Abstract
Aim
To characterize AmpC-beta lactamases among Enterobacteriaceae isolates from clinical samples at Mbarara Regional Referral Hospital.
Study Design
Laboratory-based descriptive cross-sectional study
Place and Duration of Study
Microbiology Department, Mbarara Regional Referral Hospital and MBN clinical Laboratories, between May to September 2013.
Methodology
This study included 293 Enterobacteriaceae isolates recovered from clinical specimens that included blood, urine, stool and aspirates. AmpC Beta lactamase production was determined using disc placement method for cefoxitin at a break point of <18mm. Common AmpC plasmid mediated genes were EBC, ACC, FOX, DHA, CIT and MOX were; was determined by Multiplex PCR as described by Hanson and Perez-Perez.
Results
Plasmid mediated AmpC phenotype was confirmed in 107 of the 293 (36.5%) cefoxitin resistant isolates with 30 isolates having more than one gene coding for resistance. The commonest source that harbored AmpC beta lactamases was urine and E. coli was the most common AmpC producer (59.5%). The genotypes detected in this study, included EBC (n=36), FOX (n=18), ACC (n=11), CIT (n=10), DHA (n=07) and MOX (n=1).
Conclusion
Our findings showed that prevalence of AmpC beta-lactamase at MRRH was high (39.6), with EBC as the commonest genotype among Enterobacteriaceae Urine and E. coli were the commonest source and organism respectively that harbored AmpC beta-lactamases. There‘s rational antimicrobial therapy and antibiotic susceptibility tests should be requested by health workers especially patients presenting with urinary tract infections and bacteraemias.
Keywords: Enterobacteriaceae, AmpC beta lactamase, antibiotic, resistant
1. INTRODUCTION
Enterobacteriaceae are common causes of hospital and community acquired infections. The main stay treatment of these infections is the use of antibiotics, mainly beta-lactam agents, which are the most commonly administered drugs in most resource-poor settings [1].
A key challenge in this treatment has been the tendency for these enteric bacteria to acquire plasmid genetic elements bearing genes for drug resistance. These genes encode for drug resistant proteins (beta lactamase) which have increasingly rendered beta lactam agents less useful in the treatment of the above stated infections [2,3]
Plasmid-mediated AmpC beta-lactamases have risen through the transfer of chromosomal genes for the inducible AmpC beta-lactamase onto plasmids, this transfer has resulted in plasmid-mediated AmpC beta-lactamases in isolates of E. coli, Klebsiella pneumoniae, Salmonella species, and Proteus mirabilis [4,5]
AmpC beta-lactamases which are often plasmid mediated hydrolyze all β-lactam antibiotics except cefepime and carbapenems and confer resistance to cephalothin, cefazolin, cefoxitin, most penicillins and beta-lactam inhibitor combinations (broad multidrug resistance) continue to be a major problem in health care settings[6]. Although published literature has evidence that levels of antibiotic-resistant bacteria are high and continue to rise elsewhere in Africa [7,8].
There’s insufficient information about occurrence and detection of AmpC at Mbarara Regional Referral Hospital. Knowledge of AmpC beta-lactamase occurrence is essential to guide the clinicians towards the appropriate anti-microbial treatment [9]. A serious challenge facing clinical laboratories is that clinically relevant AmpC-mediated resistance is not always detectable in routine susceptibility tests. This study evaluated presence of AmpC-beta lactamases among Enterobacteriaceae isolates from clinical samples at Mbarara Regional Referral Hospital.
2. MATERIALS AND METHODS
2.1 Study Design
This was a Laboratory based descriptive cross sectional study conducted between May to September 2013 at Mbarara Regional Referral Hospital microbiology laboratory and MBN Clinical Laboratories Kampala, Uganda.
2.2 Study Samples
These included Non-repetitive Gram negative isolates (Enterobacteriaceae) obtained from various clinical samples that were received in the Microbiology Laboratory were sub cultured on MacConkey agar and incubated at 35–37°C for 16–24 hours. In house made Triple sugar iron agar, urease, oxidase, indole, motility and citrate test were used for biochemical identifications as published by [10].
2.3 Laboratory Detection of AmpC Beta Lactamases
2.3.1 Disc diffusion test
This was performed by the Novel disc displacement method. In the center of the Muller-Hinton agar plate, imipenem (10μg) (Inducer) disc was applied. At the distance of 20 mm, the disc of cefotaxime (30μg) was placed. From this disc, in a circular manner, clockwise, the discs of cefoxitin (30μg) (Inducer), ceftriaxone (30μg), ceftazidime (30μg), ceftazidime + clavulanic acid (30/10μg), and aztreonam (30μg) were placed such that any two adjacent discs were 20mm apart from center to center. On overnight aerobic incubation at 37°C, the diameters of zones of inhibition were measured and interpreted according to Nagdeo et al., 2012 [11]. A break point <18mm zone diameter for cefoxitin was taken as resistant to cefoxitin, no increase of zone size with addition of inhibitor (ceftazidime-clavulanic acid) and flattening zone of inhibition for cefotaxime (30μg), ceftazime (30μg), ceftriazone (30μg), aztreonam (30μg), ceftazidime-clavulanic acid (30/10μg) towards imipenem (10μg) was interpreted as phenotypically positive for AmpC.
2.3.2 Genotypic characterization
All isolates were screened for the resistance genes MOX and DHA, EBC and FOX, ACC and CIT by a multiplex PCR assay using universal primers [12–14]. Criteria for multiplexing was Based on molecular weight (base pairs) and melting temperatures, and Primers were paired as follows; MOX and DHA, EBC and FOX, ACC and CIT. Total DNA targeting both genomic and plasmid DNA of the Enterobacteriacae was extracted by the boiling method as published by Perez-Perez and Hanson [15]. All PCR amplicons were verified by gel electrophoresis.
2.4 Quality Control
For phenotypic detection, Known AmpC producers or Indicator strains (E. coli ATCC 25922 and E. coli ATCC 35218 were cultured along the test organisms as negative and positive controls respectively and their zone diameters measured and interpreted according to CLSI guidelines. For genotypic detection, Negative controls were PCR reagent mixtures with the addition of sterile nuclease free PCR water in place of template DNA and positive controls were: Escherichia Coli CCUG 58543 and Escherichia Coli CCUG 62975.
2.5 Data Analysis
Data was entered in Microsoft Excel cleaned and imported to Stata version 11 (Stata Corporation, College Station, TX, USA) statistical packages for analysis. The prevalence of different AmpC Beta lactamase producing organisms and genotypes like MOX, DHA, EBC, ACC, FOX, and ACC obtained after characterization was determined using univariate analysis and cross tabulations.
3. RESULTS AND DISCUSSION
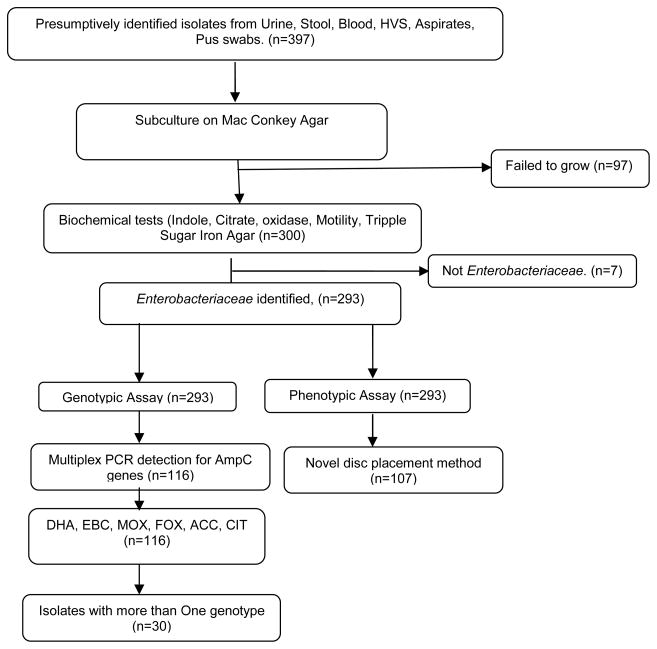
The study included 397 clinical isolates sent to the microbiology laboratory for culture and sensitivity collected from different sources, 293 out of 397 clinical isolates were clearly identified as Enterobacteriaceae according to our biochemical tests tested by disc diffusion method using Cefoxitin, 107/293 (36.5%) were identified as AmpC producers. Multiplex PCR identified 116/293 (39.6%) as AmpC producers, with 30 possessing more than one of the following genotypes; DHA, MOX, EBC, ACC, CIT and FOX as shown in Fig. 1
Fig. 1.
Showing the study profile
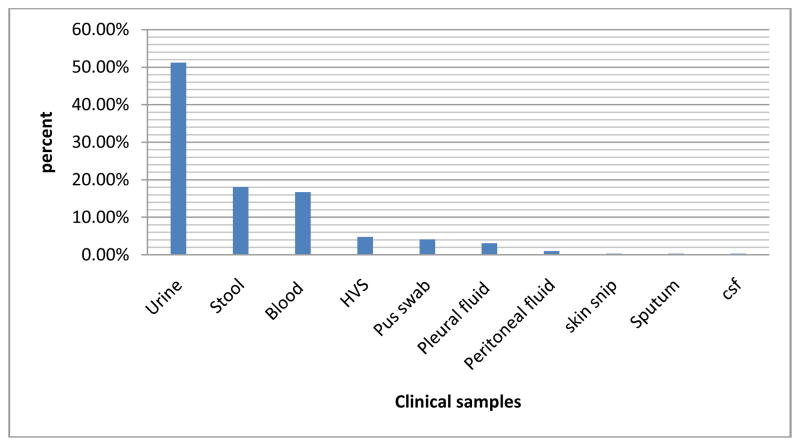
Two hundred ninety three enterobacteriaceae isolates were obtained and analysed from the following sources and the majority of the isolates were isolated from urine (51.19%) and blood (16.72%) as shown below in Fig. 2.
Fig. 2.
Showing clinical specimens from which study isolates were obtained
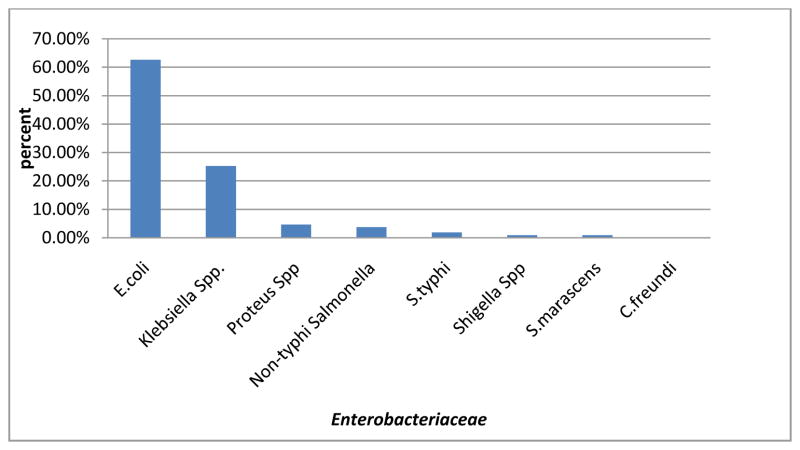
The overall phenotypic prevalence was 36.52%. Out of 107 AmpC producing Enterobacteriaceae isolates, detected phenotypically majorly were E. coli 67(62.62%), Klebsiella Spp. 27, (25.23%), and Proteus Spp. 5(4.67%). Citrobacter freundii was a non AmpC producer (Fig. 3).
Fig. 3.
Showing common AmpC producers by phenotypic detection
3.1 Prevalence of AmpC Beta Lactamases by Genotypic Assay
Based on the PCR assays, the overall prevalence by genotypic detection was 39.6% (116/293) Enterobacteriaceae bacteria isolates positive for one or more of the AmpC beta lactamase resistance genes and the most frequent AmpC gene was EBC followed by FOX (See Table 1).
Table 1.
Prevalence of AmpC beta lactamase genes among Enterobacteriaceae
| AmpC beta lactamase resistance gene | Frequency (n=293) | Percentage (%) |
|---|---|---|
| FOX | 30 | 10.24 |
| EBC | 76 | 25.94 |
| CIT | 17 | 5.8 |
| ACC | 21 | 7.17 |
| DHA | 9 | 3.07 |
| MOX | 01 | 0.34 |
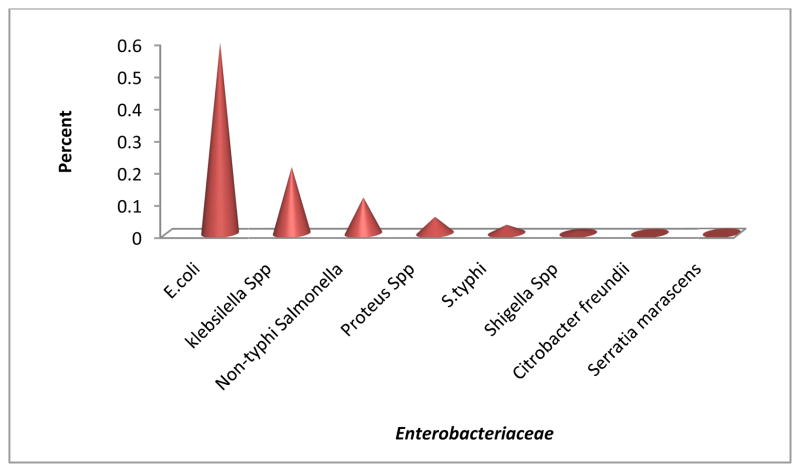
Out of 116 isolates that were genotypically positive, E. coli (59.48%) possessed most AmpC Beta lactamase resistance genes followed by Klebsiella Spp. (20.69%) and Non typhi salmonella (11.21%) as shown in Fig. 4.
Fig. 4.
Enterobacteriaceae found to harbor AmpC beta lactamase genes
Thirty Enterobacteriaceae isolates had multiple AmpC resistance genes or more than one gene coding for resistance as shown below (Table 2).
Table 2.
Enterobacteriaceae with more than one gene
| Bacteria species | No. of AmpC resistance genes per isolate | ||||
|---|---|---|---|---|---|
| S. typhi | One | Two | Three | Four | Total |
| S. marcescens | 2 | 1 | 0 | 0 | 3 |
| C. freundii | 0 | 0 | 0 | 0 | 0 |
| E. coli | 0 | 0 | 0 | 0 | 0 |
| Klebsiella Spp | 57 | 8 | 3 | 1 | 69 |
| Proteus Spp | 12 | 10 | 2 | 0 | 24 |
| Non-typhi Salmonella | 5 | 0 | 1 | 0 | 6 |
| Shigella | 9 | 4 | 0 | 0 | 13 |
| Total No. of isolates | 1 | 0 | 0 | 0 | 1 |
| S. typhi | 86 | 23 | 6 | 1 | 116 |
The major AmpC beta lactamase genes found in E. coli isolates were FOX, followed by ACC and CIT as shown above (Table 3).
Table 3.
Distribution of different AmpC beta lactamases among Enterobacteriaceae
| EBC (%) | FOX (%) | DHA (%) | MOX (%) | CIT (%) | ACC (%) | |
|---|---|---|---|---|---|---|
| Klebsiella spp (n=52) | 18(23.68) | 5(16.67) | 4(44.44) | 1(100) | 4(23.53) | 6(28.57) |
| E. coli (n=194) | 39(51.32) | 21(70.00) | 3(33.33) | 0(0) | 10(58.80) | 13(61.90) |
| S. typhi (n=5) | 2(2.63) | 0 (0) | 1(11.11) | 0(0) | 1(5.88) | 0(0) |
| Non-typhi Salmonella (n=23) | 12(15.79) | 3 (10.00) | 0(0) | 0(0) | 1(5.88) | 1(4.76) |
| C. freundii (n=2) | 0(0) | 0 (0) | 0(0) | 0(0) | 0(0) | 0(0) |
| Proteus Spp (n=14) | 4(5.26) | 1(3.33) | 1(11.11) | 0(0) | 1(5.88) | 1(4.76) |
| S. marcescens (n=1) | 0(0) | 0 (0) | 0(0) | 0(0) | 0(0) | 0(0) |
| Shigella (n=2) | 1(1.32) | 0 (0) | 0(0) | 0(0) | 0(0) | 0(0) |
| Total | 76 (100) | 30(100) | 9(100) | 1(100) | 17(100) | 21(100) |
Out of 76 isolates with EBC 39(51.3%) were E. coli, 18 (23.7%) Klebsiella Spp., and 12 (15.8%) were non-typhi Salmonella and less prevalent in the rest of the isolates.
Out of 30 isolates with FOX, 21(70.0%) were E. coli, 5 (16.7%) Klebsiella Spp, 3(10%) non-typhi Salmonella and less prevalent in other isolates.
Out of 17 isolates with CIT, 10(58.8%) were E. coli, 4 (23.5%) Klebsiella Spp, 1(5.88%) each of S. typhi, non-typhi Salmonella and Proteus Spp. The rest of the isolates had no CIT genes.
Out of 21 isolates with ACC, 13(61.9%) were E. coli, 6 (28.6%) Klebsiella Spp, 1(4.8%) each of non-typhi Salmonella and Proteus Spp. The rest of the isolates had no ACC genes.
Only one MOX gene was found in a Klebsiella Spp. Out of the 9 isolates with DHA 4(44.4%) were Klebsiella Spp., 3(33.3%) were E. coli, and each of 1(11.11%) S. typhi and Proteus Spp. (Table 3).
The commonest specimen that harbored AmpC beta lactamases was urine (61.99%) possessing mainly EBC, FOX and ACC genes. Sputum, skin snips, peritoneal and cerebral spinal fluids didn’t harbor any AmpC genes (Table 4).
Table 4.
Distribution of AmpC Beta lactamases in different clinical specimens
| Specimens | EBC | FOX | DHA | MOX | CIT | ACC |
|---|---|---|---|---|---|---|
| Urine (n=150) | 38 | 15 | 4 | 1 | 9 | 10 |
| Blood (n=49) | 17 | 7 | 0 | 0 | 6 | 3 |
| Puss wab (n=12) | 7 | 1 | 0 | 0 | 01 | 0 |
| Pleural fluid (n=9) | 03 | 01 | 0 | 0 | 0 | 02 |
| HVS (n=3) | 0 | 0 | 0 | 0 | 0 | 01 |
4. DISCUSSION
AmpC beta-lactamases mediate resistance to cephalothin, cefazolin, cefoxitin, most penicillins and beta-lactam/beta-lactam inhibitor combinations and their over expression confers resistance to broad-spectrum cephalosporins including cefotaxime, ceftazidime, and ceftriaxone [16]
We detected high prevalence (37.19%) of AmpC producers among Enterobacteriaceae isolates. The findings in this study are similar to a study carried out in India by Anand et al. [16] found the prevalence of phenotypic AmpC producers among Enterobacteriaceae strains (36.5%).
This prevalence is also higher than 10% AmpC prevalence reported by [17] from Kenya [18] and 12% AmpC prevalence reported by [19] in Brazil This can be explained that only E. coli isolates were studied as opposed to our study that included a number of other species of Enterobacteriacae. The other reason for the difference in our findings could be the different methods used., that included, combined disc diffusion, Tridimensional and Hodge test) The prevalence is higher because the genotypic method used in this study is more sensitive compared to the above methods.
Majority of the AmpC genes containing Enterobacteriacae were in urine and blood. This is consistent with the studies done by [16,19]. This implies the rational use of antibiotics especially patients with UTIs and bactermias, therefore carbapenems should be considered during the patient management.
In the study, sizeable numbers of cefoxitin resistant isolates were not positive for AmpC production by the disc placement method or multiplex PCR; this warrants further investigation into the other mechanisms of resistance and their laboratory detection.
Thirty clinical isolates expressed more than one plasmid-mediated AmpC beta-lactamases. Two reasons could explain this observation. First, the inability of current phenotypic tests to accurately detect the type of transferable AmpC beta-lactamase does not allow for the differentiation of multiple AmpC enzymes. Second, it is possible that there is a limit to the amount of AmpC β-lactamase that a bacterial cell can accommodate and still be a viable pathogen according to [16]. A single type of test (PCR) will not be able to accurately characterize the resistance mechanisms in these complex organisms.
5. CONCLUSION
Overall, prevalence of AmpC beta-lactamases was high (39.6%). The commonest genotype detected was EBC (n= 76) and FOX (n=30) and the least detected genotype was MOX (n=1). Thirty Enterobacteriaceae had more than one genotype. The common AmpC producers in this study were E. coli, (59.48%) followed by Klebsiella Spp.,(20.69%) and Non-typhi Salmonella (11.21%) and the commonest source or specimen that harbored AmpC producers was urine (47.4%). The genotypic detection was better than the phenotypic detection.
Acknowledgments
The project described was supported by the MESAU-MEPI programmatic award through award No: 1R24TW00886 from the Forgaty International Centre.
Footnotes
Authors’ contributions
This work was carried out in collaboration between all authors. Author MN designed the study, performed the statistical analysis, wrote the protocol and wrote the first draft of the manuscript. Author FB supervised and managed the analyses of the study. Authors HI, IJS and MB managed the literature searches. Author JB participated in the planning of the study, drafting and critical review of the manuscript. All authors read and approved the final manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Forgarty International Center or National Institutes of Health.
ETHICAL APPROVAL
Ethical clearance was received from the Institutional Ethical Review Committee of Mbarara University of Science and Technology and Uganda National Council for Science and Technology
COMPETING INTERESTS
Authors have declared that no competing interests exist.
References
- 1.Medeiros A. Evolution and dissemination of beta-lactamases accelerated by generations of beta-lactam antibiotics. Clin Infect Dis. 1997 Jan 24;(Suppl 1):S19–45. doi: 10.1093/clinids/24.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 2.Stokes HW, Gillings MR. Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into Gram-negative pathogens. FEMS Microbiol Rev. 2011;35:790–819. doi: 10.1111/j.1574-6976.2011.00273.x. [DOI] [PubMed] [Google Scholar]
- 3.Toleman MA, Walsh TR. Combinatorial events of insertion sequences and ICE in Gram-negative bacteria. FEMS Microbiol Rev. 2011;35:912–35. doi: 10.1111/j.1574-6976.2011.00294.x. [DOI] [PubMed] [Google Scholar]
- 4.Bauernfeind A, Chong Y, Lee K. Plasmid-encoded AmpC beta-lactamases: How far have we gone 10 years after the discovery? Yonsei Med J. 1998;39:520–5. doi: 10.3349/ymj.1998.39.6.520. [DOI] [PubMed] [Google Scholar]
- 5.Livermore DM. Beta-lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The World Health Organization, WHO. The evolving threat of antimicrobial resistance. 2012. p. 5. [Google Scholar]
- 7.Okeke IN, Aboderin OA, Byarugaba DK, Ojo KK, Opintan JA. Growing problem of multidrug-resistant enteric pathogens in Africa. Emerg Infect Dis. 2007;13:1640–1646. doi: 10.3201/eid1311.070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akinniyi AP, Oluwaseun E, Motayo BO, Adeyokinu AF. Emerging Multidrug Resistant AmpC beta-Lactamase and Carbapenamase Enteric Isolates in Abeokuta, Nigeria. Nature and Science. 2012;7(10):70. [Google Scholar]
- 9.Singh RKM, Mandira B, Soma S, Manideepa S. Surveillance on Extended Spectrum β-lactamase and AmpC β-lactamase producing gram negative isolates from nosocomial infections. Imedpub Journals. 2012;3(3:1) doi: 10.3823/252. [DOI] [Google Scholar]
- 10.Mshana SE, Kamugisha E, Mirambo M, Chakraborty T, Lyamuya E. Prevalence of multiresistant Gram-negative organisms in a tertiary hospital in Mwanza, Tanzania. BMC Research Notes. 2009;2(49) doi: 10.1186/1756-0500-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagdeo NV, Navinchandra MK, Vilas RT. Phenotypic methods for detection of various β-lactamases in Gram-negative clinical isolates. Chron Young Sci. 2012;3(4):292–298. [Google Scholar]
- 12.Paterson DL, Hujer KM, Hujer AM, Yeiser B, Bonomo MD, Rice LB, Bonomo RA. The International Klebsiella Study Group: Extended-spectrum β-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: Dominance and widespread prevalence of SHV- and CTX-M-type β-lactamases. Antimicrob Agents Chemother. 2003;47(11):3554–3560. doi: 10.1128/AAC.47.11.3554-3560.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karami N. Colonization dynamics of ampicillin resistant Escherichia coli in the infantile colonic microbiota. J Antimicrob Chemother. 2008;62:703–708. doi: 10.1093/jac/dkn263. [DOI] [PubMed] [Google Scholar]
- 14.Hujer KM, Hujer AM, Hulten EA, Bajaksouzian S, Adams J, Donskey CJ, Ecker DJ, Massire C, Eshoo MW, Sampath R, Thomson JM, Rather PN, Craft DW, Fishbain JT, Ewell AJ, Jacobs MR, Paterson DL, Bonomo RA. Analysis of antibiotic resistance genes in multidrug resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother. 2006;50(12):4114–4123. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson DN, Perez-Perez F. Detection of Plasmid-Mediated AmpC β-Lactamase Genes in Clinical Isolates by Using Multiplex PCR. J Clin Microbiol. 2002 doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand M, Madhan S, Anil K, Hepzibah J, Dilip M. Phenotypic & molecular characterization of AmpC β-lactamases among Escherichia coli Klebsiella spp. & Enterobacter spp from five Indian Medical Centers. Indian J Med Res. 2012;135(3):359–364. [PMC free article] [PubMed] [Google Scholar]
- 17.Kiiru J, Samuel K, Bruno MG, Patrick B. Analysis of β-lactamase phenotypes and carriage of selected β-lactamase genes among Escherichia coli strains obtained from Kenyan patients during an 18-year period. Biomed central Microbiology. 2012;12(155) doi: 10.1186/1471-2180-12-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubens C, Armado A, et al. Prevalence of AmpC and other beta-lactamases in enterobacteria at a large urban university hospital in Brazil. Diagn Microbiol Infect Dis. 2008;60(1):7987. doi: 10.1016/j.diagmicrobio.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasirekha B. Prevalence of ESBL, AmpC-beta lactamases and MRSA among Uropathogens and its antibiogram. EXCL journal. 2013;12:18–88. [PMC free article] [PubMed] [Google Scholar]