Abstract
The central goal of this manuscript is to survey our present knowledge of how cortical interneuron subtypes are generated. To achieve this, we will first define what is meant by subtype diversity. To this end, we begin by considering the mature properties that differentiate between the different populations of cortical interneurons. This requires us to address the difficulties involved in determining which characteristics allow particular interneurons to be assigned to distinct subclasses. Having grappled with this thorny issue, we will then proceed to review the progressive events in development involved in the generation of interneuron diversity. Starting with their origin and specification within the subpallium, we will follow them up through the first postnatal weeks during their integration into a functional network. Finally, we will conclude by calling the readers attention to the devastating consequences that result from developmental failures in the formation of inhibitory circuits within the cortex.
1. General Introduction
Alber Szent-Gyogyi once said: “If structure does not tell us anything about function, it only means that we have not looked at it correctly” (Buzsaki, Rhythms of the Brain, 2006). A century ago, our understanding of brain structure was limited to descriptions of its gross anatomy. Cajal’s recognition that the neuron was the fundamental building block of the brain provided the first meaningful step in the exploration of how brain structure at a cellular level relates to its ability to manifest complex behaviors. Since then, great strides have been made in unraveling how the topographic projections of excitatory pyramidal neurons and associated inhibitory interneurons contribute to the creation of sensory representations of the physical world. However, less progress has been made in understanding the learning and memory consolidation within the neocortex. Cortical interneurons, with their enormous diversity of subtypes, and their ability to provide feedforward and feedback information are ideally suited for this role. Each interneuron subclass likely possesses both specific connectivity and an input/output function that define their role in the cortex. Here we explore the logic by which genetic and environmental events contribute to the generation of cortical interneuron subtypes and the events leading to their subsequent integration into cortical networks.
2. The Classification of Cortical Interneuron Subtypes
Recently, a consortium of scientists specializing in development, anatomy and/or physiology convened at the birthplace of Ramon y Cajal for 3 days to discuss interneuron classification (PING, 2008). Adding to the historical spirit of this venture, the meeting was held in the fifteenth-century chapel where in his youth Cajal was no doubt compelled to languish. This committee vetted itself with the task of creating a unifying nomenclature for GABAergic interneurons in the cerebral cortex, which they ultimately designated as the Petilla terminology (Petilla Interneuron Nomenclature Group et al., 2008). Following the recommendations of this committee, we will review the different characteristics attributed to distinct subtypes of interneurons in the mature cortex. This includes consideration of the characteristic morphologies, molecular markers, and physiological properties that characterize distinct mature cortical interneuron populations.
2.1. What is an interneuron? How many subtypes? Basic properties of mature interneurons
GABAergic cortical interneuron diversity is achieved by the acquisition of different properties such as morphology, molecular, targeting, synaptic transmission and physiological character. On the basis of these traits, cortical interneurons have been subdivided into different subclasses. Ideally, all the criteria would be taken into account in determining the specific designation of particular interneurons; however, the difficulty of performing parametric analysis makes this in most cases impractical. Moreover, as scientists often disagree on the appropriate weighting of specific characteristics in classifying these cells, an interneuron’s subtype is all too often left in the eye of the beholder.
Interneurons were first identified and classified based on their morphology. In 1899, Cajal described them as “short axon cells,” whose axons project locally (however, if stretched out these axons are anything but short). Axon morphology has been historically considered to be a critical characteristic for determining interneuron subtype (with the position of the initial segment, arbor trajectory, branch metrics, and synaptic subcellular selectivity typically being used as classifiers). Emphasis on this feature seems sensible, as the extent of an axon’s ramifications will to a great extent determine its output domain. A horizontal or vertical arbor will establish if the output will influence individual or multiple cortical columns or layers. Dendrites (arborization polarity and branch metrics) and the morphology (shape and size) of the cell soma are also used for classification (Petilla Interneuron Nomenclature Group et al., 2008). Another important criteria to consider is the precise cell types a particular interneuron targets, such as pyramidal cells, other interneurons, glial cells, or cells of the vascular system, as well as the subcellular compartment (somata, dendrites, axon, etc.) that they selectively target (Petilla Interneuron Nomenclature Group et al., 2008).
In addition, molecular markers that account for many of the neuron’s emergent properties, such as neuropeptides, Ca2+ proteins, ionic channels, receptors, and transporters have been typically used for interneuron classification, (Baraban and Tallent, 2004; Goldberg et al., 2005, 2008; Lau et al., 2000; Monyer and Markram, 2004; Rudy and McBain, 2001). By combining gene expression techniques with intracellular recording, dye filling, and morphological analysis, it has been shown that some molecular markers such as parvalbumin (PV), calretinin (CR), Kv3.1, vasoactive intestinal peptide (VIP), somatostatin (SST), cholecystokinin (CCK), and neuronal nitric oxide synthase (nNOS) are good indicators of subtype identity, while others, such as calbindin (CB), neuropeptide Y (NPY), and Kv3.2, are more difficult to use due to their expression by a variety of cell types (Cauli et al., 1997; Chow et al., 1999; DeFelipe, 1993; Gerashchenko et al., 2008; Gonchar and Burkhalter, 1997; Gupta et al., 2000; Kawaguchi and Kubota, 1996; Kubota and Kawaguchi, 1994, 1997; Monyer and Markram, 2004). Comprehensive subtype analysis has become possible with the advent of microarray technology. Genome-wide profiling of small groups of interneurons (Sugino et al., 2006) or even single cells (Kamme et al., 2003) is becoming increasingly common.
A further criterion widely utilized for interneuron classification is their intrinsic firing properties, which are indicative of the activity of particular neurons and their presumed role within the cortical circuitry. Different classes of interneurons have distinct firing and subthreshold properties. For example, in response to a long depolarizing current pulse, interneurons show a variety of distinct firing characteristics, including bursting, stuttering, fast spiking, irregular spiking, and accomodation (Petilla Interneuron Nomenclature Group et al., 2008). Some caution, however, must be applied to the interpretation of these depolarization patterns, as they may not occur under physiological conditions. It is difficult to gauge the significance of a neuron stuttering when it normally only speaks in short sentences.
Interneurons are also distinguishable at the synaptic level, by their inputs, the target specificity of their output and postsynaptic responses (Somogyi and Klausberger, 2005; Somogyi et al., 1998). The response of interneurons to excitatory or inhibitory input can vary depending on the receptor subtype mediating these responses. For example, axoaxonic interneurons (or chandelier cells) despite being inhibitory produce a postsynaptic GABAergic depolarization of the initial segment of pyramidal cells. Such postsynaptic selectivity was recently shown to have dramatic functional consequences at the microcircuit level in humans (Molnar et al., 2008). Moreover, a neuron’s prior history of stimulation can affect how it responds to later synaptic events. As a consequence, repeated firing can result in facilitation or depression (Gupta et al., 2000). The intrinsic and synaptic properties are both likely a product of the molecular composition of the cell such as channels and other signaling molecules, as well as the ramification of their processes. Therefore it is not surprising that in many cases there is a correlation between the intrinsic electrophysiology and synaptic responses of an interneuron, and their molecular markers and morphology. However, this correlation is not absolute, and one cannot a priori predict the functional characteristics of an interneuron solely on the basis of their molecular signature. Thus, to both classify and assess the function of a certain interneuron subtype, it is essential to take a multifaceted approach, including as many of the features referred above (and summarized in Table 3.1) as possible.
Table 3.1.
Summary of morphological, molecular, and physiological features (from the Petilla meeting)
| Morphological features |
| Axon: initial segment, arbor trajectory, terminal shape, branch metrics, boutons, synaptic targets |
| Dendrite: arborization polarity, branch metrics, fine structure, postsynaptic element |
| Soma: shape, size, orientation |
| Connections: chemical and electrical, source, location and distribution |
| Molecular features |
| Transcription factors |
| Neurotransmitters or their synthesizing enzymes |
| Neuropeptides |
| Calcium-binding proteins |
| Receptors: ionotropic, metabotropic |
| Structural proteins |
| Cell-surface markers |
| Ion channels |
| Connexions |
| Transporters: plasma membrane, vesicular |
| Physiological features |
| Passive or subthreshold parameters: resting membrane potential, membrane time constants, input resistance, oscillation and resonance, rheobase and chronaxie, rectification |
| Action potential (AP) measurements: amplitude, threshold, half-width, afterhyperpolarization, afterdepolarization, changes in AP waveform during train |
| Dendritic backpropagation |
| Depolarizing plateaus |
| Firing pattern: oscillatory and resonant behavior, onset response to depolarizing step, steady-state response to depolarizing step |
| Response to hyperpolarizing step: rectification, rebound |
| Spiking recorded extracellularly: phase relationship to oscillations, functional response specificity, crosscorrelation and other dynamics |
| Postsynaptic responses: spontaneous and evoked, ratio of receptor subtypes, spatial and temporal summation, short- and long-term plasticity, gap junctions |
The so-called fast spiking, basket cells provide a good example of this sort of taxonomy in practice. These cells are primarily PV expressing and are classified by their innervation at the soma and proximal dendrite of target neurons (both pyramidal and GABAergic) (Freund, 2003). Among their archtypical characteristics are their fast kinetics, including their intrinsic firing (high-frequency repetitive firing, brief single spikes, a fast membrane time constant) and their synaptic outputs and inputs (Jonas et al., 2004). These to a large degree are accounted for by their expression of specific channels, including voltage-gated potassium (K+) channels (namely Kv3.1), sodium (Na+) channels with rapid activation kinetics, and AMPA receptors with fast gating kinetics (Jonas et al., 2004; Rudy and McBain, 2001). They innervate postsynaptic targets close to the site of action potential initiation, the axon initial segment. As a result they have been shown to provide strong inhibitory control over postsynaptic cell firing and are the main class of cells to provide feedforward inhibition and hence precise control of neuronal output (Pouille and Scanziani, 2001). Moreover, as a given PV basket cell contacts a vast number postsynaptic cells, this interneuron subtype has the capacity to synchronize the firing of large groups of neurons (Cobb et al., 1995; Pouille and Scanziani, 2001). Although we have chosen the FS basket cells to illustrate the mature properties of a particular interneuron subtype equally intriguing characteristics have been ascribed to many of the other major classes of cortical interneurons. Recent literature provides detailed discussions of some of the more prominent subclasses, including VIP-expressing multipolar, CR-expressing bipolar, or Sst-expressing Martinotti interneuron subclasses (Ferezou et al., 2002; Ma et al., 2006; Markram et al., 2004; Porter et al., 1998).
Despite the seductive wealth of data describing subtype complexity, in the final analysis the plaintive warning given by Dr Somogyi at the beginning of the Petilla meeting should be heeded:
The shapes, markers and firing patterns of interneurons exist as separate entities only in the collective imagination of investigators. In the end there is only the contextual input-output function of interneurons, as they perform operations via electrical and chemical signals. Thus, their reality is only tangible to the extent that they provide effective neural modulation to a functioning network, as it transits through different brain states. (P. Somogyi, personal communication).
3. Development of Cortical Interneurons
Inhibitory (GABAergic) interneurons, although far less numerous than the excitatory pyramidal neurons, are comprised by at least 15 subtypes, (Markram et al., 2004; Petilla Interneuron Nomenclature Group et al., 2008). This large variety in inhibition is thought to bestow the brain with the profound nonlinear functionality required for higher cortical processes. While to date no systematic studies have been done across various species, we predict that such a study would reveal that the diversity of interneurons compared to projection neurons has increased disproportionately during speciation. Furthermore, as we believe that much of this diversity is determined during development, examining the developmental origins of cortical interneurons should ultimately prove to be highly informative. Here, we survey the current literature and provide our present view of how genetic and environmental factors result in the generation of different cortical interneuron subtypes, as well as their subsequent integration into cortical circuitry.
3.1. The telencephalon is an alar plate derivative
Comparative fate-mapping studies in a variety of organisms indicate that the telencephalon is derived from the anterior lateral (i.e., alar) neural plate, and subdivided into specific regional territories defined by morphogenetic parameters during development (Fishell, 1997; Rubenstein et al., 1998; Shimamura et al., 1995, 1997). Pioneering efforts to understand the compartmentalization of the telencephalon have lead to the proposal of the prosomeric model (Pombal and Puelles, 1999; Puelles, 2001; Puelles and Rubenstein, 2003; Rubenstein et al., 1994). This model posits that early lineage restrictions result in the establishment of transient prosomeres (defined as segment-like divisions) within the forebrain (Rubenstein et al., 1994). The existence and exact location of the prosomeric boundaries are still a matter of debate; however, some of the divisions of the telencephalon are today universally accepted. The most prominent subdivision separates the dorsal pallium and ventral subpallium. Rather than being a result of lineage restriction, early dorsoventral patterning events appear to be mediated by extrinsic cues (for reviews, see Hérbert and Fishell, 2008; Rallu et al., 2002a; Sur and Rubenstein, 2005; Wilson and Houart, 2004; Wilson and Rubenstein, 2000).
Recent studies suggest that BMPs and Wnts bestow the pallium with dorsal character (Aboitiz and Montiel, 2007; O’Leary and Sahara, 2008; O’Leary et al., 2007). Glutamatergic cortical cells (or principal cells) originate within the ventricular and subventricular region of the pallium and migrate radially to their final destination (Kriegstein and Noctor, 2004; Marin and Rubenstein, 2003; Mission et al., 1991; Parnavelas, 2000; Rakic, 1990). By contrast, the ventral telencephalon (subpallium) responds to Shh and FGF8 by producing different types of GABAergic neurons, such as cortical interneurons and striatal medium spiny neurons. Postmitotic interneurons of all mammals studied to date (and probably many vertebrates) undergo long-range tangential migration to reach the cortex (Corbin et al., 2001; Kriegstein and Noctor, 2004; Marin and Rubenstein, 2001, 2003). Surprisingly, a possible exception to this occurs in humans, where it has been reported that interneurons undergo at least their final mitotic events in the subventricular zone (SVZ) of the pallium (Letinic et al., 2002).
3.2. The embryonic subpallium: From embryology to developmental biology
The ganglionic eminences were first described as bumps of proliferating cells within the ventral telencephalon. Later, it was discovered that these regions could be subdivided based on their expression of specific genes. Loss of function analysis of these genes, as well as fate-mapping efforts showed that these regions produce different types of neurons. The next section follows along historical lines to consider (1) the embryology, (2) molecular specification, and (3) in vivo fate mapping of the subpallium.
3.3. The ganglionic eminences were first defined by their morphology
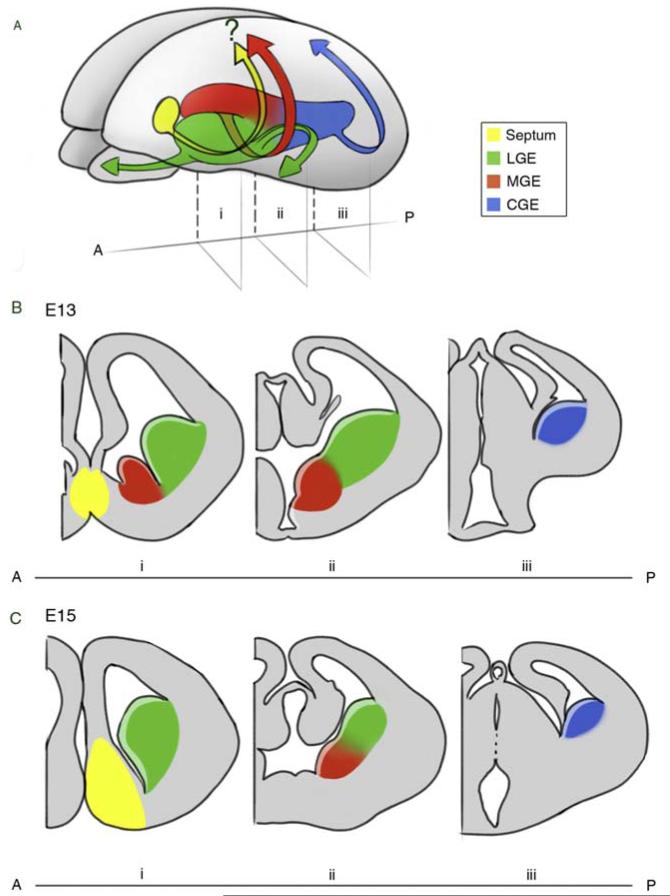
Landmark studies by Smart and Sturrock (Smart, 1976; Sturrock and Smart, 1980) subdivided the subpallium into discrete territories based on anatomical features within the neuroepithelium. They described the murine lateral (LGE) and medial (MGE) ganglionic eminences by analogy to what was first described in human embryos (Smart, 1976; Sturrock and Smart, 1980). From the beginning, it was appreciated that these ganglionic eminences arose in a discrete temporal order with the “earlier-generated ventral eminence” (the MGE) appearing at the telencephalic–diencephalic junction, followed by a “later appearing dorsal eminence,” named the lateral ganglionic eminence (LGE) (Smart, 1976). The caudal ganglionic eminence (CGE) was proposed much later as a discrete entity being first defined by Anderson et al. (2001) as the eminence that is posterior to the fusion of the MGE and LGE. However, while the MGE and LGE are clearly separated by a sulcus, a physical barrier does not exist in the case of the CGE (see figure 3.1), and therefore its identity based on embryology is controversial, a matter that we will address later. Another progenitor region within the subpallium that has received little attention to date is the septal eminence. Like the CGE, this structure arises relatively late in development. While the septal eminence has been assumed to give rise to the septal nucleus, this structure has yet to be fate mapped. Although studies are too preliminary for a definitive answer, hints exist that this eminence may also be a source for tangentially migrating cell populations, which possibly include a subset of cortical interneurons (Taglialatela et al., 2004; Xu et al., 2008). In addition, less well morphologically defined regions such as the anterior entopeduncular region (AEP) and the preoptic area (POa) may also give rise to unique populations, most likely within the amygdala complex (Bulfone et al., 1993; Cobos et al., 2001; Flames et al., 2007; Puelles et al., 2000) (Fig. 3.1).
Figure 3.1.
Schematic representation of the developing murine brain. (A) Three-dimensional view of a developing brain indicating the ventral anatomical regions that give rise to interneurons and their correspondent migratory pathways. The LGE (green) generates interneurons that migrate to the olfactory bulb and striatum. Both the MGE (red) and CGE (blue) produce cortical interneurons. Whether the septum produces cortical interneurons (yellow) is still a matter of debate. (B–C) Coronal views of the brain in (A) at three locations (i–iii) along the anterior (A)–posterior (P) axis at the embryonic ages E13 (B) and E15 (C).
3.4. The ganglionic eminences as defined by molecules
The subpallium undergoes gradual morphological changes during development. This complicates the identification of the eminences solely on the basis of anatomical features. To overcome this limitation there has been a major effort, spearheaded by the Rubenstein laboratory, to discover the molecular markers that correspond with these anatomical differences. In this section, we will present what is currently known about the molecular and genetic mechanisms that generate cell type diversity within the ventral eminences, and discuss insights regarding how specific transcription factors might regulate cell fate decisions.
Many genes are broadly expressed within the three eminences and are known to be essential for the generation of GABAergic cells, as well as more specifically for cortical interneuron development. In particular, members of the Dlx gene family, which are expressed throughout the subpallial SVZ, have been shown to be critical for interneuron specification (Anderson et al., 1997b; Petryniak et al., 2007; Pleasure et al., 2000). Mice containing compound Dlx1/Dlx2 mutations die at birth and have a severe reduction in the tangential migration of interneurons from the ventral eminences to the neocortex, resulting in a massive loss of neocortical GABAergic cells at birth (Anderson et al., 1997a). Furthermore, Dlx1/Dlx2 nulls have abnormal striatal differentiation (Anderson et al., 1997b); and virtually no olfactory bulb interneurons (Bulfone et al., 1998). Similarly, null mutations in Mash1, a proneural gene expressed throughout the subpallial SVZ (Porteus et al., 1994) display a marked loss of GABAergic cortical and olfactory bulb interneurons (Casarosa et al., 1999).
While Dlx family and Mash1 genes are broadly expressed within the subpallium, other genes show more restricted expression patterns. Loss of function of such these genes revealed that ganglionic subdivisions generate different subtypes of interneurons. It seems likely that with the discovery of novel gene expression patterns, finer graded subdivisions within the eminences will be established.
3.4.1. Molecular development of the LGE
Gene expression analyses coupled with loss of function studies have shown that the LGE, which broadly expresses Pax6 and Gsh2, can be subdivided into a dorsal and ventral domain (Flames et al., 2007; Yun et al., 2001). The dorsal domain of the LGE (dLGE) anatomically separates the pallium and the subpallium. This region expresses genes characteristic of the pallium such as Pax6, Ngn2, and Dbx1 (Flames et al., 2007; Puelles et al., 2000; Yun et al., 2001), and it gives rise to the postnatal SVZ, as well as to olfactory bulb interneurons (Stenman et al., 2003a). The ventral LGE (vLGE) expresses Gsh2, and low levels of Pax6, and primarily gives rise to the striatum (Flames et al., 2007; Puelles et al., 2000; Stenman et al., 2003a; Waclaw et al., 2006). In fact, most striatal projection neurons derive from the LGE (Anderson et al., 1997b; Deacon et al., 1994; Olsson et al., 1995, 1998; Stenman et al., 2003a; Wichterle et al., 2001).
Gsh genes are required for LGE development. In Gsh1/2 mutants, the LGE becomes dorsalized, and shows reduced expression of Mash1 and Dlx genes. By contrast, the MGE (which expresses both of these genes, albeit in the case of Gsh2, somewhat later) does not appear to be affected (Corbin et al., 2000; Toresson and Campbell, 2001; Toresson et al., 2000; Yun et al., 2001, 2003). During development, Er81 is restricted to the dLGE (also note that a more ventral region of Er81 expression is observed within the MGE) (Stenman et al., 2003a). Suggesting their genetic hierarchy, in Gsh1/2 mutants the expression of Er81 in the dLGE is lost. Similarly, Sp8 is expressed within the dLGE, in the postnatal SVZ, the rostral migratory stream (RMS), and eventually in the calretinin (CR) olfactory bulb interneurons arising from this progenitor zone. Conditional removal of this gene within the Dlx5/6 domain results in a decrease of the CR olfactory bulb interneuron subtype (Waclaw et al., 2006). Taken together, the LGE can be subdivided into a ventral part that produces projection neurons and a dorsal portion that generates interneurons. Notably, although the dLGE appears to be the primary source of interneurons for the olfactory bulb, recent analysis has indicated subsets of this population are generated within both the pallium and the septum (Merkle et al., 2007) (Fig. 3.2).
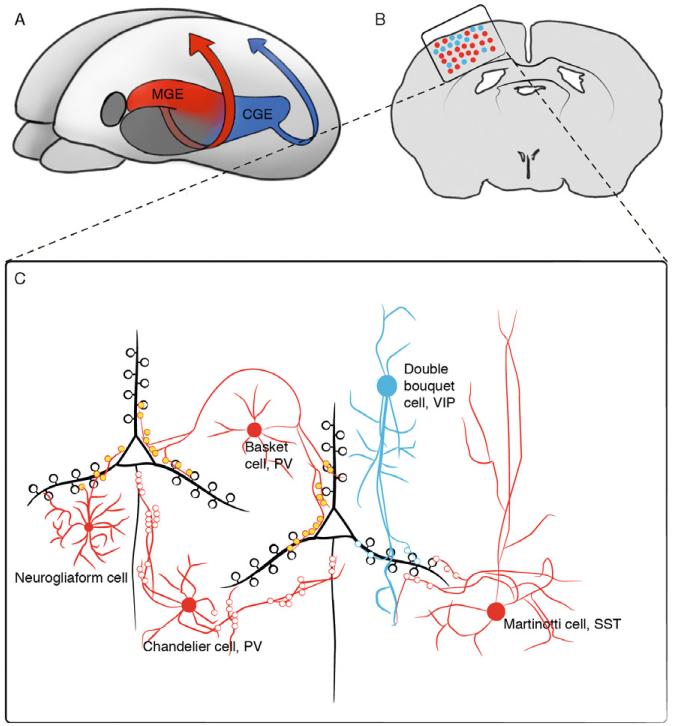
Figure 3.2.
Differential origin of cortical interneuron subtypes. (A) Three-dimensional view of an embryonic murine brain highlighting the two ventral regions that produce cortical interneurons, the MGE (red) and the CGE (blue). (B) Coronal view of an adult brain, illustrating the proportion and relative distribution of cortical interneurons derived from the MGE (red dots) and the CGE (blue dots). (C) Detailed schematic view of the boxed region in (B) illustrating the main interneuron subtypes derived from the MGE (red cells) and CGE (blue cells), and how they characteristically interact with the pyramidal cells (black cells). This figure is adapted from Kawaguchi and Kubota (2002).
3.4.2. Molecular development of the MGE
The majority of the MGE expresses Nkx2.1 (Sussel et al., 1999). By contrast, the dorsal MGE expresses Nkx6.2 and Gli1 and is partially Nkx2.1 negative (Fogarty et al., 2007; Rallu et al., 2002b; Wonders et al., 2008). Nkx2.1 null mice have an atrophied MGE and show a drastic reduction of cortical interneurons at birth (Sussel et al., 1999). Nkx2.1 is required for the correct specification of MGE-derived interneuron subtypes and for the establishment and maintenance of MGE identity (Butt et al., 2008). Conditional removal of Nkx2.1 leads to an interneuron subtype fate switch in a time-dependent manner. Similar to what was observed in Nkx2.1 nulls (Sussel et al., 1999), early conditional removal (E10.5) of Nkx2.1 leads to a decrease of cortical interneurons and a corresponding increase in striatal spiny neurons (Butt et al., 2008). It also results in the remaining cortical interneurons acquiring characteristics normally found in the CGE population. Later removal of Nkx2.1 at E12.5 resulted in a decrease of MGE-derived PV/SST cortical interneuron subtypes, and an increase in the normally CGE-derived CR/VIP population.
It has recently been shown that Nkx2.1 is upstream of the LIM-homeobox transcription factor Lhx6 and specifies interneuron fate by directly activating this gene (Du et al., 2008). MGE-derived progenitors start to express Lhx6 as soon as they leave the ventricular zone (Grigoriou et al., 1998). Lhx6 expression persists through adulthood in most parvalbumin (PV)- and somatostatin (SST)-expressing cortical interneurons (Cobos et al., 2005; Du et al., 2008; Fogarty et al., 2007; Gong et al., 2003; Lavdas et al., 1999; Liodis et al., 2007). Lhx6 loss of function analysis has shown that this gene is required for the normal specification and migration of MGE-derived GABAergic cells, and null animals exhibit a loss of PV and SST interneurons in the neocortex and hippocampus (Liodis et al., 2007). Hence, the MGE appears to be the sole source of three of the most prominent interneuron subtypes the basket, chandelier, and Martinotti cells.
3.4.3. Molecular development of the CGE is a black box
In addition to the MGE, the CGE is the other main source of cortical interneurons. Although our initial analysis at E13.5 suggested that the CGE accounted for 15% the total cortical interneuron population (Nery et al., 2002), recent results from our group suggests that its contribution may actually be far larger, and contribute as much as 40% of all cortical interneurons (G. Miyoshi et al., unpublished results). To date, no CGE specific transcription factors have been identified. However, it has been recently shown that CGE-derived interneurons specifically express the serotonin receptor 5HT3a, while Nkx6.2 and CoupTF1/2 are widely but not selectively expressed within the CGE (Sousa et al., 2009). The similarity of genes expressed in the CGE and dMGE (namely Nkx6.2 and CoupTF1/2) raises the possibility that they might be considered a common structure. Accordingly, in our Nkx2.1 loss of function analysis, we observed the expansion of both CGE and dMGE gene expression (Sousa et al., 2009). Alternatively, emerging fate-mapping studies suggest that the dMGE appears to have a hybrid identity producing both interneuron subtypes characteristic of the MGE and CGE (Fogarty et al., 2007). In summary, while the CGE is a distinct anatomical progenitor region that originates cortical interneurons, no specific molecular identity has been attributed to this structure, making its distinction a difficult task.
3.4.4. The undiscovered country
In addition, as noted above it is possible that the septum, AEP and POa may also contribute to the production of cortical interneurons (Bulfone et al., 1993; Cobos et al., 2001; Flames et al., 2007; Puelles et al., 2000). These structures express genes characteristic of the ganglionic eminences, such as Nkx2.1, Dlx1/2, and Vax1 (Puelles et al., 2000; Taglialatela et al., 2004). Vax1 null mice have a reduced MGE and virtually no septum, and show a loss of about 40% of cortical interneurons at birth (Taglialatela et al., 2004). Selective fate mapping of the septum will be necessary to definitively address this issue. It seems likely that further analysis of these structures will produce a few more surprises, such as the recent realization that the AEP generates interneurons destined to specific amygdala nuclei (Hirata et al., 2009).
3.5. Fate mapping of the ganglionic eminences
Gene expression and loss of function analyses have given us strong indications about the differential production of cortical interneuron subtypes within specific progenitor domains. However, the most direct way of addressing this issue is by selective fate mapping of these embryonic regions. In this section, we will summarize the results of different fate-mapping approaches that have been undertaken over the last decade. As will be evident, these findings both confirm and extend the fate conclusions based on expression and loss of function analyses.
Transplantation of labeled progenitors into the different eminences has shown that the MGE and LGE produce both GABAergic projection neurons (such as the spiny striatal neurons) and interneurons (cortical interneurons from the MGE and olfactory bulb interneurons from the LGE) (Nery et al., 2002; Wichterle et al., 2001; Xu et al., 2004); while the CGE produces only interneurons (Nery et al., 2002). Multiple studies have demonstrated that interneurons born in the MGE and CGE migrate tangentially to enter the cortex, while interneurons born in the LGE migrate via the RMS to reach the olfactory bulb (Batista-Brito et al., 2008a; Corbin et al., 2001; Marin and Rubenstein, 2003). Based on tracing experiments and the migration patterns of cells from the eminences into the cortex, it was initially thought that the LGE was a primary source of cortical interneurons (de Carlos et al., 1996; Tamamaki et al., 1997). While the possibility that the LGE produces a small subpopulation of cortical interneurons has not been entirely ruled out, this conclusion can be largely explained by the fact that MGE- and CGE-derived interneurons migrate through the LGE while en route to the cortex (Anderson et al., 2001; Wichterle et al., 1999, 2001).
Fate mapping of the MGE has shown that a number of distinct interneuronal populations are generated in this region and subsequently migrate tangentially to populate distant telencephalic regions, such as the striatum, cortex, and hippocampus (Anderson et al., 2001; Lavdas et al., 1999; Wichterle et al., 1999, 2001). Transplants of labeled MGE progenitors in vivo (Butt et al., 2005; Wichterle et al., 2001) and in vitro experiments (Xu et al., 2004) have confirmed that this region produces PV- and SST-expressing interneuron subtypes that account for the majority of interneurons in the cortex (see figure 3.2). In terms of their intrinsic electrophysiological properties, the most abundant interneuron subtype produced in the MGE is the fast-spiking cell (FS), while the second largest population is the burst-spiking Martinotti interneurons (BSNP) (Butt et al., 2005). In addition, regular-spiking nonpyramidal (RSNP although this particular nomenclature is likely to be soon supplanted by more refined physiological criteria, Miyoshi et al. unpublished) that are largely NPY-positive interneurons are also derived from this region, but some arise from the CGE as well.
Recently, Cre/loxP-mediated genetic fate-mapping experiments have confirmed and extended previous transplantation results by providing selective labeling of MGE and CGE lineages. By using different Cre drivers of genes expressed in the MGE such as Nkx2.1, Nkx6.2, and Lhx6 (Fogarty et al., 2007; Miyoshi et al., 2007; Xu et al., 2008) it has been possible to address some of the regional aspects of MGE-derived interneuron diversity. In concordance with the transplantation studies from the MGE, genetic fate mapping of this structure by using the allele Nkx2.1Cre shows that this region produces primarily PV, SST, and CB, and to a lesser extent NPY and CR neurons (Fogarty et al., 2007; Xu et al., 2008). Genetic fate mapping of Nkx6.2 progenitors (expressed in the sulcus) using an Nkx6.2Cre allele shows that this region, similar to the Nkx2.1 domain, produces CB, SST, PV, NPY, and CR neurons (Fogarty et al., 2007; Stenman et al., 2003b). However, fate mapping of the Nkx6.2 region yields a proportionally greater contribution to the NPY and CR subtypes than the Nkx2.1 domain fate map, suggesting that the latter two subtypes preferentially originate in the most dorsal part of the MGE (Fogarty et al., 2007; Xu et al., 2008). Fate mapping of Lhx6-expressing cells reveals a total overlap of the Lhx6 lineage with the cortical PV, SST, and CB interneurons (Fogarty et al., 2007; Xu et al., 2008), and the contribution of Lhx6 for NPY and CR subtypes is similar to that observed for Nkx6.2 (Fogarty et al., 2007; Xu et al., 2008).
Transplantation experiments have found that the E13.5 CGE contributes interneurons to different regions of both the dorsal and ventral telencephalon, such as the cortex, specific regions of the limbic system (amygdala, hippocampus, and NAc) and the striatum (Nery et al., 2002). However, in contrast to the MGE and LGE, the CGE transplants produced few GABAergic projection neurons (Nery et al., 2002). This is likely due to the fact that this eminence only develops relatively late in embryogenesis, when the switch from the production of ventral GABAergic projection neurons to cortical interneurons has already occurred. Homotopic transplants of E13.5 and E15.5 CGE progenitors have shown that the majority of the cortical cells from this region are either CR-, NPY-, or VIP-expressing interneurons (see figure 3.2). These cells have mainly bipolar or multipolar morphologies, and are RSNPs and NR-RSNPs in terms of their electrophysiological profile (Butt et al., 2005). Due to the fact that we still do not know of any gene that specifically labels the CGE, genetic fate mapping of this eminence has been hampered. Recently, the fortuitous expression of a BAC Mash1CreER transgenic line selectively in the CGE and not MGE, has allowed for a detailed genetic fate-mapping analysis of this region. This study in progress has confirmed the results from previous CGE transplant experiments, and has expanded upon them by indicating that the contribution of CGE lineages to the cortical interneuron population is higher than previously estimated (about 40% of the total). Of the cells fate mapped from the CGE, a large percentage are late-spiking interneurons expressing Reelin or bipolar VIP/calretinin RSNP populations (G. Miyoshi et al., unpublished results).
3.6. A question of semantics? Current definition of the eminences
As previously discussed, historically, the division of the ganglionic eminences has been based on anatomical landmarks, which are subjective, change over time, and may or may not reflect the local molecular identity. The lack of genetic markers that clearly differentiate the lineages that arise from the distinct progenitor pools within the eminences makes the division and definition of the ventral progenitor territories complex. Of these the best understood is the MGE, which gives rise to both SST and PV interneuron populations. While proportionally, the PV interneurons appear to be derived from the ventral portion of the MGE, and SST interneurons arise from the dorsal MGE (Flames et al., 2007), whether these are derived from a common progenitor pool or not is unclear. The distinction between the LGE and CGE is more ambiguous. This has led some scientists to integrate the CGE within the LGE domain (Flames et al., 2007). However, the CGE and LGE clearly give rise to distinct neuronal populations, despite the fact that certain neuronal subtypes are produced in both regions (Butt et al., 2005; Xu et al., 2004).
3.7. MGE- and CGE-derived cortical interneurons are generated with different temporal profiles
It has long been recognized that, as a whole, cortical interneurons show the same inside–out pattern of generation that is observed in pyramidal cells (Anderson et al., 2002; Cavanagh and Parnavelas, 1989). Different subtypes of MGE-derived interneurons are produced at particular developmental times. Early-born MGE-derived interneurons end up in deep layers, while late-born interneurons occupy superficial layers of the mature cortex, and each cohort possesses unique electrophysiological properties characteristic of their birthdate (Miyoshi et al., 2007). Moreover, heterochronic transplants of progenitors demonstrated that the fate of transplanted cells is determined by the age of the donor and not the age of the recipient (Butt et al., 2005). Both pieces of evidence suggest that MGE progenitors have different potentials at different times (see figure 3.3).
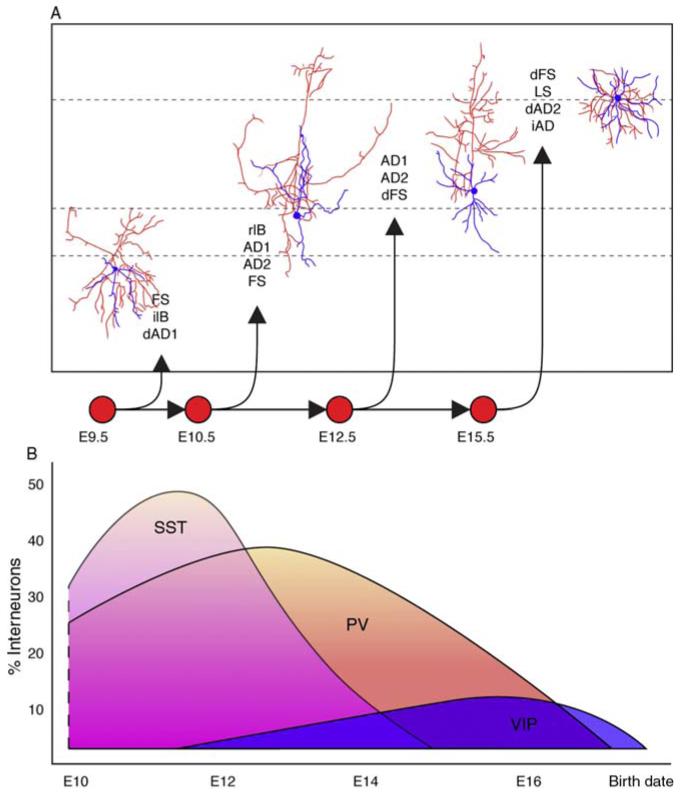
Figure 3.3.
Temporal origin of cortical interneurons. (A) Examples of the morphological and electrophysiological diversity of cortical interneurons derived from the MGE at different times. Axons and dendrites are indicated in red and blue, respectively. This figure is adapted from Miyoshi et al. (2007). (B) Diagram of temporal origin of three subtypes of MGE interneurons, the somatostatin (SST—pink), the parvalbumin (PV—orange), and a subtype of CGE-derived interneuron (VIP—blue).
While most of MGE-derived interneurons are born around E13.5, the generation of CGE-derived interneurons peaks at later ages (around E15.5) (Butt et al., 2005; Nery et al., 2002). Preliminary transplant results suggested that the interneurons generated in the CGE also differ based on birthdate (Butt et al., 2005). However, more accurate genetic fate-mapping analysis have recently shown that, contrary to the MGE, the subtypes of interneurons generated within the CGE does not significantly change over time. Furthermore, the birthdate of CGE neurons does not predict their cortical layer destination, unlike their MGE counterparts (G. Miyoshi et al., unpublished results). In fact, at least one subpopulations of CGE-derived cells, the CR interneurons, have an outside–in gradient of generation (Rymar and Sadikot, 2007), while VIP interneuron layer specificity does not appear to be correlated with their time of birth (Cavanagh and Parnavelas, 1989).
3.8. Where and when is interneuron identity specified?
As discussed earlier, there are now multiple lines of evidence showing that an interneuron’s subtype identity is related to both its temporal and spatial embryonic origin. This correlation likely reflects the logic by which the genetic program functioning in progenitors and migrating interneurons is initiated. But, how is interneuron diversity per se established? This question has been a central point of debate in the field over the last decade. Despite a multitude of attempts to reveal the mechanisms by which an interneuron acquires its mature properties, we still do not have a definitive answer to this question. However, based on the insights discussed above, it is perhaps not premature to begin to hypothesize on how this process might be regulated.
One school of thought (championed by the Rubenstein laboratory and colleagues) is that, akin to what happens in the spinal cord, the ganglionic eminences contain multiple distinct pools of progenitors, each of which gives rise to specific interneuron subtypes. In the spinal cord, analogous pools are defined by their expression of a specific combination of transcription factors established by a Shh morphogen gradient (Jessell, 2000). Even though the subpallial eminences are responsive to Shh gradients, and the MGE and CGE broadly give rise to different groups of interneuron subtypes, the identification of progenitor pools that are restricted to producing particular interneuron subtypes has yet to be definitively demonstrated. Using a combinatorial approach, based on the examination of the patterns and levels of expression of different transcription factors, Flames et al. (2007) divided both the MGE and LGE eminences into nine subdomains. However, this effort to map the eminences into microdomains is too coarse to account for the diversity of interneuron subtypes that arise from the MGE (Miyoshi et al., 2007). To test the specificity of these microdomains more directly, a micro-transplantation fate-mapping study of two of the areas within the MGE was performed. Even though there was a bias in the interneuron subtypes arising from each of the two areas transplanted, each MGE subregion still produced a mix of interneuron subtypes. Therefore, although the hypothesis of distinct MGE progenitor subdomains specified through the actions of unique transcriptional combinations is very attractive, it has yet to be established.
An alternative hypothesis is that, similar to what happens in the retina (Cepko et al., 1996), and the pyramidal cells of the cerebral cortex (Desai and McConnell, 2000; Rakic, 1974), interneuron diversity depends on changes in progenitor competence over time. In this model, progenitors within the same region go through systematic alterations in their potential as development progresses. This hypothesis is in concordance with the different electrophysiological characteristics of the MGE-derived interneurons produced at distinct developmental time points (Miyoshi et al., 2007). However, this model cannot completely account for the generation of interneuron diversity, both because the correlation between cell type and birthdate is only approximate, and because the MGE may account for the generation of as little as 60% of all cortical interneurons (Fig. 3.3).
3.9. The contribution of space and time: Lessons from the Nkx2.1 progenitors
The question of mechanism aside, the above studies provide clear evidence in favor of the idea that interneuron subtype identity is assigned, at least partially, at the progenitor level. How might a blend of these hypotheses account for the coordinated regulation of interneuron subtype identity?
In favor of transcription factors regulating spatial identity, Nkx2.1 appears to positively regulate MGE fates, while repressing LGE/CGE fates (Butt et al., 2008; Sussel et al., 1999). This suggests that Nkx2.1 might be acting as a molecular toggle switch similar to how homologous genes function in the spinal cord, where type 1 genes (repressed by Shh) and type 2 genes (induced by Shh) compete to determine cell fate. Following this idea, competitive crossrepression in the telencephalon may lead to the coalescence of distinct progenitor domains that determine specific neuronal subtypes (Jessell, 2000).
Nkx2.1 per se is unlikely to regulate changes in the temporal competence of progenitors, as its expression is relatively constant through development. Hence if the progenitors within the MGE change their potential over time, Nkx2.1 expression while being necessary cannot be sufficient. The genetic cascades regulating the temporal competence of Drosophila nerve cord progenitor cells have been shown to be regulated by the sequential expression of Hunchback, Kruppel, Pdm, and Castor, which collectively result in the ordered production of different neuronal subtypes from a common lineage (Isshiki et al., 2001). Intriguingly, homologs of many of these genes are expressed within the telencephalon but not with patterns that suggest that this genetic network is precisely mirrored in mammalian brain (Jacob et al., 2008). However, hints of analogous temporally regulated hierarchies within the telencephalon have emerged, particularly in the pallium. For instance, the production of the earliest pallial populations, the Cajal Retzius cells, appear to be negatively regulated by FoxG1 (Hanashima et al., 2004; Shen et al., 2006). Moreover, an intriguing interplay between Fezf 2, Tbr2, Sox5, and Satb2 appears to control the sequential production of deep and superficial projection neurons in the cortex (Alcamo et al., 2008; Britanova et al., 2008; Chen et al., 2008; Hevner, 2006; Lai et al., 2008; Molyneaux et al., 2005). As yet, complementary networks in progenitor cells that give rise to cortical interneurons have not been identified, but likely exist. As they emerge, the logic by which spatial identity genes such as Nkx2.1 are coregulated with temporal identity genes should be forthcoming.
One possibility is that spatial distinctions between the MGE and CGE are in fact better thought of as sequential temporal phases that sweep over these eminences as development progresses. In this view, the MGE and CGE change their competence as a result of the changing expression of genes such as Nkx2.1. Viewed in this manner, the MGE extinguishes expression of Nkx2.1 during later development, allowing cortical interneuron subtypes that we consider to be CGE-derived to be generated. Whether such an extreme view obviates the idea that cortical interneuron subtypes are at least partially depending on spatially discrete progenitor zones seems unlikely. It does, however, emphasize the point that the differences between spatial and temporal mechanisms may be somewhat semantic. Finally, in considering the intrinsic mechanisms by which cortical interneuron subtype is determined, there is the question of lineage. Specifically we must determine whether there are stereotypic clonal relationships between particular cortical interneuron subtypes that arise from a common progenitor pool. To address this question, it will be necessary to do a lineage analysis similar to what has been done previously in the retina and cortex (Cepko et al., 1996).
3.10. The influence of postmitotic environment on cortical interneuron diversity
So far, we only have addressed the mechanisms of generating interneuron diversity that result from intrinsic signaling in the progenitor pool that gives rise to these neurons. Certainly, existing data support the idea that the specification of interneuron subtypes is dependent on events occurring prior to these lineages becoming postmitotic. For example, it has been shown that heterotopic transplants of cortical interneurons at E13.5 does not alter their subtype identity, in that MGE cells transplanted in the CGE still adopt an MGE fate and CGE cells transplanted into the MGE still adopt an CGE fate (Butt et al., 2005). However, even if identity is first specified early, it is only determined postnatally, and therefore several checkpoints must exist. Alternatively, the ability of transplanted cells to maintain their normal fate may be simply a product of their ability to migrate to their appropriate destination from an ectopic location. In this model, their ability to follow the correct postmitotic cues through directed migration is the key to attaining their proper subtype identity. At least one study indicates that when their normal migratory route is disrupted, so is their laminar position. Heterochronic injections of E12.5 and E15.5 MGE cells in the telencephalic ventricles found that these transplanted cells adopted a laminar fate dependent on the age of the host rather than on their intrinsic birthdate (Valcanis and Tan, 2003). This study, however, did not explore the intrinsic properties of these transplanted interneurons and therefore it is not known whether challenging these cells in this manner altered their intrinsic subtype properties as well as their laminar distribution.
The question of the relative contribution of cell intrinsic versus nonintrinsic fate determinants has been more thoroughly explored in cortical pyramidal cells. Two opposing theories have been posited to account for pyramidal cell specification and arealization. One suggests that the mature characteristics of cortical subtypes are strongly influenced by their neocortical environment (McConnell, 1988; McConnell and Kaznowski, 1991; O’Leary and Stanfield, 1989). The alternative point of view was proposed in the “protomap hypothesis” (Rakic, 1988), in which the identity of the pyramidal cells is determined at the progenitor level. This concept implies that combinatorial gene expression during neurogenesis (Jessell, 2000) initiates an intrinsic program that ultimately results in the emergence of specific neuronal subtypes. Within the neocortex, evidence for the latter point of view came from landmark studies where the fate of pyramidal cells was challenged through heterotopic transplantation (McConnell, 1988; McConnell and Kaznowski, 1991) and from genetic studies where areal identity is maintained in the absence of thalamic afferents (Huffman et al., 2004; Nakagawa et al., 1999). Recently, genetic studies have uncovered some of the molecular effectors that mediate these events such as specific transcription factors that control pyramidal cell fate and areal identity (Chen et al., 2005a,b; Cholfin and Rubenstein, 2007; Hamasaki et al., 2004; Molyneaux et al., 2005). It is our view that even though many of the components of cell identity, such as general intrinsic physiological properties and layer position, are likely determined at the progenitor level, other components such as choice of synaptic partners are likely influenced by local environmental cues. Therefore, these two mechanisms may contribute to different aspects of cell specification. While these two models have been classically used to describe pyramidal cells, we think that cortical interneurons should be integrated within the logic of this existing framework, and are likely subject to similar rules.
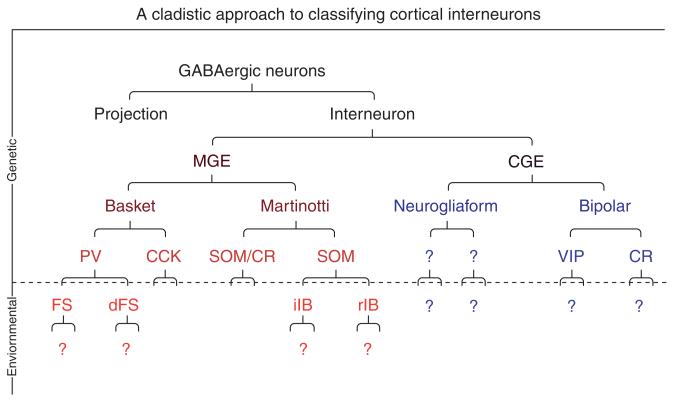
The extent to which the specific final properties of interneurons are determined at a progenitor level or acquired them later is still unclear. Taking into account the different models posited and the studies to date, we envision interneuron fate specification as a cladistic process (an idea raised in discussion between Gord Fishell and Kenneth Harris). By this we mean that the potential of progenitors narrows over time through a set of sequential restrictions that are initiated during the progenitor stage but persist postmitotically. While early decisions, such as the decision to be a PV or a SST interneuron are likely established at the progenitor level, later restrictions occur over time as they migrate, integrate into the cortex, and establish the first contacts with other cells (see figure 3.4). We think that this progression is mediated by a combination of genes expressed by the cells throughout their life. Therefore, genetic profiling of these cells at critical developmental stages will bring about new insights into interneuron development and subtype specification. Recently, we showed that migrating embryonic interneuron precursors express a variety of transcription factors and mature interneuron subtype markers, supporting the idea that significant aspects of their identity are already acquired during embryonic stages (Batista-Brito et al., 2008) (Fig. 3.4).
Figure 3.4.
Cladistic hypothesis of cortical interneuron specification. We believe the fate of inhibitory neuron subtypes are sequentially influenced by both intrinsic genetic cues within the progenitor populations and environmental signals experienced by these populations postmitotically. In this model, we suggest that while the cardinal subdivisions are dependent on an intrinsic (genetic) program, the secondary subdivisions are determined later by environmental signals.
3.11. Modes of migration of cortical interneurons—The high road and the low road
Cortical interneurons originating in the subpallium migrate tangentially to enter the cortex (Corbin et al., 2001; Marin and Rubenstein, 2001). Tangentially migrating interneurons enter the cortical plate through two different migratory routes: a superficial path within the marginal zone (MZ); and a deep route positioned at the subplate/SVZ interface (Lavdas et al., 1999). The molecular cues guiding interneurons to each of these routes are unknown, as is whether there exist preferred routes for different interneuron subtypes.
Tangential migration is mediated by a combination of molecular cues that function to both selectively repel and attract cortical interneuron populations (Marin and Rubenstein, 2003). While this process is directly influenced by the guidance molecules themselves (e.g., Semaphorins, Slits, neuregulin), the responsiveness of migrating interneurons to these cues is also regulated by subtype specific transcription factors. Recently, a wonderful example of this comes from work by the Marin group, who demonstrated that the semaphorin receptor Nrp2 is negatively regulated by Nkx2.1 (Nobrega-Pereira et al., 2008). As a result the Sema3a (the repellent ligand for the Nrp2 receptor) expression in the striatum prevents cortical interneurons from invading this structure. By contrast, the persistent Nkx2.1 expression in interneurons destined for the striatum prevents Nrp2 expression in this population and as a result permits them to invade this region. Transcription factors have also been shown to control the intracellular machinery required for migration. Dlx1/2 compound double mutants have impaired cortical interneuron migration (Anderson et al., 1997b) at least in part through their control of the p21-activated serine/threonine kinase PAK3, a downstream effector of the Rho family of GTPases (Cobos et al., 2007). Finally, ambient GABA also influences interneuron migration (Cuzon et al., 2006; Lopez-Bendito et al., 2003; Manent and Represa, 2007; Manent et al., 2005; Poluch and Juliano, 2007).
After a period of tangential migration, interneurons shift to a radial mode of migration when they enter the cortical plate (Ang et al., 2003; Polleux et al., 2002). Little is known about the mechanisms behind this switch in their mode of migration; however, the timing of this shift seems to be regulated by chemokines. Specifically, Cxcl12, a chemokine expressed by both the meninges and pyramidal cells, and Cxcr4, Cxcl12 receptors expressed in interneurons (Stumm et al., 2003; Tiveron et al., 2006) appear to jointly mediate this process. The impairment of this signaling pathway due to the either the absence of the chemokine (Cxcl12) or its receptor (Cxcr4) leads to the premature invasion of the cortical plate by migrating interneurons, and subsequent abnormal interneuron lamination (Lopez-Bendito et al., 2008; Tiveron et al., 2006). The exact mechanisms directing interneurons to stop migrating when they reach their final position are still poorly understood.
4. The Integration of Interneurons into Cortical Networks
To this point, we have examined the diversity of cortical interneurons based on their time of origin, or where within the ventral telencephalon they arise. However, at birth, the emergence of specific cortical interneuron subclasses is far from complete. In this section, we will ask how is complexity assembled, by examining emergent interneuron properties and network integration during postnatal development.
A complex set of developmental steps are involved in the integration of interneurons into the cortical network. These require the interplay of intrinsic genetic programs and their modulation by cell–cell and matrix–cell interactions. Some of the more notable of these are the development of reciprocal connections between interneurons and pyramidal cells, the transition of GABAergic signaling from excitation to inhibition and the maturation of synaptic connectivity through consolidation and refinement. Each of these contributes significantly to the establishment of a mature functional network during the first few postnatally weeks.
4.1. GABA is excitatory during development
In adult mammals, GABA is the main inhibitory neurotransmitter. GABA acts through ionotropic receptor channels, which are permeable to anions (namely Cl− and bicarbonate). In mature neurons, activation of GABAA-R leads to chloride influx, due to low intracellular chloride levels. GABA receptor activation therefore generally results in neurons becoming more hyperpolarized. For a long time, it has been known that GABA in some contexts is excitatory. The excitatory actions of GABA were documented on cultured neurons of embryonic chick spinal cord three decades ago (Obata et al., 1978). In 1984, it was shown that GABA application leads to depolarization of neonatal hippocampal pyramidal neurons (Mueller et al., 1984); however, the mechanism by which this process occurred was only discovered 5 years later. In a landmark study, Ben-Ari and colleagues showed that developing networks are spontaneously active due to the excitatory actions of GABAergic transmission in early development. This work demonstrated that at the end of the first postnatal week, GABA signaling onto hippocampal CA1 neurons resulted in hyperpolarization due to a developmental decrease of intracellular concentration of Cl− over time (leading to a switch in the Cl− reversal potential) (Ben-Ari et al., 1989). This discovery was further confirmed in multiple species demonstrating that this shift represents an evolutionarily conserved mechanism. It was also observed in neurons in other regions of the brain (Ben-Ari et al., 2007) including the cortex (Gulledge and Stuart, 2003; LoTurco et al., 1995; Luhmann and Prince, 1991; Martina et al., 2001; Owens et al., 1996), as well as in peripheral structures.
Intracellular chloride concentration is actively regulated by two transporters NKCC1 and KCC2. NKCC1 imports Cl− into the cell and is highly expressed in immature neurons. Conversely, KCC2, a Cl− exporter, expression increases after the first postnatal week (Ben-Ari, 2002; Dzhala et al., 2005). The decreased concentration of intracellular Cl− as neurons mature has been attributed to the postnatal onset of expression of the Cl− exporter KCC2. That the evolutionary alteration in the expression of a single transporter protein could have such a marked effect on GABAergic signaling is remarkable.
4.2. Early activity patterns
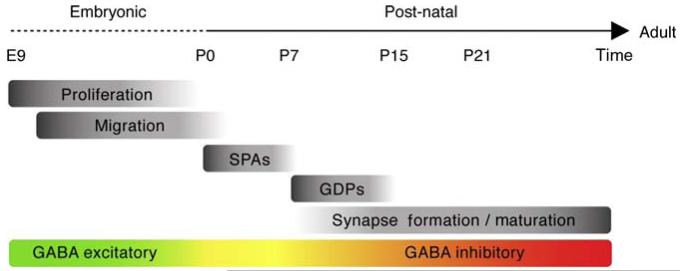
The immature cerebral cortex self-organizes into local neuronal clusters long before it is stimulated by sensory input (Katz and Crowley, 2002). Spontaneous neuronal activity is a characteristic of developing networks and is essential for their maturation (Cang et al., 2005; Kandler and Gillespie, 2005; Katz and Shatz, 1996; Khazipov et al., 2004; Nicol et al., 2007). In the neocortex, synchronous spontaneous calcium activity starts early during development, and in fact is already initiated in progenitors (Owens and Kriegstein, 1998). In postnatal animals, cortical neurons still display coherent spontaneously activity (Garaschuk et al., 2000; Khazipov et al., 2004; Yuste et al., 1992). In addition, they begin to communicate through both transmitter-gated receptors (Flint et al., 1999; Peinado, 2000), as well as electrical and chemical synapses. In the newborn mouse, the immature cortical network initiates calcium activity within cortical columns, which is at first synchronized by gap junctions (Dupont et al., 2006; Kandler and Katz, 1998), and then mediated by a synaptic network dependent on NMDA (N-methyl-d-aspartate) receptors (Dupont et al., 2006). In addition, in both the hippocampus and neocortex, synaptically mediated activity is preceded by intrinsic nonsynaptic calcium plateaus associated with membrane potential oscillations (Crepel et al., 2007). These local and synchronous activity patterns named synchronous plateau assemblies (SPAs) are also generated by neurons coupled through gap junctions (Crepel et al., 2007). SPAs are initiated around birth and are enhanced by oxytocin. SPA events begin to decline approximately coincident with the emergence of synaptic-driven network activity (Allène et al., 2008) (Fig. 3.5).
Figure 3.5.
Summary of the essential events and processes that influence interneuron development. Embryonic ages are dominated by proliferation and cell migration. During this period GABA is excitatory. The first postnatal weeks are marked by early activity patterns, including synchronous plateau assemblies (SPAs) that are preceded by giant depolarizing potentials (GDPs). During this later period GABA switches from being excitatory to be inhibitory.
So far, two different types of synapse-driven network patterns have been described, namely giant depolarizing potentials (GDPs) and cortical early network oscillations (cENOs). GDPs were the first developmental synaptic-driven cortical network oscillation discovered (Ben-Ari et al., 1989). They emerge a few days after birth and are the first synapse-driven network pattern in the developing hippocampus (Ben-Ari et al., 1989; Crepel et al., 2007; Garaschuk et al., 2000). GDPs are mediated by GABAergic synapses and ceases when GABA switches from excitatory to inhibitory (Ben-Ari et al., 1989; Garaschuk et al., 1998; Tyzio et al., 2007). Until recently, the only counterparts of GDPs described for the cortex were cENOs. These are spontaneous Ca2+ waves that are observed within the cortex immediately after birth (Garaschuk et al., 2000). Contrary to GDPs, cENOs are mediated by glutamatergic synapses and required activation of AMPA and NMDA receptors, but not GABAA receptors (Corlew et al., 2004; Garaschuk et al., 2000; McCabe et al., 2006). Based on the presence of cENOs, it has been suggested that glutamatergic synapses, rather than GABAergic ones, have a privileged role on setting up early networks in the cortex (McCabe et al., 2007). However, similarly to the hippocampus, in the developing cortex GABA is excitatory (Ben-Ari et al., 2007) and recently it has been shown that in fact the neocortex also produces GABA-driven GDPs (Allène et al., 2008). This form of cortical network activity, as in the hippocampus, occurs slightly later than cENOs (P6–P8 vs P0–P3 in rat) (Allène et al., 2008) (see figure 3.5). Thus, GABAergic transmission and hence the interneurons contribute to spontaneous network oscillations in the developing cortex. Interneurons are therefore very likely to play an important role in postnatal network construction through theses synapse-driven coordinated activity patterns. In fact, it has been recently shown that spontaneous activity regulates GABA synthesis, affecting inhibitory innervation patterns (Chattopadhyaya et al., 2007).
Major strides have been made over the last two decades. Even so the neuronal network and the cellular mechanisms underlying the process of cortical self-organization are still far from being comprehensively understood. Identification of other forms of nascent network activity and the interactions between these forms will be crucial for understanding how the establishment of cortical networks is achieved.
4.3. Interneuron development and neurological disorders
As we discussed previously, GABAergic interneurons have a powerful role in establishing networks and controlling their function. Abnormal development of GABAergic circuits and even a subtle imbalance in the ratio of excitatory versus inhibitory levels within the cerebral cortex may underlie many neurological and neurodevelopmental disorders such as epilepsy, autism spectrum disorders, schizophrenia, and Tourette’s syndrome (Baraban and Tallent, 2004; Belmonte et al., 2004; Cossart et al., 2005; Dani et al., 2005; Kalanithi et al., 2005; Levitt et al., 2004; Lewis et al., 2005; Woo and Lu, 2006). With the recent implementation of high-throughput sequence techniques, there is currently a large effort to map genes associated with neurological disorders. By crosscorrelating these studies with the genes expressed in developing cortical interneurons, we recently came to the realization that many genes linked to autism, mental retardation, epilepsy, and schizophrenia are selectively expressed in cortical interneurons precursors (Batista-Brito et al., 2008b). Consistent with this idea, a common observation associated with autistic spectrum disorders is the manifestation of “sharp spike” EEGs, suggestive of noisy and unstable cortical networks (Lewine et al., 1999; Wheless and Kim, 2002).
It is our belief that the misspecification and/or dysfunction of specific subtypes of interneurons account for a significant portion of the etiology of many neurological disorders. As a consequence, we suggest this results in the improper consolidation of activity-based brain patterning during early postnatal development. Hence, we hypothesize that abnormal development of cortical interneuron subtypes during late embryogenesis due to environment perturbations coupled with genetic abnormalities might represent a primary cause for many neurodevelopment disorders. Consistent with this idea, the postconception days 20–24 in humans (equivalent to E11.5–E14.5 in mice) appears to be a susceptibility period for environmental perturbations, such as thalidomide or valproic acid. Exposure to these factors significantly increases the risk of cerebral dysfunction in both humans and rodents (Arndt et al., 2005; Miller et al., 2005). Notably, this is the precise developmental stage during which cortical interneuron diversity is being established (Butt et al., 2005; Wonders and Anderson, 2006). Moreover, perturbation of certain cortical interneuronal subtypes (double bouquet cells and parvalbumin-positive cells) have been reported in patients afflicted with autism (Casanova et al., 2002). Additionally, GABARB3 and Arx, genes central to cortical interneuronal function, are within susceptibility loci associated with autism spectrum disorders (Chaste et al., 2007; Vincent et al., 2006). Similarly, Npas1 and Npas3 are both selectively expressed in migrating and mature cortical interneurons and have been implicated in schizophrenia (Batista-Brito et al., 2008b; Pieper et al., 2005). Another example of how developmentally expressed transcription factors may provide clues for understanding neurodevelopmental disorders comes from genetic studies of Rett’s syndrome. This syndrome leads to mental retardation and is caused by X-linked mutations in MeCP2 (Moretti and Zoghbi, 2006). It has been shown that Dlx5, a gene essential for interneuron development (Stuhmer et al., 2002), is a direct target of MeCP2 (Horike et al., 2005). Finally, Nrg1, expressed in migrating cortical interneurons (Flames et al., 2004), is located in a susceptibility locus for schizophrenia (Corfas et al., 2004).
In summary, due to the complexity and heterogeneity of GABAergic circuits, the mechanisms by which they develop and function have been difficult to study. However, with the burgeoning of sophisticated new approaches, these issues are rapidly becoming tractable. Techniques, such as the use of developmental genetics for specific interneuron subtype labeling and high-resolution imaging, are contributing toward our understanding of the principles underlying the establishment of GABAergic networks. Although the association between neurological disorders and interneuron function is already substantial, our understanding of this connection is in its nascence. Analyzing the functional and behavioral consequences of targeted modifications in these genes holds the promise of providing mouse models for the study of various neurodevelopmental disorders. It seems likely that the intrinsic programs that drive cortical interneuron development are causally linked to the integration and maintenance of these cells in cortical circuitry. This in turn will no doubt help elucidate the functions of these genes in both normal and abnormal brain function.
ACKNOWLEDGMENTS
We would like to thank Rosa Cossart, Bernardo Rudy, Robert Machold, Elsa Rossignol, Jens Herling-Leffler, Theofanis Karayannis, and Edmund Au for critically revising this manuscript. We would like to thank Karl-Johan Hjerling for his great graphic support on creating the figures of this manuscript.
REFERENCES
- Aboitiz F, Montiel J. Co-option of signaling mechanisms from neural induction to telencephalic patterning. Rev. Neurosci. 2007;18:311–342. doi: 10.1515/revneuro.2007.18.3-4.311. [DOI] [PubMed] [Google Scholar]
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Allène C, Cattani A, Ackman JB, Bonifazi P, Aniksztejn L, Ben-Ari Y, Cossart R. Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J. Neurosci. 2008;28:12851–12863. doi: 10.1523/JNEUROSCI.3733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: Dependence on Dlx genes [see comment] Science. 1997a;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Kaznowski CE, Horn C, Rubenstein JL, McConnell SK. Distinct origins of neocortical projection neurons and interneurons in vivo. Cereb. Corte. 2002;12:702–709. doi: 10.1093/cercor/12.7.702. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997b;19:27–37. doi: 10.1016/s0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- Ang ES, Jr., Haydar TF, Gluncic V, Rakic P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J. Neurosci. 2003;23:5805–5815. doi: 10.1523/JNEUROSCI.23-13-05805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt TL, Stodgell CJ, Rodier PM. The teratology of autism. Int. J. Dev. Neurosci. 2005;23:189–199. doi: 10.1016/j.ijdevneu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Tallent MK. Interneuron Diversity series: Interneuronal neuropeptides-endogenous regulators of neuronal excitability. Trends Neurosci. 2004;27:135–142. doi: 10.1016/j.tins.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Batista-Brito R, Close J, Machold R, Fishell G. The distinct temporal origins of olfactory bulb interneuron subtypes. J. Neurosci. 2008a;28:3966–3975. doi: 10.1523/JNEUROSCI.5625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Machold R, Klein C, Fishell G. Gene expression in cortical interneuron precursors is prescient of their mature function. Cereb. Corte. 2008b;18:2306–2317. doi: 10.1093/cercor/bhm258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, Cook EH, Jr., Anderson GM, Rubenstein JL, Greenough WT, Beckel-Mitchener A, Courchesne E, Boulanger LM, Powell SB, Levitt PR, Perry EK, Jiang YH, et al. Autism as a disorder of neural information processing: Directions for research and targets for therapy. Mol. Psychiatry. 2004;9:646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: The nature of the nurture. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Phys. Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, Sestan N, Molnár Z, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Puelles L, Porteus MH, Frohman MA, Martin GR, Rubenstein JL. Spatially restricted expression of Dlx-1, Dlx-2 (Tes-1), Gbx-2, and Wnt-3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J. Neurosci. 1993;13:3155–3172. doi: 10.1523/JNEUROSCI.13-07-03155.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfone A, Wang F, Hevner R, Anderson S, Cutforth T, Chen S, Meneses J, Pedersen R, Axel R, Rubenstein JL. An olfactory sensory map develops in the absence of normal projection neurons or GABAergic interneurons. Neuron. 1998;21:1273–1282. doi: 10.1016/s0896-6273(00)80647-9. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype [see comment] Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2–1 in the temporal specification of cortical interneuron subtypes [see comment] Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Renteria RC, Kaneko M, Liu X, Copenhagen DR, Stryker MP. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron. 2005;48:797–809. doi: 10.1016/j.neuron.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Casarosa S, Fode C, Guillemot F. Mash1 regulates neurogenesis in the ventral telencephalon. Development. 1999;126:525–534. doi: 10.1242/dev.126.3.525. [DOI] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J. Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh ME, Parnavelas JG. Development of vasoactive-intestinal-polypeptide-immunoreactive neurons in the rat occipital cortex: A combined immunohistochemical-autoradiographic study. J. Comp. Neurol. 1989;284:637–645. doi: 10.1002/cne.902840410. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell. fate determination in the vertebrate retina. Proc. Natl. Acad. Sci. USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaste P, Nygren G, Anckarsater H, Rastam M, Coleman M, Leboyer M, Gillberg C, Betancur C. Mutation screening of the ARX gene in patients with autism. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2007;144:228–230. doi: 10.1002/ajmg.b.30440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc. Natl. Acad. Sci. USA. 2005a;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Wang SS, Hattox AM, Rayburn H, Nelson SB, McConnell SK. The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proc. Natl. Acad. Sci. USA. 2008;105:11382–11387. doi: 10.1073/pnas.0804918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JG, Rasin MR, Kwan KY, Sestan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc. Natl. Acad. Sci. USA. 2005b;102:17792–17797. doi: 10.1073/pnas.0509032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL. Patterning of frontal cortex subdivisions by Fgf17. Proc. Natl. Acad. Sci. USA. 2007;104:7652–7657. doi: 10.1073/pnas.0702225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Erisir A, Farb C, Nadal MS, Ozaita A, Lau D, Welker E, Rudy B. K(+) channel expression distinguishes subpopulations of parvalbumin- and somatostatin-containing neocortical interneurons. J. Neurosci. 1999;19:9332–9345. doi: 10.1523/JNEUROSCI.19-21-09332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy [see comment] Nat. Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Cobos I, Puelles L, Martinez S. The avian telencephalic subpallium originates inhibitory neurons that invade tangentially the pallium (dorsal ventricular ridge and cortical areas) Dev. Bio. 2001;239:30–45. doi: 10.1006/dbio.2001.0422. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development. 2000;127:5007–5020. doi: 10.1242/dev.127.23.5007. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Nery S, Fishell G. Telencephalic cells take a tangent: Non-radial migration in the mammalian forebrain. Nat. Neurosci. 2001;4:1177–1182. doi: 10.1038/nn749. [DOI] [PubMed] [Google Scholar]
- Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat. Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- Corlew R, Bosma MM, Moody WJ. Spontaneous, synchronous electrical activity in neonatal mouse cortical neurones. J. Physiol. 2004;560:377–390. doi: 10.1113/jphysiol.2004.071621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Bernard C, Ben-Ari Y. Multiple facets of GABAergic neurons and synapses: Multiple fates of GABA signalling in epilepsies. Trends Neurosci. 2005;28:108–115. doi: 10.1016/j.tins.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Crepel V, Aronov D, Jorquera I, Represa A, Ben-Ari Y, Cossart R. A parturition-associated nonsynaptic coherent activity pattern in the developing hippocampus. Neuron. 2007;54:105–120. doi: 10.1016/j.neuron.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Cuzon VC, Yeh PW, Cheng Q, Yeh HH. Ambient. GABA promotes cortical entry of tangentially migrating cells derived from the medial ganglionic eminence. Cereb. Corte. 2006;16:1377–1388. doi: 10.1093/cercor/bhj084. [DOI] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carlos JA, Lopez-Mascaraque L, Valverde F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J. Neurosci. 1996;16:6146–6156. doi: 10.1523/JNEUROSCI.16-19-06146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon TW, Pakzaban P, Isacson O. The lateral ganglionic eminence is the origin of cells committed to striatal phenotypes: Neural transplantation and developmental evidence. Brain Research. 1994;668:211–219. doi: 10.1016/0006-8993(94)90526-6. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Neocortical neuronal diversity: Chemical heterogeneity revealed by colocalization studies of classic neurotransmitters, neuropeptides, calcium-binding proteins, and cell surface molecules. Cereb. Corte. 1993;3:273–289. doi: 10.1093/cercor/3.4.273. [DOI] [PubMed] [Google Scholar]
- Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- Du T, Xu Q, Ocbina PJ, Anderson SA. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135:1559–1567. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- Dupont E, Hanganu IL, Kilb W, Hirsch S, Luhmann HJ. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature. 2006;439:79–83. doi: 10.1038/nature04264. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain [see comment] Nat. Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J. Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G. Regionalization in the mammalian telencephalon. Curr. Opin. Neurobiol. 1997;7:62–69. doi: 10.1016/s0959-4388(97)80121-3. [DOI] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marin O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J. Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint AC, Dammerman RS, Kriegstein AR. Endogenous activation of metabotropic glutamate receptors in neocortical development causes neuronal calcium oscillations. Proc. Natl. Acad. Sci. USA. 1999;96:12144–12149. doi: 10.1073/pnas.96.21.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J. Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Hanse E, Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J. Physiol. 1998;507:219–236. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat. Neurosci. 2000;3:452–459. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Wisor JP, Burns D, Reh RK, Shiromani PJ, Sakurai T, de la Iglesia HO, Kilduff TS. Identification of a population of sleep-active cerebral cortex neurons. Proc. Natl. Acad. Sci. USA. 2008;105:10227–10232. doi: 10.1073/pnas.0803125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58:387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Watanabe S, Chang SY, Joho RH, Huang ZJ, Leonard CS, Rudy B. Specific functions of synaptically localized potassium channels in synaptic transmission at the neocortical GABAergic fast-spiking cell synapse. J. Neurosci. 2005;25:5230–5235. doi: 10.1523/JNEUROSCI.0722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb. Corte. 1997;7:347–358. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes [see comment] Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Grigoriou M, Tucker AS, Sharpe PT, Pachnis V. Expression and regulation of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain encoding genes, suggests a role in mammalian head development. Development. 1998;125:2063–2074. doi: 10.1242/dev.125.11.2063. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron. 2003;37:299–309. doi: 10.1016/s0896-6273(02)01146-7. [DOI] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex [see comment] Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Leingartner A, Ringstedt T, O’Leary DD. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors [see comment] Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Hanashima C, Li SC, Shen L, Lai E, Fishell G. Foxg1 suppresses early cortical cell fate [see comment] Science. 2004;303:56–59. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]
- Hébert JM, Fishell G. The genetics of early telencephalon patterning: Some assembly required. Nat. Rev. Neurosci. 2008 doi: 10.1038/nrn2463. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF. From radial glia to pyramidal-projection neuron: Transcription factor cascades in cerebral cortex development. Mol. Neurobiol. 2006;33:33–50. doi: 10.1385/MN:33:1:033. [DOI] [PubMed] [Google Scholar]
- Hirata T, Li P, Lanuza GM, Cocas LA, Huntsman MM, Corbin JG. Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nat. Neurosci. 2009;12:141–149. doi: 10.1038/nn.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horike S, Cai S, Miyano M, Cheng JF, Kohwi-Shigematsu T. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome [see comment] Nat. Genet. 2005;37:31–40. doi: 10.1038/ng1491. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Garel S, Rubenstein JL. Fgf8 regulates the development of intra-neocortical projections. J. Neurosci. 2004;24:8917–8923. doi: 10.1523/JNEUROSCI.2086-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki T, Pearson B, Holbrook S, Doe CQ. Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell. 2001;106:511–521. doi: 10.1016/s0092-8674(01)00465-2. [DOI] [PubMed] [Google Scholar]
- Jacob J, Maurange C, Gould AP. Temporal control of neuronal diversity: Common regulatory principles in insects and vertebrates? Development. 2008;135:3481–3489. doi: 10.1242/dev.016931. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat. Rev. Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron Diversity series: Fast in, fast out–temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 2004;27:30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc. Natl. Acad. Sci. USA. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamme F, Salunga R, Yu J, Tran DT, Zhu J, Luo L, Bittner A, Guo HQ, Miller N, Wan J, Erlander M. Single-cell microarray analysis in hippocampus CA1: Demonstration and validation of cellular heterogeneity. J. Neurosci. 2003;23:3607–3615. doi: 10.1523/JNEUROSCI.23-09-03607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Gillespie DC. Developmental refinement of inhibitory sound-localization circuits. Trends Neurosci. 2005;28:290–296. doi: 10.1016/j.tins.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Katz LC. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J. Neurosci. 1998;18:1419–1427. doi: 10.1523/JNEUROSCI.18-04-01419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Crowley JC. Development of cortical circuits: Lessons from ocular dominance columns. Nat. Rev. Neurosci. 2002;3:34–42. doi: 10.1038/nrn703. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J. Neurosci. 1996;16:2701–2715. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Corte. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Three classes of GABAergic interneurons in neocortex and neostriatum. Japanese J. Physiol. 1994;44:S145–S148. [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Two distinct subgroups of cholecystokinin-immunoreactive cortical interneurons. Brain Research. 1997;752:175–183. doi: 10.1016/s0006-8993(96)01446-1. [DOI] [PubMed] [Google Scholar]
- Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, Menezes JR, Macklis JD. SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron. 2008;57:232–247. doi: 10.1016/j.neuron.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Lau D, Vega-Saenz de Miera EC, Contreras D, Ozaita A, Harvey M, Chow A, Noebels JL, Paylor R, Morgan JI, Leonard CS, Rudy B. Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J. Neurosci. 2000;20:9071–9085. doi: 10.1523/JNEUROSCI.20-24-09071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J. Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex [see comment] Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci. 2004;27:400–406. doi: 10.1016/j.tins.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lewine JD, Andrews R, Chez M, Patil AA, Devinsky O, Smith M, Kanner A, Davis JT, Funke M, Jones G, Chong B, Provencal S, et al. Magnetoence-phalographic patterns of epileptiform activity in children with regressive autism spectrum disorders [see comment] Pediatrics. 1999;104:405–418. doi: 10.1542/peds.104.3.405. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J. Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Lujan R, Shigemoto R, Ganter P, Paulsen O, Molnar Z. Blockade of GABA(B) receptors alters the tangential migration of cortical neurons. Cereb. Corte. 2003;13:932–942. doi: 10.1093/cercor/13.9.932. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Sanchez-Alcaniz JA, Pla R, Borrell V, Pico E, Valdeolmillos M, Marin O. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J. Neurosci. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. J. Neurophys. 1991;65:247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J. Neurosci. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L, Represa A. A noncanonical release of GABA and glutamate modulates neuronal migration. J. Neurosci. 2005;25:4755–4765. doi: 10.1523/JNEUROSCI.0553-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manent JB, Represa A. Neurotransmitters and brain maturation: Early paracrine actions of GABA and glutamate modulate neuronal migration. Neuroscientist. 2007;13:268–279. doi: 10.1177/1073858406298918. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. A long, remarkable journey: Tangential migration in the telencephalon. Nat. Rev. Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell migration in the forebrain. Ann. Rev. Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Martina M, Royer S, Pare D. Cell-type-specific GABA responses and chloride homeostasis in the cortex and amygdala. J. Neurophys. 2001;86:2887–2895. doi: 10.1152/jn.2001.86.6.2887. [DOI] [PubMed] [Google Scholar]
- McCabe AK, Chisholm SL, Picken-Bahrey HL, Moody WJ. The self-regulating nature of spontaneous synchronized activity in developing mouse cortical neurones. J. Physiol. 2006;577:155–167. doi: 10.1113/jphysiol.2006.117523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe AK, Easton CR, Lischalk JW, Moody WJ. Roles of glutamate and GABA receptors in setting the developmental timing of spontaneous synchronized activity in the developing mouse cortex. Dev. Neurobio. 2007;67:1574–1588. doi: 10.1002/dneu.20533. [DOI] [PubMed] [Google Scholar]
- McConnell SK. Fates of visual cortical neurons in the ferret after isochronic and heterochronic transplantation. J. Neurosci. 1988;8:945–974. doi: 10.1523/JNEUROSCI.08-03-00945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Miller MT, Stromland K, Ventura L, Johansson M, Bandim JM, Gillberg C. Autism associated with conditions characterized by developmental errors in early embryogenesis: A mini review. Int. J. Dev. Neurosci. 2005;23:201–219. doi: 10.1016/j.ijdevneu.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Mission JP, Takahashi T, Caviness VS., Jr. Ontogeny of radial and other astroglial cells in murine cerebral cortex. GLIA. 1991;4:138–148. doi: 10.1002/glia.440040205. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J. Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar G, Olah S, Komlosi G, Fule M, Szabadics J, Varga C, Barzo P, Tamas G. Complex events initiated by individual spikes in the human cerebral cortex. Plos Biology. 2008;6:e222. doi: 10.1371/journal.pbio.0060222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Monyer H, Markram H. Interneuron Diversity series: Molecular and genetic tools to study GABAergic interneuron diversity and function. Trends Neurosci. 2004;27:90–97. doi: 10.1016/j.tins.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Moretti P, Zoghbi HY. MeCP2 dysfunction in Rett syndrome and related disorders. Curr. Opin. Genet. Dev. 2006;16:276–281. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Mueller AL, Taube JS, Schwartzkroin PA. Development of hyperpolarizing inhibitory postsynaptic potentials and hyperpolarizing response to gamma-amino-butyric acid in rabbit hippocampus studied in vitro. J. Neurosci. 1984;4:860–867. doi: 10.1523/JNEUROSCI.04-03-00860.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Johnson JE, O’Leary DD. Graded and areal expression patterns of regulatory genes and cadherins in embryonic neocortex independent of thalamocortical input. J. Neurosci. 1999;19:10877–10885. doi: 10.1523/JNEUROSCI.19-24-10877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat. Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Nicol X, Voyatzis S, Muzerelle A, Narboux-Neme N, Sudhof TC, Miles R, Gaspar P. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat. Neurosci. 2007;10:340–347. doi: 10.1038/nn1842. [DOI] [PubMed] [Google Scholar]
- Nobrega-Pereira S, Kessaris N, Du T, Kimura S, Anderson SA, Marín O. Postmitotic Nkx2–1 controls the migration of telencephalic interneurons by direct repression of guidance receptors [see comment] Neuron. 2008;59:733–745. doi: 10.1016/j.neuron.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr. Opin. Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Stanfield BB. Selective elimination of axons extended by developing cortical neurons is dependent on regional locale: Experiments utilizing fetal cortical transplants. J. Neurosci. 1989;9:2230–2246. doi: 10.1523/JNEUROSCI.09-07-02230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Oide M, Tanaka H. Excitatory and inhibitory actions of GABA and glycine on embryonic chick spinal neurons in culture. Brain Res. 1978;144:179–184. doi: 10.1016/0006-8993(78)90447-x. [DOI] [PubMed] [Google Scholar]
- Olsson M, Bjorklund A, Campbell K. Early specification of striatal projection neurons and interneuronal subtypes in the lateral and medial ganglionic eminence. Neuroscience. 1998;84:867–876. doi: 10.1016/s0306-4522(97)00532-0. [DOI] [PubMed] [Google Scholar]
- Olsson M, Campbell K, Wictorin K, Bjorklund A. Projection neurons in fetal striatal transplants are predominantly derived from the lateral ganglionic eminence. Neuroscience. 1995;69:1169–1182. doi: 10.1016/0306-4522(95)00325-d. [DOI] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J. Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Patterns of intracellular calcium fluctuation in precursor cells of the neocortical ventricular zone. J. Neurosci. 1998;18:5374–5388. doi: 10.1523/JNEUROSCI.18-14-05374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnavelas JG. The origin and migration of cortical neurones: New vistas. Trends Neurosci. 2000;23:126–131. doi: 10.1016/s0166-2236(00)01553-8. [DOI] [PubMed] [Google Scholar]
- Peinado A. Traveling slow waves of neural activity: A novel form of network activity in developing neocortex. J. Neurosci. 2000;20:RC54. doi: 10.1523/JNEUROSCI.20-02-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petilla Interneuron Nomenclature Group. Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairén A, Feldmeyer D, Fishell G, et al. Petilla terminology: Nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 2007;55:417–433. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper AA, Wu X, Han TW, Estill SJ, Dang Q, Wu LC, Reece-Fincanon S, Dudley CA, Richardson JA, Brat DJ, McKnight SL. The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc. Natl. Acad. Sci. USA. 2005;102:14052–14057. doi: 10.1073/pnas.0506713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure SJ, Anderson S, Hevner R, Bagri A, Marin O, Lowenstein DH, Rubenstein JL. Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron. 2000;28:727–740. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- Poluch S, Juliano SL. A normal radial glial scaffold is necessary for migration of interneurons during neocortical development. GLIA. 2007;55:822–830. doi: 10.1002/glia.20488. [DOI] [PubMed] [Google Scholar]
- Pombal MA, Puelles L. Prosomeric map of the lamprey forebrain based on calretinin immunocytochemistry, Nissl stain, and ancillary markers. J. Comp. Neurol. 1999;414:391–422. [PubMed] [Google Scholar]
- Porter JT, Cauli B, Staiger JF, Lambolez B, Rossier J, Audinat E. Properties of bipolar VIPergic interneurons and their excitation by pyramidal neurons in the rat neocortex. Eur. J. Neurosci. 1998;10:3617–3628. doi: 10.1046/j.1460-9568.1998.00367.x. [DOI] [PubMed] [Google Scholar]
- Porteus MH, Bulfone A, Liu JK, Puelles L, Lo LC, Rubenstein JL. DLX-2, MASH-1, and MAP-2 expression and bromodeoxyuridine incorporation define molecularly distinct cell populations in the embryonic mouse forebrain. J. Neurosci. 1994;14:6370–6383. doi: 10.1523/JNEUROSCI.14-11-06370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Puelles L. Brain segmentation and forebrain development in amniotes. Brain Res. Bulletin. 2001;55:695–710. doi: 10.1016/s0361-9230(01)00588-3. [DOI] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J. Comp. Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: Systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. Principles of neural cell migration. Experientia. 1990;46:882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- Rallu M, Corbin JG, Fishell G. Parsing the prosencephalon. Nat. Rev. Neurosci. 2002a;3:943–951. doi: 10.1038/nrn989. [DOI] [PubMed] [Google Scholar]
- Rallu M, Machold R, Gaiano N, Corbin JG, McMahon AP, Fishell G. Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development. 2002b;129:4963–4974. doi: 10.1242/dev.129.21.4963. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Martinez S, Shimamura K, Puelles L. The embryonic vertebrate forebrain: The prosomeric model. Science. 1994;266:578–580. doi: 10.1126/science.7939711. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Shimamura K, Martinez S, Puelles L. Regionalization of the prosencephalic neural plate. Annu. Rev. Neurosci. 1998;21:445–477. doi: 10.1146/annurev.neuro.21.1.445. [DOI] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: Voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- Rymar VV, Sadikot AF. Laminar fate of cortical GABAergic interneurons is dependent on both birthdate and phenotype. J. Comp. Neurol. 2007;501:369–380. doi: 10.1002/cne.21250. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells [see comment] Nat. Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JL. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Martinez S, Puelles L, Rubenstein JL. Patterns of gene expression in the neural plate and neural tube subdivide the embryonic forebrain into transverse and longitudinal domains. Dev. Neurosci. 1997;19:88–96. doi: 10.1159/000111190. [DOI] [PubMed] [Google Scholar]
- Smart IH. A pilot study of cell production by the ganglionic eminences of the developing mouse brain. J. Anat. 1976;121:71–84. [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J. Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res. - Brain Res. Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, Fishell G. Characterization of Nkx6-2-Derived Neocortical Interneuron Lineages. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp038. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: Implications for striatal and olfactory bulb neurogenesis. J. Neurosci. 2003a;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Wang B, Campbell K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J. Neurosci. 2003b;23:10568–10576. doi: 10.1523/JNEUROSCI.23-33-10568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhmer T, Anderson SA, Ekker M, Rubenstein JL. Ectopic expression of the Dlx genes induces glutamic acid decarboxylase and Dlx expression. Development. 2002;129:245–252. doi: 10.1242/dev.129.1.245. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J. Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock RR, Smart IH. A morphological study of the mouse subependymal layer from embryonic life to old age. J. Anat. 1980;130:391–415. [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB. Molecular taxonomy of major neuronal classes in the adult mouse forebrain [see comment] Nat. Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. erratum appears in Nat. Neurosci. 2006 Feb;9 (2):292. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: Evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Taglialatela P, Soria JM, Caironi V, Moiana A, Bertuzzi S. Compromised generation of GABAergic interneurons in the brains of Vax1−/− mice. Development. 2004;131:4239–4249. doi: 10.1242/dev.01299. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Fujimori KE, Takauji R. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J. Neurosci. 1997;17:8313–8323. doi: 10.1523/JNEUROSCI.17-21-08313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiveron MC, Rossel M, Moepps B, Zhang YL, Seidenfaden R, Favor J, Konig N, Cremer H. Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J. Neurosci. 2006;26:13273–13278. doi: 10.1523/JNEUROSCI.4162-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toresson H, Campbell K. A role for Gsh1 in the developing striatum and olfactory bulb of Gsh2 mutant mice. Development. 2001;128:4769–4780. doi: 10.1242/dev.128.23.4769. [DOI] [PubMed] [Google Scholar]
- Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: Opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Holmes GL, Ben-Ari Y, Khazipov R. Timing of the developmental switch in GABA(A) mediated signaling from excitation to inhibition in CA3 rat hippocampus using gramicidin perforated patch and extracellular recordings. Epilepsia. 2007;48:96–105. doi: 10.1111/j.1528-1167.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- Valcanis H, Tan SS. Layer specification of transplanted interneurons in developing mouse neocortex. J. Neurosci. 2003;23:5113–5122. doi: 10.1523/JNEUROSCI.23-12-05113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JB, Horike SI, Choufani S, Paterson AD, Roberts W, Szatmari P, Weksberg R, Fernandez B, Scherer SW. An inversion inv(4)(p12-p15.3) in autistic siblings implicates the 4p GABA receptor gene cluster. J. Med. Genet. 2006;43:429–434. doi: 10.1136/jmg.2005.039693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Allen ZJ, 2nd, Bell SM, Erdelyi F, Szabo G, Potter SS, Campbell K. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006;49:503–516. doi: 10.1016/j.neuron.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Wheless JW, Kim HL. Adolescent seizures and epilepsy syndromes. Epilepsia. 2002;43:33–52. doi: 10.1046/j.1528-1157.43.s.3.12.x. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat. Neurosci. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Wilson SW, Houart C. Early steps in the development of the forebrain. Dev. Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SW, Rubenstein JL. Induction and dorsoventral patterning of the telencephalon. Neuron. 2000;28:641–651. doi: 10.1016/s0896-6273(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat. Rev. Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev. Bio. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Lu B. Regulation of cortical interneurons by neurotrophins: From development to cognitive disorders. Neurosci. 2006;12:43–56. doi: 10.1177/1073858405284360. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J. Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J. Comp. Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Yun K, Garel S, Fischman S, Rubenstein JL. Patterning of the lateral ganglionic eminence by the Gsh1 and Gsh2 homeobox genes regulates striatal and olfactory bulb histogenesis and the growth of axons through the basal ganglia. J. Comp. Neurol. 2003;461:151–165. doi: 10.1002/cne.10685. [DOI] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- Yuste R, Peinado A, Katz LC. Neuronal domains in developing neocortex. Science. 1992;257:665–669. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]