Abstract
Afferent and efferent cardiac neurotransmission via the cardiac nerves intricately modulates nearly all physiological functions of the heart (chronotropy, dromotropy, lusitropy and inotropy). Afferent information from the heart is transmitted to higher levels of the nervous system for processing (intrinsic cardiac nervous system, extracardiac-intrathoracic ganglia, spinal cord, brain stem and higher centers) which ultimately results in efferent cardiomotor neural impulses (via the sympathetic and parasympathetic nerves). This system forms interacting feedback loops that provide physiological stability for maintaining normal rhythm and life-sustaining circulation. This system also ensures that there is fine-tuned regulation of sympathetic-parasympathetic balance in the heart under normal and stressed states in the short (beat to beat), intermediate (minutes-hours) and long term (days-years). This important neurovisceral /autonomic nervous system also plays a major role in the pathophysiology and progression of heart disease, including heart failure and arrhythmias leading to sudden cardiac death (SCD). Transdifferentiation of neurons in heart failure, functional denervation, cardiac and extra-cardiac neural remodeling have also been identified and characterized during the progression of disease. Recent advances in understanding the cellular and molecular processes governing innervation and the functional control of the myocardium in health and disease provides a rational mechanistic basis for development of neuraxial therapies for preventing SCD and other arrhythmias. Advances in cellular, molecular, and bioengineering realms have underscored the emergence of this area as an important avenue of scientific inquiry and therapeutic intervention.
Keywords: Autonomic nervous system, sudden cardiac death, arrhythmia, pathophysiology
INTRODUCTION
Cardiac autonomic dysregulation is central to the development and progression of most cardiovascular diseases (hypertension (HTN), heart failure (HF), arrhythmias, and myocardial infarction). Impaired cardiac parasympathetic responsiveness and enhanced sympathetic activity are negative prognostic indicators for both morbidity and mortality associated with arrhythmias and sudden death.1, 2 The autonomic nervous system (ANS), plays a major role in the pathophysiology of arrhythmias leading to sudden cardiac death (SCD), and neuraxial modulation is emerging as an important avenue of scientific inquiry and therapeutic intervention.3, 4 Mechanism based autonomic regulation therapy (ART) holds promise to treat both arrhythmias and HF. Improved basic scientific understanding can result in innovative low cost therapeutic options, with a global impact, that can not only prevent death but also favorably alter the course of the underlying disease. The ANS intricately regulates cardiac excitability and function. Cardiac afferents provide beat to beat sensory information of cardiac muscle activity to the neuraxis, additional information is conveyed by extra cardiac circulatory receptors (Fig 1 & 2). The processing of this afferent information at several levels (intrinsic cardiac nervous system, extracardiac-intrathoracic ganglia, spinal cord, brain stem and higher centers) provides an elegant mechanism for interacting feedback loops to provide physiological stability for maintaining normal rhythm and life-sustaining circulation. These nested feedback loops ensure that there is fine-tuned regulation of efferent (sympathetic and parasympathetic cardiomotor) neural signals to the heart in normal and stressed states. Concepts regarding cardiac neural control have been revised in recent years based on new physiological data from multiple studies that together provide an elegant framework for understanding regulatory control of the mammalian heart (Fig 2). Direct single neuron and neural network recordings from intrinsic cardiac and extra-cardiac ganglia provide the methods to study organ level physiology5–7 and a proper framework of interpretation of the neural control-myocyte interface.
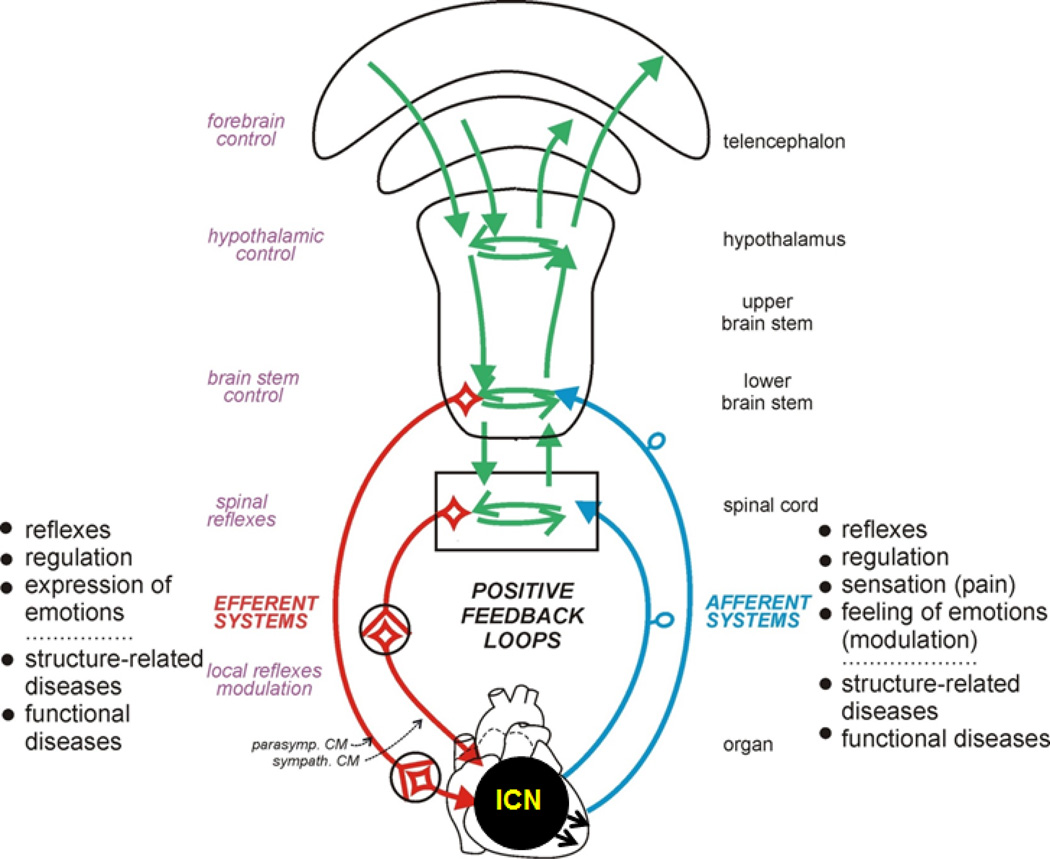
Figure 1. Cardiac Neurotransmission.
ICN (intrinsic nervous system of the heart). Figure modified from W.Jänig (with permission).
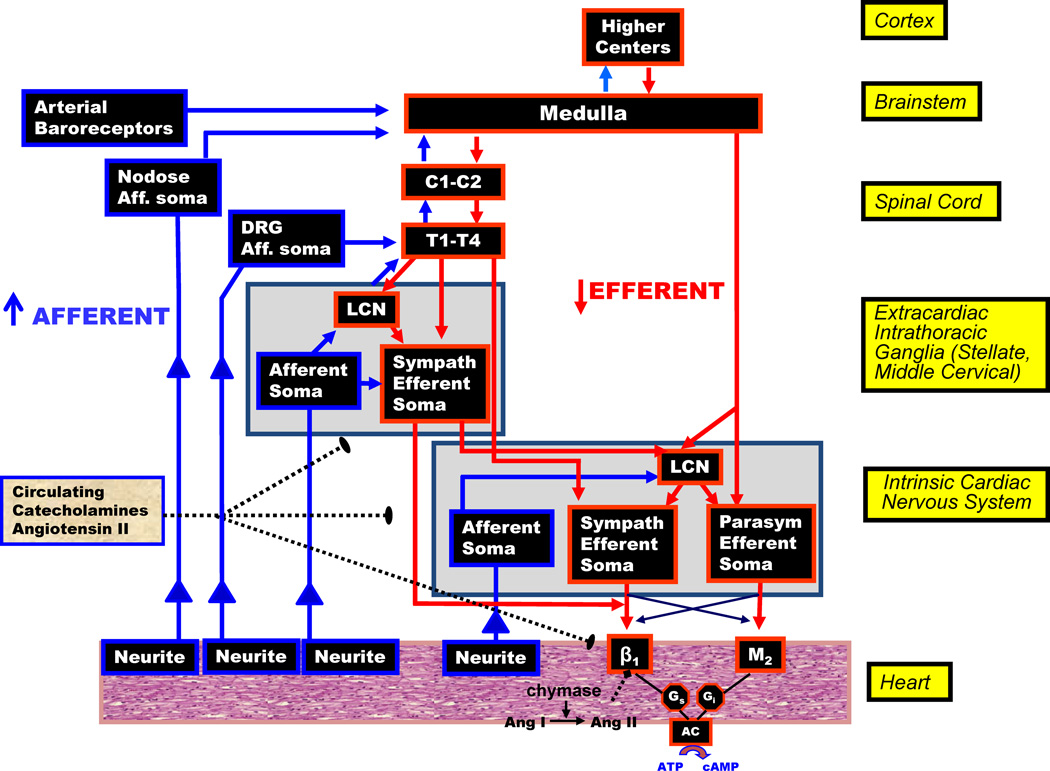
Figure 2. Neurohumoral control and anatomical organization of cardiac innervation.
LCN=local circuit neuron, DRG=dorsal root ganglion, Aff=afferent, C=cervical, T=thoracic, Ang=angiotensin
PATHOPHYSIOLOGY
Cardiac injury (e.g. infarction, focal inflammation) results in the formation of a scar at the level of the organ and likewise alters the integrative regulation of the heart.4, 8 The changes at the level of the organ result in slowed and altered paths of myocardial electrical propagation, which together creates the substrate for reentrant arrhythmias. The systemic effects of this scar are characterized by afferent-mediated activation of the neuroendocrine system, primarily sympatho-excitation in conjunction with withdrawal of central parasympathetic tone, which provides short term benefits to maintain cardiac output, but at a cost.9 The recovery from acute injury is characterized by a state wherein there is continued abnormal cardiac afferent signaling (cardio-centric afferents).10 Mechanistically, such dysregulation reflects reactive and adaptive responses of the cardiac neural hierarchy leading to excessive neuronal interactive excitability and network interconnectivity from the intrinsic cardiac nervous system up to and including the insular cortex.7 This reorganization ultimately leads to conflict between central and peripheral aspects of the hierarchy. This leads to a maladaptive response of excessive sympatho-excitation contributing to the evolution of cardiac disease and fatal arrhythmias.
Cortical and sub-cortical control of the heart can be demonstrated experimentally in animal models11 and in humans12, 13 and has been implicated in arrhythmias.14, 15 The efficacy of ART such as cardiac afferent denervation after myocardial infarction16 and sympathectomy to treat ventricular tachycardia (VT) storm could be due to a reduction of these conflicts between levels of the cardiac nervous system.17, 18 In these settings it is very likely that the intrinsic nervous system of the heart is able to provide the neural coordination and ensure electrical stability without the interference of central input. The electrical stability of the transplanted heart is a clear manifestation of this principle.19
1. Determination of cardiac innervation patterning by both neural chemo attractants & chemo repellents in the heart. (Figure 3)
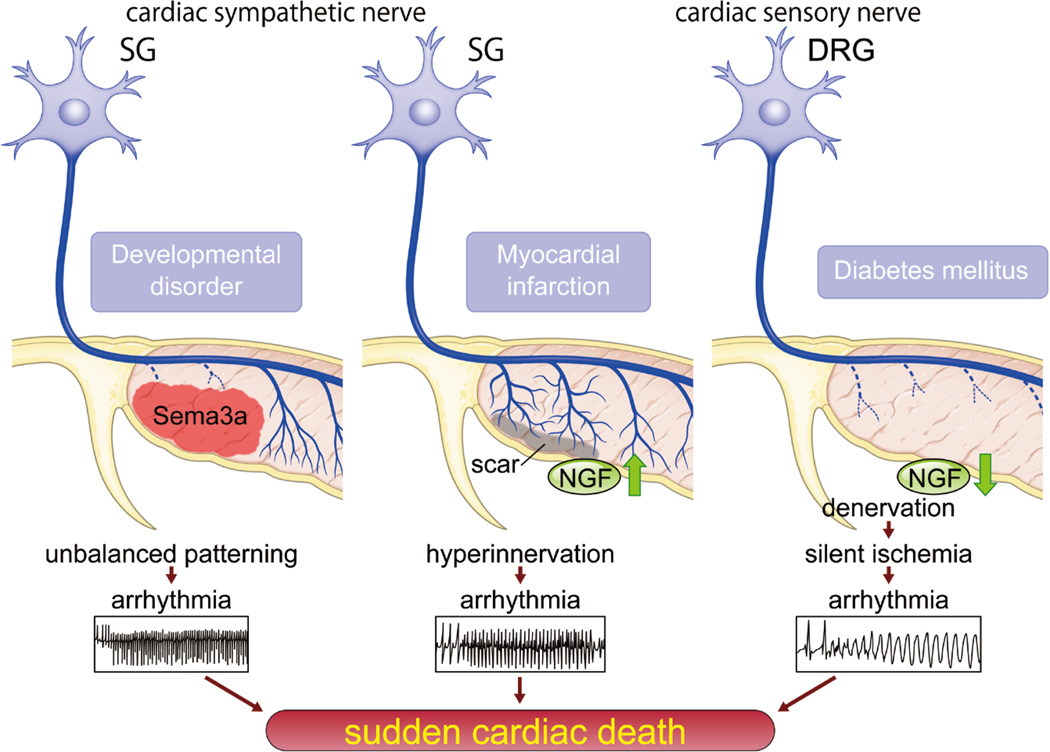
Figure 3. Regulation of cardiac innervation patterning and sudden cardiac death.
Left, Overexpression or lack of sema3A in endocardium causes unbalanced patterning of sympathetic nerves, which alters the potential for lethal arrhythmia. Appropriate sema3A-mediated sympathetic innervation is crucial for maintenance of arrhythmia-free heart. Middle, Up-regulation of secreted nerve growth factor (NGF) from cardiomyocytes in diseased heart may cause lethal arrhythmia and sudden cardiac death (SCD). Right, Down-regulation of NGF in diabetic heart induces denervation of cardiac sensory nerve, which leads to silent ischemia and lethal arrhythmia.
The heart is abundantly innervated and its performance is tightly controlled by the both of sympathetic and parasympathetic efferent nerves (Figure 2). Cardiac innervation density including sensory nerves is altered in diseased hearts, which can lead to unbalanced neural activation and lethal arrhythmias. In this section, we focus on the regulatory mechanisms controlling cardiac innervation and the critical roles of these processes on cardiac performance.
-Semaphorin 3A reduces arrhythmia potential through modulation of sympathetic innervation patterning
Semaphorin 3A (sema3A) is a Class 3 secreted semaphorin that has been cloned and identified as a potent neural chemorepellent and a directional guidance molecule for nerve fibers.20 Initially, we analyzed the kinetics and distribution of cardiac sympathetic innervation in developing mouse ventricles.21
By analyzing sema3A knocked-in lacZ mice (SemaTG), we found that sema3A is strongly expressed in the developing heart at embryonic day (E) 12 with sema3A expression in the subendocardium, but not subepicardium, of the atria and ventricles. SemaTG mice have reduced sympathetic innervation and attenuation of the epicardial to endocardial innervation gradient.21 The expression of sema3A in developing hearts revealed a linear decrease from E 12 that corresponded with an increase in sympathetic innervation density. Thus, the spatial and temporal expression pattern of sema3A directly mirrors the patterning of sympathetic innervation in developing hearts. These results indicate that sema3A is a negative regulator of cardiac innervation. We also analyzed sema3A-deficient mice and found that the sympathetic nerve density is lower in the subepicardium and higher in the subendocardium. As such, these changes resulted in disruption of the innervation gradient in the ventricles. Overall, these results indicate that cardiomyocyte-derived sema3A plays a critical role in cardiac sympathetic innervation by inhibiting neural growth.
Most sema3A-deficient mice died within the first postnatal week due to sinus bradycardia and abrupt sinus arrest. By comparison, SemaTG mice died suddenly without any symptoms at 10 months of age. Sustained ventricular tachyarrhythmia was induced in SemaTG mice, but not in wild-type (WT) mice, after epinephrine administration. Programmed electrical stimulation also revealed that SemaTG mice were highly susceptible to ventricular tachyarrhythmias.
Together, this data indicates that the highly organized innervation patterning mediated by sema3A is critical for the maintenance of arrhythmia-resistant hearts. From the clinical perspective, consistent with our data, Stramba-Badiale et al. report that developmental abnormalities in cardiac innervation may play a role in the genesis of some cases of sudden infant death syndrome.22 In a recent study of unexplained cardiac arrest Nakano and colleagues23 demonstrated that a polymorphism of SEMA3A(I334V) diminishes the cardiac sympathetic innervation gradient and partially contributes to the etiology of SCD with ventricular fibrillation (VF). These findings are important in elucidating the pathogenesis of cardiac sudden death and indicates the dynamic synergism between neural and cardiac development in control of cardiac electrical stability.
-Nerve growth factor up-regulation causes nerve sprouting and sudden cardiac death
Sympathetic activation is important in the genesis of SCD in diseased hearts. It has been known for decades that β-blocker therapy prevents SCD secondary to VT in ischemic heart disease or congestive heart failure (CHF). It is further recognized that beta blockers (BBs) exert this effect by targeting both cardiac myocytes and elements of the cardiac nervous system.24–26
Nerve growth factor (NGF) is a prototypic member of the neurotrophin family, the members of which are critical for the differentiation, survival, and synaptic activity of the peripheral sympathetic and sensory nervous systems.27 The level of NGF expression within innervated tissue corresponds approximately to innervation density. Previous studies show that NGF expression increases during development and is altered in diseased hearts.28, 29 Zhou et al. showed that NGF, which is critical for sympathetic nerve sprouting, is upregulated after myocardial infarction in animal models, resulting in the regeneration of cardiac sympathetic nerves and heterogeneous innervation.30
It has also been reported that NGF is upregulated in cardiac hypertrophy, leading to sympathetic hyperinnervation.31 In addition, Cao et al reported that NGF infusion after MI enhances myocardial nerve sprouting and results in a dramatic increase in SCD and a high incidence of ventricular tachyarrhythmias.28 Chen and colleagues have shown that overexpression of sema3A in MI border zone could reduce the inducibility of ventricular arrhythmias by reducing sympathetic hyper-innervation after infarction.32 These results demonstrate that NGF-induced augmentation of sympathetic nerve sprouting in diseased hearts leads to lethal arrhythmias and SCD.
-Nerve growth factor down-regulation is critical for diabetic neuropathy and silent myocardial ischemia
Cardiac autonomic neuropathy is a frequent complication of diabetes mellitus (DM), and diabetic patients are at high risk for developing arrhythmias, silent myocardial ischemia and SCD.33
The cardiac ANS is composed of efferent and afferent nerves. In contrast to sympathetic innervation, very little is known about sensory innervation and how it is altered in diseased hearts. A subset of the cardiac sensory innervation is responsible for pain perception. Activation of these nociceptive afferents results in multiple somatic and visceral responses during myocardial ischemia.34 Cardiac sensory nerve impairment causes silent myocardial ischemia and this is a likely a major cause of sudden death in DM patients.35 Further, there is data which indicates nerve sprouting induced by a potent stimulator of NGF after myocardial injury increases the incidence of ventricular tachyarrhythmias.36
A screen of several neurotrophic factors found that the development of cardiac sensory nerves parallels the production of NGF in the heart.37 Cardiac nociceptive sensory nerves that are immunopositive for calcitonin gene-related peptide (CGRP) (including the dorsal root ganglia and the dorsal horn) are markedly retarded in NGF-deficient mice and rescued in mice overexpressing NGF specifically in the heart. Thus, NGF synthesis in the heart is critical for the development of the cardiac sensory innervation.38
To investigate whether NGF is involved in diabetic neuropathy, type I diabetes was induced with streptozotocin in WT and transgenic mice overexpressing NGF in the heart.39 DM-induced WT mice show downregulation of NGF, CGRP-immunopositive cardiac sensory denervation, and atrophic changes in the dorsal root ganglia. These defects are prevented in DM-induced NGF-transgenic mice. Cardiac sensory function, as measured by myocardial ischemia-induced c-Fos expression in the dorsal root ganglia, is also downregulated by DM in WT mice, but not by DM in NGF-transgenic mice. Direct gene transfer of NGF into diabetic rat hearts improves the impaired cardiac sensory innervation and function, as determined by the electrophysiological activity of cardiac afferent nerves during myocardial ischemia. These findings demonstrate that development of the cardiac sensory nervous system depends on the synthesis of NGF in the heart, and that DM-induced suppression of NGF expression may lead to cardiac sensory neuropathy.39 In human clinical trials of recombinant human NGF administered to diabetic patients with polyneuropathy, none of adverse events such as ventricular arrhythmias were reported.40 However, a better understanding of the regulation of these pathways and precise studies on reliable and efficient methods of gene therapy and optimal dosage or route of administration are required for further clinical trials.
2. Abnormalities and alteration of cardiac sympathetic nerve profile in heart failure. (Figure 4)
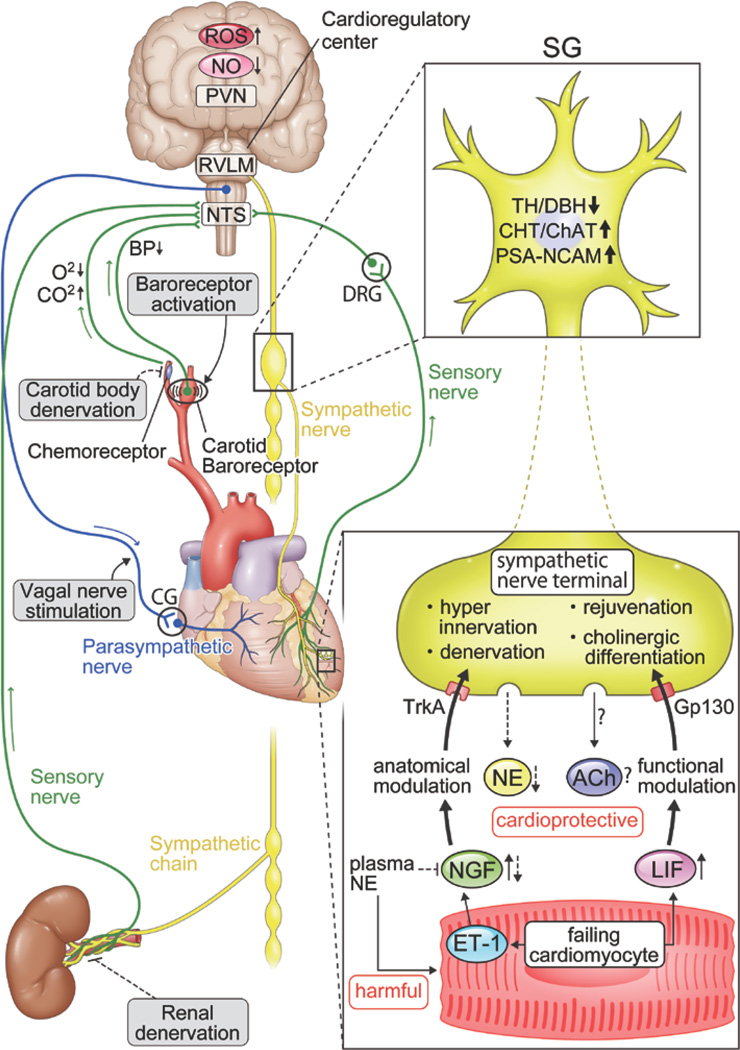
Figure 4. Systemic autonomic interactions and crosstalk between cardiomyocyte and sympathetic nerve terminal via humoral factors in diseased heart.
This figure shows that central and peripheral mechanism of the heart and brain interaction including the cardiac autonomic efferent (sympathetic & parasympathetic), and sensory nerves (sympathetic & parasympathetic) but only the sympathetic afferents (labeled sensory nerve) is shown in this figure for an illustration. See Figure 2 for a full schematic. Representative promising interventional therapies are also described in the figure. In addition, alteration of cardiac sympathetic nerves occur in post ganglionic fiber. Failing cardiomyocytes induces NGF via endothelin-1 (ET-1) mediated pathway and leukemia inhibitory factor (LIF). NGF and LIF leads to hyper-innervation (anatomical modulation) and rejuvenation/ cholinergic differentiation (functional modulation), respectively. This phenomenon shows the expression of catecholaminergic marker such as tyrosine hydroxylase (TH) and dopamine-β-hydroxylase (DBH) reduced, and of cholinergic (CHT, ChAT) and juvenile (PSA-NCAM) increase.
Abbreviations, CHT, choline transporter; ChAT, choline acetyltransferase; PSA-NCAM, polysialylated neural cell adhesion molecule; NE, norepinephrine; ROS, reactive oxygen species; NO, nitric oxide; PVN, paraventricular nucleus; RVLM, rostral ventrolateral medulla; NTS, solitary tract; SG, stellate ganglia; DRG, dorsal root ganglia; CG, cardiac ganglia. Details of these pathways are referenced in the text21, 36, 142
Recently, crosstalk, through various humoral factors, between cardiomyocyte and cardiac sympathetic nerves has been demonstrated. Axon growth, denervation and functional alternation of sympathetic nerves has been noted in HF. Utilizing molecular biological approaches, a new concept about the adaptation mechanism of the ANS in HF has been developed. With this understanding, new interventional therapies targeted for the ANS and based on a concept with multiple organ linkage have emerged. In this section, we focus on a framework to understand cardiac sympathetic nerve abnormalities in HF and implications for therapy of HF and SCD prevention strategies that target autonomic nerves.
-Systemic autonomic nerve dysfunction as related to central & peripheral neural interactions
There is strong evidence that sympathetic efferent neuronal activity is increased in CHF.41 Such sympathetic activation in HF can also trigger malignant arrhythmias. One of the mechanisms proposed to explain sympathetic activation in HF involves abnormalities in baroreceptors. Signals from baroreceptors are transmitted to the central nervous system (CNS) via afferent nerves, and after central processing is transduced back to the heart to suppress sympathetic efferent activity.42 An impairment of carotid baroreflex sensitivity (BRS) has been shown to be a marker of the risk of mortality or a cardiovascular event in HF.43, 44 Baroreceptor activation has been thought to confer benefits in prevention of SCD which is prevalent in patients with HF.45 The HOPE4HF trial (ClinicalTrials.gov Identifier: NCT00957073) is a prospective randomized trial, where patients will be randomized in a 2:1 ratio to receive baroreceptor activation therapy (device arm) or optimal medical therapy alone (medical arm).44
Mechanisms mediated by the chemoreceptor reflex that sense hypoxic and hypercapnic conditions could also be involved in sympathetic activation.46 Recently Rio et al. showed that carotid chemoreceptor ablation reduces cardiorespiratory dysfunction and improves survival during HF in rats.47 In addition, Niewiński et al. showed that surgical removal of the carotid body from a patient with systolic HF significantly decreased sympathetic tone.48
Brainstem and suprabulbar regions of the CNS are critical elements for integrated cardiovascular contol.49 It is well established that the paraventricular nucleus of the hypothalamus and the rostral ventrolateral medulla (RVLM) are involved in the enhanced central sympathetic outflow in HF.50 Reduced nitric oxide, increased oxidative stress, and activation of angiotensin II type 1 receptors in the RVLM all contribute to sympathetic drive.51 Further oxidative stress can alters cardiac cholinergic control.52 It is important to note that cardiac afferents are activated after cardiac injury and play a major role in cardiac dysfunction and remodeling. Wang and colleagues, utilizing resiniferatoxin a potent analog of capsaicin to delete transient receptor potential vanilloid 1 receptor-expressing cardiac afferent nerves, have demonstrated attenuation of remodeling and fibrosis in a rat HF model.16
Animal and human studies suggest that activation of both efferent and afferent renal nerves play a role in the pathogenesis and progression of disease states such as HTN and CHF. Renal denervation (RDN), which is a novel catheter-based ablation therapy, interrupts efferent sympathetic and afferent renal sensory nerves. It is being studied as an option for patients with resistant HTN and HF.53 Physiological cardiovascular control involves afferent signals from the kidneys which are processed in the hypothalamus54, 55 as well as in the nucleus of the solitary tract, insular cortex, anterior cingulate cortex and based on functional MRI studies in the infra-limbic cortex.56 Alterations in afferent input would be expected to alter set-points and sensitivities for reflex control of blood pressure.
Treatment of drug-resistant HTN was the initial therapeutic use of RDN. Both pre-clinical57 and clinical trials58–61 demonstrated decrease ambulatory blood pressure in medication refractory HTN. However, the recently reported prospective Symplicity HTN-3 trial did not meet the expected anti-hypertensive endpoints62, and was terminated early. Currently, RDN is being evaluated as a potential adjunctive therapy in a spectrum of sympathetically modulated cardiovascular diseases, including left ventricular hypertrophy and diastolic dysfunction,63 CHF,64 obstructive sleep apnea,65 and atrial fibrillation (AF).66 RDN has also been proposed as a possible treatment strategy in patients with recurrent ventricular arrhythmias.67–69 Consequently, there are several ongoing clinical trials (Symplicity-HF and REACH) investigating the safety and efficacy of RDN in patients with CHF and VT (Reset VT).70
Finally, considering its systemic relationship with the peripheral sympathetic nerves, the involvement of the CNS in sympathetic dysfunction is of considerable interest and further studies in this area are anticipated in the future.51
-Crosstalk between cardiomyocyte and cardiac sympathetic nerves mediated by humoral factors. (Figure 5)
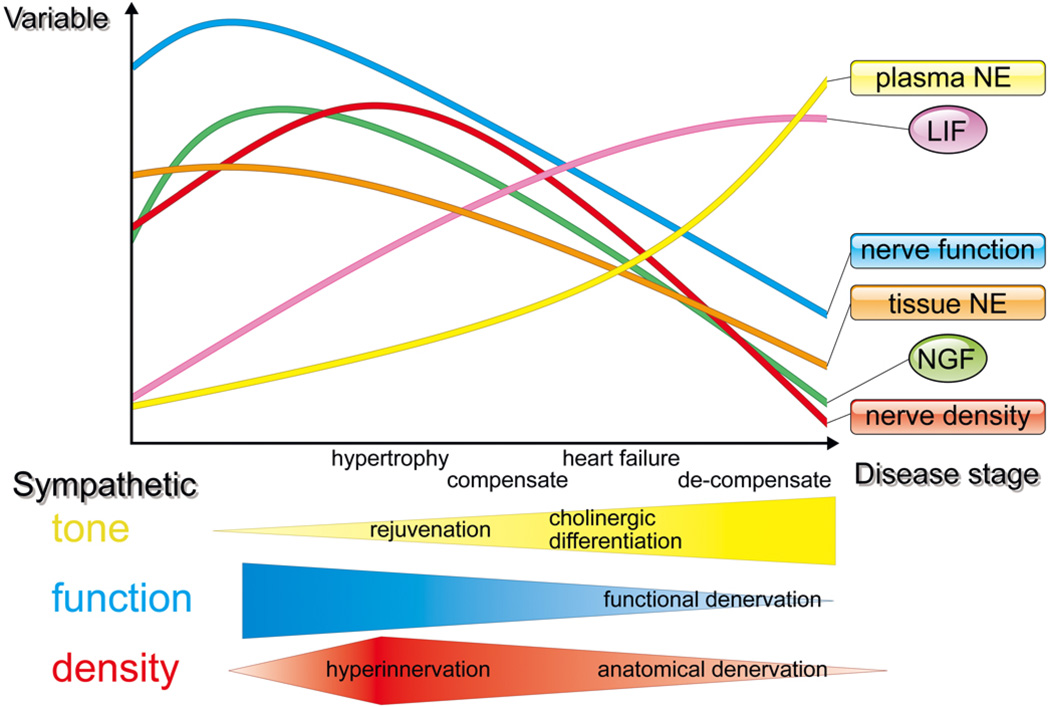
Figure 5. Temporal changes in cardiac innervation with disease progression.
NGF= nerve growth factor, LIF=leukemia inhibitory factor, NE=nor-epinephrine
The pathology of HF involves various abnormalities in sympathetic nerve terminals. During the transition to overt HF, sympathetic neural tone is upregulated, and NGF expression is elevated contributing to hyperinnervation of cardiac sympathetic nerves.31 On the other hand, there is a paradoxical reduction in norepinephrine (NE) synthesis concomitant with down regulation of tyrosine hydroxylase (TH), the rate-limiting enzyme in innervated neurons, NE reuptake into the sympathetic nerve terminals and depression of NE levels in the myocardium.31, 71–73 This discrepancy between anatomical or functional integrity and the catecholaminergic properties of the cardiac sympathetic nervous system (SNS) in HF is long-standing. However, the molecular mechanisms underlying the reduction in catecholaminergic characteristics of cardiac sympathetic nerve system in HF remain poorly understood.
CHF leads to upregulation of a range of growth factors and cytokines in the heart. Leukemia inhibitory factor (LIF) and other members of the interleukin-6 (IL-6) family, which can induce fetal gene expression (so-called rejuvenation) in adult cardiomyocytes are upregulated during CHF.31 In the cardiac SNS in CHF, strong expression of growth-associated protein 43 (GAP-43) and highly polysialylated neural cell adhesion molecule has been noted.31 We also found that this neural rejuvenation can be induced by LIF expressed in hypertrophied hearts (unpublished data). These observations are supported by several other studies, which showed that LIF changes the neuropeptide phenotype74, 75 or increases the degradation of TH through the ubiquitin- proteasome system.76 Taken together, these results suggests that cardiac SNS dysfunction is accompanied by neuronal rejuvenation, the so-called “functional denervation due to rejuvenation” mechanism.31
Cardiac sympathetic properties are also altered in CHF as mediated by changes in cardiac-derived humoral factors. In a HF model using Dahl salt-sensitive rats and in autopsy specimens from patients with HF, decreased density of TH-positive neurons were seen.77 Further, many neurons in the stellate ganglia and left ventricle also expressed parasympathetic markers such as choline transporter and choline acetyltransferase. This was thought to represent cholinergic transdifferentiation of cardiac adrenergic neurons into cholinergic neurons, induced by LIF via a gp130 signaling pathway.77 The diverse potential of sympathetic neurons in terms of plasticity (adaptability to changes in the environment) is implied by the functional changes to cardiac sympathetic neurons in HF.
It remains controversial whether cardiac sympathetic differentiation induced by gp130-mediated cytokines in CHF is a favorable or unfavorable event for cardiac performance and prognosis. We found significantly improved survival rate and ventricular function in reference mice when compared with sympathetic nerve specific, gp130-deficient mice, suggesting a protective role for the transdifferentiation seen in the model of hypoxia-induced HF mice.77 Together, these results indicate that IL-6 family cytokines secreted from the failing myocardium act as negative modulators of sympathetic function by rejuvenation and cholinergic differentiation via a gp130 signaling pathway, possibly affecting cardiac performance and prognosis.77
Modulation of parasympathetic function can exert profound effects on sympathetic function in the heart. Previous reports have demonstrated that the vagal nerve stimulation suppresses arrhythmia and prevents sudden death in CHF after myocardial infarction with dogs or rats.78, 79 Indeed, in recent reported clinical trial, vagal nerve stimulation improves cardiac function and quality of life with tolerable safety profile in CHF patients.80 However the recent results of another multicenter trial of vagal stimulation failed to demonstrate benefits with regards to cardiac remodeling and functional capacity despite improvement in quality-of-life measures.81
Long-term exposure of high plasma NE concentration caused a reduction in myocardial NGF and associated sympathetic fiber loss in severe decompensated HF animals, the so-called “anatomical denervation due to depletion of NGF”.73 Recently, Rana et al showed that mechanical stretch and α-1 adrenergic stimulation attenuated NGF expression via the calcineurin-nuclear factor of activated T-cells signaling pathway in cultured neonatal cardiomyocytes.82 The spatial and temporal innervation pattern and activity of sympathetic nerves directly impact the pathogenesis in HF (Figure 3). We believe that better understanding of the mechanisms of cardiac sympathetic anatomical and functional innervation patterning represents an important approach for future development of therapies to avoid SCD.
3. Translational Relevance of Cardiac and Extra-cardiac Neural Remodeling (Figure 6)
Figure 6. Functional remodeling of cardiac innervation in an experimental infarct model and humans with post-infarct cardiomyopathy.
Innervation patterns of the mammalian heart are altered following myocardial infarction. Left upper panel: polar maps of global epicardial activation recovery intervals (ARIs) recorded from a control and an infarcted porcine heart at baseline (BL), and during stimulation of the right, left, and bilateral stellate ganglion (RSG, LSG, & BSG, respectively). The focal region of myocardial infarction in the antero-apical left ventricle is indicated by the dashed circle in bottom row. The altered pattern of ARI distribution in the infarcted heart extends beyond the region of focal myocardial infarction. Right upper panel: graphical representation of the regional responses of the porcine heart to stimulation of RSG, LSG, and BSG in control and infarcted hearts respectively. The anterior and posterior predominance of RSG and LSG stimulations respectively, are completely lost following infarction. Left lower panel: ARIs recorded from a patient with ischemic cardiomopathy and a large antero-apical scar. The location of the recording multi-electrode catheter on fluoroscopy in the right and left anterior oblique (RAO and LAO, respectively) projections; and the corresponding electroanatomic map are shown. On the electroanatomic map, the purple regions indicate tissue with normal voltage (non-scar tissue), while the dense grey regions represent dense scar. All other colors represent border zones (tissue with voltage ≥0.5 mV but ≤1.5 mV). Right lower panel: The degree of change in ARI from baseline in response to direct (isoproterenol) and indirect reflex-mediated (nitroprusside) sympathetic stimulation in cardiomyopathic and normal hearts is shown. With isoproterenol, ARI shortening is exaggerated in CM-NL (normal voltage regions in cardiomyopathic hearts) and CM-scar (scarred tissue) regions of the cardiomyopathic heart. Border zone regions are slightly less responsive to isopreterenol. With nitroprusside, CM-NL and CM-Scar zones paradoxically demonstrate ARI increase compared with the border zone regions. These observations, when compared to normal hearts, indicate the severe degree of adrenergic nerve dysfunction in human hearts with ischemic cardiomyopathy. AICD=automatic internal cardioverter defibrillator lead, CS=coronary sinus electrode, RV= right ventricular lead
The electrophysiological effects of neural remodeling have been the subject of recent human and animal studies. Data from epicardial and endocardial recordings in patients referred for interventional cardiac electrophysiology procedures, demonstrate there is global cardiac remodeling in humans (analysis of infarcted regions, peri-infarct regions and ‘remote/normal’ parts of the ventricle).83 This study showed that in humans sympathetic stimulation increased regional differences in repolarization. The myocardium remote from the infract demonstrated abnormal neural control consistent with denervation (lack of action potential shortening with neural stimulation). This fucntional ‘denervation’ is also seen in experimental infarction replicating the human condition.83, 84 Dispersion of action potential duration in response to sympathetic stimulation (heterogeneity in response) is significantly increased in cardiomyopathic hearts which explains the proclivity to lethal arrhythmias. To mechanistically evaluate this disease induced remodeling, a porcine model of myocardial infarction was developed that reproduces all key aspects of disease observed in humans.84 From these animal models, we found that ‘remote’ (non-infarcted) myocardium in these hearts shows abnormal regulation and the stellate ganglia show neuronal remodeling and adrenergic ‘trans-differentiation’ (greater TH-positive cells in stellate ganglia).85 We have recently extended this work to human and have found that in addition to cardiac changes, extra-cardiac neural structures undergo significant neural remodeling in in the presence of myocardial dysfunction.86 Evaluating the stellate ganglia removed from patients with refractory arrhythmias, we found morphological changes (enlargement of neurons), as well as changes in GAP-43 and synaptophysin consistent with increased activity.86 Such changes likely reflect the pathophysiological changes in response to neural transduction in the stressed heart and the removal of these structures is beneficial by interrupting efferent and afferent pathways.17, 87 It is of interest that vagal stimulation, which is being evaluated as a treatment for HF to prevent sudden death, leads to cholinergic transdifferentiation of stellate ganglion in dogs.88
CLINICAL CORRELATES
Several studies have highlighted the value of autonomic indices to identify patients at risk for sudden death. These typically have related to measurable indices of sympathetic and parasympathetic function. Although several tests are valuable, they have not surpassed simpler measures of risk such as ventricular function assessment. However, the physiological basis of these tests will be alluded to briefly.
1. Identifying high risk patients for sudden cardiac death in diseased heart by evaluation of the autonomic nervous system (Table 1)
Table 1.
Cardiac autonomic testing
| Heart rate variability (HRV) |
| Baroreflex sensitivity (BRS) |
| Heart rate turbulence (HRT) |
| Heart rate deceleration capacity (HRDC) |
| T wave alternans (TWA) |
Higher sympathetic tone and lower parasympathetic tone promotes fatal arrhythmias by multiple mechanisms including reducing ventricular refractory period and VF threshold, promoting triggered activity and automaticity. To identify patients at high risk for SCD, evaluation of the ANS has received attention over the years primarily due to the limitations of only using left ventricular ejection fraction (LVEF). Multifaceted evaluation using different risk markers is expected to increase the accuracy for detecting cardiac risk and also provides opportunities to initiate protective therapy and continues to be a matter of clinical debate. In this section we summarize available cardiac autonomic testing strategies including heart rate variability (HRV), BRS, heart rate turbulence (HRT), heart rate deceleration capacity (HRDC) and T wave alternans (TWA) to place them in the context of cardiac innervation.
Heart rate variability
Sinus node automaticity is modulated by both sympathetic and parasympathetic nervous systems. Modulation of heart rate by respiration is well known phenomenon mediated by cardiopulmonary afferent inputs and central interactions between cardiovascular and respiratory networks.89, 90 Alterations of the heart rate is easily measured clinically from ECG recordings and is used to quantify cardiac autonomic modulations as HRV. HRV is measured by multiple different methods. The most popular methods are time domain or frequency domain analysis.
Baroreflex sensitivity
BRS is an index of autonomic input to the sinus node and measured by the reflex changes in R-R interval in response to induced changes in blood pressure. It is usually measured by characterizing the magnitude of induced bradycardia in response to a pressor (phenylephrine) challenge. BRS decreases with advancing age and is reduced in patients with HTN or HF.43, 91 The ATRAMI study showed that, after myocardial infarction, the standard deviation of the average of normal sinus to normal sinus intervals (SDANN) <70ms or BRS<3.0ms/mmHg with LVEF below 35% carried a significant risk of cardiac mortality.43 Daily exercise prevents VF induced by acute myocardial infarction by decreasing sympathetic and/or increasing parasympathetic tone.92
Heart rate turbulence
HRT is an index of changes in sinus rate after a premature ventricular complex (PVC) followed by a compensatory pause. Normally, the sinus rate initially accelerates and slows thereafter but this phenomenon is disturbed in various heart diseases. Abnormal HRT is associated with increased total mortality and sudden death in patients with coronary artery disease and dilated cardiomyopathy.93 From the substudy of ATRAMI study, relative risk for abnormal values of HRT was a strong predictor.94
Heart rate deceleration capacity
HRDC capacity is based on a signal processing algorithm to separately characterize deceleration and acceleration of heart rate, which in turn distinguish between vagal and sympathetic factors. HRDC is reported to be a better predictor of mortality after myocardial infarction than LVEF and standard deviation of normal sinus to normal sinus.95
T wave alternans
TWA is beat-to-beat variability in the amplitude or morphology of T-waves. TWA reflects temporal heterogeneity or dispersion in ventricular repolarization. TWA is primary used as a tool for the risk stratification for SCD in patients with ischemic and nonischemic heart diseases. Negative predictive value of this test is high and a negative test strongly predicts freedom from VT and VF.96
2. Disease specific treatment by sympathetic or parasympathetic modulation for patients at high risk for sudden cardiac death (Table 2)
Table 2.
Disease specific treatment by sympathetic of parasympathetic modulation to prevent sudden cardiac death
| Disease | Trigger | Treatment | |
|---|---|---|---|
| Ventircular tachyarrhythmias | |||
| Ischemia & myocardial infarction | Sympathetic | BB, ARB, ACE-I, aspirin, RFCA, ICD, BCSD | |
| Cardiomyopathy | |||
| DCM | Sympathetic | BB, ARB, ACE-I, III, ICD, BCSD | |
| HCM | Sympathetic | BB, Ca-B, III, ICD, BCSD | |
| ARVC | Sympathetic | BB, ARB, ACE-I, III, ICD, BCSD | |
| Arrhythmia | |||
| Long QT syndrome | |||
| LQT1 | Sympathetic | BB, LCSD | |
| LQT2 | Sympathetic | BB, LCSD? | |
| LQT3 | Parasympathetic | Mexiletine, pacemaker, ICD | |
| Brugada Syndrome | Parasympathetic | Quinidine, isoproterenol, ICD BB?, ICD | |
| Idiopathic VF | Sympathetic/Parasympathetic | BB?, ICD Quinidine, isoproterenol, ICD | |
| CPVT | Sympathetic | BB, flecainide, LCSD | |
BB=beta blocker, Ca-B= calcium channel blocker, ACE-I=angiotensin converting enzyme inhibitor, ICD=implantable cardioverter defibrillator, I,II= class I II and III anti arrhythmics, RFCA=radiofrequency catheter ablation, BCSD=bilateral cardiac sympathectomy, LCSD=left cardiac sympathectomy,
Atrial fibrillation
Several mechanisms have been proposed to explain the pathogenesis of AF suggesting a strong link to the ANS.97 The clinical correlation of an autonomic influence was noted by Coumel et al and has since then been the subject of several studies.98 Electrical stimulation of autonomic nerves during the atrial refractory period has been shown to produce rapid ectopic beats from the pulmonary veins (PVs) and superior vena cava, which in turn can initiate AF.99–101 It is now generally accepted that the ANS has an important contribution to the pathogenesis of AF.102, 103. However, AF still remains poorly understood and the specific mechanisms underlying the relationship between the ANS and AF have yet to be fully elucidated. Imbalances in the intrinsic nervous system of the heart (Figure 2) are thought to be involved in the pathogenesis of AF and therefore strategies have been developed to modify the synaptic efficacy of these structures by spinal cord stimulation,104 ablate ganglionated plexi105 or the vein of Marshall.106 Recent studies have utilized an alternative neuromodulation based strategy for control of the atrial arrythmogenic substrate, spinal cord stimulation.104 High thoracic spinal cord stimulation stabilizes neural processing within the intrinsic cardiac nervous system, reducing the potential for neurally induced AF. Moreover the efficacy of such therapy increases with time and is related to induced changes in intrinsic cardiac neural network function.7, 101, 104 Ganglionated plexus ablation has been proposed as a strategy for management of AF based on experimental models and human studies. These treatments have the potential to impact ventricular electrophysiology and arrhythmogenesis. Studies have shown significantly increased risk of ventricular arrhythmias in the setting of acute myocardial ischemia heart compared with normal hearts107 and there is some evidence of this having relevance to humans post ablation suggesting the need for careful follow up.108
Ventricular tachyarrhythmias related to ischemia and infarction
In patients with myocardial infarction, ventricular arrhythmias develop during the acute and chronic phase. In the acute phase of coronary occlusion, reentry caused by heterogeneity of the ischemic myocardium is considered as major mechanism. Reperfusion arrhythmias are caused by washout of various ions such as lactate, potassium and toxic metabolic substances from the ischemic zone109 and also oxidative stress alters autonomic function.52 Reflex activation of the cardiac nervous system, leading to heterogeneous sympathetic activation, contributes to the arrhythmogenic substrate.
VT is often encountered in patients with a healed myocardial infarction.110 These VTs are mostly monomorphic and caused by reentry involving a region of infarcted scar. Myocardial scars are most commonly caused by an old myocardial infarction, but can also be seen in arrhythmogenic right ventricular cardiomyopathy (ARVC), sarcoidosis and other non-ischemic cardiomyopathies.111 Fibrotic scar creates areas of slow conduction or block between surviving myocytes and promotes reentry. Schwartz et al reported that the presence of a reduced BRS is associated with a greater susceptibility to VF in a canine model of healed myocardial infarction.112 This data indicates that there are inherent and acquired differences in the neural substrate for cardiac control that contribute to the potential for SCD in the setting of acute and chronic ischemic heart disease.
BBs are essential pharmacological treatment in patients with coronary artery disease and HF. BBs reduce O2 requirements in myocardium by decreasing heart rate and exercise induced increases in blood pressure. Since BBs block arrhythmogenic sympathetic myocardial stimulation, antiarrhythmic effects also contribute to a favorable outcome. BBs exert this cardioprotective effect by targeting elements of the cardiac nervous system as well as the end-effectors of the heart.24–26
Cardiomyopathy
Cardiomyopathies including dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy and ARVC can be associated with VTs. The mechanism of VTs in these patients is reentry, involving the fibrotic scar with slow conduction. Although TWA may be a useful marker of risk stratification in patients with DCM,113, 114 it is difficult to predict patients at high risk of sudden death. The effect of antiarrhythmic drugs is uncertain and implantable cardioverter defibrillator are indicated for primary and secondary prevention of SCD in these patients. In addition to BBs, neuraxial therapy has been valuable in the management of arrhythmias in these patients.87
Heart Failure
Increased sympathetic tone in the failing heart causes diastolic Ca++ leak through ryanodine receptor 2 (RyR2) resulting in localized and transient increases in Ca++ in cardiomyocytes. Focally increased Ca++ initiates more Ca++ release and propagates as Ca++ waves. These Ca++ waves can cause delayed after depolarizations resulting in a ventricular premature beats and sustained VT. The effect of beta-adrenergic receptor blockers on the survival in patients with HF was proven by multiple placebo-controlled multicenter trials.115–117 BB combined with angiotensin converting enzyme-I produce reverse remodeling of LV, improve patient symptoms, lower hospitalization and prolong survival.118
Inherited arrhythmia
The ANS plays an important role in the development of various inherited arrhythmias.
Long QT syndrome
Long QT (LQT) syndromes are characterized by a prolonged QT interval on the ECG and an increased risk of sudden death by polymorphic VT/torsades de pointes. In congenital LQT syndrome, several clinical phenotypes have been well described including the autosomal dominant Romano-Ward syndrome and the autosomal recessive Jervell and Lange-Nielsen syndrome with or without associated deafness. So far, more than 17 genotypes have identified but great majority (90%) of cases are LQT1–3. Phenotype-genotype relationships are well studied and the onset of syncope or TdP is initiated by exercise in LQT1 and by noise, sudden wakening from sleep by an alarm clock or telephone rings in LQT2; there are also cardiac changes associated with sleep stages.119–121 Patients with LQT3 develop events when at rest or asleep. In LQT1 and LQT2, beta-adrenergic stimulation enhances transmural dispersion of repolarization and induced TdP. BBs are effective especially in LQT1 but indicated in all LQT patients including genotyped patients with normal QTc. Therapeutic importance of cardiac innervation is evidenced by the fact left cardiac sympathetic denervation (LCSD) is valuable in high risk patients who are intolerant or refractory to BBs alone.122
Brugada syndrome
Brugada syndrome (BS) is characterized by ST elevation in the precordial leads and associated with syncope or sudden death due to VF.123 VF in BS patients is known to develop more frequently at night than during the remainder of the day. Enhanced vagal tone including a full stomach provokes ST elevation. A decreased nocturnal SDANN measured in Holter recordings is one of the markers of risk stratification of BS. Sympathetic stimulation such as exercise, isoproterenol infusion improves ST elevation and suppresses syncopal or fibrillatory events.
Idiopathic ventricular fibrillation
Diagnosis of idiopathic ventricular fibrillation (IVF) is made if patients survive cardiac arrest and the etiology cannot be determined by all available testing. Clinical evaluation should be performed to exclude coronary artery disease, cardiomyopathy, or primary electrical disease including BS, LQT or catecholaminergic polymorphic ventricular tachycardia (CPVT). Although IVF patients are heterogeneous, among the non-Brugada IVF patient, some patients demonstrate similar phenotype with Brugada syndrome. Such patients have higher incidence of J-waves. IVF patients with J-waves were highlighted as early repolarization syndrome and isoproterenol and quinidine is effective in suppressing VF episodes. There exists a circadian pattern of VF in idiopathic VF patients and the presence of J waves was associated with nocturnal occurrence.124
Catecholaminergic polymorphic ventricular tachycardia
CPVT is characterized by adrenergically induced polymorphic ventricular tachycardia which can be reproducibly induced by physical or emotional stress. Mutations in the cardiac RyR2 gene underlie autosomal dominant CPVT125, while cardiac calsequestrin mutations underlie autosomal recessive CPVT.126 Intracellular calcium overload triggered by adrenergic stimulation is the disease mechanism. Discontinuation of exercise is required and beta-blocking agents are the first line of therapy. Flecainide is alternative pharmacological therapy for patients when cardiac events are not controlled with BBs alone.127 LCSD has been reported to be effective in patients with drug refractory ventricular arrhythmias.128
Ventricular Tachycardia and Fibrillation Storm in Patients with Structural Heart Disease
Patients with a wide variety of cardiac structural disease present with VT and sometimes this occurs in a cluster (storm) which is associated with a high mortality.129 Typically these patients are managed with supportive measures, anti-arrhythmic drugs and catheter ablation. The presence of a scar in the heart provides the substrate for VT, but it is not always seen and the pathophysiological role is unclear in patients with dilated cardiomyopathies suggesting a role for functional factors that govern impulse propagation.111 However, even scar based reentrant arrhythmias require obligate areas of functional block/conduction changes that allow impulse propagation in preferential directions.84, 130, 131 Thus, clinical occurrence of VT reflects the balance between macro structure and functional control. The importance of understanding why only some VTs are clinically encountered when a scar can have multiple circuits is highlighted by the clinical data showing that targeting of the clinical VT is crucial for improved outcomes (not just an arbitrary circuit modification achieved by catheter ablation).132 In instances when the cardiac substrate is not amenable to catheter modification or refractory to such approaches, neuraxial strategies such as thoracic epidural anesthesia and bilateral cardiac sympathetic denervation have been beneficial.17, 87, 133 Patients who undergo such procedures can show changes in cardiac interoception and objective measures of reduced sympathetic outflow to the heart.18 This again highlights another aspect of the brain heart connection.12, 13
A perspective on neuromodulation to prevent sudden cardiac death based on improved understanding of cardiac innervation
Cardiac disease results in adaptations of afferent and efferent input to various levels of the neuraxis.2, 10 Such adaptations result in changes to the integrated neural function within central and peripheral aspects of the cardiac nervous system. For stress-induced changes in cardiac electrical stability, there are inter-dependent interactions within the nervous system and at the neural-myocyte interface. The following points summarize the current state of the field for neurocardiology with respect to the evolving potential for neuromodulation based anti-arrhythmic therapy based on a better understanding of cardiac innervation.
-
➢
Afferent sensory transduction of the pathologically stressed heart results in a reflex driven adrenergic efferent postganglionic neuronal output to the heart.
-
➢
The reflex response of the higher centers to the sensory inputs from stressed heart, especially from ischemic myocardium, is inherently pro-arrhythmic resulting in augmented NE release.
-
➢
Chronic heart disease adversely remodels multiple levels of the cardiac neuraxis with a resultant shift towards discordant cardio-cardiac reflexes, an adaptation by itself that can be pro-arrhythmic.
-
➢
Cardiac neuromodulation/ART at different levels of the cardiac neuraxis has the potential to exert anti-arrhythmic effects while still preserving basic integrated reflex control the heart.
Recent work has demonstrated that targeting select elements within the cardiac nervous system by electrical stimulation or transection and pharmacological manipulation is effective in select cardiac disease states including myocardial ischemia/infarction,134–136 atrial arrhythmias,101, 104, 137, 138 and ventricular arrhythmias.3, 87, 134 With appropriate neuro-modulation therapy, myocytes are rendered stress resistant, autonomic responsiveness for control of the heart is preserved, and the potential for fatal arrhythmias is reduced.134–136, 139–141 Current ART therapies are delivered in the open-loop configuration (no feedback) and with the cardiac nervous system considered a ‘black box’. To rectify this critical deficit in knowledge, future studies should evaluate reactive and adaptive changes in network function from successive levels for the cardiac neuraxis. This is likely to help develop approaches to mechanism-based targeted neuromodulation for cardiac therapeutics.
Conclusions
The emerging field of neurocardiology is predicated on the dynamic interactions between the substrate of the heart and the neurohumoral control systems that regulate it. As detailed herein, there are inherent and acquired adaptions in both the heart and the nervous system that impact the progression of cardiac disease. With each year new insights are gained into these adaptations at the molecular, cellular, organ and whole body level. Such information is critical to: 1) identifying patients at high risk for future adverse outcome; 2) providing novel targets to pre-emptively manage such patients. Neuromodulation strategies show promise of sustaining cardiac function while maintaining electrical stability.
Supplementary Material
Acknowledgments
Sources of funding:
KS is supported by the NHLBI (R01HL084261) and JLA was supported by NHLBI (R01 HL071830)
Disclosures:
JLA has grant funding from St. Jude Medical, Glaxo Smith Klein, and Cyberonics Inc. JLA serves as a consultant to Cyberonics Inc.
Nonstandard Abbreviations and Acronyms
- HTN
hypertension
- HF
heart failure
- ANS
autonomic nervous system
- SCD
sudden cardiac death
- ART
autonomic regulation therapy
- VT
ventricular tachycardia
- Sema3A
semaphoring 3A
- SemaTG
transgenic mouse expressing semaphorin
- E
embryonic
- WT
wild-type
- VF
ventricular fibrillation
- CHF
congestive heart failure
- BBs
beta blockers
- NGF
nerve growth factor
- DM
diabetes mellitus
- CGRP
Calcitonin gene-related peptide
- CNS
central nervous system
- BRS
baroreflex sensitivity
- RVLM
rostral ventrolateral medulla
- RDN
renal denervation
- AF
atrial fibrillation
- NE
norepinephrine
- TH
tyrosine hydroxylase
- SNS
sympathetic nervous system
- LIF
leukemia inhibitory factor
- IL-6
interleukin-6
- GAP43
growth-associated protein 43
- LVEF
left ventricular ejection fraction
- HRV
heart rate variability
- HRT
heart rate turbulence
- HRDC
heart rate deceleration capacity
- TWA
T wave alternans
- SDANN
standard deviation of the average of normal sinus to normal sinus
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- DCM
dilated cardiomyopathy
- RyR2
ryanodine receptor 2
- LQT
long QT
- LCSD
left cardiac sympathetic denervation
- BS
Brugada syndrome
- IVF
idiopathic ventricular fibrillation
- CPVT
catecholaminergic polymorphic ventricular tachycardia
REFERENCES
- 1.Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, Mariani R, Gunson K, Jui J. Epidemiology of sudden cardiac death: Clinical and research implications. Prog Cardiovasc Dis. 2008;51:213–228. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–1021. doi: 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz PJ. Cardiac sympathetic denervation to prevent life-threatening arrhythmias. Nat Rev Cardiol. 2014;11:346–353. doi: 10.1038/nrcardio.2014.19. [DOI] [PubMed] [Google Scholar]
- 4.Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis. 2008;50:404–419. doi: 10.1016/j.pcad.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armour JA. Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol. 2004;287:R262–R271. doi: 10.1152/ajpregu.00183.2004. [DOI] [PubMed] [Google Scholar]
- 6.Armour JA. Potential clinical relevance of the 'little brain' on the mammalian heart. Exp Physiol. 2008;93:165–176. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- 7.Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: Implications for reflex control of regional cardiac function. J Physiol. 2013;591:4515–4533. doi: 10.1113/jphysiol.2013.259382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115:2305–2315. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kember G, Armour JA, Zamir M. Neural control hierarchy of the heart has not evolved to deal with myocardial ischemia. Physiol Genomics. 2013;45:638–644. doi: 10.1152/physiolgenomics.00027.2013. [DOI] [PubMed] [Google Scholar]
- 10.Zucker IH, Patel KP, Schultz HD. Neurohumoral stimulation. Heart Fail Clin. 2012;8:87–99. doi: 10.1016/j.hfc.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oppenheimer SM, Cechetto DF. Cardiac chronotropic organization of the rat insular cortex. Brain Res. 1990;533:66–72. doi: 10.1016/0006-8993(90)91796-j. [DOI] [PubMed] [Google Scholar]
- 12.Child N, Hanson B, Bishop M, Rinaldi CA, Bostock J, Western D, Cooklin M, O'Neil M, Wright M, Razavi R, Gill J, Taggart P. Effect of mental challenge induced by movie clips on action potential duration in normal human subjects independent of heart rate. Circ Arrhythm Electrophysiol. 2014;7:518–523. doi: 10.1161/CIRCEP.113.000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray MA, Taggart P, Sutton PM, Groves D, Holdright DR, Bradbury D, Brull D, Critchley HD. A cortical potential reflecting cardiac function. Proc Natl Acad Sci U S A. 2007;104:6818–6823. doi: 10.1073/pnas.0609509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oppenheimer S. Cerebrogenic cardiac arrhythmias: Cortical lateralization and clinical significance. Clin Auton Res. 2006;16:6–11. doi: 10.1007/s10286-006-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laowattana S, Zeger SL, Lima JA, Goodman SN, Wittstein IS, Oppenheimer SM. Left insular stroke is associated with adverse cardiac outcome. Neurology. 2006;66:477–483. doi: 10.1212/01.wnl.0000202684.29640.60. [DOI] [PubMed] [Google Scholar]
- 16.Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension. 2014;64:745–755. doi: 10.1161/HYPERTENSIONAHA.114.03699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourke T, Vaseghi M, Michowitz Y, Sankhla V, Shah M, Swapna N, Boyle NG, Mahajan A, Narasimhan C, Lokhandwala Y, Shivkumar K. Neuraxial modulation for refractory ventricular arrhythmias: Value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation. 2010;121:2255–2262. doi: 10.1161/CIRCULATIONAHA.109.929703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalsa SS, Shahabi L, Ajijola OA, Bystritsky A, Naliboff BD, Shivkumar K. Synergistic application of cardiac sympathetic decentralization and comprehensive psychiatric treatment in the management of anxiety and electrical storm. Front Integr Neurosci. 2014;7:98. doi: 10.3389/fnint.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaseghi M, Lellouche N, Ritter H, Fonarow GC, Patel JK, Moriguchi J, Fishbein MC, Kobashigawa JA, Shivkumar K. Mode and mechanisms of death after orthotopic heart transplantation. Heart rhythm. 2009;6:503–509. doi: 10.1016/j.hrthm.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puschel AW, Adams RH, Betz H. Murine semaphorin d/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron. 1995;14:941–948. doi: 10.1016/0896-6273(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 21.Ieda M, Kanazawa H, Kimura K, Hattori F, Ieda Y, Taniguchi M, Lee JK, Matsumura K, Tomita Y, Miyoshi S, Shimoda K, Makino S, Sano M, Kodama I, Ogawa S, Fukuda K. Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat Med. 2007;13:604–612. doi: 10.1038/nm1570. [DOI] [PubMed] [Google Scholar]
- 22.Stramba-Badiale M, Lazzarotti M, Schwartz PJ. Development of cardiac innervation, ventricular fibrillation, and sudden infant death syndrome. Am J physiol. 1992;263:H1514–H1522. doi: 10.1152/ajpheart.1992.263.5.H1514. [DOI] [PubMed] [Google Scholar]
- 23.Nakano Y, Chayama K, Ochi H, Toshishige M, Hayashida Y, Miki D, Hayes CN, Suzuki H, Tokuyama T, Oda N, Suenari K, Uchimura-Makita Y, Kajihara K, Sairaku A, Motoda C, Fujiwara M, Watanabe Y, Yoshida Y, Ohkubo K, Watanabe I, Nogami A, Hasegawa K, Watanabe H, Endo N, Aiba T, Shimizu W, Ohno S, Horie M, Arihiro K, Tashiro S, Makita N, Kihara Y. A nonsynonymous polymorphism in semaphorin 3a as a risk factor for human unexplained cardiac arrest with documented ventricular fibrillation. PLoS Genet. 2013;9:e1003364. doi: 10.1371/journal.pgen.1003364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hankes GH, Ardell JL, Tallaj J, Wei CC, Aban I, Holland M, Rynders P, Dillon R, Cardinal R, Hoover DB, Armour JA, Husain A, Dell'Italia LJ. Beta1-adrenoceptor blockade mitigates excessive norepinephrine release into cardiac interstitium in mitral regurgitation in dog. Am J Physiol Heart Circ Physiol. 2006;291:H147–H151. doi: 10.1152/ajpheart.00951.2005. [DOI] [PubMed] [Google Scholar]
- 25.Hardwick JC, Southerland EM, Girasole AE, Ryan SE, Negrotto S, Ardell JL. Remodeling of intrinsic cardiac neurons: Effects of beta-adrenergic receptor blockade in guinea pig models of chronic heart disease. Am J Physiol Regul Integr Comp Physiol. 2012;303:R950–R958. doi: 10.1152/ajpregu.00223.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tallaj J, Wei CC, Hankes GH, Holland M, Rynders P, Dillon AR, Ardell JL, Armour JA, Lucchesi PA, Dell'Italia LJ. Beta1-adrenergic receptor blockade attenuates angiotensin ii-mediated catecholamine release into the cardiac interstitium in mitral regurgitation. Circulation. 2003;108:225–230. doi: 10.1161/01.CIR.0000079226.48637.5A. [DOI] [PubMed] [Google Scholar]
- 27.Snider WD. Functions of the neurotrophins during nervous system development: What the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 28.Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86:816–821. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 29.Kaye DM, Vaddadi G, Gruskin SL, Du XJ, Esler MD. Reduced myocardial nerve growth factor expression in human and experimental heart failure. Circ Res. 2000;86:E80–E84. doi: 10.1161/01.res.86.7.e80. [DOI] [PubMed] [Google Scholar]
- 30.Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B, Chen PS. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004;95:76–83. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]
- 31.Kimura K, Ieda M, Kanazawa H, Yagi T, Tsunoda M, Ninomiya S, Kurosawa H, Yoshimi K, Mochizuki H, Yamazaki K, Ogawa S, Fukuda K. Cardiac sympathetic rejuvenation: A link between nerve function and cardiac hypertrophy. Circ Res. 2007;100:1755–1764. doi: 10.1161/01.RES.0000269828.62250.ab. [DOI] [PubMed] [Google Scholar]
- 32.Chen RH, Li YG, Jiao KL, Zhang PP, Sun Y, Zhang LP, Fong XF, Li W, Yu Y. Overexpression of sema3a in myocardial infarction border zone decreases vulnerability of ventricular tachycardia post-myocardial infarction in rats. J Cell Mol Med. 2013;17:608–616. doi: 10.1111/jcmm.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol. 2012;8:405–416. doi: 10.1038/nrendo.2012.21. [DOI] [PubMed] [Google Scholar]
- 34.Hua F, Harrison T, Qin C, Reifsteck A, Ricketts B, Carnel C, Williams CA. C-fos expression in rat brain stem and spinal cord in response to activation of cardiac ischemia-sensitive afferent neurons and electrostimulatory modulation. Am J Physiol. Heart circ physiol. 2004;287:H2728–H2738. doi: 10.1152/ajpheart.00180.2004. [DOI] [PubMed] [Google Scholar]
- 35.Faerman I, Faccio E, Milei J, Nunez R, Jadzinsky M, Fox D, Rapaport M. Autonomic neuropathy and painless myocardial infarction in diabetic patients. Histologic evidence of their relationship. Diabetes. 1977;26:1147–1158. doi: 10.2337/diab.26.12.1147. [DOI] [PubMed] [Google Scholar]
- 36.Lu J, Gao X, Gu J, Zhou L, Guo S, Hao W, Zhou Z, Cao JM. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48:448–455. doi: 10.1007/s12031-012-9720-x. [DOI] [PubMed] [Google Scholar]
- 37.Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez CF. Peripheral expression and biological activities of gdnf, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of trka trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 39.Ieda M, Kanazawa H, Ieda Y, Kimura K, Matsumura K, Tomita Y, Yagi T, Onizuka T, Shimoji K, Ogawa S, Makino S, Sano M, Fukuda K. Nerve growth factor is critical for cardiac sensory innervation and rescues neuropathy in diabetic hearts. Circulation. 2006;114:2351–2363. doi: 10.1161/CIRCULATIONAHA.106.627588. [DOI] [PubMed] [Google Scholar]
- 40.Apfel SC, Schwartz S, Adornato BT, Freeman R, Biton V, Rendell M, Vinik A, Giuliani M, Stevens JC, Barbano R, Dyck PJ. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: A randomized controlled trial. Rhngf clinical investigator group. JAMA. 2000;284:2215–2221. doi: 10.1001/jama.284.17.2215. [DOI] [PubMed] [Google Scholar]
- 41.Chidsey CA, Harrison DC, Braunwald E. Augmentation of the plasma nor-epinephrine response to exercise in patients with congestive heart failure. The New Engl J Med. 1962;267:650–654. doi: 10.1056/NEJM196209272671305. [DOI] [PubMed] [Google Scholar]
- 42.Cody RJ, Franklin KW, Kluger J, Laragh JH. Mechanisms governing the postural response and baroreceptor abnormalities in chronic congestive heart failure: Effects of acute and long-term converting-enzyme inhibition. Circulation. 1982;66:135–142. doi: 10.1161/01.cir.66.1.135. [DOI] [PubMed] [Google Scholar]
- 43.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Atrami (autonomic tone and reflexes after myocardial infarction) investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 44.Georgakopoulos D, Little WC, Abraham WT, Weaver FA, Zile MR. Chronic baroreflex activation: A potential therapeutic approach to heart failure with preserved ejection fraction. J Card Fail. 2011;17:167–178. doi: 10.1016/j.cardfail.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Doumas M, Faselis C, Tsioufis C, Papademetriou V. Carotid baroreceptor activation for the treatment of resistant hypertension and heart failure. Curr Hypertens Rep. 2012;14:238–246. doi: 10.1007/s11906-012-0258-7. [DOI] [PubMed] [Google Scholar]
- 46.Chua TP, Clark AL, Amadi AA, Coats AJ. Relation between chemosensitivity and the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol. 1996;27:650–657. doi: 10.1016/0735-1097(95)00523-4. [DOI] [PubMed] [Google Scholar]
- 47.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: Rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol. 2013;62:2422–2430. doi: 10.1016/j.jacc.2013.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niewinski P, Janczak D, Rucinski A, Jazwiec P, Sobotka PA, Engelman ZJ, Fudim M, Tubek S, Jankowska EA, Banasiak W, Hart EC, Paton JF, Ponikowski P. Carotid body removal for treatment of chronic systolic heart failure. Int J Cardiol. 2013;168:2506–2509. doi: 10.1016/j.ijcard.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Leenen FH. Brain mechanisms contributing to sympathetic hyperactivity and heart failure. Circ Res. 2007;101:221–223. doi: 10.1161/CIRCRESAHA.107.158261. [DOI] [PubMed] [Google Scholar]
- 50.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]
- 51.Hirooka Y. Brain perivascular macrophages and central sympathetic activation after myocardial infarction: Heart and brain interaction. Hypertension. 2010;55:610–611. doi: 10.1161/HYPERTENSIONAHA.109.145128. [DOI] [PubMed] [Google Scholar]
- 52.Dawson TA, Li D, Woodward T, Barber Z, Wang L, Paterson DJ. Cardiac cholinergic no-cgmp signaling following acute myocardial infarction and nnos gene transfer. Am J Physiol. Heart Circ Physiol. 2008;295:H990–H998. doi: 10.1152/ajpheart.00492.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sobotka PA, Krum H, Bohm M, Francis DP, Schlaich MP. The role of renal denervation in the treatment of heart failure. Curr Cardiol Rep. 2012;14:285–292. doi: 10.1007/s11886-012-0258-x. [DOI] [PubMed] [Google Scholar]
- 54.Calaresu FR, Ciriello J. Renal afferent nerves affect discharge rate of medullary and hypothalamic single units in the cat. J Auton Nerv Syst. 1981;3:311–320. doi: 10.1016/0165-1838(81)90072-2. [DOI] [PubMed] [Google Scholar]
- 55.Ciriello J, Calaresu FR. Central projections of afferent renal fibers in the rat: An anterograde transport study of horseradish peroxidase. J Auton Nerv Syst. 1983;8:273–285. doi: 10.1016/0165-1838(83)90110-8. [DOI] [PubMed] [Google Scholar]
- 56.Cechetto DF. Cortical control of the autonomic nervous system. Exp Physiol. 2014;99:326–331. doi: 10.1113/expphysiol.2013.075192. [DOI] [PubMed] [Google Scholar]
- 57.Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: Chronic preclinical evidence for renal artery safety. Clin Res Cardiology. 2011;100:1095–1101. doi: 10.1007/s00392-011-0346-8. [DOI] [PubMed] [Google Scholar]
- 58.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 59.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity htn-2 trial): A randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 60.Krum H, Schlaich MP, Sobotka PA, Bohm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: Final 3-year report of the symplicity htn-1 study. Lancet. 2014;383:622–629. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 61.Vogel B, Kirchberger M, Zeier M, Stoll F, Meder B, Saure D, Andrassy M, Mueller OJ, Hardt S, Schwenger V, Strothmeyer A, Katus HA, Blessing E. Renal sympathetic denervation therapy in the real world: Results from the heidelberg registry. Clin Res Cardiology. 2014;103:117–124. doi: 10.1007/s00392-013-0627-5. [DOI] [PubMed] [Google Scholar]
- 62.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL. A controlled trial of renal denervation for resistant hypertension. New Engl J Med. 2014 doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 63.Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Bohm M, Hoppe UC. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901–909. doi: 10.1016/j.jacc.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 64.Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, Hamady M, Hughes AD, Sever PS, Sobotka PA, Francis DP. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: Primary outcome from reach-pilot study. Int J Cardiol. 2013;162:189–192. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 65.Witkowski A, Prejbisz A, Florczak E, Kadziela J, Sliwinski P, Bielen P, Michalowska I, Kabat M, Warchol E, Januszewicz M, Narkiewicz K, Somers VK, Sobotka PA, Januszewicz A. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 66.Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, Shirokova N, Karaskov A, Mittal S, Steinberg JS. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–1170. doi: 10.1016/j.jacc.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 67.Remo BF, Preminger M, Bradfield J, Mittal S, Boyle N, Gupta A, Shivkumar K, Steinberg JS, Dickfeld T. Safety and efficacy of renal denervation as a novel treatment of ventricular tachycardia storm in patients with cardiomyopathy. Heart Rhythm. 2014;11:541–546. doi: 10.1016/j.hrthm.2013.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ukena C, Bauer A, Mahfoud F, Schreieck J, Neuberger HR, Eick C, Sobotka PA, Gawaz M, Bohm M. Renal sympathetic denervation for treatment of electrical storm: First-in-man experience. Clin Res Cardiol. 2012;101:63–67. doi: 10.1007/s00392-011-0365-5. [DOI] [PubMed] [Google Scholar]
- 69.Bradfield JS, Vaseghi M, Shivkumar K. Renal denervation for refractory ventricular arrhythmias. Trends Cardiovasc Med. 2014;24:206–213. doi: 10.1016/j.tcm.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reddy V. Renal sympathetic denervation to suppress ventricular tachyarrhythmias (rescue-vt), nct01747837 [Google Scholar]
- 71.Chidsey CA, Braunwald E, Morrow AG, Mason DT. Myocardial norepinephrine concentration in man. Effects of reserpine and of congestive heart failure. New Engl J Med. 1963;269:653–658. doi: 10.1056/NEJM196309262691302. [DOI] [PubMed] [Google Scholar]
- 72.Himura Y, Felten SY, Kashiki M, Lewandowski TJ, Delehanty JM, Liang CS. Cardiac noradrenergic nerve terminal abnormalities in dogs with experimental congestive heart failure. Circulation. 1993;88:1299–1309. doi: 10.1161/01.cir.88.3.1299. [DOI] [PubMed] [Google Scholar]
- 73.Kimura K, Kanazawa H, Ieda M, Kawaguchi-Manabe H, Miyake Y, Yagi T, Arai T, Sano M, Fukuda K. Norepinephrine-induced nerve growth factor depletion causes cardiac sympathetic denervation in severe heart failure. Auton neurosci. 2010;156:27–35. doi: 10.1016/j.autneu.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Zigmond RE, Hyatt-Sachs H, Mohney RP, Schreiber RC, Shadiack AM, Sun Y, Vaccariello SA. Changes in neuropeptide phenotype after axotomy of adult peripheral neurons and the role of leukemia inhibitory factor. Perspect Dev Neurobiol. 1996;4:75–90. [PubMed] [Google Scholar]
- 75.Sun Y, Zigmond RE. Involvement of leukemia inhibitory factor in the increases in galanin and vasoactive intestinal peptide mrna and the decreases in neuropeptide y and tyrosine hydroxylase mrna in sympathetic neurons after axotomy. J Neurochem. 1996;67:1751–1760. doi: 10.1046/j.1471-4159.1996.67041751.x. [DOI] [PubMed] [Google Scholar]
- 76.Shi X, Habecker BA. Gp130 cytokines stimulate proteasomal degradation of tyrosine hydroxylase via extracellular signal regulated kinases 1 and 2. J Neurochem. 2012;120:239–247. doi: 10.1111/j.1471-4159.2011.07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanazawa H, Ieda M, Kimura K, Arai T, Kawaguchi-Manabe H, Matsuhashi T, Endo J, Sano M, Kawakami T, Kimura T, Monkawa T, Hayashi M, Iwanami A, Okano H, Okada Y, Ishibashi-Ueda H, Ogawa S, Fukuda K. Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. J Clin Invest. 2010;120:408–421. doi: 10.1172/JCI39778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- 79.Zheng C, Li M, Inagaki M, Kawada T, Sunagawa K, Sugimachi M. Vagal stimulation markedly suppresses arrhythmias in conscious rats with chronic heart failure after myocardial infarction. Conf Proc IEEE Eng Med Biol Soc. 2005;7:7072–7075. doi: 10.1109/IEMBS.2005.1616135. [DOI] [PubMed] [Google Scholar]
- 80.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K, Schwartz PJ CardioFit Multicenter Trial I. Chronic vagus nerve stimulation: A new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 81.Zannad F, De Ferrari GM, Tuinenburg AE, Wright D, Brugada J, Butter C, Klein H, Stolen C, Meyer S, Stein KM, Ramuzat A, Schubert B, Daum D, Neuzil P, Botman C, Caste MA, D'Onofrio A, Solomon SD, Wold N, Ruble SB. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: Results of the neural cardiac therapy for heart failure (nectar-hf) randomized controlled trial. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu345. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rana OR, Saygili E, Meyer C, Gemein C, Kruttgen A, Andrzejewski MG, Ludwig A, Schotten U, Schwinger RH, Weber C, Weis J, Mischke K, Rassaf T, Kelm M, Schauerte P. Regulation of nerve growth factor in the heart: The role of the calcineurin-nfat pathway. J Mol Cell Cardiol. 2009;46:568–578. doi: 10.1016/j.yjmcc.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 83.Vaseghi M, Lux RL, Mahajan A, Shivkumar K. Sympathetic stimulation increases dispersion of repolarization in humans with myocardial infarction. Am J Physiol Heart Circ Physiol. 2012;302:H1838–H1846. doi: 10.1152/ajpheart.01106.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ajijola OA, Yagishita D, Patel KJ, Vaseghi M, Zhou W, Yamakawa K, So E, Lux RL, Mahajan A, Shivkumar K. Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: Neural remodeling in a spatial context. Am J Physiol Heart Circ Physiol. 2013;305:H1031–H1040. doi: 10.1152/ajpheart.00434.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ajijola OA, Yagashita D, Reddy N, Yamakawa K, Zhou W, Vaseghi M, Takemoto M, Mahajan A, Shivkumar K. Neurochemical remodeling of left and right stellate ganglion neurons after myocardial infarction. Heart rhythm. 2013;10:s446. doi: 10.1016/j.hrthm.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ajijola OA, Wisco JJ, Lambert HW, Mahajan A, Stark E, Fishbein MC, Shivkumar K. Extracardiac neural remodeling in humans with cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5:1010–1116. doi: 10.1161/CIRCEP.112.972836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vaseghi M, Gima J, Kanaan C, Ajijola OA, Marmureanu A, Mahajan A, Shivkumar K. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: Intermediate and long-term follow-up. Heart Rhythm. 2014;11:360–366. doi: 10.1016/j.hrthm.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen MJ, Hao-Che C, Park HW, George Akingba A, Chang PC, Zheng Z, Lin SF, Shen C, Chen LS, Chen Z, Fishbein MC, Chiamvimonvat N, Chen PS. Low-level vagus nerve stimulation upregulates small conductance calcium-activated potassium channels in the stellate ganglion. Heart Rhythm. 2013;10:910–915. doi: 10.1016/j.hrthm.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: So near, yet so far. Annu Rev Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB. C1 neurons: The body's emts. Am J Physiol Regul Integr Comp Physiol. 2013;305:R187–R204. doi: 10.1152/ajpregu.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.La Rovere MT, Specchia G, Mortara A, Schwartz PJ. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. A prospective study. Circulation. 1988;78:816–824. doi: 10.1161/01.cir.78.4.816. [DOI] [PubMed] [Google Scholar]
- 92.Billman GE, Schwartz PJ, Stone HL. The effects of daily exercise on susceptibility to sudden cardiac death. Circulation. 1984;69:1182–1189. doi: 10.1161/01.cir.69.6.1182. [DOI] [PubMed] [Google Scholar]
- 93.Schmidt G, Malik M, Barthel P, Schneider R, Ulm K, Rolnitzky L, Camm AJ, Bigger JT, Jr, Schomig A. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999;353:1390–1396. doi: 10.1016/S0140-6736(98)08428-1. [DOI] [PubMed] [Google Scholar]
- 94.Ghuran A, Reid F, La Rovere MT, Schmidt G, Bigger JT, Jr, Camm AJ, Schwartz PJ, Malik M. Heart rate turbulence-based predictors of fatal and nonfatal cardiac arrest (the autonomic tone and reflexes after myocardial infarction substudy) Am J Cardiol. 2002;89:184–190. doi: 10.1016/s0002-9149(01)02198-1. [DOI] [PubMed] [Google Scholar]
- 95.Bauer A, Kantelhardt JW, Barthel P, Schneider R, Makikallio T, Ulm K, Hnatkova K, Schomig A, Huikuri H, Bunde A, Malik M, Schmidt G. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: Cohort study. Lancet. 2006;367:1674–1681. doi: 10.1016/S0140-6736(06)68735-7. [DOI] [PubMed] [Google Scholar]
- 96.Ikeda T, Yoshino H, Sugi K, Tanno K, Shimizu H, Watanabe J, Kasamaki Y, Yoshida A, Kato T. Predictive value of microvolt t-wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction: Results of a collaborative cohort study. J Am Coll Cardiol. 2006;48:2268–2274. doi: 10.1016/j.jacc.2006.06.075. [DOI] [PubMed] [Google Scholar]
- 97.Wickramasinghe SR, Patel VV. Local innervation and atrial fibrillation. Circulation. 2013;128:1566–1575. doi: 10.1161/CIRCULATIONAHA.113.001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coumel P, Attuel P, Lavallée J, Flammang D, Leclercq JF, Slama R. the atrial arrhythmia syndrome of vagal origin. Arch Mal Coeur Vaiss. 1978;71:645–656. [PubMed] [Google Scholar]
- 99.Schauerte P, Scherlag BJ, Patterson E, Scherlag MA, Matsudaria K, Nakagawa H, Lazzara R, Jackman WM. Focal atrial fibrillation: Experimental evidence for a pathophysiologic role of the autonomic nervous system. J Cardiovasc Electrophysiol. 2001;12:592–599. doi: 10.1046/j.1540-8167.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- 100.Shivkumar K, Buch E, Boyle NG. Nonpharmacologic management of atrial fibrillation: Role of the pulmonary veins and posterior left atrium. Heart rhythm. 2009;6:S5–S11. doi: 10.1016/j.hrthm.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gibbons DD, Southerland EM, Hoover DB, Beaumont E, Armour JA, Ardell JL. Neuromodulation targets intrinsic cardiac neurons to attenuate neuronally mediated atrial arrhythmias. Am J Physiol Regul Integr Comp Physiol. 2012;302:R357–R364. doi: 10.1152/ajpregu.00535.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arora R. Recent insights into the role of the autonomic nervous system in the creation of substrate for atrial fibrillation: Implications for therapies targeting the atrial autonomic nervous system. Circ Arrhythm Electrophysiol. 2012;5:850–859. doi: 10.1161/CIRCEP.112.972273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ng J, Villuendas R, Cokic I, Schliamser JE, Gordon D, Koduri H, Benefield B, Simon J, Murthy SN, Lomasney JW, Wasserstrom JA, Goldberger JJ, Aistrup GL, Arora R. Autonomic remodeling in the left atrium and pulmonary veins in heart failure: Creation of a dynamic substrate for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:388–396. doi: 10.1161/CIRCEP.110.959650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ardell JL, Cardinal R, Beaumont E, M V, Smith FM, Armour JA. Chronic spinal cord stimulation modifies intrinsic cardiac synaptic efficacy in the suppression of atrial fibrillation. Auton Neurosci. 2014 doi: 10.1016/j.autneu.2014.09.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: A randomized study. Heart Rhythm. 2011;8:672–678. doi: 10.1016/j.hrthm.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 106.Baez-Escudero JL, Keida T, Dave AS, Okishige K, Valderrabano M. Ethanol infusion in the vein of marshall leads to parasympathetic denervation of the human left atrium: Implications for atrial fibrillation. J Am Coll Cardiol. 2014;63:1892–1901. doi: 10.1016/j.jacc.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He B, Lu Z, He W, Wu L, Cui B, Hu X, Yu L, Huang C, Jiang H. Effects of ganglionated plexi ablation on ventricular electrophysiological properties in normal hearts and after acute myocardial ischemia. Int J Cardiol. 2013;168:86–93. doi: 10.1016/j.ijcard.2012.09.067. [DOI] [PubMed] [Google Scholar]
- 108.Osman F, Kundu S, Tuan J, Jeilan M, Stafford PJ, Ng GA. Ganglionic plexus ablation during pulmonary vein isolation--predisposing to ventricular arrhythmias? Indian Pacing Electrophysiol J. 2010;10:104–107. [PMC free article] [PubMed] [Google Scholar]
- 109.Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69:1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- 110.Ajijola OA, Tung R, Shivkumar K. Ventricular tachycardia in ischemic heart disease substrates. Indian Heart J. 2014;66(Suppl 1):S24–S34. doi: 10.1016/j.ihj.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakahara S, Tung R, Ramirez RJ, Michowitz Y, Vaseghi M, Buch E, Gima J, Wiener I, Mahajan A, Boyle NG, Shivkumar K. Characterization of the arrhythmogenic substrate in ischemic and nonischemic cardiomyopathy implications for catheter ablation of hemodynamically unstable ventricular tachycardia. J Am Coll Cardiol. 2010;55:2355–2365. doi: 10.1016/j.jacc.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation. 1988;78:969–979. doi: 10.1161/01.cir.78.4.969. [DOI] [PubMed] [Google Scholar]
- 113.Sakabe K, Ikeda T, Sakata T, Kawase A, Kumagai K, Tezuka N, Takami M, Nakae T, Noro M, Enjoji Y, Sugi K, Yamaguchi T. Predicting the recurrence of ventricular tachyarrhythmias from t-wave alternans assessed on antiarrhythmic pharmacotherapy: A prospective study in patients with dilated cardiomyopathy. Ann Noninv Electrocardiol. 2001;6:203–208. doi: 10.1111/j.1542-474X.2001.tb00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sakabe K, Ikeda T, Sakata T, Kawase A, Kumagai K, Tezuka N, Takami M, Nakae T, Noro M, Enjoji Y, Sugi K, Yamaguchi T. Comparison of t-wave alternans and qt interval dispersion to predict ventricular tachyarrhythmia in patients with dilated cardiomyopathy and without antiarrhythmic drugs: A prospective study. Jap heart J. 2001;42:451–457. doi: 10.1536/jhj.42.451. [DOI] [PubMed] [Google Scholar]
- 115.The cardiac insufficiency bisoprolol study ii (cibis-ii): A randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 116.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol heart failure study group. New Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 117.Effect of metoprolol cr/xl in chronic heart failure: Metoprolol cr/xl randomised intervention trial in-congestive heart failure (merit-hf) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 118.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 accf/aha guideline for the management of heart failure: Executive summary: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 119.Garcia-Touchard A, Somers VK, Kara T, Nykodym J, Shamsuzzaman A, Lanfranchi P, Ackerman MJ. Ventricular ectopy during rem sleep: Implications for nocturnal sudden cardiac death. Nat Clin Pract Cardiovasc Med. 2007;4:284–288. doi: 10.1038/ncpcardio0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goldenberg I, Thottathil P, Lopes CM, Moss AJ, McNitt S, J OU, Robinson JL, Zareba W, Ackerman MJ, Kaufman ES, Towbin JA, Vincent M, Barsheshet A. Trigger-specific ion-channel mechanisms, risk factors, and response to therapy in type 1 long qt syndrome. Heart Rhythm. 2012;9:49–56. doi: 10.1016/j.hrthm.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 121.Lanfranchi PA, Ackerman MJ, Kara T, Shamsuzzaman AS, Wolk R, Jurak P, Amin R, Somers VK. Gene-specific paradoxical qt responses during rapid eye movement sleep in women with congenital long qt syndrome. Heart Rhythm. 2010;7:1067–1074. doi: 10.1016/j.hrthm.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Collura CA, Johnson JN, Moir C, Ackerman MJ. Left cardiac sympathetic denervation for the treatment of long qt syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm. 2009;6:752–759. doi: 10.1016/j.hrthm.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 123.Brugada P, Brugada J. Right bundle branch block, persistent st segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 124.Aizawa Y, Sato M, Ohno S, Horie M, Takatsuki S, Fukuda K, Chinushi M, Usui T, Aonuma K, Hosaka Y, Haissaguerre M, Aizawa Y. Circadian pattern of fibrillatory events in non-brugada-type idiopathic ventricular fibrillation with a focus on j waves. Heart Rhythm. 2014 doi: 10.1016/j.hrthm.2014.08.022. in press. [DOI] [PubMed] [Google Scholar]
- 125.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hryr2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 126.Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of casq2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in bedouin families from israel. Am J Hum Gen. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, Duff HJ, Roden DM, Wilde AA, Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, Ferrandi C, Koolbergen DR, Odero A, Schwartz PJ. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. New Engl J Med. 2008;358:2024–2029. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- 129.Verma A, Kilicaslan F, Marrouche NF, Minor S, Khan M, Wazni O, Burkhardt JD, Belden WA, Cummings JE, Abdul-Karim A, Saliba W, Schweikert RA, Tchou PJ, Martin DO, Natale A. Prevalence, predictors, and mortality significance of the causative arrhythmia in patients with electrical storm. J Cardiovasc Electrophysiol. 2004;15:1265–1270. doi: 10.1046/j.1540-8167.2004.04352.x. [DOI] [PubMed] [Google Scholar]
- 130.Tung R, Mathuria N, Michowitz Y, Yu R, Buch E, Bradfield J, Mandapati R, Wiener I, Boyle N, Shivkumar K. Functional pace-mapping responses for identification of targets for catheter ablation of scar-mediated ventricular tachycardia. Circ Arrhythm Electrophysiol. 2012;2:264–272. doi: 10.1161/CIRCEP.111.967976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tung R, Mathuria NS, Nagel R, Mandapati R, Buch EF, Bradfield JS, Vaseghi M, Boyle NG, Shivkumar K. Impact of local ablation on interconnected channels within ventricular scar: Mechanistic implications for substrate modification. Circ Arrhythm Electrophysiol. 2013;6:1131–1138. doi: 10.1161/CIRCEP.113.000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Della Bella P, Baratto F, Tsiachris D, Trevisi N, Vergara P, Bisceglia C, Petracca F, Carbucicchio C, Benussi S, Maisano F, Alfieri O, Pappalardo F, Zangrillo A, Maccabelli G. Management of ventricular tachycardia in the setting of a dedicated unit for the treatment of complex ventricular arrhythmias: Long-term outcome after ablation. Circulation. 2013;127:1359–1368. doi: 10.1161/CIRCULATIONAHA.112.000872. [DOI] [PubMed] [Google Scholar]
- 133.Pedersen CT, Kay GN, Kalman J, Borggrefe M, Della-Bella P, Dickfeld T, Dorian P, Huikuri H, Kim YH, Knight B, Marchlinski F, Ross D, Sacher F, Sapp J, Shivkumar K, Soejima K, Tada H, Alexander ME, Triedman JK, Yamada T, Kirchhof P, Lip GY, Kuck KH, Mont L, Haines D, Indik J, Dimarco J, Exner D, Iesaka Y, Savelieva I. Ehra/hrs/aphrs expert consensus on ventricular arrhythmias. Heart rhythm. 2014;10:e166–e196. doi: 10.1016/j.hrthm.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 134.Lopshire JC, Zhou X, Dusa C, Ueyama T, Rosenberger J, Courtney N, Ujhelyi M, Mullen T, Das M, Zipes DP. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120:286–294. doi: 10.1161/CIRCULATIONAHA.108.812412. [DOI] [PubMed] [Google Scholar]
- 135.Shinlapawittayatorn K, Chinda K, Palee S, Surinkaew S, Thunsiri K, Weerateerangkul P, Chattipakorn S, KenKnight BH, Chattipakorn N. Low-amplitude, left vagus nerve stimulation significantly attenuates ventricular dysfunction and infarct size through prevention of mitochondrial dysfunction during acute ischemia-reperfusion injury. Heart Rhythm. 2013;10:1700–1707. doi: 10.1016/j.hrthm.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 136.Southerland EM, Milhorn DM, Foreman RD, Linderoth B, DeJongste MJ, Armour JA, Subramanian V, Singh M, Singh K, Ardell JL. Preemptive, but not reactive, spinal cord stimulation mitigates transient ischemia-induced myocardial infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physiol. 2007;292:H311–H317. doi: 10.1152/ajpheart.00087.2006. [DOI] [PubMed] [Google Scholar]
- 137.Bernstein SA, Wong B, Vasquez C, Rosenberg SP, Rooke R, Kuznekoff LM, Lader JM, Mahoney VM, Budylin T, Alvstrand M, Rakowski-Anderson T, Bharmi R, Shah R, Fowler S, Holmes D, Farazi TG, Chinitz LA, Morley GE. Spinal cord stimulation protects against atrial fibrillation induced by tachypacing. Heart Rhythm. 2012;9:1426–1433. e1423. doi: 10.1016/j.hrthm.2012.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: Pathophysiology and therapy. Circ Res. 2014;114:1500–1515. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lopshire JC, Zipes DP. Device therapy to modulate the autonomic nervous system to treat heart failure. Curr Cardiol Rep. 2012;14:593–600. doi: 10.1007/s11886-012-0292-8. [DOI] [PubMed] [Google Scholar]
- 140.Odenstedt J, Linderoth B, Bergfeldt L, Ekre O, Grip L, Mannheimer C, Andrell P. Spinal cord stimulation effects on myocardial ischemia, infarct size, ventricular arrhythmia, and noninvasive electrophysiology in a porcine ischemia-reperfusion model. Heart Rhythm. 2011;8:892–898. doi: 10.1016/j.hrthm.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 141.Southerland EM, Gibbons DD, Smith SB, Sipe A, Williams CA, Beaumont E, Armour JA, Foreman RD, Ardell JL. Activated cranial cervical cord neurons affect left ventricular infarct size and the potential for sudden cardiac death. Auton Neurosci. 2012;169:34–42. doi: 10.1016/j.autneu.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rajab M, Jin H, Welzig CM, Albano A, Aronovitz M, Zhang Y, Park HJ, Link MS, Noujaim SF, Galper JB. Increased inducibility of ventricular tachycardia and decreased heart rate variability in a mouse model for type 1 diabetes: Effect of pravastatin. Am J Physiol Heart Circ Physiol. 2013;305:H1807–H1816. doi: 10.1152/ajpheart.00979.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.