Abstract
Polydipsic hyponatremic schizophrenic (PHS) patients exhibit altered neuroendocrine activity that has been linked to their life-threatening water imbalance, as well as to impaired function and reduced volume of the anterior hippocampus. Polydipsic patients without hyponatremia (polydipsic normonatremic schizophrenics: PNS) exhibit similar, albeit less marked, changes in neuroendocrine activity and anterior hippocampal function, but not reduced anterior hippocampal volume. Indeed, reduced anterior hippocampal volume is seen in patients with normal water balance (nonpolydipsic normonatremic schizophrenics: NNS) whose neuroendocrine activity and anterior hippocampal function differ markedly from those with polydipsia. In an effort to reconcile these findings we measured hippocampal, amygdala and 3rd ventricle shapes in 26 schizophrenic patients (10 PNS, 7 PHS, 9 NNS) and 12 healthy controls matched for age and gender. Bilateral inward deformations were localized to the anterior lateral hippocampal surface (part of a neurocircuit which modulates neuroendocrine responses to psychological stimuli) in PHS and to a lesser extent in PNS, while deformations in NNS were restricted to the medial surface. Proportional deformations of the right medial amygdala, a key segment of this neurocircuit, were seen in both polydipsic groups, and correlated with the volume of the 3rd ventricle, which lies adjacent to the neuroendocrine nuclei. Finally, these structural findings were most marked in those with impaired hippocampal-mediated stress responses. These results reconcile previously conflicting data, and support the view that anterior lateral hippocampal pathology disrupts neuroendocrine function in polydipsic patients with and without hyponatremia. The relationship of these findings to the underlying mental illness remains to be established.
Keywords: hyponatremia, polydipsia, morphometry, 3rd ventricle, amygdala, stress diathesis, shape analysis, LDDMM
1. Introduction2
Increased water intake (Hoskins and Sleeper, 1933) and impaired water excretion (Targowla, 1923) have been linked to chronic psychotic disorders since the 1930's. In many, water excretory capacity deteriorates during psychotic exacerbations (Targowla, 1923) producing symptomatic hyponatremia (Barahal, 1938) and fatal water intoxication (Hobson and English, 1963; Raskind et al., 1975; Vieweg et al., 1985). About 3% of schizophrenic patients exhibit both increased intake and impaired excretion (polydipsic hyponatremic schizophrenia: PHS)) while another 15% exhibit increased intake alone (polydipsic normonatremic schizophrenics: PNS) (DeLeon et al., 1994; Goldman, 2009).
PHS have subsequently been shown to have unexplained increases in basal antidiuretic hormone (arginine vasopressin: AVP) activity (Goldman et al., 1988) which are further aggravated by acute psychosis (Goldman et al., 1997). Both PHS and PNS also exhibit persistent cortisol resistance to dexamethasone (`DST nonsuppression') (Goldman et al., 1993), which, along with the AVP dysfunction in PHS, appear attributable to a failure of the anterior hippocampus to normally restrain hormonal responses to psychological stress (Goldman et al., 2007a; Goldman, 2009). Interestingly, AVP and cortisol responses to psychological stress in patients without water imbalance (nonpolydipsic normonatremic schizophrenics: NNS) are indicative of enhanced hippocampal restraint (Goldman et al., 2007a).
PHS also exhibit reduced anterior hippocampal volume (Goldman et al., 2007b; Luchins et al., 1997) further linking this segment to the neuroendocrine findings. The anterior hippocampus, and specifically its lateral aspect containing the CA1 and subicular subfields, normally restrain AVP and HPAA responses to psychological stress (Cullinan et al., 1993; Eisenberg and Berman, 2010; Herman et al., 2006; Nettles et al., 2000; Poletti et al., 1973; Risold and Swanson, 1996). Indeed, disruption of the neurodevelopment of this region in an animal model of schizophrenia reproduces the neuroendocrine findings (Mitchell and Goldman, 2004). Preliminary data show oxytocin, a closely related neuropeptide, is diminished in PHS proportional to their anterior hippocampal volume and hippocampal-mediated neuroendocrine dysfunction (Goldman et al., 2007c; 2008). Finally, diminished oxytocin activity has been tentatively linked to clinical features of these patients psychotic illness (Goldman, 2009). In summary, these data suggest imagable anterior hippocampal pathology contributes to both the life-threatening neuroendocrine dysfunction and the underlying psychiatric disorder in hyponatremic schizophrenic patients.
Other data appear to conflict with this hypothesis. Thus, diminished anterior hippocampal volume has been linked to schizophrenia (Harrison et al, 2004; Schobel et al., 2009a; 2009b; Zhou et al., 2008) and is likely found in the majority of patients (Steen et al., 2006) who are not polydipsic and whose neuroendocrine function differs markedly from the polydipsic groups (i.e. NNS: Goldman, 2009 ; van Venrooij et al., 2010). Furthermore, anterior hippocampal volume is not reduced in PNS (Goldman et al., 2007b; Luchins et al., 1997) whose neuroendocrine function is intermediate to the hyponatremic (PHS) and nonpolydipsic (NNS) patients.
High dimensional brain mapping (HDBM) supplemented by principal components analysis of the resulting patterns (Csernanasky et al., 2004) can potentially reconcile these conflicting data by determining 1) if deformations in PHS overlie lateral subfields which modulate neuroendocrine responses to stress; 2) if similar but less pronounced deformations are seen in PNS; and 3) if deformations in NNS are restricted to the medial anterior surfaces. Shape changes on both the medial and lateral anterior hippocampus have been previously described (Csernanasky et al., 1998; 2002; Ho and Magnotta, 2010; Narr et al., 2004) in schizophrenia. In addition, we also analyzed amygdala and 3rd ventricle shapes because of the roles of the medial amygdala and the periventricular nuclei in modulating neuroendocrine responses to psychological stress (Aggleton, 1986; Cullinan et al., 1993; Herman and Mueller, 2006; Jankford and Herman, 2008) as well as in schizophrenia (Benes et al., 2010; Goldstein et al., 2007; Shenton et al., 2001).
Methods
2.1 Subjects
MRI scans from twenty six schizophrenic patients and 12 healthy controls matched for age and gender were reanalyzed from a previous study which found reduced anterior hippocampal volume in PHS, but not PNS, relative to other schizophrenic groups and controls (Goldman et al., 2007b). In addition, we report the relationship of structural findings in a subset of these subjects to their previously published neuroendocrine responses to both a psychological (cold pressor) and physiological (postural stimulus) stressor (Goldman et al., 2007a; 2008).
Psychiatric subjects with a previous diagnosis of schizophrenia or schizoaffective disorder were selected in a two step process in which grouping for water balance was first done by review of the previous medical laboratory records, and then confirmed with measures of urine and plasma osmolality obtained three times/weekly over a three week period. Mean plasma osmolality provides a reliable index of impaired water excretion (Goldman et al., 1996), while mean morning (0700h) and afternoon (1600) urine osmolality provide a reliable index of polydipsia in schizophrenic patients (Goldman et al., 1992).
All were on clinically determined doses of antipsychotic medications and several were on benztropine and valproic acid (Table 1). None was taking lithium, carbamazepine or thiazide diuretics, or had alcohol/substance abuse or dependence in the past year. Psychiatric diagnosis was confirmed at discharge in a multidisciplinary conference and relied largely on the Structured Clinical Interview for DSM-IV. Patients with schizoaffective disorder were included because identical disorders of water balance are frequently seen in these patients (De Leon et al., 1994; 1996), and recent evidence suggests schizophrenia and schizoaffective disorder share common neural and genetic mechanisms (Heckers, 2009). Healthy comparison subjects were recruited by advertisements placed around the University community. None had a personal or first-degree relative with a history of a DSM-IV Axis I disorder based on the SCID-NP (Version 2.0). All subjects provided informed witnessed consent to a protocol approved by the IRBs at University of Chicago and University of Illinois at Chicago.
Table 1.
Clinical measures and indices of water imbalance in patient groups
| Polydipsic | Nonpolydipsic | ||
|---|---|---|---|
| Hyponatremic (PHS) | Normonatremic (PNS) | Normonatremic (NNS) | |
| AM Plasma Osmolality, mmol/kg*(SD) | 279.4 (6.2) | 291.7 (4.5) | 289.5 (3.4) |
| PM Urine Osmolality, mmol/kg^ (SD) | 140 (154) | 258 (101) | 746 (368) |
| Age at onset, y (SD) | 23.8 (10.0) | 21.0 (4.3) | 20.6 (3.7) |
| PANSS Positive (SD) | 13.7 (5.7) | 16.4 (4.3) | 17.3 (7.5) |
| PANSS Negative (SD) | 18.4 (9.3) | 13.5 (4.2) | 13.8 (4.7) |
| PANSS General (SD) | 28.6 (11.1) | 27.0 (5.6) | 29.3 (10.3) |
| GAF (SD) | 35.8 (8.7) | 42.5 (7.2) | 41.1 (4.1) |
| Chlorpromazine Equiv., mgs/day@ (SD) | 482 (395) | 425 (259) | 532 (330) |
| Schizoaffective, No. (%) | 1 (14) | 1 (10) | 1 (11) |
| 2nd generation antipsychotic (%) | 3 (43) | 7 (70) | 3 (33) |
| Smoker (%)# | 4 (57) | 8 (80) | 3 (33) |
Group effect for plasma osmolality: P <.05; Post-hoc tests for hyponatremic polydipsic vs other two groups: each P<.05
Group effect for urine osmolality: P<.01; Post-hoc tests for nonpolydipsic normonatremic vs other two groups: each P < .01.
Three PHS, four PNS, and five NNS were receiving benztropine. Four patients PHS, four PNS, and one NNS received valproic acid.
There was a trend toward more smokers in the polydipsic normonatremic group than the other patient groups. No healthy controls were smokers.
All other group effects were not significant.
SD = Standard deviation
2.2 MRI acquisition
Whole brain images were obtained on a 3.0T-GE Signa LX scanner (GE Medical Systems, Milwaukee, WI). 3D T1 weighted images were acquired using a spoiled gradient-recalled echo sequence (TE = min-full; TR = 20 ms; 1.5 mm coronal slices, flip angle = 30°, FOV = 160 mm, matrix = 256 × 256). T2 weighted images were acquired using a fast spin-echo sequence (TE = 8ms, TR = 4,800 ms, 1.8 mm interleaved coronal slices, FOV = 160 mm, matrix = 256 × 256, echo train length = 8). Post acquisition processing was performed with BRAINS2 software (Magnotta et al., 2002). Tissue classification into white matter, gray matter and CSF was performed in 6 PHS, 6 PNS, 6 NNS and 10 healthy controls. Classification could not be performed in remaining subjects due to image artifacts. In addition to the shape analyses described below, parcellated hippocampal volumes (gray, white matter, CSF) in this subset were also not previously reported.
2.3 Shape Analysis
Right and left hippocampus (ICC = 0.72, n = 20), right and left amygdala (ICC = 0.77, n = 10) and 3rd ventricle (ICC = .99, n = 6) were manually segmented (Kates et al., 1997; Pantel et al., 2000) in the 38 subjects, as well as a separate healthy subject who provided the template structures. Large-deformation diffeomorphic metric mapping (LDDMM) (Beg et al., 2005) was used to inject the templates into the manual tracings in each subject (Qui and Miller, 2008). To quantify shape, a triangulated graph of points was superimposed onto the surface of each template and carried along as the template was transformed onto each target segmentation. An average surface constructed from all subjects was used as a reference from which linear displacements were calculated at each surface vertex and compared between groups to generate surface maps. To definitively compare shapes between subject groups, a pooled within-group covariance matrix was derived from the resulting transformation vectors, and the dimensionality of this matrix was reduced by using principal components analysis (PCA) revealing the major dimensions of shape variation (i.e., eigenvectors). We limited ourselves a priori to the first 5 eigenvectors for each surface (which generally capture 75 to 85% of the total shape variance). To visualize the overlap between the structural deformation defined by significant eigenvectors and the shape differences generated by the initial surface maps, we mapped the maximum and minimal deviations from the template surface defined by significant eigenvectors. This technique allows one to make shape comparisons independent of volume differences and thus there is no need to covary for overall hippocampal size/brain size, intracranial cavity, or height.
2.4 Statistics
Statistical analyses were performed by analysis of variance. Our primary outcome measures were the first five eigenvectors for the hippocampus, each defining unique aspects of deformations from the template surface. We transformed the main effect of group into polynomial contrasts in order to test the specific linear contrast interest: PHS>PNS>HC>NNS which we reasoned would be most sensitive to the predicted pattern of shape differences. This rank ordering (i.e. HC group between PHS/PNS and NNS) does not imply that healthy controls have greater deformations than NNS subjects, but instead that structural variations in patients with and without polydipsia are divergent (i.e. analogous to their neuroendocrine responses to stress) and more marked in PHS than PNS. The associated quadratic and cubic group contrasts were also assessed to help assure the specificity of the findings. Identical analyses were conducted on the amygdala and 3rd ventricle. Type I error for these ANOVAs was addressed by applying a Bonferroni correction. Neuroendocrine responses and their association with the structural findings were assessed with mixed effects linear regression as previously described (Goldman et al., 2007a, 2008) and employed the group contrasts defined above (see legend Table 2). Medication types were added separately as dummy variables (or chlorpromazine equivalents in the case of antipsychotics) in all these analyses to examine their contribution to the findings.
Table 2.
Association between neuroendocrine levels following a cold pressor test and indices of water intake to structural findings
| Neuroendocrine responses |
Water intake |
||||
|---|---|---|---|---|---|
| Vasopressin (Z, P) | ACTH (Z, P) | Oxytocin (Z,P) | UOsmAM (t,P) | UOsmPM (t,P) | |
| Linear group contrast w/o covariate # | 1.85, .06 | 2.23, .03 | 2.95, .004 | 4.9, .0001 | 6.7, .0001 |
| Covariate effects: | |||||
|
| |||||
| 3rd Ventricle volume | 1.40, .15 | 3.74,.0005 * | .66, .51 | .76, .45 | 1.63, .11 |
| Resulting linear group contrast | 1.76, .07 | 2.09, .03 | .74, .45 | 3.8, .001 | 5.0, .0001 |
|
| |||||
| Left hippocampal 5th eigenvector | 1.98, .05 | .15,.87 | 3.13, .001 * | .52, .86 | .24, .81 |
| Resulting linear group contrast | 1.78, .07 | 2.35, .01 | 3.48, .0005 | 3.8, .001 | 5.0, .0001 |
|
| |||||
| Right hippocampal 5th eigenvector | 1.37, .16 | .26,.79 | 1.60,.11 | .30, .76 | .51, .60 |
| Resulting linear group contrast | 1.80, .07 | 2.36, .018 | 3.43, .0006 | 4.0, .001 | 4.8,.0001 |
|
| |||||
| Left amygdala 5th eigenvector | 3.65, .0002 * | .30,.76 | 2.28, .02 | .03, .97 | .10, .91 |
| Resulting linear group contrast | 2.34, .019 | 3.24, .001 | 4.83, .0000 | 3.3, .003 | 5.34, .0001 |
|
| |||||
| Right amygdala 5th eigenvector | 1.95, .05 | .76, .44 | 2.36, .02 | .87, .39 | .82,.41 |
| Resulting linear group contrast | 2.33, .02 | 2.84, .004 | 5.65, .0000 | 3.6, .002 | 5.34, .0001 |
The table shows whether the structural measures defined in the text were significantly associated with neuroendocrine responses to a cold pressor test and to the indices of water intake in the psychiatric patients. The first row shows the significance of the linear group contrasts (PHS>PNS>HC>NNS) in the regression models for each of the dependent measures listed in the five vertical columns (see Supplemental Figure 2 for vasopressin and ACTH). The subsequent rows show the significance of each of the structural measures when added as covariates to these models, as well as the impact of their addition to the linear group contrast. A reduction in statistical significance of the group contrast indicates the covariate effect is not independent of the group effect, and thus is more likely an epiphenomenon, while an increase suggests an independent contribution.
AM and PM urine osmolality were analyzed by ANCOVA. Neuroendocrine responses were analyzed by mixed effects linear regression as described in Goldman et al. 2007a, 2008. This approach better accounts for the correlation between repeated measures and the variability in individual responses. For vasopressin and ACTH the statistic is for the interaction between the linear group contrast and the quadratic time trend (i.e. rise and fall). Other statistics are mean levels (i.e. there was no oxytocin response)
P<.05 after Bonferroni correction (i.e. 15 comparisons).
3. Results
3.1 Clinical data and parcellated hippocampal volumes
Subject groups consisted of seven polydipsic hyponatremic (PHS: 5 male, 45 ± 3 years), ten polydipsic normonatremic (PNS: 6 male, 42 ± 7 years), nine nonpolydipsic normonatremic (NNS: 6 male, 37 ± 11 years) schizophrenic patients and twelve healthy normals (HC: 8 male, 42 ± 12 years). Clinical indices of water imbalance differed significantly, as expected given the selection criteria (Table 1). In contrast to patients, none of the healthy controls smoked cigarettes nor were they taking psychotropic medications. Estimated pre-morbid IQ was higher in healthy controls but did not differ across patient groups (Table1). Other clinical (Table 1) and demographic measures did not differ across groups (Supplemental Table 1).
Left hippocampal gray matter volume differed (F3,24=3.0, P=.05: PHS:1.82ccs ± .21, PNS: 2.18 ±.23, NNS: 2.07 ±.27 ccs, HC: 2.18 ± .26), and post-hoc testing identified significant as well as trend-level reductions in PHS relative to HC (P <.05), and PNS (P = .08) respectively.
3.2 Surface Maps
3.21 Hippocampus
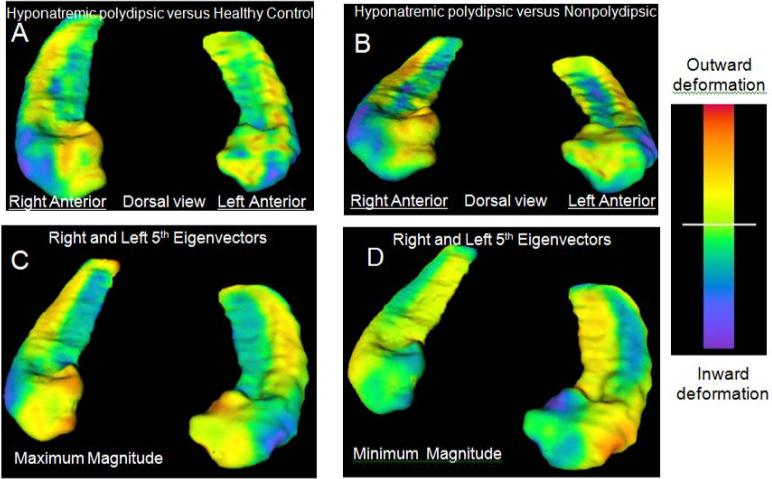
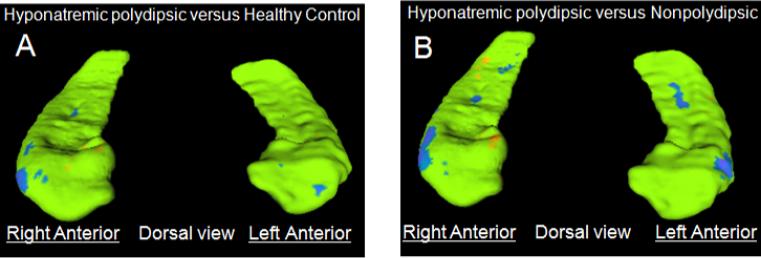
Inward deformations in PHS relative to HC (blue: Figure 1A) were, as predicted, centered on the dorsal anterior lateral surface of the hippocampus bilaterally (overlying the CA1 and subiculum) (Csernanasky et al., 2005). Less pronounced diffuse inward deformations were also seen on the medial surface of the hippocampal body, while diffuse outward deformations (orange) were apparent on the opposing surfaces. Significant differences between these two groups were largely limited, however, to the anterior lateral surface (blue: Figure 2A). Inward deformations in PHS relative NNS were similar (blue: Figure 1B), and as predicted even more extensive on the anterior lateral surfaces (blue: Figure 2B). In addition, there was, as predicted, an outward deformation in PHS on the right medial anterior surface (orange: Figure 1B, 2B) indicative of the inward deformation in NNS.
Figure 1.
Hippocampal shape differences. Panel A shows surface map of polydipsic hyponatremic schizophrenic patients (PHS) compared to healthy controls and Panel B is PHS compared to nonpolydipsic patients (NNS). Inward deformations in PHS patients are in blue in Panels A, B, inward deformations in NNS patients are in orange (minimal apparent) in panel B. See Figure 2 for surface map restricted to significant group differences. Panel C shows maximal (illustrative of PHS group) and Panel D shows minimal (illustrative of NNS group) deformations captured by the 5th eigenvector. Note the blue on the anterior lateral surfaces in C overlaps the blue in A, B and in Figure 2 A,B; and that blue on the anterior medial surface in D overlaps the orange in B and in Figure 2B. The range in A,B is 0 to ±3 mm, and in C,D is 0 to ±1 mm.
Figure 2.
Hippocampal shape differences showing statistically significant (P <.05: surface point-wise, without multiple comparison correction) deformations in the surface maps of PHS compared to healthy controls (Panel A) and PHS compared to nonpolydipsic patients (Panel B) Note the inward deformations on the bilateral anterior lateral surfaces in PHS (A,B: blue), and on the right anterior medial surface in NNS (B: orange).
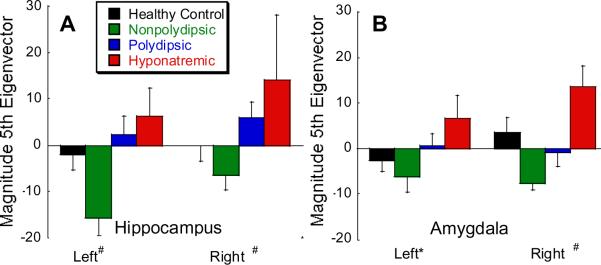
Mean eigenvectors capture discrete shape differences across groups. The figure shows group mean (± SD) magnitude of the 5th eigenvectors for the hippocampus (A) and amygdala (B). Note the magnitudes parallel the neuroendocrine responses in Figure 4 which were the basis for the predicted linear contrast (PHS>PNS>HC>NNS). See Figures 1C,D and 3 C,D for maps of the extreme values of these eigenvectors and to assess their overlap with the corresponding surface maps. Vector magnitude PHS>PNS>HC>NNS: #P < .01; * p < .10 (Bonferonni corrected for multiple comparisons).
Shape differences were objectified and compared across groups by reducing each subjects' variation from the template surface into unique elements with principal components analysis. The first five eigenvectors on each side accounted for 80% of the shape variance for all subjects. The 5th eigenvectors differed across groups (Figure 3A: left: F3,34= 4.90, P = .006, Bonferonni corrected P = .03; right: F3,34= 4.37, P = .010, Bonferonni corrected P = .05) and were ordered in the predicted manner (PHS>PNS>HC>NNS; left F1,34= 12.4, P = .001, Bonferonni corrected P = .005; right F1,34= 13.0, P = .001, Bonferroni corrected P = .005;). Neither the quadratic nor cubic group contrasts approached significance. The maximum extent of these eigenvectors (blue in Figure 1C: illustrative of the PHS group) overlap the inward deformations in PHS while the minimal extent (blue in Figure 1D: illustrative of NNS group) overlap the inward deformation seen on the right medial surface in NNS (orange in Figure 1B and Figure 2B). Indeed Figure 1A closely resembles 1C, and, if one inverts the colors (i.e blue for orange) Figure 1B resembles Figure 1D. Thus the signifcant vector differences appear to encompass the above described predicted differences on the surface maps.
Figure 3.
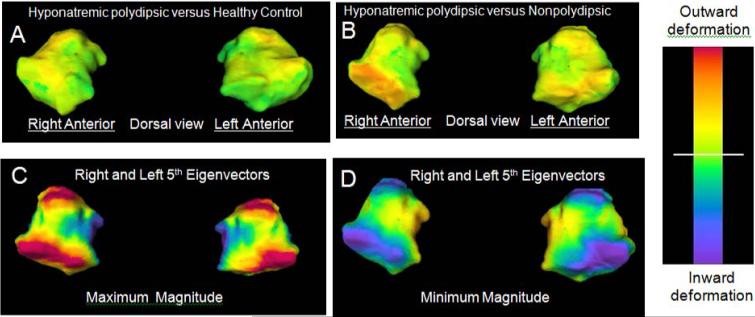
Amygdala shape differences. Note the minimal shape differences between PHS and HC in Panel A. Red/orange colors in Panel B reflect inward deformations on the anterior surface in NNS relative to PHS. Panel C shows maximal (illustrative of PHS group) and Panel D shows minimal (illustrative of NNS group) deformations captured by the 5th eigenvector. Note the blue on the medial surface in C, D reflecting inward deformations in PHS, and the blue on the anterior and posterior lateral surfaces reflecting inward deformations in NNS.
3.21 Amygdala and 3rd ventricle
Amygdala surface maps comparing PHS relative to HC (Figure 4A) and PHS relative to NNS (Figure 4B) were unremarkable, except for an outward deformation on the anterior lateral surface in PHS relative to NNS (orange: Figure 4B), indicative of a relative inward deformation in NNS. Still, the fifth eigenvector on the right differed across groups (F3,29= 5.92, P = .003, Bonferonni corrected P < .02) and the group linear contrast defined above was again significant (Figure 2B: F1,29= 11.0, Bonferonni corrected P = .002) suggesting that there were more subtle shape differences paralleling those in the hippocampus. The 5th eigenvector on the left showed similar but insignificant group differences (Figure 3B: F3,29= 2.27, P = .10; left linear contrast F1,29= 6.7, Bonferonni corrected: P = .07). The maximum extent of these eigenvectors (Figure 4C: illustrative of PHS group) demonstrated outward deformations on the anterior and posterior surfaces (4C: red) and inward deformations (blue) on the medial surface. The latter overlies the medial nucleus (Heimer et al., 1999) which is innervated by the anterior lateral hippocampus and modulates HPAA responses to stress (Benes, 2010; Jankford and Herman, 2008). The minimum extent (Figure 4D: illustrative of NNS group) demonstrated outward deformations on both the anterior and posterior surfaces and overlapped with the outward deformations on the surface map (orange: Figure 4B) indicative of inward deformations in NNS.
Figure 4.
Arginine vasopressin (AVP: Panel A) and adrenocorticotropin (ACTH: Panel B) responses to a psychological (cold pressor) and physiologic (postural) stressor (inset). Note that group responses to the cold pressor, but not the postural stimulus, generally parallel group differences in the structural measures shown in Figure 2. See Goldman et al., 2007a for further information on this study.
Outward deformations of the 3rd ventricle were seen in the surface map comparing PHS and NNS. These deformations (orange: Supplemental Figure 1) were located adjacent to the supraoptic and paraventricular nuclei, less marked differences were seen in the PHS relative to HC groups (not shown). None of the first five eigenvectors, however, differed across groups for this structure.
3.3 Correlations between structural data
The 5th eigenvector from the left hippocampus was correlated with the 5th eigenvector from the right amygdala (r = .463, df = 33, P = .007), which in turn was correlated with 3rd ventricle volume (r = .504, df = 33, P = .003). Both the hippocampal eigenvectors were correlated with 3rd ventricle volume (left: r = .550, df = 38, P < .001; right: r = .571, df = 38, P < .001). These correlations appear robust as they were largely independent of predicted group effects (L hippocampus/R amygdala: partial r controlling for predicted group contrast = .445, df = 30, P = .011; R amygdala/3rd ventricle partial r = .469, df = 30, P = .007; Left hippocampus/3rd ventricle partial r =.464, df = 35, P =.004; R hippocampus/3rd ventricle partial r = .489, df = 35, P = .002).
3.4 Relationship of neuroendocrine responses to structural findings
Twenty-five subjects (7 PHS, 6 PNS, 5 NNS, 5 HC) in this study also participated in a study characterizing neuroendocrine responses to a cold pressor (a primarily psychological stressor) (Supplemental Figure 2 A,B) and to a postural stimulus (a primarily physiological stressor) (Supplemental Figure 2 insets) (Goldman et al., 2007a; Goldman et al., 2008). Differences in the group responses to these stimuli (or, in the case of oxytocin, absolute levels) in this subset resembled those seen in the entire sample, that is AVP and ACTH responses to the cold pressor were enhanced in PHS, intermediate in PNS and blunted in NNS relative to healthy controls (Supplemental Figure 2A,B). While there was not oxytocin response to the cold pressor, absolute levels were ordered in the same manner (Goldman et al., 2008).
The first row of Table 2 (first three columns) shows the extent that the rise and fall in AVP and ACTH (i.e. quadratic component of time response) and absolute oxytocin levels differ across groups in the previously described order (PHS>PNS>HC>NNS) and recapitulate findings of the previously summarized studies. Subsequent rows show the effect of adding 3rd ventricle volume and the hippocampal and amygdala eigenvectors as covariates to each of the statistical models for these hormones and thus assesses if the structural measures make an independent contribution beyond that associated with the predicted group effect. Seven of 15 covariates were significant (Table 2). In contrast, structural measures did not correlate with the two indices of water intake (Table 2, last column) suggesting shape differences were not predictive of polydipsia.
Discussion
These results reconcile conflicting evidence regarding whether neuroendocrine dysfunction in schizophrenic patients with water imbalance is attributable to anterior hippocampal pathology. Thus, vasopressin, adrenocorticotropin and oxytocin functioning are altered in polydipsic hyponatremic (PHS) and, to a lesser extent, in polydipsic normonatremic schizophrenic (PNS) patients. The hormonal findings are suggestive of anterior hippocampal dysfunction, and in PHS are accompanied by evidence of structural pathology (i.e. reduced anterior hippocampal volume). Anterior hippocampal volume in PNS, however, resembles that of healthy controls, while the majority of schizophrenic patients, who are neither polydipsic nor hyponatremic (NNS), have consistently been reported to have diminished anterior hippocampal volumes, yet NNS exhibit neuroendocrine responses that diverge from those of the two polydipsic groups.
The greater structural detail provided by high definition brain mapping has reconciled this discrepancy: PHS, and to a lesser extent, PNS exhibit bilateral inward deformations on the anterior lateral hippocampal surface (Figure 1, 2A; Figure 3) which overlies subfields known to modulate neuroendocrine responses to psychological stress while nonpolydipsic patients (NNS) show anterior hippocampal deformations restricted to the medial surface (Figure 1 B,D; Figure 2B). These findings were predicted from the previous literature, apparent on surface maps and confirmed with principal components analysis to be significantly different across groups. Furthermore, the findings were robust in that they were apparent after controlling for multiple comparisons.
Additional support for the involvement of the anterior hippocampus in the neuroendocrine dysfunction comes from examining the shapes of associated structures and assessing whether these are also correlated with each other and the neuroendocrine dysfunction. While the findings were not predicted as a result of a prior literature or as robust or consistent, distinct patterns of shape deformations in the right medial amygdala (Figure 3B) overlay an area closely associated with anterior lateral hippocampal function (Figure 4 C,D) (Benes, 2010; Cullinan et al., 1993; Herman et al., 2006; Jankford and Herman, 2008) and were correlated with the shape changes in the left hippocampus and volume of the 3rd ventricle. Finally, the structural findings in the left hippocampus, right amygdala and 3rd ventricle volume correlated with the previously characterized neuroendocrine dysfunction (Table 2), further supporting the view that anterior hippocampal pathology is responsible for the findings in the polydipsic groups.
Alternative interpretations of the data are possible. Group differences in indices in water balance (Table 1) could theoretically be a cause rather than a consequence of the difference in structural shapes. While to our knowledge such selective structural deformations have not been previously observed, this possibility must be considered. A previous history of alcoholism could play a role since polydipsic patients are more likely to have been alcoholic (Poirer et al., 2010), which in turn can disrupt water balance and brain structure. Most of the effects of acute ingestion and withdrawal on water balance resolve rapidly (Mander et al., 1989;Taivainen et al., 1995), however, and those that persist are opposite to what is seen in the polydipsic groups (Doring et a., 2003). Alcohol also causes brain volume loss in limbic areas which may be permanent (Rosenbloom et al., 2003), leaving open the possibility that a prior history of alcoholism could explain the findings. Finally, group differences in psychological stress or excessive HPAA activity could theoretically produce the structural changes (Koenig et al., 2002) though evidence indicates stress alters the medial, not lateral, hippocampus (Wang et al., 2010), and the effect of stress on hippocampal structure remains controversial.
Differences between healthy controls and patients in smoking, pre-morbid IQ and neuroleptic treatment are possible, but unlikely explanations, since healthy controls' structural and functional findings were generally intermediate to those of the patient groups. Psychotropic medication, per se, also appears to be an unlikely explanation because medication exposure did not differ across patient groups (Table 1); other studies indicate antipsychotic medication cannot account for the neuroendocrine or structural findings in schizophrenia (Csernansky et al., 2004; Jessani et al., 2006; Goldman, 2009; Ho and Magnotta, 2010); inclusion of medication type or dose as a covariate in the analysis did not alter the key findings; and the fundamental aspects of the water imbalance (i.e. impaired water excretion which worsens during psychotic exacerbations, life threatening water intoxication) (Targowla, 1923; Barahal, 1938) were described prior to the discovery of this class of medicines. Finally, measures associated with differences in brain volume are unlikely to account for the findings, not only because they did not differ across groups (Supplemental Table 1), but because they are not known to effect shape, per se.
There are a number of limitations in the interpretation of the data. Foremost, is the small sample size leaving open the possibility that the findings may be difficult to reproduce, and making it difficult to conclusively identify or exclude explanatory variables. Differences in a structure's shape cannot be taken to represent pathologic changes intrinsic to that structure, and, may instead, for instance, reflect changes in neighboring structures. Other important limitations regard the applicability of discoveries regarding rodent structure/function relationships to humans. The study does not address or resolve other important issues. Some studies (Narr et al., 2004), including our own (Csernanasky et al., 1998), have identified shape changes in the anterior lateral hippocampus in schizophrenic patients most of whom were presumably nonpolydipsic. Perhaps a larger sample would identify discrete differences in PHS and NNS subjects on the lateral surface, but currently this issue is unresolved. The significance of the deformations in the amygdala and the medial surface of the anterior hippocampus in NNS is unclear, as are group differences in the body of the hippocampus. The latter could relate to previously observed differences in cognition in polydipsic and nonpolydipsic patients (Torres et al., 2009), but according to our data is unlikely to account for the polydipsia, per se.
Despite accumulating evidence that schizophrenia reflects altered neural circuit function, pinning down the pathophysiology by linking structural and functional pathology together remains a huge challenge. Many studies rely on poorly understood and complicated CNS functions that are difficult to model in animals. The current work raises the possibility that structural changes in a relatively well-characterized and accessible neurocircuit found in all mammals may induce functional changes which contribute to the psychiatric disorder in a subset of patients. In particular, the findings identified here could be part of the hippocampal dysfunction (Harrison et al, 2004; Schobel et al., 2009a; 2009b; Zhou et al., 2008) which has been proposed to induce an increased vulnerability to psychological stress (Gray, 1998; Mednick and Schulsinger, 2005) in schizophrenia. Whether these patients represent a distinct subtype of schizophrenia attributable to a distinct pathophysiology or have the same illness affecting different limbic neurocircuits is an open question.
Supplementary Material
Acknowledgements
The authors would like to thank the patients and staff of the Psychiatric Institute, University of Illinois at Chicago. Beth Beenken, Ph.D., Ana Solodkin, Ph.D, Steve Small, MD, Ph.D, and the staff of the Brain Research Imaging Center at the University of Chicago for their assistance with the study.
Funded by the Brain Research Foundation and NIH R01 56525 (MG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: PHS: polydipsic hyponatremic schizophrenics; PNS: polydipsic normonatremic schizophrenics; NNS: nonpolydipsic normonatremic schizophrenics; DST: dexamethasone suppression test.
Disclosure/Conflict of Interest The authors have no conflicts of interest regarding the material presented here.
References
- Aggleton JP. A description of the amygdalo-hippocampal interconnections in the maca,que monkey. Exp. Brain. Res. 1986;64:515–526. doi: 10.1007/BF00340489. [DOI] [PubMed] [Google Scholar]
- Barahal HS. Water intoxication in a mental case. J. Psych. Quat. 1938;12:767–771. [Google Scholar]
- Beg MF, Miller MI, Trouve A, Younes L. Computing large deformation metric mappings via geodesic flows of diffeomorphisms. Int. J. Comp. Vision. 2005;61:139–157. [Google Scholar]
- Benes FM. Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropsychopharm. 2010;35:239–257. doi: 10.1038/npp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Jones D, Rastogi-Cruz D, Posener JA, Heydebrand G, et al. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am. J. Psychiatry. 2002;159:2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, et al. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc. Natl. Acad. Sci. U S A. 1998;95:11406–11411. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Joshi SC, Ratnanather JT, Miller MI. Computational anatomy and neuropsychiatric disease: Probabilistic assessment of variation and statistical inference of group difference, hemispheric asymmetry, and time-dependent change. Neuroimage. 2004;23(Suppl 1):S56–S68. doi: 10.1016/j.neuroimage.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Swank J, Haller JW, Gado M, Miller JP, et al. Preclinical detection of Alzheimer's disease: hippocampal shape and volume predict dementia onset in the elderly. NeuroImage. 2005;25:783–792. doi: 10.1016/j.neuroimage.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J. Comp. Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- de Leon J, Dadvand M, Canuso C, Odom-White A, Stanilla J, Simpson GM. Polydipsia and water intoxication in a long-term psychiatric hospital. Biol. Psychiatry. 1996;40:28–34. doi: 10.1016/0006-3223(95)00353-3. [DOI] [PubMed] [Google Scholar]
- de Leon J, Verghese C, Tracy JI, Josiassen RC, Simpson GM. Polydipsia and water intoxication in psychiatric patients: a review of the epidemiological literature. Biol. Psychiatry. 1994;35:408–419. doi: 10.1016/0006-3223(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Döring WK, Herzenstiel MN, Krampe H, Jahn H, Pralle L, Sieg S, et al. Persistent alterations of vasopressin and N-terminal proatrial natriuretic peptide plasma levels in long-term abstinent alcoholics. Alcohol Clin. Exp. Res. 2003;27:849–861. doi: 10.1097/01.ALC.0000065433.17403.DE. [DOI] [PubMed] [Google Scholar]
- Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35:258–277. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB. The mechanism of life-threatening water imbalance in schizophrenia and its relationship to the underlying psychiatric illness. Brain Res. Rev. 2009;61:210–220. doi: 10.1016/j.brainresrev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB, Blake L, Marks RC. Association of nonsuppression of cortisol on the DST with primary polydipsia in chronic schizophrenia. Am. J. Psychiatry. 1993;50:653–655. doi: 10.1176/ajp.150.4.653. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Hussain N, Gnerlich J. Neuroendocrine responses to a cold pressor stimulus in polydipsic hyponatremic and in matched schizophrenic patients. Neuropsychopharmacology. 2007a;32:1611–1621. doi: 10.1038/sj.npp.1301282. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Luchins DJ, Robertson GL. Mechanisms of altered water metabolism in psychotic patients with polydipsia and hyponatremia. N. Engl. J. Med. 1988;318:397–403. doi: 10.1056/NEJM198802183180702. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Marlow-O'Connor M, Torres I, Carter CS. Preliminary data on plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophrenia Res. 2008;98:247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB, Marks RC, Blake L, Petkovic M, Hedeker D, Luchins DJ. Estimating daily urine volume in psychiatric patients: Empiric conformation. Biol. Psychiatry. 1992;31:1228–1231. doi: 10.1016/0006-3223(92)90343-x. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Robertson GL, Luchins DJ, Hedeker D, Pandey GN. Psychotic exacerbations and enhanced vasopressin secretion in schizophrenics with hyponatremia and polydipsia. Arch. Gen. Psychiatry. 1997;54:443–449. doi: 10.1001/archpsyc.1997.01830170069010. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Robertson GL, Luchins DJ, Hedeker D. The influence of polydipsia on water excretion in hyponatremic, polydipsic schizophrenic patients. J. Clin. Endocrinol. Metab. 1996;81:1465–1470. doi: 10.1210/jcem.81.4.8636352. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Torres IJ, Keedy S, Marlow-O'Connor M, Beenken B, Pilla R. Reduced anterior hippocampal formation volume in hyponatremic schizophrenic patients. Hippocampus. 2007b;17:554–562. doi: 10.1002/hipo.20292. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Wood G, Goldman MB, Gavin M, Paul S, Zaheer S, et al. Diminished glucocorticoid negative feedback in polydipsic hyponatremic schizophrenic patients. J. Clin. Endocrinol. Metab. 2007c;92:698–704. doi: 10.1210/jc.2006-1131. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Makris N, Ahern T, O'Brien LM, Caviness VS, Jr, et al. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol. Psychiatry. 2007;61:935–945. doi: 10.1016/j.biopsych.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Gray JA. Integrating schizophrenia. Schizophr. Bull. 1998;24:249–266. doi: 10.1093/oxfordjournals.schbul.a033324. [DOI] [PubMed] [Google Scholar]
- Heimer L, de Olmos JS, Alheid GF, Pearson J, Sakamoto N, Shinoda K, et al. The human basal forebrain. Part II. In: Bloom FE, Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy: The Primate Nervous System, Part III. Vol. 15. Elsevier Science; New York: 1999. pp. 57–210. [Google Scholar]
- Hariprasad MK, Eisinger RP, Nadler IM, Padmanabhan CS, Nidus BD. Hyponatremia in psychogenic polydipsia. Arch. Intern. Med. 1980;140:1639–1642. [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Heckers S. Is schizoaffective disorder a useful diagnosis? Curr. Psychiatry Rep. 2009;11:332–337. doi: 10.1007/s11920-009-0048-3. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav. Brain. Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Ho BC, Magnotta V. Hippocampal volume deficits and shape deformities in young biological relatives of schizophrenia probands. Neuroimage. 2010;149:3385–3393. doi: 10.1016/j.neuroimage.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson JA, English JT. Self-induced water intoxication. Ann. Int. Med. 1963;58:324–332. doi: 10.7326/0003-4819-58-2-324. [DOI] [PubMed] [Google Scholar]
- Hoskins RG, Sleeper FH. Organic functions in schizophrenia. Arch. Neurol. Psychiatry. 1923;30:123–140. [Google Scholar]
- Jankford R, Herman JP. Limbic regulation of hypthalamo-pituitary-adrenocortical function during acute and chronic stress. Ann. N. Y. Acad. Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessani M, Montgomery J, Fedde JD, Josiassen RC. Lack of association between antipsychotics and hyponatremia in chronic schizophrenia. Schizophr. Res. 2006;83:307–309. doi: 10.1016/j.schres.2005.12.859. [DOI] [PubMed] [Google Scholar]
- Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27:309–318. doi: 10.1016/S0893-133X(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Luchins DJ, Nettles KW, Goldman MB. Anterior medial temporal lobe volumes in polydipsic schizophrenic patients with and without hypo-osmolemia: A pilot study. Biol. Psychiatry. 1997;42:767–770. doi: 10.1016/s0006-3223(96)00491-x. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O'Leary DS, Yuh WT, Heckel Structural MR image processing using the BRAINS2 toolbox. Comput. Med. Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Mander AJ, Young A, Merrick MV, Morton JJ. Fluid balance, vasopressin and withdrawal symptoms during detoxification from alcohol. Drug Alcohol Depend. 1989;24:233–237. doi: 10.1016/0376-8716(89)90060-4. [DOI] [PubMed] [Google Scholar]
- Mednick S, Schulsinger F. Some premorbid characteristics related to breakdown in children with schizophrenic mothers. In: Rosenthal D, Kety S, editors. The Transmission of Schizophrenia. Pergamon; Oxford, UK: 2005. pp. 267–293. [Google Scholar]
- Mitchell CP, Goldman MB. Neonatal lesions of the ventral hippocampal formation disrupt neuroendocrine responses to auditory stress in the adult rat. Psychoneuroendocrinology. 2004;29:1317–1325. doi: 10.1016/j.psyneuen.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Nettles KW, Pesold C, Goldman MB. Influence of the ventral hippocampal formation on plasma vasopressin, hypothalamic–pituitary–adrenal axis, and behavioral responses to novel acoustic stress. Brain. Res. 2000;858:181–190. doi: 10.1016/s0006-8993(99)02281-7. [DOI] [PubMed] [Google Scholar]
- Pantel J, O'Leary DS, Cretsinger K, Bockholt HJ, Keefe H, Magnotta VA, et al. A new method for the in vivo volumetric measurement of the human hippocampus with high neuroanatomical accuracy. Hippocampus. 2000;10:752–758. doi: 10.1002/1098-1063(2000)10:6<752::AID-HIPO1012>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Poirier S, Legris G, Tremblay P, Michea R, Viau-Guay L, Mérette C, et al. Schizophrenia patients with polydipsia and water intoxication are characterized by greater severity of psychotic illness and a more frequent history of alcohol abuse. Schizophr. Res. 2010;118:285–291. doi: 10.1016/j.schres.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Poletti CE, Kinnard MA, MacLean PD. Hippocampal influence on unit activity of hypothalamus, preoptic region, and basal forebrain in awake, sitting squirrel monkeys. J. Neurophysiol. 1973;36:308–324. doi: 10.1152/jn.1973.36.2.308. [DOI] [PubMed] [Google Scholar]
- Qiu A, Miller MI. Multi-structure network shape analysis via normal surface momentum maps. Neuroimage. 2008;42:1430–1438. doi: 10.1016/j.neuroimage.2008.04.257. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Orenstein H, Christopher TG. Acute psychosis, increased water ingestion, and inappropriate antidiuretic hormone secretion. Am. J. Psychiatry. 1975;132:907–910. doi: 10.1176/ajp.132.9.907. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Science. 1996;272:1484–1486. doi: 10.1126/science.272.5267.1484. [DOI] [PubMed] [Google Scholar]
- Rosenbloom M, Sullivan EV, Pfefferbaum A. Using magnetic resonance imaging and diffusion tensor imaging to assess brain damage in alcoholics. Alcohol Res. Health. 2003;27:146–152. [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Kelly MA, Corcoran CM, Van Heertum K, Seckinger R, Goetz R, et al. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophr. Res. 2009a;114:110–118. doi: 10.1016/j.schres.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch. Gen. Psychiatry. 2009b;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr. Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br. J. Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Taivainen H, Laitinen K, Tähtelä R, Kilanmaa K, Välimäki MJ. Role of plasma vasopressin in changes of water balance accompanying acute alcohol intoxication. Alcohol Clin. Exp. Res. 1995;19:759–t62. doi: 10.1111/j.1530-0277.1995.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Targowla R. Des troubles fonctionnel du rein dans les maladies mentales. L'excretion del'eau (Kidney malfunction and mental illness: water excretion) Bulletins et mémoires de la Société médicale des hôpitaux de Paris. 1923;47:711–715. [Google Scholar]
- Torres IJ, Keedy S, Marlow-O'Connor M, Beenken B, Goldman MB. Neuropsychological impairment in patients with schizophrenia and evidence of hyponatremia and polydipsia. Neuropsychology. 2009;23:307–314. doi: 10.1037/a0014481. [DOI] [PubMed] [Google Scholar]
- van Venrooij JA, Fluitman SB, Lijmer JG, Kavelaars A, Heijnen CJ, Westenberg G, et al. Impaired neuroendocrine and immune response to acute stress in medication-naive patients with a first episode of psychosis. Schizophr. Bull. 2010 doi: 10.1093/schbul/sbq062. PMID: 20558533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieweg WV, David JJ, Rowe WT, Wampler GJ, Burns WJ, Spradlin WW. Death from self-induced water intoxication among patients with schizophrenic disorders. J. Nerv. Ment. Dis. 1985;1173:161–165. doi: 10.1097/00005053-198503000-00005. [DOI] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch. Gen. Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Shu N, Liu Y, Song M, Hao Y, Liu H, et al. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr. Res. 2008;100:120–132. doi: 10.1016/j.schres.2007.11.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.