Abstract
Celiac disease is a T cell mediated immune disorder characterized by the loss of oral tolerance to dietary gluten and the licensing of intraepithelial lymphocytes to kill intestinal epithelial cells, leading to villous atrophy. Innate immunity plays a critical role in both of these processes and cytokines such as interleukin-15 and interferon-α can modulate innate processes such as polarization of dendritic cells as well as intraepithelial lymphocyte function. These cytokines can be modulated by host microbiota, which can also influence dendritic cell function and intraepithelial lymphocyte homeostasis. We will elaborate on the role of interleukin-15, interferon-α, and the microbiota in modulating the processes that lead to loss of tolerance to gluten and tissue destruction in celiac disease.
Keywords: Celiac Disease, Innate Immunity, Immune Tolerance, Interleukin-15, Interferons, Microbiota, Dysbiosis
Introduction
Celiac disease (CD) is a T cell-mediated enteropathy with an autoimmune component that occurs in the small intestine of genetically predisposed individuals in response to gluten protein found in wheat, barley, and rye. The critical events required for disease pathogenesis are the induction of a gluten-specific inflammatory T helper-1 (Th1) CD4 T cell response as well as the targeted killing of intestinal epithelial cells (IEC) by licensed T cell receptor (TCR) αβ intestinal epithelial lymphocytes (TCR αβ IEL) [1]. These immunological events translate into a wide disease spectrum, ranging from potential CD to refractory sprue [2].
The role of innate immunity is highlighted by the enrichment of CD susceptibility loci in gene ontology pathways associated with innate immune processes [3]. Understanding the role of innate immunity in the inductive and effector phases of disease is critical to understanding the disease as a whole. To that effect, we structure our discussion on innate immunity in CD around the two central processes implicated in disease pathogenesis: (A) the triggers that implicate innate immunity in the loss of tolerance to gluten, and (B) the factors that can unleash the innate-like lymphokine killer activity (LAK activity) of TCR αβ IEL.
Defining the players
Our view of innate immunity in the small intestine is one that encompasses dynamic interactions between cytokines, antigen presenting cells (APCs), microbiota, IEC, and IEL. In the context of CD this takes the form of interrogating the role of interleukin 15 (IL-15) and type I interferons (type-1 IFNs), two cytokines produced primarily by innate immune cells, in polarizing innate immune responses by acting on dendritic cells (DCs), IEC, and or IEL. We do not discuss IL21 below, despite its potential impact on IEL and regulatory T cells, as it is a cytokine produced by adaptive gluten-specific CD4 T cells in CD [4] and acts downstream of type-1 IFNs [5] and IL-15 [6]. In addition, we comment on the potential for the microbiota to influence the intestinal microenvironment in a manner that would favor either the loss of tolerance to gluten or the activation of TCR αβ IEL. We are aware of the studies demonstrating the innate stimulatory properties of gluten and wheat components, but we do not discuss these studies as they are reviewed in detail in another section of this issue [7,8].
Innate cytokines and their role in CD
The immune system is a complex entity that is constantly integrating signals from a multitude of sources. The language that is understood by all immune cells is cytokines and under homeostatic conditions in the intestine the conversation being dictated by these cytokines is one of a neutral nature. In CD this conversation takes a different tone as the immune system initiates processes that lead to the loss of tolerance to gluten and licensing of TCR αβ IEL to kill IEC. The observation that negatively charged gluten peptides generated by active transglutaminase 2 (TG2) [9,10] have a higher affinity for binding HLA-DQ2 and HLA-DQ8 [11,12] is critical but does not explain why an inflammatory, rather than a regulatory, immune response to gluten is induced in CD. Furthermore, it has become evident, from both human and mouse studies, that TCR αβ IEL are the effector cells mediating IEC destruction [1]. Yet TCR αβ IEL are not gluten-specific, suggesting they destroy IEC based on recognition of stress signals/molecules. We believe these processes are in particular dictated by IL-15 and type-1 IFNs, two cytokines produced by innate cells that have the potential to influence multiples levels of the system by acting on IEC, APCs, and IEL.
IL-15
The dysregulated expression of IL-15 has been implicated in CD for quite some time [13-16]. Of particular importance to the topic of this review is IL-15's ability to polarize DCs and impact the function of TCR αβ IEL. Importantly, IL-15 acts in a cell contact dependent manner [17] and is upregulated in active CD in both the lamina propria (LP) and epithelium, where it exerts its effects on DCs and TCR αβ IEL, respectively [15].
A. LP IL-15 and loss of oral tolerance to gluten
DCs are the primary cells responsible for translating innate immune messages into adaptive immune responses. The loss of tolerance to gluten in CD is characterized by the expansion of gluten specific CD4+ TCR αβ T cells that exhibit a Th1 polarization, which is characterized by the ability of these cells to produce IFN-γ when activated via their TCR [1]. The challenge then becomes explaining which factors dictate to a DC when to induce a tolerogenic Foxp3+ regulatory T cell (Tregs) response to one oral antigen versus an inflammatory Th1 response to another such as gluten. To that point, it was shown that DCs treated with IL-15 in the presence of retinoic acid, which is a vitamin A metabolite enriched in the intestinal microenvironment [18], promoted differentiation of Th1 CD4+ T cells producing IFN-γ via induction of the cytokine IL-12p70 [19]. Importantly, mice expressing both the HLA-DQ8 molecule and transgenic IL-15 in the LP exhibited all features of potential CD [19], a form of CD characterized by the presence of adaptive anti-gluten immunity in the absence of villous atrophy [20]. These experiments collectively showed that IL-15 has the potential to polarize adaptive immune responses by acting on DCs and more importantly, that IL-15 can influence the events that lead to the development of inflammatory T cell responses directed against dietary antigens. These findings are relevant in humans given there is a correlation between IL-15 and IL-12 expression in the LP [19]. Also, the finding that retinoic acid acquires adjuvant properties in the presence of IL-15 [19] provides a scientific basis for why administration of retinoic acid is associated with an increased risk of inflammatory bowel disease [21].
While there is widespread agreement among the field's investigators that CD patients develop an intestinal inflammatory immune response against gluten, while healthy individuals do not [22], it remains controversial as to whether healthy individuals develop a Foxp3+ regulatory anti-gluten T cell response and whether this is disrupted by IL-15, as shown in mice [19]. The phenomenon of oral tolerance, which is thought to be associated with the induction of a Treg response against dietary antigens, has been reported in humans [23]. That being said, identification of gluten specific Tregs in healthy individuals has been elusive. The difficulty resides in that we can apply neither our current knowledge regarding immunodominant gluten peptides, nor the tetramer tools developed in CD, to healthy individuals. It is indeed likely that the anti-gluten T cell response in healthy individuals is directed against different peptides and not restricted by HLA-DQ2 or DQ8 because transglutaminase 2 (TG2), which generates gluten peptides with higher affinity for HLA-DQ2 or DQ8 by introducing negative charges through a process called deamidation [9,10], is not active in healthy non-inflamed intestine [24]. Finally, whether IL-15 can drive TG2 activation remains to be determined.
B. Epithelial IL-15 and TCR αβ IEL
While upregulation of IL15 in the LP may initiate loss of tolerance to gluten through its effect on DCs, upregulation of IL-15 in IEC has been extensively shown to influence the function of TCR αβ IEL [15,25]. Of relevance to this review is the impact IL-15 has on triggering the innate-like properties of TCR αβ IEL that result in the eventual destruction of intestinal tissue via directed killing of IEC, without the need for these cells to be gluten-specific [15].
To gain insight into the roles of IEL at the intestinal barrier, transcriptional studies were conducted on TCR αβ IEL in mice. The conclusion drawn from the data is TCR αβ IEL are in an “activated yet resting” state [26]. This suggests that, although these cells are widely considered effector cells, they still require signals that license them to engage target cells with the intention of killing. In addition, the observation that IEL do not recognize gluten implies that they will mediate killing of IEC based on direct or indirect recognition of stress signals, that include upregulation of non-classical MHC class I molecules and IL-15 [1]. Expression of natural killer receptors [13,27-31] and their regulation by IL-15 [13,15,16,28,32] on the surface of IEL has been well documented in humans.
The means by which these IEL kill, and whether the TCR is involved in these events, is still being actively investigated. IL-15 has been suggested to provide one of the signals that can license TCR αβ IEL to kill IEC. Specifically, IL-15 upregulates the activating natural killer receptor NKG2D on TCR αβ IEL, and licenses these cells to kill IEC expressing the stress induced ligand MICA [28,32]. The induction of this LAK activity was independent of TCR specificity. However, in addition to inducing LAK activity, IL-15 was shown to lower TCR activation threshold in vitro [27], which can help explain both CD8+ TCR αβ T cell mediated destruction of solid tumors lacking expression of cognate antigen [33] as well as the acquired ability to recognize self-antigens [34]. For IEL in CD, these antigens may take the form of microbiota derived antigens and/or IEC self-antigens. These studies provide evidence to help explain how IL-15 can influence killing of IEC by TCR αβ IEL even though no gluten specific TCR αβ IEL have been identified.
In addition to its ability to induce LAK activity in T cells, IL-15 was shown to increase expression of anti-apoptotic molecules such as B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma-extra large (Bcl-xL) [35] that are thought to contribute to the survival and expansion of IEL in CD [36], particularly in complications of CD associated with cryptic or overt T cell lymphomas that arise from IEL [2,37]. Finally, it was reported that IL-15 renders IEL resistant to TGF-β signaling by counteracting Smad3, hence preventing TGF-β from exerting its anti-inflammatory effects on IEL [38].
C. Summary
It is clear that IL-15 acts on the two central components of innate immunity in the context of CD by targeting the polarization of DCs or the function of TCR αβ IEL, depending on whether it is upregulated in the LP or IEC. Exactly when and how IL-15 is dysregulated are the biggest unknowns remaining about the role of IL-15 in CD. The observation that IL-15 remains upregulated in IEC but not in the LP of a subset of CD patients on a gluten-free diet ([16] and unpublished data) raises the question of what causes constitutively dysregulated expression of IL-15 in IEC in these individuals, and whether this dysregulated expression impacts mucosal healing and/or the development of refractory sprue and T cell lymphomas. Furthermore, the nature of the cross-talk between IL-15, adaptive anti-gluten immunity, and TCR αβ IEL activation remains to be further dissected and understood. It is likely that they synergize and amplify each other, ultimately inducing villous atrophy. For example, the ability of IL-15 to render effector T cells resistant to Fox3+ regulatory T cells was shown in the context of juvenile rheumatoid arthritis [39] and confirmed in CD [40]. The importance of understanding the role of IL-15 in CD cannot be overstated. Insights gained in the field will have a broad impact, given the association of IL-15 with rheumatoid arthritis [41], multiple sclerosis [42], and type-I diabetes [43].
Type-1 IFNs
The evidence for type-1 IFNs playing a role in CD stems from studies documenting dysregulated expression of IFN-α in CD patients [44-46], that treatment of hepatitis C patients with IFN-α leads to some patients developing CD [47], and that rotavirus infections that induce type-1 IFN are associated with increased incidence of CD [48]. Taken together, these studies suggest a role for type-1 IFNs in the loss of tolerance to gluten. This is further supported by studies showing that type-1 IFNs induce activation of DC [49]. More direct evidence for a role of type-1 IFNs in CD is provided by the observation that blocking IFN-α inhibits IFN-γ transcripts in ex vivo organ culture of CD biopsies challenged with gluten [45]. Furthermore, polycytidylic acid (poly I:C), a double-stranded RNA virus mimic that induces type-1 IFNs, was shown to induce TG2 activation [24]. Hence, type-1 IFNs could lead to loss of tolerance to gluten through their effect on DCs and/or TG2 activation. In addition to their role in the loss of oral tolerance, the hypothesis that type-1 IFNs may license IEL is plausible based on a studies showing type-1 IFNs can promote NK cell activity [50] and cytolytic properties of CD8 T cells [51].
More work is needed to better understand the role of type-1 IFNs in CD by focusing on how they polarize DCs and license TCR αβ IEL to become pro-inflammatory and killers of IEC, respectively. Furthermore, it remains to be determined what upregulates type-1 IFNs in CD patients. To that extent, the role of viral infection in particular needs to be further assessed. The knowledge that viruses induce type-1 IFNs, identification by GWAS of the TLR7/TLR8 locus as a risk factor for CD [3], as well as the observation that multiple rotavirus infections increase the incidence of CD [48], stresses the potential role of viral infections in CD pathogenesis.
An interesting concept to consider is the possibility that type-1 IFNs may act in synergy with IL-15 to break the threshold for disease and initiate the processes that lead to the eventual destruction of tissue. Furthermore, it is possible that IL-15 induction in some patients results from type-1 IFN signaling [52]. Finally, dissecting IL-15 and type-1 IFN expression in CD patients will help determine whether CD is a heterogeneous disease with different pathways leading to loss of oral tolerance and activation of IEL.
Dysbiosis in Celiac Disease
The relationship between the gut microbiota and CD is a topic that has been widely studied [53,54], and at the same time one that requires more work before we can make satisfying conclusions that can lead to therapeutic interventions. Here we will discuss some of these studies in light of the potential role of the microbiota in the regulation of IL-15 and type-1 IFNs, and in the induction of loss of oral tolerance and IEL activation.
The microbiome of CD
Epidemiological and clinical studies have led to long-standing speculation that the gut microbiota plays a role in CD. The evidence that supports this hypothesis includes, but is not limited to the following: i. There has been a rapid increase in the prevalence of CD over the last two decades, much shorter than the conceivable rate of genetic drift [55]; ii. Only a small proportion of the total pool of genetically predisposed individuals develop active disease [2,3]; iii. Delivery by cesarean section leads to an increase in susceptibility of CD [56] and delivery mode has a large impact on the gut microbial composition of newborns [57]; and iv. There is a positive correlation between the early use of antibiotics and CD development [58].
Recent advances in sequencing have made the investigation of gut microbial composition increasingly straightforward, and this has enabled studies demonstrating that the dysregulation of the gut microbiota (dysbiosis) accompanies, and may also play a role in the pathogenesis of gastrointestinal diseases such as inflammatory bowel disease (IBD) as well as autoimmune disorders such as type 1 diabetes, and rheumatoid arthritis [59].
Multiple sequencing efforts have been made on intestinal biopsies and feces of adult and juvenile CD patients [53,54]. Thus far, there does not appear to be a uniform “CD microbiome” to be found from these studies, with complicating factors being the variations in anatomical location from which samples were acquired, experimental methodology, and the inherent heterogeneity found in CD. In fact, one study has highlighted the differences that are found in patients depending on the presence of extraintestinal symptoms [60], while others have demonstrated differences between active CD patients and patients on a gluten free diet [61]. Below we discuss how having a dysregulated microbiome can aid in reaching the threshold necessary to develop CD.
Dysbiosis as a driver for the CD cytokine response
One way in which dysregulated microbiota could trigger CD is by driving the production of the known inducers of disease. We have discussed the known and potential roles of IL-15 and Type I IFNs, however it is not known what drives their excessive production in the context of CD, and it could be that dysbiosis plays a role.
Innate immune signaling has been shown to directly influence IL-15 expression. Activation of toll-like receptor 4 (TLR4) by bacterial membrane component LPS has been shown to induce upregulation of IL-15 and IL-15Rα in DCs [62] and Myd88 was shown to regulate IL-15 expression in IEC [63]. This provides a potential link between microbiota and the levels of IL-15 in the gut, however no studies have directly assessed the role of the microbiota in the regulation of IL-15 in IEC and the LP.
Although people often associate the production of type-1 IFNs with recognition of viruses, the molecular sensors of virus and bacteria often share common components of signaling, and it is known that bacteria can drive the production of type-1 IFNs [64]. In the context of the gut immune system, it was demonstrated that germ-free or antibiotic-treated mice have a significantly dampened type-1 IFN response to systemic viral infection, and gene expression analysis showed that in the context of a healthy gut, the microbiota maintains a tonic level of type-1 IFN production from antigen presenting cells that “poises” them to secrete the required amounts of IFN to control intestinal viral infection [65,66]. Another study showed that commensal lactic acid bacteria can drive the production of IFN-β from gut DCs in a TLR3 dependent manner [67].
Altogether, these observations suggest that a dysregulated microbiome could play a role in CD pathogenesis by driving aberrant type-1 IFN and/or IL15 expression. However, evidence for this hypothesis is mostly indirect and we lack studies analyzing how dysbiosis could drive dysregulated expression of these cytokines in the intestinal mucosa.
Microbial products in the loss of oral tolerance and activation of IEL
Another way in which the microbiota could drive disease is by directly affecting the loss of oral tolerance and the activation of IEL through bacterial products and metabolites.
A. Loss of oral tolerance
During times of health, the microbiome plays an essential role in the metabolism of certain foods and the development of the mucosal immune system, and helps to maintain the gut environment in a tolerogenic state that does not mount inflammatory responses against food or bacterial antigens [59]. Based on current studies and the known mechanisms underlying tolerance to dietary antigens, we can imagine two key scenarios by which dysbiosis could promote loss of oral tolerance. The first involves a decrease in short chain fatty acid (SCFA) production, and the second an expansion of Proteobacteria.
SCFA are molecules that are derived from the breakdown of dietary fiber by specific microbiota in the gut. SCFA can exert their function by signaling through G-protein coupled receptors (GPCRs) and by modulating gene expression via receptor-independent histone deacetylase (HDAC) inhibitory activity [68]. It was recently demonstrated that commensal-derived butyrate can directly lead to the differentiation of Tregs in the gut through HDAC inhibition [69,70]. The majority of butyrate producing bacteria belong to the Clostridia clusters IV and XIVa [71], suggesting that these bacteria may exert immune regulatory properties. In accordance, a more recent study identified 17 strains of Clostridia from healthy human feces that belong to clusters IV, XIVa, and XVIII and drive the production of SCFA and TGF-β, leading to the differentiation of colonic Tregs in germ-free mice [72]. Of relevance to CD, it was reported that SCFA may be decreased in CD patients [73]. Furthermore, studies in untreated CD children showed a decrease in the Faecalibacterium prausnitzii group (Clostridia cluster IV) that almost exclusively comprises butyrate producing bacteria [74]. However, it is important to point out that studies conducted in CD patients thus far have been conflicted, reporting both increases and decreases in different Clostridia species [74,75] and SCFA production [73,76]. This suggests that future studies should be designed to revisit these specific questions.
While the production of SCFA and presence of particular Clostridia species have been associated with promoting immune tolerance, expansion of Proteobacteria is often associated with intestinal inflammation [59]. A direct role for Proteobacteria in the induction of Th1 immunity was shown for Bilophila wadsworthia, which blooms in animals fed with a diet high in saturated fat [77]. This study also illustrates how diet, independent of gluten, can promote dysbiosis and create the conditions that lead to disease in genetically susceptible individuals. A relative increase in Proteobacteria was reported in CD patients [73,78], suggesting a potential role of Proteobacteria in CD pathogenesis. The increased burden of Escherichia coli in CD patients compared to controls [78] was further characterized to carry a higher number of virulence genes when compared to strains from controls [79]. Because the increase in E. coli was not found in children on a gluten-free diet it poses the question of whether its expansion was secondary to inflammation and not a driver of disease [61]. However, another study reported that there was a higher relative abundance of Protoebacteria in CD patients suffering from persistent symptoms on a gluten-free diet [80]. In addition to Proteobacteria, other bacteria could also play a pathogenic role. For instance, Staphylococcus epidermidis, found in children with active CD and on a gluten free diet [81], and Actinomyces graevenitzii, which was identified in duodenal biopsies of children with CD during the Swedish CD epidemic [82], are potential candidates that warrant more studies.
B. IEL
There have been several studies in murine models that have demonstrated a direct effect of microbiota on IEL function. Studies in germ-free mice show a severe reduction in the number of IEL, in particular TCR αβ IEL, and indicate that the microbiota plays a critical role in the recruitment and expansion of these cells [25]. Furthermore, a global transcriptional comparison of TCR γδ IEL in conventional and germ-free mice demonstrated that the microbiota contributes to the regulation of their transcriptional program [83]. Finally, other studies showed that both TCR αβ and TCR γδ IEL can receive signals from the microbiota through NOD2 [84], and receptors that utilize MyD88, presumably TLRs [85]. Given these direct lines of communication between bacteria and lymphocyte, and the demonstrated effects of bacteria on IEL effector function, it would be interesting to study the effect that a dysregulated microbiome could have on IEL killing in the context of CD.
Summary
A major challenge with studying and discussing a system as complex as the microbiome in the context of a multifactorial and heterogeneous disease is determining whether the microbiome is causative for disease, whether disease is causative for dysbiosis, or whether it is a combination of both. Utilization of gnotobiotic technologies in conjunction with additional human studies that investigate the CD microbiota using “-omic” approaches will facilitate the experimental validation of the potential pathogenic or therapeutic power of particular bacterial families and species in CD.
General conclusion
Increasingly, the need to identify treatments that could substitute for a gluten free diet is being acknowledged [86]. A characterization of the regulation of and interplay between IL-15, type-1 IFNs, and the microbiota will allow us to better understand the natural history and spectrum of CD (Figure 1). Furthermore, defining the role of key innate pathways in the development of loss of oral tolerance and villous atrophy will help identify therapies aimed at preventing and treating CD. Future clinical trials in CD will benefit from subphenotyping patients based on their cytokine and microbial profile, which will help establish the role of these innate players in CD pathogenesis.
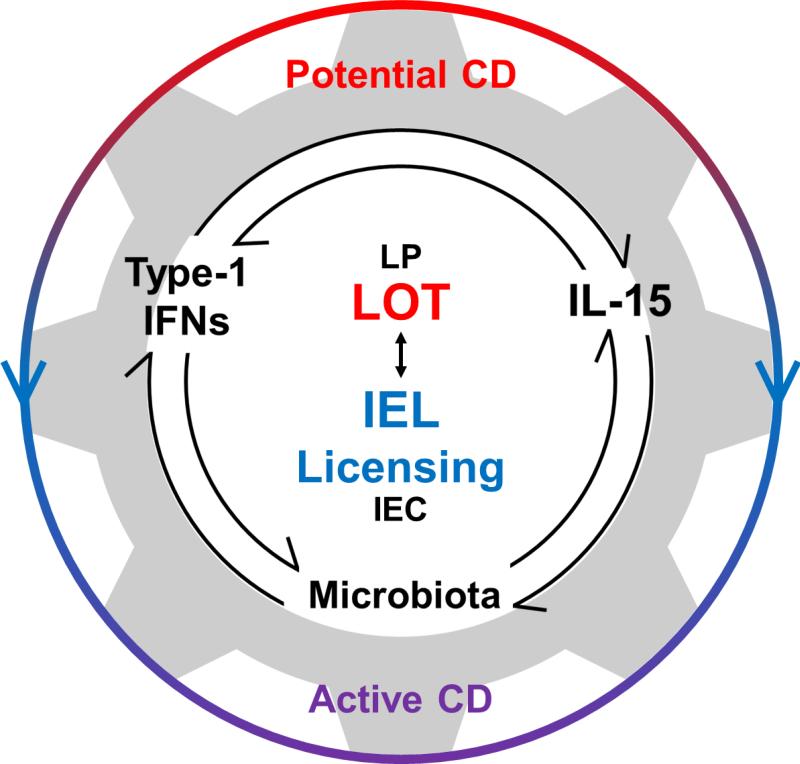
Figure 1. Innate actuators of CD pathogenesis.
IL-15, type-1 IFNs, and the microbiota can impact processes that are involved in the loss of oral tolerance (LOT) to gluten in the lamina propria (LP) and licensing of intraepithelial lymphocytes (IEL licensing) in the epithelium (IEC). In addition to the independent contributions of each factor, we appreciate the potential interplay between them. A better understanding of this interplay will help explain how potential CD patients can have LOT in the absence of IEL licensing whereas both LOT and IEL licensing are required for IEC destruction and villous atrophy in active CD.
Practice points.
IL-15 and type-1 IFNs are upregulated in the intestinal mucosa of CD patients. These cytokines, alone or in synergy may be sufficient to drive loss of oral tolerance and activation of IEL in genetically susceptible individuals. Hence targeting these cytokines may constitute a treatment for CD.
The microbiota is dysregulated in active CD and in patients on a GFD, thus altering its composition/function could help restore tolerance to gluten and prevent villous atrophy.
Induction of inflammatory anti-gluten T cell responses and CD-associated antibodies (anti-gluten and anti-TG2) can occur in the absence of villous atrophy. This provides a basis for the existence of potential CD and circumvents the requirement of villous atrophy for the diagnosis of CD.
Research agenda.
Define the drivers of IL-15 and type-1 IFNs in the epithelium and the LP
Determine whether CD is a heterogeneous disorder with distinct pathways involving IL-15, type-1 IFNs, or yet to be defined cytokines leading ultimately to loss of oral tolerance to gluten and/or villous atrophy.
Determine the crosstalk between epithelial stress and adaptive anti-gluten immunity in the development of villous atrophy.
Assess the potential role of dysbiosis and the interplay with gluten in CD. In particular determine whether dysbiosis drives dysregulation of IL-15 and type-1 IFN expression, and assess the potential direct pathogenic impact of CD dysbiosis on loss of oral tolerance and activation of IEL.
Acknowledgments
We would like to thank Christopher Carmean for critical reading of the manuscript. This work was supported by grants from the Digestive Diseases Research Core Center (DK42086) at the University of Chicago and from the US National Institutes of Health (RO1DK67180 and R01DK098435) to Bana Jabri.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none
Contributor Information
Sangman Michael Kim, Department of Medicine; Committee on Immunology, University of Chicago, Chicago, IL 60637, USA.
Toufic Mayassi, Department of Medicine; Committee on Immunology, University of Chicago, Chicago, IL 60637, USA.
Bana Jabri, Department of Pathology; Department of Pediatrics; Department of Medicine; Committee on Immunology, University of Chicago, Chicago, IL 60637, USA.
References
- 1.Jabri B, Sollid LM. Tissue-mediated control of immunopathology in coeliac disease. Nat Rev Immunol. 2009;9:858–70. doi: 10.1038/nri2670. [DOI] [PubMed] [Google Scholar]
- 2.Tack GJ, Verbeek WHM, Schreurs MWJ, Mulder CJJ. The spectrum of celiac disease: epidemiology, clinical aspects and treatment. Nat Rev Gastroenterol Hepatol. 2010;7:204–13. doi: 10.1038/nrgastro.2010.23. [DOI] [PubMed] [Google Scholar]
- 3*.Abadie V, Sollid LM, Barreiro LB, Jabri B. Integration of Genetic and Immunological Insights into a Model of Celiac Disease Pathogenesis. Annu. Rev. Immunol. 2011;29:493–525. doi: 10.1146/annurev-immunol-040210-092915. [DOI] [PubMed] [Google Scholar]
- 4.Bodd M, Ráki M, Tollefsen S, Fallang LE, Bergseng E, Lundin KEA, et al. HLA-DQ2-restricted gluten-reactive T cells produce IL-21 but not IL-17 or IL-22. Mucosal Immunology. 2010;3:594–601. doi: 10.1038/mi.2010.36. [DOI] [PubMed] [Google Scholar]
- 5.Strengell M, Julkunen I, Matikainen S. IFN-alpha regulates IL-21 and IL-21R expression in human NK and T cells. J. Leukoc. Biol. 2004;76:416–22. doi: 10.1189/jlb.1003488. [DOI] [PubMed] [Google Scholar]
- 6.Sarra M, Cupi ML, Monteleone I, egrave EF, Ronchetti G, Di Sabatino A, et al. IL-15 positively regulates IL-21 production in celiac disease mucosa. 2012;6:244–55. doi: 10.1038/mi.2012.65. [DOI] [PubMed] [Google Scholar]
- 7.Junker Y, Zeissig S, Kim S- J, Barisani D, Wieser H, Leffler DA, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. Journal of Experimental Medicine. 2012;209:2395–408. doi: 10.1084/jem.20102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barone M, Troncone R, Auricchio S. Gliadin Peptides as Triggers of the Proliferative and Stress/Innate Immune Response of the Celiac Small Intestinal Mucosa. IJMS. 2014;15:20518–37. doi: 10.3390/ijms151120518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molberg O, Mcadam SN, Körner R, Quarsten H, Kristiansen C, Madsen L, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nature Medicine. 1998;4:713–7. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 10.van de Wal Y, Kooy Y, van Veelen P, Peña S, Mearin L, Papadopoulos G, et al. Cutting Edge: Selective Deamidation by Tissue Transglutaminase Strongly Enhances Gliadin-Specific T Cell Reactivity. J Immunol. 1998;161(4):1585–8. [PubMed] [Google Scholar]
- 11.Kim CY, Quarsten H, Bergseng E. Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Proc Natl Acad Sci U S A. 2004;101(12):4175–9. doi: 10.1073/pnas.0306885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson KN, Tye-Din JA, Reid HH, Chen Z, Borg NA, Beissbarth T, et al. A Structural and Immunological Basis for the Role of Human Leukocyte Antigen DQ8 in Celiac Disease. Immunity. 2007;27:23–34. doi: 10.1016/j.immuni.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Jabri B, De Serre N, Cellier C, Evans K, Gache C. Selective expansion of intraepithelial lymphocytes expressing the HLA-E–specific natural killer receptor CD94 in celiac disease. Gastroenterology. 2000;118(5):867–79. doi: 10.1016/S0016-5085(00)70173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maiuri L, Ciacci C, Auricchio S, Brown V, Quaratino S, Londei M. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology. 2000;119:996–1006. doi: 10.1053/gast.2000.18149. [DOI] [PubMed] [Google Scholar]
- 15.Abadie V, Jabri B. IL-15: a central regulator of celiac disease immunopathology. Immunol. Rev. 2014;260:221–34. doi: 10.1111/imr.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Mention J- J, Ben Ahmed M, Bègue B, Barbe U, Verkarre V, Asnafi V, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–45. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 17.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 18.Mora JR, Andrian von UH. Retinoic acid: an educational “vitamin elixir” for gut-seeking T cells. Immunity. 2004;21:458–60. doi: 10.1016/j.immuni.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 19*.DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–4. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the Diagnosis of Coeliac Disease. Journal of Pediatric Gastroenterology and Nutrition. 2012;54:136–60. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 21.Dai C, Jiang M, Sun M-J. Isotretinoin and Risk of Inflammatory Bowel Disease. Am J Gastroenterology. 2014;109:1493–4. doi: 10.1038/ajg.2014.184. [DOI] [PubMed] [Google Scholar]
- 22.Molberg O, Kett K, Scott H, Thorsby E, Sollid LM, Lundin KE. Gliadin specific, HLA DQ2-restricted T cells are commonly found in small intestinal biopsies from coeliac disease patients, but not from controls. Scand. J. Immunol. 1997;46:103–9. doi: 10.1046/j.1365-3083.1997.d01-93.x. [DOI] [PubMed] [Google Scholar]
- 23.Kraus TA, Toy L, Chan L, Childs J, Cheifetz A, Mayer L. Failure to induce oral tolerance in Crohn's and ulcerative colitis patients: possible genetic risk. Ann. N. Y. Acad. Sci. 2004;1029:225–38. doi: 10.1196/annals.1309.054. [DOI] [PubMed] [Google Scholar]
- 24*.Siegel M, Strnad P, Watts RE, Choi K, Jabri B, Omary MB, et al. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS ONE. 2008;3:e1861. doi: 10.1371/journal.pone.0001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abadie V, Discepolo V, Jabri B. Intraepithelial lymphocytes in celiac disease immunopathology. Semin Immunopathol. 2012;34:551–66. doi: 10.1007/s00281-012-0316-x. [DOI] [PubMed] [Google Scholar]
- 26.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 27.Roberts AI, Lee L, Schwarz E, Groh V, Spies T, Ebert EC, et al. Cutting Edge: NKG2D Receptors Induced by IL-15 Costimulate CD28-Negative Effector CTL in the Tissue Microenvironment. The Journal of Immunology. 2001;167:5527–30. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- 28*.Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–66. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Hüe S, Mention J-J, Monteiro RC, Zhang S, Cellier C, Schmitz J, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–77. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Jabri B, Selby JM, Negulescu H, Lee L, Roberts AI, Beavis A, et al. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity. 2002;17:487–99. doi: 10.1016/s1074-7613(02)00427-2. [DOI] [PubMed] [Google Scholar]
- 31.Meresse B. Reprogramming of CTLs into natural killer-like cells in celiac disease. Journal of Experimental Medicine. 2006;203:1343–55. doi: 10.1084/jem.20060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang F, Chen Z, Ciszewski C, Setty M, Solus J, Tretiakova M, et al. Cytosolic PLA2 is required for CTL-mediated immunopathology of celiac disease via NKG2D and IL-15. Journal of Experimental Medicine. 2009;206:707–19. doi: 10.1084/jem.20071887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu RB, Engels B, Schreiber K, Ciszewski C, Schietinger A, Schreiber H, et al. IL-15 in tumor microenvironment causes rejection of large established tumors by T cells in a noncognate T cell receptor-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 2013;110:8158–63. doi: 10.1073/pnas.1301022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deshpande P, Cavanagh MM, Le Saux S, Singh K, Weyand CM, Goronzy JJ. IL-7- and IL-15-Mediated TCR Sensitization Enables T Cell Responses to Self-Antigens. The Journal of Immunology. 2013;190:1416–23. doi: 10.4049/jimmunol.1201620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu T-S, Lee J-M, Lai Y-G, Hsu J-C, Tsai C-Y, Lee Y-H, et al. Reduced expression of Bcl-2 in CD8+ T cells deficient in the IL-15 receptor alpha-chain. J. Immunol. 2002;168:705–12. doi: 10.4049/jimmunol.168.2.705. [DOI] [PubMed] [Google Scholar]
- 36*.Malamut G, Machhour El R, Montcuquet N, Martin-Lannerée S, Dusanter-Fourt I, Verkarre V, et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease–associated inflammation and lymphomagenesis. J. Clin. Invest. 2010;120:2131–43. doi: 10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Malamut G, Machhour El R, Montcuquet N, Martin-Lannerée S, Dusanter-Fourt I, Verkarre V, et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J. Clin. Invest. 2010;120:2131–43. doi: 10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benahmed M, Meresse B, Arnulf B, Barbe U, Mention J-J, Verkarre V, et al. Inhibition of TGF-beta signaling by IL-15: a new role for IL-15 in the loss of immune homeostasis in celiac disease. Gastroenterology. 2007;132:994–1008. doi: 10.1053/j.gastro.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 39.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J. Exp. Med. 2005;201:1793–803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed MB, Belhadj Hmida N, Moes N, Buyse S, Abdeladhim M, Louzir H, et al. IL-15 Renders Conventional Lymphocytes Resistant to Suppressive Functions of Regulatory T Cells through Activation of the Phosphatidylinositol 3-Kinase Pathway. The Journal of Immunology. 2009;182:6763–70. doi: 10.4049/jimmunol.0801792. [DOI] [PubMed] [Google Scholar]
- 41.Asquith DL, McInnes IB. Emerging cytokine targets in rheumatoid arthritis. Current Opinion in Rheumatology. 2007;19:246–51. doi: 10.1097/BOR.0b013e3280eec78c. [DOI] [PubMed] [Google Scholar]
- 42.Rentzos M, Rombos A. The role of IL-15 in central nervous system disorders. Acta Neurol. Scand. 2012;125:77–82. doi: 10.1111/j.1600-0404.2011.01524.x. [DOI] [PubMed] [Google Scholar]
- 43*.Chen J, Feigenbaum L, Awasthi P, Butcher DO, Anver MR, Golubeva YG, et al. Insulin-dependent diabetes induced by pancreatic beta cell expression of IL-15 and IL-15Rα. Proc. Natl. Acad. Sci. U.S.A. 2013;110:13534–9. doi: 10.1073/pnas.1312911110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monteleone G. Role of interferon alpha in promoting T helper cell type 1 responses in the small intestine in coeliac disease. Gut. 2001;48:425–9. doi: 10.1136/gut.48.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Sabatino A, Pickard KM, Gordon JN, Salvati V, Mazzarella G, Beattie RM, et al. Evidence for the Role of Interferon-alfa Production by Dendritic Cells in the Th1 Response in Celiac Disease. Gastroenterology. 2007;133:1175–87. doi: 10.1053/j.gastro.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 46.Ráki M, Beitnes A-CR, Lundin KEA, Jahnsen J, Jahnsen FL, Sollid LM. Plasmacytoid dendritic cells are scarcely represented in the human gut mucosa and are not recruited to the celiac lesion. Mucosal Immunology. 2013;6:985–92. doi: 10.1038/mi.2012.136. [DOI] [PubMed] [Google Scholar]
- 47*.Cammarota G, Cuoco L, Cianci R, Pandolfi F, Gasbarrini G. Onset of coeliac disease during treatment with interferon for chronic hepatitis C. Lancet. 2000;356:1494–5. doi: 10.1016/S0140-6736(00)02880-4. [DOI] [PubMed] [Google Scholar]
- 48.Stene LC, Honeyman MC, Hoffenberg EJ, Haas JE, Sokol RJ, Emery L, et al. Rotavirus Infection Frequency and Risk of Celiac Disease Autoimmunity in Early Childhood: A Longitudinal Study. Am J Gastroenterology. 2006;101:2333–40. doi: 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 49.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: Endogenous activators of dendritic cells. Nature Medicine. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 50.Biron CA, Nguyen KB, Pien GC. Innate immune responses to LCMV infections: natural killer cells and cytokines. Curr. Top. Microbiol. Immunol. 2002;263:7–27. doi: 10.1007/978-3-642-56055-2_2. [DOI] [PubMed] [Google Scholar]
- 51.Hervas-Stubbs S, Riezu-Boj J-I, Gonzalez I, Mancheño U, Dubrot J, Azpilicueta A, et al. Effects of IFN-α as a signal-3 cytokine on human naïve and antigen-experienced CD8+ T cells. Eur. J. Immunol. 2010;40:3389–402. doi: 10.1002/eji.201040664. [DOI] [PubMed] [Google Scholar]
- 52.Colpitts SL, Stoklasek TA, Plumlee CR, Obar JJ, Guo C, Lefrançois L. Cutting edge: the role of IFN-α receptor and MyD88 signaling in induction of IL-15 expression in vivo. The Journal of Immunology. 2012;188:2483–7. doi: 10.4049/jimmunol.1103609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanz Y, De Pama G, Laparra M. Unraveling the ties between celiac disease and intestinal microbiota. Int. Rev. Immunol. 2011;30:207–18. doi: 10.3109/08830185.2011.599084. [DOI] [PubMed] [Google Scholar]
- 54.Galipeau HJ, Verdu EF. Gut microbes and adverse food reactions: Focus on gluten related disorders. Gut Microbes. 2015;5:594–605. doi: 10.4161/19490976.2014.969635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, et al. Increasing prevalence of coeliac disease over time. Aliment. Pharmacol. Ther. 2007;26:1217–25. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 56.Decker E, Engelmann G, Findeisen A, Gerner P, Laass M, Ney D, et al. Cesarean Delivery Is Associated With Celiac Disease but Not Inflammatory Bowel Disease in Children. PEDIATRICS. 2010;125:e1433–40. doi: 10.1542/peds.2009-2260. [DOI] [PubMed] [Google Scholar]
- 57.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U.S.A. 2010;107(26):11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Canova C, Zabeo V, Pitter G, Romor P, Baldovin T, Zanotti R, et al. Association of maternal education, early infections, and antibiotic use with celiac disease: a population-based birth cohort study in northeastern Italy. Am. J. Epidemiol. 2014;180:76–85. doi: 10.1093/aje/kwu101. [DOI] [PubMed] [Google Scholar]
- 59.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–41. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wacklin P, Kaukinen K, Tuovinen E, Collin P, Lindfors K, Partanen J, et al. The duodenal microbiota composition of adult celiac disease patients is associated with the clinical manifestation of the disease. Inflammatory Bowel Diseases. 2013;19:934–41. doi: 10.1097/MIB.0b013e31828029a9. [DOI] [PubMed] [Google Scholar]
- 61.Collado MC, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalances in faecal and duodenal Bifidobacterium species composition in active and non-active coeliac disease. BMC Microbiol. 2008;8:232. doi: 10.1186/1471-2180-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Re F, Strominger JL. Heterogeneity of TLR-induced responses in dendritic cells: from innate to adaptive immunity. Immunobiology. 2004;209:191–8. doi: 10.1016/j.imbio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Yu Q, Tang C, Xun S, Yajima T, Takeda K, Yoshikai Y. MyD88-Dependent Signaling for IL-15 Production Plays an Important Role in Maintenance of CD8 TCR and TCR Intestinal Intraepithelial Lymphocytes. The Journal of Immunology. 2006;176:6180–5. doi: 10.4049/jimmunol.176.10.6180. [DOI] [PubMed] [Google Scholar]
- 64.Perry AK, Chen G, Zheng D, Tang H, Cheng G. The host type I interferon response to viral and bacterial infections. Cell Res. 2005;15:407–22. doi: 10.1038/sj.cr.7290309. [DOI] [PubMed] [Google Scholar]
- 65.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–70. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37:171–86. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 67.Kawashima T, Kosaka A, Yan H, Guo Z, Uchiyama R, Fukui R, et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-β. Immunity. 2013;38:1187–97. doi: 10.1016/j.immuni.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 68.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nature Publishing Group. 2013;14:676–84. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 70.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hold GL, Schwiertz A, Aminov RI, Blaut M, Flint HJ. Oligonucleotide probes that detect quantitatively significant groups of butyrate-producing bacteria in human feces. Appl. Environ. Microbiol. 2003;69:4320–4. doi: 10.1128/AEM.69.7.4320-4324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–6. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 73.Di Cagno R, De Angelis M, De Pasquale I, Ndagijimana M, Vernocchi P, Ricciuti P, et al. Duodenal and faecal microbiota of celiac children: molecular, phenotype and metabolome characterization. BMC Microbiol. 2011;11:219. doi: 10.1186/1471-2180-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74*.De Palma G, Nadal I, Medina M, Donat E, Ribes-Koninckx C, Calabuig M, et al. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010;10:63. doi: 10.1186/1471-2180-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tjellström B, Stenhammar L, Högberg L, Fälth-Magnusson K, Magnusson K-E, Midtvedt T, et al. Gut microflora associated characteristics in children with celiac disease. Am J Gastroenterology. 2005;100:2784–8. doi: 10.1111/j.1572-0241.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- 76.Tjellström B, Stenhammar L, Högberg L, Fälth-Magnusson K, Magnusson K-E, Midtvedt T, et al. Gut microflora associated characteristics in first-degree relatives of children with celiac disease. Scand J Gastroenterol. 2007;42:1204–8. doi: 10.1080/00365520701320687. [DOI] [PubMed] [Google Scholar]
- 77.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487:104–8. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Collado MC, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J. Clin. Pathol. 2009;62:264–9. doi: 10.1136/jcp.2008.061366. [DOI] [PubMed] [Google Scholar]
- 79.Sánchez E, Nadal I, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Reduced diversity and increased virulence-gene carriage in intestinal enterobacteria of coeliac children. BMC Gastroenterol. 2008;8:50. doi: 10.1186/1471-230X-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wacklin P, Laurikka P, Lindfors K, Collin P, Salmi T, Lähdeaho M-L, et al. Altered duodenal microbiota composition in celiac disease patients suffering from persistent symptoms on a long-term gluten-free diet. Am J Gastroenterology. 2014;109:1933–41. doi: 10.1038/ajg.2014.355. [DOI] [PubMed] [Google Scholar]
- 81.Sánchez E, Ribes-Koninckx C, Calabuig M, Sanz Y. Intestinal Staphylococcus spp. and virulent features associated with coeliac disease. J. Clin. Pathol. 2012;65:830–4. doi: 10.1136/jclinpath-2012-200759. [DOI] [PubMed] [Google Scholar]
- 82.Ou G, Hedberg M, Hörstedt P, Baranov V, Forsberg G, Drobni M, et al. Proximal small intestinal microbiota and identification of rod-shaped bacteria associated with childhood celiac disease. Am J Gastroenterology. 2009;104:3058–67. doi: 10.1038/ajg.2009.524. [DOI] [PubMed] [Google Scholar]
- 83.Ismail AS, Behrendt CL, Hooper LV. Reciprocal Interactions between Commensal Bacteria and γδ Intraepithelial Lymphocytes during Mucosal Injury. The Journal of Immunology. 2009;182(5):3047–54. doi: 10.4049/jimmunol.0802705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang W, Wang X, Zeng B, Liu L, Tardivel A, Wei H, et al. Recognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytes. Journal of Experimental Medicine. 2013;210:2465–76. doi: 10.1084/jem.20122490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ismail AS, Severson KM, Vaishnava S. γδ intraepithelial lymphocytes are essential mediators of host–microbial homeostasis at the intestinal mucosal surface. 2011 doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gottlieb K, Dawson J, Hussain F, Murray JA. Development of drugs for celiac disease: review of endpoints for Phase 2 and 3 trials. Gastroenterol Rep (Oxf) 2015 doi: 10.1093/gastro/gov006. [DOI] [PMC free article] [PubMed] [Google Scholar]