Abstract
BONDI, C.O., B.D. Semple, L.J. Noble-Haeusslein, N.D. Osier, S.W. Carlson, C.E. Dixon, C.C. Giza and A.E. Kline. Found in translation: understanding the biology and behavior of experimental traumatic brain injury. NEUROSCI BIOBEHAV REV. The aim of this review is to discuss in greater detail the topics covered in the recent symposium entitled “Traumatic brain injury: laboratory and clinical perspectives,” presented at the 2014 International Behavioral Neuroscience Society annual meeting. Herein we review contemporary laboratory models of traumatic brain injury (TBI) including common assays for sensorimotor and cognitive behavior. New modalities to evaluate social behavior after injury to the developing brain, as well as the attentional set-shifting test (AST) as a measure of executive function in TBI, will be highlighted. Environmental enrichment (EE) will be discussed as a preclinical model of neurorehabilitation, and finally, an evidence-based approach to sports-related concussion will be considered. The review consists predominantly of published data, but some discussion of ongoing or future directions is provided.
Keywords: Attentional set-shifting test (AST), closed head injury, concussion, controlled cortical impact (CCI), environmental enrichment (EE), fluid percussion (FP), neurorehabilitation, pediatrics, social behavior
1. Introduction
Traumatic brain injury (TBI) affects approximately 10 million individuals worldwide each year. Two million of those occur in the United States (U.S.), making it one of the most prevalent of all neurological disorders (Goldstein 1990; Selassie et al., 2008; Faul et al., 2010). In the U.S. approximately 300,000 of the TBI cases are severe enough to warrant hospitalization, where roughly 50,000 patients die (Sosin et al., 1995; Faul et al., 2010). Of the 250,000 survivors, 70,000 to 90,000 endure long-term disabilities leading to costly medical and rehabilitative care (Max et al., 1991; Thurman et al., 1999; Selassie et al., 2008; Summers et al., 2009). In addition to physical impairments that limit routine daily function, other pervasive and persistent challenges faced by TBI survivors are significant disturbances in cognitive function, such as memory loss, poor response inhibition, distractibility and the inability to acquire or store new information, particularly as it relates to prior experience or environmental feedback (Busch et al., 2005; Horneman and Emanuelson, 2009; Walker and Tesco, 2013). These disturbances occur not only in moderate to severe TBI cases, but also in patients with mild TBI who are reported to develop post-concussion symptoms characterized by cognitive impairments comorbid with other neurobehavioral symptoms such as emotional alterations (Levin and Robertson, 2012).
Unfortunately, treatment options for brain injury are limited and therefore continued research is critical. In this vein, several experimental models have been developed with the intent to better comprehend the pathophysiology and neurological sequelae of TBI. These models are utilized extensively to induce brain injury replicating features and outcomes that are seen clinically. The expectation is that understanding pathological mechanisms through empirical research may lead to therapeutic interventions capable of attenuating motor and/or cognitive dysfunction. The objective of this review is to provide a broad perspective of the contemporary models of TBI that are in use today, as well as to present data on neurobehavioral and social deficits resulting from their use in pediatric and adult rodents. The review will also discuss the value of pharmacological and environmental interventions in promoting recovery, and will conclude with a bench to bedside perspective on concussion.
2. Contemporary laboratory models of traumatic brain injury
Experimental models of TBI have been developed to study injury biomechanics, discover pathological mechanisms, and develop therapies with the goal of reducing TBI-induced human suffering. This section will describe contemporary animal models of TBI and will focus on the neuropathology and relevant neurobehavioral outcomes. Regarding the latter, commonly used behavioral tasks presented at the symposium will also be highlighted as we consider them to be integral components of TBI models. While other less common behavioral tasks are available to assess function after TBI, they were not discussed at the symposium and hence are not considered germane to this review.
2.1. Fluid percussion
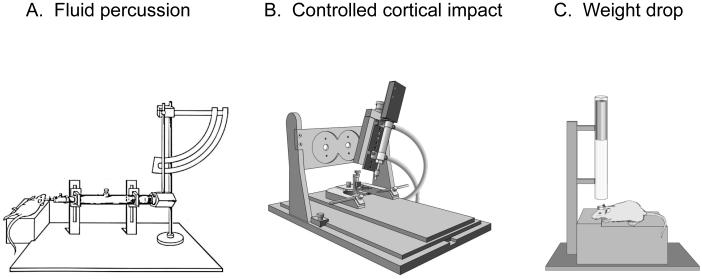
First described by Lindgren and Rinder in a rabbit model of TBI (Lindgren and Rinder, 1966), the fluid percussion (FP) device has been used in several other animal species, including cats (Sullivan et al., 1976), rats (Dixon et al. 1987; McIntosh et al., 1989), pigs (Armstead and Kurth, 1994), and mice (Carbonell et al., 1998). The FP device (Figure 1A) consists of a Plexiglas cylinder filled with physiologic saline and enclosed at one end by a male Luer-Lock fitting that is subsequently paired with a female fitting. Injury is produced when a metal pendulum strikes the piston of the device from a predetermined height and causes the rapid injection of saline into the closed cranium. The resulting pulse induces a brief increase in intracranial pressure with associated displacement and deformation of neural tissue. The severity of injury is regulated by varying the height of the pendulum, which corresponds to variations in extracranial pressure pulses expressed in atmospheres.
Figure 1.
Modified illustrations of the three most commonly used rodent models of traumatic brain injury (TBI). Fluid percussion (A), controlled cortical impact (B), and closed head impact-acceleration/weight drop (C).
2.1.1. Rat midline fluid percussion
Midline FP in rats is produced by placing the injury screw along the central sagittal suture midway between bregma and lambda. Midline FP has been used to induce concussive injuries (Dixon et al., 1987) and can produce cognitive deficits in the absence of overt hippocampal cell death (Lyeth et al., 1990). Although the lateral FP model has been more popular for studying neuronal cell death mechanisms, there is a recent resurgence of interest in midline FP because of the increased interest in diffuse brain injury associated with sports concussions and blast-induced TBI.
2.1.2. Rat lateral fluid percussion
Lateral FP in rats is produced by placing the injury screw over the parietal cortex midway between bregma and lambda. Lateral FP is advantageous for producing hippocampal cell death and cortical contusions (McIntosh et al., 1989). Furthermore, lateral FP injury in the rat has been shown to disrupt the blood-brain barrier (Cortez et al., 1989) and reduce cerebral blood flow (Yamakami and McIntosh, 1991). Other established consequences of lateral FP injury in rats include increased cerebral edema, tissue shearing, and intraparenchymal hemorrhage (Graham et al., 2000). Taken together, these consequences contribute to the formation of a focal lesion in the injured cortex (Tanno et al., 1992; Dietrich et al., 1998).
2.2. Controlled cortical impact
Experimental TBI induced with a pneumatic impactor was first introduced for use in laboratory ferrets by Lighthall and colleagues (Lighthall, 1988; Lighthall et al., 1990) and subsequently adapted for rats by Dixon and associates in an attempt to better control the biomechanical parameters of brain injury (Dixon et al., 1991). Unlike the FP injury device that disperses a stream of solution intracranially that cannot be readily quantified, the controlled cortical impact (CCI) model of experimental TBI takes advantage of biomechanical events contributing to injury (Figure 1B). These events can be analyzed by establishing a quantifiable relationship between measurable engineering parameters, such as force, velocity, and tissue deformation, and the magnitude of tissue damage or functional impairment. These controlled mechanical variables enable accurate, reliable, and independent control of the deformation parameters over a wide range of contact velocities.
The CCI injury device typically consists of a rod that is actuated either 1) pneumatically using a small-bore (1.975 cm), double-acting stroke-constrained pneumatic cylinder with a 5.0-cm stroke (Dixon et al., 1991), or 2) electromagnetically (Brody et al., 2007). The actuators are rigidly mounted on either a crossbar or stereotaxic manipulator arm in either an angled (perpendicular to the dura surface) or vertical position. The lower rod end has an impact tip attached that varies in geometry (rounded or flat edge), material (rigid metal or soft tip), and diameter (commonly 5 to 6 mm for rat CCI). CCI devices typically contain a velocity-measuring sensor system. The velocity of the impacting shaft is controlled by varying the gas pressure, in the case of pneumatic pistons (Dixon et al., 1991) or by varying electrical power in electromagnetic actuators (Brody et al., 2007). The impactor tip is driven at a predetermined velocity, to a target depth, and for a set duration of tissue deformation.
For mild or concussive TBI, the tip can strike the intact skull. For more severe TBI, the brain is impacted directly at a greater velocity through a craniectomy. Some research teams produce increasingly severe injury by driving the impactor tip deeper into the tissue. In rats, a depth of penetration of 2.6 to 2.8 mm with a velocity of 4.0 m/sec and a dwell time of 50 to 150 msec consistently produces an injury of moderate severity. However, it is important to note that the abovementioned guidelines regarding how to produce moderate injury are based on those used by the authors; the exact injury parameters depend on the particular laboratory. For example, Washington et al. (2012) used a Leica Impact One electromechanical CCI to produce mild, moderate, and severe TBI in mice, by keeping the velocity (5.25 m/sec), tip size (3.5 mm diameter) and dwell time (0.1 sec) constant, while scaling the depth as follows: 1.5 mm depth for mild-, 2.0 mm for moderate-, and 2.5 mm for severe-injury. Budinich et al. (2012) used the same CCI device (Leica Impact One) to produce moderate and severe injury in mice; notably, in this study while severe injury was produced with a 2.5 mm depth, moderate injury was induced with a depth of merely 1.0 mm. In the Budinich et al. (2012) study, the size of the impact tip and velocity were also different from the Washington et al. (2012) study; a 3.0 mm impact tip was driven at a velocity of 5m/s, with a dwell time of 0.1 seconds. The differences in injury parameters used in the Washington et al. (2012) and Budinich et al. (2012) studies highlight the variability across labs and the importance of making thoughtful choices in study design as they relate to injury severity. It is also important to note that injury severity can be selected or validated using physiologic criteria such as blood pressure and breathing outcomes. For example, Igarashi et al. (2007) consider severe injury to be characterized by apnea as well as transient post-injury hypertension followed by hypotension, whereas lower-grade injuries do not result in apnea and are characterized by immediate and prolonged hypotension. Other research teams use other physiological outcomes to set and refine their injury parameters to achieve the desired extent of injury. Researchers can also base injury severity on the nature or extent of functional deficit observed. Notably, there is no consensus regarding how to equate histopathological or behavioral outcomes or injury severity. Injury parameter settings should be informed by the research goals, a thorough review the literature, and, when possible, evidence from pilot studies.
2.2.1. Rat controlled cortical impact
The rat CCI model also produces morphologic and cerebrovascular injury responses that resemble certain aspects of human TBI. Commonly observed are graded histologic and axonal derangements (Lighthall 1988; Dixon et al., 1991; Goodman et al., 1994; Meaney et al., 1994), as well as disruption of the blood-brain barrier (Dhillon et al., 1994; Kochanek et al., 1995), subdural and intraparenchymal hematoma, edema, inflammation, and alterations in cerebral blood flow (Hall et al., 2005). Similarly, the CCI model also produces neurobehavioral and cognitive impairments similar to those observed in human patients. For example, Briones and colleagues (2013) found the CCI significantly impaired spatial memory, as evidenced by increased latency to find the escape platform in a Morris water maze (MWM) task. Working memory deficits are common after human brain injury and also been reported following rat CCI injury (Kline et al. 2002a). As will be discussed in greater detail in section 4.1, frontal lobe function has also been observed to be impaired in rats following CCI when evaluated using the attentional set-shifting test (AST) 4 weeks post-injury (Bondi et al. 2014). In contrast to other TBI models, the CCI device produces a pronounced cortical contusion, which is calculated by measuring tissue loss in serial coronal sections stained with hematoxylin and eosin or cresyl violet (Kline et al., 2004; Olsen et al., 2012; Monaco et al., 2013; Bondi et al., 2014). Hippocampal neuronal loss/survival is measured semi-quantitatively by counting healthy-appearing neurons or by using unbiased stereologic methods (Baldwin et al., 1997; Kline et al., 2004; Olsen et al., 2012; Monaco et al., 2013). Studies of CCI with the de Olmos amino-cupric-silver staining method have indicated that CCI can induce neurodegeneration in regions distant from the site of impact in rats (Hall et al., 2005).
2.2.2. Mouse controlled cortical impact
With the development of mutant strains of mice, including both gene knockout and transgenic lines, a version of the CCI model in mice has logically followed. Smith and colleagues characterized this model in the C57BL6 mouse - the background commonly used to produce relevant mutant strains (Smith et al., 1995). While the depth of deformation is scaled down to 1.0 to 1.2 mm based on the cortical thickness of the mouse versus rat, and for a given impact velocity, an insult of similar severity to that commonly seen in rats is produced. Because mice are steadily becoming more widely used, the mouse CCI model is taking on greater importance in the field of TBI. Neurobehavioral deficits in the mouse are similar to those seen in rat and many of the behavioral testing strategies are identical or similar, but on a smaller scale. For example, spatial memory deficits have been observed following CCI when evaluated using both the MWM and the Barnes maze (Fox et al., 1998, 1999). More recently, dysfunctions in social interaction, such as impaired social investigation, loss of preference for sociability, loss of presence for social novelty, and increased aggression were reported for adult mice who received a pediatric CCI injury (Thau-Zuchman et al., 2012).
2.2.3. Pig controlled cortical impact
In general, large-animal models of TBI are justified for complex physiologic and biomechanical studies that require large brain mass, more white matter content, or greater cortical complexity. By increasing the size of the impact tip and depth of impact, the CCI model has easily been scaled up to larger animals such as the pig. Using a direct focal impact method, Duhaime and associates demonstrated in piglets of different ages a vulnerability to mechanical trauma that increased progressively during maturation (Duhaime et al., 2000). CCI produced in swine with a pneumatic impactor has been shown to result in clinically relevant pathophysiology such as edema, cell death, white matter damage, and cerebrovascular dysregulation (Alessandre et al., 2003; Manley et al., 2006). Pig CCI models may be the most useful TBI model to mimic the neurological intensive care unit environment due to the ease of monitoring clinically-relevant physiological parameters in these larger animals (Zhou et al., 2009).
2.3. Closed head injury
Closed head injury models are thought to produce injury by transmitting mechanical forces through the skull to the brain. There are three main variations of closed head injury models. The first is a focal impact applied to the intact skull. Hall and coworkers produced concussive injury in mice by dropping a 50-g weight 18 cm onto the intact skull (Hall et al., 1988). Other investigators have characterized and applied a closed head impact model in both rats (Shapira et al., 1988) and mice (Chen et al., 1996) that produces edema, functional deficits, and significant hippocampal neuronal cell death. For weight drop models, the height and mass of the falling weight are adjusted according to the desired severity of injury, with farther distances and increased weight producing more injury than less distant and lighter weights.
The second type of closed head model is the Marmarou impact acceleration model (Marmarou et al., 1994) (Figure 1C). In this model, a weight is attached to a string, and when the predetermined height above the cranium is obtained, the weight is released through a guide tube that subsequently strikes a cemented disk (“helmet”) on the rat skull to prevent fractures (Marmarou et al., 1994). During impact the head is rapidly accelerated downward onto a foam pad, which facilitates relatively slower head deceleration. Impact acceleration produces a diffuse injury without noticeable contusions or hippocampal cell loss. However, brainstem and/or diffuse axonal injury (Okonkwo and Povlishock, 1999) and elevations in intracranial pressure have been observed after closed head impact acceleration injury (Engleborghs et al., 1998).
The third type of closed head TBI models is the head rotation model. Initially characterized in non-human primates (Gennarelli et al., 1982), it was later successfully adapted to swine (Ross et al., 1994). Diffuse axonal injury and transient posttraumatic unconsciousness are produced in miniature swine by rapid acceleration and deceleration of the head in the coronal plane, without impact (Ross et al., 1994). When applied to immature piglets this model produces a range of clinically relevant functional deficits that correlate with neuropathologic axonal damage (Friess et al., 2007). For example, piglets exposed to moderate injury had impaired visual-based problem solving and were less interested in exploring their environments (Friess et al., 2007). Sullivan and colleagues (2013) developed a highly sensitive and specific battery of neurobehavioral assessments to facilitate quantification of cognitive, motor, and other behavioral changes after experimental TBI in piglets.
2.4. Blast relevant TBI
Animal models have been developed to simulate elements of blast-relevant TBI (bTBI) including explosive or pressurized gas-driven shock tubes to generate pressure waves similar to those produced by explosives (Svetlov et al., 2010). bTBI models have focused on mimicking the primary overpressure shock wave as it is a key aspect of explosive blast that differentiates itself from civilian and penetrating models of TBI. The classic method of evaluating blast on living tissue is to produce a controlled detonation with animals placed at standardized standoff distances. While such models are clinically and biomechanically relevant to military bTBI (Bauman et al., 2009) their accessibility is limited to only a few facilities and thus they are not yet available for widespread investigation. Laboratory models of bTBI typically expose anesthetized animals to a controlled generated Friedlander waveform that is characterized by an initial peak pressure followed by a negative pressure.
Non-explosive shock tubes are the most common methods to generate shockwaves in laboratory environments. These tubes are typically comprised of a driver section that is separated by a longer driven section by a diaphragm. The driver section is pressurized until the diaphragm bursts allowing rapid expansion of the gas into the driven section producing a pressure waveform that mimics elements of an explosive shockwave. Varying the thickness of the diaphragm material can control the pressure of the burst. The test animal can be placed at varying distances from the end of the tube, or inside the tube. Furthermore, the torso can be shielded to mimic body armor and minimize non-cerebral damage. Another way to produce bTBI to the head only is to use smaller shock tubes where the expelled gas is limited to the head (Svetlov et al., 2010). In general, these models not only mimic the primary blast shock waves, but also produce clinically relevant cognitive and neuropathological responses (Long et al., 2009; Elder et al., 2012).
2.5. Motor assessment tests
2.5.1. Beam-balance and beam-walk tests
Motor function is a common deficit in TBI patients. These deficits include difficulty with balance and fine motor performance, increased dizziness, and reductions in muscle strength. Clinically relevant rodent models of TBI have utilized complex vestibulomotor and strength tasks to evaluate similar deficits in experimental TBI. The beam-balance task is used to assess motor and vestibular functioning by observing the rodents’ ability to balance on a stationary beam. Briefly, it consists of placing the rat on an elevated beam (1.5 cm wide) and recording the time it remains on for a maximum of 60 sec. The beam-walk task, originally described by Feeney and colleagues (1982), consists of training/assessing rats using a negative-reinforcement paradigm to escape ambient light and white noise by traversing an elevated narrow beam (2.5 × 100 cm) and entering a darkened goal box situated at the opposite end. When the rat enters the goal box the adverse stimuli (light and noise) are terminated thus serving as reinforcement (reward) for completing the task. Performance is assessed by recording the elapsed time to traverse the beam. Both beam tasks (beam-walking and beam-balance) were used to characterize the FP (Dixon et al., 1987) and CCI (Dixon et al., 1991) models of TBI and are used extensively after TBI to evaluate therapeutic manipulations (Fujimoto et al., 2004; Kline et al., 2004a,b, 2007, 2008, 2010; Cheng et al., 2007, 2008; Sozda et al., 2010).
2.5.2. Rotarod test
The rotarod test has been used to assess fine motor function in both rats (Hamm et al., 1994; Hamm, 2001; Monaco et al., 2013) and mice (Mouzon et al., 2012). As is the case for the beam-walk and beam-balance, the rats are trained prior to TBI or sham injury to perform the task. Typically, training begins two to three days prior to surgery and consists of both fixed rate and accelerating protocols until the rats are able to perform proficiently (Monaco et al., 2013). On the day of surgery the rats are provided three trials according to the accelerating protocol, which may vary from laboratory to laboratory, to establish baseline performance. Motor assessment is then conducted at prescribed time points after TBI. The testing procedure evaluates the time and/or speed of revolutions before the rat loses its balance and falls off the accelerating rotating rod.
2.6. Cognitive assessment tests
2.6.1. Acquisition of spatial learning and memory
The Morris water maze (MWM), developed by Morris et al., (1982) was first applied to in vivo TBI research in a study of CCI conducted by Hamm and colleagues (1992). Several studies have found long-term deficits in the acquisition of spatial learning that persist from 6 months after CCI injury (Cheng et al., 2012) to one year after either CCI injury (Lindner et al., 1998; Dixon et al., 1999) or lateral FP brain injury (Pierce et al., 1998). It has since become one of the most widely used tools for assessing the potential benefits of various therapeutic manipulations on spatial learning in preclinical TBI research (Bramlett et al., 1995; Scheff et al., 1997; Kline et al., 2002a,b; Cheng et al., 2007, 2008; Olsen et al., 2012; Yelleswarapu et al., 2012). Briefly, the maze consists of a pool (typically 180 cm diameter; 60 cm high) filled with tap water (26 ± 1°C) to a depth of 28 cm and is situated in a room with salient visual cues that remain constant throughout the study. The platform is a clear Plexiglas stand (10 cm diameter, 26 cm high) that is positioned 26 cm from the maze wall in the southwest quadrant and is held constant for each rat. Spatial learning typically begins on post-operative day 14 and consists of providing a block of four daily trials (4-min inter-trial interval) for five consecutive days (14–18) to locate the platform when it is submerged 2 cm below the water surface (i.e., invisible to the rat). For each daily block of trials the rats are placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a randomized manner. Each trial lasts until the rat climbs onto the platform or until 120 sec have elapsed, whichever occurs first. Rats that fail to locate the goal within the allotted time are manually guided to it. All rats remain on the platform for 30 sec before being placed in a heated incubator between trials. The times of the 4 daily trials for each rat are averaged and used in the statistical analyses.
Another common use of the MWM is to assess memory retention via a probe trial. In this subtest, the platform is removed from the water maze and the percent time the rodent swims in the quadrant of the pool that contained the platform during acquisition is measured. The rationale is that a rat that has learned and remembers the location of the hidden platform should spend more time in the target zone searching for it. Numerous studies have shown benefits with various therapies in memory retention during a relative acute time period of 3 weeks (Kline et al., 2004a,b, 2007, 2010, 2012; Monaco et al., 2013). However, the task is also sensitive to long-term effects (i.e., 1 year) as demonstrated by Dixon and colleagues (Dixon et al., 1999). A more recent study by Byrnes et al. (2012) found that performance on the MWM probe trial was poorer among TBI-exposed mice than their control (sham or naïve) counterparts.
The MWM can also be used to assess working memory after experimental TBI. Hamm and colleagues (1996) have shown working memory deficits following FP injury and Fox et al. (1998) have reported similar working memory deficits in mice following CCI injury. Kline and colleagues (2002a) demonstrated working memory deficits in male rats after TBI. In the latter study, spatial working memory was assessed on postoperative days 11–15. Briefly, each rat received eight pairs of trials per day with a maximum of 120 sec to find the escape platform on each trial and a 10-sec delay between trial 1 (information trial) and trial 2 (retention trial). The start points (North, East, South, and West) and goal locations (1, 2, 3, and 4) were quasi-randomly assigned each day with the requirement that during the 8 pairs of trials each rat was tested twice from each start position and goal location.
2.6.2. Novel object recognition memory
Recognition memory is defined as the ability to successfully match an encounter (e.g., person, object, location) to a representation stored as memory upon re-experience (Norman and O’Reilly, 2003). Recognition memory represents an important component of human long-term memory and is of interest to researchers using data from both humans (Kelly et al., 1996; Wright et al., 2014) and animals (Bevins and Besheer, 2006). TBI patients have impaired recognition memory (Hannay et al., 1979; Millis and Dijkers, 1993), a finding that has been replicated in animal models using a variety of assessments. For example, the novel-object recognition (NOR) test has been used in a number of in vivo TBI studies (Ji et al., 2012; Yang et al., 2013; Scafidi et al., 2010; Zhao et al., 2012). However, in our experience, subtle changes in the NOR paradigm with respect to the nature of the objects used (e.g., size, shape, color) and proximity of the objects to one another may affect NOR performance. A review of the methodological modifications of the NOR test and its application to behavioral pharmacology research was recently published (Antunes and Biala, 2012).
2.6.3. Contextual fear conditioning
Assessment of post-injury fear responses can be evaluated using contextual fear conditioning (CFC). Originally developed by Michael Faneslow (Faneslow and Tighe, 1988), CFC is rooted in Pavlovian fear conditioning in that through repeated pairing with a threatening exposure (e.g., loud noise), a non-threatening stimulus begins to elicit fear responses (Kim et al., 1992; Phillips and LeDoux, 1992; Rudy and O’Reilly, 1999). Brain regions responsible for CFC include the hippocampus and amygdala. CFC has been widely used in diverse in vivo studies (Young et al., 1994). CFC was only recently applied to a few TBI specific studies as an outcome measure (Klemenhagen et al, 2013; Titus et al., 2013; Luo et al., 2014).
2.7. Limitations of TBI models
Overall, the TBI devices discussed mimic many of the important histopathological changes (e.g., axonal injury, cell death, inflammation) and functional deficits (e.g. spatial memory problems, social dysfunction) seen following human TBI. However, an important caveat is that experimental TBI models do not fully capture the breadth and complexity of human brain injury outcomes with respect to the neurobehavioral correlates, particularly those functions involving communication and language. Moreover, some neurobehavioral outcomes are less well studied in experimental TBI. For example, frontal lobe dysfunction is understudied in this context because of challenges associated with designing analogs of complex frontal lobe functions such as impulse control and language, although as described later in section 4 of this review executive function can be assessed quite sensitively. Similarly, while adolescent TBI survivors have been found to display impaired empathy and moral reasoning compared to their non-injured peers (Beauchamp et al., 2013), there are no clear analogous outcomes for empathy and moral reasoning in animal models.
3. Social behavior after injury to the developing brain
TBI at a young age may have adverse consequences on the development of normal social behavior(s); however, few preclinical studies have addressed this important outcome measure. In this section we describe the application of behavioral assays for social investigation, socio-sexual behaviors, and social communication in experimental TBI models, and present recently identified social changes in a mouse model of pediatric TBI. Factors that potentially impact social outcomes will also be considered, including age-at-injury, severity of injury, and time post-injury.
3.1. Social deficits in young brain-injured patients
TBI is the primary cause of morbidity and mortality among young children and older adolescents (Schneier et al., 2006). Within these populations, brain injury results in a wide range of physical, neurological, cognitive, and psychosocial difficulties, which may persist long-term (Beauchamp et al., 2013; Karver et al., 2012; Levin et al., 1992). Compared to the adult, the pediatric brain shows some unique responses to TBI. For example, a greater head-to-torso ratio and higher brain water content in the child compared to adult brain contribute to an injury that may be more diffuse in nature (Kochanek, 2006). Indeed, both clinical (Adelson et al., 1997; Koskiniemi et al., 1995) and experimental studies (Bittigau et al., 2004; Fan et al., 2003; Tsuru-Aoyagi et al., 2009) have demonstrated that the developing brain is inherently more vulnerable to injury compared to the adult (Anderson et al., 2005; Bauer and Fritz, 2004; Potts et al., 2006). Further, functional deficits may emerge over time post-injury as survivors fail to reach age-appropriate milestones (Anderson et al., 2005; Anderson and Moore, 1995; Koskiniemi et al., 1995).
The normally developing brain, characterized by complex changes in both structure and composition, is likewise associated with the emergence of temporally specific behaviors. Developmentally-regulated processes of myelination, synaptic pruning, and structural reorganization continue throughout early childhood and adolescence in a region-specific manner, while physiological measures such as intracranial, arterial, and cerebral perfusion pressures also vary with age (Adelson et al., 2003; Donders and Warschausky, 2007; Kochanek et al., 2012). It has become clear that the developing brain exhibits age-dependent vulnerability to TBI, with children under four years of age typically exhibiting poorer outcomes compared to older children (Anderson and Moore, 1995; Karver et al., 2012). To better understand the pathobiological mechanisms underlying this vulnerability, several of the abovementioned experimental models of TBI have been adapted for use in rodents at an early post-natal age (Adelson et al., 1996; Adelson et al., 1998; Prins et al., 1996; Tong et al., 2002). One such model utilizes a unilateral CCI in mice at postnatal day (PND)-21, approximating a toddler-aged child (Semple et al., 2013; Yager and Thornhill, 1997). This model generates a distinct, reproducible pattern of neuronal loss in the injured frontoparietal cortex and underlying subcortical structures, associated with widespread white matter damage (Tong et al., 2002). Progressive neuronal loss results in an increase in tissue atrophy over time (Claus et al., 2010; Pullela et al., 2006), consistent with ongoing neurodegeneration seen after TBI in young patients (Keightley et al., 2014). Functionally, brain-injured mice display a phenotype of transient anxiolysis and persistent hyperactivity, as well as spatial memory deficits that are delayed in onset (Pullela et al., 2006). These features are consistent with neurobehavioral sequelae seen in brain-injured children (Konrad et al., 2000; Levin, 1998; Max et al., 2011).
Problems with social functioning are common after TBI in both adults and children, and have widespread quality of life consequences including academic achievement, relationships and re-integration into society (Yeates et al., 2007; Rosema et al., 2012; Bigler et al., 2013; Rosema et al., 2014). While motor and physical symptoms tend to stabilize or diminish over time after injury, functional impairments including cognitive and emotional disturbances cause the greatest long-term distress (Yeates et al., 2004; Catroppa et al., 2008; Chapman et al., 2010; Catroppa et al., 2012). In children, severe TBI is associated with low peer acceptance and high levels of rejection and victimization in the classroom (Bigler et al., 2013; Rosema et al., 2014). The maturation of social behaviors is protracted through childhood and adolescence in a normally-developing child, suggesting that TBI at a young age is likely to adversely affect the acquisition and establishment of social skills (Wells et al., 2009). In addition, brain injury at a young age is predictive of poorer language and non-verbal communication (Ewing-Cobbs et al., 1997; Wells et al., 2009; Sullivan and Riccio, 2010), which may complicate the ongoing development of social skills (Didus et al., 1999) and persist up to 20 years after severe TBI during childhood (Cattelani et al., 1998; Hoofien et al., 2001; Ryan et al., 2013).
To address the increasing clinical data indicating the prevalence of post-traumatic social dysfunction and its importance in long-term prognosis, behavioral tests to assay social function are beginning to be incorporated into preclinical TBI studies. Much can be learned from paradigms developed and validated by colleagues in autism research, where assessment of social behaviors forms the basis of disease diagnosis (Crawley, 2012).
3.2. Behavioral assays of social behavior in rodents
Social behavior is a complex collection of actions including approach, affiliation and agonistic encounters. Many of these interactions are readily observable in rodents, and mouse models have been developed to study the core symptoms seen in autism spectrum disorders as well as other neurodevelopmental conditions such as fragile × syndrome (Kooy et al., 1996; McFarlane et al., 2008; Young et al., 2010). Laboratory Mus musculus display a broad repertoire of quantifiable social behaviors including approach to olfactory pheromones and other mice, ultrasonic vocalizations, communal nesting, scent marking, aggression, sexual and play behaviors (Terranova and Laviola, 2005; Silverman et al., 2010; Crawley, 2012). The most common paradigms to assess social behavior are described here briefly. Behaviors are typically scored from videotapes by investigators blinded to genotype, treatment or injury group, or by software linked to video-tracking or photocell detection systems (Spencer et al., 2005; Ohayon et al., 2013).
3.2.1. Reciprocal social interactions
Observation of reciprocal social interactions is currently the most common experimental paradigm for social behavior. Two unfamiliar rodents are permitted free exploration within a specified arena, and social investigation is quantified (Wöhr and Scattoni, 2013). Different interactive actions including sniffing, body contact, aggressive behaviors, climbing, mounting, huddling, close following and play behaviors may be assessed, and parameters such as the session length, arena size, time of day and prior social experiences can be modified according to the experimental aims (Terranova and Laviola, 2005; An et al., 2011). Repeated testing of the same mice is usually possible, allowing for the evaluation of developmental trajectories across age (Silverman et al., 2010). The resident-intruder test is an adaption of this test, typically conducted in the home cage of the experimental animal, to which an unfamiliar stimulus is introduced (Duvoisin et al., 2011; Koolhaas et al., 2013). The stimulus rodent may be male or female, to examine male-to-male interactions or sociosexual behaviors, respectively. Further, the stimulus is typically either naïve or an experimental animal matched to the condition of the animal being tested, and may be of a younger age to minimize antagonistic behaviors. Another adaption of this paradigm is the partition test, where the test and stimulus mice are physically separated by a perforated partition. Investigation close to the partition is quantified as a measure of social interest towards the stimulus mouse (Kudryavtseva, 2003; Silverman et al., 2010).
3.2.2. Three-chamber social approach task
The three-chamber social approach test, established by Crawley and colleagues, is now considered the ‘gold-standard’ for evaluating social deficits in autism mouse models (Nadler et al., 2004; Silverman et al., 2010; Yang et al., 2011). In this paradigm, the test mouse has a choice of spending time in the central (neutral) chamber or two adjacent chambers, one containing a wire cup-enclosed stimulus mouse and the other containing an empty cup. A mouse exhibiting normal sociability typically favors proximity of the stimulus mouse in preference over the empty cup (Moy et al., 2004, 2008). In contrast to tests of free social exploration, the physical confinement of the stimulus mouse in a wire cup permits olfactory, visual and auditory cues while preventing aggressive and sexual behaviors, and limits the initiation of social contact to the test mouse only. A subsequent test stage involves the introduction of a second novel mouse into the previously empty chamber, thereby presenting the test mouse with a choice of social partners. Typically, mice will spend more time in proximity of the novel stimulus, indicating social recognition and social memory of the now-familiar mouse (Silverman et al., 2010). An alternative test for social recognition is the habituation-dishabituation paradigm. This design consists of short, sequential encounters between pairs of mice, whereby habituation usually occurs and the time spent engaged in social investigation declines over time. During the final encounter, a novel stimulus mouse is instead introduced, and social recognition of this individual stimulates an increase in social interest (Guan and Dluzen, 1994).
3.2.3. Social aggression
Social aggression, typically exhibited by paired males, can be evaluated during resident-intruder scenarios as described above, by quantifying the frequency, duration, temporal and sequential patterns of antagonistic behaviors (Miczek, 1979; Miczek et al., 2001). An alternative measure is the tube-dominance task that evaluates the dominant-submissive relationship established between male mice during a brief encounter enclosed within a narrow tube in which they cannot turn around (Molina et al., 2008; Wang et al., 2011). The establishment of dominance in this test is thought to reflect a tendency for aggression, although it may also be dependent upon social anxiety (Spencer et al., 2005).
3.2.4. Social communication
The social transmission of food preference is another useful test of olfactory-based social memory in both rats and mice (Galef et al., 1983; Wrenn, 2004). In this paradigm, the test mouse interacts with a "demonstrator" mouse that has recently eaten a novel food. As a result, the test mouse will eat more of the now-familiar food compared to a completely new food when presented with the choice. This phenomenon depends on the test mouse detecting meaningful olfactory cues from the demonstrator during their interaction, and the subsequent food preference serves as a measure of social memory (Wrenn et al, 2003; Wrenn 2004). This task has not yet been evaluated in experimental TBI.
In addition to the evaluation of social investigation and contact, novel methods to assess social communication between rodents have also emerged from the autism field. Urinary scent marking is performed by male mice in a context-specific manner to mark territories, attract mates, and communicate health status information (Hurst and Beynon, 2004; Wöhr and Scattoni, 2013). A reduction in scent marking has been observed in several mouse strains characterized by low sociability (Wöhr et al., 2011a,b). Male mice will deposit scent marks onto absorbent paper in response to a female mouse or female urine, and these can be readily quantified (Arakawa et al., 2007, 2008).
Another means of communication between rodents is by ultrasonic vocalizations (USV), which are produced by male mice in response to the presence of a female or female scent (Whitney and Nyby, 1979; Bean, 1982). Male-emitted USV’s have been described as an index of social and sexual interest, recognition and motivation (Hammerschmidt et al., 2012; Wöhr and Scattoni, 2013), and changes have been reported in the context of autism-like social deficits (Scattoni et al., 2011; Wöhr et al., 2011a,b; Yang et al., 2012).
3.2.5. Considerations for study design
Experimental design and conditions can profoundly influence behavioral results from these tests; potentially more so for subtle changes in social behaviors compared to when assaying for motor or cognitive functions. Social behaviors in rodents are predominantly mediated by olfactory cues and are therefore dependent upon intact olfactory function (Ryan et al., 2008). Thus evaluating the ability of experimental mice to detect volatile odors, for example, in a buried food olfactory discrimination task, is advisable (Yang and Crawley, 2009). In addition, social behaviors are particular sensitive to handling and housing conditions, as well as previous life experiences, particularly social isolation (Pietropaoloa et al., 2004; Terranova and Laviola, 2005; Crawley, 2007). Test variables including lighting, background noise, cage/arena size, the length of the habituation period and test duration, vary widely across studies and make comparisons somewhat difficult between different laboratories. As such, the evaluation of social parameters in experimental TBI requires meticulous control and evaluation of many subtle factors that may influence mouse behaviors and interfere with assessments.
3.3. Social deficits in experimental models of brain injury
Despite the above mentioned readily available assays of social behavior, there is a paucity of research examining social changes in the context of neurotrauma, a field that traditionally has focused on measures of cognitive and sensorimotor outcomes. Studies that have incorporated social tests into the study design to date (Table 1) provide some insight into the mechanisms and comorbidities associated with social dysfunction. Here, we will consider social tests and findings from different models of TBI that have been described in detail in the preceding section.
Pandey and colleagues (2009) examined depression-like and anxiety-like behaviors in rats at 14 days after diffuse impact-acceleration injury. Social interactions were assessed in an open field arena during a 5 minute session, and unfamiliar pairs of TBI rats were found to engage in a shorter duration of social investigation compared to paired sham-operated rats. In concert with abnormal behavior in the marble-burying test (Njung'e and Handley, 1991), the authors interpreted this finding as the manifestation of anxiety-like behavior (Pandey et al., 2009). In addition, sociosexual interactions were assessed in response to a sexually-receptive naïve female rat during a 20 min session in a neutral arena. Here, TBI rats showed a significant reduction in the number of genital probing, thrusting and pursuit episodes, and an increased latency to perform such behaviors (Pandey et al., 2009). The authors interpret this to reflect depressive-like behavior, based upon the understanding that sexual anhedonia is one of the key features of clinical depression (Kennedy, 2008).
Another recent study, using the midline FP injury model of diffuse TBI, examined ‘social withdrawal’ as the degree of social engagement with a novel juvenile mouse introduced into the home cage of the experimental animal (Wohleb et al., 2012; Fenn et al., 2013). After a peripheral inflammatory challenge at 30 days post-injury, brain-injured mice showed a reduction in total social exploration, which coincided with the presence of primed microglia and an exacerbated cytokine response (Fenn et al., 2013). This finding was again interpreted as a depressive-like phenotype, a concept first postulated by File and Hyde (1978), who observed that social interactions between rats often correlated with measures of emotionality (File and Hyde, 1978).
Several studies in models of mild or concussive brain injuries have recently considered social behavior as a phenotype independent of anxiety. Shultz and colleagues found that neither single nor repeated mild injuries at adulthood affected rat social behavior when tested at either 24 h or 4 weeks post-injury, despite evidence of a persistent cerebral inflammatory response (Shultz et al., 2011, 2012). Here, they employed a lateral FP injury to deliver 1, 3, or 5 insults, spaced 5 days apart, and an automated video-analysis system determined the distance between paired experimental rats in a neutral arena. In another study, two closed-skull impacts timed 24 hr apart were paired with a stressor (foot shock fear-conditioning paradigm) to examine the potential consequences of post-traumatic stress on TBI outcomes, including social behavior in a habituation-dishabituation paradigm at 10 days post-injury (Klemenhagen et al., 2013). They found that TBI (non-stressed) mice showed normal habituation towards an unfamiliar stimulus mouse across 9 consecutive trials; however, when a novel mouse was subsequently introduced, TBI mice interacted significantly less than sham animals. In comparison, TBI mice that were also stressed by foot shock additionally exhibited decreased interactions with an unfamiliar mouse during the habituation phase, suggesting that post-traumatic stress worsens the severity of social deficits (Klemenhagen et al., 2013). Together, these studies also suggest that social deficits may be less prevalent or less apparent after mild concussive TBI compared to more severe injuries, although further research is clearly needed to confirm this assertion.
Only one study to date has examined social dysfunction specifically after bTBI. Koliatsos and colleagues (2011) used a shock tube to generate shock waves in mice that are comparable to open-field blast waves. In addition to motor abnormalities and spatial memory deficits, mice underwent a habituation-dishabituation paradigm in a neutral arena across a series of 3 × 2 min exposures with the same mouse, followed by a final 2 min session with a novel stimulus mouse. The initial level of social exploration was similar between sham and bTBI mice, indicating no effect of bTBI on baseline sociability. However, blast-injured mice failed to show habituation to a familiar mouse across repeated exposures, suggesting that they failed to recognize or remember the stimulus mouse. These deficits were transient, detected at 1 week post-injury, but normalized by 2 weeks, and are in contrast to spatial memory deficits that still persisted at this latter time point (Koliatsos et al., 2011). Further research is needed to delineate potential correlations between comorbid functional deficits after TBI, such as the relationship between cognitive, emotional and social functioning (Milders et al., 2008; Pepin et al., 2000; Spikeman et al., 2012).
3.4. Social deficits after pediatric TBI in mice
To date, only two published studies have examined social behaviors after TBI to the developing brain, using the CCI model in male mice at PND-21 (Tong et al., 2002). In the first study, brain-injured and sham-operated control mice underwent the partition test, resident-intruder paradigm, Crawley three-chamber social approach, and tube-dominance tasks during adolescence (PND 35-42) and again at early adulthood (PND 60-70), in encounters with naïve age-matched stimulus mice (Semple et al., 2012). Despite normal social behaviors at adolescence, by adulthood, brain-injured mice showed reduced social investigation towards stimulus mice during the resident-intruder task, as well as a loss of preference for sociability in the three-chamber task. TBI mice also lacked a preference for social novelty, indicative of deficit in social recognition or memory. Also at adulthood, early-life TBI was associated with more frequent exertion of dominance in the tube task compared to sham-operated controls, a finding suggestive of increased aggressive tendencies. Of note, these deficits were evident despite normal olfactory function, as determined during the buried food test (Semple et al., 2012). Together, these findings reveal an emergence of aberrant social behavior over time after injury, a trajectory that parallels the development of cognitive deficits in this model, as well as behaviors seen in brain-injured children.
To address the extent to which the age at injury influences social behaviors, a subsequent study from the same investigators recently compared mice injured at PND 21 or PND 35 (Semple et al., 2014). Mice injured at a young postnatal age (PND 21), when tested at adulthood, exhibited reduced scent marking behavior and an alteration in context-dependent USVs in response to female stimuli. In contrast, mice injured at adolescence (PND 35) showed remarkable resilience to develop social deficits at adulthood. Together, these findings are in line with accumulating clinical evidence suggesting that the younger brain may show greater vulnerability to social deficits compared to older children or adults (Didus et al., 1999; Wells et al., 2009; Anderson et al., 2010).
3.5. The challenge: understanding mechanisms of social dysfunction
While social dysfunction is evident after TBI in patients and experimental models, the question inevitably arises - what is the pathobiology contributing to the manifestation of such deficits? Evidence from clinical neuroimaging, combined with animal models involving region-specific experimental lesions, is being utilized to address this issue. A better understanding of the mechanisms underlying social deficits may aid in the development of symptom-specific interventions for brain-injured patients.
Social behaviors in humans are mediated by the ‘social brain,’ comprised of the superior temporal sulcus, fusiform gyrus, temporal pole, medial prefrontal cortex, orbitofrontal cortex, amygdala, temporoparietal junction, and inferior parietal cortex (Beauchamp and Anderson, 2010; Blakemore, 2008; Yeates et al., 2007). Neuroimaging has revealed ongoing reorganization and connectivity of these structures during childhood and adolescence, indicating considerable development during brain maturation (Heyes et al., 2012). After TBI, psychosocial dysfunction has been adversely associated with frontal lobe pathology in patients (Levin et al., 2004; Wilde et al., 2005), particularly to regions implicated in social cognition and information processing (Eslinger et al., 1992; Spikeman et al., 2012; Wilde et al., 2005).
However, the relationship between regional neuroanatomy and social deficits is likely to be more complex, particularly in the context of a brain that is still undergoing maturational processes. Although adult models of frontal injury exist (Hoane et al., 2004; Kilbourne et al., 2009; Lindner et al., 1998), few have considered a juvenile age of injury or examined social functions. By adapting the standard CCI model to the parietal lobe of juvenile mice (Semple et al., 2012; Tong et al., 2002), to instead impact the unilateral left frontal lobe, Chen and colleagues hypothesized that injury to this location would also result in notable social dysfunction (Chen et al., 2013). Contrary to expectations, however, frontally-injured mice were indistinguishable from sham-operated controls in a battery of social tests at adolescence and adulthood after PND-21 injury; instead, the predominant phenotype was a persistent motor deficit, likely attributed to volumetric loss of the motor cortex (Chen et al., 2013). This surprising lack of social dysfunction may result from lesion laterality or age-dependent vulnerability to social deficits, based upon the region-specific maturational state at the time of injury. These findings support a more global mechanism underlying abnormal social function after TBI. It has also been proposed that the developing brain is less functionally-specific compared to the mature brain, such that regional localization of social functions may not yet be complete at an early postnatal age, rendering the young brain primed for more rapid establishment of compensatory connections (Spencer-Smith and Anderson, 2009).
In line with these findings, there is increasing evidence that diffuse axonal injury, which disrupts white matter connectivity between regions of the social brain network, may underlie many TBI-induced social deficits (Bigler et al., 2013; Ryan et al., 2013). Changes in the corpus callosum in terms of volumetric atrophy, microstructural integrity or connectivity (Ewing-Cobbs et al., 2008; Wu et al., 2010; Ewing-Cobbs et al., 2012; Semple et al., 2014) have been associated with poorer social outcomes in both adults and children after TBI (Beauchamp et al., 2009; Ryan et al., 2013). In children, volumetric changes of both grey and white matter structures after injury are likely to reflect not only ongoing neurodegeneration, but also result from the retardation of normal growth trajectories. Further, there is the potential for compensatory mechanisms that may result in aberrant regrowth. Techniques such as susceptibility-weighted, diffusion, and functional magnetic resonance imaging may be required to elucidate the complex relationship between neural circuitry, connectivity of specific pathways, and social behaviors.
4. The attentional set-shifting test as a novel behavioral paradigm for TBI
Brain-injury models in the laboratory, whose aims are to target relevant injury-related symptomatology and mimic neurochemical, motor and cognitive recovery after TBI, have been associated for decades with decline in long-term learning and memory. Nevertheless, the types of behavioral tests performed to date, albeit important for providing validity to experimental models of injury, such as the MWM for reference memory, the Barnes maze test for working memory, a combination of both in the radial arm maze test, as well as the novel object recognition test for declarative memory, have failed to focus on the complex attention impairments related to the frontal lobe, which are common in many if not most TBIs throughout all injury severities.
4.1. Neuropsychological tests of executive function
Executive function and cognitive flexibility represent sophisticated brain capabilities to use environmental feedback to “unlearn” a previously valid set of rules, switch gears and filter unwanted distractions, in order to acquire a new rule by shifting attention from a salient stimulus dimension to a previously irrelevant one (Bondi et al., 2008). In 1948, the Wisconsin Card Sorting Test (WCST) was developed as a multi-factorial test to assess strategy-switching deficits as an index of behavioral flexibility in healthy young subjects (Berg, 1948; Grant and Berg, 1948), and subsequently demonstrated to detect deficits in patients with frontal lobe damage or dysfunction, age- or neurodegenerative illness-related cognitive impairments or dementia, as well as schizophrenia or attention deficit hyperactivity disorder (Tait et al., 2014). This neuropsychological test requires subjects to orient themselves based on a “correct” rule in order to match a deck of cards displaying a number of colored objects to a set of exemplars based on three dimensions: color, number, and shape. Subjects must acquire the sorting rule (color, number, or shape) based on trial-by-trial feedback indicating whether the previous response is correct or incorrect. Once a given contingency is learned, the rules are changed without the knowledge of the subject, who must then identify and interpret an error, suppress a previously successful but now incorrect approach, and adapt to the new sorting rule by switching attention from the previously salient dimension to a previously irrelevant dimension (Lapiz-Bluhm et al., 2008). An assessment of executive function and behavioral flexibility can be derived from the number of trials to reach criterion as well as number of errors required to learn the new rule after a shift (Tait et al., 2014).
Attentional sets represent information “stores” mediated by top-down control mechanisms that prioritize stimuli based on perceptual, reward-predicting features within a salient dimension identified via environmental and trial-by-trial feedback (Leber and Egeth, 2006; Tait et al., 2014). As new dimensions become relevant, the salience of stimulus features needs to be updated accordingly in order to receive positive feedback and receive a reward. Upon formation of an attentional set, discriminations that are congruent with the relevant dimension will be achieved faster, even with presentation of novel stimuli, whereas tasks requiring an attentional set-shift will take more trials to appropriately solve (Tait et al., 2014). In order to overcome certain drawbacks of the WCST regarding its dependence on multiple cognitive mechanisms, such as subjects’ performance being affected by trying to recall previously salient rules or an inherent difficulty shifting attention from one perceptual feature of the cards (e.g., shape) to another (e.g., color), another neuropsychological test, the intradimensional (ID)/extradimensional (ED) task in the Cambridge Neuropsychological Automated Testing Battery (Sahakian and Owen., 1992), has also been successfully used in both human and non-human primates to assess attentional set-shifting capabilities. This task employs visual discriminations between pictorial features of the stimuli presented on a touchscreen (i.e., line segments superimposed onto abstract shapes) and has provided valuable information about executive function impairments in a plethora of mental and neurological disorders (Tait et al., 2014), as well as in TBI (Moore et al., 2010). Studies have shown that the WCST and ID/ED tasks recruit a widespread neural network of brain regions (Stuss et al., 2000) such as the prefrontal (PFC) and parietal cortices, basal ganglia, and hippocampus (Nyhus and Barcelo, 2009), all of which are vulnerable to injury and play a direct role in measured neurobehavioral sequelae (McAllister 2008; Schwarzbold et al., 2008; McAllister 2011). Therefore, neuropsychological difficulties associated with damage of PFC regions or afferent innervations comprise specific deficits in planning, cognitive flexibility, behavioral inhibition, poor organization of learning, memory and language formation. Considering the intricate direct or indirect connectivity of the PFC with a variety of brain regions, such as hippocampus, hypothalamus, amygdala, striatum or thalamus, there is a growing interest to investigate pathological mechanisms through which TBI results in abnormalities in the coordinated activation among these brain regions. Integrating an animal model of cognitive flexibility in the standard neurotrauma measures of behavior after brain injury is paramount to investigating complex cognitive problems and in finding therapies more relevant to the clinic.
4.2. The attentional set-shifting test: a measure of executive function in rodents
To specifically explore similar behavioral flexibility capabilities assessed in such neuropsychological tasks administered to humans and primates, an analogous ID/ED behavioral test was developed to measure attentional set-shifting and stimulus reversal learning performance in rats and demonstrated a pivotal role played by the rodent PFC in mediating such complex cognitive processes (Birrell and Brown, 2000; McAlonan and Brown, 2003). Moreover, this rodent task employs the natural foraging behavioral tendencies of rats by asking them to dig in small terracotta bowls to obtain a food reward, rather than learning visual discriminations. Using odor and tactile cues provides for fast acquisition of successive two-choice discriminations within a few hours, which is considerably faster than hundreds of trials of training and testing across multiple days for visual discrimination tasks (Tait et al., 2014). The attentional set-shifting test (AST) is characterized by a series of increasingly difficult perceptual discriminations to obtain a food reward that requires rodents (rats and mice) to form and maintain an attentional set or subsequently shift from one stimulus dimension to another that previously served as an overlapping distractor. Subjects are trained to retrieve a food reward from small terracotta pots containing distinctive digging media and marked with different odors on the upper rim. After they acquire the contingency on each stage to a criterion of six consecutive correct responses, the rules are changed and they progress to the next stage (Lapiz-Bluhm et al., 2008; Bondi et al., 2014). Subjects must learn to recognize the salient stimulus dimension (odor or digging medium) associated with the reward, based on a predetermined randomized order of stimulus presentation and overlying secondary dimension distractors, while facing rule changes as in the WCST or ID/ED task upon learning a given contingency (Bondi et al., 2008, 2014). In the first stage, the rats must learn to locate the reward based only on a single salient cue, either the odors applied to the rims of the two pots, or the texture of the digging material in each pot (simple discrimination). Then, the second dimension is introduced in a stage called the compound discrimination, serving as a distractor, but the salient cue remains the same. For example, if odor was the commencing relevant dimension for discriminating towards a reward, then two different digging media are introduced into the pots with odor remaining the salient cue. The third stage is a reversal, in which the negative cue from the relevant dimension (e.g. odor) in the previous stage becomes positive, and the positive cue from the previous stage is negative. The fourth stage is the ID shift, with all new stimuli (odors and media) being used, but in this case odor would still be relevant and media would still be irrelevant. After the subjects complete this new discrimination, a second reversal ensues. In all stages up to this point, the rats will have learned repeatedly that one stimulus dimension is informative when the rules are changed and an error is encountered (i.e. forming a cognitive set). The sixth stage (ED set shift) introduces an additional degree of difficulty, in that all new stimuli are again introduced, but the previously irrelevant dimension (e.g., medium) becomes relevant, and the previously informative dimension (e.g., odor) becomes irrelevant. The last stage is a final reversal (Table 2). The dependent measure in this test is the number of trials required to reach criterion on each stage (Lapiz-Bluhm et al., 2008).
4.3. The neurobiological basis of attentional set-shifting
Validity of the AST as a model of prefrontal cortical cognitive function in rats has been demonstrated initially using electrolytic or neurotoxic lesions to specific sub-regions of the prefrontal cortex. For example, lesions of medial PFC selectively disrupted ED set-shifting (Birrell and Brown, 2000), whereas orbitofrontal cortex (OFC) lesions impaired reversal learning (McAlonan and Brown, 2003). These results are in line with the brain region-related effect specificity using similar tests of ED set-shifting and reversal learning in primates (Dias et al., 1996, 1997). Cognitive inflexibility and perseveration in the AST have been previously described in rodent models of various neuropsychiatric and emotional disorders, such as in the spontaneously hypertensive rat, a genetic model of attention-deficit hyperactivity disorder (Cao et al., 2012), the neonatal ventral hippocampal lesion model (Brooks et al., 2012), the 6-hydroxydopamine model of Parkinson’s disease (Tait et al., 2007), in glutamate N-methyl-D-aspartate receptor antagonist-induced schizophrenia-like symptomatology (Broberg et al., 2009; Goetghebeur et al., 2010; Kos et al., 2011), aging-induced cognitive decline (Young et al., 2010) or chronic unpredictable (Bondi et al., 2008, 2010) or restraint stress (Liston et al., 2006) exposure. The neurobiological circuitry that facilitates attentional set-shifting in rats is primarily mediated in the PFC by ascending projections from various neurotransmitter systems, such as norepinephrine, but also dopamine (DA), serotonin, and acetylcholine (Aston-Jones and Cohen, 2005; Chudasama and Robbins, 2006; Robbins and Arnsten, 2009). Therefore, TBI-induced secondary and long-term sequelae involving disruption of endogenous neurotransmitter influences in the mPFC and OFC could be responsible for the impaired cognitive performance revealed by Bondi and colleagues (Bondi et al., 2014). This is consistent with a growing body of experimental TBI literature suggesting alterations in multiple neural pathways, such as neurotransmitter synthesis, release, or signaling, as well as effects on neurobehavioral outcome post-injury (Dixon et al., 1999; Cheng et al., 2008; Wagner et al., 2009; Shin et al., 2011; Olsen et al., 2012; Bondi et al., 2014; Wang et al., 2014).
4.4. Novel assessment of executive function in experimental TBI
Therefore, in order to begin to address alterations in frontal cortex-mediated executive function in a rodent model of TBI, Bondi and colleagues (2014) set out to investigate whether a CCI injury of various cortical deformation depths (2.6, 2.8, or 3.0 mm) would induce cortical impact depth-dependent cognitive deficits in the attentional performance of adult male rats on the AST (Bondi et al., 2014). TBI produced impact depth-dependent impairments in ED set-shifting and stimulus reversals, seen as a significant increase in the number of trials to reach criterion, as well as increased total response errors and set loss errors (i.e., failure to maintain acquisition of correct stimulus contingency), when assessed at 4 weeks post injury. Specifically, rats subjected to the milder TBI condition (2.6 mm impact depth) did not display cognitive deficits in the AST; however, rats in the TBI (2.8 mm) group showed impaired cognitive performance throughout all the measures evaluated in this task, such as trials to reach criterion, total errors, and set-loss errors, whereas rats in the TBI (3.0 mm) group also exhibited selective ED set-shifting deficits in trials to criterion and set-loss errors. Moreover, as a validation of surgery methodology and cortical impact depth-dependent injury severity, the TBI (3.0 mm) group displayed a significant increase in return to righting ability post-anesthesia, as well as significantly larger cortical lesion volumes, in comparison to the TBI (2.6 mm) group (Bondi et al., 2014). Also, there were no differences between sham (i.e., rats that received craniectomies) and naive (i.e., no manipulation) animals, suggesting that this task is not differentially impacted by anesthesia and/or craniectomy.
Taken together, these results provide a novel assessment of complex, frontal cortex-mediated cognitive processes, specifically executive function and behavioral flexibility, which were sensitively and reliably disrupted in this model of TBI (Bondi et al., 2014). Future studies could further evaluate pharmacological and cognitive rehabilitation therapies alone and in combination as a relevant preclinical paradigm, as well as elucidating mechanisms underlying the neuropsychological deficits. This is relevant considering that, similar to the study by Bondi et al. (2014) a growing body of clinical research also suggests that executive dysfunction is associated with the level of brain injury severity in TBI patients (Ord et al., 2010; Finnanger et al., 2013; Sorg et al., 2014). Moreover, cognitive recovery after TBI occurs in a variable time-dependent manner across individuals and may continue for years after injury (Millis et al., 2001).
5. Environmental enrichment: a preclinical model of neurorehabilitation after TBI
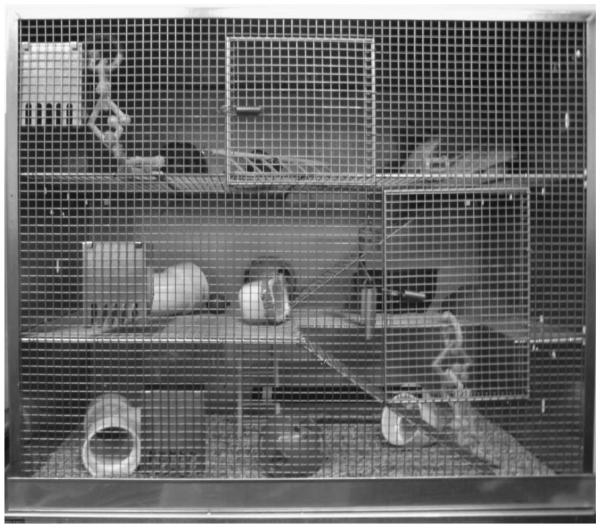
Environmental enrichment (EE) is an experimental strategy where housing is substantially different from that of usual experimental laboratory rats. Specifically, in the EE condition the rats are group-housed in an expansive living space and exposed to multiple objects of various shapes and sizes (Figure 2). The environment affords enrichment and integration of exploratory, physical, and social elements (Kline et al., 2007, Sozda et al., 2010; for comprehensive review see Bondi et al., 2014). This distinctive milieu is in marked contrast to standard (STD) housing where single or paired subjects live in traditional laboratory-sized cages and receive only basic amenities (i.e., food and water).
Figure 2.
Photograph of the environmental enrichment (EE) cage with multiple levels and wide array of sensory stimuli (e.g., balls, ramps, tubes, and nesting materials). Typically 10-12 rats, which include a subset of all experimental groups (i.e., both TBI and sham controls) are housed together to ensure equal conditions. The continuously housed rats are removed only briefly for behavioral assessments, weighing, and if applicable, drug administrations, while those in abbreviated rehabilitation paradigms are removed after their prescribed time and returned to STD laboratory cages.
As previously indicated, TBI produces a loss of cortical and hippocampal neurons, changes in neurotransmitter expression and function, and a downregulation of neurotrophin expression, all of which have been reported to correlate with behavioral dysfunction (McIntosh et al., 1994; Thompson et al., 2005; Bales et al., 2009). Because EE has been shown to affect neuroanatomical and neurochemical changes (Bennett et al., 1964; Diamond et al., 1966, 1976; La Torre 1968; Walsh et al., 1969) it seemed reasonable to theorize that these neuroplastic changes could affect the brain’s response to injury and perhaps serve as a potential rehabilitative therapy. Indeed, EE has consistently been shown to enhance motor and cognitive performance, as well as reduce histological damage after TBI (Passineau et al., 2001; Kline et al., 2007; 2010, 2012; Hoffman et al., 2008; Sozda et al., 2010; de Witt et al., 2011; Matter et al., 2011; Cheng et al., 2012) and thus has been likened to physiotherapy (Will et al., 2004; Kline et al., 2010).
5.1. Early and continuous enrichment: typical paradigm
5.1.1. Effects of EE in males after TBI
The typical EE protocol consists of placing rats (or mice), usually male, in the environment immediately after TBI with continuous exposure. Several studies have shown that this paradigm is beneficial after either FP or CCI injury. The first study to assess the potential benefits of EE after TBI was carried out by Hamm and colleagues (1996). Following a moderate FP injury or sham injury, the rats were placed in either EE or STD cages. Cognitive performance (i.e., acquisition of spatial learning) was measured using a MWM task on post-operative days 11-15. Rats housed in EE performed significantly better than STD controls, and did not differ from uninjured sham controls (Hamm et al., 1996). Passineau and colleagues (2001) and Hicks and coworkers (2002) observed similar behavioral benefits after FP injury. Moreover, EE lead to a reduction in cortical cavitation, suggesting a neuroprotective effect (Passineau et al., 2001). Adding multimodal early onset stimulation (i.e., auditory, motor, olfactory, and visual stimuli) to the EE paradigm was found to enhance motor, cognitive, and histological outcome after FP injury beyond that of EE alone (Maegele et al., 2005a,b; Lippert-Gruner et al., 2007). Regarding the effects of EE in pediatric rats, the behavioral outcomes after FP or CCI injury have been mixed. Specifically, no cognitive benefit was observed after a FP injury in PND 19 rats, despite evidence of plasticity (Fineman et al., 2000; Ip et al., 2002), while after CCI injury in PND 17 rats a robust cognitive benefit was observed (Monaco et al., 2014).
Following CCI injury Smith and colleagues (2007) reported faster recovery of skilled forelimb function in rats chronically exposed to EE post injury. Recently, Briones et al. (2013) showed that 4 weeks of EE significantly improved performance in a non-matching-to-sample task. EE has also been shown to provide benefit after bTBI. Kovesdi et al. (2011) used a rodent model of bTBI via a compression-driven shock tube (as described in a previous section) and assessed the rats on the elevated plus-maze and Barnes maze tests on days 15, 44 and 66 days post injury. While EE did not affect anxiety relative to STD, it did significantly improve spatial memory in the Barnes maze (Kovesdi et al., 2011).
EE-induced benefits have also been reported in adult male C57BL/J6 mice subjected to a FP injury. The mice exhibited enhanced locomotive activity (i.e., number of visits and nose pokes) at 24 hr post injury and complete restoration at 72 hr. Moreover, striatal cell loss at 72 hr post injury was significantly reduced compared to STD housing (Muthuraju et al., 2012).
5.1.2. Effects of EE in females after TBI
The first study assessing the potential efficacy of EE in females reported no significant benefit in the acquisition of spatial learning (Wagner et al., 2002). However, in a recent study designed to address this disparity, Monaco et al (2013) used experimental procedures that were similar to those of Wagner et al. (2002), which included adult female rats receiving a CCI injury at various phases of the estrous stage to mimic clinically relevant scenarios. Behaviorally, EE improved beam-balance, beam-walk, and rotarod performance as well as spatial learning vs. STD-housed females. Histologically, EE increased hippocampal CA1/3 cell survival and decreased cortical lesion volume (Monaco et al., 2013). The marked difference between the two CCI studies may be due to the inclusion of the rotarod task in the Monaco et al (2013) study, which may have augmented rehabilitation.
5.1.3. Endurance of EE benefits
While the aforementioned studies have shown for several years that EE confers benefits after TBI induced by various models, it was unknown until recently whether the benefits could be maintained after EE (i.e., rehabilitation) is discontinued. Demonstrating effect persistence is crucial for any therapeutic approach if it is to be successful in clinical translation. Hence, to evaluate the endurance of EE, Cheng et al. (2012) subjected rats to a CCI or sham injury and then during phase 1 of the experiment randomly assigned them to 3 weeks of EE or STD housing. Motor and cognitive assessments were performed using the standard beam and MWM tests described previously (in the models section). The findings showed that EE significantly improved both motor and cognition relative to STD controls, which replicated previous studies (Kline et al., 2007, 2010, 2012; Hoffman 2008). During phase 2 of the experiment, which was designed to mimic a real-world rehabilitative strategy, half of the improved rats in the EE condition were transferred back to STD housing conditions, thus mimicking patients completing rehabilitation. The rats were subsequently retested for motor and cognition at 1-month intervals for 6 months. The EE-continuous and EE-withdrawal groups performed better than the continuous STD-housed group, but did not differ from one another, which indicates that EE-induced motor and cognitive benefits are maintained for up to 6 months following the cessation of EE (Cheng et al., 2012). The long-term efficacy of EE further supports this paradigm as a potential preclinical model of neurorehabilitation.
5.2. Delayed and abbreviated EE
5.2.1. Delayed EE
While EE exposure immediately after TBI has shown marked benefits, realistically this paradigm is not clinically relevant because TBI patients will not engage in rehabilitation until after acute critical care. Hence, two studies in adult male rats were conducted to determine whether the EE-mediated benefits after TBI are dependent on early and constant exposure or whether delayed initiation could still provide benefits (Hoffman et al., 2008; Matter et al., 2011). Briefly, the findings from two separate studies showed that delaying EE by a week after TBI enhanced cognition to the same extent as the early and continuous paradigm. However, providing EE immediately after TBI for only one week was not beneficial. These findings suggest that there is a threshold of EE that is necessary to confer benefits (Hoffman et al., 2008; Matter et al., 2011). The timing of EE initiation is also important as shown by Giza and colleagues (2005) who subjected PND-19 rats to a FP injury and then assigned them to two different EE conditions. Specifically, one group received EE for 17 days from PND 20-37 and the other received the same amount of EE, but not until PND 34-50. The group exposed to EE sooner after FP injury performed significantly better in the MWM than those who received it later (Giza et al., 2005).
5.2.2. Abbreviated EE
In addition to the requisite delay in rehabilitation, another important timing issue to consider in modeling EE as a rehabilitative paradigm is that once rehabilitation is initiated the duration of the therapy will be limited. Typical time frames of rehabilitation in the clinical setting vary widely, but usually 3-8 hr is the norm (Blackerby 1990; Shiel et al., 2001; Zhu et al., 2007; Vanderploeg et al., 2008). Thus, to more accurately mimic the clinical scenario, de Witt et al. (2011) evaluated the efficacy of an abbreviated enrichment paradigm that consisted of 2, 4, or 6 hr of EE per day and compared those times to either continuous EE or STD housing. The findings revealed two important outcomes. First, providing only 2 or 4 hr of EE was ineffective as evidenced by the two groups not differing from the STD-housed group. Second, providing EE for 6 hr per day conferred significant improvements in motor and cognitive performance, and importantly for the validity of the model did not differ from the continuous EE group (de Witt et al., 2011) These findings suggest that abbreviated EE is sufficient for promoting optimal functional benefits after moderate TBI in rats, but also indicate that a minimum threshold of daily enrichment exposure may be required to elicit neurobehavioral recovery following TBI, as previously reported (Matter et al., 2011).
5.3. Combining EE with pharmacotherapies
5.3.1. EE plus 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT)
Successful translation of preclinical findings to the bench side may be, at least partially, attributed to the use of mono-therapies, as opposed to a more realistic approach of combining therapies with rehabilitation. Hence, the following section describes the effects of combining EE with acute or chronic administration of 8-OH-DPAT. In an initial study evaluating the effect of 8-OH-DPAT, it was shown that acute treatment (single administration at 15 min after TBI) promoted behavioral recovery and restored neuropathological alterations post TBI (Kline et al., 2002b, 2004a). Subsequently, Kline et al. (2007) sought to investigate whether combining 8-OH-DPAT with EE would confer additional benefit relative to either treatment alone. As per the acute pharmacotherapy regimen, the rats received a single administration of 8-OH-DPAT after TBI or sham injury and then were provided early and continuous EE. Both 8-OH-DPAT and EE exposure enhanced behavioral recovery and rescued hippocampal CA3 cell loss following TBI, which replicated previous studies (Kline et al., 2002b, 2004a). Cognitive differences were also observed following the combination of EE and 8-OH-DPAT vs. the STD plus 8-OH-DPAT group, with the former being significantly better, suggesting that EE enhanced the effect of 8-OH-DPAT. However, an additive effect of combined treatments could not be claimed because there were no significant differences between the combination treatment group and the EE alone group (Kline et al., 2007).
Chronic administration of 8-OH-DPAT has also been shown to reduce histopathology and improve neurobehavioral recovery after CCI injury (Cheng et al., 2008). Hence, to mimic the clinic where TBI patients may be provided pharmacotherapies chronically in concert with rehabilitation a subsequent study combined chronic 8-OH-DPAT with EE (Kline et al., 2010). Motor and cognitive recovery as well as an attenuation of hippocampal CA3 and choline acetyltransferase (ChAT) medial septal cell loss were reported when each therapy was provided singly. In combination, the therapies reduced TBI-induced ChAT cell loss, but did not enhance hippocampal cell survival or neurobehavioral performance beyond that of either treatment alone. The authors suggested that a possible explanation for the lack of an additive behavioral effect might be that the EE paradigm is a robust treatment and thus there may have been a ceiling effect that precluded 8-OH-DPAT from conferring additional statistically significant improvement.
5.3.2. EE plus buspirone
The 5-HT1A receptor agonist buspirone has also been shown to enhance spatial learning and reduce histopathology after TBI (Olsen et al., 2012). Moreover, this therapy is already used in the clinical setting to treat anxiety and depression, hence safety and tolerability parameters are well characterized (Chew and Zafonte, 2009). To determine whether the benefits of buspirone can be augmented further, Kline and colleagues (2012) administered buspirone to TBI or sham rats and then provided them rehabilitation in the form of EE (Kline et al., 2012). Buspirone alone also provided significant neurobehavioral recovery in injured rats. Furthermore, the TBI animals in EE, regardless of whether buspirone or vehicle-treated, had less CA3 hippocampal cell loss, improved motor function, and enhanced spatial learning and memory retention compared to STD-housed controls. Overall, these results demonstrated significant benefits by buspirone and EE. However, the prediction that greater benefits would ensue with the combination paradigm was not supported. The lack of an additive effect in adult rats may once again be attributable to a ceiling effect produced by an early-and-continuous EE paradigm.
In contrast, the combination of buspirone and EE after a TBI in PND-17 male rats produced an additive effect as revealed by the combined treatment group performing significantly better in the acquisition of spatial learning and memory relative to the buspirone rats housed in STD conditions and the EE rats receiving no other treatment (Monaco et al., 2014). A potential explanation for the differences in additive effects between the adult and immature rats is that in the latter there is a robust surge in synaptogenesis and subsequent changes in synaptic efficacy that is associated with cognitive processing (Teyler et al., 1989), which may make the developing brain more sensitive to the combination of EE and buspirone. These novel findings in pediatric rats may have potential rehabilitative implications for clinical pediatric TBI.
5.4. Potential mechanisms for EE-induced benefits after TBI
In addition to the reduction in ChAT-positive cell loss after TBI (Kline et al., 2010), EE induces additional neuroplastic changes after trauma that may mediate the behavioral benefits observed. For instance, several studies have reported significant changes in neurotrophin expression after TBI (Yang et al., 1996; Hicks et al., 1997, 1998, 1999, 2002; Chen et al., 2005). Using the fluid percussion model of TBI that was described earlier in this review, Hicks and colleagues (1997) reported increased expression of brain-derived neurotrophic factor (BDNF) mRNA in the CA3 and dentate gyrus at various time points after injury. Neurotrophin-3 (NT-3) mRNA was also significantly reduced in the dentate at 6 and 24 hr post injury, supporting the hypothesis that secondary events following TBI are multifaceted. Based on findings that long-term exposure to EE in a non-TBI setting modulates mRNA brain expression for these neurotrophins (Falkenberg et al., 1992; Torasdotter et al., 1998; Ickes et al., 2000), Hicks and colleagues (2002) sought to evaluate the relationship between EE and neurotrophin gene expression after TBI. It was found that 2 weeks of EE exposure facilitated the acquisition of spatial learning in the MWM task, but did not change BDNF, tyrosine receptor kinase-B, and NT-3 mRNA levels in hippocampal sub-regions.
EE has also been reported to alter DA after CCI injury (Yan et al., 2001, 2002; Wilson et al., 2005; Bales et al., 2009). Hence, Wagner and colleagues sought to further investigate gender-dependent responsiveness to EE and DA regulation post injury. It was found that dopamine transporter (DAT) protein expression in males undergoes larger decreases after CCI injury compared to females, but EE exposure has larger effects on post injury DAT expression in females vs. males (Wagner et al., 2005). Subsequent studies evaluating the effects of EE on the dopaminergic system found a downregulation of tyrosine hydroxylase (Shin et al., 2012).
In addition to changes in neurotrophins and dopaminergic markers that may mediate some of the benefits of EE, other potential mechanisms were recently elucidated by Briones and colleagues who showed that continuous EE reverses CCI-induced increases of the pro-inflammatory cytokines interleukin-1 beta and tumor necrosis factor alpha. EE also decreases interleukin-10 in the prefrontal cortex and hippocampus. Lastly, markers of brain energy homeostasis, such as the ratio of phosphorylated adenosine monophosphate-activated protein kinase and the ubiquitous mitochondrial creatine kinase were decreased post injury in the same brain regions, an effect significantly normalized by EE exposure (Briones et al., 2013).
Together, these studies reveal complex, region-specific, and potential gender differences in potential molecular mechanisms mediating EE-induced recovery; this elucidation may provide new avenues for pharmaceutical targets and rehabilitative approaches after TBI.
6. Evidence-based approach to sports concussion management
6.1. Sports concussions
Concussions are a form of mild TBI resulting in pathophysiological disturbances and neurological dysfunction imparted by biomechanical forces to the brain (McCrory et al., 2013). Common acute signs and symptoms include headache, dizziness, disorientation, memory impairment, nausea, light and noise sensitivity, dizziness, incoordination, vomiting, behavioral changes and rarely, loss of consciousness. It is estimated that over 3 million sports-related concussions occur annually in the U.S. (Langlois et al., 2006). Important considerations when investigating concussions in sport include the widespread occurrence of these injuries in youth, whose brains are still undergoing development, and the fact that after injury, there is an expectation of eventual return to play and potential risk of recurrent or repetitive injury. Understanding the science of sports concussions requires both a strong understanding of basic animal TBI models as well as a robust translational perspective to link neurobiological mechanisms to real-world clinical problems.
6.2. Post-concussive dysfunction
6.2.1. Clinical concussion and acute management
Following human concussion there is ongoing evidence of neural dysfunction. This can take the form of subjectively reported symptoms such as headache and dizziness, or observable clinical signs like amnesia, incoordination, or unconsciousness. In general these signs and symptoms peak within the first hours after injury and then gradually resolve over 7-10 days (McCrea et al., 2003). There is ample evidence that different domains of impairment may not resolve simultaneously, and most recent clinical guidelines advocate detecting and monitoring post-concussion dysfunction using multiple metrics, such as symptoms, cognition, balance, and reaction time. Multiple tools have been devised in an attempt to quantify these deficits, with each having its own advantages and challenges (Giza et al., 2013).
It is also increasingly recognized that there are a number of individual risk factors for more severe symptoms or more prolonged recovery after concussion. These include a prior history of concussions (Guskiewicz et al., 2003; Eisenberg et al., 2013), younger age (Field et al., 2003; Covassin et al., 2012), headache/migraine symptoms (Mihalik et al., 2005), acute mental status symptoms (amnesia, confusion or loss of consciousness) and premorbid behavioral or cognitive challenges. Other risk factors with varying degrees of supporting evidence include specific symptoms (e.g., fogginess or dizziness), family history of neurobehavioral disorders, family history of migraine and probably genetic factors.
The knowledge of the natural course of post-concussion recovery and the relationship of recovery to clinically measurable parameters has led to individualized concussion management, and abandonment of prior concussion severity scales (Giza et al., 2013). Multiple tools have been developed to standardize evaluation of symptoms, attention, memory, balance/coordination and reaction time. Some of these are part of the clinical history and examination, some are paper and pencil or computerized tests, and some require other devices. Furthermore, the determination of a given patient’s risk factors is a clinically useful way of anticipating who may require more time to recover.
6.2.2. Animal models and outcome measures
Animal models have significant challenges to accurately model all of the biomechanical parameters of human concussion. The mass and orientation of the human brain is different from all quadrupedal animals. Most animal investigations are done in rodents, which are lissencephalic and have relatively small proportions of white matter, while human brains are gyrencephalic and have proportionately large amounts of white matter.
Most animal TBI models attempt to quantitate injury by histological evidence that is not typically seen in human concussion and mild TBI. However, animal models may also quantitate post-TBI dysfunction using physiological parameters, such as glucose metabolism, cerebral blood flow, or electrophysiology. While older methods of measuring metabolism or blood flow may have required cross-sectional terminal studies, newer modalities of imaging are allowing longitudinal examination of brain water content, white matter integrity and even neural activation. Outcome assessments in animal models that are translatable to humans include behavioral tests of working and spatial memory, among others as previously described.
6.3. Vulnerability to second injury
6.3.1. Clinical vulnerability and repeat concussion
As mentioned above, having had a concussion is both a risk factor for sustaining another concussion and a risk factor for more severe symptoms or longer recovery after a second or recurrent concussion. It is known from multiple studies that a history of prior concussion increases the risk for subsequent concussion by a factor of about 3-fold (Guskiewicz et al., 2000). Several prospective studies have found that 75-90% of in-season repeat concussions happen within 7-10 days of the initial one (Guskiewicz et al., 2003; McCrea et al., 2009).
Other clinical studies have examined the severity or duration of symptoms. Again, evidence here suggests that recurrent concussions manifest either more severe acute symptoms or a more prolonged recovery (Guskiewicz et al., 2003; Eisenberg et al., 2013). These facts led to the recommendation that any player suspected of having a concussion be removed from contact-risk activity until evaluated by a health care professional with expertise and training in concussion.
While previous human correlates with the neurometabolic cascade of concussion involved intracranial sensors utilized in comatose patients with severe TBI, advanced neuroimaging now allows clinical researchers to noninvasively evaluate neurobiological changes after concussion/mild TBI. Diffusion tensor imaging (DTI) examines directional water diffusion which is used as a surrogate for integrity of white matter tracts. DTI measures change after TBI of all severities, although the exact time course, tract location and correlation with measurable symptoms or neurocognitive impairments varies between studies (Wilde et al., 2008; Mayer et al., 2012). Magnetic resonance spectroscopy provides a window into cerebral metabolism. Reductions of N-acetylaspartate/creatine (NAA/Cr) ratio have been reported after human sports concussion and may take 30 days to resolve. During this time, however, correlation with clinical symptoms is loose. Interestingly, for a small subset of subjects who sustained a second concussion before recovery from the first one, the reductions of NAA/Cr were larger and took 45 days until resolution (Vagnozzi et al., 2008, 2010). These data suggest there may be a biological window of vulnerability, but again, correlation with signs and symptoms is still needed to determine whether these imaging changes are indicative of clinically important dysfunction.
6.3.2. Animal models of repeat injury and impairment
There are an increasing number of experimental models producing repeated TBI. Most involve rodents, although a few studies of repeat injury in piglets have also been reported. More traditional experimental TBI models suffer from the limitation of requiring a craniectomy (i.e., FP and CCI) or having a significant risk for skull fracture (i.e., weight drop). Newer variations have used less severe weight drops or closed skull pneumatic impactors to induce less severe TBI that, in some cases, demonstrates minimal impairment after a single injury but significant cognitive dysfunction after repeated injury (Longhi et al., 2005; Prins et al., 2010).
Some of these models also show more severe histopathology after repeat concussive injury, including evidence of greater traumatic axonal injury. Experimental studies examining levels of energy metabolites have suggested a metabolic window of vulnerability during which repeated concussions have worse pathophysiology, but outside of which a second concussion behaves similarly to an isolated one (Vagnozzi et al., 2007). More recently, using 2-deoxyglucose autoradiography and carefully controlled timing of repeated injury in a rat closed head injury model, two injuries within 24 hours resulted in both worse metabolic activity and memory impairment, while if separated by 5 days metabolism and memory deficits were similar to a single injury (Prins et al., 2013). These animal studies strongly support the concept of a biological window of vulnerability and help support the clinical management strategy of keeping concussed athletes from returning to contact play early after injury.
6.4. Activating the injured brain
6.4.1. Recovery and return to activity in humans
Beyond the increased risk for repeat or more severe concussion attributable to premature return to play, there is a growing body of research investigating whether early neural stimulation itself may exacerbate or alleviate impairments. Initially based upon clinical observations that acute symptoms and signs of concussion may be exacerbated with increased cognitive effort, busy environments, sustained attention or stress, clinical management of post-concussive activity has run the gamut from the old school approach of back to normal activity right away to severe restriction of activities (e.g., including texting, studying, watching TV, or computer time) for a few days or as long as weeks-months in symptomatic individuals.
Beyond anecdotes, clinical studies suggest that extremes of activity were associated with greater subacute symptom burden, lower computerized cognitive test scores or prolonged recovery (Majerske et al., 2008; Brown et al., 2014). These data have led to most concussion management strategies to include a brief period of cognitive rest, followed by a gradual return to school/work, which is limited if symptoms increase. There is no controlled evidence that complete cognitive rest for more than a couple of days enhances recovery. Moreover, clinical studies of return to activity for other conditions (e.g., low back pain, chronic headache) suggest that bed rest or inactivity may actually prolong recovery (Malmivaara et al., 1995; Milde-Busch et al., 2010).
6.4.2. Neural activation following experimental injury
Early studies utilized a cortical lesion that resulted in contralateral motor impairment. If the unimpaired limb was restrained then the animal was forced to rely upon the impaired limb, a paradigm termed ‘forced overuse’. If the restraint was applied within the first week after the lesion, the increased activation was associated with a significantly larger cortical lesion. If the restraint were applied >1 week after the lesion, then lesion size was unaffected (Humm et al., 1998).
Animal models using non-lesion FP injury have also examined the timing of motor activation induced by voluntary wheel running. In these experiments, early running resulted in lower levels of hippocampal neurotrophic factors and worse cognition. However, if access to the running wheel were delayed until 1 week post-concussion, then exercise enhanced neurotrophin production and facilitated cognitive recovery (Griesbach et al., 2004; 2007). These data support an initial rest period, followed by a gradual increase in neural activation. Determining the window for optimal return to activity remains a challenge, but appears to loosely parallel the time course of neurometabolic recovery.
Lastly, in pediatric models of mild TBI, an impairment of experience dependent neuroplasticity in response to rearing in an enriched environment has been demonstrated (Fineman et al., 2000). Injured animals appear to interact normally with the novel environment, but fail to demonstrate the microanatomical changes (e.g., increased dendritic branching; Ip et al., 2002) or behavioral enhancements, such as improved spatial learning and memory (Giza et al., 2005) typically induced by such experience (as described in the preceding section). Interestingly, this period of altered responsiveness largely resolves after a couple weeks, but subtle deficits are still detectable. These data also support a window of deficient cerebral activation after concussion, and have the same potential significance and challenges as mentioned above.
6.5. Long-term consequences
6.5.1. Chronic cognitive impairment or chronic traumatic encephalopathy?
There is conflicting clinical evidence regarding the long-term consequences of repeated concussion, although in general the data support a dose-dependent cognitive impairment. Multiple studies of professional athletes, taking neurocognitive tests as well as some determination of the dose of their exposure to contact, show measurable neurocognitive impairments. Six of eight studies measured the ‘dose’ of concussion exposure in some way and in all six there was a dose-dependent relationship with chronic neurocognitive impairment, suggesting causality (Jordan et al., 1997; Kutner et al., 2000; Matser et al., 1998; Wall et al., 2006; Guskiewicz et al., 2005, 2007). However, in a much larger group of studies in amateur athletes, only half were found to exhibit chronic impairment. In any case, these data as a whole would indicate that repeated high level exposure to concussions may have a long-term cognitive cost (Giza et al., 2013). None of these studies specifically looked at neurodegeneration or progressive impairments.
There is also a high profile series of autopsy cases, mostly from professional athletes, but including amateurs as well and even high school athletes, where post-mortem pathological findings of neurodegeneration have been reported. The largest series proposes a condition termed chronic traumatic encephalopathy (CTE), which is characterized by aggregated tau deposits perivascularly and in the depths of cortical sulci in milder cases, and more pervasive and involving hippocampus/amygdala with evidence of atrophy in more severe cases (McKee et al., 2012). Not all reported cases had tauopathy, but the vast majority did, and in a pattern that was distinct from other known neurodegenerative conditions. A smaller case series of pathological specimens found that only half (3 of 6) had evidence of CTE-like tauopathy, but all also had evidence of another neurodegenerative disease at the same time (Hazrati et al., 2013).
Overall, there is credible evidence of some cognitive cost to high levels of exposure to collision/contact sports, with a dose-dependent relationship in multiple professional sports. This indicates there is a risk for chronic neurocognitive impairment associated with repeated concussions, but there are currently no longitudinal data to definitively prove that these impairments are degenerative/progressive. The case series suggest there is a subset of individuals who will develop pathological evidence of neurodegeneration, such as CTE, and these individuals are often symptomatic; however, the incidence and relative risk of developing CTE are unable to be determined from existing published reports.
6.5.2. Biological linkage of early pathophysiology with neurodegeneration
While extensive animal models exist for both TBI and for neurodegeneration, the biological linkage between the two remains to be clearly elucidated. Several hypotheses exist and studies are ongoing to better delineate these relationships.
Acute energy crisis is a fundamental component of TBI pathophysiology. While it is rarely lethal to the cell in mild forms of TBI, repeated injury, genetic susceptibilities or other factors may tip the balance where metabolically compromised cells pass a ‘point-of-no-return’. This could be mediated through excessive calcium flux, mitochondrial dysfunction, and activation of proteases. Protease activation can then lead to cytoskeletal disruption and triggering of delayed cell death pathways. There are multiple longitudinal studies after moderate-severe experimental TBI that demonstrate ongoing cell death and atrophy (Kochanek et al., 2002; Pullela et al., 2006; Immonen et al., 2009); long-term studies using models of repeated mild TBI/concussive injury are underway.
It is also known that there can be abnormal accumulation of proteins after TBI. Tau deposition has been described after FP and bTBI in rodents, but not chronically after repetitive mild TBI. Amyloid precursor protein accumulation is a commonly used measure for impaired axonal transport/axonal injury acutely/subacutely after experimental TBI, but chronic deposition of amyloid is distinct from this phenomenon. There is some evidence for chronic accumulation of amyloid in transgenic animals vulnerable to neurodegeneration (e.g., 3xTG-apoE4; Tran et al., 2011; Bennett et al., 2013).
Normal cellular protein homeostasis requires energy and a complex called the proteasome (Tanaka et al., 2012). Dysfunction of the proteasome can be triggered by acute or chronic oxidative stress. Proteasome dysfunction has been linked to abnormal/toxic accumulation of proteins, which in turn, have been implicated in various forms of neurodegeneration such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (Martinez et al., 2010). A few laboratory studies have demonstrated post-TBI proteasomal dysfunction; in one study it was also associated with increased apoptosis (Wan et al., 2014).
7. Conclusions
In this review, we discussed contemporary models of TBI and how their application alters behavioral responses, be it social, motor, or cognitive. We also described potential therapies that have been shown to attenuate TBI-induced behavioral deficits. Lastly, we provided a clinical perspective tying in the experimental models with the clinic, especially concussion. As a caveat, it should be noted that although the terms “model” and “device” were used interchangeably, it is not the intention of the authors to imply that a model of TBI is only a means of producing an injury. Rather, we believe that a model of TBI consists of a combination of device, species of animals used, location and severity of injury, choice of anesthesia, and the behavioral assessment tests employed.
While there are several differences and similarities between the in vivo models that have been presented, we contend that the observed differences between them are associated mainly with differences in the primary injury. This is most apparent in the morphologic response to experimental TBI where some models produce extensive cortical cavitation (e.g., CCI) while others do not (e.g., FP). We further state that the similarities between the models are associated with a relatively universal cascade of secondary injury processes that occur regardless of the physical/mechanical initiator of TBI. Despite not mimicking all the characteristics of human TBI, each of the in vivo models discussed have been instrumental in enhancing our knowledge about potential mechanisms of TBI. They have also afforded the opportunity to screen potential pharmacotherapies and rehabilitative approaches.
The identification of social deficits in animal models of TBI paves the way for the elucidation of underlying cellular and genetic mechanisms that may be targeted to improve long-term psychosocial outcomes and quality of life. Future studies to examine social behaviors relative to advanced neuroimaging correlates are warranted, to better understand the neuroanatomical basis underlying social dysfunction following childhood TBI. Consideration of potential sex-related differences in social behavior is also needed, with all experimental TBI studies to date focusing exclusively on males. As we move towards the goal of optimizing recovery after TBI, the inclusion of sociability measures when assessing candidate therapeutics will likely result in more rigorous standards for defining efficacy.
Current and ongoing findings employing the AST as a task of executive function and behavioral flexibility after brain injury are of significant relevance for expanding the validity of experimental models of TBI, by mimicking higher function cognitive impairments in humans, with the ultimate goal of improving rehabilitation and pharmacological treatment paradigms in the clinic. AST is a novel task to the neurotrauma field and warrants integration in the standard neurotrauma behavioral battery after TBI in order to evaluate sensitive cognitive dysfunction and subsequent relevant therapies.
From a rehabilitation perspective, the conclusions regarding EE as a therapy is that it benefits behavioral and histological outcome in male and female as well as adult and pediatric rats after brain injury produced by various models. Taken together, these findings provide the impetus for considering EE as a generalized and robust preclinical model of neurorehabilitation. The section has highlighted possible pharmacotherapies that impact dopaminergic and inflammatory pathways that can be coupled with EE. Development and refinement of these applicable variables will advance a clinically relevant experimental model of rehabilitation that can be applied to the TBI setting to facilitate rehabilitation research that can lead to increased translatability. The success of the transition from bed to bedside will be strengthened further by continuing to expand the model in males and females, as well as developing it further in pediatric and aged populations.
Current clinical management of sports-related concussions is still evolving, but good consensus and evidence-based guidelines are available. The focus is on removal of athletes with suspected concussion from play, to reduce the risk for repeat concussion, which is increased early after an initial concussive injury. Neural dysfunction after concussion manifests as symptoms such as headache and neurological impairments in cognition, reaction time, and balance. Multimodal clinical tests are available to assess these functions and may provide clinical guidance in the diagnosis of, and recovery from, concussion, but there is no single test to diagnose concussion accurately. Clinically identifiable risk factors for prolonged recovery have been identified (repeated concussion, younger age, migraine, etc), and have support from our understanding of basic neurobiology. Neural activation is impaired in the acute/subacute period of concussion recovery, suggesting that the highest level of activity may be associated with worse outcomes. However, translational studies also suggest that properly timed moderate activity may ultimately prove to facilitate recovery. Chronic neurocognitive impairment appears to be a consequence of higher levels of repetitive exposure to biomechanical force, and chronic neurodegeneration (i.e., CTE) has been described in case series of autopsy specimens, but the relative risk for CTE is unknown. Ongoing translational studies are underway to help better determine the biological linkage between acute TBI and chronic neurodegeneration.
Supplementary Material
Highlights.
in vivo TBI models provide essential understanding of mechanisms and recovery
Multiple deficits in social behavior can be detected after TBI in young rodents
The AST is a sensitive measure of executive function after experimental TBI
Environmental enrichment is a preclinical model of neurorehabilitation
No single test exists to diagnose concussion accurately
Acknowledgements
The authors appreciate the detailed work of Michael Farmer in recreating the Marmarou TBI model depicted in Figure 1C. This work was supported, in part, by National Institutes of Health grants HD069620, NS060005, NS084967 (AEK), NS050159, NS077767 (LJN-H), NS060672, NS079061 (CED), NS027544, HD061504 (CCG), Veterans Affairs B6761R (CED), a CJ Martin Overseas Biomedical Postdoctoral Fellowship from the National Health and Medical Research Council of Australia #1052505 (BDS), the Joseph Drown Foundation, NCAA, Today's and Tomorrow's Children Fund, UCLA BIRC, UCLA Steve Tisch BrainSPORT Program (CCG).
Abbreviations
- AST
attentional set-shifting test
- bTBI
blast traumatic brain injury
- BDNF
brain-derived neurotrophic factor
- CCI
controlled cortical impact
- CD
compound discrimination
- CFC
contextual fear conditioning
- ChAT
choline acetyltransferase
- Cr
creatine
- CTE
chronic traumatic encephalopathy
- DA
dopamine
- DAT
dopamine transporter
- DTI
diffusion tensor imaging
- ED
extradimensional
- EE
environmental enrichment
- FP
fluid percussion
- ID
intradimensional
- MWM
Morris water maze
- NAA
N-acetylaspartate
- NOR
novel-object recognition
- NT-3
neurotrophin-3
- OFC
orbitofrontal cortex
- PFC
prefrontal cortex
- PND
postnatal day
- R1
first reversal
- R2
second reversal
- R3
third reversal
- SD
simple discrimination
- STD
standard
- TBI
traumatic brain injury
- USV
ultrasonic vocalizations
- WCST
Wisconsin Card Sorting Test
- 5-HT1A
serotonin1A
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino) tetralin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR, Warden CR, Wright DW. Threshold for treatment of intracranial hypertension. Pediatr. Crit. Care. Med. 2003;4:S25–27. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 6. [PubMed] [Google Scholar]
- Adelson PD, Clyde B, Kochanek PM, Wisniewski SR, Marion DW, Yonas H. Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr. Neurosurg. 1997;26:200–207. doi: 10.1159/000121192. [DOI] [PubMed] [Google Scholar]
- Adelson PD, Robichaud P, Hamilton RL, Kochanek PM. A model of diffuse traumatic brain injury in the immature rat. J. Neurosurg. 1996;85:877–884. doi: 10.3171/jns.1996.85.5.0877. [DOI] [PubMed] [Google Scholar]
- Adelson PD, Whalen MJ, Kochanek PM, Robichaud P, Carlos TM. Blood brain barrier permeability and acute inflammation in two models of traumatic brain injury in the immature rat: a preliminary report. Acta Neurochir. Suppl. 1998;71:104–106. doi: 10.1007/978-3-7091-6475-4_31. [DOI] [PubMed] [Google Scholar]
- Alessandri B, Heimann A, Filippi R, Kopacz L, Kempski O. Moderate controlled cortical contusion in pigs: effects on multi-parametric neuromonitoring and clinical relevance. J. Neurotrauma. 2003;20:1293–1305. doi: 10.1089/089771503322686094. [DOI] [PubMed] [Google Scholar]
- An XL, Zou JX, Wu RY, Yang Y, Tai FD, Zeng SY, Jia R, Zhang X, Liu EQ, Broders H. Strain and sex differences in anxiety-like and social behaviors in C47BL/6J and BALB/cJ mice. Exper. Anim. 2011;60:111–123. doi: 10.1538/expanim.60.111. [DOI] [PubMed] [Google Scholar]
- Anderson V, Brown S, Newitt H. What contributes to quality of life in adult survivors of childhood traumatic brain injury? J. Neurotrauma. 2010;27:863–870. doi: 10.1089/neu.2009.1169. [DOI] [PubMed] [Google Scholar]
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374–1382. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
- Anderson V, Moore C. Age at injury as a predictor of outcome following pediatric head injury: a longitudinal perspective. Child Neuropsychol. 1995;1:187–202. [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Scent marking behavior in male C57Bl/6J mice: sexual and developmental determination. Behav. Brain Res. 2007;182:73–79. doi: 10.1016/j.bbr.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. A new test paradigm for social recognition evidenced by urinary scent marking behavior in C57BL/6J mice. Behav. Brain Res. 2008;190:97–104. doi: 10.1016/j.bbr.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead WM, Kurth CD. Different cerebral hemodynamic responses following fluid percussion brain injury in the newborn and juvenile pig. J. Neurotrauma. 1994;11:487–497. doi: 10.1089/neu.1994.11.487. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Gibson T, Callihan CT, Sullivan PG, Palmer E, Scheff SW. Neuronal cell loss in the CA3 subfield of the hippocampus following cortical contusion utilizing the optical dissector method for cell counting. J. Neurotrauma. 1997;14:385–398. doi: 10.1089/neu.1997.14.385. [DOI] [PubMed] [Google Scholar]
- Bales JW, Wagner AK, Kline AE, Dixon CE. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neurosci. Biobehav. Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Fritz H. Pathophysiology of traumatic injury in the developing brain: an introduction and short update. Exp. Toxicol. Pathol. 2004;56:65–73. doi: 10.1016/j.etp.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Bauman RA, Ling G, Tong L, Januszkiewicz A, Agoston D, Delanerolle N, Kim Y, Ritzel D, Bell R, Ecklund J, Armonda R, Bandak F, Parks S. An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J. Neurotrauma. 2009;26:841–860. doi: 10.1089/neu.2008.0898. [DOI] [PubMed] [Google Scholar]
- Bean NJ. Olfactory and vomeronasal mediation of ultrasonic vocalizations in male mice. Physiol. Behav. 1982;28:31–37. doi: 10.1016/0031-9384(82)90097-x. [DOI] [PubMed] [Google Scholar]
- Beauchamp KM, Anderson AJ. SOCIAL: an integrative framework for the development of social skills. Psychol. Bull. 2010;136:39–64. doi: 10.1037/a0017768. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Anderson VA, Catroppa C, Maller JJ, Godfrey C, Rosenfeld JV, Kean M. Implications of reduced callosal area for social skills after severe traumatic brain injury in children. J. Neurotrauma. 2009;26:1645–1654. doi: 10.1089/neu.2009.0916. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Beare R, Ditchfield M, Coleman L, Babl FE, Kean M, Crossley L, Catroppa C, Yeates KO, Anderson V. Susceptibility weighted imaging and its relationship to outcome after pediatric traumatic brain injury. Cortex. 2013;49:591–598. doi: 10.1016/j.cortex.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Dooley JJ, Anderson V. A preliminary investigation of moral reasoning and empathy after traumatic brain injury in adolescents. Brain Inj. 2013;27:896–902. doi: 10.3109/02699052.2013.775486. [DOI] [PubMed] [Google Scholar]
- Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and anatomical plasticity brain. Science. 1964;146:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- Bennett RE, Esparza TJ, Lewis HA, Kim E, Mac Donald CL, Sullivan PM, Brody DL. Human apolipoprotein E4 worsens acute axonal pathology but not amyloid-beta immunoreactivity after traumatic brain injury in 3xTG-AD mice. J. Neuropathol. Exp. Neurol. 2013;72:396–403. doi: 10.1097/NEN.0b013e31828e24ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg EA. A simple objective technique for measuring flexibility in thinking. J. Gen. Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching- to-sample learning task to study “recognition memory”. Nat. Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Yeates KO, Dennis M, Gerhardt CA, Rubin KH, Stancin T, Taylor HG, Vannatta K. Neuroimaging and social behavior in children after traumatic brain injury: Findings from the social outcomes of brain injury in kids (SOBIK) study. NeuroRehabilitation. 2013;32:707–720. doi: 10.3233/NRE-130896. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Felderhoff-Mueser U, Ikonomidou C. Apoptotic neurodegeneration in the context of traumatic injury to the developing brain. Exp. Toxicol. Pathol. 2004;56:83–89. doi: 10.1016/j.etp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blackerby WF. Intensity of rehabilitation and length of stay. Brain Inj. 1990;4:167–173. doi: 10.3109/02699059009026162. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Cheng JP, Tennant HM, Monaco CM, Kline AE. Old dog, new tricks: the attentional set-shifting test as a novel cognitive behavioral task after controlled cortical impact injury. J. Neurotrauma. 2014;31:926–937. doi: 10.1089/neu.2013.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Jett JD, Morilak DA. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:913–923. doi: 10.1016/j.pnpbp.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Green EJ, Dietrich WD, Busto R, Globus MY, Ginsberg MD. Posttraumatic brain hypothermia provides protection from sensorimotor and cognitive behavioral deficits. J. Neurotrauma. 1995;12:289–298. doi: 10.1089/neu.1995.12.289. [DOI] [PubMed] [Google Scholar]
- Briones TL, Woods J, Rogozinska M. Decreased neuroinflammation and increased brain energy homeostasis following environmental enrichment after mild traumatic brain injury is associated with improvement in cognitive function. Acta Neuropathol. Commun. 2013;1:57. doi: 10.1186/2051-5960-1-57. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Broberg BV, Glenthoj BY, Dias R, Larsen DB, Olsen CK. Reversal of cognitive deficits by an ampakine (CX516) and sertindole in two animal models of schizophrenia—sub-chronic and early postnatal PCP treatment in attentional set-shifting. Psychopharmacol (Berl.) 2009;206:631–640. doi: 10.1007/s00213-009-1540-5. [DOI] [PubMed] [Google Scholar]
- Brody DL, MacDonald C, Kessens CC, Yuede C, Parsadanian M, Spinner M, Kim E, Schwetye KE, Holtzman DM, Bayly PV. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J. Neurotrauma. 2007;24:657–673. doi: 10.1089/neu.2006.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JM, Pershing ML, Thomsen MS, Mikkelsen JD, Sarter M, Bruno JP. Transient inactivation of the neonatal ventral hippocampus impairs attentional set-shifting behavior: reversal with an alpha7 nicotinic agonist. Neuropsychopharmacology. 2012;37:2476–2486. doi: 10.1038/npp.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NJ, Mannix RC, O'Brien MJ, Gostine D, Collins MW, Meehan WP., III. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics. 2014;133:e299–304. doi: 10.1542/peds.2013-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budinich CS, Chen H, Lowe D, Rosenberger JG, Bernstock JD, McCabe JT. Mouse brain PSA-NCAM levels are altered by graded-controlled cortical impact injury. Neural Plast. 2012:378307. doi: 10.1155/2012/378307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch RM, McBride A, Curtiss G, Vanderploeg RD. The components of executive functioning in traumatic brain injury. J. Clin. Exp. Neuropsychol. 2005;27:1022–1032. doi: 10.1080/13803390490919263. [DOI] [PubMed] [Google Scholar]
- Byrnes KR, Loane DJ, Stoica BA, Zhang J, Faden AI. Delayed mGluR5 activation limits neuroinflammation and neurodegeneration after traumatic brain injury. J. Neuroinflammation. 2012;9:43. doi: 10.1186/1742-2094-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao AH, Yu L, Wang YW, Wang JM, Yang LJ, Lei GF. Effects of methylphenidate on attentional set-shifting in a genetic model of attention-deficit/hyperactivity disorder. Behav. Brain Funct. 2012;8:10. doi: 10.1186/1744-9081-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell WS, Maris DO, McCall T, Grady MS. Adaptation of the fluid percussion injury model to the mouse. J. Neurotraum. 1998;15:217–229. doi: 10.1089/neu.1998.15.217. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson V, Morse S, Haritou F, Rosenfeld J. Outcome and predictors of functional recovery 5 years following pediatric traumatic brain injury (TBI) J. Pediatr. Psychol. 2008;33:707–718. doi: 10.1093/jpepsy/jsn006. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Godfrey C, Rosenfeld JV, Hearps SS, Anderson VA. Functional recovery ten years after pediatric traumatic brain injury: outcomes and predictors. J. Neurotrauma. 2012;29:2539–2547. doi: 10.1089/neu.2012.2403. [DOI] [PubMed] [Google Scholar]
- Cattelani R, Lombardi F, Brianti R, Mazzucchi A. Traumatic brain injury in childhood: intellectual, behavioural and social outcome into adulthood. Brain Inj. 1998;12:283–296. doi: 10.1080/026990598122584. [DOI] [PubMed] [Google Scholar]
- Chapman LA, Wade SL, Walz NC, Taylor HG, Stancin T, Yeates KO. Clinically significant behavior problems during the initial 18 months following early childhood traumatic brain injury. Rehabil. Psychol. 2010;55:48–57. doi: 10.1037/a0018418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Noble-Haeusslein LJ, Ferriero D, Semple BD. Traumatic injury to the immature frontal lobe: A new murine model of long-term motor impairment in the absence of psychosocial or cognitive deficits. Dev. Neurosci. 2013;35:474–490. doi: 10.1159/000355874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li Y, Kline AE, Dixon CE, Zafonte RD, Wagner AK. Gender and environmental effects on regional brain-derived neurotrophic factor expression after experimental traumatic brain injury. Neuroscience. 2005;135:11–17. doi: 10.1016/j.neuroscience.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Chen Y, Constantini S, Trembovler V, Weinstock M, Shohami E. An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma. 1996;13:557–568. doi: 10.1089/neu.1996.13.557. [DOI] [PubMed] [Google Scholar]
- Cheng JP, Aslam HA, Hoffman AN, Zafonte RD, Kline AE. The neurobehavioral benefit conferred by a single systemic administration of 8-OH-DPAT after brain trauma is confined to a narrow therapeutic window. Neurosci. Lett. 2007;416:165–168. doi: 10.1016/j.neulet.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JP, Hoffman AN, Zafonte RD, Kline AE. A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav. Brain Res. 2008;194:79–85. doi: 10.1016/j.bbr.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JP, Shaw KE, Monaco CM, Hoffman AN, Sozda CN, Olsen AS, Kline AE. A relatively brief exposure to environmental enrichment after experimental traumatic brain injury confers long-term cognitive benefits. J. Neurotrauma. 2012;29:2684–2688. doi: 10.1089/neu.2012.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury-a state-of-the-art review. J. Rehabil. Res. Dev. 2009;46:851–879. doi: 10.1682/jrrd.2008.09.0120. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol. Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Claus CP, Tsuru-Aoyagi K, Adwanikar H, Walker B, Whetstone W, Noble-Haeusslein LJ. Age is a determinant of the inflammatory response and loss of cortical volume after traumatic brain injury. Dev. Neurosci. 2010;32:454–465. doi: 10.1159/000316805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez SC, McIntosh TK, Noble LJ. Experimental fluid percussion brain injury: vascular disruption and neuronal and glial alterations. Brain Res. 1989;482:271–282. doi: 10.1016/0006-8993(89)91190-6. [DOI] [PubMed] [Google Scholar]
- Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am. J. Sports Med. 2012;40:1303–1312. doi: 10.1177/0363546512444554. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin. Neurosci. 2012;14:293–305. doi: 10.31887/DCNS.2012.14.3/jcrawley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witt BW, Ehrenberg KM, McAloon RL, Panos AH, Shaw KE, Raghavan PV, Skidmore ER, Kline AE. Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury. Neurorehabil. Neural Repair. 2011;25:343–350. doi: 10.1177/1545968310390520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon HS, Donaldson D, Dempsey RJ, Prasad MR. Regional levels of free fatty acids and Evans blue extravasation after experimental brain injury. J. Neurotrauma. 1994;11:405–415. doi: 10.1089/neu.1994.11.405. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Ingham CA, Johnson RE, Bennett EL, Rosenzweig MR. Effects of environment on morphology of rat cerebral cortex and hippocampus. J. Neurobiol. 1976;7:75–85. doi: 10.1002/neu.480070108. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Law F, Rhodes H, Lindner B, Rosenzweig MR, Krech D, Bennett EL. Increases in cortical depth and glia numbers in rats subjected to enriched environment. J. Comp. Neurol. 1966;128:117–126. doi: 10.1002/cne.901280110. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex of the marmoset. Behav. Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from ‘on-line’ processing. J. Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didus E, Anderson VA, Catroppa C. The development of pragmatic communication skills in head injured children. Pediatr. Rehabil. 1999;3:177–186. doi: 10.1080/136384999289441. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R, Prado R, Zhao W, Dewanjee MK, Ginsberg MD. Posttraumatic cerebral ischemia after fluid percussion brain injury: an autoradiographic and histopathological study in rats. Neurosurgery. 1998;43:585–594. doi: 10.1097/00006123-199809000-00105. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Kochanek PM, Yan HQ, Schiding JK, Griffith RG, Baum E, Marion DW, DeKosky ST. One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J. Neurotrauma. 1999;16:109–122. doi: 10.1089/neu.1999.16.109. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, Hayes RL. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Donders J, Warschausky S. Neurobehavioral outcomes after early versus late childhood traumatic brain injury. J. Head Trauma Rehabil. 2007;22:296–302. doi: 10.1097/01.HTR.0000290974.01872.82. [DOI] [PubMed] [Google Scholar]
- Duhaime AC, Margulies SS, Durham SR, O'Rourke MM, Golden JA, Marwaha S, Raghupathi R. Maturation-dependent response of the piglet brain to scaled cortical impact. J. Neurosurg. 2000;93:455–462. doi: 10.3171/jns.2000.93.3.0455. [DOI] [PubMed] [Google Scholar]
- Duvoisin RM, Villasana L, Davis MJ, Winder DG, Raber J. Opposing roles of mGluR8 in measures of anxiety involving non-social and social challenges. Behav. Brain Res. 2011;221:50–54. doi: 10.1016/j.bbr.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg MA, Andrea J, Meehan W, Mannix R. Time interval between concussions and symptom duration. Pediatrics. 2013;132:8–17. doi: 10.1542/peds.2013-0432. [DOI] [PubMed] [Google Scholar]
- Elder GA, Dorr NP, De Gasperi R, Gama Sosa MA, Shaughness MC, Maudlin-Jeronimo E, Hall AA, McCarron RM, Ahlers ST. Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J. Neurotrauma. 2012;29:2564–2575. doi: 10.1089/neu.2012.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelborghs K, Verlooy J, Van Reempts J, Van Deuren B, Van de Ven M, Borgers M. Temporal changes in intracranial pressure in a modified experimental model of closed head injury. J. Neurosurg. 1998;89:796–806. doi: 10.3171/jns.1998.89.5.0796. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Grattan LM, Damasio H, Damasio AR. Developmental consequences of childhood frontal lobe damage. Arch. Neurol. 1992;49:764–769. doi: 10.1001/archneur.1992.00530310112021. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Fletcher JM, Levin HS, Francis DJ, Davidson K, Miner ME. Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. J. Int. Neuropsychol. Soc. 1997;3:581–591. [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Swank P, Kramer L, Cox CS, Jr., Fletcher JM, Barnes M, Zhang X, Hasan KM. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage. 2008;42:1305–1315. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Swank P, Kramer L, Mendez D, Treble A, Payne C, Bachevalier J. Social communication in young children with traumatic brain injury: Relations with corpus callosum morphometry. Int. J. Dev. Neurosci. 2012;30:247–254. doi: 10.1016/j.ijdevneu.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg T, Mohammed AK, Henriksson B, Persson H, Winblad B, Lindefors N. Increased expression of brain-derived neurotrophic factor mRNA in rat hippocampus is associated with improved spatial memory and enriched environment. Neurosci. Lett. 1992;138:153–156. doi: 10.1016/0304-3940(92)90494-r. [DOI] [PubMed] [Google Scholar]
- Fan P, Yamauchi T, Noble L, Ferriero D. Age-dependent differences in glutathione peroxidase activity after traumatic brain injury. J. Neurotrauma. 2003;20:437–445. doi: 10.1089/089771503765355513. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Tighe TJ. Contextual conditioning with massed versus distributed unconditional stimuli in the absence of explicit conditional stimuli. J. Exp. Psychol. Anim. Behav. Process. 1988;14:187–199. [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta (GA): 2010. [Google Scholar]
- Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP. Immune Activation Promotes Depression 1 Month After Diffuse Brain Injury: A Role for Primed Microglia. Biol Psychiatry. 2013;25:00950–00955. doi: 10.1016/j.biopsych.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J. Pediatr. 2003;142:546–553. doi: 10.1067/mpd.2003.190. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? Brit. J. Pharmacology. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineman I, Giza CC, Nahed BV, Lee SM, Hovda DA. Inhibition of neocortical plasticity during development by a moderate concussive brain injury. J. Neurotrauma. 2000;17:739–749. doi: 10.1089/neu.2000.17.739. [DOI] [PubMed] [Google Scholar]
- Finnanger TG, Skandsen T, Andersson S, Lydersen S, Vik A, Indredavik M. Differentiated patterns of cognitive impairment 12 months after severe and moderate traumatic brain injury. Brain Inj. 2013;27:1606–1616. doi: 10.3109/02699052.2013.831127. [DOI] [PubMed] [Google Scholar]
- Fox GB, Fan L, LeVasseur RA, Faden AI. Effect of traumatic brain injury on mouse spatial and nonspatial learning in the Barnes circular maze. J. Neurotrauma. 1998;15:1037–1046. doi: 10.1089/neu.1998.15.1037. [DOI] [PubMed] [Google Scholar]
- Fox GB, LeVasseur RA, Faden AI. Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to traumatic brain injury: implications for gene targeting approaches to neurotrauma. J. Neurotrauma. 1999;16:377–389. doi: 10.1089/neu.1999.16.377. [DOI] [PubMed] [Google Scholar]
- Friess SH, Ichord RN, Owens K, Ralston J, Rizol R, Overall KL, Smith C, Helfaer MA, Margulies SS. Neurobehavioral functional deficits following closed head injury in the neonatal pig. Exp. Neurol. 2007;204:234–243. doi: 10.1016/j.expneurol.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto ST, Longhi L, Saatman KE, Conte V, Stocchetti N, McIntosh TK. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 2004;28:365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Galef BG, Jr., Wigmore SW, Kennett DJ. A failure to find socially mediated taste aversion learning in Norway rats (R. norvegicus). J. Comp. Psychol. 1983;97:358–363. [PubMed] [Google Scholar]
- Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP. Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol. 1982;12:564–574. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- Giza CC, Griesbach GS, Hovda DA. Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav. Brain Res. 2005;157:11–22. doi: 10.1016/j.bbr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TS, Gioia GA, Gronseth GS, Guskiewicz K, Mandel S, Manley G, McKeag DB, Thurman DJ, Zafonte R. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the guideline development subcommittee of the american academy of neurology. Neurology. 2013;80:2250–2257. doi: 10.1212/WNL.0b013e31828d57dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetghebeur PJ, Lerdrup L, Sylvest A, Dias R. Erythropoietin reverses the attentional set-shifting impairment in a rodent schizophrenia disease-like model. Psychopharmacology (Berl.) 2010;212:635–642. doi: 10.1007/s00213-010-1990-9. [DOI] [PubMed] [Google Scholar]
- Goldstein M. Traumatic brain injury: a silent epidemic. Ann. Neurol. 1990;27:327. doi: 10.1002/ana.410270315. [DOI] [PubMed] [Google Scholar]
- Goodman JC, Cherian L, Bryan RM, Jr, Robertson CS. Lateral cortical impact injury in rats: pathologic effects of varying cortical compression and impact velocity. J. Neurotrauma. 1994;11:587–597. doi: 10.1089/neu.1994.11.587. [DOI] [PubMed] [Google Scholar]
- Graham DI, Raghupathi R, Saatman KE, Meaney D, McIntosh TK. Tissue tears in the white matter after lateral fluid percussion brain injury in the rat: relevance to human brain injury. Acta. Neuropathol. 2000;99:117–124. doi: 10.1007/pl00007414. [DOI] [PubMed] [Google Scholar]
- Grant DA, Berg EA. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type cardsorting problem. J. Exp. Psychol. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004;1016:154–162. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Gomez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J. Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- Guan X, Dluzen DE. Age related changes of social memory/recognition in male Fischer 344 rats. Behav. Brain. Res. 1994;61:87–90. doi: 10.1016/0166-4328(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP, Jr., Matthews A, Mihalik JR, Cantu RC. Recurrent concussion and risk of depression in retired professional football players. Med. Sci. Sports Exerc. 2007;39:903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, Onate JA, Kelly JP. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2549–2555. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Weaver NL, Padua DA, Garrett WE., Jr. Epidemiology of concussion in collegiate and high school football players. Am. J. Sports Med. 2000;28:643–650. doi: 10.1177/03635465000280050401. [DOI] [PubMed] [Google Scholar]
- Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Hall ED, Yonkers PA, McCall JM, Braughler JM. Effects of the 21-aminosteroid U74006F on experimental head injury in mice. J. Neurosurg. 1988;68:456–461. doi: 10.3171/jns.1988.68.3.0456. [DOI] [PubMed] [Google Scholar]
- Hamm RJ. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J. Neurotrauma. 2001;18:1207, 1216. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Temple MD, O’Dell DM, Pike BR, Lyeth BG. Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. J. Neurotrauma. 1996;13:41–47. doi: 10.1089/neu.1996.13.41. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. The structure and usage of female and mouse ultrasonic vocalizations reveal only minor differences. PLoS ONE. 2012;7:e41133. doi: 10.1371/journal.pone.0041133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannay HJ, Levin HS, Grossman RG. Impaired recognition memory after head injury. Cortex. 1979;15:269–283. doi: 10.1016/s0010-9452(79)80031-3. [DOI] [PubMed] [Google Scholar]
- Hazrati LN, Tartaglia MC, Diamandis P, Davis KD, Green RE, Wennberg R, Wong JC, Ezerins L, Tator CH. Absence of chronic traumatic encephalopathy in retired football players with multiple concussions and neurological symptomatology. Front. Hum. Neurosci. 2013;7:222. doi: 10.3389/fnhum.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes SB, Sebastian CL, Cohen Kadosh K. Brain Development and the Emergence of social function. In: Anderson V, Beauchamp MH, editors. Developmental Social Neuroscience and Childhood Brain Insult. The Guilford Press; New York: 2012. pp. 325–349. [Google Scholar]
- Hicks RR, Martin VB, Zhang L, Seroogy KB. Mild experimental brain injury differentially alters the expression of neurotrophin and neurotrophin receptor mRNAs in the hippocampus. Exp. Neurol. 1999;160:469–478. doi: 10.1006/exnr.1999.7216. [DOI] [PubMed] [Google Scholar]
- Hicks RR, Numan S, Dhillon HS, Prasad MR, Seroogy KB. Alterations in BDNF and NT-3 mRNAs in rat hippocampus after experimental brain trauma. Brain Res. Mol. Brain Res. 1997;48:401–406. doi: 10.1016/s0169-328x(97)00158-7. [DOI] [PubMed] [Google Scholar]
- Hicks RR, Zhang L, Atkinson A, Stevenon M, Veneracion M, Seroogy KB. Environmental enrichment attenuates cognitive deficits, but does not alter neurotrophin gene expression in the hippocampus following lateral fluid percussion brain injury. Neuroscience. 2002;112:631–637. doi: 10.1016/s0306-4522(02)00104-5. [DOI] [PubMed] [Google Scholar]
- Hicks RR, Zhang L, Dhillon HS, Prasad MR, Seroogy KB. Expression of trkB mRNA is altered in rat hippocampus after experimental brain trauma. Brain Res. Mol. Brain Res. 1998;59:264–268. doi: 10.1016/s0169-328x(98)00158-2. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Lasley LA, Akstulewicz SL. Middle age increases tissue vulnerability and impairs sensorimotor and cognitive recovery following traumatic brain injury in the rat. Behav. Brain Res. 2004:153. doi: 10.1016/j.bbr.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Malena RR, Westergom BP, Luthra P, Cheng JP, Aslam HA, Zafonte RD, Kline AE. Environmental enrichment-mediated functional improvement after experimental traumatic brain injury is contingent on task-specific neurobehavioral experience. Neurosci. Lett. 2008;431:226–230. doi: 10.1016/j.neulet.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofien D, Gilboa A, Vakil E, Donovick PJ. Traumatic brain injury (TBI) 10-20 years later: a comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Inj. 2001;15:189–209. doi: 10.1080/026990501300005659. [DOI] [PubMed] [Google Scholar]
- Horneman G, Emanuelson I. Cognitive outcome in children and young adults who sustained severe and moderate traumatic brain injury 10 years earlier. Brain Inj. 2009;23:907–914. doi: 10.1080/02699050903283239. [DOI] [PubMed] [Google Scholar]
- Humm JL, Kozlowski DA, James DC, Gotts JE, Schallert T. Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res. 1998;783:286–292. doi: 10.1016/s0006-8993(97)01356-5. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signalling in mice. BioEssays. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp. Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Potts MB, Noble-Haeusslein LJ. Injury severity determines Purkinje cell loss and microglial activation in the cerebellum after cortical contusion injury. Exp. Neurol. 2007;203:258–268. doi: 10.1016/j.expneurol.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Immonen RJ, Kharatishvili I, Grohn H, Pitkanen A, Grohn OH. Quantitative MRI predicts long-term structural and functional outcome after experimental traumatic brain injury. Neuroimage. 2009;45:1–9. doi: 10.1016/j.neuroimage.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Ip EY, Giza CC, Griesbach GS, Hovda DA. Effects of enriched environment and fluid percussion injury on dendritic arborization within the cerebral cortex of the developing rat. J. Neurotrauma. 2002;19:573–585. doi: 10.1089/089771502753754055. [DOI] [PubMed] [Google Scholar]
- Ji J, Kline AE, Amoscato A, Samhan-Arias AK, Sparvero LJ, Tyurin VA, Tyurina YY, Fink B, Manole MD, Puccio AM, Okonkwo DO, Cheng JP, Alexander H, Clark RS, Kochanek PM, Wipf P, Kagan VE, Bayır H. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat. Neurosci. 2012;15:1407–1413. doi: 10.1038/nn.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, Gandy S. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278:136–140. [PubMed] [Google Scholar]
- Karver CL, Wade SL, Cassedy A, Taylor HG, Stancin T, Yeates KO, Walz NC. Age at injury and long-term behavior problems after traumatic brain injury in young children. Rehabil. Psychol. 2012;57:256–265. doi: 10.1037/a0029522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley ML, Sinopoli KJ, Davis KD, Mikulis DJ, Wennberg R, Tartaglia MC, Chen JK, Tator CH. Is there evidence for neurodegenerative change following traumatic brain injury in children and youth? A scoping review. Front. Hum. Neurosci. 2014;8:139. doi: 10.3389/fnhum.2014.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Johnson CT, Govern JM. Recognition memory test: validity in diffuse traumatic brain injury. Appl. Neuropsychol. 1996;3:147–154. doi: 10.1080/09084282.1996.9645379. [DOI] [PubMed] [Google Scholar]
- Kennedy SH. Core symptoms of major depressive disorder: relevance to diagnosis and treatment. Dialogues Clin. Neurosci. 2008;10:271–277. doi: 10.31887/DCNS.2008.10.3/shkennedy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne M, Kuehn R, Tosun C, Caridi J, Keledjian K, Bochicchio G, Scalea T, Gerzanich V, Simard JM. Novel model of frontal impact closed head injury in the rat. J. Neurotrauma. 2009;26:2233–2243. doi: 10.1089/neu.2009.0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS, DeCola JP, Landeira-Fernandez J. Selective impairment of long-term but not short-term conditional fear by the N-methyl-D-aspartate antagonist APV. Behav. Neurosci. 1992;106:591–596. doi: 10.1037//0735-7044.106.4.591. [DOI] [PubMed] [Google Scholar]
- Klemenhagen KC, O'Brien SP, Brody DL. Repetitive concussive traumatic brain injury interacts with post-injury foot shock stress to worsen social and depression-like behavior in mice. PLoS ONE. 2013;8:e74510. doi: 10.1371/journal.pone.0074510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, Hoffman AN, Cheng JP, Zafonte RD, Massucci JL. Chronic administration of antipsychotics impede behavioral recovery after experimental traumatic brain injury. Neurosci. Lett. 2008;448:263–267. doi: 10.1016/j.neulet.2008.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, Massucci JL, Dixon CE, Zafonte RD, Bolinger BD. The therapeutic efficacy conferred by the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J. Neurotrauma. 2004a;21:175–185. doi: 10.1089/089771504322778631. [DOI] [PubMed] [Google Scholar]
- Kline AE, Massucci JL, Ma X, Zafonte RD, Dixon CE. Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J. Neurotrauma. 2004b;21:1712–1722. doi: 10.1089/neu.2004.21.1712. [DOI] [PubMed] [Google Scholar]
- Kline AE, Massucci JL, Marion DW, Dixon CE. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J. Neurotrauma. 2002a;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- Kline AE, McAloon RL, Henderson KA, Bansal UK, Ganti BM, Ahmed RH, Gibbs RB, Sozda CN. Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma. 2010;27:2021–2032. doi: 10.1089/neu.2010.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, Olsen AS, Sozda CN, Hoffman AN, Cheng JP. Evaluation of a combined treatment paradigm consisting of environmental enrichment and the 5-HT1A receptor agonist buspirone after experimental traumatic brain injury. J. Neurotrauma. 2012;29:1960–1969. doi: 10.1089/neu.2012.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, Wagner AK, Westergom BP, Malena RR, Zafonte RD, Olsen AS, Sozda CN, Luthra P, Panda M, Cheng JP, Aslam HA. Acute treatment with the 5-HT1A receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav. Brain Res. 2007;177:186–194. doi: 10.1016/j.bbr.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, Yu J, Massucci JL, Zafonte RD, Dixon CE. Protective effects of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) against traumatic brain injury-induced cognitive deficits and neuropathology in adult male rats. Neurosci. Lett. 2002b;333:179–182. doi: 10.1016/s0304-3940(02)01101-1. [DOI] [PubMed] [Google Scholar]
- Kochanek PM. Pediatric traumatic brain injury: Quo vadis? Dev. Neurosci. 2006;28:244–255. doi: 10.1159/000094151. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, Carson S, Chesnut RM, Ghajar J, Goldstein B, Grant GA, Kissoon N, Peterson K, Selden NR, Tasker RC, Tong KA, Vavilala MS, Wainwright MS, Warden CR. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr. Crit. Care Med. 2012;13(Suppl. 1):S1–82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Hendrich KS, Dixon CE, Schiding JK, Williams DS, Ho C. Cerebral blood flow at one year after controlled cortical impact in rats: assessment by magnetic resonance imaging. J. Neurotrauma. 2002;19:1029–1037. doi: 10.1089/089771502760341947. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Marion DW, Zhang W, Schiding JK, White M, Palmer AM, Clark RS, O'Malley ME, Styren SD, Ho C, DeKosky ST. Severe controlled cortical impact in rats: assessment of cerebral edema, blood flow, and contusion volume. J. Neurotrauma. 1995;12:1015–1025. doi: 10.1089/neu.1995.12.1015. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Cernak I, Xu L, Song Y, Savonenko A, Crain BJ, Eberhart CG, Frangakis CE, Melnikova T, Kim H, Lee D. A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J. Neuropathol. Exp. Neurol. 2011;70:399–416. doi: 10.1097/NEN.0b013e3182189f06. [DOI] [PubMed] [Google Scholar]
- Konrad K, Gauggel S, Manz A, Schöll M. Inhibitory control in children with traumatic brain injury (TBI) and children with attention deficit/hyperactivity disorder (ADHD) Brain Inj. 2000;14:859–875. doi: 10.1080/026990500445691. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J. Vis. Exp. 2013;4:e4367. doi: 10.3791/4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooy RF, D'Hooge R, Reyniers E, Bakker CE, Nagels G, De Boulle K, Storm K, Clincke G, De Deyn PP, Oostra BA, Willems PJ. Transgenic mouse model for the fragile X syndrome. Am. J. Med. Genet. 1996;64:241–245. doi: 10.1002/(SICI)1096-8628(19960809)64:2<241::AID-AJMG1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Kos T, Nikiforuk A, Rafa D, Popik P. The effects of NMDA receptor antagonists on attentional set-shifting task performance in mice. Psychopharmacol (Berl.) 2011;214:911–921. doi: 10.1007/s00213-010-2102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskiniemi M, Kyykkä T, Nybo T, Jarho L. Long-term outcome after severe brain injury in preschoolers is worse than expected. Arch. Pediatr. Adolesc. Med. 1995;149:249–254. doi: 10.1001/archpedi.1995.02170150029004. [DOI] [PubMed] [Google Scholar]
- Kovesdi E, Gyorgy AB, Kwon SK, Wingo DL, Kamnaksh A, Long JB, Kasper CE, Agoston DV. The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, proteomics, and histological study. Front. Neurosci. 2011;5:42. doi: 10.3389/fnins.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryavtseva NN. Use of the 'partition' test in behavioral and pharmacological experiments. Neurosci. Behav. Physiol. 2003;35:461–471. doi: 10.1023/a:1023411217051. [DOI] [PubMed] [Google Scholar]
- Kutner KC, Erlanger DM, Tsai J, Jordan B, Relkin NR. Lower cognitive performance of older football players possessing apolipoprotein E epsilon4. Neurosurgery. 2000;47:651–657. doi: 10.1097/00006123-200009000-00026. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MDS, Bondi CO, Doyen J, Rodriguez GA, Bedard-Arana T, Morilak DA. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J. Neuroendocrinol. 2008;20:1115–1137. doi: 10.1111/j.1365-2826.2008.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber AB, Egeth HE. Attention on autopilot: past experience and attentional set. Visual Cog. 2006;14:565–583. [Google Scholar]
- Levin H, Robertson CS. Mild TBI in translation. J. Neurotrauma. 2012;30:610–617. doi: 10.1089/neu.2012.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS. Cognitive function outcomes after traumatic brain injury. Curr. Opin. Neurol. 1998;11:643–646. doi: 10.1097/00019052-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Levin HS, Aldrich EF, Saydjari C, Eisenberg HM, Foulkes MA, Bellefleur M, Luerssen TG, Jane JA, Marmarou A, Marshall LF. Severe head injury in children: experience of the Traumatic Coma Data Bank. Neurosurgery. 1992;31:435–443. doi: 10.1227/00006123-199209000-00008. [DOI] [PubMed] [Google Scholar]
- Levin HS, Zhang L, Dennis M, Ewing-Cobbs L, Schachar R, Max J, Landis JA, Roberson G, Scheibel RS, Miller DL, Hunter JV. Psychosocial outcome of TBI in children with unilateral frontal lesions. J. Int. Neuropsychol. Soc. 2004;10:305–316. doi: 10.1017/S1355617704102129. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- La Torre JC. Effect of differential environmental enrichment on brain weight and on acetylcholinesterase and cholinesterase activities in mice. Exp. Neurol. 1968;22:493–503. doi: 10.1016/0014-4886(68)90144-1. [DOI] [PubMed] [Google Scholar]
- Lighthall JW. Controlled cortical impact: a new experimental brain injury model. J. Neurotrauma. 1988;5:1–15. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- Lighthall JW, Goshgarian HG, Pinderski CR. Characterization of axonal injury produced by controlled cortical impact. J. Neurotrauma. 1990;7:65–76. doi: 10.1089/neu.1990.7.65. [DOI] [PubMed] [Google Scholar]
- Lindgren S, Rinder L. Experimental studies in head injury. I. Pressure propagation in “percussion concussion.”. Biophysik. 1966;3:174–180. doi: 10.1007/BF01191611. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Plone MA, Cain CK, Frydel B, Francis JM, Emerich DF, Sutton RL. Dissociable long-term cognitive deficits after frontal versus sensorimotor cortical contusions. J. Neurotrauma. 1998;15:199–216. doi: 10.1089/neu.1998.15.199. [DOI] [PubMed] [Google Scholar]
- Lippert-Gruner M, Maegele M, Pokorny J, Angelov DN, Svestkova O, Wittner M, Trojan S. Early rehabilitation model shows positive effects on neural degeneration and recovery from neuromotor deficits following traumatic brain injury. Physiol. Res. 2007;56:359–368. doi: 10.33549/physiolres.930971. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JB, Bentley TL, Wessner KA, Cerone C, Sweeney S, Bauman RA. Blast overpressure in rats: recreating a battlefield injury in the laboratory. J. Neurotrauma. 2009;26:827–840. doi: 10.1089/neu.2008.0748. [DOI] [PubMed] [Google Scholar]
- Longhi L, Saatman KE, Fujimoto S, Raghupathi R, Meaney DF, Davis J, McMillan BSA, Conte V, Laurer HL, Stein S, Stocchetti N, McIntosh TK. Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery. 2005;56:364–374. doi: 10.1227/01.neu.0000149008.73513.44. [DOI] [PubMed] [Google Scholar]
- Luo J, Nguyen A, Villeda S, Zhang H, Ding Z, Lindsey D, Bieri G, Castellano JM, Beaupre GS, Wyss-Coray T. Long-term cognitive impairments and pathological alterations in a mouse model of repetitive mild traumatic brain injury. Front. Neurol. 2014;5:12. doi: 10.3389/fneur.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyeth BG, Jenkins LW, Hamm RJ, Dixon CE, Phillips LL, Clifton GL, Young HF, Hayes RL. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- Maegele M, Lippert-Gruener M, Ester-Bode T, Garbe J, Bouillon B, Neugebauer E, Klug N, Lefering R, Neiss WF, Angelov DN. Multimodal early onset stimulation combined with enriched environment is associated with reduced CNS lesion volume and enhanced reversal of neuromotor dysfunction after traumatic brain injury in rats. Eur. J. Neurosci. 2005a;21:2406–2418. doi: 10.1111/j.1460-9568.2005.04070.x. [DOI] [PubMed] [Google Scholar]
- Maegele M, Lippert-Gruener M, Ester-Bode T, Sauerland S, Schafer U, Molcanyi M, Lefering R, Bouillon B, Neiss WF, Angelov DN, Klug N, McIntosh TK, Neugebauer EA. Reversal of neuromotor and cognitive dysfunction in an enriched environment combined with multimodal early onset stimulation after traumatic brain injury in rats. J. Neurotrauma. 2005b;22:772–782. doi: 10.1089/neu.2005.22.772. [DOI] [PubMed] [Google Scholar]
- Majerske CW, Mihalik JP, Ren D, Collins MW, Reddy CC, Lovell MR, Wagner AK. Concussion in sports: postconcussive activity levels, symptoms, and neurocognitive performance. J. Athl. Train. 2008;43:265–274. doi: 10.4085/1062-6050-43.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmivaara A, Hakkinen U, Aro T, Heinrichs ML, Koskenniemi L, Kuosma E, Lappi S, Paloheimo R, Servo C, Vaaranen V. The treatment of acute low back pain--bed rest, exercises, or ordinary activity? N. Engl. J. Med. 1995;332:351–355. doi: 10.1056/NEJM199502093320602. [DOI] [PubMed] [Google Scholar]
- Manley GT, Rosenthal G, Lam M, Morabito D, Yan D, Derugin N, Bollen A, Knudson MM, Panter SS. Controlled cortical impact in swine: pathophysiology and biomechanics. J. Neurotrauma. 2006;23:128–139. doi: 10.1089/neu.2006.23.128. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J. Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- Martinez A, Portero-Otin M, Pamplona R, Ferrer I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol. 2010;20:281–297. doi: 10.1111/j.1750-3639.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matser JT, Kessels AG, Jordan BD, Lezak MD, Troost J. Chronic traumatic brain injury in professional soccer players. Neurology. 1998;51:791–796. doi: 10.1212/wnl.51.3.791. [DOI] [PubMed] [Google Scholar]
- Matter AM, Folweiler KA, Curatolo LM, Kline AE. Temporal effects of environmental enrichment-mediated functional improvement after experimental traumatic brain injury in rats. Neurorehabil. Neural Repair. 2011;25:558–564. doi: 10.1177/1545968310397206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max JE, Keatley E, Wilde EA, Bigler ED, Levin HS, Schachar RJ, Saunders A, Ewing-Cobbs L, Chapman SB, Dennis M, Yang TT. Anxiety disorders in children and adolescents in the first six months after traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2011;23:29–39. doi: 10.1176/jnp.23.1.jnp29. [DOI] [PubMed] [Google Scholar]
- Max W, Mackenzie EJ, Rice DP. Head injuries: costs and consequences. J. Head Trauma Rehabil. 1991;6:76–91. [Google Scholar]
- Mayer AR, Ling JM, Yang Z, Pena A, Yeo RA, Klimaj S. Diffusion abnormalities in pediatric mild traumatic brain injury. J. Neurosci. 2012;32:17961–17969. doi: 10.1523/JNEUROSCI.3379-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW. Neurobehavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatry. 2008;7:3–10. doi: 10.1002/j.2051-5545.2008.tb00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin. Neurosci. 2011;13:287–300. doi: 10.31887/DCNS.2011.13.2/tmcallister. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav. Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, Kelly JP. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz K, Randolph C, Barr WB, Hammeke TA, Marshall SW, Kelly JP. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65:876–882. doi: 10.1227/01.NEU.0000350155.89800.00. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvorak J, Echemendia RJ, Engebretsen L, Johnston K, Kutcher JS, Raftery M, Sills A, Benson BW, Davis GA, Ellenbogen RG, Guskiewicz K, Herring SA, Iverson GL, Jordan BD, Kissick J, McCrea M, McIntosh AS, Maddocks D, Makdissi M, Purcell L, Putukian M, Schneider K, Tator CH, Turner M. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br. J. Sports Med. 2013;47:250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenic JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McIntosh TK. Neurochemical sequelae of traumatic brain injury: therapeutic implications. Cerebrovasc. Brain Metab. Rev. 1994;6:109–162. [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, Lee HS, Hall G, Wojtowicz SM, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney DF, Ross DT, Winkelstein BA, Brasko J, Goldstein D, Bilston LB, Thibault LE, Gennarelli TA. Modification of the cortical impact model to produce axonal injury in the rat cerebral cortex. J. Neurotrauma. 1994;11:599–612. doi: 10.1089/neu.1994.11.599. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: Differential effects of d-amphetamine and cocaine. Psychopharmacology. 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav. Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Mihalik JP, Stump JE, Collins MW, Lovell MR, Field M, Maroon JC. Posttraumatic migraine characteristics in athletes following sports-related concussion. J. Neurosurg. 2005;102:850–855. doi: 10.3171/jns.2005.102.5.0850. [DOI] [PubMed] [Google Scholar]
- Milde-Busch A, Blaschek A, Borggrafe I, Heinen F, Straube A, von Kries R. Associations of diet and lifestyle with headache in high-school students: results from a cross-sectional study. Headache. 2010;50:1104–1114. doi: 10.1111/j.1526-4610.2010.01706.x. [DOI] [PubMed] [Google Scholar]
- Milders M, Ietswaart M, Crawford JR, Currie D. Social behavior following traumatic brain injury and its association with emotion recognition, understanding of intentions, and cognitive flexibility. J. Int. Neuropsychol. Soc. 2008;14:318–326. doi: 10.1017/S1355617708080351. [DOI] [PubMed] [Google Scholar]
- Millis SR, Dijkers M. Use of the recognition memory test in traumatic brain injury: preliminary findings. Brain Inj. 7:53–58. doi: 10.3109/02699059309008156. [DOI] [PubMed] [Google Scholar]
- Millis SR, Rosenthal M, Novack TA, Sherer M, Nick TG, Kreutzer JS, High WM, Jr., Ricker JH. Long-term neuropsychological outcome after traumatic brain injury. J. Head Trauma Rehabil. 2001;16:343–355. doi: 10.1097/00001199-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Molina J, Carmona-Mora P, Chrast J, Krall PM, Canales CP, Lupski JR, Reymond A, Walz K. Abnormal social behaviors and altered gene expression rates in a mouse model for Potocki-Lupski syndrome. Hum. Mol. Genet. 2008;17:2486–2495. doi: 10.1093/hmg/ddn148. [DOI] [PubMed] [Google Scholar]
- Monaco CM, Gebhardt KM, Chelbowski SM, Shaw KE, Cheng JP, Henchir JJ, Zupa MF, Kline AE. A combined therapeutic regimen of buspirone and environmental enrichment is more efficacious than either alone in enhancing spatial learning in brain-injured pediatric rats. J. Neurotrauma. 2014 doi: 10.1089/neu.2014.3541. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco CM, Mattiola VV, Folweiler KA, Tay JK, Yelleswarapu NK, Curatolo LM, Matter AM, Cheng JP, Kline AE. Environmental enrichment promotes robust functional and histological benefits in female rats after controlled cortical impact injury. Exp. Neurol. 2013;247:410–418. doi: 10.1016/j.expneurol.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DW, Ashman TA, Cantor JB, Krinick RJ, Spielman LA. Does gender influence cognitive outcome after traumatic brain injury? Neuropsychol. Rehabil. 2010;20:340–354. doi: 10.1080/09602010903250928. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mouzon B, Chaytow H, Crynen G, Bachmeier C, Stewart J, Mullan M, Stewart W, Crawford F. Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J. Neurotrauma. 2012;29:2761–2673. doi: 10.1089/neu.2012.2498. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav. Brain Res. 2008;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuraju S, Pati S, Rafiqul M, Abdullah JM, Jaafar H. IntelliCage provides voluntary exercise and an enriched environment, improving locomotive activity in mice following fluid percussion injury. Basal Ganglia. 2012;2:143–151. [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol. Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Njung'e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol. Biochem. Behav. 1991;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- Nyhus E, Barcelo F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn. 2009;71:437–451. doi: 10.1016/j.bandc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Ohayon S, Avni O, Taylor AL, Perona P, Egnor SER. Automated multi-day tracking of marked mice for the analysis of social behavior. J. Neurosci. Methods. 2013;219:10–19. doi: 10.1016/j.jneumeth.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo DO, Povlishock JT. An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J. Cereb. Blood Flow Metab. 1999;19:443–451. doi: 10.1097/00004647-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Olsen AS, Sozda CN, Cheng JP, Hoffman AN, Kline AE. Traumatic brain injury-induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT1A receptor agonist buspirone. J. Neurotrauma. 2012;29:1898–1907. doi: 10.1089/neu.2012.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord JS, Greve KW, Bianchini KJ, Aguerrevere LE. Executive dysfunction in traumatic brain injury: the effects of injury severity and effort on the Wisconsin Card Sorting Test. J. Clin. Exp. Neuropsychol. 2010;32:132–140. doi: 10.1080/13803390902858874. [DOI] [PubMed] [Google Scholar]
- Pandey DK, Yadav SK, Mahesh R, Rajkumar R. Depression-like and anxiety-like behavioural aftermaths of impact accelerated traumatic brain injury in rats: A model of comorbid depression and anxiety? Behav. Brain Res. 2009;205:436–442. doi: 10.1016/j.bbr.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Passineau MJ, Green EJ, Dietrich WD. Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Exp. Neurol. 2001;168:373–384. doi: 10.1006/exnr.2000.7623. [DOI] [PubMed] [Google Scholar]
- Pepin M, Dumont C, Hopps S. Relationship between cognitive capabilities and social participation among people with traumatic brain injury. Brain Inj. 2000;14:563–572. doi: 10.1080/026990500120466. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pierce JE, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87:359–369. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Pietropaoloa S, Branchia I, Cirullia F, Chiarottia F, Aloeb L, Alleva E. Long-term effects of the periadolescent environment on exploratory activity and aggressive behaviour in mice: social versus physical enrichment. Physiol. Behav. 2004;81:443–453. doi: 10.1016/j.physbeh.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Potts M, Koh S-E, Whetstone W, Walker B, Yoneyama T, Claus C, Manvelyan H, Noble-Haeusslein L. Traumatic injury to the immature brain: inflammation, oxidative injury, and iron-mediated damage as potential therapeutic targets. Neuroreport. 2006;3:143–153. doi: 10.1016/j.nurx.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Alexander D, Giza CC, Hovda DA. Repeated mild traumatic brain injury: mechanisms of cerebral vulnerability. J. Neurotrauma. 2013;30:30–38. doi: 10.1089/neu.2012.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Hales A, Reger M, Giza CC, Hovda DA. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev. Neurosci. 2010;32:431–441. doi: 10.1159/000316800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Lee SM, Cheng CL, Becker DP, Hovda DA. Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res. Dev. Brain Res. 1996;95:272–282. doi: 10.1016/0165-3806(96)00098-3. [DOI] [PubMed] [Google Scholar]
- Pullela R, Raber J, Pfankuch T, Ferriero DM, Claus CP, Koh S-E, Yamauchi T, Rola R, Fike JR, Noble-Haeusslein LJ. Traumatic injury to the immature brain results in progressive neuronal loss, hyperactivity and delayed cognitive impairments. Dev. Neurosci. 2006;28:396–409. doi: 10.1159/000094166. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu. Rev. Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosema S, Crowe L, Anderson V. Social function in children and adolescents after traumatic brain injury: a systematic review 1989-2011. J. Neurotrauma. 2012;29:1277–1291. doi: 10.1089/neu.2011.2144. [DOI] [PubMed] [Google Scholar]
- Rosema S, Muscara F, Anderson V, Godfrey C, Eren S, Catroppa C. Agreement on and predictors of long term psychosocial development 16 years post childhood traumatic brain injury. J. Neurotrauma. 2014;13:13. doi: 10.1089/neu.2013.3226. [DOI] [PubMed] [Google Scholar]
- Ross DT, Meaney DF, Sabol MK, Smith DH, Gennarelli TA. Distribution of forebrain diffuse axonal injury following inertial closed head injury in miniature swine. Exp. Neurol. 1994;126:291–299. doi: 10.1006/exnr.1994.1067. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav. Neurosci. 1999;113:867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Moy SS, Crawley JN. Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57Bl/6J mice. Behav. Brain Res. 2008;193:235–242. doi: 10.1016/j.bbr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan NP, Anderson V, Godfrey C, Beauchamp MH, Coleman L, Eren S, Rosema S, Taylor K, Catroppa C. Predictors of very long-term socio-cognitive function after pediatric traumatic brain injury: support for the vulnerability of the immature 'social brain. J. Neurotrauma. 2013;31:649–657. doi: 10.1089/neu.2013.3153. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J. R. Soc. Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- Scafidi S, Racz J, Hazelton J, McKenna MC, Fiskum G. Neuroprotection by acetyl-L-carnitine after traumatic injury to the immature rat brain. Dev. Neurosci. 2010;32:480–487. doi: 10.1159/000323178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Baldwin SA, Brown RW, Kraemer PJ. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J. Neurotrauma. 1997;14:615–627. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- Schneier AJ, Shields BJ, Hostetler SG, Xiang H, Smith GA. Incidence of pediatric traumatic brain injury and associated hospital resource utilization in the United States. Pediatrics. 2006;118:483–492. doi: 10.1542/peds.2005-2588. [DOI] [PubMed] [Google Scholar]
- Schwarzbold M, Diaz A, Martins ET, Rufino A, Amante LN, Thais ME, Quevedo J, Hohl A, Linhares MN, Walz R. Psychiatric disorders and traumatic brain injury. Neuropsychiatr. Dis. Treat. 2008;4:797–816. doi: 10.2147/ndt.s2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 2008;23:123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013;106-107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Canchola SA, Noble-Haeusslein L. Deficits in social behavior emerge during development after pediatric traumatic brain injury in mice. J. Neurotrauma. 2012;29:2672–2683. doi: 10.1089/neu.2012.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Noble-Hauesslein LJ, Jun Kwon Y, Sam PN, Gibson AM, Grissom S, Brown S, Adahman Z, Hollingsworth CA, Kwakye A, Gimlin K, Wilde EA, Hanten G, Levin HS, Schenk AK. Age-dependent sociosexual and communication deficits after traumatic injury to the developing murine brain. PLoS ONE. 20142014 doi: 10.1371/journal.pone.0103386. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira Y, Shohami E, Sidi A, Soffer D, Freeman S, Cotev S. Experimental closed head injury in rats: mechanical, pathophysiologic, and neurologic properties. Crit. Care Med. 1988;16:258–265. doi: 10.1097/00003246-198803000-00010. [DOI] [PubMed] [Google Scholar]
- Shiel A, Burn JP, Henry D, Clark J, Wilson BA, Burnett ME, McLellan DL. The effects of increased rehabilitation therapy after brain injury: results of a prospective controlled trial. Clin. Rehabil. 2001;15:501–514. doi: 10.1191/026921501680425225. [DOI] [PubMed] [Google Scholar]
- Shin SS, Bales JW, Yan HQ, Kline AE, Wagner AK, Lyons-Weiler J, Dixon CE. The effect of environmental enrichment on substantia nigra gene expression after traumatic brain injury in rats. J. Neurotrauma. 2013;30:259–270. doi: 10.1089/neu.2012.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SS, Bray ER, Zhang CQ, Dixon CE. Traumatic brain injury reduces striatal tyrosine hydroxylase activity and potassium-evoked dopamine release in rats. Brain Res. 2011;1369:208–215. doi: 10.1016/j.brainres.2010.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SR, Bao F, Omana V, Chiu C, Brown A, Cain DP. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal of repeated concussion. J. Neurotrauma. 2012;29:281–294. doi: 10.1089/neu.2011.2123. [DOI] [PubMed] [Google Scholar]
- Shultz SR, MacFabe DF, Foley KA, Taylor R, Cain DP. A single mild fluid percussion injury induces short-term behavioral and neuropathological changes in the Long-Evans rat: support for an animal model of concussion. Behav. Brain Res. 2011;224:326–335. doi: 10.1016/j.bbr.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioral phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J. Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- Smith JM, Lunga P, Story D, Harris N, Le Belle J, James MF, Pickard JD, Fawcett JW. Inosine promotes recovery of skilled motor function in a model of focal brain injury. Brain. 2007;130:915–925. doi: 10.1093/brain/awl393. [DOI] [PubMed] [Google Scholar]
- Sorg SF, Delano-Wood L, Luc N, Schiehser DM, Hanson KL, Nation DA, Lanni E, Jak AJ, Lu K, Meloy MJ, Frank LR, Lohr JB, Bondi MW. White matter integrity in veterans with mild traumatic brain injury: associations with executive function and loss of consciousness. J. Head Trauma Rehabil. 2014;29:21–32. doi: 10.1097/HTR.0b013e31828a1aa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosin DM, Sniezek JE, Waxweiler RJ. Trends in death associated with traumatic brain injury, 1979 through 1992. JAMA. 1995;273:1778–1780. [PubMed] [Google Scholar]
- Sozda CN, Hoffman AN, Olsen AS, Cheng JP, Zafonte RD, Kline AE. Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J. Neurotrauma. 2010;27:1047–1057. doi: 10.1089/neu.2010.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Spencer-Smith M, Anderson V. Healthy and abnormal development of the prefrontal cortex. Dev. Neurorehabil. 2009;12:279–297. doi: 10.3109/17518420903090701. [DOI] [PubMed] [Google Scholar]
- Spikeman JM, Timmerman ME, Milders MV, Veenstra WS, van der Naalt J. Social cognition impairments in relation to general cognitive deficits, injury severity, and prefrontal lesions in traumatic brain injury patients. J. Neurotrauma. 2012;29:101–111. doi: 10.1089/neu.2011.2084. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, Murphy KJ, Izukawa D. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38:388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Sullivan HG, Martinez J, Becker DP, Miller JD, Griffith R, Wist AO. Fluid percussion model of mechanical brain injury in the cat. J. Neurosurg. 1976;45:521–534. [PubMed] [Google Scholar]
- Sullivan JR, Riccio CA. Language functioning and deficits following pediatric traumatic brain injury. Appl. Neuropsychol. 2010;17:93–98. doi: 10.1080/09084281003708852. [DOI] [PubMed] [Google Scholar]
- Sullivan S, Friess SH, Ralston J, Smith C, Propert KJ, Rapp PE, Margulies SS. Improved behavior, motor, and cognition assessments in neonatal piglets. J. Neurotrauma. 2013;30:1770–1779. doi: 10.1089/neu.2013.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CR, Ivins B, Schwab KA. Traumatic brain injury in the United States: an epidemiologic overview. Mt. Sinai J. Med. 2009;76:105–110. doi: 10.1002/msj.20100. [DOI] [PubMed] [Google Scholar]
- Svetlov SI, Prima V, Kirk DR, Gutierrez H, Curley KC, Hayes RL, Wang KK. Morphologic and biochemical characterization of brain injury in a model of controlled blast overpressure exposure. J. Trauma. 2010;69:795–804. doi: 10.1097/TA.0b013e3181bbd885. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur. J. Neurosci. 2007;25:3719–3724. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- Tait DS, Chase EA, Brown VJ. Attentional set-shifting in rodents: a review of behavioural methods and pharmacological results. Curr. Pharm. Des. 2014 doi: 10.2174/1381612819666131216115802. PMID: 24345263 (E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- Tanaka K, Mizushima T, Saeki Y. The proteasome: molecular machinery and pathophysiological roles. Biol. Chem. 2012;393:217–234. doi: 10.1515/hsz-2011-0285. [DOI] [PubMed] [Google Scholar]
- Tanno H, Nockels RP, Pitts LH, Noble LJ. Breakdown of the blood-brain barrier after fluid percussive brain injury in the rat. Part 1: distribution and time course of protein extravasation. J. Neurotrauma. 1992;9:21–32. doi: 10.1089/neu.1992.9.21. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Laviola G. Scoring of social interactions and play in mice during adolescence. Curr. Prot. Toxicol. 2005:13.10.1–13.10.11. doi: 10.1002/0471140856.tx1310s26. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, Perkins AT, Harris KM. The development of long-term potentiation in hippocampus and neocortex. Neuropsychologia. 1989;27:31–39. doi: 10.1016/0028-3932(89)90088-2. [DOI] [PubMed] [Google Scholar]
- Thau-Zuchman O, Shohami E, Alexandrovich AG, Trembovler V, Leker RR. The anti-inflammatory drug carprofen improves long-term outcome and induces gliogenesis after traumatic brain injury. J. Neurotrauma. 2012;29:375–384. doi: 10.1089/neu.2010.1673. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Titus DJ, Sakurai A, Kang Y, Furones C, Jergova S, Santos R, Sick TJ, Atkins CM. Phosphodiesterase inhibition rescues chronic cognitive deficits induced by traumatic brain injury. J. Neurosci. 2013;33:5216–5226. doi: 10.1523/JNEUROSCI.5133-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Igarashi T, Ferriero DM, Noble LJ. Traumatic brain injury in the immature mouse brain: characterization of regional vulnerability. Exp. Neurol. 2002;176:105–116. doi: 10.1006/exnr.2002.7941. [DOI] [PubMed] [Google Scholar]
- Torasdotter M, Metsis M, Henriksson BG, Winblad B, Mohammed AH. Environmental enrichment results in higher levels of nerve growth factor mRNA in the rat visual cortex and hippocampus. Behav. Brain Res. 1998;93:83–90. doi: 10.1016/s0166-4328(97)00142-3. [DOI] [PubMed] [Google Scholar]
- Tran HT, Sanchez L, Esparza TJ, Brody DL. Distinct temporal and anatomical distributions of amyloid-beta and tau abnormalities following controlled cortical impact in transgenic mice. PLoS One. 2011;6:e25475. doi: 10.1371/journal.pone.0025475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuru-Aoyagi K, Potts M, Trivedi A, Pfankuch T, Raber J, Wendland M, Claus C, Koh S-E, Ferriero D, Noble-Haeusslein L. Glutathione peroxidase activity modulates recovery in the injured immature brain. Ann. Neurol. 2009;65:540–549. doi: 10.1002/ana.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgro E, Ria A, Marziale S, Zoccatelli G, Tavazzi B, Del Bolgia F, Sorge R, Broglio SP, McIntosh TK, Lazzarino G. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133:3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Tavazzi B, Floris R, Ludovici A, Marziali S, Tarascio G, Amorini AM, Di Pietro V, Delfini R, Lazzarino G. Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes--part III. Neurosurgery. 62:1286–1295. doi: 10.1227/01.neu.0000333300.34189.74. [DOI] [PubMed] [Google Scholar]
- Vanderploeg RD, Schwab K, Walker WC, Fraser JA, Sigford BJ, Date ES, Scott SG, Curtiss G, Salazar AM, Warden DL, Defense and Veterans Brain Injury Center Study, G. Rehabilitation of traumatic brain injury in active duty military personnel and veterans: Defense and Veterans Brain Injury Center randomized controlled trial of two rehabilitation approaches. Arch. Phys. Med. Rehabil. 2008;89:2227–2238. doi: 10.1016/j.apmr.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Chen X, Kline AE, Li Y, Zafonte RD, Dixon CE. Gender and environmental enrichment impact dopamine transporter expression after experimental traumatic brain injury. Exp. Neurol. 2005;195:475–483. doi: 10.1016/j.expneurol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Drewencki LL, Chen X, Santos FR, Khan AS, Harun R, Torres GE, Michael AC, Dixon CE. Chronic methylphenidate treatment enhances striatal dopamine neurotransmission after experimental traumatic brain injury. J. Neurochem. 2009;108:986–997. doi: 10.1111/j.1471-4159.2008.05840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Kline AE, Sokoloski J, Zafonte RD, Capulong E, Dixon CE. Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neurosci. Lett. 2002;334:165–168. doi: 10.1016/s0304-3940(02)01103-5. [DOI] [PubMed] [Google Scholar]
- Walker KR, Tesco G. Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front. in Aging Neurosci. 2013;5:1–25. doi: 10.3389/fnagi.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall SE, Williams WH, Cartwright-Hatton S, Kelly TP, Murray J, Murray M, Owen A, Turner M. Neuropsychological dysfunction following repeat concussions in jockeys. J. Neurol. Neurosurg. Psychiatry. 2006;77:518–520. doi: 10.1136/jnnp.2004.061044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RN, Budtz-Olsen OE, Penny JE, Cummins RA. The effects of environmental complexity on the histology of the rat hippocampus. J. Comp. Neurol. 1969;137:361–366. doi: 10.1002/cne.901370309. [DOI] [PubMed] [Google Scholar]
- Wan C, Chen J, Hu B, Zou H, Li A, Guo A, Jiang J. Downregulation of UBE2Q1 is associated with neuronal apoptosis in rat brain cortex following traumatic brain injury. J. Neurosci. Res. 2014;92:1–12. doi: 10.1002/jnr.23305. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. Bidirectional control of social hierachy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- Wang T, Huang XJ, Van KC, Went GT, Nguyen JT, Lyeth BG. Amantadine improves cognitive outcome and increases neuronal survival after fluid percussion traumatic brain injury in rats. J. Neurotrauma. 2014;31:370–377. doi: 10.1089/neu.2013.2917. [DOI] [PubMed] [Google Scholar]
- Washington PM, Forcelli PA, Wilkins T, Zapple DN, Parsadanian M, Burns MP. The effect of injury severity on behavior: a phenotypic study of cognitive and emotional deficits after mild, moderate, and severe controlled cortical impact injury in mice. J. Neurotrauma. 2012;29:2283–2296. doi: 10.1089/neu.2012.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R, Minnes P, Phillips M. Predicting social and functional outcomes for individuals sustaining pediatric traumatic brain injury. Dev. Neurorehab. 2009;12:12–23. doi: 10.1080/17518420902773109. [DOI] [PubMed] [Google Scholar]
- Whitney G, Nyby J. Cues that elicit ultrasounds from adult male mice. Am Zoologist. 1979:19. [Google Scholar]
- Wilde EA, Hunter JV, Newsome MR, Scheibel RS, Bigler ED, Johnson JL, Fearing MA, Cleavinger HB, Li X, Swank PR, Perdoza C, Roberson GS, Bachevalier J, Levin HS. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J. Neurotrauma. 2005;22:333–344. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990-2002) Prog. Neurobiol. 2004;72:167–182. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Wilson MS, Chen X, Ma X, Ren D, Wagner AK, Reynolds IJ, Dixon CE. Synaptosomal dopamine uptake in rat striatum following controlled cortical impact. J. Neurosci. Res. 2005;80:85–91. doi: 10.1002/jnr.20419. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011a;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Roullet FI, Hung AY, Sheng M, Crawley JN. Communication impairments in mice lacking Shank1: Reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS ONE. 2011b;6:e20631. doi: 10.1371/journal.pone.0020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Scattoni ML. Behavioural methods used in rodent models of autism spectrum disorders: Current standards and new developments. Behav. Brain Res. 2013;251:5–17. doi: 10.1016/j.bbr.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Wrenn CC. Social transmission of food preference in mice. Curr Protoc Neurosci. 2004:8. doi: 10.1002/0471142301.ns0805gs28. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Harris AP, Saavedra MC, Crawley JN. Social transmission of food preference in mice: methodology and application to galanin-overexpressing transgenic mice. Behav. Neurosci. 2003;117:21–31. [PubMed] [Google Scholar]
- Wright MJ, Wong AL, Obermeit LC, Woo E, Schmitter-Edgecombe M, Fuster JM. Memory for performed and observed activities following traumatic brain injury. J. Clin. Exp. Neuropsychol. 2014;36:268–277. doi: 10.1080/13803395.2014.884543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TC, Wilde EA, Bigler ED, Li X, Merkley TL, Yallampalli R, McCauley SR, Schnelle KP, Vasquez AC, Chu Z, Hanten G, Hunter JV, Levin HS. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev. Neurosci. 2010;32:361–373. doi: 10.1159/000317058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager JY, Thornhill JA. The effect of age on susceptibility to hypoxic-ischemic brain damage. Neurosci. Biobehav. Rev. 1997;21:167–174. doi: 10.1016/s0149-7634(96)00006-1. [DOI] [PubMed] [Google Scholar]
- Yamakami I, McIntosh TK. Alterations in regional cerebral blood flow following brain injury in the rat. J. Cereb. Blood Flow Metab. 1991;11:655–660. doi: 10.1038/jcbfm.1991.117. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Kline AE, Ma X, Hooghe-Peters EL, Marion DW, Dixon CE. Tyrosine hydroxylase, but not dopamine beta-hydroxylase, is increased in rat frontal cortex after traumatic brain injury. Neuroreport. 2001;12:2323–2327. doi: 10.1097/00001756-200108080-00009. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Kline AE, Ma X, Li Y, Dixon CE. Traumatic brain injury reduces dopamine transporter protein expression in the rat frontal cortex. Neuroreport. 2002;13:1899–1901. doi: 10.1097/00001756-200210280-00013. [DOI] [PubMed] [Google Scholar]
- Yang K, Perez-Polo JR, Mu XS, Yan HQ, Xue JJ, Iwamoto Y, Liu SJ, Dixon CE, Hayes RL. Increased expression of brain-derived neurotrophic factor but not neurotrophin-3 mRNA in rat brain after cortical impact injury. J. Neurosci. Res. 1996;44:157–164. doi: 10.1002/(SICI)1097-4547(19960415)44:2<157::AID-JNR8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, Wohr M, Roullet FI, Katz AM, Abrams DN, Kalikhman D, Simon H, Woldeyohannes L, Zhang JY, Harris MJ, Saxena JL, Silverman JL, Buxbaum JD, Crawley JN. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J. Neurosci. 2012;32:6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr. Prot. Neurosci. 2009:8.21.1–8.24.12. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Curr. Protoc. Neurosci. 2011 doi: 10.1002/0471142301.ns0826s56. Chapter 8, Unit 8.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Gustafson J, Gangidine M, Stepien D, Schuster R, Pritts TA, Goodman MD, Remick DG, Lentsch AB. A murine model of mild traumatic brain injury exhibiting cognitive and motor deficits. J. Surg. Res. 2013;184:981–988. doi: 10.1016/j.jss.2013.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, Taylor HG, Vannatta K. Social outcomes in childhood brain disorder: a heuristic integration of social neuroscience and developmental psychology. Psychol. Bull. 2007;133:535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Swift E, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N. Short- and long-term social outcomes following pediatric traumatic brain injury. J. Int. Neuropsychol. Soc. 2004;10:412–426. doi: 10.1017/S1355617704103093. [DOI] [PubMed] [Google Scholar]
- Yelleswarapu NK, Tay JK, Fryer WM, Shah MA, Garcia AN, Cheng JP, Kline AE. Elucidating the role of 5-HT1A and 5-HT7 receptors on 8-OH-DPAT-induced behavioral recovery after experimental traumatic brain injury. Neurosci Lett. 2012;515:153–156. doi: 10.1016/j.neulet.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DM, Schenk AK, Yang SB, Jan YN, Jan LY. Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. PNAS. 2010;107:11074–11079. doi: 10.1073/pnas.1005620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Geyer MA, Jeste DV, Risbrough VB. The mouse attentional-set-shifting task: a method for assaying successful cognitive aging? Cogn. Affect. Behav. Neurosci. 2010;10:243–251. doi: 10.3758/CABN.10.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SL, Bohenek DL, Fanselow MS. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behav. Neurosci. 1994;108:19–29. doi: 10.1037//0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Loane DJ, Murray MG, Stoica BA, Faden AI. Comparing the predictive value of multiple cognitive, affective, and motor tasks after rodent traumatic brain injury. J. Neurotrauma. 2012;29:2475–2489. doi: 10.1089/neu.2012.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Eucker SA, Durduran T, Yu G, Ralston J, Friess SH, Ichord RN, Margulies SS, Yodh AG. Diffuse optical monitoring of hemodynamic changes in piglet brain with closed head injury. J. Biomed Opt. 2009;14:034015. doi: 10.1117/1.3146814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XL, Poon WS, Chan CC, Chan SS. Does intensive rehabilitation improve the functional outcome of patients with traumatic brain injury (TBI)? A randomized controlled trial. Brain Inj. 2007;21:681–690. doi: 10.1080/02699050701468941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.