Abstract
Objective
To compare outcomes of patients with retroperitoneal or pelvic sarcoma (RPPS) treated with versus without perioperative radiation therapy (RT).
Summary Background Data
Radiation therapy for RPPS is controversial, and few studies have compared outcomes with and without RT.
Methods
Prospectively-maintained databases were reviewed to retrospectively compare patients with primary RPPS treated during 2003-2011. Multivariate Cox regression models were used to assess associations with the primary endpoints: local recurrence-free survival (LRFS) and disease-specific survival (DSS).
Results
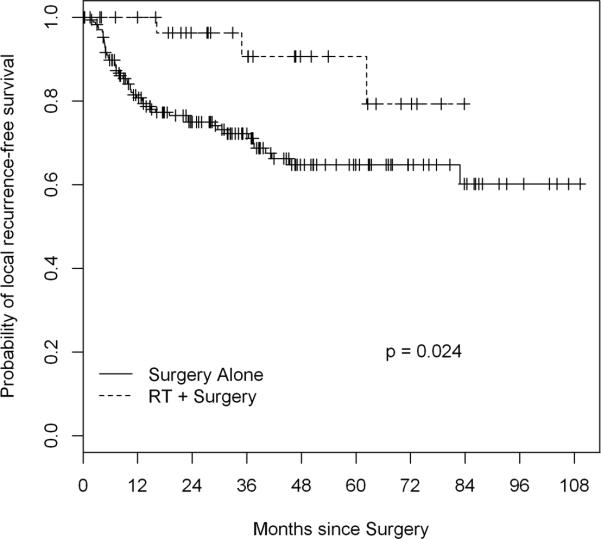
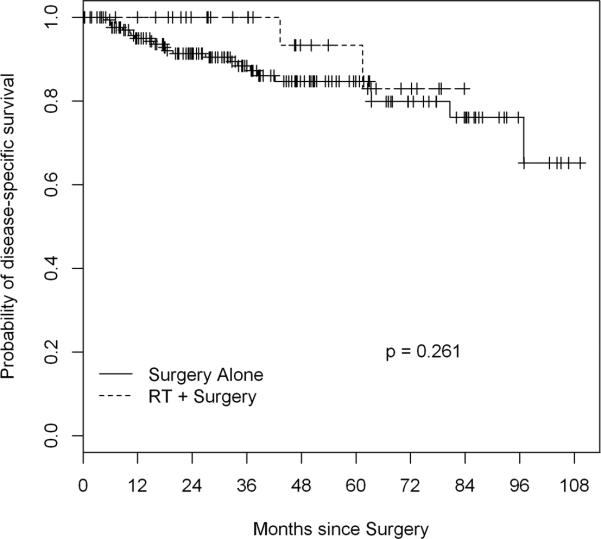
At one institution 172 patients were treated with surgery alone while at another institution 32 patients were treated with surgery and perioperative proton beam RT or intensity-modulated RT with or without intraoperative RT. The groups were similar in age, tumor size, grade, and margin status (all p>0.08). The RT group had a higher percentage of pelvic tumors (p=0.03) and a different distribution of histologies (p=0.04). Perioperative morbidity was higher in the RT group (44% vs 16% of patients; p=0.004). After a median follow-up of 39 months, 5-year LRFS was 91% (95% CI, 79-100%) in the RT group and 65% (57-74%) in the surgery only group (p=0.02). On multivariate analysis, RT was associated with better LRFS (hazard ratio 0.26; p=0.03). Five-year DSS was 93% (95% CI, 82-100%) in the RT group and 85% (78-92%) in the surgery-only group (p=0.3).
Conclusions
The addition of advanced-modality RT to surgery for primary RPPS was associated with improved LRFS, although this did not translate into significantly better DSS. This treatment strategy warrants further investigation in a randomized trial.
Introduction
Retroperitoneal and pelvic sarcomas (RPPSs) constitute approximately 15% of all soft tissue sarcomas. RPPSs encompass a diverse group of histologic types and subtypes, all with different biologic behaviors.1 This histologic diversity, as well as the low incidence, make RPPS difficult to study. For soft tissue sarcomas of the extremity and superficial trunk, which are more common, randomized, prospective trials have demonstrated that addition of radiation therapy (RT) to surgery improves local control.2-4 However, this improved local control has not been shown to translate into improved disease-specific survival, with distant metastases accounting for most disease-related deaths.
RPPSs differ from extremity and superficial trunk soft tissue sarcomas in that local disease progression, rather than distant metastasis, is the most common cause of disease-related death.5 The primary treatment modality for RPPS is surgery, but complete resection is often difficult or impossible due to large tumor size and involvement of adjacent vital structures. Even when complete gross resection is achieved, local recurrence is common, occurring in more than 40% of patients in studies with median follow-up of at least 3 years.6
Because of the high rate of local recurrence after surgery for RPPS, attempts have been made to improve local control with more aggressive local therapies. One strategy is the addition of radiation therapy (RT) to surgery. The initial studies evaluating the role of RT for RPPS used external beam radiation (EBRT) in the neoadjuvant or adjuvant setting.7, 8 In 1993, a randomized, prospective trial was reported comparing 20 patients treated with postoperative EBRT (50 to 55 Gy) with 15 patients treated with postoperative EBRT (35 to 40 Gy) plus intraoperative radiation therapy (20 Gy).9 At a median follow-up of 8 years, this study demonstrated lower local recurrence (40% versus 80%) with intraoperative RT plus postoperative EBRT but no significant difference in median survival. Both arms, however, had substantial toxicity (50-60%). Given the small size of this trial, the toxicity in both arms, and the lack of a survival benefit, post-operative EBRT alone or in combination with intraoperative RT was not widely adopted.
Since the time of that trial, better targeting of RPPS with sparing of adjacent normal tissues has become feasible through the use of advanced RT modalities. Intensity-modulated radiation therapy (IMRT) uses computerized, 3-dimensional planning to contour the radiation beams to the tumor shape based on pretreatment cross-sectional images. IMRT also allows for targeting of specific, high-risk areas such as the posterior margins. Intraoperative electron beam radiation therapy (IOERT) involves the use of a linear accelerator to deliver high-dose RT to high-risk surgical margins while protecting adjacent radiation-sensitive organs with retraction. Proton beam radiation therapy (PBRT) utilizes protons rather than photons. Protons expel their energy over a shorter distance, which is advantageous as it minimizes entry doses and essentially eliminates exit doses. However, PBRT is not widely available. Multiple single-arm studies of perioperative RT using IMRT, IOERT, and more recently PBRT have been published with promising results in terms of local control; however, to our knowledge no publications have compared outcomes of RPPS patients treated with these techniques to patients treated with no radiation therapy.10-14 As a result, the role of advanced modality RT in the treatment of RPPS is not well defined. RT is offered routinely at some centers and not at all at others due to institutional biases and differences in resources.
The current study sought to compare RPPS patients treated with modern perioperative RT and surgical resection at one institution with patients treated with surgery alone at another institution during the same time period to determine if the addition of advanced-modality RT was associated with a difference in local recurrence-free survival (LRFS) or disease-specific survival (DSS).
Methods
Patients
Among patients treated at Massachusetts General Hospital (MGH) between 2003 and 2011, with institutional review board (IRB) approval, those patient with primary RPPS treated with perioperative radiation therapy (IMRT, PBRT, and/or IOERT) and complete gross resection of disease were identified from a prospectively-maintained sarcoma database. Patients with multifocal or recurrent disease at the time of presentation were excluded, as were patients who did not undergo complete gross resection. In total, 32 patients met these criteria and were included in the study.
Patients presenting with primary RPPS at Memorial Sloan Kettering Cancer Center (MSKCC) during this same time period were similarly identified from an IRB-approved, prospectively-maintained sarcoma database. As at MGH, patients were excluded if they had multifocal disease, recurrent disease at presentation, or grossly incomplete (R2) resection. Patients were also excluded if they would not have met eligibility requirements for perioperative RT per the protocol at MGH. These exclusion criteria included tumors too large or too symptomatic at presentation for RT, or RT-induced tumors where further RT could not be given. Lastly, patients were excluded if they had other active malignancies for which they were undergoing treatment, if they received any perioperative RT, and if they had gastrointestinal stromal tumor (GIST) histology. In total, 172 patients met these criteria and were included in the study.
Radiation Therapy
For patients who were given preoperative radiation therapy, an image-guided biopsy was performed via a retroperitoneal approach to obtain a tissue diagnosis. Once a diagnosis of RPPS was established, patients received IMRT, PBRT, or a combination of the two per a previously described protocol.10 PBRT was preferentially given to patients with larger tumors and to those with tumors adjacent to highly radiation-sensitive organs (liver, small intestine). Due to a waiting list for PBRT, many patients designated for PBRT started their treatment with IMRT to avoid treatment delay, then transitioned to PBRT. Following preoperative RT, patients underwent surgery. The tumor was resected and taken by the operating surgeon to the frozen section laboratory, where the closest margin was analyzed. When the margin was microscopically close or positive, IORT was given. For one patient who was too symptomatic to receive preoperative radiation and for one patient in whom the biopsy was nondiagnostic, surgery was performed first, followed by IORT and postoperative RT.
Complete surgical resection followed by IOERT and/or postoperative RT was given to those patients who were too symptomatic for preoperative RT or in whom a diagnosis could not be established by biopsy.
Surgery
All patients at both institutions underwent surgical resection of their tumors to achieve grossly negative margins. Dissection was carried out circumferentially from anterior to posterior with en bloc resection of adjacent organs that showed evidence of invasion or direct involvement. Where there was no invasion, the operating surgeon could choose to resect adjacent organs to obtain a negative microscopic margin. Posterior dissection was carried out sharply under direct vision whenever possible. Major vascular resection and reconstruction was performed when necessary because of direct invasion. Patients who received preoperative RT underwent surgery 5-7 weeks after completion of RT.
Clinical Data
Clinical and follow-up data were obtained from chart review and from prospectively-maintained sarcoma databases at the respective institutions. Complications were defined according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.15 Briefly, a complication was defined as any unfavorable and unintended sign, symptom, or disease occurring within 30 days of surgery, whether or not it was considered related to surgery or RT. Complications were graded in severity from 1 to 5 according to the following definitions: Grade 1: Mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated. Grade 2: Moderate; minimal, local or noninvasive intervention indicated. Grade 3: Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated. Grade 4: Life-threatening consequences; urgent intervention indicated. Grade 5: Death related to complication.
Follow-up
For high grade tumors, patients received a chest/abdomen/pelvis CT scan every 3-4 months for the first 2 years after treatment, every 6 months in the 3rd, 4th, and 5th years, and yearly thereafter. For low grade tumors, patients received an abdomen/pelvis CT scan every 6 months for the first 2 years after treatment and yearly thereafter along with a yearly chest x-ray. Eight patients (4%) in the surgery alone group and one patient (3%) in the combined therapy group were lost to follow-up.
Statistical Analysis
Patients with and without perioperative RT were compared by Kruskal-Wallis test for continuous variables and Fisher's test for categorical variables. Kaplan-Meier analysis was used to assess distant recurrence–free survival, LRFS, and DSS. Time to event was calculated from date of surgery to date of event or last follow-up. Patients without the event of interest at last follow-up were censored. The log-rank test was used to compare survival outcomes between the two patient groups. Univariate and multivariate Cox proportional hazards regression was used to assess the association between patient characteristics and the event of interest.
For local recurrence, crude cumulative incidence curves were calculated with death and distant metastasis as competing risks as described by Gray.16 In addition, univariate and multivariate analyses for factors associated with local recurrence were performed with death and distant metastases as competing risks.17
Results
The study included 204 patients with primary RPPS treated at two institutions with surgery alone or with combined surgery and perioperative RT. Their characteristics are shown in Table 1. The median age was 61 years (range 26 - 92), and 101 (50%) were female. The most common histologies were liposarcoma (67%) and leiomyosarcoma (28%). Most tumors (88%) were located primarily in the retroperitoneum above the pelvic brim, whereas 12% were located primarily in the pelvis. The groups with and without perioperative RT were similar for age, gender, and tumor size. The two treatment groups differed in the distribution of tumor histology (p=0.04), with the combined therapy group having a lower frequency of liposarcomas and higher frequency of tumors classified as “other. The combined therapy group also had a higher frequency of pelvic tumors (25% versus 9%, p=0.03).
Table 1.
Demographic, clinicopathologic, and operative characteristics of the patients. Data shown are number (%) or median (range).
| Variable | All Patients | Surgery Alone | RT + Surgery | p value |
|---|---|---|---|---|
| (n = 204) | (n = 172) | (n = 32) | ||
| Age, years | 61 (26-92) | 62 (26-92) | 57 (41-85) | 0.08 |
| Female gender | 101 (50%) | 84 (49%) | 17 (53%) | 0.70 |
| Grade | 0.54 | |||
| High | 135 (66%) | 112 (65%) | 23 (72%) | |
| Low | 69 (34%) | 60 (35%) | 9 (28%) | |
| Histology | 0.040 | |||
| Leiomyosarcoma | 57 (28%) | 49 (29%) | 8 (25%) | |
| WDLS | 60 (29%) | 54 (31%) | 6 (19%) | |
| DDLS | 76 (37%) | 63 (37%) | 13 (41%) | |
| MPNST | 4 (2%) | 3 (2%) | 1 (3%) | |
| Other | 7 (3%) | 3 (2%)a | 4 (14%)b | |
| Site | 0.03 | |||
| Pelvis | 24 (12%) | 16 (9%) | 8 (25%) | |
| Retroperitoneum | 180 (88%) | 156 (91%) | 24 (75%) | |
| Size | 0.18 | |||
| <18 cm | 102 (50%) | 82 (48%) | 20 (63%) | |
| ≥18 cm | 101 (50%) | 89 (52%) | 12 (38%) | |
| Estimated blood loss, mL | 400 (0-5100) | 400 (0-5100) | 415 (50-4000) | 0.99 |
| Operative time, minutes | 206 (48-569) | 195 (48-528) | 262 (140-569) | <0.001 |
| Adjacent organs resected | 0.16 | |||
| 0 | 28 (14%) | 25 (15%) | 3 (9%) | |
| 1 | 82 (40%) | 73 (42%) | 9 (28%) | |
| >1 | 94 (46%) | 74 (43%) | 20 (62%) | |
| Inferior vena cava resection | 20 (10%) | 16 (9%) | 4 (12%) | 0.53 |
| Margin status | 0.12 | |||
| R0 | 119 (58%) | 105 (61%) | 14 (45%) | |
| R1 | 84 (41%) | 67 (39%) | 17 (55%) | |
| Length of stay, days | 7 (1-31) | 7 (1-31) | 7 (2-15) | 0.26 |
| Patients with complications within 30 days | 42 (21%) | 29 (17%) | 13 (41%) | 0.004 |
RT, radiation therapy; WDLS, well-differentiated liposarcoma; DDLS, dedifferentiated liposarcoma; MPNST, malignant peripheral nerve sheath tumor
3 cases of sarcoma not otherwise specified
2 myofibroblastic sarcomas, 1 malignant fibrous histiocytoma, and 1 pleomorphic fibroblastic sarcoma
Two patients in the perioperative RT group received chemotherapy. One received a course of ifosfamide as a radiation-sensitizer; the other received bevacizumab as part of a clinical trial. In the surgery-only group, one patient received doxorubicin prior to referral for surgical evaluation. No patients in either group were given adjuvant chemotherapy.
Operative characteristics of the patients are shown in Table 1. The majority of patients (86%) required resection of one or more adjacent organs. Inferior vena cava resection was performed in 20 patients (10%). R0 resection was achieved in 119 patients (58%), while the remaining 84 patients (41%) had microscopically-positive margins. Adjacent organ resection, inferior vena cava resection, margin status, estimated blood loss, and length of stay were similar between the two groups. Operative time was longer for the combined therapy group (median 262 minutes versus 195 minutes, p<0.001).
Table 2 summarizes the RT delivered to patients. RT was preoperative in 30 patients and postoperative in 2 patients. One of the 2 patients did not receive preoperative RT because a diagnosis could not be established by image-guided biopsy. The other patient presented with incomplete resection of a pelvic, inguinal, and scrotal liposarcoma and subsequently received a complete resection, IOERT, and postoperative RT. External beam RT was delivered by IMRT, PBRT, or both with a median dose of 50 Gy. IOERT was given to nearly half of patients with a median dose of 10 Gy.
Table 2.
Details of Perioperative Radiation Therapy
| Variable | Value |
|---|---|
| Patients treated with RT, n | 32 |
| EBRT median dose, Gy (range) | 50 (45.0-58.1) |
| Type of EBRT, n (%) | |
| Intensity-modulated | 19 (59%) |
| Proton beam | 6 (19%) |
| Both intensity-modulated and proton beam | 7 (22%) |
| Timing of EBRT, n (%) | |
| Preoperative | 30 (94%) |
| Postoperative | 2 (6%) |
| IOERT | |
| Patients, n (%) | 15 (47%) |
| Median dose, Gy (range) | 10.0 (6.0-15.0) |
RT, radiation therapy; EBRT, external beam radiation therapy; IMRT, intensity-modulated radiation therapy; IOERT, intraoperative electron beam radiation therapy
Local Recurrence-Free Survival
At a median follow-up of 38.7 months (36.9 for RT group, 38.8 for surgery alone), 52 patients had experienced local recurrence. Five-year estimated LRFS was 91% (95% CI, 79-100%) in the RT group and 65% (57-74%) in the surgery-only group (log-rank p=0.024) (Figure 1). On univariate analysis (Table 3), perioperative RT was associated with a lower risk of local recurrence (hazard ratio [HR] 0.28). High grade, larger size (≥18 cm), and positive resection margin (R1) were also associated with a higher risk of local recurrence. Of the various histologies, dedifferentiated liposarcoma (DDLS) was associated with a higher risk of local recurrence than well-differentiated liposarcoma (WDLS). On multivariate analysis adjusting for univariate predictors, perioperative RT and non-DDLS histology were independently associated with better LRFS (Table 3). Grade and histology were not included in the same multivariate model because they were confounding. When grade replaced histology in the multivariate model, perioperative RT and low grade were independently associated with improved LRFS (perioperative RT [yes vs. no] hazard ratio [HR] 0.28, 95% C.I. 0.09-0.90, p=0.033; grade [high vs. low] HR 3.16, 95% C.I. 1.56-6.41, p=0.001). Five-year distant recurrence-free survival was 92% (95% CI, 81-100%) in the RT group and 79% (72-88%) in the surgery-only group (p=0.4).
Figure 1.
Kaplan Meier curve and log-rank analysis of local recurrence free survival in RPPS patients treated with versus without perioperative RT at a median follow-up of 38.7 months. . Perioperative RT was associated with a statistically significant increase in local recurrence free survival.
Table 3.
Univariate and Multivariate Analysis of Factors Potentially Associated with Local Recurrence
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p value | HR | 95% CI | p value |
| Age | 1.00 | 0.98-1.02 | 0.93 | - | - | - |
| Gender (male vs. female) | 1.49 | 0.85-2.58 | 0.16 | - | - | - |
| RT (yes vs. no) | 0.28 | 0.09-0.91 | 0.035 | 0.26 | 0.08-0.86 | 0.026 |
| Grade (high vs. low) | 2.37 | 1.19-4.73 | 0.014 | - | - | - |
| Site (pelvis vs. retroperitoneum) | 0.39 | 0.12-1.24 | 0.11 | 0.87 | 0.26-2.97 | 0.824 |
| Size (≥ 18 cm vs. < 18 cm) | 2.59 | 1.43-4.67 | 0.002 | 1.78 | 0.96-3.29 | 0.065 |
| Margin status (R1 vs. R0) | 1.95 | 1.13-3.37 | 0.017 | 1.75 | 1.00-3.08 | 0.051 |
| Histology | - | - | - | |||
| DDLS vs WDLS | 3.17 | 1.56-6.44 | 0.001 | *3.19 | 1.73-5.88 | <0.001 |
| Leiomyosarcoma vs. WDLS | 0.49 | 0.17-1.45 | 0.199 | - | - | - |
| MPNST vs. WDLS | 3.04 | 0.66-13.91 | 0.153 | - | - | - |
| Other vs. WDLS | 1.83 | 0.40-8.39 | 0.434 | - | - | - |
HR, hazard ratio; CI, confidence interval; RT, radiation therapy; WDLS, well-differentiated liposarcoma; DDLS, dedifferentiated liposarcoma; ; MPNST, malignant peripheral nerve sheath tumor
DDLS vs others
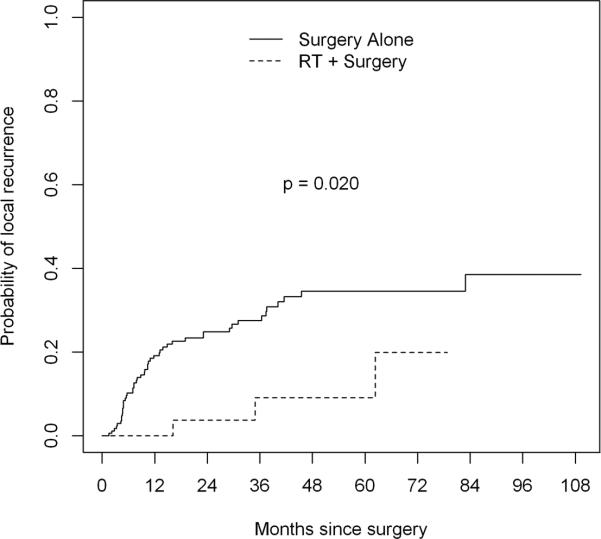
The above analysis of local recurrence does not take into account competing risks such as death or distant metastasis. There were 52 local recurrences, 7 deaths without local recurrence, and 12 distant recurrences without local recurrence. We thus calculated crude cumulative incidence curves for the RT plus surgery group and the surgery alone group (Figure 2). The RT plus surgery group had a statistically lower risk of local recurrence in this analysis (Gray's test p value 0.020). We also repeated the univariate analysis for factors potentially associated with local recurrence using the Fine and Gray model (which accounts for competing risks) instead of the Cox model. In this analysis, radiation was still significantly associated with reduced local recurrence on both univariate (p=0.026) and multivariate (p=0.018) analyses.
Figure 2.
Cumulative incidence curve and Gray's test for local recurrence in RPPS patients treated with versus without perioperative RT. Excluding competing risks included in an analysis of local recurrence free survival (i.e. death, distant recurrence), perioperative RT was associated with a statistically significant decrease in the rate of local recurrence.
Disease-Specific Survival
Twenty-five deaths of disease were recorded among patients in this study, and 5-year DSS was 93% (95% CI, 82-100%) in the RT group and 85% (78-92%) in the surgery-only group (p=0.28) (Figure 3). On univariate analysis, increasing age and DDLS histology (vs. leiomyosarcoma) were significantly associated with DSS (Table 4). No patient with low- grade disease or WDLS experienced disease-related death within the follow-up period, so grade and WDLS histology could not be assessed for association with DSS. In a multivariate model including age and perioperative RT, age was the only variable independently associated with DSS (H.R. 1.04; p=0.019) (Table 4).
Figure 3.
Kaplan Meier curve and log-rank analysis disease-specific survival in RPPS patients treated with versus without perioperative RT. There was not a statistically significant difference in disease specific survival between the two treatment modalities.
Table 4.
Univariate and Multivariate Analysis of Factors Potentially Associated with Disease-specific Survival
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% C.I. | p value | HR | 95% C.I. | p value |
| Age | 1.04 | 1.01-1.07 | 0.015 | 1.04 | 1.01-1.07 | 0.019 |
| Gender | 1.39 | 0.62-3.11 | 0.418 | - | - | - |
| RT (yes vs. no) | 0.46 | 0.11-1.95 | 0.291 | 0.52 | 0.12-2.22 | 0.376 |
| Grade (high vs. low) | NA | NA | NA | - | - | - |
| Site (pelvis vs. retroperitoneum) | 0.86 | 0.26-2.88 | 0.808 | - | - | - |
| Size (≥ 18 cm vs. <18 cm) | 1.74 | 0.78-3.88 | 0.178 | - | - | - |
| Margin status (R1 vs. R0) | 1.31 | 0.59-2.89 | 0.506 | - | - | - |
| Histology | - | - | - | |||
| WDLS vs. DDLS | NA | NA | NA | - | - | - |
| Leiomyosarcoma vs. DDLS | 0.30 | 0.11-0.83 | 0.020 | - | - | - |
| MPNST vs. DDLS | 0.60 | 0.08-4.74 | 0.630 | - | - | - |
| Other vs. DDLS | 1.33 | 0.31-5.81 | 0.700 | - | - | - |
HR, hazard ratio; CI, confidence interval; RT, radiation therapy; WDLS, well-differentiated liposarcoma; DDLS, dedifferentiated liposarcoma; ; MPNST, malignant peripheral nerve sheath tumor
Perioperative Morbidity
Complications occurring within 30 days of surgery are summarized in Table 5. There were no operative morbidities and no perioperative deaths in either group. Perioperative morbidity affected more patients in the combined therapy group (41%) than in the surgery-alone group (17%; p=0.004). The majority of complications in both groups were minor (grade 1 or 2). The most common complication in the RT group was ileus (16%) followed by urinary tract infection (13%). The most common complication in the surgery-alone group was superficial site infection (8%), followed by ileus (3%). In both groups, the only grade 4 complication was postoperative hemorrhage, which occurred in 2 patients (6%) in the RT group and 2 patients (1%) in the surgery-alone group. Despite the increased incidence of complications in the RT group, the median length of stay was 7 days in both groups.
Table 5.
Perioperative Morbidity
| Complication Grade and Type | Surgery Alone | RT + Surgery |
|---|---|---|
| n = 172 | n = 32 | |
| Grade 1 | ||
| Pneumothorax | 0 | 1 (3%) |
| Peripheral neuropathy | 0 | 1 (3%) |
| Grade 2 | ||
| Arrhythmia | 2 (1%) | 1 (3%) |
| Myocardial infarction | 1 (<1%) | 0 |
| Chyle leak | 0 | 1 (3%) |
| Ileus | 6 (3%) | 5 (16%) |
| Cellulitis | 2 (1%) | 0 |
| Superficial site infection | 13 (8%) | 1 (3%) |
| Urinary tract infection | 5 (3%) | 4 (13%) |
| Grade 3 | ||
| Wrist abscess | 0 | 1 (3%) |
| Bladder leak | 0 | 1 (3%) |
| Deep space infection / abscess | 4 (2%) | 0 |
| GI anastomotic leak | 1 (<1%) | 0 |
| Dehiscence | 1 (<1%) | |
| Enterocutaneous fistula | 1 (<1%) | 0 |
| Grade 4 | ||
| Postoperative hemorrhage | 2 (1%) | 2 (6%) |
GI, gastrointestinal; RT, radiation therapy
Discussion
Few studies have tested whether modern RT improves outcomes for patients with RPPS. The current study compared patients with primary RPPS who were treated with surgery alone to patients treated with surgery plus advanced-modality perioperative RT during the same time period. Both participating institutions were sarcoma referral centers, and 5-year LRFS and DSS were excellent for both groups. However, estimated 5-year LRFS was significantly higher in the RT group than in the surgery-only group (91% vs. 65%). This association of RT with improved LRFS remained significant in a multivariate analysis accounting for potential confounding variables. Other variables independently associated with local recurrence were DDLS histology and high grade. A subset analysis of the 136 patients with liposarcoma histology was performed, and RT, along with histology (DDLS versus WDLS) remained an independent predictor of LRFS (data not shown).
Despite the association with LRFS, RT was not associated with improved DSS. The DSS analysis was limited in that no patients with low-grade disease or WDLS histology died of disease during follow-up, so these variables could not be analyzed for association with DSS. The only variable independently associated with DSS was age. RT was significantly associated with a higher frequency of perioperative complications; therefore, the potential benefit of RT must be weighed against increased toxicity.
For extremity soft tissue sarcoma, surgical resection with negative microscopic margins and adjuvant radiation can result in local recurrence rates of less than 10%.4 Even with positive microscopic margins, adequate doses of radiation (>64 Gy) can result in local recurrence rates of less than 20%18. However, the role of RT for RPPS is less clear. RPPSs are on average substantially larger than extremity sarcomas and arise adjacent to radiation-sensitive organs (e.g. kidney, liver, small bowel). Thus, toxicity often limits the deliverable dose of radiation.
In this study, IMRT, PBRT, and IOERT were used to deliver radiation predominantly preoperatively. Doses of radiation equivalent to at least 64 Gy of fractionated radiation were given for a close or positive microscopic margin whenever possible. Postoperative EBRT has fallen out of favor for RPPS, and most radiation oncologists with expertise in RPPS prefer to deliver EBRT preoperatively. Preoperative radiation therapy has several potential advantages including (1) gross tumor volume can clearly be demarcated, (2) the tumor acts a tissue expander, displacing adjacent normal tissue including small bowel that may lie in the treatment field postoperatively, (3) tissue oxygenation is better, and (4) the risks of tumor seeding and consequent peritoneal sarcomatosis are lower.19, 20
Combining preoperative and intraoperative RT may more reliably deliver enough radiation dose to sterilize microscopic residual disease. In the present study 47% of the RT group received IOERT, and, although this study was not designed to compare preoperative radiation with and without IOERT, prior studies suggest that the combination may be beneficial. For example, in a report from MGH of 29 patients treated with preoperative radiation (45 Gy) and complete gross resection, those who also received IOERT (10-20 Gy) had 83% local control at 5 years, compared to 61% for those who did not receive IOERT.21 The Mayo Clinic has reported similar good results with IOERT.22
Although RT was associated with increased toxicity in this study, modified RT techniques may be able to reduce toxicity for patients with RPPS. Helical tomography dosimetry for IMRT would allow the reduction of toxic doses to the kidney compared with that offered by the traditional step-and-shoot IMRT dosimetry.23 In another approach, IMRT was administered preoperatively to deliver 45 Gy to the entire tumor bed with a boost to 57.5 Gy to the margins considered to be at highest risk for recurrence.24 Of 16 patients who completed these radiation treatments, only a single patient experienced greater than a grade 1 toxicity and only 2 patients experienced late toxicity. Twelve of 16 tumors decreased in size in response to radiation and 2-year recurrence-free survival was 80%. In an effort to further minimize toxicity, Bossi et al. from Belgium used IMRT to deliver neoadjuvant radiation (50 Gy) only to the posterior margin of retroperitoneal liposarcomas in 18 patients.25 In this group, all patients completed the course of radiation and toxicity was minimal, but, after a median follow up of 84 months, local control and disease-specific survival were no better than in matched controls who received no radiation therapy.26
Despite these advances in RT technique and technology, randomized trials to define the role of modern RT in the treatment of RPPS are lacking. In 2005, the American College of Surgeons Oncology Group initiated a randomized, prospective trial to compare preoperative IMRT plus surgery versus surgery alone for retroperitoneal sarcoma.27 Due to poor accrual, however, the trial was never completed. The European Organization for Research and Treatment of Cancer is currently conducting a randomized trial of surgery plus preoperative RT (3D-conformal RT or IMRT) versus surgery alone (EORTC 62092-22092).28 The study was powered to detect a 20% difference in abdominal recurrence at 5 years. If this study is completed, it will help to define the role of preoperative RT in the treatment of primary RPPS.
A major limitation of this study is its retrospective design. There was unavoidable selection bias in the patients who were included in each group. Although the two patient groups were similar in many clinicopathologic characteristics, the surgery-alone group contained significantly more patients with retroperitoneal tumors and liposarcoma histology. Liposarcoma histology is known to be associated with high rates of local recurrence compared to other histologic types, so the higher proportion of liposarcoma in the surgery-alone group could have contributed to this group's worse LRFS.29, 30 In addition, the surgery-alone patients tended to be older (p=0.08) and to have larger tumors (p=0.18), although these differences did not reach statistical significance. Thus, even though we attempted to adjust for the imbalance in these prognostic factors, the lower local recurrence rates found in the RT group may also be explained by the selection of younger patients who tended to have non-liposarcoma histology and smaller tumors than in the surgery–alone group. In addition, there are likely unmeasurable patient, surgeon, and hospital-related differences between the two groups beyond the receipt of perioperative RT that we were unable to capture in this analysis.
To minimize selection bias, case-matching was attempted; however, only 21 patients from the perioperative RT group could be matched for age, histology, tumor size, and tumor site with patients in the surgery-alone group. This small number of patients would have limited the data analyses, so we proceeded with the current study design that included all patients and used multivariate analysis to adjust for prognostic factor imbalance between treatment groups.
An additional limitation of this retrospective study is that the assessment of surgical margins and the recording of complications were not standardized at the two institutions. Both margin assessment and complication coding were more rigorous for the patients receiving perioperative RT, as that was administered via a prospectively designed protocol.10 Finally, the size of this study was relatively small because of the rarity of the disease, and the small size limits the ability to find statistically significant associations. Nevertheless, this study is larger than most prior studies of RT for RPPS.
In conclusion, this study demonstrated that advanced-modality, perioperative RT has acceptable toxicity and is associated with improved LRFS, possibly due to patient selection. Advanced-modality RT is therefore a safe and reasonable option for highly selected patients with primary RPPS. Future randomized trials will help define those patients most likely to benefit from advanced-modality, perioperative RT. Until this definitive evidence clarifies the role of RT for RPPS, patients should be treated at sarcoma referral centers with multidisciplinary expertise to guide management decisions on a case-by-case basis.
Acknowledgments
The manuscript was edited by Janet Novak of MSKCC.
Source of funding: This work was supported by the SPORE in Soft Tissue Sarcoma (P50 CA140146, S.S., and L.Q.) and by an NIH/NCI Cancer Center Support Grant P30 CA08748.
Footnotes
No conflicts of interest
References
- 1.Taylor BS, Barretina J, Maki RG, et al. Advances in sarcoma genomics and new therapeutic targets. Nat Rev Cancer. 2011;11:541–57. doi: 10.1038/nrc3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–41. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 3.Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–68. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 4.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JJ, Leung D, Woodruff JM, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–65. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett E, Yoon SS. Current treatment for the local control of retroperitoneal sarcomas. J Am Coll Surg. 2011;213:436–46. doi: 10.1016/j.jamcollsurg.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Glenn J, Sindelar WF, Kinsella T, et al. Results of multimodality therapy of resectable soft-tissue sarcomas of the retroperitoneum. Surgery. 1985;97:316–25. [PubMed] [Google Scholar]
- 8.Tepper JE, Suit HD, Wood WC, et al. Radiation therapy of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 1984;10:825–30. doi: 10.1016/0360-3016(84)90383-3. [DOI] [PubMed] [Google Scholar]
- 9.Sindelar WF, Kinsella TJ, Chen PW, et al. Intraoperative radiotherapy in retroperitoneal sarcomas. Final results of a prospective, randomized, clinical trial. Arch Surg. 1993;128:402–10. doi: 10.1001/archsurg.1993.01420160040005. [DOI] [PubMed] [Google Scholar]
- 10.Yoon SS, Chen YL, Kirsch DG, et al. Proton-beam, intensity-modulated, and/or intraoperative electron radiation therapy combined with aggressive anterior surgical resection for retroperitoneal sarcomas. Ann Surg Oncol. 2010;17:1515–29. doi: 10.1245/s10434-010-0935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McBride SM, Raut CP, Lapidus M, et al. Locoregional recurrence after preoperative radiation therapy for retroperitoneal sarcoma: adverse impact of multifocal disease and potential implications of dose escalation. Ann Surg Oncol. 2013;20:2140–7. doi: 10.1245/s10434-013-2868-y. [DOI] [PubMed] [Google Scholar]
- 12.Pawlik TM, Pisters PW, Mikula L, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. 2006;13:508–17. doi: 10.1245/ASO.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Alektiar KM, Hu K, Anderson L, et al. High-dose-rate intraoperative radiation therapy (HDR-IORT) for retroperitoneal sarcomas. Int J Radiat Oncol Biol Phys. 2000;47:157–63. doi: 10.1016/s0360-3016(99)00546-5. [DOI] [PubMed] [Google Scholar]
- 14.Alford S, Choong P, Chander S, et al. Outcomes of preoperative radiotherapy and resection of retroperitoneal sarcoma. ANZ J Surg. 2013;83:336–41. doi: 10.1111/j.1445-2197.2012.06211.x. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute . Common Terminology Criteria for Adverse Events. Version 4.0. National Cancer Institute, National Institutes of Health; 2009. [Google Scholar]
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 18.Delaney TF, Kepka L, Goldberg SI, et al. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007;67:1460–9. doi: 10.1016/j.ijrobp.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 19.Herman K, Kusy T. Retroperitoneal sarcoma--the continued challenge for surgery and oncology. Surg Oncol. 1998;7:77–81. doi: 10.1016/s0960-7404(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 20.Pawlik TM, Ahuja N, Herman JM. The role of radiation in retroperitoneal sarcomas: a surgical perspective. Curr Opin Oncol. 2007;19:359–66. doi: 10.1097/CCO.0b013e328122d757. [DOI] [PubMed] [Google Scholar]
- 21.Gieschen HL, Spiro IJ, Suit HD, et al. Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2001;50:127–31. doi: 10.1016/s0360-3016(00)01589-3. [DOI] [PubMed] [Google Scholar]
- 22.Petersen IA, Haddock MG, Donohue JH, et al. Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2002;52:469–75. doi: 10.1016/s0360-3016(01)02595-0. [DOI] [PubMed] [Google Scholar]
- 23.Pezner RD, Liu A, Han C, et al. Dosimetric comparison of helical tomotherapy treatment and step-and-shoot intensity-modulated radiotherapy of retroperitoneal sarcoma. Radiother Oncol. 2006;81:81–7. doi: 10.1016/j.radonc.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Tzeng CW, Fiveash JB, Popple RA, et al. Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer. 2006;107:371–9. doi: 10.1002/cncr.22005. [DOI] [PubMed] [Google Scholar]
- 25.Bossi A, De Wever I, Van Limbergen E, et al. Intensity modulated radiation-therapy for preoperative posterior abdominal wall irradiation of retroperitoneal liposarcomas. Int J Radiat Oncol Biol Phys. 2007;67:164–70. doi: 10.1016/j.ijrobp.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 26.De Wever I, Laenen A, Van Limbergen E. Pre-operative irradiation for retroperitoneal liposarcoma: results of a pilot study. Acta Chir Belg. 2013;113:315–21. [PubMed] [Google Scholar]
- 27.ClinicalTrials.gov. [6 August, 2013];A Prospective Randomized Trial of Pre-Operative IMRT+Surgery Versus Surgery Alone For Primary Retroperitoneal Sarcoma 2005. Available at: http://clinicaltrials.gov/ct2/show/NCT00131898.
- 28.ClinicalTrials.gov. [19 February, 2014];Surgery with or without radiation therapy in treating patients with previously untreated nonmetastatic retroperitoneal soft tissue sarcoma (STRASS) 2011. Available at: http://clinicaltrials.gov/show/NCT01344018.
- 29.Linehan DC, Lewis JJ, Leung D, et al. Influence of biologic factors and anatomic site in completely resected liposarcoma. J Clin Oncol. 2000;18:1637–43. doi: 10.1200/JCO.2000.18.8.1637. [DOI] [PubMed] [Google Scholar]
- 30.Singer S, Antonescu CR, Riedel E, et al. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358–70. doi: 10.1097/01.sla.0000086542.11899.38. discussion 370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]