leaves of the tree Moringa oleifera, widely consumed in some tropical regions, also have anti-ulcer activity (reviewed in [149]), and certain glucosinolates/isothiocyanates from Moringa have strong antibacterial activity in vitro against H. pylori and a variety of other human pathogens [118,149]. Okra also has anti-H. pylori activity in vitro, thought to be an anti-adhesive effect of okra polysaccharides [150], and it is reported to provide some protection against duodenal ulcer [154]. Even a seaweed, the brown alga Cladosiphon fucoidan, has anti-H. pylori activity in vitro and in gerbils [136], also speculated to be due to the anti-adhesive effect of the alga’s glucuronic acid-rich polysaccharide (fucoidan).
4.3.3. Garlic
A number of studies have demonstrated the anti-H. pylori activity of garlic in vitro [43,78], but the results of in vivo studies in humans have not been encouraging [43,44,78,152]. Garlic has antimicrobial activity against other bacteria [121] and possesses antioxidant activity [153]. This work has stimulated considerable debate in the literature on the efficacy of dietary treatment of H. pylori infection.
4.4. Fruits
A number of fruits, their juices and their extracts, inhibit growth of H. pylori in vitro. Among them, blueberry, bilberry, elderberry, cranberry, raspberry, and strawberry inhibit H. pylori, and enhance the susceptibility of H. pylori to clarithromycin, one of the synthetic antibiotics most commonly used to eradicate human infections with this microbe [125]. Cranberry juice has been the focus of particular attention for its ability to inhibit the growth of H. pylori and other bacteria (E. coli and Streptococcus) in vitro [51,122,123] and for its anti-adhesion activity against H. pylori [124]. There are at least two published studies in which the effects of cranberry juice was evaluated in colonized human beings. In the first, a double-blind, randomized, placebo-controlled trial sponsored by a cranberry juice company, the authors concluded that there was a significant effect of regular consumption of cranberry juice on a Chinese cohort of 189 subjects with positive urea breath test (UBT) results [81]. The second study, by Gottland and colleagues in Chile, showed that administration of cranberry juice for three weeks inhibited H. pylori about 15% of asymptomatic, colonized children, and that in most subjects who became negative (as measured by UBT), the clearing effect did not persist following cessation of consumption [80]. Furthermore, grape skin/seed extracts and wine preparations [128], (recently reviewed by Friedman [127]), pomegranate fruit and ellagic acid-rich juice (recently reviewed by Colombo [52]), apple [126], and Rubus idaeus and R. occicentalis fruit [154], have all been shown to have antimicrobial activity against H. pylori in vitro. Whereas animal models have been used to demonstrate efficacy of some of these fruit products, no trials currently listed on the USA government website ClinicalTrials.gov, propose to evaluate effects on H. pylori colonization.
4.5. Oils
Considerable work on naturally occurring H. pylori therapeutics has focused upon the truly lipoidal oils which include the short-, medium-, and long-chain fatty acids, monoglycerides, and the polyunsaturated fatty acids (commonly referred to as PUFAs). Fish oil [129], garlic oil [121], and blackcurrant seed oil (rich in ω-3 and ω-6 unsaturated fatty acids) [82,94], all have in vitro antibiotic activity against H. pylori. Fish oil [83] shows activity against Helicobacter in humans. Evening primrose oil (rich in the ω-6 unsaturated linoleic acid) heals ulcers in rats [155] and consumption of fish oil is inversely associated with the prevalence of duodenal ulcer [129]. Numerous investigators have reported in vitro effects of specific PUFAs against H. pylori [156–158] but studies in humans have been mixed, with some studies showing anti H. pylori activity [82,83] and others showing none [156,159]. Anti-inflammatory activity has been reported [160], as has protection against ulcer formation [151], reduced risk of atrophic gastritis [161], and suppression of gastric acid secretion [83,162]. Shorter chain fatty acid activity against H. pylori has only been demonstrated in vitro [163,164].
4.6. Essential oils, herbs, spices, and food components
There has been considerable interest over the past decade or more in the utility of a variety of essential oils to eradicate or reduce H. pylori infection, and to ameliorate the symptoms of infection. The term “essential oils” refers to the mixture of low molecular weight compounds, typically rich in terpenes, aldehydes, ketones, or phenols, which is usually obtained from plants by steam extraction and distillation. The essential oils of a variety of herbs have antimicrobial, antioxidant, anti-inflammatory, and immune stimulatory activity, and the anti-H. pylori properties of a variety of these extracts have been comprehensively reviewed [53]. The essential oils from carrot seed, cloves, manuka, and lemon verbena have in vitro anti-H. pylori activity and the activity of lemongrass essential oil was demonstrated in an in vivo mouse model [54]. Peppermint oil has anti-inflammatory activity and reduces symptoms of dyspepsia in combination with caraway oil [55]. Lack of in vivo results from essential oils and other herb and spice extracts has led the authors of a recent review on the subject of their antibiotic activity to comment:
Perhaps the most striking result of this review is the extreme paucity of controlled clinical trials testing herbal antibiotics. In light of the long history and present popularity of their use, it is surprising that so few trials have tested the efficacy of herbal antibiotics. One obvious reason is the lack of patent rights on herbal medicines. Another reason could be that traditionally, herbal medicine has been hesitant to embrace modern methods for efficacy testing [56].
Other essential oils have shown efficacy against H. pylori, including that from the fungus, Hericium, in a randomized trial [84], and from chili peppers [79]. A green tea extract (Camillia sinensis) rich in 3′-sialyllactose had anti-H. pylori adhesion effects in a very small trial with Rhesus monkeys [165].
Extracts of cinnamon [56,87], rosemary, turmeric, fingerroot, nutmeg, ginger, [133] and licorice [23], all inhibit H. pylori growth in vitro, as does mastic gum (from the Mediterranean shrub Pistacia lentiscus) [134], vitamin C [166–168], a variety of flavonoids [169–171], berberine (from the barberry bush and goldenseal), a fermented rice extract, numerous traditional Chinese medicine (TCM) components [138,139], and a sulphated polysaccharide from brown seaweed. A few of these have additional activities such as improvement in gastritis or ulcer healing, but by and large there have been no notable successes in in vivo eradication of H. pylori or in eradication of its symptoms with these compounds, preparations, herbs or extracts. On the other hand, anti-inflammatory activity has been reported with ginger, turmeric, licorice [131,132], and nutmeg [133], but the connection between this response and modulation of the sequelae of H. pylori infection has not been adequately probed. Inhibition of urease activity was reported from green tea extracts [137]. The sole reported clinical trial of a spice against H. pylori used cinnamon [87], and although there was no effect overall on UBT, the authors of this study point out that in patients whose initial UBT was exceptionally high, there was a decline in UBT following cinnamon ingestion [87].
Mastic gum (an inexpensive exudate obtained from the food plant Pistacia lentiscus) previously documented in the in the clinical trial literature in the mid-1980’s for its effect on treatment of duodenal and gastric ulcers, was shown to kill H. pylori in 1998 [133]. It has since been the subject of clinical trials with both negative [86] and positive [85] outcomes.
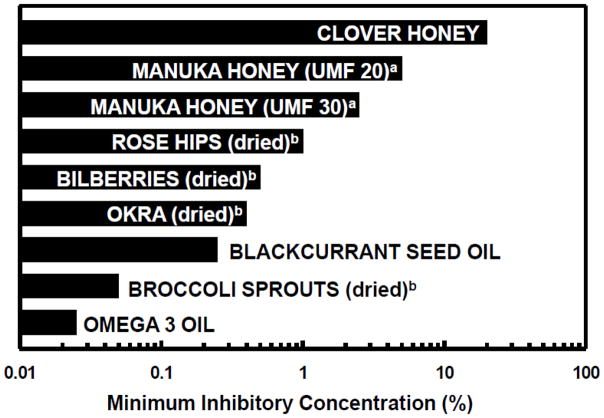
Recent studies reported by Keenan and colleagues in New Zealand show significant synergy or enhanced effects of multiple foods (e.g. blackcurrant oil and broccoli sprouts) on a number of metrics of colonization and inflammation (see Fig. 2) [88,94]. When these studies are examined in the context of the theses discussed in Sections 2 and 3, a more concerted search for truly synergistic effects of food components may be warranted.
Figure 2.
Effects of foods, and food components/ingredients on the growth of H. pylori were determined by the minimum inhibitory concentration (MIC) of that component, expressed as a weight per volume percentage. aUMF (“unique manuka factor”) is an index of potency that is used in the manuka honey trade. b”Dried” foods were lyophilized. Redrawn from Keenan et al. (2010) [94]
5. Conclusions, Gaps in Knowledge and Future Research Needs
The concept of dietary treatment of H. pylori infections both for prophylactic and therapeutic purposes has been the subject of considerable discussion and debate in recent years. By the year 2005, a comprehensive review of the subject had been published [4], a Consensus Conference (13–14 February, 2005, Honolulu, HI, USA) was organized by two of the authors (JWF and AJW), and a variety of foods and dietary ingredients were evaluated for their potential to ameliorate infection with Helicobacter pylori. The value of amelioration compared to complete eradication were discussed by the conferees, who then agreed to a draft consensus stating that “the concept that a diet-based treatment could reduce levels of H. pylori colonization without completely eradicating the organism in treated individuals is attractive in terms of cost, treatment, tolerability and cultural acceptability”. In light of the ongoing and voluminous research into this organism-disease complex, and into the clearly overwhelming effects that modification of our gastrointestinal microflora (in particular its diversity) has on health and wellness, there is now evidence that reducing colonization and its sequelae can be achieved with certain, perhaps many dietary ingredients. At this time the dietary components for treatment of H. pylori infection that have the greatest evidence to support them are broccoli sprouts, cranberry juice, essential oils of a number of spices (e.g. cloves, and blackcurrant), and some probiotic formulations. Based upon the current state of the science, a dietary approach to reduce the inflammatory response to H. pylori infection appears plausible.
Nonetheless, the huge body of in vitro and pre-clinical evidence has not received sufficient follow-up attention with rationally designed, unambiguous human trials.
Given the suggestion that complete eradication may even be detrimental, a food based approach may provide a useful adjunct or alternative to conventional drug treatments, and should be explored in colonized human subjects. These food-based approaches operate at levels of intake consistent with levels of consumption in the general population. They might require shifts in food consumption patterns but these would be consistent with adequate nutrition and good health. Moreover, avoidance of excessive antibiotic use could have profound long-term human health effects ranging from reduced development of antibiotic resistance, to maintenance of existing levels of human microbiome diversity, reduction of the risk of pathogen outbreaks, and reduced risk for a variety of non-communicable (chronic) diseases that may ultimately be proven to be influenced by the human microbiome.
Acknowledgments
Funding for the Consensus Conference held in Honolulu Hawaii on February 13–14, 2005, that is referred to in the paper, and which inspired this review, came from The New Zealand Foundation for Science, Research and Technology, Comvita New Zealand, Ltd., and the Brassica Foundation, Inc. We gratefully acknowledge the Lewis B. and Dorothy Cullman Foundation and the National Cancer Institute (CA 93780-03) for financial support during manuscript preparation.
Abbreviations
- OR
odds ratio
- PPI
proton pump inhibitor
- PUD
peptic ulcer disease
- PUFA
poly unsaturated fatty acid
- UBT
urea breath test
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–15. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Whary MT, Fox JG. Natural and experimental Helicobacter infections. Comp Med. 2004;54:128–58. [PubMed] [Google Scholar]
- 3.Parsonnet J. Clinician-discoverers - Marshall, Warren, and H. pylori. New Engl J Med. 2005;353:2421–23. doi: 10.1056/NEJMp058270. [DOI] [PubMed] [Google Scholar]
- 4.Mahady GB. Medicinal plants for the prevention and treatment of bacterial infections. Curr Pharmaceut Design. 2005;11:2405–27. doi: 10.2174/1381612054367481. [DOI] [PubMed] [Google Scholar]
- 5.Kusters JG, van Vliet AHM, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19(3):449–90. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 7.Holcombe C. The African enigma. Gut. 1992;33:429–31. doi: 10.1136/gut.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agha A, Graham DY. Evidence-based examination of the African enigma in relation to Helicobacter pylori infection. Scand J Gastroenterol. 2005;40:523–9. doi: 10.1080/00365520510012280. [DOI] [PubMed] [Google Scholar]
- 9.Bravo LE, van Doom L-J, Realpe JL, Correa P. Virulence-associated genotypes of Helicobacter pylori: do they explain the African enigma? Am J Gastroenterol. 2002;97:2839–42. doi: 10.1111/j.1572-0241.2002.07031.x. [DOI] [PubMed] [Google Scholar]
- 10.Kodama N, Pazos A, Schneider GB, Piazuelo MB, Mera R, Sobota RS, et al. Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proc Natl Acad Sci USA. 2014;111(4):1455–60. doi: 10.1073/pnas.1318093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiota S, Suzuki R, Yamaoka Y. The significance of virulence factors in Helicobacter pylori. J Dig Dis. 2013;14:341–49. doi: 10.1111/1751-2980.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Global health observatory data repository. Number of deaths (World) by cause. 2011 Available from: http://apps.who.int/gho/data/node.main.
- 13.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2014;5 doi: 10.1002/ijc.29210136:E359-E386. [DOI] [PubMed] [Google Scholar]
- 14.Correa P. Helicobacter pylori infection and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:238s–241s. [PubMed] [Google Scholar]
- 15.Greenberg ER, Alberts DS, Potter JD. Introduction: What should we do now about H. pylori? Cancer Epidemiol Biomarkers Prev. 2005;14:1851–52. doi: 10.1158/1055-9965.EPI-05-0455. [DOI] [PubMed] [Google Scholar]
- 16.Arora S, Czinn SJ. Vaccination as a method of preventing Helicobacter pylori associated gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1890–1. doi: 10.1158/1055-9965.EPI-05-0110. [DOI] [PubMed] [Google Scholar]
- 17.Zawahir S, Czinn SJ, Nedrud JG, Blanchard TG. Vaccinating against Helicobacter pylori in the developing world. Gut Microbes. 2013;4(6):568–76. doi: 10.4161/gmic.27093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham KS, Graham DY. Handbooks in Healthcare. 2. Newtown PA: 2002. Contemporary Diagnosis and Management of H pylori-Associated Gastrointestinal Diseases. [Google Scholar]
- 19.Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: Meeting the challenge of antimicrobial resistance. World J Gastroenterol. 2014;20(29):9898–911. doi: 10.3748/wjg.v20.i29.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaser MJ. Not all Helicobacter pylori strains are created equal: Should all be eliminated? Lancet. 1997;349:1020–2. doi: 10.1016/S0140-6736(96)09133-7. [DOI] [PubMed] [Google Scholar]
- 21.Blaser MJ, Berg DE. Helicobacter pylori genetic diversity and risk of human disease. J Clin Invest. 2001;107:767–73. doi: 10.1172/JCI12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makobongo MO, Gilbreath JJ, Merrell DS. Nontraditional therapies to treat Helicobacter pylori infection. J Microbiol. 2014;52(4):259–272. doi: 10.1007/s12275-014-3603-5. [DOI] [PubMed] [Google Scholar]
- 23.Fukai T, Marum A, Kaitou K, Kanda T, Terada S, Nomura T. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002;71:1449–63. doi: 10.1016/s0024-3205(02)01864-7. [DOI] [PubMed] [Google Scholar]
- 24.Manyi-Loh CE, Clarke AM, Mkwetshana NF, Ndip RN. Treatment of Helicobacter pylori infections: Mitigating factors and prospective natural remedies. Afr J Biotechnol. 2010;9(14):2032–42. [Google Scholar]
- 25.Cwikla C, Schmidt K, Matthias A, Bone KM, Lehmann R, Tiralongo E. Investigations into the antibacterial activities of phytotherapeutics against Helicobacter pylori and Campylobacter jejuni. Phytother Res. 2010;24(5):649–56. doi: 10.1002/ptr.2933. [DOI] [PubMed] [Google Scholar]
- 26.Bakhtaoui F-Z, Lakmichi H, Megraud F, Chait A, Gadhi C-EA. Gastro-protective, anti-Helicobacter pylori and antioxidant properties of Moroccan Zizyphus lotus L. J Appl Pharm Sci. 2014;4(10):81–7. [Google Scholar]
- 27.Eng-Chong T, Yean-Kee L, Chin-Fei C, Choon-Han H, Sher-Ming W, Li-Ping CT, et al. Boesenbergia rotunda: From ethnomedicine to drug discovery. Evidence-Based Compl Altern Med. 2012:Article ID 473637, 25. doi: 10.1155/2012/473637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadekar R, Singour PK, Chaurasiya PK, Pawar RS, Patil UK. A potential of some medicinal plants as an antiulcer agents. Pharmacog Rev. 2010;4(8):136–46. doi: 10.4103/0973-7847.70906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rastall RA, Gibson GR. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr Opin Biotechnol. 2015;32:42–6. doi: 10.1016/j.copbio.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Lawal TO, Soni KK, Doyle BJ, Adeniyi BA, Mahady GB. Susceptibility of Helicobacter pylori to natural products: Can past research direct future drug development? Curr Bioactive Com. 2012;8:266–76. [Google Scholar]
- 31.Manyi-Loh CE, Clarke AM, Munzhelele T, Green E, Mkwetshana NF, Ndip RN. Selected South African honeys and their extracts possess in vitro anti-Helicobacter pylori activity. Arch Med Res. 2010;41:324–32. doi: 10.1016/j.arcmed.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Fahey JW, Kensler TW. Health span extension through green chemoprevention. Am Med Assoc Virtual Mentor. 2013;15(4):311–8. doi: 10.1001/virtualmentor.2013.15.4.stas1-1304. [DOI] [PubMed] [Google Scholar]
- 33.Blaser MJ. An endangered species in the stomach. Sci Am. 2005 Feb;:38–45. doi: 10.1038/scientificamerican0205-38. [DOI] [PubMed] [Google Scholar]
- 34.Whitfield J. The ulcer bug: Gut reaction. Nature. 2003;423:583–4. doi: 10.1038/423583a. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Blaser MJ. Association between gastric Helicobacter pylori colonization and glycated haemoglobin levels. J Infect Dis. 2012;205:1195–202. doi: 10.1093/infdis/jis106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holster IL, Vila AMJ, Caudri D, den Hoed CM, Perez-Perez GI, Blaser MJ, et al. The impact of Helicobacter pylori on atopic disorders in childhood. Helicobacter. 2012;17:232–7. doi: 10.1111/j.1523-5378.2012.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gotteland M, Cruchet S, Brunser O. Can the amount of Helicobacter pylori in the stomach be kept low throught probiotic intake? Am J Clin Nutr. 2005;81:939. doi: 10.1093/ajcn/81.4.939. [DOI] [PubMed] [Google Scholar]
- 38.Gaby AR. Helicobacter pylori eradication: are there alternatives to antibiotics? Alternative Med Rev. 2001;6:355–66. [PubMed] [Google Scholar]
- 39.Blaser MJ. The Jeremiah Metzger lecture: Global warming redux: The disappearing microbiota and epidemic obesity. Trans Am Clin Climatolog Assoc. 2012;123:230–41. [PMC free article] [PubMed] [Google Scholar]
- 40.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nature Rev Genet. 2012;13:260–70. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooke CL, Torres J, Solnick JV. Biomarkers of Helicobacter pylori-associated gastric cancer. Gut Microbes. 2013;4(6):532–40. doi: 10.4161/gmic.25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu M-S, Chow L-P, Lin J-T, Chiou S-H. Proteomic identification of biomarkers related to Helicobacter pylori-associated gastroduodenal disease: Challenges and opportunities. J Gastroenterol Hepatol. 2008;23(11):1657–61. doi: 10.1111/j.1440-1746.2008.05659.x. [DOI] [PubMed] [Google Scholar]
- 43.Graham DY, Osato M. Response to Drs. Mahady and Pendland - pretreatment testing for H. pylori in duodenal ulcer disease. Am J Gastroenterol. 2000;95:309–10. doi: 10.1111/j.1572-0241.2000.01704.x. [DOI] [PubMed] [Google Scholar]
- 44.Mahady GB, Pendland S. Garlic and Helicobacter pylori. Am J Gastroenterol. 2000;95:309. doi: 10.1111/j.1572-0241.2000.01713.x. [DOI] [PubMed] [Google Scholar]
- 45.Yanaka A, Zhang S, Tauchi M, Nakahara A, Tanaka N. Daily intake of sulforaphane-rich broccoli sprouts prevents progression of high salt diet-induced gastric atrophy in Helicobacter pylori-infected C57/BL6 mice in vivo. Jpn J Helicobacter Res. 2004;5:9–13. [Google Scholar]
- 46.Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, et al. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res. 2009;2(4):353–60. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 47.Camargo MC, Garcia A, Riquelme A, Otero W, Camargo CA, Hernandez-Garcia T, et al. The problem of Helicobacter pylori resistance to antibiotics: A systematic review in Latin America. Am J Gastroenterol. 2014;109:485–95. doi: 10.1038/ajg.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fahey JW, Talalay P, Kensler TW. Notes from the field: “green” chemoprevention as frugal medicine. Cancer Prev Res. 2012;5(2):179–88. doi: 10.1158/1940-6207.CAPR-11-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu H, Dinkova-Kostova AT, Talalay P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc Natl Acad Sci USA. 2008;105(41):15926–31. doi: 10.1073/pnas.0808346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y. Molecular mechanism of rapid cellular accumulation of anticarcinogenic isothiocyanates. Carcinogenesis. 2001;22(3):425–31. doi: 10.1093/carcin/22.3.425. [DOI] [PubMed] [Google Scholar]
- 51.Carson CF, Riley TV. Non-antibiotic therapies for infectious diseases. Commun Dis Intel. 2003;27:S144–7. doi: 10.33321/cdi.2003.27.38. [DOI] [PubMed] [Google Scholar]
- 52.Colombo E, Sangiovanni E, Dell’Agli M. A review on the anti-inflammatory activity of pomegranate in the gastrointestinal tract. Evid-Based Compl Alt Med. 2013;247145:1–11. doi: 10.1155/2013/247145. ( http://dx.dot.org/10.1155/2013/247145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bergonzelli GE, Donnicola D, Porta N, Corthesy-Theulaz IE. Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob Agents Chemother. 2003;47:3240–6. doi: 10.1128/AAC.47.10.3240-3246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohno T, Kita M, Yamaoka Y, et al. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter. 2003;8:207–15. doi: 10.1046/j.1523-5378.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 55.Holtmann G, Haag S, Adam B, Funk P, Wieland V, Heydenreich CR. Effects of a fixed combination of peppermint oil and caraway oil on symptoms and quality of life in patients suffering from functional dyspepsia. Phytomedicine. 2003;10:56–7. doi: 10.1078/1433-187x-00310. [DOI] [PubMed] [Google Scholar]
- 56.Martin KW, Ernst E. Herbal medicines for treatment of bacterial infections: a review of controlled clinical trials. J Antimicrob Chemother. 2003;51:241–6. doi: 10.1093/jac/dkg087. [DOI] [PubMed] [Google Scholar]
- 57.Chun KS, Kim EH, Lee S, Hahm KB. Chemoprevention of gastrointestinal cancer: The reality and the dream. Gut Liver. 2013;7(2):137–49. doi: 10.5009/gnl.2013.7.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. [Accessed Dec 8, 2014]; http://clinicaltrials.gov/ct2/results/map/click?map.x=100&map.y=360&term=helicobacter.
- 59.McGovern DPB, Abbas SZ, Vivian G, Dalton HR. Manuka honey against Helicobacter pylori. J Royal Soc Med. 1999;92:439. doi: 10.1177/014107689909200832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zou J, Dong J, Yu X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:97–107. doi: 10.1111/j.1523-5378.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 61.Yoon H, Kim N, Kim JY, Park SY, Park JH, Jung HC, et al. Effects of multistrain probiotic-containing yogurt on second-line triple therapy for Helicobacter pylori infection. J Gastroenterol Hepatol. 2011;26(1):44–8. doi: 10.1111/j.1440-1746.2010.06477.x. [DOI] [PubMed] [Google Scholar]
- 62.Iannitti T, Palmieri B. Therapeutical use of probiotic formulations in clinical practice. Clin Nutr. 2010;29:701–25. doi: 10.1016/j.clnu.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Da Silva Medeiros JA, Gonçalves TMFO, Boyanova L, De Correia Pereira MI, Da Silva Paiva De Carvalho JN, De Sousa Pereira AM, et al. Evaluation of Helicobacter pylori eradication by triple therapy plus Lactobacillus acidophilus compared to triple therapy alone. Eur J Clin Microbiol Infect Dis. 2011;30(4):555–9. doi: 10.1007/s10096-010-1119-4. [DOI] [PubMed] [Google Scholar]
- 64.Lionetti E, Indrio F, Pavone L, Borrelli G, Cavallo L, Francavilla R. Role of probiotics in pediatric patients with Helicobacter pylori infection: A comprehensive review of the literature. Helicobacter. 2010;15(2):79–87. doi: 10.1111/j.1523-5378.2009.00743.x. [DOI] [PubMed] [Google Scholar]
- 65.Song MJ, Park DI, Park JH, Kim HJ, Cho YK, Sohn CI, et al. The effect of probiotics and mucoprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Helicobacter. 2010;15(3):206–13. doi: 10.1111/j.1523-5378.2010.00751.x. [DOI] [PubMed] [Google Scholar]
- 66.Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: The effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Therapeut. 2010;32:1069–79. doi: 10.1111/j.1365-2036.2010.04457.x. [DOI] [PubMed] [Google Scholar]
- 67.Boonyaritichaikij S, Kuwabara K, Nagano J, Kobayashi K, Koga Y. Long-term administration of probiotics to asymptomatic pre-school children for either the eradication or the prevention of Helicobacter pylori infection. Helicobacter. 2009;14(3):202–7. doi: 10.1111/j.1523-5378.2009.00675.x. [DOI] [PubMed] [Google Scholar]
- 68.Sachdeva A, Rawat S, Nagpal J. Efficacy of fermented milk and whey proteins in Helicobacter pylori eradication: A review. World J Gastroenterol. 2014;20(3):724–37. doi: 10.3748/wjg.v20.i3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: A systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21(1):45–53. doi: 10.1097/MEG.0b013e32830d0eff. [DOI] [PubMed] [Google Scholar]
- 70.Okuda M, Nakazawa T, Yamauchi K, Miyashiro E, Koizumi R, Booka M, et al. Bovine lactoferrin is effective to suppress Helicobacter pylori colonization in the human stomach: a randomized, double-blind, placebo controlled study. J Infect Chemother. 2005;11:265–9. doi: 10.1007/s10156-005-0407-x. [DOI] [PubMed] [Google Scholar]
- 71.Okuda M, Nakazawa T, Yamauchi K, et al. Bovine lactoferrin is effective to suppress Helicobacter pylori colonization in the human stomach: a randomized, double-blind, placebo controlled study . J Infect Chemother. 2005;11:265–9. doi: 10.1007/s10156-005-0407-x. [DOI] [PubMed] [Google Scholar]
- 72.Zullo A, De Francesco V, Scaccianoce G, Hassan C, Panarese A, Piglionica D, et al. Quadruple therapy with lactoferrin for Helicobacter pylori eradication: a randomised, multicentre study. Dig Liver Dis. 2005;37:496–500. doi: 10.1016/j.dld.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 73.Guttner Y, Windsor HM, Viiala CH, Marshall BJ. Human recombinant lactoferrin is ineffective in the treatment of human Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:125–9. doi: 10.1046/j.1365-2036.2003.01395.x. [DOI] [PubMed] [Google Scholar]
- 74.Fahey JW, Stephenson KK, Wade KL, Talalay P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochem Biophys Res Commun. 2013;435:1–7. doi: 10.1016/j.bbrc.2013.03.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bahadoran Z, Mirmiran P, Yeganeh MZ, Hosseinpanah F, Zojaji H, Azizi F. Complementary and alternative medicinal effects of broccoli sprouts powder on Helicobacter pylori eradication rate in type 2 diabetic patients: A randomized clinical trial. J Funct Foods. 2014;7(1):390–7. [Google Scholar]
- 76.Opekun AR, Yeh CW, Opekun JL, Graham DY. In vivo tests of natural therapy, Tibetan yogurt or fresh broccoli for Helicobacter pylori infection. Methods Find Exp Clin Pharmacol. 2005;27:327–9. doi: 10.1358/mf.2005.27.5.896760. [DOI] [PubMed] [Google Scholar]
- 77.Hara M, Hanaoka T, Kobayashi M, Otani T, Yukari A, Montani A, et al. Cruciferous vegetables, mushrooms, and gastrointestinal cancer risks in a multicenter, hospital-based case-control study in Japan. Nutr Cancer. 2003;46:138–47. doi: 10.1207/S15327914NC4602_06. [DOI] [PubMed] [Google Scholar]
- 78.Graham DY, Anderson S-Y, Lang T. Garlic or jalapeño peppers for treatment of Helicobacter pylori infection. Am J Gastroenterol. 1999;94:1200–2. doi: 10.1111/j.1572-0241.1999.01066.x. [DOI] [PubMed] [Google Scholar]
- 79.Mhaskar RS, Ricardo I, Azliyati A, Laxminarayan R, Amol B, Santosh W, Boo K. Assessment of risk factors of Helicobacter pylori infection and peptic ulcer disease. J Global Infect Dis. 2013;5(2):60–7. doi: 10.4103/0974-777X.112288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gotteland M, Andrews M, Toledo M, Munoz L, Caceres P, Anziani A, et al. Modulation of Helicobacter pylori colonization with cranberry juice and Lactobacillus johnsonii La1 in children. Nutrition. 2008;24:421–426. doi: 10.1016/j.nut.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 81.Zhang L, Ma J, Pan K, Go VL, Chen J, You WC. Efficacy of cranberry juice on Helicobacter pylori infection: a double-blind, randomized placebo-controlled trial. Helicobacter. 2005;10:139–45. doi: 10.1111/j.1523-5378.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 82.Frieri G, Pimpo MT, Palombieri A, Melideo D, Marcheggiano A, Caprilli R, et al. Polyunsaturated fatty acid dietary supplementation: an adjuvant approach to treatment of Helicobacter pylori infection. Nutr Res. 2000;20:907–16. [Google Scholar]
- 83.Meier R, Wettstien A, Drewe J, Geiser HR. Fish oil (Eicosapen) is less effective than metronidazole, in combination with pantoprazole and clarithromycin, for Helicobacter pylori eradication. Aliment Pharmacol Therapeut. 2001;15:851–5. doi: 10.1046/j.1365-2036.2001.00989.x. [DOI] [PubMed] [Google Scholar]
- 84.Donatini B. Randomized study comparing the efficacy of hericium versus essential oils against Helicobacter pylori (HP) infection. [Étude randomisée comparant l’efficacité d’Hericium erinaceus versus huiles essentielles sur Helicobacter pylori (HP)] Phytotherapie. 2014;12(1):3–5. [Google Scholar]
- 85.Dabos KJ, Sfika E, Vlatta LJ, Giannikopoulos G. The effect of mastic gum on Helicobacter pylori: A randomized pilot study. Phytomedicine. 2010;17 :296–9. doi: 10.1016/j.phymed.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 86.Bebb JR, Bailey-Flitter N, Ala’Aldeen D, Atherton JC. Mastic gum has no effect on Helicobacter pylori load in vivo. J Antimicrob Chemother. 2003;52:522–3. doi: 10.1093/jac/dkg366. [DOI] [PubMed] [Google Scholar]
- 87.Nir Y, Potasman I, Stermer E, Tabak M, Neeman I. Controlled trial of the effect of cinnamon extract on Helicobacter pylori. Helicobacter. 2000;5:94–7. doi: 10.1046/j.1523-5378.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 88.Keenan JI, Salm N, Wallace AJ, Hampton MB. Using food to reduce H. pylori-associated inflammation. Phytotherapy Res. 2012;26(11):1620–5. doi: 10.1002/ptr.4618. [DOI] [PubMed] [Google Scholar]
- 89.Osato MS, Reddy SG, Graham DY. Osmotic effect of honey on growth and viability of Helicobacter pylori. Dig Dis Sci. 1999;44:462–4. doi: 10.1023/a:1026676517213. [DOI] [PubMed] [Google Scholar]
- 90.Namias N. Honey in the management of infections. Surg Infect. 2003;4:219–26. doi: 10.1089/109629603766957022. [DOI] [PubMed] [Google Scholar]
- 91.Miorin PL, Levy NCJ, Custodio AR, Bretz WA, Marcucci MC. Antibacterial activity of honey and propolis from Apis mellifera and Tetragonisca angustula against Staphylococcus aureus. J Appl Microbiol. 2003;95:913–20. doi: 10.1046/j.1365-2672.2003.02050.x. [DOI] [PubMed] [Google Scholar]
- 92.Cooper RA, Molan PC, Harding KG. Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J Royal Soc Med. 1999;92:283–5. doi: 10.1177/014107689909200604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molan P. Honey: Antimicrobial actions and role in disease management. In: Ahmad I, Aqil F, editors. New Strategies Combating Bacterial Infection. Weinheim: Wiley VCH; 2009. pp. 229–253. [Google Scholar]
- 94.Keenan JI, Salm N, Hampton MB, Wallace AJ. Individual and combined effects of foods on Helicobacter pylori growth. Phytotherapy Res. 2010;24(8):1229–33. doi: 10.1002/ptr.3167. [DOI] [PubMed] [Google Scholar]
- 95.Al Somal N, Coley KE, Molan PC, Hancock BM. Susceptibility of Helicobacter pylori to the antibacterial activity of manuka honey. J Royal Soc Med. 1994;87:9–12. doi: 10.1177/014107689408700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Banskota AH, Tezuka Y, Adnyana IK, et al. Hepatoprotective and anti-Helicobacter pylori activities of constituents from Brazilian propolis. Phytomedicine. 2001;8:16–23. doi: 10.1078/0944-7113-00004. [DOI] [PubMed] [Google Scholar]
- 97.Boyanova L, Derejian S, Koumanova R, et al. Inhibition of Helicobacter pylori growth in vitro by Bulgarian propolis: preliminary report. J Med Microbiol. 2003;52:417–9. doi: 10.1099/jmm.0.04895-0. [DOI] [PubMed] [Google Scholar]
- 98.Gill HS. Probiotics to enhance anti-infective defences in the gastrointestinal tract. Best Practice Res Clin Gastroenterol. 2003;17:755–73. doi: 10.1016/s1521-6918(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 99.Pagliaro G, Battino M. The use of probiotics in gastrointestinal diseases. Mediterr J Nutr Metab. 2010;3:105–13. [Google Scholar]
- 100.Felley C, Michetti P. Probiotics and Helicobacter pylori. Best Practice Res Clin Gastroenterol. 2003;17:785–91. doi: 10.1016/s1521-6918(03)00070-2. [DOI] [PubMed] [Google Scholar]
- 101.Johnson-Henry KC, Mitchell DJ, Avitzur Y, Galindo-Mata E, Jones NL, Sherman PM. Probiotics reduce bacterial colonization and gastric inflammation in H.pylori-infected mice. Dig Dis Sci. 2004;49:1095–102. doi: 10.1023/b:ddas.0000037794.02040.c2. [DOI] [PubMed] [Google Scholar]
- 102.Dang Y, Reinhardt JD, Zhou X, Zhang G. The effect of probiotics supplementation on Helicobacter pylori eradication rates and side effects during eradication therapy: A meta-analysis. PLoS ONE. 2014;9(11):e111030. doi: 10.1371/journal.pone.0111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sgouras D, Maragkoudakis P, Petraki K, Martinez-Gonzalez B, Eriotou E, Michopoulos S, et al. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environm Microbiol. 2004;71:518–26. doi: 10.1128/AEM.70.1.518-526.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamilton-Miller JMT. The role of probiotics in the treatment and prevention of Helicobacter pylori infection. Int J Antimicrob Agents. 2003;22:360–6. doi: 10.1016/s0924-8579(03)00153-5. [DOI] [PubMed] [Google Scholar]
- 105.Dial EJ, Hall LR, Serna H, Romero JJ, Fox JG, Lictenbergerger LM. Antibiotic properties of bovine lactoferrin on Helicobacter pylori. J Clin Microbiol. 1998;34:2593–4. doi: 10.1023/a:1026675916421. [DOI] [PubMed] [Google Scholar]
- 106.Husson MO, Legrand D, Spik G, Leclerc H. Iron acquisition by Helicobacter pylori: importance of human lactoferrin. Infect Immunol. 1993;61:2694–7. doi: 10.1128/iai.61.6.2694-2697.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miehkle S, Reddy R, Osato MS, Ward PP, Conneely OM, Graham DY. Direct activity of recombinant human lactoferrin against Helicobacter pylori. J Clin Microbiol. 1996;34:2593–4. doi: 10.1128/jcm.34.10.2593-2594.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dial EJ, Lictenbergerger LM. Effect of lactoferrin on Helicobacter felis induced gastritis. Biochem Cell Biol. 2002;80:113–7. doi: 10.1139/o01-205. [DOI] [PubMed] [Google Scholar]
- 109.Dial EJ, Romero JJ, Headon DR, Lictenbergerger LM. Recombinant human lactoferrin is effective in the treatment of Helicobacter felis-infected mice. J Pharm Pharmacol. 2000;52:1541–6. doi: 10.1211/0022357001777595. [DOI] [PubMed] [Google Scholar]
- 110.Akedo I, Tatsuta M, Narahara H, Iishi H, Uedo N, Yano H, et al. Prevention by bovine milk against Helicobacter pylori-associated atrophic gastritis through its adherence inhibition. Hepato-Gastroenterology. 2004;51:277–81. [PubMed] [Google Scholar]
- 111.Jantscher-Krenn E, Bode L. Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatrica. 2012;64(1):83–99. [PubMed] [Google Scholar]
- 112.Yanaka A. Sulforaphane enhances protection and repair of gastric mucosa against oxidative stress via nrf2-dependent mechanisms. Front Gastrointest Res. 2012;30:170–80. [Google Scholar]
- 113.Yanaka A, Zhang S, Tauchi M, Suzuki H, Shibahara T, Matsui H, et al. Role of nrf-2 gene in protection and repair of gastric mucosa against oxidative stress. Inflammopharmacology. 2005;13:83–90. doi: 10.1163/156856005774423863. [DOI] [PubMed] [Google Scholar]
- 114.Yamada T, Wei M, Toyoda T, Yamano S, Wanibuchi H. Inhibitory effect of Raphanobrassica on Helicobacter pylori-induced gastritis in Mongolian gerbils. Food Chem Toxicol. 2014;70:107–13. doi: 10.1016/j.fct.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 115.Yanaka A, Tauchi M, Yamamoto M. Effects of sulforaphane-rich broccoli sprouts on H. pylori-infected gastric mucosa. Nippon Rinsho. 2005;63:582–6. [PubMed] [Google Scholar]
- 116.Yanaka A, Zhang S, Yamamoto M, Fahey JW. Daily intake of sulforaphane-rich broccoli sprouts improves gastritis in H. pylori infected human subjects. Cancer Epidemiol Biomark Prevent. 2005;14:2754s. [Google Scholar]
- 117.Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Nat Acad Sci USA. 2002;99:7610–5. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Haristoy X, Fahey JW, Scholtus I, Lozniewski A. Evaluation of the antimicrobial effects of several isothiocyanates on Helicobacter pylori. Planta Med. 2005;71:326–30. doi: 10.1055/s-2005-864098. [DOI] [PubMed] [Google Scholar]
- 119.Haristoy X, Angioi-Duprez K, Duprez A, Lozniewski A. Efficacy of sulforaphane in eradicating Helicobacter pylori in human gastric xenografts implanted in nude mice. Antimicrob Agents Ch. 2003;47:3982–4. doi: 10.1128/AAC.47.12.3982-3984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Galan MV, Kishan AA, Silverman AL. Oral broccoli sprouts for the treatment of Helicobacter pylori infection: a preliminary report. Dig Dis Sci. 2004;48:1088–90. doi: 10.1023/b:ddas.0000037792.04787.8a. [DOI] [PubMed] [Google Scholar]
- 121.Canizares P, Gracia I, Gomez LA, de Argila CM, de Rafael L, Garcia A. Optimization of Allium sativum solvent extraction for the inhibition of in vitro growth of Helicobacter pylori. Biotechnol Prog. 2002;18:1227–32. doi: 10.1021/bp025592z. [DOI] [PubMed] [Google Scholar]
- 122.Burger O, Weiss E, Sharon N, Tabak M, Neeman I, Ofek I. Inhibition of Helicobacter pylori adhesion to human gastric mucus by a high-molecular weight constituent of cranberry juice. Crit Rev Food Sci Nutr. 2002;42:279–84. doi: 10.1080/10408390209351916. [DOI] [PubMed] [Google Scholar]
- 123.Sharon N, Ofek I. Fighting infectious disease with inhibitors of microbial adhesion to host tissues. Crit Rev Food Sci Nutr. 2002;42:267–72. doi: 10.1080/10408390209351914. [DOI] [PubMed] [Google Scholar]
- 124.Shmuely H, Burger O, Neeman I, Yahav J, Samra Z, Niv Y, et al. Susceptibility of Helicobacter pylori isolates to the antiadhesion activity of a high-molecular-weight constituent of cranberry. Diagn Microbiol Infect Dis. 2004;50:231–5. doi: 10.1016/j.diagmicrobio.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 125.Chatterjee A, Yasmin T, Bagchi D, Stohs SJ. Inhibition of Helicobacter pylori in vitro by various berry extracts, with enhanced susceptibility to clarithromycin. Mol Cell Biochem. 2004;265:19–26. doi: 10.1023/b:mcbi.0000044310.92444.ec. [DOI] [PubMed] [Google Scholar]
- 126.Pastene E, Speisky H, Garcia A, Moreno J, Troncoso M, Figueroa G. In vitro and in vivo effects of apple peel polyphenols against Helicobacter pylori. J Agric Food Chem. 2010;58:7172–9. doi: 10.1021/jf100274g. [DOI] [PubMed] [Google Scholar]
- 127.Friedman M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J Agric Food Chem. 2014;62(26):6025–42. doi: 10.1021/jf501266s. [DOI] [PubMed] [Google Scholar]
- 128.Ghaedi M, Darvishi S, Rezaee MA, Motaharinia Y, Ghavami K, Rahmani MR. Antibacterial activity of extracts of halgho and rashe grape cultivars against Helicobacter pylori. J Pure Appl Microbiol. 2013;7(3):1895–901. [Google Scholar]
- 129.Drago L, Mombelli B, Ciardo G, De Vecchi E, Gismondo MR. Effects of three different fish oil formulations on Helicobacter pylori growth and viability: in vitro study. J Chemother. 1999;11:207–10. doi: 10.1179/joc.1999.11.3.207. [DOI] [PubMed] [Google Scholar]
- 130.Ozen F, Ekinci FY, Korachi M. The inhibition of Helicobacter pylori infected cells by Origanum minutiflorum. Indust Crops Prod. 2014;58:329–34. [Google Scholar]
- 131.Park C-G, Lee AY, Lee JH, Lee JM, Park JY, Lee S-H, et al. Biological activities of licorice f1 lines and content analysis of phytochemical constituents. Natural Prod Sci. 2014;20(3):137–45. [Google Scholar]
- 132.Park J-M, Park S-H, Hong K-S, Han Y-M, Jang S-H, Kim E-H, et al. Special licorice extracts containing lowered glycyrrhizin and enhanced licochalcone A prevented Helicobacter pylori-initiated, salt diet-promoted gastric tumorigenesis. Helicobacter. 2014;19(3):221–36. doi: 10.1111/hel.12121. [DOI] [PubMed] [Google Scholar]
- 133.Bhamarapravati S, Pendland SL, Mahady GB. Extracts of spice and food plants from Thai traditional medicine inhibit the growth of the human carcinogen Helicobacter pylori. In Vivo. 2003;17:541–4. [PubMed] [Google Scholar]
- 134.Huwez FU, Thirlwell D, Cockayne A, Ala’Aldeen DA. Mastic gums kills Helicobacter pylori. New Engl J Med. 1998;339:1946. doi: 10.1056/NEJM199812243392618. [DOI] [PubMed] [Google Scholar]
- 135.Wittschier N, Faller G, Hensel A. Aqueous extracts and polysaccharides from liquorice roots (Glycyrrhiza glabra L. ) inhibit adhesion of Helicobacter pylori to human gastric mucosa. J Ethnopharmacol. 2009;125:218–23. doi: 10.1016/j.jep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 136.Shibata H, Iimuro M, Uchiya N, Tshihiko K, Nagaoka M, Ueyama S, et al. Preventive effects of Cladosiphon fucoidan against Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2003;8:59–65. doi: 10.1046/j.1523-5378.2003.00124.x. [DOI] [PubMed] [Google Scholar]
- 137.Matsubara S, Shibata H, Ishikawa F, Yokokura T, Takahashi M, Sugimura T, et al. Suppression of Helicobacter pylori-induced gastritis by green tea extract in Mongolian gerbils. Biochem Biophys Res Commun. 2003;310:715–9. doi: 10.1016/j.bbrc.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 138.Li Y, Xu C, Zhang Q, Kiu JY, Tan RX. In vitro anti-Helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J Ethnopharmacol. 2005;98:329–33. doi: 10.1016/j.jep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 139.Ma F, Chen Y, Li J, Qing H-P, Wang J-D, Zhang Y-L, et al. Screening test for anti-Helicobacter pylori activity of traditional Chinese herbal medicines. World J Gastroenterol. 2010;16(44):5629–34. doi: 10.3748/wjg.v16.i44.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Haniandka R, Saldanha E, Sunita V, Palatty PL, Rayad R, Baliga MS. A review of the gastroprotective effects of ginger (Zingiber officinale Roscoe) Food Funct. 2013;4:845–55. doi: 10.1039/c3fo30337c. [DOI] [PubMed] [Google Scholar]
- 141.Lutay N, Nilsson I, Wadström T, Ljungh Å. Effect of heparin, fucoidan and other polysaccharides on adhesion of enterohepatic Helicobacter species to murine macrophages. Appl Biochem Biotechnol. 2011;164(1):1–9. doi: 10.1007/s12010-010-9109-7. [DOI] [PubMed] [Google Scholar]
- 142.Molan PC. Re-introducing honey in the management of wounds and ulcers - theory and practice. Ostomy Wound Mngmnt. 2002;48:28–40. [PubMed] [Google Scholar]
- 143.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 144.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon ATR, Bazzoli F, et al. Management of Helicobacter pylori infection – the Maastricht IV Florence consensus report. Gut. 2012;61:646–64. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 145.Fahey JW, Muñoz A, Matsuzaki Y, Suzuki H, Talalay P, Tauchi M, et al. Dietary amelioration of Helicobacter pylori infection: Design criteria for a clinical trial. Cancer Epidemiol Biomarkers Prevent. 2004;13:1610–6. [PubMed] [Google Scholar]
- 146.Herr I, Büchler MW. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat Rev. 2010;36(5):377–83. doi: 10.1016/j.ctrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 147.Øverby A, Zhao C-M, Chen D. Plant phytochemicals: Potential anticancer agents against gastric cancer. Curr Opinion Pharmacol. 2014;19:6–10. doi: 10.1016/j.coph.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 148.Fahey JW. Brassicas. In: Caballero B, Trujo LL, Finglas PM, editors. Encyclopedia of Food Science and Nutrition. Academic Press; London: 2003. pp. 606–13. [Google Scholar]
- 149.Fahey JW. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1 Trees for Life J. 2005;1:5. http://www.tfljournal.org/article.php/20051201124931586. [Google Scholar]
- 150.Lengsfeld C, Titgemeyer F, Faller G, Hensel A. Glycosylated compounds from okra inhibit adhesion of Helicobacter pylori to human gastric mucosa. J Ag Food Chem. 2004;52:1495–503. doi: 10.1021/jf030666n. [DOI] [PubMed] [Google Scholar]
- 151.Jayaraj AP, Tovey FI, Lewin MR, Clark CG. Duodenal ulcer prevalence: experimental evidence for the possible role of dietary lipids. J Gastroenterol Hepatol. 2000;15:610–6. doi: 10.1046/j.1440-1746.2000.02214.x. [DOI] [PubMed] [Google Scholar]
- 152.Bhandari PR. Garlic (Allium sativum L): A review of potential therapeutic applications. Int J Green Pharm. 2012;6(2):118–29. [Google Scholar]
- 153.Serafini M, Bellocco R, Wolk A, Ekstrom AM. Total antioxidant potential of fruit and vegetables and risk of gastric cancer. Gastroenterology. 2002;123:985–91. doi: 10.1053/gast.2002.35957. [DOI] [PubMed] [Google Scholar]
- 154.Krauze-Baranowska M, Majdan M, Hałasa R, Głód D, Kula M, Fecka I, Orzeł A. The antimicrobial activity of fruits from some cultivar varieties of Rubus idaeus and Rubus occidentalis. Food Funct. 2014;5(10):2536–41. doi: 10.1039/c4fo00129j. [DOI] [PubMed] [Google Scholar]
- 155.al-Shabanah OA. Effect of evening primrose oil on gastric ulceration and secretion induced by various ulcerogenic and necrotizing agents in rats. Food Chem Toxicol. 1997;35:769–75. doi: 10.1016/s0278-6915(97)00046-x. [DOI] [PubMed] [Google Scholar]
- 156.Duggan AE, Atherton JC, Cockayne A. Clarification of the link between polyunsaturated fatty acids and Helicobacter pylori-associated duodenal ulcer disease: a dietary intervention study. Br J Nutr. 1997;78:515–22. doi: 10.1079/bjn19970171. [DOI] [PubMed] [Google Scholar]
- 157.Thompson L, Cockayne A, Spiller RC. Inhibitory effect of polyunsaturated fatty acids on the growth of Helicobacter pylori: a possible explanation of the effect of diet on peptic ulceration. Gut. 1994;35:1557–61. doi: 10.1136/gut.35.11.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Khulusi S, Ahmed HA, Patel P, Mendall MA, Northfield TC. The effects of unsaturated fatty acids on Helicobacter pylori in vitro. J Med Microbiol. 1995;42:276–82. doi: 10.1099/00222615-42-4-276. [DOI] [PubMed] [Google Scholar]
- 159.Aldoori WH, Giovannucci EL, Stampfer MJ, Rimm EB, Wing AL, Willett WC. Prospective study of diet and the risk of duodenal ulcer in men. Am J Epidemiol. 1997;145:42–50. doi: 10.1093/oxfordjournals.aje.a009030. [DOI] [PubMed] [Google Scholar]
- 160.Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343–52. doi: 10.1007/s11745-003-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ito Y, Suzuki K, Ichino N, et al. The risk of Helicobacter pylori infection and atrophic gastritis from food and drink intake: a cross-sectional study in Hokkaido, Japan. Asian Pacific J Cancer Prevent. 2000;1:147–56. [PubMed] [Google Scholar]
- 162.Das UN. Hypothesis: cis-unsaturated fatty acids as potential anti-peptic ulcer drugs. Prostaglandins Leukot Essent Fatty Acids. 1998;58:377–80. doi: 10.1016/s0952-3278(98)90074-6. [DOI] [PubMed] [Google Scholar]
- 163.Petschow BW, Batema RP, Ford LL. Susceptibility of Helicobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids. Antimicrob Agents Chemother. 1996;40:302–6. doi: 10.1128/aac.40.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Petschow BW. Helicobacter. Europe: Squibb Bristol-Myers Co; 1996. Use of lauric acid or a monoglyceride of a C8–C16 fatty acid for inhibiting. esp@cenet. [Google Scholar]
- 165.Mysore JV, Wigginton T, Simon PM, Zopf D, Heman-Achah LM, Dubois A. Treatment of Helicobacter pylori infection in rhesus monkeys using a novel antiadhesion compound. Gastroenterology. 1999;117(6):1316–25. doi: 10.1016/s0016-5085(99)70282-9. [DOI] [PubMed] [Google Scholar]
- 166.Tabak M, Armon R, Rosenblat G, Stermer E, Neeman I. Diverse effects of ascorbic acid and palmitoyl ascorbate on Helicobacter pylori survival and growth. FEMS Microbiol Lett. 2003;224:247–53. doi: 10.1016/S0378-1097(03)00439-7. [DOI] [PubMed] [Google Scholar]
- 167.Phull PS, Green CJ, Jacyna MR. A radical view of the stomach: the role of oxygen-derived free-radicals and anti-oxidants in gastroduodenal disease. Eur J Gastroenterol Hepatol. 1995;7:265–74. [PubMed] [Google Scholar]
- 168.Lacy BE, Rosemore J. Helicobacter pylori: ulcers and more: the beginning of an era. J Nutr. 2001;131:2789S–93S. doi: 10.1093/jn/131.10.2789S. [DOI] [PubMed] [Google Scholar]
- 169.Yoshida T, Hatano T, Ito H. Chemistry and function of vegetable polyphenols with high molecular weights. Biofactors. 2000;13:121–5. doi: 10.1002/biof.5520130120. [DOI] [PubMed] [Google Scholar]
- 170.Beil W, Birkholz C, Sewing KF. Effects of flavonoids on parietal cell acid secretion, gastric mucosal prostaglandin production and Helicobacter pylori growth. Arzneimittelforschung. 1995;45:697–700. [PubMed] [Google Scholar]
- 171.Bae E-A, Han MJ, Kim D-H. In vitro anti-Helicobacter pylori activity of some flavonoids and their metabolites. Planta Med. 1999;6:442–3. doi: 10.1055/s-2006-960805. [DOI] [PubMed] [Google Scholar]