Abstract
OBJECTIVE
We sought to compare fundal height and handheld ultrasound–measured fetal abdominal circumference (HHAC) for the prediction of fetal growth restriction (FGR) or large for gestational age.
STUDY DESIGN
This was a diagnostic accuracy study in nonanomalous singleton pregnancies between 24 and 40 weeks’ gestation. Patients underwent HHAC and fundal height measurement prior to formal growth ultrasound. FGR was defined as estimated fetal weight less than 10%, whereas large for gestational age was defined as estimated fetal weight greater than 90%. Sensitivity and specificity were calculated and compared using methods described elsewhere.
RESULTS
There were 251 patients included in this study. HHAC had superior sensitivity and specificity for the detection of FGR (sensitivity, 100% vs 42.86%) and (specificity, 92.62% vs 85.24%). HHAC had higher specificity but lower sensitivity when screening for LGA (specificity, 85.66% vs 66.39%) and (sensitivity, 57.14% vs 71.43%).
CONCLUSION
HHAC could prove to be a valuable screening tool in the detection of FGR. Further studies are needed in a larger population.
Keywords: fetal growth abnormalities, fundal height, ultrasound
Identification of abnormal fetal growth is a critical component of prenatal care and failure of antenatal diagnosis can result in increased perinatal morbidity and mortality as well as affect the long-term health of the neonate. As such, reliable screening methods to detect fetuses with potential fetal growth restriction (FGR) and large for gestational age (LGA) are essential to prevent poor perinatal outcomes.
FGR refers to a weight below the 10th percentile for gestational age, although other definitions using a variety of criteria have been advocated.1 Inadequate fetal growth affects up to 10% of all pregnancies. Growth-restricted fetuses have an increased risk for meconium aspiration syndrome, neurological injury, acidosis, and fetal demise during the peripartum period with subsequent risks of hypertension, diabetes, coronary artery disease, and stroke in adulthood.2-4 Studies show that FGR is often undetected antenatally by current methods, and in recent studies up to 50-90% of these fetuses are not diagnosed until delivery.5-8
At the other end of the spectrum is fetal macrosomia, defined as a birthweight greater than 4000-4500 g. Antenatally, these fetuses are classified as LGA with an estimated fetal weight (EFW) greater than the 90th percentile for gestational age. Approximately 10% of all live-born infants in the United States weigh more than 4000 g, putting these infants at risk for shoulder dystocia and resulting injuries and increased admissions to neonatal intensive care units.9,10 Maternal morbidity is also increased because of the higher rates of cesarean delivery and severe perineal lacerations. Unfortunately, an accurate diagnosis of LGA or macrosomia can be made only after weighing the infant at birth because clinical estimates and ultrasonography have proven to be unreliable.11
Clinical estimation of fetal weight is most commonly performed using symphysis–fundal height measurement. Numerous studies have shown that fundal height (FH) has a poor positive predictive value for identifying abnormally grown fetuses, and the increasing incidence of maternal obesity further confounds this clinical estimation.12-17 Current literature supports the theory that measurement of the abdominal circumference (AC) in the fetus is the most sensitive single indicator of fetal growth abnormalities.18-21
Our study investigates the ability of portable handheld ultrasound measurements of fetal AC to more accurately screen for fetal growth abnormalities compared with FH measurement. Our group hypothesized that height and handheld ultrasounde–measured fetal abdominal circumference (HHAC) would be a superior screening modality for the detection of abnormal EFW and birthweight (BW) compared with FH.
Materials and Methods
Study design
This study is a diagnostic accuracy study as defined by the Standards for Reporting of Diagnostic Accuracy statement22 comparing the diagnostic accuracy of FH measurements with fetal AC measurements obtained by handheld ultrasound to identify fetal growth abnormalities. In addition, the abdominal circumference obtained from the formal ultrasound (USAC) was also included in our investigation of screening modalities. All ultrasounds and measurements were performed at the American Institute of Ultrasound in Medicine–certified Prenatal Wellness Center at the Medical University of South Carolina.
Following institutional review board approval and informed consent, patients were eligible for the study if they were between 24 and 40 weeks’ gestational age with a singleton pregnancy and undergoing a scheduled ultrasound to assess fetal growth. Gestational age was based on the last menstrual period confirmed by dating ultrasound done at less than 20 weeks’ gestational age.
Patients were excluded if their fetus had known congenital anomalies, aneuploidy, or an unsure estimated date of conception. Data abstracted from patient charts included maternal demographics, maternal indication for ultrasound, maternal weight, and maternal comorbidities including hypertension, tobacco use, and pregestational diabetes.
Prior to undergoing their formal growth ultrasound by registered diagnostic medical sonographers, an obstetrics and gynecology resident or maternal-fetal medicine specialist measured both FH and fetal AC using the portable handheld ultrasound device (GE Vscan, version 1.1; GE Healthcare, Indianapolis, IN).
FH was measured from the pubis symphysis to the top of the uterine fundus using a paper measuring tape in centimeters. Size greater than dates was recorded if the measurement was 3 cm or greater above the patient’s gestational age. Similarly, size less than dates was recorded if the measurement was 3 cm or less below the patient’s gestational age.
To measure the HHAC, the fetal abdomen was visualized in a crosssectional view using the handheld ultrasound at the level of the stomach and umbilical vein. Figures 1 and 2 depict the image quality obtained with HHAC compared with USAC. The fetal AC was obtained by placing the ultrasound cursor directly in the middle of the fetal abdomen and expanding a circle to encompass the entire abdominal circumference using the radius feature on the device. The radius measurement obtained from the handheld ultrasound device was then used to calculate the total abdominal circumference using the following formula: circumference = 2πr, with r meaning the radius.
FIGURE 1. Fetal abdominal circumference obtained with USAC.
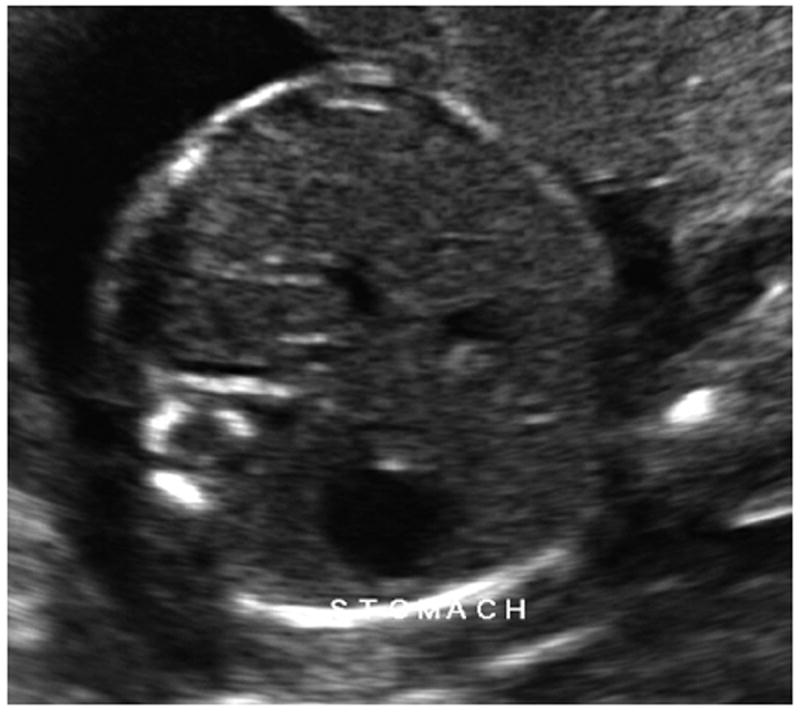
USAC, ultrasound abdominal circumference.
Haragan. Handheld abdominal circumference v fundal height. Am J Obstet Gynecol 2015.
FIGURE 2. Fetal abdominal circumference obtained with HHAC.
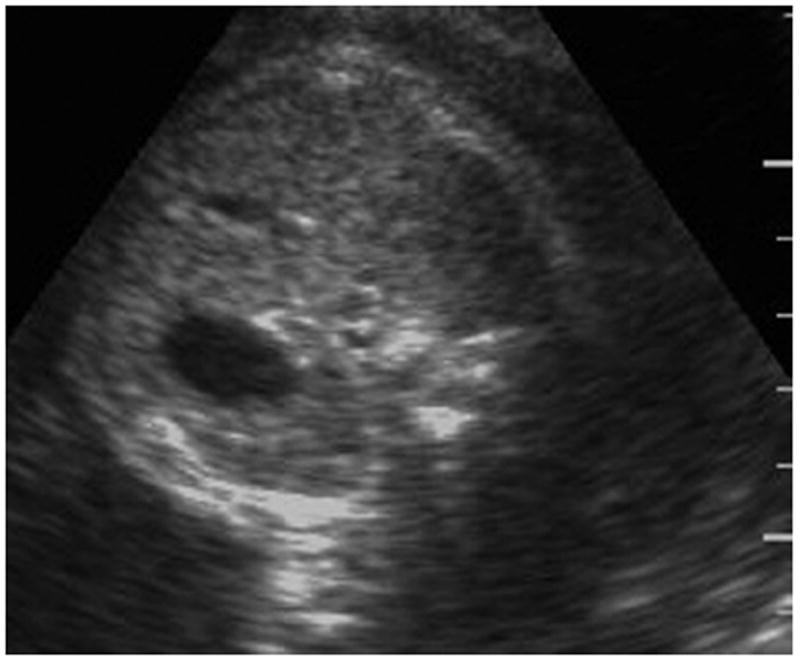
HHAC, handheld ultrasound abdominal circumference.
Haragan. Handheld abdominal circumference v fundal height. Am J Obstet Gynecol 2015.
A positive screen for FGR was recorded as an HHAC less than the 5th percentile and a positive screen for LGA was an HHAC greater than the 95th percentile. This cutoff was chosen to avoid an overestimation of FGR and LGA and to support the data showing an AC less than the 5th percentile is more closely associated with an increased perinatal morbidity and mortality.23,24
For the current study, FGR was defined as an EFW less than the 90th percentile, and LGA was defined as an EFW greater than the 90th percentile because macrosomia cannot be diagnosed until a fetus reaches greater than 4000 g. Therefore, LGA will refer to both an EFW greater than the 90th percentile and a BW greater than the 90th percentile.
After completion of the formal growth scan, measurements from this study were recorded into the patient’s research chart. EFW and percentiles were calculated automatically by the GE Voluson machines in our office using the formula of Hadlock et al.25 Fetal AC measurement percentiles using the handheld ultrasound device were also calculated using the Hadlock formula for consistency.
A total of 251 patients were consecutively enrolled throughout the study period. The patients were subsequently scheduled for follow-up growth ultrasound at intervals determined by their primary provider. After delivery, the sex of the infant, gestational age at delivery, and BW were recorded. Birthweight percentiles were then calculated using the growth curves of Olsen et al26 and recorded into the patient’s research chart. Small for gestational age (SGA) at the time of delivery was defined as a BW less than the 10th percentile, and LGA was defined as a BW greater than the 90th percentile.
Sensitivity and specificity of the screening tests were then calculated using a Fisher exact test using SAS 9.3 statistical software (SAS Institutes, Carey, NC) with the EFWand BW as the gold standard for which these were compared. These values were then analyzed using methods previously described by Hawass27 and McNemar.28 Statistical significance was established based on a value of P <.05.
Results
A total of 251 patients were enrolled in our study between April 2013 and October 2013. Table 1 describes the demographic and obstetric characteristics of our study population. The average gestational age at enrollment was approximately 32 weeks’ gestation and ranged from 24 weeks’ gestation to 40 weeks’ gestation. As shown, a high proportion of our patients had significant comorbidities including diabetes, hypertension, and tobacco use, making our population relatively high risk for fetal growth abnormalities. Of the patients enrolled, 145 of 151 (96%) had a body mass index greater than 30 kg/m2.
TABLE 1.
Demographic and obstetric characteristics
| Demographic | Average | Range or % |
|---|---|---|
| Age, y | 28.3 | 18–41 |
| Gestational age, wks | 31 6/7 | 24 0/7–40 0/7 |
| Parity | 1 | 0–4 |
| BMI | 33.9 | 18.1–71.6 |
| EFW, g | 1961.3 | 600–4732 |
| Race | ||
| Black | 113 | 45% |
| White | 131 | 52% |
| Other | 7 | 3% |
| Prevalence of conditions | ||
| Hypertension | n = 65 | 25.9% |
| Diabetes | n = 52 | 20.7% |
| Smoking | n = 29 | 11.5% |
BMI, body mass index; EFW, estimated fetal weight.
Haragan. Handheld abdominal circumference v fundal height. Am J Obstet Gynecol 2015.
To validate the handheld ultrasound device used in our study, a correlation between HHAC and AC measurement from the formal growth scan is presented in Figure 3. There is a highly significant correlation between HHAC and AC determined at the time of a formal ultrasound (R = 0.939; P < .001), leading us to accept that the AC measurements made by the clinicians using the handheld ultrasound device were overall comparable with those measurements performed by the registered diagnostic medical sonographere–certified ultrasonographers.
FIGURE 3. Correlation between HHAC and AC from formal growth ultrasound.
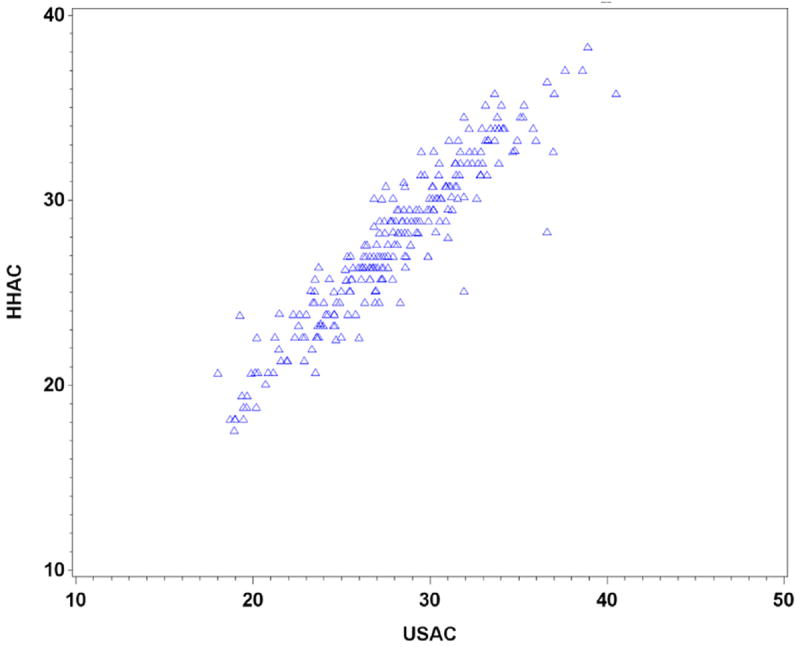
AC, abdominal circumference; HHAC, handheld ultrasound abdominal circumference.
Haragan. Handheld abdominal circumference v fundal height. Am J Obstet Gynecol 2015.
In our study population, we found that antenatally we had 7 fetuses (2.79%) that met criteria for FGR, defined as an EFW less than the 10th percentile on the scheduled growth scan. Similarly, we found that 7 fetuses (2.79%) met criteria for LGA, measuring greater than the 90th percentile at the time of the scheduled ultrasound screening. However, once the BWs were collected and the percentiles calculated using gestational age at delivery and sex of the infant by the method of Olsen et al,26 we found that 27 infants (10.76%) were considered SGA, defined as a BW below the 10th percentile. On the other hand, 24 infants (9.56%) were found to be LGA, or a BW greater than the 90th percentile.
We first looked at FH, HHAC, and USAC as screening modalities to detect an EFW less than the 10th percentile. These data are broken down in Tables 2, 3, and 4. Using FH as a screening test, 3 of 7 fetuses (42.86%) with an EFW less than the 10th percentile were detected using FH (size less than the dates). Using HHAC, all 7 fetuses (100%) with an EFW less than the 10th percentile had a positive screen using HHAC less than the 5th percentile. Finally, USAC less than the 5th percentile was tested as a screening tool for EFW less than the 10th percentile, with detection of 6 of 7 fetuses (85.71%).
TABLE 2.
Use of FH to screen for EFW and BW less than the 10th percentile or greater than the 90th percentile
| Variable | Size less than dates | Normal FH | Size greater than dates | Totals |
|---|---|---|---|---|
| EFW less than 10th percentile | 3 (1.2%) | 4 (1.59%) | 0 | 7 (2.79%) |
| Normal EFW | 18 (7.17%) | 137 (54.58%) | 82 (32.67%) | 237 (94.42%) |
| EFW greater than 90th percentile | 0 | 2 (0.8%) | 5 (1.99%) | 7 (2.79%) |
| Totals | 21 (8.37%) | 143 (56.97%) | 87 (34.66%) | 251 (100%) |
| BW less than 10th percentile | 10 (3.98%) | 13 (5.18%) | 4 (1.59%) | 27 (10.76%) |
| Normal BW | 11 (4.38%) | 118 (47.01%) | 71 (28.29%) | 200 (79.68%) |
| BW greater than 90th percentile | 0 | 12 (4.78%) | 12 (4.78%) | 24 (9.56%) |
| Totals | 21 (8.37%) | 143 (56.97%) | 87 (34.66%) | 251 (100%) |
BW, body weight; EFW, estimated fetal weight; FH, fundal height.
Haragan. Handheld abdominal circumference v fundal height. Am J Obstet Gynecol 2015.
TABLE 3.
Use of HHAC to screen for EFW and BW less than the 10th percentile or greater than the 90th percentile
| Variable | HHAC less than 5th percentile | Normal HHAC | HHAC greater than 95th percentile | Totals |
|---|---|---|---|---|
| EFW less than 10th percentile | 7 (2.79%) | 0 | 0 | 7 (2.79%) |
| Normal EFW | 35 (13.94%) | 167 (66.53%) | 35 (13.94%) | 237 (94.42%) |
| EFW greater than 90th percentile | 1 (0.4%) | 2 (0.8%) | 4 (1.59%) | 7 (2.79%) |
| Totals | 43 (17.13%) | 143 (56.97%) | 39 (15.54%) | 251 (100%) |
| BW less than 10th percentile | 20 (7.97%) | 7 (2.79%) | 0 | 27 (10.76%) |
| Normal BW | 22 (8.76%) | 155 (61.75%) | 23 (9.16%) | 200 (79.68%) |
| BW greater than 90th percentile | 1 (0.4%) | 7 (2.79%) | 16 (6.37%) | 24 (9.56%) |
| Totals | 43 (17.13%) | 143 (56.97%) | 39 (15.54%) | 251 (100%) |
BW, body weight; EFW, estimated fetal weight; HHAC, handheld ultrasound–measured fetal abdominal circumference.
Haragan. Handheld abdominal circumference v fundal height. Am J Obstet Gynecol 2015.
TABLE 4.
Use of USAC to screen for EFW less than the 10th percentile or greater than the 90th percentile
| Variable | USAC less than 5th percentile | Normal USAC | USAC greater than 95th percentile | Totals |
|---|---|---|---|---|
| EFW less than 10th percentile | 6 (2.4%) | 1 (0.4%) | 0 | 7 (2.8%) |
| Normal EFW | 18 (7.2%) | 190 (75.7%) | 29 (11.5%) | 237 (94.4%) |
| EFW greater than 90th percentile | 0 | 0 | 7 (2.8%) | 7 (2.8%) |
| Totals | 24 (9.6%) | 191 (76.1%) | 36 (14.3%) | 251 (100%) |
| BW less than 10th percentile | 15 (6%) | 12 (4.8%) | 0 | |
| Normal BW | 9 (3.6%) | 173 (68.9%) | 18 (7.2%) | 200 (79.7%) |
| BW greater than 90th percentile | 0 | 6 (2.4%) | 18 (7.2%) | 24 (9.5%) |
| Totals | 24 (9.6%) | 191 (76.1%) | 36 (14.3%) | 251 (100%) |
BW, birthweight; EFW, estimated fetal weight; USAC, ultrasound abdominal circumference.
Haragan. Handheld abdominal circumference v fundal height. Am J Obstet Gynecol 2015.
We then looked at the ability of the same screening modalities to detect an EFW greater than the 90th percentile, which is again shown in Tables 2, 3, and 4. Using FH as a screening test, 5 of 7 fetuses (71.42%) with an EFW greater than the 90th percentile were detected using FH (size greater than the dates).
Using HHAC, 4 of 7 fetuses (57.14%) with an EFW greater than the 90th percentile had a positive screen using a cutoff of HHAC greater than the 95th percentile. Finally, USAC greater than the 95th percentile was tested as a screening tool for EFW greater than the 90th percentile, with detection of 7 of 7 fetuses (100%).
Once BWs were collected, we then looked at our screening modalities with the addition of EFW obtained from the scheduled ultrasound to screen for SGA (BWless than the 10th percentile). These data are outlined in Tables 2, 3, 4, and 5. Using FH as a screening test, 10 of 27 fetuses (37.04%) with a BW less than the 10th percentile were detected using FH (size less than the dates). Using HHAC, 20 of 27 SGA neonates (74.07%) had a positive screen using HHAC less than the 5th percentile. Next, USAC less than the 5th percentile was tested as a screening tool for SGA, with detection of 15 of 27 fetuses (55.56%). Finally, EFW was investigated as a screening modality to detect SGA. Of the neonates with a BW less than the 10th percentile, 6 of 27 (21.43%) were detected at the time of the scheduled ultrasound examination using EFW less than the 10th percentile as the screening modality.
TABLE 5.
Diagnostic performance of FH, HHAC, USAC, and EFW to predict EFW and BW less than the 10th percentile or greater than the 90th percentile
| Variable | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|
| EFW less than 10th percentile | ||||
| Fundal height (size less than dates) | 42.86 (41.68–44.03) | 92.62 (92.52–92.73) | 14.29 (13.81–14.77) | 98.26 (98.21–98.32) |
| HHAC less than 5th percentile | 100 (100–100) | 85.25 (85.1–85.39) | 16.28 (15.93–16.63) | 100 (100–100) |
| USAC less than 5th percentile | 85.71 (84.88–86.54) | 92.62 (92.52–92.73) | 25 (24.44–25.55) | 99.56 (99.53–99.59) |
| EFW greater than 90th percentile | ||||
| Fundal height (size greater than dates) | 71.43 (70.36–72.49) | 66.39 (66.2–66.58) | 5.75 (5.59–5.9) | 98.78 (98.72–98.93) |
| HHAC greater than 95th percentile | 57.14 (55.97–58.32) | 85.66 (85.52–85.79) | 10.2 (9.95–10.56) | 98.5 (98.53–98.64) |
| USAC greater than 95th percentile | 100 (100–100) | 88.11 (87.99–88.25) | 19.44 (19.03–19.86) | 100 (100–100) |
| BW less than 10th percentile | ||||
| Fundal height (size less than dates) | 37.03 (36.45–37.62) | 95.09 (94.99–95.18) | 47.62 (46.93–48.3) | 92.61 (92.5–92.72) |
| HHAC less than 5th percentile | 74.07 (73.54–74.6) | 89.73 (89.61–89.86) | 46.51 (46.04–46.99) | 96.63 (96.56–96.71) |
| USAC less than 5th percentile | 55.56 (54.96–56.16) | 95.98 (95.9–96.06) | 62.50 (61.88–63.12) | 94.71 (94.62–94.81) |
| EFW less than 10th percentile | 21.43 (20.94) | 99.55 (99.52–99.58) | 85.71 (84.88–86.54) | 90.98 (90.87–91.1) |
| BW greater than 90th percentile | ||||
| Fundal height (size greater than dates) | 50 (49.36–50.64) | 66.96 (66.77–67.16) | 13.79 (13.56–14.03) | 92.68 (92.56–92.81) |
| HHAC greater than 95th percentile | 66.67 (66.06–67.27) | 89.87 (89.74–89.99) | 41.03 (40.53–41.52) | 96.23 (96.14–96.31) |
| USAC greater than 95th percentile | 75 (74.45–75.55) | 92.07 (91.96–92.18) | 50 (49.48–50.52) | 97.21 (97.14–97.28) |
| EFW greater than 90th percentile | 25 (24.45–25.55) | 99.56 (99.53–99.59) | 85.71 (84.89–86.54) | 92.62 (92.52–92.73) |
Data are in percentages unless otherwise specified.
BW, birthweight; CI, confidence interval; EFW, estimated fetal weight; FH, fundal height; HHAC, handheld ultrasound abdominal circumference; NPV, negative predictive value; PPV, positive predictive value; USAC, ultrasound abdominal circumference.
Haragan. Handheld abdominal circumference v fundal height. Am J Obstet Gynecol 2015.
Next, we investigated the use of FH, HHAC, USAC, and EFW to screen for LGA (BW greater than the 90th percentile). These data are again outlined in Tables 2, 3, 4, and 5. Using FH as a screening test, 12 of 24 fetuses with a BW greater than the 90th percentile (50%) were detected using FH (size greater than the dates). Using HHAC, 16 of 24 LGA neonates (66.67%) had a positive screen using HHAC greater than the 95th percentile. Next, USAC greater than the 95th percentile was tested as a screening tool for LGA, with detection of 18 of 24 fetuses (75%).
Finally, oval ultrasound EFW was investigated as a screening modality to detect LGA. Of the LGA neonates, only 6 of 24 (25%) were detected at the time of the scheduled ultrasound examination using EFW greater than the 90th percentile as the screening modality.
Table 5 summarizes the screening performance of all the investigated screening modalities. When screening for EFW less than the 10th percentile, HHAC had the highest sensitivity when compared with USAC and FH (100% vs 84.71% vs. 42.86%), but lower specificity (85.25% vs 92.62% vs 92.62%). All of the screening modalities had poor positive predictive value (PPV), but of the modalities investigated, USAC had the highest PPV compared with HHAC and FH (25% vs 16.28% vs 14.29%). Conversely, the negative predictive value (NPV) of FH, HHAC, and USAC were all very favorable (98.26% vs 100% vs 99.56%).
When screening for EFW greater than the 90th percentile, HHAC had the lowest sensitivity when compared with USAC and FH (57.14% vs 100% vs 71.43%), and comparable specificity with USAC (85.66% vs 88.11% vs 66.39%). All of the screening modalities again had poor PPV, but of the modalities investigated, USAC had the highest PPV compared with HHAC and FH (19.44% vs 10.26% vs 5.75%). Conversely, the NPVof FH, HHAC, and USAC were all very favorable (98.78% vs 98.58% vs 100%).
Table 5 also summarizes the validity of the selected screening modalities when screening for SGA and LGA. When screening for SGA (BW less than the 10th percentile), HHAC had superior sensitivity compared with FH, USAC, and EFW (74.07% vs 37.03% vs 55.56% vs 21.43%) but lower specificity (89.73% vs 95.09% vs 95.98% vs 99.55%).
EFW had the highest PPV compared with FH, HHAC and USAC (84.71% vs 47.62% vs 46.51 vs 62.5%). Again, the NPV of FH, HHAC, USAC, and EFW were all very favorable (92.61% vs 96.63% vs 94.71% vs 90.98%). When screening for LGA (BW greater than the 90th percentile), USAC had the highest sensitivity compared with FH, HHAC, and EFW (75% vs 50% vs 66.67% vs 25%), whereas EFW had the highest specificity compared with FH, HHAC, and USAC (99.56%vs 66.96%vs 89.87% vs 92.07%). EFW had a much higher PPV compared with the other screening modalities (85.71% vs 13.79% vs 41.03% vs 50%), and all modalities had similar NPV (92.68% vs 96.23% vs 97.21% vs 92.62%). Confidence intervals were calculated for all the screening modalities and are reflected in Table 5.
Comment
This study illustrates that HHAC has the potential to be a valuable screening tool for FGR. As previously discussed, failure to diagnose FGR in the prenatal period can lead to poor perinatal outcomes, and to date, our current detection rates are rather dismal, with up to 75-90% of cases going undiagnosed until the time of birth.4
Previous studies have shown that with EFW less than the 10th percentile, the risk of fetal death is approximately 1.5%, which is twice the rate of normally grown fetuses.2 This, along with the substantial increase in poor perinatal morbidity associated with FGR, should prompt efforts to improve our screening methods.
This is the first study investigating the use of a handheld ultrasound to screen for fetal growth abnormalities using a simple abdominal circumference measurement. Previously in our clinic, these pocket ultrasounds had been used solely to confirm fetal cardiac activity in early gestations and confirm fetal presentation. The software installed on the machines allows for the measurement of a circumference and thus the ability to estimate fetal AC. The potential benefits of this screening modality are numerous, from improved accuracy in screening for FGR to the ease of use and dramatic costsavings benefits.
Previous studies have compared FH with traditional measurement of fetal AC in term pregnancies to predict high and low BWs with the conclusion that fetal AC measurement by ultrasound is superior to clinical examination.6 Our study differs in that we looked at a variety of gestational ages and the ability of several different screening modalities to detect SGA and LGA at the time of delivery.
We were able to validate the clinicianperformed HHAC measurements with a strong correlation between the abdominal circumference we obtained with our portable device and the measurements obtained during the scheduled ultrasound. These data were able to be reproduced, regardless of maternal body mass index or gestational age.
Several interesting findings surfaced once all the screening modalities were compared. Notably, although HHAC had excellent sensitivity in the detection of FGR, it performed rather poorly when screening for LGA. This may represent a device failure because the largest measurable circumference is approximately 36 cm, which is smaller than many of the macrosomic fetuses included in our study.
Looking at the results, one can see that USAC performed very well when screening for LGA, leading one to believe that, overall, the AC measurements performed by either device would detect the vast majority of fetuses with growth abnormalities, allowing obstetricians a rapid screening modality to ascertain which patients require further diagnostic imaging, fetal surveillance, and/or umbilical artery Doppler studies.29
Also worth mentioning is when using EFW as a screening modality for SGA or LGA, it had the highest PPV but also the lowest sensitivity. In the current study, EFW is considered a diagnostic test to which the investigated screening modalities (FH, HHAC, and USAC) are compared. In turn, we also examined EFW as a screening modality for abnormal BWs. This study’s findings confirm that although EFW is the current gold standard in the diagnosis of fetal growth abnormalities, other screening tests need to be considered to increase sensitivity in the detection of these fetuses.
In the era of increasing maternal obesity, screening for growth abnormalities using traditional means (FH, Leopold maneuvers) is becoming increasingly difficult and inaccurate.14 Our study supports these findings, with our results confirming the poor PPV of FH when a patient measures size greater than the dates. This is largely attributed to maternal obesity.
Current literature does not support routine third-trimester screening biometry because it has not been shown to decrease perinatal morbidity and mortality, and consequently, it is not time or cost effective in an unselected population.30,31 However, at our institution, we routinely screen our morbidly obese population with serial ultrasounds because of the inability to detect growth abnormalities in their fetuses.
Our study investigates a new bedside approach that may provide a better method to clinically screen for fetal growth abnormalities, even in the patient with morbid obesity. Because of its minimal cost and the fact it can be performed by clinicians at the bedside, this approach could prove cost effective in its application to low-risk women as a screen for possible growth abnormalities. This would avoid unnecessary ultrasounds in this large, low-risk population.
Our study had several limitations, the first being that all of our patients were already undergoing a growth ultrasound for other indications, selecting out a higher-risk population. Interestingly, this result did not identify a greater frequency of FGR or LGA than would be expected in the general population. As a result, these results may remain applicable to the general population.
Because of the wide variety of gestational ages enrolled in the study, there is the possibility that several of our patients went on to develop growth abnormalities later in gestation and were not captured at their current gestational age. In addition, only 251 patients were enrolled in our study to provide preliminary data to assess the validity of using a handheld ultrasound as a screening modality. This sample size did allow us to confirm the strong correlation between the HHAC and the USAC. However, our small sample size created difficulties in our statistical analysis when comparing sensitivities and specificities between the various screening tests.
Unfortunately, because of the low prevalence of FGR (2.79%) and LGA (2.79%) in our patient population, the difference between the sensitivities and specificities were not statistically significant. However, when looking at the results of our study, the outcomes would likely be clinically significant if applied to a larger population.
In conclusion, our investigation provides preliminary evidence that HHAC could prove to be a useful bedside screening tool for obstetricians, particularly when screening for growth abnormalities in the obese population. Further studies in a larger, low-risk population could provide valuable insight into the cost-savings benefits and feasibility of HHAC as a routine prenatal evaluation instead of the fundal height measurement. Future studies of value would also include investigation of birth outcomes in this population related to a missed diagnosis of FGR or LGA.
Footnotes
The authors report no conflict of interest.
Presented at the 77th annual meeting of the South Atlantic Association of Obstetricians and Gynecologists, Asheville, NC, Jan. 18-21, 2015.
References
- 1.Unterscheider J, Daly S, Geary MP, et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO Study. Am J Obstet Gynecol. 2013;208:290.e1–6. doi: 10.1016/j.ajog.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Getahun D, Ananth CV, Kinzler WL. Risk factors for antepartum and intrapartum stillbirth: a population-based study. Am J Obstet Gynecol. 2007;196:499–507. doi: 10.1016/j.ajog.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49:270–83. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 4.McIntire D, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–8. doi: 10.1056/NEJM199904223401603. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan S, Beydoun H, Chang E, et al. Prenatal detection of fetal growth restriction in newborns classified as small for gestational age: correlates and risk of neonatal morbidity. Am J Perinatol. 2014;31:187–94. doi: 10.1055/s-0033-1343771. [DOI] [PubMed] [Google Scholar]
- 6.Kayem G, Grangé G, Bréart G, Goffinet F. Comparison of fundal height measurement and sonographically measured fetal abdominal circumference in the prediction of high and low birth weight at term. Ultrasound Obstet Gynecol. 2009;34:566–71. doi: 10.1002/uog.6378. [DOI] [PubMed] [Google Scholar]
- 7.Warsof S, Cooper DJ, Little D, Campbell S. Routine ultrasound screening for antenatal detection of intrauterine growth retardation. Obstet Gynecol. 1986;67:33. [PubMed] [Google Scholar]
- 8.Kean LH, Liu DT. Antenatal care as a screening tool for the detection of small for gestational age babies in the low risk population. J Obstet Gynaecol. 1996;16:77–82. [Google Scholar]
- 9.Martin J, Hamilton BE, Ventura SJ, Osterman MJ, Wilson EC, Mathews TJ. Births: final data for 2010. Natl Vital Stat Rep. 2012;60:1–100. [PubMed] [Google Scholar]
- 10.Gordon M, Rich H, Deutschberger J, Green M. The immediate and long-term outcome of obstetric birth trauma. Am J Obstet Gynecol. 1973;117:51–6. doi: 10.1016/0002-9378(73)90727-8. [DOI] [PubMed] [Google Scholar]
- 11.Chauhan SP, Hendrix NW, Magann EF, Morrison JC, Kenney SP, Devoe LD. Limitations of clinical and sonographic estimates of birth weight: experience with 1034 parturients. Obstet Gynecol. 1998;91:72–7. doi: 10.1016/s0029-7844(97)00590-5. [DOI] [PubMed] [Google Scholar]
- 12.Sparks TN, Cheng YW, McLaughlin B, Esakoff TF, Caughey AB. Fundal height: a useful screening tool for fetal growth? J Matern Fetal Neonatal Med. 2011;24:708–12. doi: 10.3109/14767058.2010.516285. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor C, Stuart B, Fitzpatrick C, Turner MJ, Kennelly MM. A review of contemporary modalities for identifying abnormal fetal growth. J Obstet Gynaecol. 2013;33:239–45. doi: 10.3109/01443615.2012.753423. [DOI] [PubMed] [Google Scholar]
- 14.Goetzinger KR, Tuuli MG, Odibo AO, Roehl KA, Macones GA, Cahill AG. Screening for fetal growth disorders by clinical exam in the era of obesity. J Perinatol. 2013;33:352–7. doi: 10.1038/jp.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert Pter J, Ho JJ, Valliapan J, Sivasangari S. Symphysial hundal height measurement in pregnancy for detecting abnormal fetal growth. Cochrane Database Syst Rev. 2012;7 doi: 10.1002/14651858.CD008136.pub2. CD008136. [DOI] [PubMed] [Google Scholar]
- 16.Persson B, Stangenberg M, Lunell NO, Brodin U, Holmberg NG, Vaclavinkova V. Prediction of size of infants at birth by measurement of symphysis fundus height. Br J Obstet Gynaecol. 1986;93:206. doi: 10.1111/j.1471-0528.1986.tb07894.x. [DOI] [PubMed] [Google Scholar]
- 17.Harding K, Evans S, Newnham J. Screening for the small fetus: a study of the relative efficacies of ultrasound biometry and symphysiofundal height. Aust N Z J Obstet Gynaecol. 1995;35:142–50. doi: 10.1111/j.1479-828x.1995.tb01859.x. [DOI] [PubMed] [Google Scholar]
- 18.Smith GC, Smith MF, McNay MB, Fleming JE. The relation between fetal abdominal circumference and birth weight: findings in 3512 pregnancies. Br J Obstet Gynaecol. 1997;104:186–90. doi: 10.1111/j.1471-0528.1997.tb11042.x. [DOI] [PubMed] [Google Scholar]
- 19.Niknafs P, Sibbald J. Accuracy of single ultrasound parameters in detection of fetal growth restriction. Am J Perinatal. 2001;18:325–34. doi: 10.1055/s-2001-17856. [DOI] [PubMed] [Google Scholar]
- 20.Chang TC, Robson SC, Boys RJ, Spencer JA. Prediction of the small for gestational age infant: which ultrasonic measurement is best? Obstet Gynecol. 1992;80:1030. [PubMed] [Google Scholar]
- 21.Chauhan S, Cole J, Sanderson M, Magann EF, Scardo JA. Suspicion of intrauterine growth restriction: use of abdominal circumference alone or estimated fetal weight below 10% J Matern Fetal Neonatal Med. 2006;19:557–62. doi: 10.1080/14767050600798267. [DOI] [PubMed] [Google Scholar]
- 22.Bossuyt PM, Reitsma JB, Bruns DE, et al. Standards for reporting of diagnostic accuracy. Toward complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Ann Intern Med. 2003;138:40–4. doi: 10.7326/0003-4819-138-1-200301070-00010. [DOI] [PubMed] [Google Scholar]
- 23.Hecher K, Snijders R, Campbell S, Nicolaides K. Fetal venous, intracardiac, and arterial blood flow measurements in intrauterine growth retardation: relationship with fetal blood gases. Am J Obstet Gynecol. 1995;173:10. doi: 10.1016/0002-9378(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 24.Turitz AL, Quant H, Schwartz N, Elovitz M, Bastek JA. Isolated abominal circumference <5% or estimated fetal weight 10-19% as predictors of small of gestational age infants. Am J Perinatol. 2014;31:469–76. doi: 10.1055/s-0033-1353438. [DOI] [PubMed] [Google Scholar]
- 25.Hadlock FP, Harrist RB, Sharman RS, et al. Estimation of fetal weight with the use of head, body, and femur measurements–a prospective study. Am J Obstet Gynecol. 1985;151:333. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 26.Olsen I, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:214–24. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 27.Hawass NED. Comparing the sensitivities and specificities of two diagnostic procedures performed on the same group of patients. Br J Radiol. 1997;70:360–6. doi: 10.1259/bjr.70.832.9166071. [DOI] [PubMed] [Google Scholar]
- 28.McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12:153–7. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]
- 29.Figueras F, Gardosi J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstst Gynecol. 2011;204:288–300. doi: 10.1016/j.ajog.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 30.Skråstad RB, Eik-Nes SH, Sviggum O, et al. A randomized controlled trial of third-trimester routine ultrasound in a non-selected population. Acta Obstet Gynecol Scand. 2013;92:1353–60. doi: 10.1111/aogs.12249. [DOI] [PubMed] [Google Scholar]
- 31.Sylvan K, Ryding EL, Rydhstroem H. Routine ultrasound screening in the third trimester: a population-based study. Acta Obstet Gynecol Scand. 2005;84:1154–8. doi: 10.1111/j.0001-6349.2005.00649.x. [DOI] [PubMed] [Google Scholar]


