Summary
Streptococcus mutans, a key etiological agent of the human dental caries, lives primarily on the tooth surface in tenacious biofilms. The SMU864 locus, designated pdxR, is predicted to encode a member of the novel MocR/GabR family proteins, which are featured with a winged helix DNA-binding N-terminal domain and a C-terminal domain highly homologous to the pyridoxal phosphate-dependent aspartate aminotransferases. A pdxR-deficient mutant, TW296, was constructed using allelic exchange. PdxR deficiency in S. mutans had little effect on cell morphology and growth when grown in brain heart infusion. However, when compared with its parent strain, UA159, the PdxR-deficient mutant displayed major defects in acid tolerance response and formed significantly fewer biofilms (P < 0.01). When analyzed by realtime polymerase chain reaction, PdxR deficiency was found to drastically reduce expression of an apparent operon encoding a pyridoxal kinase (SMU865) and a pyridoxal permease (SMU866) of the salvage pathway of vitamin B6 biosynthesis. In addition, PdxR deficiency also altered the expression of genes for ClpL protease, glucosyl-transferase B and adhesin SpaP, which are known to play important roles in stress tolerance and biofilm formation. Consistently, PdxR-deficiency affected the growth of the deficient mutant when grown in defined medium with and without vitamin B6. Further studies revealed that although S. mutans is known to require vitamin B6 to grow in defined medium, B6 vitamers, especially pyridoxal, were strongly inhibitory at millimolar concentrations, against S. mutans growth and biofilm formation. Our results suggest that PdxR in S. mutans plays an important role in regulation of vitamin B6 metabolism, acid tolerance response and biofilm formation.
Keywords: dental caries, MocR/GabR family proteins, Streptococcus mutans, vitamin B6
Introduction
Streptococcus mutans, a key aetiological agent of dental caries, lives primarily on the tooth surface in a dynamic environment that has frequent, often drastic, fluctuations in conditions, such as the source and availability of nutrients, pH, temperature, oxygen tension and antimicrobial agents (Burne, 1998). This bacterium is known for its ability to colonize the tooth surface, to survive and adapt to various environmental conditions, and to become numerically significant under certain conditions, leading to carious lesions (Burne, 1998; Lemos & Burne, 2008). Besides molecular chaperones and the Clp proteases (Lemos et al., 2001; Lemos & Burne, 2008; Kajfasz et al., 2009), multiple networks including two-component signal transduction systems (Ahn et al., 2006; Biswas et al., 2008; Senadheera et al., 2009; Suntharalingam et al., 2009; Burne et al., 2011; Stipp et al., 2013), the Com system (Li et al., 2002b), AI-2 mediated signaling (Wen & Burne, 2004; Wen et al., 2011), and cell envelope associated transcriptional regulator BrpA (Wen et al., 2006; Bitoun et al., 2012a), have been shown to play integral roles in survival and adaptation to low pH, reactive oxygen species and cell envelope stresses and are required for biofilm formation by S. mutans.
The MocR/GabR family proteins belong to the GntR super-family of transcriptional regulators (Rigali et al., 2002). They are chimeric proteins that incorporate a short N-terminal helix-turn-helix-containing domain with DNA-binding properties (Rigali et al., 2002; Lee et al., 2003; Wiethaus et al., 2008) and a long C-terminal domain closely related in amino acid sequence to full-length aminotransferases or to the pyridoxal-5′-phosphate (PLP)-binding domain of aminotransferases (Schneider et al., 2000; Belitsky & Sonenshein, 2002; Wiethaus et al., 2008; Bramucci et al., 2011; Edayathumangalam et al., 2013). GabR in Bacillus subtilis is the first member of the family shown to function as a transcriptional regulator (Belitsky & Sonenshein, 2002; Belitsky, 2004; Edayathumangalam et al., 2013). In the presence of γ-aminobutyric acid, GabR activates transcription of the gabTD operon. It also negatively regulates the divergently transcribed gabR gene. In vitro transcription reactions showed that GabR alone repressed expression from the gabR promoter but activated expression from the gabT promoter only in the presence of gamma-aminobutyric acid and PLP (Belitsky, 2004). MocR in Sinorhizobium meliloti is known to be required for rhizopine utilization (Rossbach et al., 1994), although no further information is available. PdxR, a MocR/GabR homologue in Corynebacterium glutamicum, controls expression of divergently oriented pdxST genes coding for the subunits of PLP synthase whose deficiency caused vitamin B6 auxotrophy in this bacterium (Jochmann et al., 2011). Similar results were also recently reported in Listeria monocytogenes (Belitsky, 2014). However, the presence and the possible role of the aminotransferase domain in MocR/GabR-like proteins remain unclear (Belitsky, 2004; Bramucci et al., 2011).
Pyridoxal (PL), pyridoxamine (PM) and pyridoxine (PN) are collectively referred to as vitamin B6, although PLP is considered as the only active form (Fitzpatrick et al., 2007; El Qaidi et al., 2013). PLP is an essential cofactor of numerous enzymes catalyzing a variety of biochemical reactions primarily in amino acid metabolism (Fitzpatrick et al., 2007; El Qaidi et al., 2013). Besides, PLP is also shown to function as an inhibitor of PdxR-mediated activation of the deoxyxylulose 5-phosphate (DXP)-independent pathway of vitamin B6 biosynthesis in Streptococcus pneumoniae and Corynebacterium glutamicum (Fitzpatrick et al., 2007; Jochmann et al., 2011; El Qaidi et al., 2013) and GabR-regulated utilization of γ-aminobutyrate in B. subtilis (Belitsky & Sonenshein, 2002; Belitsky, 2004).
The SMU864 locus, herein designated pdxR, is annotated as a member of the GntR superfamily of regulatory proteins. As revealed by BLAST search, the pdxR product contains, in its N-terminal region, a GntR-type of winged helix-turn-helix DNA-binding domain and a large C-terminal domain that is similar to the PLP-dependent aspartate aminotransferase (AST). It was found recently by DNA microarray analysis to be down-regulated more than twofold in response to deficiency of LuxS, an enzyme known to be responsible for AI-2-mediated signaling in S. mutans and many other bacterial species (Wen & Burne, 2004; Wen et al., 2011). In this study, a PdxR-deficient mutant was constructed by allelic replacement. Characterization of the resulting mutant revealed that PdxR is required for the expression of PL kinase, PdxK and PL permease, PdxU. Deficiency of PdxR affected growth of the deficient mutant when grown in defined medium and caused major defects in acid tolerance response and biofilm formation. These results suggest that PdxR in S. mutans plays an important role in vitamin B6 and amino acid metabolism and in regulation of stress tolerance response and biofilm formation.
Methods
Plasmids, bacterial strains and growth conditions
Streptococcus mutans UA159 and its derivatives used in this study are listed in Table 1. They were maintained in brain heart infusion (BHI, Difco Laboratories, Detroit, MI). For growth characterization, chemically defined medium FMC was also used (Terleckyj et al., 1975). For biofilm growth, a modified semi-defined biofilm medium (BM) was used with glucose (20 mM, BMG), sucrose (10 mM, BMS), and glucose plus sucrose (18 and 2 mM, respectively) (BMGS) as the supplemental carbohydrate and energy sources (Loo et al., 2000; Li et al., 2002a; Wen & Burne, 2002b). All solid media were prepared similarly, but agar (Difco Laboratories) was added at a concentration of 1.5% (w/v). When needed, kanamycin (1 mg ml−1) and/or spectinomycin (1 mg ml−1) were added. Unless stated otherwise, S. mutans strains were grown at 37°C in an aerobic chamber with 5% CO2. For growth studies, Bioscreen C (Oy Growth Curves AB Ltd, Finland) was also used to culture cells at 37°C with and without an overlay of mineral oil, and the optical density (OD) at 600 nm was monitored automatically every 30 minutes following moderate shaking for 10 seconds. Escherichia coli strains were grown in Luria–Bertani medium, with or without the addition of kanamycin (40 μg ml−1) and/or ampicillin (100 μg ml−1) (Table 1).
Table 1. Bacterial strains, plasmids and primers used in this study.
| Strains/plasmid | Relevant characteristics | References/sources | |
|---|---|---|---|
| S. mutans UA159 | Wild-type | Ajdic et al. (2002) | |
| S. mutans TW296 | ΔSMU864, Kanr | This study | |
| S. mutans TW296C | TW296/pDL278:pdxR, Kanr and Spcr | This study | |
| S. mutans TW372 | ΔpdxK, Kanr | This study | |
| S. mutans TW373 | ΔpdxU, Spcr | This study | |
| S. mutans TW374 | ΔpdxKU, Spcr | This study | |
| E. coli BL2 | New England Biolabs | ||
| pDL278 | Shuttle vector, Spcr | LeBanc & Lee (1991) | |
| pMALc2x | Expression vector | New England Biolabs | |
|
| |||
| Primers | Sequence (5′–3′) | Sequence (5′–3′) | Application |
|
| |||
| pdxR 5F | 55-atccaagtatcattcaaacgattc | 53-agaaaactggcagaattcgctaac | 5′, pdxR for mutation |
| pdxR 3F | 35-tgcagtggaattcttggctcagttg | 33-tcaagtaaatgcggttcataacaaagac | 3′, pdxR for mutation |
| pdxR C | C5-actagactgaggatccttctc | C3-agtaagattgaattctcaccaaagagc | For complementation |
| pdxR E | E5: ggacggatccgttagtaaatatcatgaa | E3: tcaccaaagagctctagtacttttaag | For SMU.953 expression |
| 16S-1 rRNA | Fw: cacaccgcccgtcacacc | Rv: cagccgcaccttccgatacg | 16S rRNA qPCR, 160 bp |
| pdxR | Fw: cagccaacaagccctttacatcc | Rv: caaggtgttcatgcgatggtagg | pdxR qPCR, 100 bp |
| gtfB | Fw: agcaatgcagccatctacaaat | Rv: acgaactttgccgttattgtca | gtfB qPCR, 98 bp |
| gtfC | Fw: atggcgacaatatgatta | Rv: cggatgaaggaataagaa | gtfC qPCR, 172 bp |
| gtfD | Fw: tgacttctgttcgttatg | Rv: ggttattgctggtaatga | gtfD qPCR, 98 bp |
| SMU860 | Fw: attatccgtgaagaacta | Rv: tgattatagacagcaact | SMU860 qPCR, 143 bp |
| SMU861 | Fw: acaatgcttctattcttctt | Rv: cctaacagcgtattgataa | SMU861 qPCR, 124 bp |
| SMU862 | Fw: ttgttgtagtctggttgt | Rv: atagcataatcgtaatgtaagg | SMU862 qPCR, 135 bp |
| SMU863 | Fw: atagaagaagtggctgaa | Rv: tatctgttatatcggcaatc | SMU863 qPCR, 118 bp |
| pdxK | Fw: ttgaagcagataagattggtt | Rv: taagcggaagacaggatt | pdxK qPCR, 133 bp |
| pdxU | Fw: ttagaatcactgcttgtt | Rv: attgagaataatcttcgttac | pdxU qPCR, 126 bp |
| SMU867 | Fw: cggctatgatgataacag | Rv: ttgaaggagaagagtgat | SMU867 qPCR, 118 bp |
Kanr and Spcr, for kanamycin and spectinomycin resistance, respectively. Sequences underlined are restriction sites engineered for cloning.
Construction of mutants and complement strains
Streptococcus mutans strains deficient for pdxR were generated using a polymerase chain reaction (PCR)-ligation-mutation strategy as described elsewhere (Lau et al., 2002; Wen & Burne, 2004). Briefly, a 990-bp fragment 5′ of pdxR and a 30 fragment of 1010 bp were amplified by PCR using high-fidelity DNA polymerase Phusion (New England Biolabs, Ipswich, MA) with gene specific primers (Table 1). Following proper digestions to generate compatible ends, the PCR amplicons were ligated with a non-polar kanamycin resistance cassette (aphA encoding aminoglycoside 3′-phosphotransferase) (Zeng et al., 2006), and the resulting ligation mix was used to transform S. mutans UA159. Putative mutants were selected on BHI-kanamycin plates, and further analyzed by PCR and DNA sequencing to verify the deficiency and sequence accuracy. For mutant complementation, the coding sequence plus its putative promoter region were amplified by PCR and directly cloned into shuttle vector pDL278 (LeBanc & Lee, 1991). Following sequence confirmation, the resulting construct, pDL278:pdxR, was transformed into the mutant, and transformants carrying with the wild-type pdxR were isolated from BHI-Spc.
Biofilm formation and microscopic analyses
For biofilm assays, S. mutans was grown in biofilm medium BMG, BMS and BMGS as described above (Loo et al., 2000; Li et al., 2002a; Wen & Burne, 2002b). For confocal laser scanning microscopy (CLSM), biofilms were grown on hydroxylapatite (HA) disks (Clarkson Chromatography Products Inc., South Williamsport, PA) that were vertically deposited in 24-well culture plates (Corning, NY) (Lemos et al., 2010) or plain glass slides deposited in 50-ml Falcon tubes (Wen et al., 2010) for 24 and 48 h, and were then stained with Live/Dead BacLight fluorescent dye (Invitrogen, Carlsbad, CA) before optical dissection using an Olympus Fluoview BX61 confocal laser scanning microscope (Olympus, Tokyo, Japan) at 6009 magnification (Bitoun et al., 2011; Wen et al., 2011). At least five random areas were scanned for each sample. For post-acquisition analysis, simulated xyz three-dimensional images were generated using SLIDEBOOK 5.0 (Olympus) (Bitoun et al., 2011; Wen et al., 2011). Quantitative analysis, such as biovolume, biomass, surface area and mean height, was carried out using COMSTAT 2.0 with at least five independent scans for each sample (Heydorn et al., 2000; Wen et al., 2011). The biovolume is defined as the volume (μm3) of the biomass per μm2 of substratum area. Field-emission-scanning electron microscopy (FE-SEM) was carried out similarly as described previously (Wen & Burne, 2004; Bitoun et al., 2011).
Acid killing and hydrogen peroxide challenge assays
To evaluate the ability of S. mutans strains to withstand acid and oxidative stress, planktonic cultures were prepared from mid-exponential phase (OD600 nm ≅ 0.5) cultures grown in BHI broth. For sessile populations, BMGS was used to support bacterial growth and glass slides were used as substratum, as detailed elsewhere (Wen et al., 2010, 2011). Acid and hydrogen peroxide killing assays were carried out as described previously (Wen & Burne, 2004; Wen et al., 2006).
Glycolytic pH drop
The glycolytic pH drop assay was carried out as described by Belli & Marquis (1991). Briefly, S. mutans strains were grown in BHI broth until mid-exponential phase (OD600 nm ≅ 0.5), washed twice with ice-cold de-ionized water by centrifugation at 2737×g at 4°C for 10 min, and the cells were then resuspended in 50 mM KCl and 1 mM MgCl2. The pH was adjusted to 7.2 with 0.1 M KOH before the addition of 50 mM glucose. The pH drop was monitored continuously for a period of 30 min.
Expression and purification of recombinant PdxR
For recombinant protein rPdxR, pdxR-coding sequence was PCR amplified with gene-specific primers (Table 1) and then directly cloned in expression vector pMAL-c2X, generating an in-frame fusion behind E. coli malE sequence that encodes an engineered maltose-binding protein domain (New England Biolabs) (Zeng et al., 2006). The resulting construct was transformed to and amplified in E. coli BL21 (New England Biolabs). Following sequence confirmation by sequencing, the fusion protein was expressed by Isopropyl β-D-1-thiogalactopyranoside induction and purified using amylose affinity columns by following the manufacturer's recommendation (New England Biolabs) (Zeng et al., 2006).
Aspartate aminotransferase assay
For AST assay, purified rPdxR was washed and reconstituted in 50 mM sodium phosphate buffer, pH 7.4, using Protein Desalting Spin Columns (#89849, Pierce Biotechnology, Rockford, IL). The S. mutans whole cell lysates were prepared from mid-exponential phase cultures grown in BHI and homogenized using a glass bead beater (Biospec, Bartlesville, OK) as described previously (Burne et al., 1999; Wen & Burne, 2002a). AST activity was measured in a coupled assay with malate dehydrogenase and aspartate plus 2-oxoglutarate as substrates (Karmen et al., 1955; Ziak et al., 1990). Malate dehydrogenase catalyzed oxidation of NADH, which is proportional to AST product, oxaloacetate, was monitored by the change of absorbance at 340 nm using a Bio-Tek microplate reader (Synergy II™; Bio-Tek, Winooski, VT). Porcine AST (Sigma, St Louis, MO) was used as a positive control, and maltose-binding protein domain (MalE) (New England Biolabs) expressed and purified using similar strategies, was used as a negative control. Protein concentration was assayed using a BCA™ protein assay kit (Pierce) as recommended by the manufacturer. AST activity was further normalized by protein concentration and is expressed as units per milligram of protein.
RNA isolation and real-time PCR assay
For RNA extraction, mid-exponential phase (OD600 nm ≅ 0.5) S. mutans cultures were treated with RNAProtect (Qiagen, Inc., Hilden, Germany), and total RNAs were extracted using hot phenol as described previously (Browngardt et al., 2004; Wen et al., 2006, 2011). To remove residual DNA, RNA samples were treated with RNase-free DNase I (Ambion, Inc., Austin, TX) and total RNA was retrieved with the RNeasy purification kit (Qiagen, Inc.). For real-time PCR analysis, cDNA was synthesized with 1 lg of total RNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) by following the procedures recommended by the supplier. Real-time PCR was carried out with a Bio-Rad iCycler using procedures detailed elsewhere (Ahn et al., 2006; Wen et al., 2006).
Statistical analysis
Comparisons between different strains and different conditions were carried out using paired Student's t-test. A difference at P < 0.05 or less is considered statistically significant.
Results
PdxR-deficiency affects vitamin B6 metabolism
Genetic analysis revealed that the pdxR (SMU864) locus is flanked by genes encoding proteins with potential roles in amino acid and vitamin B6 metabolism (see Supporting information, Fig. S1). Downstream of pdxR in opposite orientation are mmuM (SMU863) for a putative homocysteine S-methyltransferase and ybgF (SMU862) for a putative histidine permease. Upstream of pdxR are two divergently transcribed genes in an apparent operon coding for a putative PL kinase (PdxK, SMU865) and a putative PL transporter (PdxU, SMU866), respectively. A PdxR-deficient mutant, TW296, was constructed by allelic exchange using a non-polar kanamycin resistance marker (Table 1). PdxR-deficiency did not have a major effect on cell morphology under the optical microscope (data not shown) and growth rate during growth in regular BHI (Fig. 1A). Relative to the parent strain, UA159, the deficient mutant only slightly extended its lag phase and slightly reduced its final OD, as measured using Bioscreen C. However, when incubated in BHI adjusted to pH 6.0 (Fig. 1A) and lower (data not shown), the growth rate of the deficient mutant was significantly reduced when compared with the parent strain (P < 0.05). Inclusion of methyl viologen (also paraquat, Sigma) in the growth medium also slightly reduced the growth rate of the PdxR-deficient mutant compared with parent strain, UA159 (data not shown), although the differences were not statistically significant. Similar trends were also obtained when grown in chemically defined medium FMC, although the impact of PdxR-deficiency on the culture OD was much more significant (P < 0.05) (Fig. 1B). Complementation in TW296C via a shuttle vector carrying a wild-type copy of pdxR plus its cognate promoter was able to restore the phenotypes to a level similar to the wild-type during growth in both BHI (Fig. 1A) and in FMC (Fig. 1B).
Figure 1.
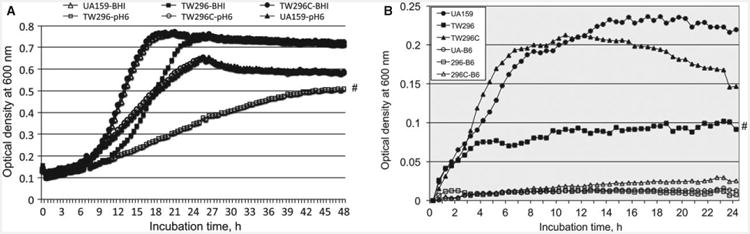
Growth studies in brain–heart infusion (BHI) (A) and chemically defined medium FMC (B). (A) Streptococcus mutans UA159, its PdxR-deficient mutant, TW296, and the complement strain, TW296C were grown in BHI adjusted to pH 7.0 and 6.0, and the culture optical density at 600 nm was continuously measured using Bioscreen C. Our results showed that PdxR-deficiency significantly affects the growth rate of the mutant, TW296 at pH 6.0. (B) Streptococcus mutans UA159, the PdxR-deficient mutant, TW296 and its complement strain, TW296C were grown in defined medium FMC with (solid symbols) and without (open symbols) inclusion of vitamin B6 at 5 μM. Results showed that S. mutans requires vitamin B6 for growth and PdxR-deficiency causes major growth defects under the conditions studied. Data presented here are representatives of three independent experiments. Symbol # indicates differences in growth rate between the wild-type, UA159 and the PdxR mutant, TW296 at P < 0.05.
PL kinase and PL permease are part of the salvage pathway of vitamin B6 biosynthesis (Fitzpatrick et al., 2007; Mukherjee et al., 2011). To further investigate the role of the pdxKU operon in vitamin B6 metabolism, mutants deficient for PdxK, PdxU, and both PdxK and PdxU were generated using similar strategies as detailed in Methods, and further evaluated using modified FMC medium with or without inclusion of B6 vitamer PL, PN, PM or PLP (all from Sigma) at 5 μM (final concentration). The results showed that mutants deficient for PdxK, PdxU or both PdxK and PdxU grew as well as the parent strain, UA159 when PL, PM, PN or PLP was the B6 vitamin (data not shown). In FMC without inclusion of B6 vitamers, both S. mutans UA159 and the PdxR mutant were able to continue to grow albeit at extremely low rates, yielding very limited increases in optical density (Fig. 1B and see Supporting information, Fig. S2). Similar, but slightly better performance was seen with the complement strain. In contrast, however, all three PdxK-, PdxU- and PdxKU-deficient mutants displayed significantly better growth rates and final ODs (P < 0.001) (Fig. S2).
The PdxR-deficiency affects acid tolerance response
Reduction of growth rate during growth in BHI adjusted to lower pH indicates a reduced glycolytic activity and/or weakened acid tolerance. When analyzed using glycolytic pH drop experiments, it was shown that relative to wild-type, the rate (slope) of glucose-induced pH drop of the PdxR-deficient mutant was significantly reduced and the resting pH of the PdxR mutant was significantly higher (P < 0.05) (Fig. 2). As expected, complementation of the mutant with the wild-type coding sequence plus its cognate promoter region in TW296C was able to partly restore these phenotypes to levels more resembling the wild-type. When analyzed by acid-killing by incubation in a buffer of pH 2.8, PdxR-deficient mutant, TW296, displayed more than 1-log reduction in survival rate (P < 0.001) compared with the wild-type, UA159 (Fig. 3). Complementation with the wild-type copy of pdxR in trans increased the resistance moderately, but not to a level similar to the wild-type. When subjected to oxidative challenge by incubation in buffer containing 0.2% (or 58 mM) of hydrogen peroxide, no significant differences were measured between UA159 and TW296 (data not shown), which is consistent with results of the growth study with inclusion of paraquat (data not shown).
Figure 2.
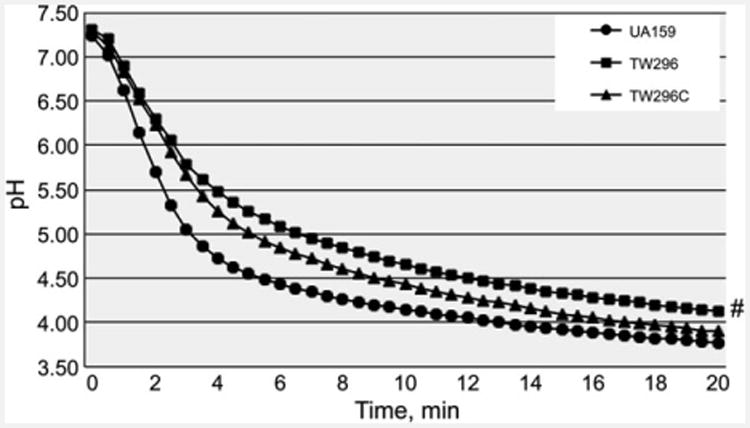
Glycolytic pH drop. Results of pH drop experiments showed that relative to Streptococcus mutans UA159, the PdxR-deficient mutant, TW296, had a slower pH drop and a higher resting pH after 20 min (#P < 0.05). Complementation with wild-type pdxR in TW296C was able to partly restore the phenotype to the wild-type, UA159.
Figure 3.
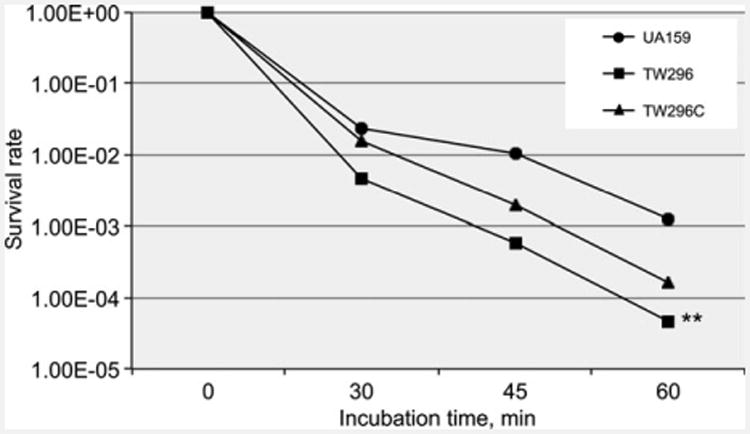
Acid killing assay. Panel shows survival rates of sessile populations of S. mutans wild-type UA159, PdxR-deficient mutant TW296, and its complement strain, TW296C, after incubation in buffer of pH 2.8 for periods of time indicated. Results showed that PdxR-deficiency significantly reduced the ability of the deficient mutant to survive low pH challenge (**P < 0.001), compared with the parent strain, UA159.
PdxR-deficiency reduces biofilm formation
No significant differences in growth rate were measured when grown planktonically in BM medium (data not shown). However, when compared with the parent strain, UA159, the PdxR-deficient mutant, TW296, showed a significant reduction in its ability to form biofilms. During biofilm growth in BMG, the PdxR-deficient mutant, TW296 formed < 43% biofilms (P < 0.05) after 24 h compared with the parent strain, with TW296 averaging 1.9E7 colony-forming-units (CFU) vs. 3.4E7 CFU for the parent strain, UA159 (P < 0.05). When grown in BMGS, the mutant biofilms decreased by >62% averaging 2.35E7 CFU, compared with 6.15E7 CFU for the parent strain (P < 0.05). When analyzed using FE-SEM, S. mutans UA159 developed substantial biofilms after 24 h (Fig. 4A). In contrast, the mutant biofilms were flat and tight and contained little or no glucans (Fig. 4A). Similar results were also obtained using CLSM (Fig. 4B). CLSM also showed that complementation with a wild-type copy of the genes plus its cognate promoter was able to restore the ability to form biofilms to a level similar to the wild-type. As revealed by comstat analysis, the wild-type and the PdxR-deficient mutant biofilms did not have major differences in surface areas (data not shown), suggesting that the PdxR-deficient mutant colonized similarly well on the HA disks. The biovolume of the PdxR-deficient mutant biofilms was reduced, averaging only 1.14 (± 1.04) μm3 μm−2, compared with 5.73 (± 3.9) μm3 μm−2 for the parent strain, although the differences were not statistically significant (P = 0.06). When analyzed using colorimetric assay (Bitoun et al., 2012b), the PdxR-deficient mutant, TW296, had only 11.49 (± 0.46) ng μl−1 of glucans (Fig. 4C), compared with 49.18 (± 5.42) (P < 0.01) ng μl−1 of the wild-type, UA159, and 31.67 (± 5.09) ng μl−1 (P<0.05) by the complement strain, TW296C. These results corroborate well with what were seen under both FE-SEM and CLSM.
Figure 4.
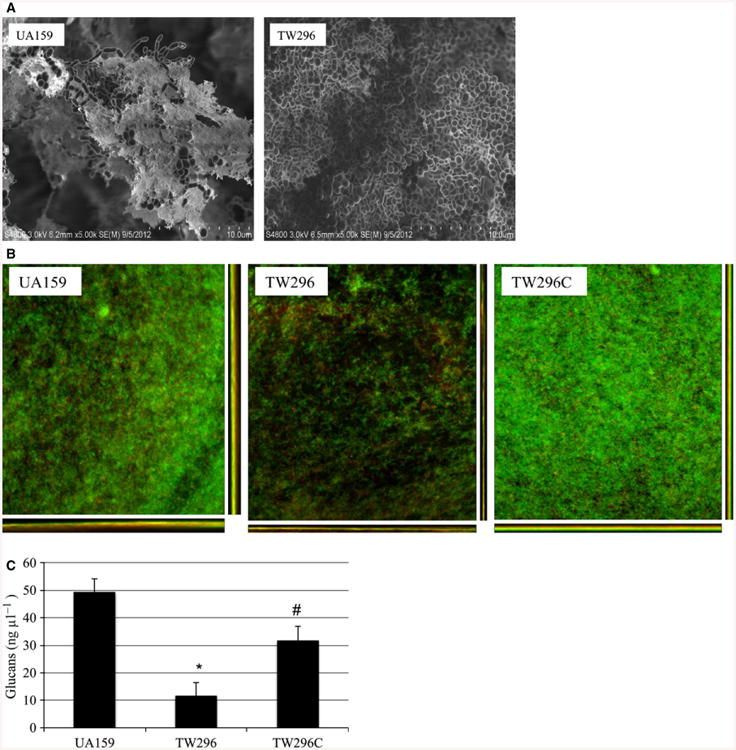
Field-emission-scanning electron microscopy (FE-SEM) (A) and confocal laser scanning microscopy (CLSM) (B) analysis of biofilms and Glucan measurement (C). (A) For FE-SEM analysis, Streptococcus mutans wild-type UA159 and the PdxR mutant, TW296 were grown in biofilm medium (BM) sucrose on hydroxylapatite (HA) disks for 24 h. Relative to the wild-type, biofilms of the PdxR-deficient mutant appeared to be tight and flat, and contained little or no extracellular glucans. Images were taken at 5000×. (B) CLSM images show 24-h biofilms grown on HA disks with BM glucose and stained with Live/Dead Baclight bacterial viability kit (Invitrogen). Results showed PdxR-deficiency significantly decreased biofilm formation. Images presented here are representatives of xyz, xz and yz images (512 × 512). (C) Glucans in the biofilms of different strains were measured using a phenol–sulfuric acid assay. The results showed that relative to the wild-type, PdxR-deficiency in TW296 significantly reduced glucan production (*P < 0.01). Complementation in TW296C partly restored the production (#P < 0.05).
PdxR-deficiency affects expression of genes in vitamin B6 metabolism
Real-time PCR was used to analyze the effect of PdxR-deficiency on the expression of flanking genes and those known to be associated with biofilm formation. Of the genes downstream pdxR, pdxK and pdxU were found to be down-regulated by 15.30-fold and 3.80-fold, respectively. Relative to the wild-type, transcription of clpL (SMU867), for a ClpL protease, was also down-regulated by more than seven-fold. Of the upstream genes analyzed, ybgF (SMU861) for an S-methyl methionine transporter, and mmuM (SMU863) for a putative homocysteine S-methyltransferase were upregulated by 1.87-fold and 3.37-fold, respectively. Of the genes known to be critical to S. mutans biofilm formation, spaP, for high-affinity adhesin SpaP, was found to be upregulated fourfold, and gtfB, for glucosyltransferase B, was increased by 3.94-fold in the PdxR-deficient mutant, relative to the wild-type, UA159 (Table 2). No differences were measured in expression of gtfC and gbpB.
Table 2. Analysis of selected genes by real-time polymerase chain reaction.
| Gene/locus | Description and putative function1 | UA1592 | TW2962 | Ratio (TW/UA)3 | P value |
|---|---|---|---|---|---|
| SMU860 | Putative ATP-dependent Clp protease, subunit X | 1.17E+06 | 9.88E+05 | 1.18 | >0.05 |
| SMU861 | Putative GTP-binding protein | 8.61E+05 | 9.89E+05 | 1.15 | >0.05 |
| SMU862 | Putative methionine permease | 3.70E+06 | 1.96E+06 | 1.87 | <0.01 |
| SMU863 | Putative homocysteine S-methyltransferase | 7.18E+05 | 2.46E+06 | 3.37 | <0.01 |
| SMU865 | Putative pyridoxal kinase, PdxK | 2.80E+06 | 1.83E+05 | –15.3 | <0.001 |
| SMU.866 | Putative pyridoxal transporter, PdxU | 1.75E+06 | 4.60E+05 | –3.80 | <0.001 |
| SMU867 | ATP-dependent Clp protease, ATP-binding subunit | 2.08E+06 | 2.96E+5 | –7.01 | <0.001 |
| GbpB | Glucan binding protein B | 6.22E+07 | 5.8E+07 | 1.07 | >0.05 |
| SpaP | Multi-function adhesin, also AgI/II and P1 | 4.42E+06 | 1.76E+07 | 4.0 | <0.001 |
| GtfC | Glucosyltransferase C | 3.16E+07 | 3.95E+07 | 1.25 | >0.05 |
| GtfB | Glucosyltransferase B | 1.86E+06 | 7.33E+06 | 3.94 | <0.001 |
Description and putative function of the selected genes are based upon the published Streptococcus mutans database.
Expressed as copy number of respective transcripts per lg of total RNA. Real-time PCR were repeated at least three times using total RNA from four separate sets of cultures. Data presented here are average of different sets of experiments.
Defined as levels of expression in the SMU864-deficient mutant, TW296, relative to those of the wild-type, UA159, with “–” indicating downregulation.
B6 vitamers affect growth and biofilm formation
It is well documented that micromolar vitamin B6 is required for S. mutans to grow in chemically defined medium (Carlsson, 1970; Terleckyj et al., 1975). However, there is also evidence that pyridine analogs, including B6 vitamers, could reduce glucose uptake and inhibit glucosyltransferase activity (Thaniyavarn et al., 1982). In an effort to further investigate the effects of vitamin B6 on growth and biofilm formation by S. mutans, various concentrations of PL, PM and PN were included in BHI for growth studies and in BMGS for biofilm formation on 96-well plates. Our studies so far have shown that PL, PM and PN all displayed inhibitory effects against S. mutans growth and biofilm formation, and such effects were concentration dependent. Of the B6 vitamers tested, PL was the most potent with a minimal inhibitory concentration (MIC) at 10 mM (Fig. 5A). No effect on growth was measured at 0.75 mM and below. No biofilms were measured when S. mutans was grown with PL at 5 mM or above (Fig. 5B). However, relative to the control that received no PL, more than 40% (P < 0.05) reduction of biofilms was still measured at 0.16 mM. Similar effects on growth and biofilm formation were also seen with PM and PN, albeit to a much lesser degree (see Supporting information, Fig. S3–S6).
Figure 5.
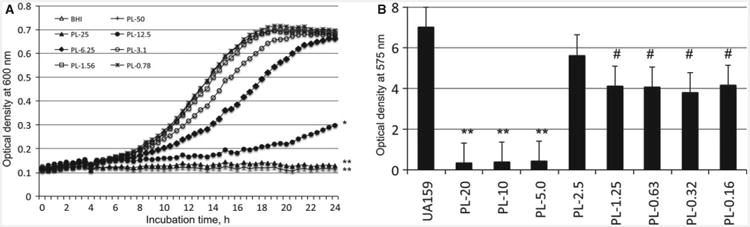
Effects of pyridoxal (PL) on growth (A) and biofilm formation (B). For growth effects, Streptococcus mutans was grown in brain heart infusion broth with different concentrations (mM) of PL, and the culture optical density was continuously monitored using Bioscreen C. For biofilm formation, S. mutans was grown in biofilm medium with glucose and sucrose (BMGS) with different concentrations of PL (mM) using 96-well plates. After 24 h, biofilms were stained with 0.1% crystal violet and then measured by spectrophotometry. Symbols **, * and # indicate differences in growth rate (A) or biofilms (B) at P < 0.001, P < 0.01 and P < 0.05, respectively, when compared with controls that received no PL.
Discussion
Unlike S. pneumoniae and many others, S. mutans lacks the DXP-independent de novo pathway of vitamin B6 biosynthesis, but does contain the salvage pathway(s) and two genes encoding enzymes of the DXP-dependent de novo pathway can be identified in the genome (http://www.genome.jp/kegg-bin/show_pathway?smu00750) (Ajdic et al., 2002; El Qaidi et al., 2013). In the salvage pathway, PL kinase catalyzes the transfer of a phosphate group from ATP to the 5-hydroxylmethyl group of B6 vitamers, leading to the generation of PLP (Fitzpatrick et al., 2007; Mukherjee et al., 2011). It is apparent that PdxR-deficiency drastically reduces the expression of PdxK (SMU865) and PdxU (SMU866), although the deficient mutant was still capable of growing in defined medium FMC with the B6 vitamers tested, albeit in a reduced growth rate. Interestingly and surprisingly, the PdxK and PdxU single and double mutants grew similarly well compared with the wild-type in FMC with inclusion of B6 vitamers but grew a lot better than the wild-type in medium without addition of B6 vitamers. A BLAST search of the genome database (www.theseed.org) revealed that S. mutans possesses at least another two-gene operon in loci SMU77 and SMU78 that are predicted to code for a PL kinase, PdxK2 (SMU77) of the ThiD family and a PL permease, PdxU2 (SMU78), respectively. It is possible that the expression of PdxK2 and PdxU2 is PLP-dependent and becomes de-repressed in response to the diminished intracellular pool of PLP in the PdxK and PdxU mutants. Such a de-repression could compensate the deficiency of PdxK and PdxU in the respective mutants, and at least partly explain why the PdxK and PdxU mutants were able to grow like the wild-type when B6 vitamers were included in the growth medium. On the other hand, when grown in FMC without inclusion of any vitamin B6, the intracellular PLP became quickly depleted in the PdxK-, PdxU-, and PdxKU-deficient mutants. It is possible that a DXP-dependent pathway exists in S. mutans and that PLP depletion triggered the activation of this not-yet characterized alternative de novo pathway, enabling the respective mutants to grow in the absence of exogenous B6 vitamers.
Widespread in both gram-positive and gram-negative bacteria (Belitsky & Sonenshein, 2002; Belitsky, 2004, 2014; Bramucci et al., 2011), the GabR/MocR sub-family proteins all possess a C-terminal domain with high similarity to AST. The AST catalyzes the interconversion of aspartate and a-ketoglutarate to oxaloacetate and glutamate. It contains PLP as a co-factor essential for catalytic activity. As revealed recently by crystal structure analysis, the C-terminal region of Bacillus subtilis GabR shows high similarity to many aminotransferases, and PLP appears to be a tightly bound covalently linked component of the protein, similar to the case of aminotransferases and other PLP-dependent enzymes (Edayathumangalam et al., 2013). While the GabR/MocR family proteins have been well-documented for their roles as transcriptional activators and/or repressors and for their dependence on PLP for regulatory activities (Belitsky & Sonenshein, 2002; Belitsky, 2004, 2014; Wiethaus et al., 2008; Jochmann et al., 2011; El Qaidi et al., 2013), there is currently no report that these proteins actually possess any aminotransferase activity. In an effort to see whether PdxR in S. mutans contains any aminotransferase activity, the recombinant protein, rPdxR was expressed, purified and analyzed for transaminase activity using a coupled assay (Karmen et al., 1955; Ziak et al., 1990). The results so far indicated that rPdxR possessed little or no detectable transaminase activity (data not shown). Protease Factor Xa (New England Biolabs) was also used to cleave the fusion domain, MalE, but such cleavage did not seem to cause any major differences in enzyme activity (data not shown). These results suggest that PdxR in S. mutans does not possess aminotransferase activity under the conditions studied, consistent with several other MocR/GabR homologues in other bacterial species (Belitsky, 2004; Bramucci et al., 2011). It is worth noting that when analyzed using whole cell lysates, the transaminase activity of the PdxR-deficient mutant was decreased by more than 40% (P < 0.05) when compared with the wild-type, UA159 (data not shown), and that complementation with the wild-type pdxR plus its cognate promoter in a shuttle vector restored the level to be similar to that of the wild-type. The mechanism that underlies the modulated transaminase activity in response to PdxR-deficiency awaits further investigation.
Streptococcus mutans, a well recognized key etiological agent of human dental caries, has evolved a variety of adaptive mechanisms that contribute to its abilities to tolerate and survive various detrimental conditions such as low pH and nutrient deficiency (Lemos & Burne, 2008; Lemos et al., 2013). LuxS/AI-2-mediated signaling is an intercellular communication system that has been shown to play an important role in S. mutans pathophysiology, regulating carbohydrate and amino acid metabolism, acid and oxidative stress tolerance and biofilm formation (Wen & Burne, 2004; Sztajer et al., 2007; Wen et al., 2011). Consistent with DNA microarray analysis, SMU864 is confirmed by real-time PCR as part of the LuxS-regulon (Wen et al., 2011). Our results presented here showed that PdxR-deficiency caused alteration in expression of genes known to be involved in the stress tolerance response and biofilm formation.
Glucosyltransferase GtfB produces primarily α1,3-linked water-insoluble glucans that are known to play an essential role in S. mutans adherence, biofilm accumulation and structure (Bowen & Koo, 2011; Gregoire et al., 2011). Our results showed that PdxR-deficiency reduced S. mutans biofilm formation. Consistently, both FE-SEM analysis and colorimetric assay demonstrated that the PdxR-deficient mutant had significantly less glucans than the parent strain, UA159, which could be at least in part attributed to the structure and quantitative characteristics of the deficient mutant biofilms. In contrast, however, realtime PCR revealed that gtfB transcription, but not gtfC, was elevated in response to PdxR-deficiency. The gtfB and gtfC genes are known to be linked, but as indicated in multiple reports (Goodman & Gao, 2000; Wen et al., 2005; Bitoun et al., 2013), our results again showed that these two genes could be differentially transcribed under the conditions studied. PLP at 10 mM was shown by Thaniyavarn et al. (1982) to reduce Gtf activity in vitro (see below for more), although whether it affects GtfB activity and glucan production at micromolar levels remains unclear. It is also possible that PdxR-deficiency influences the conformation and/or stability of GtfB protein, which consequently can affect the assembly of glucose polymer and biofilm formation of the deficient mutant. Of the genes/proteins altered in TW296, ClpL is an ATP-dependent Clp protease that has been shown to play a role in the stress tolerance response (Kajfasz et al., 2009). Major reduction in expression of ClpL is likely a part of the contributing factors to the weakened tolerance to low pH, which in turn likely contributes to the reduced biofilm formation. Considering the role of PLP as an essential cofactor of numerous enzymes, dysregulation of vitamin B6 metabolism as a result of PdxR-deficiency will also likely cause defects that can be attributed to the phenotypes observed with the deficient mutant.
Results presented here also show that vitamin B6, especially PL at higher concentrations, can strongly inhibit S. mutans growth. This is consistent with the finding by Thaniyavarn et al. (1982) that S. mutans significantly reduced its D-glucose uptake following pre-incubation of S. mutans cells with several other pyridine analogs, including PL, PM, PN and PLP (Thaniyavarn et al., 1982). Besides, pyridine analogs were also shown to inhibit glucan production by purified Gtf enzyme in vitro (Thaniyavarn et al., 1982). Consistently, our studies showed that vitamin B6, especially PL, are potent inhibitors of S. mutans biofilm formation. Besides growth defects, the influences on Gtf enzymes and glucan production are likely part of the underlying mechanisms. It awaits further investigation if any other factors are involved. However, it is unlikely that cytotoxicity played a role in the growth defects observed with the respective mutants. First, the concentration (5 μM) used in the growth studies, such as those shown in Fig. 1B and the supporting information, Fig. S2, is well below the threshold of toxicity measured, especially for PM and PN. Second, the fact that the PdxR-deficient mutant had a decreased growth rate relative to the wild-type also supports this notion. It is also interesting to note that PL was effective against S. mutans' biofilm formation at concentrations higher and lower than 2.5 mM, but not as significant at 2.5 mM tested. The underlying mechanism awaits further investigation.
In summary, PdxR in S. mutans is a member of the MocR/GabR family of proteins and regulates expression of genes in vitamin B6 metabolism and those known to play a role in stress tolerance response and biofilm formation. However, it remains unclear whether other genes are affected by PdxR and how exactly PdxR regulates gene expression in response to environmental conditions in S. mutans. Current effort is being directed to elucidation of the roles of PdxK2/U2 in vitamin B6 metabolism and to determination of the DXP-dependent de novo pathway in PLP biosynthesis in S. mutans.
Supplementary Material
Acknowledgments
This project is partly supported by NIDCR grant DE019452 to ZTW. We would like to thank Dr Jibao He at Tulane University Electron Microscope Laboratory for the FE-SEM analysis.
Footnotes
Supporting Information: Additional supporting information may be found in the online version of this article at the publisher's web-site.
References
- Ahn SJ, Wen ZT, Burne RA. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect Immun. 2006;74:1631–1642. doi: 10.1128/IAI.74.3.1631-1642.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajdic D, McShan WM, McLaughlin RE, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A. 2002;99:14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belitsky BR. Bacillus subtilis GabR, a protein with DNA-binding and aminotransferase domains, is a PLP-dependent transcriptional regulator. J Mol Biol. 2004;340:655–664. doi: 10.1016/j.jmb.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Belitsky BR. Role of PdxR in the activation of vitamin B6 biosynthesis in Listeria monocytogenes. Mol Microbiol. 2014;92:1113–1128. doi: 10.1111/mmi.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belitsky BR, Sonenshein AL. GabR, a member of a novel protein family, regulates the utilization of gamma-aminobutyrate in Bacillus subtilis. Mol Microbiol. 2002;45:569–583. doi: 10.1046/j.1365-2958.2002.03036.x. [DOI] [PubMed] [Google Scholar]
- Belli WA, Marquis RE. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol. 1991;57:1134–1138. doi: 10.1128/aem.57.4.1134-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas I, Drake L, Erkina D, Biswas S. Involvement of sensor kinases in the stress tolerance response of Streptococcus mutans. J Bacteriol. 2008;190:68–77. doi: 10.1128/JB.00990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitoun JP, Nguyen AH, Fan Y, Burne RA, Wen ZT. Transcriptional repressor Rex is involved in regulation of oxidative stress response and biofilm formation by Streptococcus mutans. FEMS Microbiol Lett. 2011;320:110–117. doi: 10.1111/j.1574-6968.2011.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitoun JP, Liao S, Yao X, et al. BrpA is involved in regulation of cell envelope stress responses in Streptococcus mutans. Appl Environ Microbiol. 2012a;78:2914–2922. doi: 10.1128/AEM.07823-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitoun JP, Liao S, Yao X, Xie GG, Wen ZT. The redox-sensing regulator Rex modulates central carbon metabolism, stress tolerance response and biofilm formation by Streptococcus mutans. PLoS ONE. 2012b;7:e44766. doi: 10.1371/journal.pone.0044766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitoun JP, Liao S, McKey BA, et al. Psr is involved in regulation of glucan production, and double deficiency of BrpA and Psr is lethal in Streptococcus mutans. Microbiology. 2013;159:493–506. doi: 10.1099/mic.0.063032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramucci E, Milano T, Pascarella S. Genomic distribution and heterogeneity of MocR-like transcriptional factors containing a domain belonging to the superfamily of the pyridoxal-5′-phosphate dependent enzymes of fold type I. Biochem Biophys Res Comm. 2011;415:88–93. doi: 10.1016/j.bbrc.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Browngardt CM, Wen ZT, Burne RA. RegM is required for optimal fructosyltransferase and glucosyltransferase gene expression in Streptococcus mutans. FEMS Microbiol Lett. 2004;240:75–79. doi: 10.1016/j.femsle.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Burne RA. Oral streptococci…products of their environment. J Dent Res. 1998;77:445–452. doi: 10.1177/00220345980770030301. [DOI] [PubMed] [Google Scholar]
- Burne RA, Wen ZT, Chen YM, Penders JEC. Regulation of expression of the fructan hydrolase gene of Streptococcus mutans GS-5 by induction and carbon catabolite repression. J Bacteriol. 1999;181:2863–2871. doi: 10.1128/jb.181.9.2863-2871.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne RA, Abranches J, Ahn SJ, Lemos JA, Wen ZT, Zeng L. Functional Genomics of Streptococcus mutans. In: Kolenbrander PE, editor. Oral Microbial Communities: Genomic Inquires and Interspecies Communication. Washington, DC: ASM Press; 2011. pp. 185–204. [Google Scholar]
- Carlsson J. Nutritional requirements of Streptococcus mutans. Caries Res. 1970;4:305–320. doi: 10.1159/000259653. [DOI] [PubMed] [Google Scholar]
- Edayathumangalam R, Wu R, Garcia R, et al. Crystal structure of Bacillus subtilis GabR, an autorepressor and transcriptional activator of gabT. Proc Natl Acad Sci U S A. 2013;110:17820–17825. doi: 10.1073/pnas.1315887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Qaidi S, Yang J, Zhang JR, Metzger DW, Bai G. The vitamin B6 biosynthesis pathway in Streptococcus pneumoniae is controlled by pyridoxal 5′-phosphate and the transcription factor PdxR and has an impact on ear infection. J Bacteriol. 2013;195:2187–2196. doi: 10.1128/JB.00041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick TB, Amrhein N, Kappes B, Macheroux P, Tews I, Raschle T. Two independent routes of de novo vitamin B6 biosynthesis: not that different after all. Biochem J. 2007;407:1–13. doi: 10.1042/BJ20070765. [DOI] [PubMed] [Google Scholar]
- Goodman SD, Gao Q. Characterization of the gtfB and gtfC promoters from Streptococcus mutans GS-5. Plasmid. 2000;43:85–98. doi: 10.1006/plas.1999.1444. [DOI] [PubMed] [Google Scholar]
- Gregoire S, Xiao J, Silva BB, et al. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl Environ Microbiol. 2011;77:6357–6367. doi: 10.1128/AEM.05203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A, Nielsen AT, Hentzer M, et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146(Pt 10):2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Jochmann N, Gotker S, Tauch A. Positive transcriptional control of the pyridoxal phosphate biosynthesis genes pdxST by the MocR-type regulator PdxR of Corynebacterium glutamicum ATCC 13032. Microbiology. 2011;157:77–88. doi: 10.1099/mic.0.044818-0. [DOI] [PubMed] [Google Scholar]
- Kajfasz JK, Martinez AR, Rivera-Ramos I, et al. Role of Clp proteins in expression of virulence properties of Streptococcus mutans. J Bacteriol. 2009;191:2060–2068. doi: 10.1128/JB.01609-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmen A, Wroblewski F, Ladue JS. Transaminase activity in human blood. J Clin Investig. 1955;34:126–131. doi: 10.1172/JCI103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau PCY, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J Microbiol Methods. 2002;49:193–201. doi: 10.1016/s0167-7012(01)00369-4. [DOI] [PubMed] [Google Scholar]
- LeBanc D, Lee L. Replication function of pVA380-1. In: Dunny G, Cleary PP, Mckay LL, editors. Genetics and Molecular Biology of Streptococci, Lactococci, and Enterococci. Washington, DC: ASM Press; 1991. pp. 235–239. [Google Scholar]
- Lee MH, Scherer M, Rigali S, Golden JW. PlmA, a new member of the GntR family, has plasmid maintenance functions in Anabaena sp. strain PCC 7120. J Bacteriol. 2003;185:4315–4325. doi: 10.1128/JB.185.15.4315-4325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JA, Burne RA. A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology. 2008;154:3247–3255. doi: 10.1099/mic.0.2008/023770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JA, Chen YY, Burne RA. Genetic and physiologic analysis of the groE operon and role of the HrcA repressor in stress gene regulation and acid tolerance in Streptococcus mutans. J Bacteriol. 2001;183:6074–6084. doi: 10.1128/JB.183.20.6074-6084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JA, Abranches J, Koo H, Marquis RE, Burne RA. Protocols to study the physiology of oral biofilms. Methods Mol Biol. 2010;666:87–102. doi: 10.1007/978-1-60761-820-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JA, Quivey RG, Jr, Koo H, Abranches J. Streptococcus mutans: a new Gram-positive paradigm? Microbiology. 2013;159:436–445. doi: 10.1099/mic.0.066134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Lau PC, Tang N, Svensater G, Ellen RP, Cvitkovitch DG. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J Bacteriol. 2002a;184:6333–6342. doi: 10.1128/JB.184.22.6333-6342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Tang N, Aspiras MB, et al. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J Bacteriol. 2002b;184:2699–2708. doi: 10.1128/JB.184.10.2699-2708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo CY, Corliss DA, Ganeshkumar N. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J Bacteriol. 2000;182:1374–1382. doi: 10.1128/jb.182.5.1374-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee T, Hanes J, Tews I, Ealick SE, Begley TP. Pyridoxal phosphate: biosynthesis and catabolism. Biochim Biophys Acta. 2011;1814:1585–1596. doi: 10.1016/j.bbapap.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Rigali S, Derouaux A, Giannotta F, Dusart J. Subdivision of the helix-turn-helix GntR family of bacterial regulators in the FadR, HutC, MocR, and YtrA subfamilies. J Biol Chem. 2002;277:12507–12515. doi: 10.1074/jbc.M110968200. [DOI] [PubMed] [Google Scholar]
- Rossbach S, Kulpa DA, Rossbach U, de Bruijn FJ. Molecular and genetic characterization of the rhizopine catabolism (mocABRC) genes of Rhizobium meliloti L5-30. Mol Gen Genetics. 1994;245:11–24. doi: 10.1007/BF00279746. [DOI] [PubMed] [Google Scholar]
- Schneider G, Kack H, Lindqvist Y. The manifold of vitamin B6 dependent enzymes. Structure. 2000;8:R1–R6. doi: 10.1016/s0969-2126(00)00085-x. [DOI] [PubMed] [Google Scholar]
- Senadheera D, Krastel K, Mair R, et al. Inactivation of VicK affects acid production and acid survival of Streptococcus mutans. J Bacteriol. 2009;191:6415–6424. doi: 10.1128/JB.00793-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipp RN, Boisvert H, Smith DJ, Hofling JF, Duncan MJ, Mattos-Graner RO. CovR and VicRK regulate cell surface biogenesis genes required for biofilm formation in Streptococcus mutans. PLoS ONE. 2013;8:e58271. doi: 10.1371/journal.pone.0058271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam P, Senadheera MD, Mair RW, Levesque CM, Cvitkovitch DG. The LiaFSR system regulates the cell envelope stress response in Streptococcus mutans. J Bacteriol. 2009;191:2973–2984. doi: 10.1128/JB.01563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztajer H, Lemme A, Vilchez R, et al. Autoinducer-2 regulated genes in Streptococcus mutans UA159 and global metabolic effect of the luxS mutation. J Bacteriol. 2007;190:401–415. doi: 10.1128/JB.01086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B, Willett NP, Shockman GD. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975;11:649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaniyavarn S, Taylor KG, Singh S, Doyle RJ. Pyridine analogs inhibit the glucosyltransferase of Streptococcus mutans. Infect Immun. 1982;37:1101–1111. doi: 10.1128/iai.37.3.1101-1111.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZT, Burne RA. Analysis of cis- and trans-acting factors involved in regulation of the Streptococcus mutans fructanase gene (fruA) J Bacteriol. 2002a;184:126–133. doi: 10.1128/JB.184.1.126-133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZT, Burne RA. Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl Environ Microbiol. 2002b;68:1196–1203. doi: 10.1128/AEM.68.3.1196-1203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZT, Burne RA. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J Bacteriol. 2004;186:2682–2691. doi: 10.1128/JB.186.9.2682-2691.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen TZ, Suntharaligham P, Cvitkovitch DG, Burne RA. Trigger factor in Streptococcus mutans is involved in stress tolerance, competence development, and biofilm formation. Infect Immun. 2005;73:219–225. doi: 10.1128/IAI.73.1.219-225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZT, Baker HV, Burne RA. Influence of BrpA on critical virulence attributes of Streptococcus mutans. J Bacteriol. 2006;188:2983–2992. doi: 10.1128/JB.188.8.2983-2992.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZT, Yates D, Ahn SJ, Burne RA. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol. 2010;10:111. doi: 10.1186/1471-2180-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZT, Nguyen AH, Bitoun JP, Abranches J, Baker HV, Burne RA. Transcriptome analysis of LuxS-deficient Streptococcus mutans grown in biofilms. Mol Oral Microbiol. 2011;26:2–18. doi: 10.1111/j.2041-1014.2010.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiethaus J, Schubert B, Pfander Y, Narberhaus F, Masepohl B. The GntR-like regulator TauR activates expression of taurine utilization genes in Rhodobacter capsulatus. J Bacteriol. 2008;190:487–493. doi: 10.1128/JB.01510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Wen ZT, Burne RA. A novel signal transduction system and feedback loop regulate fructan hydrolase gene expression in Streptococcus mutans. Mol Microbiol. 2006;62:187–200. doi: 10.1111/j.1365-2958.2006.05359.x. [DOI] [PubMed] [Google Scholar]
- Ziak M, Jaussi R, Gehring H, Christen P. Aspartate aminotransferase with the pyridoxal-5′-phosphate-binding lysine residue replaced by histidine retains partial catalytic competence. Eur J Biochem FEBS. 1990;87:329–333. doi: 10.1111/j.1432-1033.1990.tb15309.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


