Summary
Radical cystectomy is a standard treatment for non-metastatic, muscle-invasive bladder cancer. Treatment with trimodality therapy consisting of maximal transurethral resection of the bladder tumor (TURBT) followed by concurrent chemotherapy and radiation has emerged as a method to preserve the native bladder in highly motivated patients. A number of factors can impact the likelihood of long term bladder preservation after trimodality therapy, and therefore should be taken into account when selecting patients. New radiation techniques such as intensity modulated radiation therapy and image guided radiation therapy may decrease the toxicity of radiotherapy in this setting, but remain an area of active study. Novel chemotherapy regimens may improve response rates and minimize toxicity.
Keywords: radiation, bladder preservation, muscle-invasive bladder cancer, chemoradiation, urothelial carcinoma of the bladder
Introduction
An estimated 74,690 cases of urinary bladder cancer will be diagnosed in the United States in 2014, (1) of which 30% will be muscle-invasive. The current standard of care for the treatment of muscle-invasive bladder cancer (MIBC) is neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy (RC) with pelvic lymph node dissection. (2) In appropriately selected patients, bladder preservation can be an effective alternative to RC. The term “bladder preservation” can include trans-urethral resection of the bladder tumor (TURBT), limited surgery, chemotherapy, radiation therapy, or various combinations of one or more of these modalities; however, the best outcomes have consistently been seen with trimodality therapy (TMT) including maximal TURBT followed by concurrent chemoradiation. This review will focus on TMT for bladder preservation and will not detail other therapeutic combinations for bladder preservation.
A number of prospective trials have been completed evaluating TMT as a means of bladder preservation. The purpose of these studies has been to define the rate of bladder preservation and survival with this approach and to improve the tolerability and efficacy of TMT regimens. The aim of this review is to provide an overview of modern TMT bladder-preservation strategies, focusing on important criteria for patient selection, the integration of novel radiation techniques, commonly used and new chemotherapies for TMT, and the role of chemoradiation for T1 disease.
Discussion
Trimodality Therapy Treatment Approach
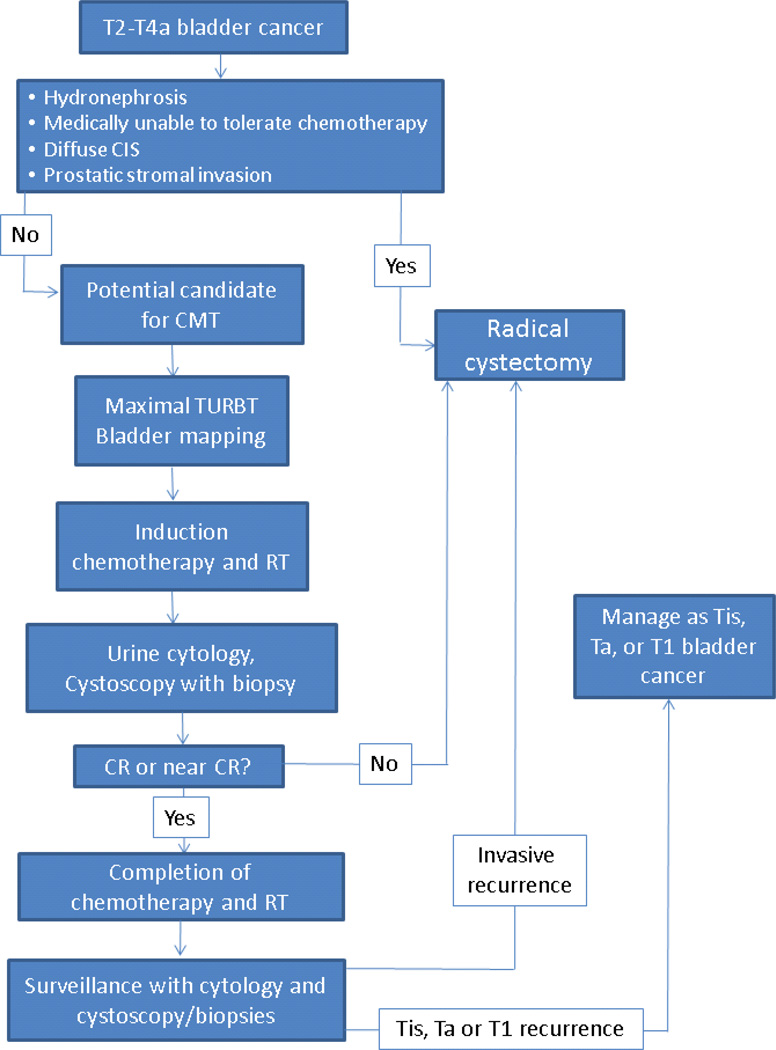
TMT includes the combination of maximal tumor debulking and concurrent chemoradiotherapy. The optimal radiation target volume, radiation fractionation, chemotherapy, and sequencing remain areas of active study. In general, the patient undergoes a maximal, preferably visually complete TURBT, ideally with bladder mapping (Figure 1), followed by the delivery of cisplatin-based chemoradiotherapy to a dose of approximately 40–45Gy. If no evidence of disease or minimal residual disease is noted at cystoscopic reassessment, the final consolidative phase of chemoradiotherapy is initiated. If progressive or unresponsive disease is found, the patient proceeds to radical cystectomy. After completion of therapy, patients are closely surveilled with cystoscopy and urine cytology.
Figure 1. Sequencing of trimodality therapy for bladder preservation.
Patients undergoing TMT for bladder preservation undergo a maximal TURBT followed by induction chemoradiation. Patients with a CR to induction therapy proceed to consolidative chemoradiation, whereas evidence of progression results in immediate cystectomy. Following therapy, a strict schedule of surveillance is undertaken. Evidence of invasive recurrence is treated with cystectomy. Non-invasive recurrences are managed with TURBT and intravesicle therapy.
Patient selection
Patient selection is a key component to bladder preservation (Table 1). Most criteria used to select appropriate patients for TMT predict for a high rate of response or the ability to safely tolerate therapy. Factors predicting for increased rates of distant metastases are important for predicting overall survival (OS) after TMT.
Table 1.
Patient Selection For Bladder Preservation
| Preferred or Ideal | Less than Ideal | Relative Contraindications |
Absolute Contraindications |
|---|---|---|---|
|
|
|
|
A complete response (CR) to induction therapy with concurrent chemoradiation has typically been defined as negative urine cytology as well as no visible tumor and negative biopsies at cystoscopy. Achieving a CR to induction therapy is required to avoid salvage cystectomy and has been associated with improved disease-free and OS after TMT. The CR rate for patients with T2-T4a disease treated with TMT is approximately 70%. (3) Factors that may impact the likelihood of achieving a CR after TMT and should be considered when selecting patients include completeness of TURBT, tumor stage, hydronephrosis, multifocality and CIS, and baseline bladder function. It is important to consider that the rate of response to induction therapy may not always be known, as many recent trials, such as BC2001 and RTOG 0926, do not include cystoscopic reassessment after induction. For these studies, careful patient selection becomes increasingly important as a full radiotherapy dose will be delivered before response to therapy is assessed.
Completeness of TURBT
A recent pooled analysis of 314 patients treated on six RTOG trials found that a visibly complete TURBT was associated with a significantly higher rate of CR to TMT on multivariate analaysis. (3) Similarly, the Erlangen series showed that completeness of resection after initial TURBT was an independent predictor of CR. (4) Likewise, a series of 348 patients from Massachusetts General Hospital found that visibly complete TURBT was associated with higher CR rates (79% with visibly complete TURBT vs 57% without). (5) Thus, a visibly complete TURBT is ideal. A less than complete TURBT is not an absolute contraindication to s bladder preservation as several trials have demonstrated acceptable CR rates without a visibly complete TURBT.
Tumor Stage
Most TMT trials include patients with clinical T2-T4a disease. In RTOG 85-12, RTOG 88-02, RTOG 97-06, and the Erlangen series, tumor stage was not significantly associated with the rate of CR to TMT on multivariate analysis. (4, 6–8) A pooled analysis of 361 patients treated on RTOG trials confirmed that T stage did not predict for the likelihood of CR to TMT on multivariate analysis. (3) In contrast, increasing T stage is reproducibly associated with reduced long term survival after TMT. (4, 5, 9)
In surgical series, the presence of prostate invasion by urothelial carcinoma is associated with a higher risk of lymph node metastases and reduced 5 year survival. (10) The decrement in survival is greatest for patients with prostatic stromal invasion or extraprostatic invasion compared to patients with more limited mucosal involvement. Patients with prostatic stromal invasion are excluded from many trials of TMT for bladder preservation, however prostatic urethral invasion is not generally an exclusion criteria if it is amenable to visibly complete resection.
Little data exists regarding the treatment of patients with involved lymph nodes with TMT. These patients have in some cases been included in RTOG trials of TMT if the lymph nodes are located below the bifurcation of the iliac vessels. The presence of lymph node involvement is a poor prognostic indicator in regards to OS, and in general these patients are counseled to undergo neoadjuvant chemotherapy and RC.
Hydronephrosis
Tumor-related hydronephrosis has been an exclusion criteria for several trials of TMT. RTOG 89-03 found CR rates with and without hydronephrosis to be 38% vs 64%, respectively. A series from the Massachusetts General Hospital found a CR rate of 52% in those with hydronephrosis and 77% in those without. (11) Importantly, hydronephrosis not only predicts for a reduced likelihood of CR, but is also a predictor for advanced stage and decreased survival. In RTOG 89-03, the 5 year OS for patients with and without hydronephrosis was 33% vs 54%, respectively. (12) Likewise, in RTOG 88-02, hydronephrosis was the only analyzed factor to be significantly associated with probability of distant metastases and death. (7) Patients with tumor-related hydronephrosis are poor candidates for bladder preservation and are usually excluded from trials of TMT.
Multifocality and carcinoma in situ
Multiple tumors or multifocal disease has been suggested as a predictive factor for decreased response rates to TMT, and these patients are excluded from most trials of bladder preservation. Multifocality may not predict for lower rates of CR but is associated with a higher risk for local relapse. (4) In general, TMT is not advocated in those with diffuse multifocal disease.
Similarly, the presence of extensive CIS prior to therapy has been associated with lower rates of CR to TMT and radiotherapy alone, and higher rates of recurrence after TMT. (13–16) A panel convened by the Société Internationale d’Urologie suggested that the presence of extensive CIS should be considered a relative and not absolute contraindication to TMT because the presence of CIS effects only the risk of recurrence after TMT and not survival. (17)
Baseline Bladder Function
The rationale of bladder preservation therapy is to preserve a functional bladder. A subset of patients who preserve their bladders with TMT may develop symptoms such as urgency and control problems. (18) Therefore, baseline dysfunction in these areas should be considered when determining if a patient is a candidate for TMT.
Chemotherapy for TMT
Concurrent chemotherapy has been shown in randomized trials to improve local and regional control compared to RT alone. (19, 20) Although these trials did not demonstrate an OS advantage with the addition of concurrent chemotherapy, several large retrospective series have found concurrent chemotherapy to be associated with improved survival. (9, 21) The majority of bladder preservation trials utilizing concurrent chemoradiation have used cisplatin-based chemotherapy regimens. Cisplatin based combination regimens have been tested with the aim of improving response rates. The RTOG has evaluated cisplatin based chemotherapy combinations including cisplatin with 5-fluorouracil (5-FU) (RTOG 9506) and paclitaxel with cisplatin (RTOG 9906). (22, 23) The recently published RTOG 0233 trial compared paclitaxel with cisplatin to 5-FU with cisplatin with concurrent radiation in patients with mostly T2 disease (95%). (24) Following TMT patients received adjuvant gemcitabine, cisplatin, and paclitaxel. Both regimens showed similar rates of CR (62%–72%), 5 year OS (71–75%), and 5 year survival with an intact bladder with moderate toxicity.
Candidates for TMT may have comorbidities that preclude the delivery of concurrent cisplatin, and a number of studies have evaluated alternative regimens. The BC2001 trial tested the use of 5-FU with mitomycin-C (MMC) concurrently with radiotherapy with excellent response rates and impressive tolerability. (19) This regimen can be particularly useful in those with renal dysfunction prohibiting the use of cisplatin. Gemcitabine is a potent radiation sensitizer and has shown activity in the setting of metastatic urothelial cancers. The use of 100 mg/m2 weekly gemcitabine during radiotherapy as a component of TMT was tested in a recently completed Phase II trial. (25) The regimen resulted in an 88% cystoscopic response rate, and a 3 year OS of 75%. Bowel toxicity resulted in four of 50 patients stopping chemotherapy, and in one late bowel resection. There were two treatment related deaths.
Single insititution Phase I data supporting twice weekly gemcitabine concurrent with radiotherapy as part of TMT is available. A study from the University of Michigan of TMT with radiotherapy delivered to a total dose of 60 Gy found the MTD of twice weekly gemcitabine to be 27 mg/m2. (26) Twenty one of the 23 patients treated on this trial obtained a CR. At a median follow up of 43 months, 65% of patients were alive with no evidence of recurrence and intact bladders. Twice-weekly low-dose gemcitabine (27 mg/m2) was recently compared to a regimen of twice daily radiation with 5-FU and cisplatin in the recently closed RTOG 0712 trial. Results for this trial are pending.
The target volume of radiation is an important consideration when comparing chemotherapy regimens for bladder cancer. Some trials testing alternative regimens have evaluated bladder only radiation and have not included an initial pelvic radiation field. (19) Thus, toxicity for these chemotherapy regimens may be greater if extrapolated to a setting where a pelvic field is included.
The use of neoadjuvant cisplatin based chemotherapy in the setting of cystectomy has shown improvements in survival compared to cystectomy alone, likely through early treatment of micrometastatic disease. (27, 28) As distant failure remains a concern after bladder preservation, this approach has been tested in several trials of TMT. Unfortunately, neoadjuvant or adjuvant chemotherapy with TMT has not improved survival or bladder preservation rates. However, the data available is limited to older regimes, with only two cycles of chemotherapy delivered instead of the standard three to four, and many of these studies were underpowered. As more effective chemotherapeutic regimens are developed, this strategy is likely to be further explored. (29)
Targeted agents have been an area of interest in efforts to improve TMT outcomes.
The Epidermal Growth Factor Receptor (EGFR) family of receptors has been of particular interest in this regard. HER2/Neu is overexpressed in bladder cancers, particularly in metastatic or LN positive tumors, (30) and overexpression may be correlated to worse outcomes after chemoradiotherapy. (31) These findings suggest that targeting HER2/Neu in the context of TMT may provide a therapeutic opportunity. RTOG 0524 evaluated the addition of trastuzumab to paclitaxel and daily radiation following TURBT in the treatment of non-cystectomy candidates with MIBC. (32) The study has been reported in abstract form and showed a favorable response rate but with an increase in hematologic toxicity and other adverse events.
Radiation techniques
Radiotherapy is a critical component of TMT. A range of doses of irradiation, fractionation schedules, sequences of treatment, and treatment volumes have been applied in the treatment of bladder cancer. Attempts to improve radiotherapy have been geared towards enhancing bladder preservation rates while minimizing the toxicity of therapy. Recently, newer technologies, such as intensity modulated radiotherapy (IMRT) and image guided radiotherapy (IGRT) are being integrated into the clinic. We will outline general concepts regarding radiotherapy field design and discuss current areas of research in radiotherapy technique.
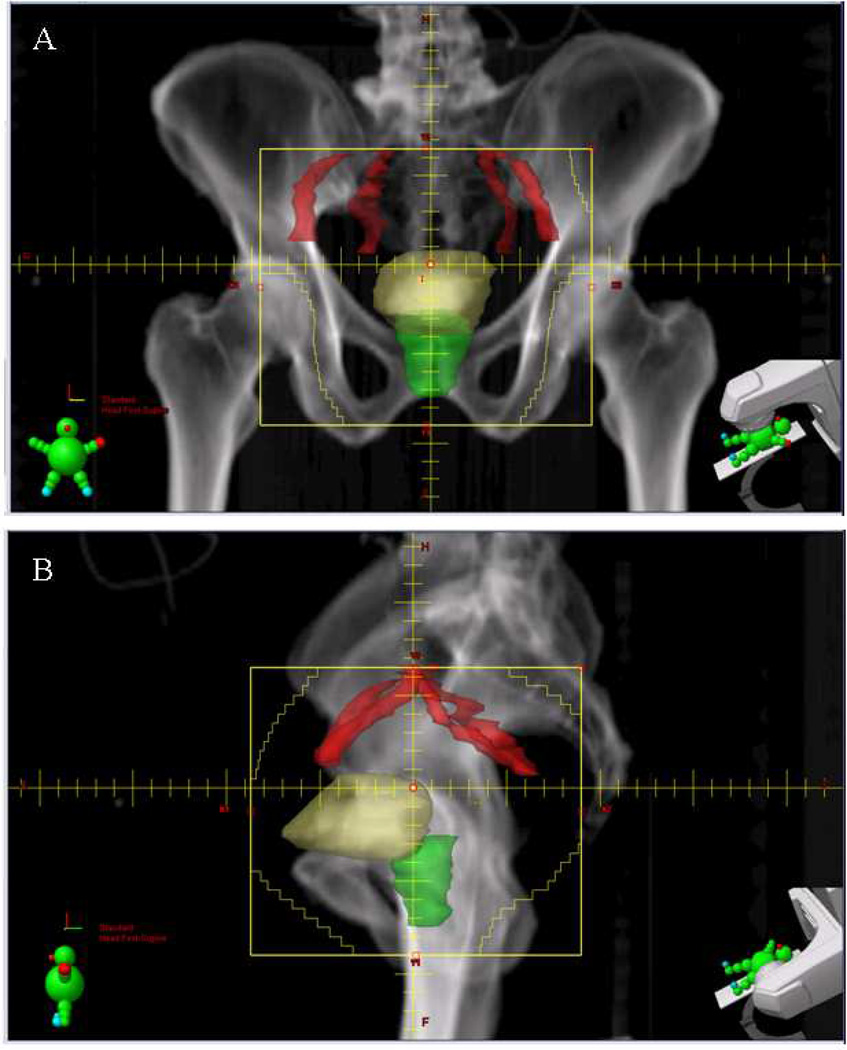
The optimal volume of irradiation is one area of controversy. In most North American Trials, treatment includes an initial course of radiotherapy to ta toal dose of 39.6–45 Gy directed at the pelvic lymph nodes below the bifurcation of the common iliac vessels, the prostate in males, and the whole bladder (Figure 2, 3). A margin surrounding the bladder is included to account for daily variation in bladder filling, visceral organ motion, and setup error. To minimize the field size and to increase the reproducibility of daily treatment, patients are simulated and treated with an empty bladder (immediately post-void) with a small amount of bladder contrast.
Figure 2. Pelvic radiation field for bladder preservation.
A) Anterior-to-posterior and B) left lateral field. The fields extend from the top of the bifurcation of the iliac vessels to the bottom of the pelvis. The bladder (yellow) and prostate (green) with margin are included in the target volume. Field edges are noted in yellow.
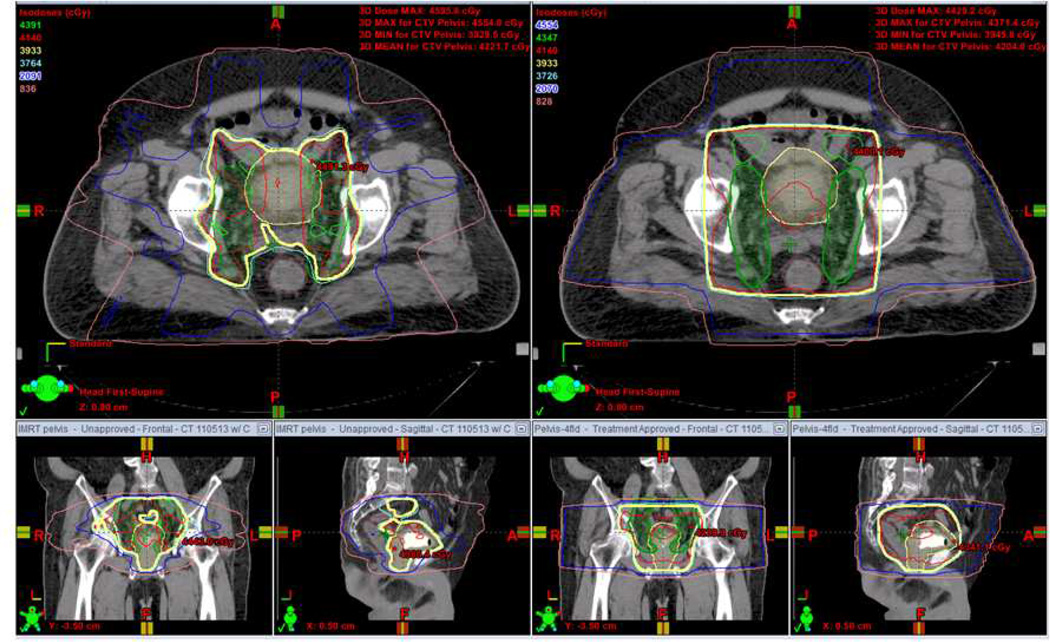
Figure 3. IMRT for bladder preservation.
A comparison of IMRT (left panels) and standard 4 field plan (right) for treatment of the pelvis as a component of bladder preservation. The bladder and prostate (shaded yellow) and lower pelvic lymph nodes (shaded green) are targeted. Radiation isodose levels are noted in the top left and are represented by the corresponding colored line. Note the superior sparing of the rectum and non-target tissues with the IMRT approach.
The rationale for including pelvic lymph nodes in the initial portion of the radiation treatment field relates to the relatively high rate of occult lymph node involvement in regions typically targeted with pelvic radiotherapy, (33) and the finding that extensive lymphadenectomy at the time of radical cystectomy improves survival, suggesting that treatment of these lymph nodes has therapeutic efficacy. (34) The rationale for not specifically targeting pelvic lymph nodes includes improving tolerability of therapy by excluding more normal tissue from the treatment volume and the low rate of nodal failure when not specifically targeted. A small, single institution trial including patients with T2-T4N0 disease treated with maximal TURBT followed by chemoradiation with weekly cisplatin randomized patients between whole pelvis RT vs. bladder only RT. (35) At a median follow up of 5 years, no difference in 5 year disease-free survival, bladder preservation rates, regional nodal failure rates, or 5 year OS was observed. In the randomized BC2001 trial comparing radiation alone to radiation with chemotherapy, pelvic lymph nodes were not specifically targeted, and pelvic relapses were seen in only 5.8% of patients, suggesting that treatment of the pelvic lymph nodes may not be necessary. (19) Although the authors concluded that bladder alone RT was as effective as whole pelvis RT with less toxicity in their trial, a randomized trial comparing these techniques directly is warranted.
If the pelvic lymph nodes are irradiated, one or two reductions in the size of the fields are performed after the pelvic nodal portion of the treatment. A further area of controversy and ongoing evolution in treatment practices in the volume included in the reduced treatment field, which may include the entire bladder for all or part of the treatment, or only the tumor with margin. The rationale for using a tumor only boost is the ability to reduce the volume of bladder receiving the higher doses of radiation, thus potentially reducing long term urinary and gastrointestinal toxicity. Although the most common approach is to treat only the residual tumor/tumor bed to the high dose of radiation (~64Gy) with partial bladder irradiation after delivery of approximately 54Gy to the whole bladder, this can be challenging for several reasons. For one, after complete TURBT it can be difficult to know exactly where the pre-TURBT tumor was located despite using the operative-report and bladder mapping. Additionally, targeting the region accurately can be difficult on a day-to-day basis as the bladder can have significant inter- and intra-fraction movement due to differences in bladder filling, rectal volume changes, and other variations in organ motion. (36, 37) If a tumor bed boost technique is employed, accurate tumor delineation and accurate treatment delivery are critical.
Data supporting a more limited boost include the BC2001 trial, in which 219 of the patients were randomized to receive full dose radiation to the bladder alone vs. 80% of the dose to the bladder with full dose to the bladder tumor/tumor bed. (19) There was no significant difference in toxicity or local control, however they concluded noninferiority of locoregional control could not be concluded formally. Additional trials have demonstrated comparable control rates with radiotherapy directed at the tumor as opposed to the whole bladder, suggesting that this approach does not compromise tumor control. (38, 39)
New Radiation Techniques
Recent advances in radiotherapy delivery have included the development of IMRT, in which the beam is modulated over the course of the treatment, resulting in improved conformality and a reduction in the amount of normal tissues exposed to higher doses. This technique has been adopted in a wide range of tumor types as a method to improve the conformality of treatment and reduce toxicity, and there have been some reports of the use of IMRT for the treatment of bladder cancer. (40, 41) Although a reduction in toxicity that may be afforded by IMRT is welcome, one of the concerns using IMRT is the possibility of marginal misses in regions where there can be considerable organ/target motion. Thus, inclusion of advanced imaging techniques for daily radiation treatment set up is encouraged if IMRT is employed.
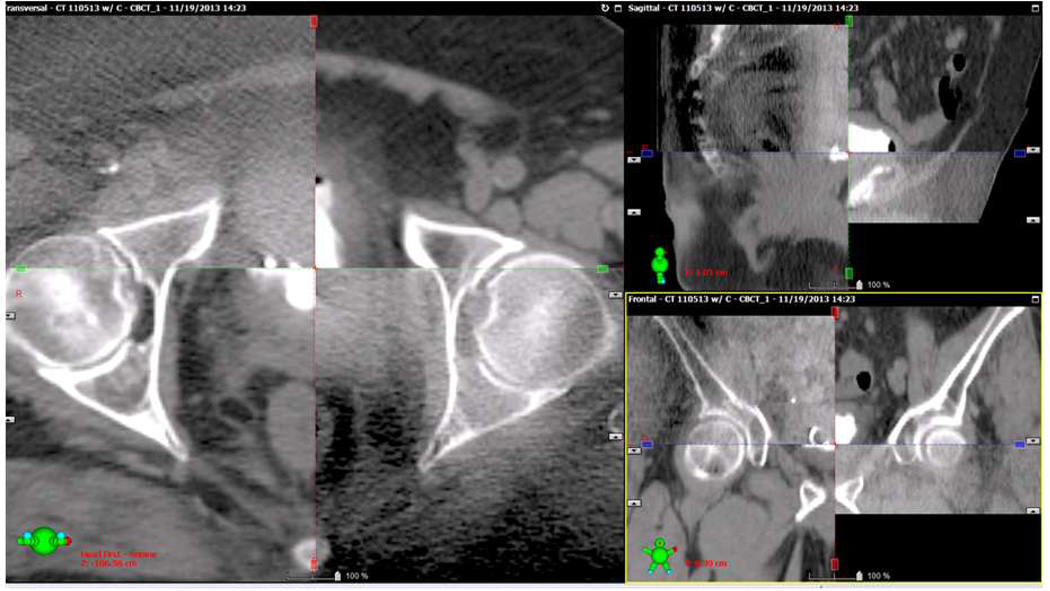
The ability to accurately define the high risk region for a whole bladder and partial bladder boost on a daily basis may improve normal tissue sparing by allowing a reduction in the additional margin of normal tissues that must be included to account for set up error. The use of frequent 2-dimensional (D) or 3D imaging to align patients for daily treatment is known as image guided radiation therapy (IGRT). IGRT can be accomplished with daily in-room CT scanning (on-board imaging with cone beam CT, Figure 4) or daily imaging of fiducial markers implanted within or near the target volume (Figure 3). Although fiducials have not been widely used in the treatment of bladder cancer, Garcia et al. recently published their experience with fiducial marker placement in the bladder wall fro daily localization. In this small series, fiducial marker placement was feasible and associated with excellent retention and no complications. (42) Alternatives to fiducials, such as lipiodol injections in the bladder wall have also been employed as a fiducial to assist with accurate daily alignment. (43)
Figure 4. Cone beam CT fused with a treatment planning image.
A cone beam CT was obtained on a patient receiving TMT to verify alignment prior to radiation treatment. In each panel, the cone beam CT image is in the upper left and bottom right portion of the image, while the planning CT image is in the upper right and lower left portion. Note that cone beam CT image quality is inferior to the diagnostic image. Overlay of the images allows comparison of the anatomy between scans to verify alignment of bony and soft tissue structures. Transverse, sagittal, and coronal reconstructions are presented.
Outcomes of Trimodality therapy
Although no randomized trials compare radical cystectomy to bladder preservation in patients with MIBC, many trials, prospective and retrospective, show outcomes similar to those obtained after radical cystectomy in regards to OS (Table 2). Following maximal TURBT, definitive treatment with concurrent chemoradiation leads to a CR in approximately 70% of patients. (3) The five year OS and disease specific survival with TMT in a series of prospective RTOG trials was 57% and 71%, respectively. (3) In patients alive at 5 years after TMT, approximately 80% will preserve an intact bladder. (3)
Table 2.
Prospective Trails of Bladder Preservation
| Trial | Phase/Design | Stage | Number | Radiation (Gy) | Neoadjuvant Chemotherapy |
Concurrent Chemotherapy |
Adjuvant Chemotherapy |
Complete Response |
Bladder intact survival |
5-yr Overall Survival |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BC2001 | III | T2–T4a | 360 | 55 of 64 | 5FU and Mitomycin | 48% | James, et al (19) |
||||
| RTOG 02–33 | Randomized phase II |
T2–4a | 93 | 40.3 + 24 | Paclitaxel/Cisplatin 5FU/Cisplatin |
Cisplatin gemcitabine paclitaxel x 4 |
72% Pac 62% 5FU |
67% paclitaxel 71% 5FU |
71% paclitaxel 75% 5FU |
Mitin, et al (24) |
|
| RTOG 99-06 | I/II | T2–T4a | 80 | 40.3 + 24 | Cisplatin + Paclitaxel | GC x 4 | 81 | 47% | 56% | Kaufman et al (23) |
|
| RTOG 97-06 | I/II | T2–T4a | 46 | 40.8 + 24 | Cisplatin | MCV x 3 | 74 | 47 (3 yr) | 61 (3 yr) | Hagan et al (6) |
|
| RTOG 95-06 | I/II | T2–T4a | 34 | 24 + 40 | Cisplatin + 5-FU | 67 | 66 (3 yr) | 83 (3 yr) | Kaufman et al. (22) |
||
| RTOG 89-03 | III | T2–T4a | 123 | 39.6 + 25.2 | MCV x 2 None |
Cisplatin | 61 51 |
36 40 |
49 48 |
Shipley et al (12) |
|
| RTOG 88-02 | II | T2–T4a | 91 | 39.6 + 25.2 | MCV x 2 | Cisplatin | 75 | 44 (4 yr) | 62 (4 yr) | Tester et al (7) |
|
| RTOG 85-12 | II | T2–T4 | 42 | 40 + 24 | Cisplatin | 66 | 52 | Tester et al (25) |
|||
| Erlangan | N/A | T1–T4 | 415 (89 T1) |
Median 54 (45–69.4) |
Various: cisplatin, carboplatin, cisplatin + 5-FU |
72 | 42 | 51% | Rodel et al (4) |
||
| MGH | N/A | T2–T4a | 106 | 39.6 + 25.2 | MCV x 2 | Cisplatin | 66 | 43 | 52 | Kachnic et al (11) |
|
| Paris | N/A | T2–T4 | 54 | 24 +20 | Cisplatin + 5-FU | 74 | 59 (3 yr) | Housset et al (53) |
|||
| Italy | N/A | T2–T4 | 121 | Median 65 (34–57) |
MCV x 2 | Cisplatin or carboplatin | 86 | 51 | 68 | Perdona et al (9) |
An important consideration is the ability of TMT to preserve a functional bladder. Although data are limited, single insitition studies suggest normal bladder function in 75% of conserved bladders as assessed by questionnaire and urodynamic studies. (18) A series of 112 patients treated with TMT-based bladder preservation reported that 79% of survivors with an intact bladder were “delighted” or “pleased” with urinary function. (44) The need for cystectomy to deal with complications of radiotherapy is uncommon, and ranges from 0–2% in several series, (3, 4, 44, 45) although bladder contracture not requiring cystectomy is reported in a subset of patients.
Other possible complications of TMT include gastrointestinal complaints and sexual dysfunction. The rate of these complications varies greatly by study, largely due to the method of collection of data. A review of late toxicity in 157 patients treated on RTOG trials who survived at least two years from the intiation of treatment found RTOG/EORTC Grade 3 late genitourinary toxicity in 5.7% and RTOG/EORTC Grade 3 late gastrointestinal toxicity in 1.9%. (46) Importantly, no Grade 4 or 5 toxicities were reported in this series. Single institution series using patient questionnaires have reported gastrointestinal complaints of any severity (10–32% of patients) and sexual dysfunction (8–38%) in a greater number of patients (18, 47, 48)
Post-therapy surveillance remains a critical component of bladder preservation with TMT. Approximately 60% of recurrences after TMT will be CIS and will arise at the site of the original invasive tumor. (16) The risk of non-invasive failure is higher in patients with a component of CIS in the original tumor. Conservative management of these patients, with TURBT and intravesicle therapy is recommended. Invasive recurrence is managed with salvage cystectomy.
Special Considerations
Response after induction
The presence of a CR to induction TMT is associated with an excellent long-term likelihood of bladder preservation. Importantly, obtaining less than a CR after induction therapy and undergoing immediate RC does not impact OS compared to those who require RC for late recurrences after achieving a CR. There is data to support that less than a CR to a complete course of TMT is associated with worse outcomes despite immediate RC, suggesting that reassessment after about 40Gy is important to detect non-responders. (4, 29)
Both RTOG 99-06 and RTOG 02-33 allowed patients with a “near-complete response,” including Tis and Ta, at the time of assessment following induction therapy to continue with definitive chemoradiation. (23, 24) Among 119 patients in these trials, 85% achieved a T0 “complete response” and 15% achieved a Ta or Tis (near-complete response). Although only presented in abstract form, (49) with a median follow up 5.9 years, 36% of T0 patients versus 28% of Ta or Tis patients experienced a bladder tumor recurrence (non-invasive and muscle-invasive). Fourteen patients among 101 complete responders eventually required RC for salvage, in comparison to one patient among 18 near-complete responders (p=0.47). There was no difference in disease-specific, bladder-intact, or OS. The authors concluded that it is appropriate to recommend that patients with Ta or Tis after induction TMT continue with bladder preservation. The numbers of patients included in this series are small, and confirmation will be useful.
TMT for refractory/recurrent T1 disease
Bladder-preservation trials have focused on patients with MIBC, thus, data supporting TMT for T1 disease (lamina propria invasion) is limited. Treatment of T1 tumors typically involves conservative therapy with TURBT followed by intravesical BCG, with RC reserved for recurrent or refractory disease. Recent data indicates that concurrent chemoradiation may be an effective alternative to RC in patients with recurrent high grade T1 tumors after failure of BCG treatment. (50, 51)
One argument supporting the use of TMT for patients with refractory or recurrent high grade T1 bladder tumors is the high rate of clinical-pathologic stage discrepancy. As many as 46% of patients with T1 disease who undergo RC will be upstaged pathologically at surgery. (34) Given the high risk of occult MIBC in these patients, it has been suggested that more aggressive treatment with TMT may be warranted to avoid possible undertreatment.
The University of Erlangen reported their experience in 141 patients with high risk T1 disease treated with bladder preservation as the initial treatment (80% received concurrent chemoradiation). (50) Sixty percent had T1 Grade 3 (T1G3) disease; the others were high risk due to multifocal disease or CIS. Overall, there was an 88% CR rate and a progression rate of 19%. Of the patients with T1G3 tumors, the 10 year progression-free rate of the bladder tumor was 71% and 10 year disease-specific survival was 70%. This compares favorably to most contemporary series of T1G3 patients treated with TURBT and BCG.
A mutlicenter trial conducted in the United Kingdom evaluated whole bladder radiation versus conservative therapy (observation or BCG depending on risk factors) after visibly complete TURBT in patients with previously untreated T1G3 bladder cancer to determine the efficacy of radiation in reducing the incidence of progression to MIBC. (52) There was no evidence of an advantage to the addition of radiation alone (no chemotherapy) in progression-free interval, progression-free survival, or OS. Importantly, a complete TURBT was not required prior to radiation therapy, which may explain higher than expected rates of recurrence. Although this trial suggested that RT alone may not be better than the current standard of care, the role of RT alone or concurrent with chemotherapy for recurrent disease after failure of BCG is undefined.
As previously noted, a wealth of data including two randomized trials demonstrate significantly better outcomes with the addition of radiosensitizing concurrent chemotherapy in the setting of bladder preservation in patients with MIBC. The current RTOG 0926 phase II trial is evaluating the role of bladder preservation with concurrent chemoradiation (cisplatin or 5-FU with MMC with 61.2Gy) in operable patients with T1G3 disease in whom RC is the next conventional step in therapy.
Future directions
Bladder preservation with trimodality therapy continues to evolve with refinements in radiation techniques and chemotherapy delivery. Improved ability to visualize and target the bladder tumor promises to minimize the margin of normal tissue treated, and as a result, to minimize toxicity. Novel targeted agents are being explored to reduce the risk of distant failure and to enhance the local effects of radiotherapy. Enrollment of patients interested in TMT bladder preservation on clinical trials is important.
Patient selection for bladder preservation has to this point largely been accomplished by reviewing patient and tumor related factors that predict for outcome after TMT. Recently, a number of prognostic and predictive biomarkers have been described that may allow improved pateint selection and avoidance of TMT in patients at high risk of bladder tumor recurrence. (31, 53) Several markers have been explored in this context, however the majority of markers studies are most predictive of distant metastases and cause specific survival, suggesting that they may have prognostic, not predictive efficacy. (31, 53) One notable exception is expression of Mre11, which is predictive for cause specific survival after radical radiotherapy. (54) The identification of such markers may allow selection of therapy most appropriate based on rates of expected control with each treatment option.
Key Points.
Bladder preservation with maximal TURBT, concurrent chemotherapy, and radiation can result in approximately 75% of long-term survivors maintaining a functional bladder.
The ideal patient for bladder preservation has a clinical T2 unifocal tumor, a visibly complete TURBT, no carcinoma in situ (CIS), no tumor-related hydronephrosis, with good pre-treatment bladder function.
Participation in a bladder preservation approach requires a highly motivated patient that is a good candidate for radiation and chemotherapy and is committed to long-term cystoscopic surveillance.
Acknowledgement
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication., As a service to our customers we are providing this early version of the manuscript., The manuscript will undergo copyediting, typesetting, and review of the resulting proof, before it is published in its final citable form. Please note that during the production, process errors may be discovered which could affect the content, and all legal disclaimers, that apply to the journal pertain.
Disclosures and Conflicts of Interests: The authors have no conflicts of interests or relationships to disclose.
Contributor Information
Christopher Premo, Radiation Oncology Branch, Center for Cancer Research, National Cancer Institute, 10 CRC, B2-3500, Bethesda, MD 20892, Christopher.Premo@nih.gov, Phone: (301) 496-5457, Fax (301) 480-5439.
Andrea B. Apolo, Bladder Cancer Section, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, 10 Center Dr.12N226, MSC 1906, Bethesda, MD 20892, Tel: 301-451-1984, Fax: 301-402-0172, andrea.apolo@nih.gov.
Piyush K. Agarwal, Bladder Cancer Section, Urologic Oncology Branch, National Cancer Institute, NIH, Building 10, Room 2W-5940, Bethesda, MD 20892-1210, Office: 301-496-6353, Fax: 301-480-5626, piyush.agarwal@nih.gov.
Deborah Citrin, Radiation Oncology Branch, Center for Cancer Research, National Cancer Institute, 10 CRC, B2-3500, Bethesda, MD 20892, citrind@mail.nih.gov, Phone: (301) 496-5457, Fax (301) 480-5439.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, et al. Bladder cancer. J Natl Compr Canc Netw. 2013;11:446–475. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 3.Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of radiation therapy oncology group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014;32:3801–3809. doi: 10.1200/JCO.2014.57.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodel C, Grabenbauer GG, Kuhn R, Papadopoulos T, Dunst J, Meyer M, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol. 2002;20:3061–3071. doi: 10.1200/JCO.2002.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Efstathiou JA, Spiegel DY, Shipley WU, Heney NM, Kaufman DS, Niemierko A, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur Urol. 2012;61:705–711. doi: 10.1016/j.eururo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Hagan MP, Winter KA, Kaufman DS, Wajsman Z, Zietman AL, Heney NM, et al. RTOG 97-06: initial report of a phase I-II trial of selective bladder conservation using TURBT, twice-daily accelerated irradiation sensitized with cisplatin, and adjuvant MCV combination chemotherapy. Int J Radiat Oncol Biol Phys. 2003;57:665–672. doi: 10.1016/s0360-3016(03)00718-1. [DOI] [PubMed] [Google Scholar]
- 7.Tester W, Caplan R, Heaney J, Venner P, Whittington R, Byhardt R, et al. Neoadjuvant combined modality program with selective organ preservation for invasive bladder cancer: results of Radiation Therapy Oncology Group phase II trial 8802. J Clin Oncol. 1996;14:119–126. doi: 10.1200/JCO.1996.14.1.119. [DOI] [PubMed] [Google Scholar]
- 8.Tester W, Porter A, Asbell S, Coughlin C, Heaney J, Krall J, et al. Combined modality program with possible organ preservation for invasive bladder carcinoma: results of RTOG protocol 85-12. Int J Radiat Oncol Biol Phys. 1993;25:783–790. doi: 10.1016/0360-3016(93)90306-g. [DOI] [PubMed] [Google Scholar]
- 9.Perdona S, Autorino R, Damiano R, De Sio M, Morrica B, Gallo L, et al. Bladder-sparing, combined-modality approach for muscle-invasive bladder cancer: a multi-institutional, long-term experience. Cancer. 2008;112:75–83. doi: 10.1002/cncr.23137. [DOI] [PubMed] [Google Scholar]
- 10.Shen SS, Lerner SP, Muezzinoglu B, Truong LD, Amiel G, Wheeler TM. Prostatic involvement by transitional cell carcinoma in patients with bladder cancer and its prognostic significance. Hum Pathol. 2006;37:726–734. doi: 10.1016/j.humpath.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Kachnic LA, Kaufman DS, Heney NM, Althausen AF, Griffin PP, Zietman AL, et al. Bladder preservation by combined modality therapy for invasive bladder cancer. J Clin Oncol. 1997;15:1022–1029. doi: 10.1200/JCO.1997.15.3.1022. [DOI] [PubMed] [Google Scholar]
- 12.Shipley WU, Winter KA, Kaufman DS, Lee WR, Heney NM, Tester WR, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: initial results of Radiation Therapy Oncology Group 89-03. J Clin Oncol. 1998;16:3576–3583. doi: 10.1200/JCO.1998.16.11.3576. [DOI] [PubMed] [Google Scholar]
- 13.Gospodarowicz MK, Hawkins NV, Rawlings GA, Connolly JG, Jewett MA, Thomas GM, et al. Radical radiotherapy for muscle invasive transitional cell carcinoma of the bladder: failure analysis. J Urol. 1989;142:1448–1453. doi: 10.1016/s0022-5347(17)39122-x. discussion 1453–1444. [DOI] [PubMed] [Google Scholar]
- 14.Wolf H, Olsen PR, Hojgaard K. Urothelial dysplasia concomitant with bladder tumours: a determinant for future new occurrences in patients treated by full-course radiotherapy. Lancet. 1985;1:1005–1008. doi: 10.1016/s0140-6736(85)91612-5. [DOI] [PubMed] [Google Scholar]
- 15.Fung CY, Shipley WU, Young RH, Griffin PP, Convery KM, Kaufman DS, et al. Prognostic factors in invasive bladder carcinoma in a prospective trial of preoperative adjuvant chemotherapy and radiotherapy. J Clin Oncol. 1991;9:1533–1542. doi: 10.1200/JCO.1991.9.9.1533. [DOI] [PubMed] [Google Scholar]
- 16.Zietman AL, Grocela J, Zehr E, Kaufman DS, Young RH, Althausen AF, et al. Selective bladder conservation using transurethral resection, chemotherapy, and radiation: management and consequences of Ta, T1, and Tis recurrence within the retained bladder. Urology. 2001;58:380–385. doi: 10.1016/s0090-4295(01)01219-5. [DOI] [PubMed] [Google Scholar]
- 17.Milosevic M, Gospodarowicz M, Zietman A, Abbas F, Haustermans K, Moonen L, et al. Radiotherapy for bladder cancer. Urology. 2007;69:80–92. doi: 10.1016/j.urology.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 18.Zietman AL, Sacco D, Skowronski U, Gomery P, Kaufman DS, Clark JA, et al. Organ conservation in invasive bladder cancer by transurethral resection, chemotherapy and radiation: results of a urodynamic and quality of life study on long-term survivors. J Urol. 2003;170:1772–1776. doi: 10.1097/01.ju.0000093721.23249.c3. [DOI] [PubMed] [Google Scholar]
- 19.James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366:1477–1488. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 20.Coppin CM, Gospodarowicz MK, James K, Tannock IF, Zee B, Carson J, et al. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1996;14:2901–2907. doi: 10.1200/JCO.1996.14.11.2901. [DOI] [PubMed] [Google Scholar]
- 21.Krause FS, Walter B, Ott OJ, Haberle L, Weiss C, Rodel C, et al. 15-year survival rates after transurethral resection and radiochemotherapy or radiation in bladder cancer treatment. Anticancer Res. 2011;31:985–990. [PubMed] [Google Scholar]
- 22.Kaufman DS, Winter KA, Shipley WU, Heney NM, Chetner MP, Souhami L, et al. The initial results in muscle-invading bladder cancer of RTOG 95-06: phase I/II trial of transurethral surgery plus radiation therapy with concurrent cisplatin and 5-fluorouracil followed by selective bladder preservation or cystectomy depending on the initial response. Oncologist. 2000;5:471–476. doi: 10.1634/theoncologist.5-6-471. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman DS, Winter KA, Shipley WU, Heney NM, Wallace HJ, 3rd, Toonkel LM, et al. Phase I-II RTOG study (99-06) of patients with muscle-invasive bladder cancer undergoing transurethral surgery, paclitaxel, cisplatin, and twice-daily radiotherapy followed by selective bladder preservation or radical cystectomy and adjuvant chemotherapy. Urology. 2009;73:833–837. doi: 10.1016/j.urology.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 24.Mitin T, Hunt D, Shipley WU, Kaufman DS, Uzzo R, Wu CL, et al. Transurethral surgery and twice-daily radiation plus paclitaxel-cisplatin or fluorouracil-cisplatin with selective bladder preservation and adjuvant chemotherapy for patients with muscle invasive bladder cancer (RTOG 0233): a randomised multicentre phase 2 trial. Lancet Oncol. 2013;14:863–872. doi: 10.1016/S1470-2045(13)70255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choudhury A, Swindell R, Logue JP, Elliott PA, Livsey JE, Wise M, et al. Phase II study of conformal hypofractionated radiotherapy with concurrent gemcitabine in muscle-invasive bladder cancer. J Clin Oncol. 2011;29:733–738. doi: 10.1200/JCO.2010.31.5721. [DOI] [PubMed] [Google Scholar]
- 26.Kent E, Sandler H, Montie J, Lee C, Herman J, Esper P, et al. Combined-modality therapy with gemcitabine and radiotherapy as a bladder preservation strategy: results of a phase I trial. J Clin Oncol. 2004;22:2540–2545. doi: 10.1200/JCO.2004.10.070. [DOI] [PubMed] [Google Scholar]
- 27.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 28.Sherif A, Holmberg L, Rintala E, Mestad O, Nilsson J, Nilsson S, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol. 2004;45:297–303. doi: 10.1016/j.eururo.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Ploussard G, Daneshmand S, Efstathiou JA, Herr HW, James ND, Rodel CM, et al. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol. 2014;66:120–137. doi: 10.1016/j.eururo.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 30.Bolenz C, Shariat SF, Karakiewicz PI, Ashfaq R, Ho R, Sagalowsky AI, et al. Human epidermal growth factor receptor 2 expression status provides independent prognostic information in patients with urothelial carcinoma of the urinary bladder. BJU Int. 2010;106:1216–1222. doi: 10.1111/j.1464-410X.2009.09190.x. [DOI] [PubMed] [Google Scholar]
- 31.Chakravarti A, Winter K, Wu CL, Kaufman D, Hammond E, Parliament M, et al. Expression of the epidermal growth factor receptor and Her-2 are predictors of favorable outcome and reduced complete response rates, respectively, in patients with muscle-invading bladder cancers treated by concurrent radiation and cisplatin-based chemotherapy: a report from the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2005;62:309–317. doi: 10.1016/j.ijrobp.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 32.Michaelson MD, Hu C, Pham HT, Dahl DM, Wu C-L, Whittington RM, et al. The initial report of RTOG 0524: Phase I/II trial of a combination of paclitaxel and trastuzumab with daily irradiation or paclitaxel alone with daily irradiation following transurethral surgery for noncystectomy candidates with muscle-invasive bladder cancer. J Clin Oncol. 2014;32(suppl 4) abstr LBA287. [Google Scholar]
- 33.Goldsmith B, Baumann BC, He J, Tucker K, Bekelman J, Deville C, et al. Occult pelvic lymph node involvement in bladder cancer: implications for definitive radiation. Int J Radiat Oncol Biol Phys. 2014;88:603–610. doi: 10.1016/j.ijrobp.2013.11.211. [DOI] [PubMed] [Google Scholar]
- 34.Gray PJ, Lin CC, Jemal A, Shipley WU, Fedewa SA, Kibel AS, et al. Clinical-pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the national cancer data base. Int J Radiat Oncol Biol Phys. 2014;88:1048–1056. doi: 10.1016/j.ijrobp.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Tunio MA, Hashmi A, Qayyum A, Mohsin R, Zaeem A. Whole-pelvis or bladder-only chemoradiation for lymph node-negative invasive bladder cancer: single-institution experience. Int J Radiat Oncol Biol Phys. 2012;82:e457–e462. doi: 10.1016/j.ijrobp.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 36.Foroudi F, Pham D, Bressel M, Gill S, Kron T. Intrafraction bladder motion in radiation therapy estimated from pretreatment and posttreatment volumetric imaging. Int J Radiat Oncol Biol Phys. 2013;86:77–82. doi: 10.1016/j.ijrobp.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 37.Yee D, Parliament M, Rathee S, Ghosh S, Ko L, Murray B. Cone beam CT imaging analysis of interfractional variations in bladder volume and position during radiotherapy for bladder cancer. Int J Radiat Oncol Biol Phys. 2010;76:1045–1053. doi: 10.1016/j.ijrobp.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 38.Cowan RA, McBain CA, Ryder WD, Wylie JP, Logue JP, Turner SL, et al. Radiotherapy for muscle-invasive carcinoma of the bladder: results of a randomized trial comparing conventional whole bladder with dose-escalated partial bladder radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:197–207. doi: 10.1016/j.ijrobp.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Mangar SA, Foo K, Norman A, Khoo V, Shahidi M, Dearnaley DP, et al. Evaluating the effect of reducing the high-dose volume on the toxicity of radiotherapy in the treatment of bladder cancer. Clin Oncol (R Coll Radiol) 2006;18:466–473. doi: 10.1016/j.clon.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Turgeon GA, Souhami L, Cury FL, Faria SL, Duclos M, Sturgeon J, et al. Hypofractionated intensity modulated radiation therapy in combined modality treatment for bladder preservation in elderly patients with invasive bladder cancer. Int J Radiat Oncol Biol Phys. 2014;88:326–331. doi: 10.1016/j.ijrobp.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh CH, Chung SD, Chan PH, Lai SK, Chang HC, Hsiao CH, et al. Intensity modulated radiotherapy for elderly bladder cancer patients. Radiat Oncol. 2011;6:75. doi: 10.1186/1748-717X-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia MM, Gottschalk AR, Brajtbord J, Konety BR, Meng MV, Roach M, 3rd, et al. Endoscopic gold fiducial marker placement into the bladder wall to optimize radiotherapy targeting for bladder-preserving management of muscle-invasive bladder cancer: feasibility and initial outcomes. PLoS One. 2014;9:e89754. doi: 10.1371/journal.pone.0089754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumgarten AS, Emtage JB, Wilder RB, Biagioli MC, Gupta S, Spiess PE. Intravesical lipiodol injection technique for image-guided radiation therapy for bladder cancer. Urology. 2014;83:946–950. doi: 10.1016/j.urology.2013.09.058. [DOI] [PubMed] [Google Scholar]
- 44.Weiss C, Engehausen DG, Krause FS, Papadopoulos T, Dunst J, Sauer R, et al. Radiochemotherapy with cisplatin and 5-fluorouracil after transurethral surgery in patients with bladder cancer. Int J Radiat Oncol Biol Phys. 2007;68:1072–1080. doi: 10.1016/j.ijrobp.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 45.Shipley WU, Kaufman DS, Zehr E, Heney NM, Lane SC, Thakral HK, et al. Selective bladder preservation by combined modality protocol treatment: long-term outcomes of 190 patients with invasive bladder cancer. Urology. 2002;60:62–67. doi: 10.1016/s0090-4295(02)01650-3. discussion 67–68. [DOI] [PubMed] [Google Scholar]
- 46.Efstathiou JA, Bae K, Shipley WU, Kaufman DS, Hagan MP, Heney NM, et al. Late pelvic toxicity after bladder-sparing therapy in patients with invasive bladder cancer: RTOG 89-03, 95-06, 97-06, 99-06. J Clin Oncol. 2009;27:4055–4061. doi: 10.1200/JCO.2008.19.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henningsohn L, Wijkstrom H, Dickman PW, Bergmark K, Steineck G. Distressful symptoms after radical radiotherapy for urinary bladder cancer. Radiother Oncol. 2002;62:215–225. doi: 10.1016/s0167-8140(01)00455-8. [DOI] [PubMed] [Google Scholar]
- 48.Caffo O, Fellin G, Graffer U, Luciani L. Assessment of quality of life after cystectomy or conservative therapy for patients with infiltrating bladder carcinoma. A survey by a self-administered questionnaire. Cancer. 1996;78:1089–1097. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1089::AID-CNCR20>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 49.Mitin T, George A, Zietman AL, Kaufman DS, Uzzo RG, Dreicer R, et al. Long-term outcomes among patients who achieve complete or near-complete responses after the induction phase of bladder-preserving combined modality therapy for muscle-invasive bladder cancer: A pooled analysis of RTOG 9906 and 0233. J Clin Oncol. 2014;32 doi: 10.1016/j.ijrobp.2015.09.030. abstract 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss C, Wolze C, Engehausen DG, Ott OJ, Krause FS, Schrott KM, et al. Radiochemotherapy after transurethral resection for high-risk T1 bladder cancer: an alternative to intravesical therapy or early cystectomy? J Clin Oncol. 2006;24:2318–2324. doi: 10.1200/JCO.2006.05.8149. [DOI] [PubMed] [Google Scholar]
- 51.Wo JY, Shipley WU, Dahl DM, Coen JJ, Heney NM, Kaufman DS, et al. The results of concurrent chemo-radiotherapy for recurrence after treatment with bacillus Calmette-Guerin for non-muscle-invasive bladder cancer: is immediate cystectomy always necessary? BJU Int. 2009;104:179–183. doi: 10.1111/j.1464-410X.2008.08299.x. [DOI] [PubMed] [Google Scholar]
- 52.Harland SJ, Kynaston H, Grigor K, Wallace DM, Beacock C, Kockelbergh R, et al. A randomized trial of radical radiotherapy for the management of pT1G3 NXM0 transitional cell carcinoma of the bladder. J Urol. 2007;178:807–813. doi: 10.1016/j.juro.2007.05.024. discussion 813. [DOI] [PubMed] [Google Scholar]
- 53.Lautenschlaeger T, George A, Klimowicz AC, Efstathiou JA, Wu CL, Sandler H, et al. Bladder preservation therapy for muscle-invading bladder cancers on Radiation Therapy Oncology Group trials 8802, 8903, 9506, and 9706: vascular endothelial growth factor B overexpression predicts for increased distant metastasis and shorter survival. Oncologist. 18:685–686. doi: 10.1634/theoncologist.2012-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choudhury A, Nelson LD, Teo MT, Chilka S, Bhattarai S, Johnston CF, et al. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 70:7017–7026. doi: 10.1158/0008-5472.CAN-10-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]