Abstract
Introduction
Erectile dysfunction is a serious complication of diabetes mellitus. Apart from the peripheral actions, central mechanisms are also responsible for penile erection.
Aim
To determine the contribution of angiotensin (ANG) II in the dysfunction of central N-methyl-D-aspartic acid (NMDA)-nitric oxide (NO)-induced erectile responses in streptozotocin-induced type 1 diabetic (T1D) rats.
Methods
Three weeks after streptozotocin injections, rats were randomly treated with the angiotensin-converting enzyme inhibitor-enalapril, or the ANG II type 1 receptor blocker, losartan, or the superoxide dismutase mimetic, tempol or vehicle via chronic intracerebroventricular infusion by osmotic mini-pump for 2 weeks.
Main Outcome Measure
Central NMDA receptor stimulation or the administration of the NO donor, sodium nitroprusside (SNP)-induced penile erectile responses and concurrent behavioral responses were monitored in conscious rats.
Results
Two weeks of enalapril, losartan or tempol treatment significantly improved the erectile responses to central microinjection of both NMDA and SNP in the paraventricular nucleus (PVN) of conscious T1D rats (NMDA responses – T1D+enalapril: 1.7 ± 0.6, T1D+losartan: 2.0 ± 0.3, T1D+tempol: 2.0 ± 0.6 vs. T1D+vehicle: 0.6 ± 0.3 penile erections/rat in the first 20 min, P < 0.05; SNP responses – T1D+enalapril: 0.9 ± 0.3, T1D+losartan: 1.3 ± 0.3, T1D+tempol: 1.4 ± 0.4 vs. T1D+vehicle: 0.4 ± 0.2 penile erections/rat in the first 20 min, P < 0.05). Concurrent behavioral responses including yawning and stretching, induced by central NMDA and SNP microinjections were also significantly increased in T1D rats after enalapril, losartan or tempol treatments. Neuronal NO synthase expression within the PVN was also significantly increased and superoxide production was reduced in T1D rats after these treatments.
Conclusions
These data strongly support the contention that enhanced ANG II mechanism/s within the PVN of T1D rats contributes to the dysfunction of central NMDA-induced erectile responses in T1D rats via stimulation of superoxide.
Keywords: type 1 diabetes, central nervous system, central mechanisms of penile erection, erectile dysfunction
Introduction
Sexual dysfunction is a well known consequence of diabetes mellitus in men1, 2. Approximately 35% to 75% of men with diabetes mellitus have erectile dysfunction3. It is generally accepted that different central and peripheral neural and/or humoral endocrine mechanisms participate in the regulation of the sexual response. Although there has been considerable advance in elucidating the peripheral component of the response, the central mechanisms remain relatively unexplored. With regard to the central mechanism/s, several neurotransmitters that control erectile function, including excitatory amino acid N-methyl-D-aspartic acid (NMDA) and nitric oxide (NO), have been identified4–6. These compounds act in several brain areas, including the paraventricular nucleus (PVN) of the hypothalamus5, 6, which transmits this information to the genital organs via projections to the spinal cord.
The PVN is involved in numerous functions including feeding, metabolic balance, cardiovascular regulation, as well as erectile function and sexual behavior. In the PVN, NMDA-induced penile erection and yawning is mediated by an increased NO synthesis (NOS)6. Our previous study also demonstrated that penile erection occurred in response to administration of NMDA directly into the PVN. This response appeared to be linked to the release of NO within the PVN7. In streptozotocin (STZ)-induced type 1 diabetic (T1D) rats, the responses of penile erection, yawning and stretching induced by NMDA within the PVN were decreased7. These data suggest that the central component of the erectile response is blunted in T1D rats. We further observed that the level of neuronal NOS (nNOS) protein in the PVN was decreased in T1D rats. In T1D rats, restoration of nNOS with viral transfection within the PVN improved the behavioral responses (erection and yawning) mediated by NMDA. These data suggest an abnormal NO mechanism in the central nervous system, specifically within the PVN, may be involved in the altered erectile responses in rats with T1D.
The brain angiotensin (ANG) II system plays an important role in cardiovascular control8, vasopressin secretion9 and certain behaviors including thirst and salt appetite10. ANG II has been specifically identified as a neuromodulator in the central nervous system, and is known to be involved in neuroendocrine and autonomic function11, 12. ANG II type 1 (AT1) receptors are known to be present on PVN neurons13, 14. Functional studies have shown that AT1 receptors are involved in PVN-mediated autonomic outflow changes and consequent cardiovascular functions15, 16. Our previous data has shown an augmented AT1 receptor function and expression in the PVN of T1D rats17. We also found that ANG II down-regulated nNOS expression in cultured neuronal cells18. This data corroborates and further supports the concept of a link between ANG II and NO signaling.
Oxidative stress-mediated neurovascular alteration at the level of the penis plays an integral role in the development of erectile dysfunction in the diabetic population19. The pathogenesis of diabetes-associated erectile dysfunction is related to the endothelial damage resulting from impaired NO production, which is closely related to oxidative stress19. In STZ-induced T1D rats, Jeremy et al. reported that superoxide (O2•−) production was markedly elevated in the cavernosum20. Over-expressing superoxide dismutase (SOD) via adenoviral-mediated gene transfer results in increased bioavailability of NO by reducing corporal O2•− levels, and thus restoring erectile function in T1D rats21. In addition, NADPH oxidase inhibitor apocynin can ameliorate STZ-induced diabetes-related erectile dysfunction by reducing the reactive oxygen species (ROS) production and inhibiting the activity of RhoA/ROCK signaling pathway22. Interestingly, ANG II is known to induce endothelial dysfunction via the ROS, which can counteract the vasodilating, and vaso-protective effects of NO. It is thought that one of the mechanisms for restoration of erection function by ANG II blockade is the alleviation of endothelial dysfunction. Together, these previous studies focused on the peripheral mechanisms suggesting a link between ANG II, O2•, and NO in mediating erectile dysfunction. However, the precise details for the role of central mechanism/s that contribute to erectile dysfunction during T1D remain to be examined.
Aims
The aims of the current study were to determine 1) whether central inhibition of angiotensin-converting enzyme (ACE) or ANG II AT1 receptor improves NMDA-induced erectile responses in rats with T1D; 2) whether central administration of the SOD mimetic, tempol improves NMDA-induced erectile responses in rats with T1D; and 3) whether nNOS expression in the PVN was restored in rats with T1D after central enalapril, losartan or tempol treatments; 4) whether O2•− production in the PVN was reduced in rats with T1D after central enalapril, losartan or tempol treatments.
Methods
Animal and Treatment
This study was approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and conforms to the guidelines for the care and use of laboratory animals according to the National Institutes of Health and the American Physiological Society. Male Sprague-Dawley rats (200–220 g, Sasco) were randomly injected with STZ (65mg/kg, i.p, in a 2 % solution of 0.1 M citrate buffer, Sigma, MO) to induce diabetes or vehicle (citrate buffer) for the control group. The percentage of animals that were diabetic after STZ injection was approximately, 85 %. Onset of diabetes was identified by polydipsia, polyuria, and blood glucose levels >250mg/dl. The mortality rate of the STZ rats was 15%. Rats that exhibited ruffled hair, poor appearance, vocalizations, and lack of appetite were considered to be of poor health and were euthanized. Body weight and blood glucose was monitored weekly. Experiments were performed 6–7 weeks after the injection of STZ or vehicle.
Three weeks after STZ or vehicle injections, rats were assigned to eight groups: control+vehicle (VE), T1D+VE, control+enalapril (ENL), T1D+ENL, control+losartan (LOS), T1D+LOS, control+tempol (TEM), T1D+TEM, n=6–7/group. Experiments for erectile function and evaluation of nNOS expression were performed after 2 weeks of treatment with enalapril, losartan, tempol or vehicle. The total number of rats in the study was 130.
Chronic Intracerebroventricular (ICV) Infusion
Rats were anesthetized with a ketamine/xylazine mixture (90 mg/kg and 5 mg/kg, i.p.) and placed in a stereotaxic apparatus (Davis Kopf instruments, CA). After the bregma was identified, a sterile brain cannula using the Alzet Brain Infusion Kit II connected to an osmotic mini-pump (model 1003D, Alzet, CA) was inserted into the right lateral cerebral ventricle and fixed to the skull with dental cement. The periostia on both sides were sutured together to fasten the brain cannulae. The coordinates were determined from the Paxinos and Watson rat atlas23, which were 0.8 mm posterior, 1.5 mm lateral to the bregma and 3.8 mm ventral to the 0 level. The brain cannulae, connecting catheter and mini-pump, was pre-filled with ACE inhibitor, enalapril maleate (0.5 mg/ml, Sigma, MO)24, ANG II AT1 receptor antagonist, losartan (2 mg/ml, Merck, NJ)25, 26, SOD mimetic, tempol (50 mg/ml, Sigma, MO)26, or artificial spinal fluid (aCSF, composition in mM: 132 NaCl, 3.0 KCl, 0.65 MgCl2, 1.5 CaCl2, 24.6 NaHCO3, and 3.3 glucose adjusted to pH 7.4) as vehicle. All drugs were dissolved in aCSF and infused at 1 μl/hr through the mini-pumps for 2 weeks.
Guide Cannula Implantation for PVN Microinjection
After 2 weeks of treatment, each animal was implanted with a stainless steel cannula aimed at the PVN as described previously7. The rats were anesthetized with a ketamine/xylazine mixture (90 mg/kg and 5 mg/kg, i.p.) and then placed in a stereotaxic apparatus. Previously implanted ICV cannula was removed prior to the implantation of the PVN cannula. The coordinates for the PVN were determined according to the atlas of Paxinos and Watson23. A small burr hole was made in the skull. A stainless steel guide cannula (500 μm outer diameter; Microdialysis AB, Solna, Sweden) was implanted stereotaxically at the following coordinates: 1.5 mm posterior to the bregma, 0.4 mm lateral to midline, and 7.8 mm ventral to the dura. Two stainless-steel anchoring screws were fixed to the skull, and the cannula was secured in place by acrylic dental cement. The animals were then returned to their cages and allowed to recover for 2–3 days.
Erectile Function Protocol
Three days after surgery, penile erection, yawning and stretching were induced by NMDA or sodium nitroprusside (SNP) microinjections in the PVN in 8 groups of freely moving, conscious rats (control+VE, T1D+VE, control+ENL, T1D+ENL, control+LOS, T1D+LOS, control+TEM, T1D+TEM, n=6-7/group). Rats were placed individually into a Plexiglas cage and injected with NMDA (50 ng, Millipore, MA) or SNP (50 ng, Sigma, MO) into the PVN unilaterally in a volume of 100 nL using microsyringe (0.5 μL, Hamilton 7000 series, NV), respectively. After microinjection, the tip of the needle was left in place for at least 30s in order to allow the spread of the injectate. The vehicle solution was aCSF. After NMDA or SNP injections, the rats were monitored to quantify the number of episodes of penile erection, yawning and stretching over 20 min intervals for the next 80 min. Penile erection, yawing and stretching episodes were scored by an observer blind to the treatment.
In separate groups of control and T1D (n=3/group), only aCSF (100 nL) was injected into the PVN as a vehicle control. aCSF injection into the PVN did not induce penile erection, yawning and stretching responses in both control and T1D rats.
Microdissection of the PVN
In separate groups of rats (control+VE, T1D+VE, control+ENL, T1D+ENL, control+LOS, T1D+LOS, control+TEM, T1D+TEM, n=5/group), after euthanasia by pentobarbital (150 mg/kg, i.p.), brains were removed and frozen on dry ice. Six serial coronal sections (100 μm/section) were cut through the hypothalamus at the level of the PVN with a cryostat. The PVN was punched out bilaterally with a blunt 18-gauge needle according to the Palkovits and Brownstein technique27. Totally, 12 punches for each brain were placed in 100 L of protein extraction buffer (10 mM Tris, 1 mM EDTA, 1% SDS, 0.1% Triton X-100 and 1 mM phenylmethylsulfonyl fluoride), sonicated, and incubated for 30 min at 37°C to extract the protein.
Western Blot Analysis for nNOS Protein
The protein extraction was used for Western blot analysis of nNOS in samples obtained from the protocols above. The samples were loaded onto a 7.5% SDS-PAGE gel for electrophoresis. The fractionated proteins on the gel were electrophoretically transferred onto the polyvinylidene diflouoride membrane. The membrane was then incubated with primary antibody (rabbit anti-rat nNOS polyclonal antibody, 1:1,000, Santa Cruz Biotechnology, CA) overnight. Then, the membrane was incubated with secondary antibody (goat anti-rabbit IgG, peroxidase conjugated, 1:5,000, Thermo Scientific, IL) for 1 h. An enhanced chemiluminescence substrate (Thermo Scientific, IL) was applied to the membrane, followed by an exposure within an Epi Chemi II Darkroom (UVP BioImaging, CA) for visualization and capture of the images with the Worklab digital imaging system. Kodak 1D software was used to quantify the signal. The expression of nNOS was calculated as the ratio of intensity of the nNOS band relative to the intensity of the β tubulin band.
Dihydroethidium (DHE) Staining
In separate groups of rats (control+VE, T1D+VE, T1D+ENL, T1D+LOS, T1D+TEM, n=4/group), after euthanasia by pentobarbital (150 mg/kg, i.p.), brains were removed and frozen on dry ice. Serial coronal sections (30μm/section) were cut through the hypothalamus at the level of the PVN with a cryostat. To evaluate the O2•− production in the PVN, sections were incubated with DHE (2 μmol/L, Molecular Probes, CA) for 30 min in the dark at 37°C. Sections were evaluated under a fluorescent microscope (Leica, Germany) and digitally photographed. Openlab 4.0.3 (Improvision, MA) software was used to identify the total intensity of positive staining. Three alternate sections (1.5 ± 0.1 mm posterior to bregma) representing the PVN were analyzed, and then the mean data from these three images were calculated.
Data Analysis
Data are presented as mean ± SE. For comparison of the measured parameters in the four groups for each treatment (control+VE, T1D+VE, control+treatment and T1D+treatment), two by two ANOVA analysis was performed. When F values were significant, Newman–Keuls test was applied to identify differences between individual groups. Statistical significance was defined as P < 0.05. Table 2 shows the F values for erection, yawning and stretching responses to NMDA and SNP microinjection in the first 20 min.
Table 2.
F values for erection, yawning and stretching responses to NMDA and SNP microinjection in the first 20 min
| Erection | Yawning | Stretching | |
|---|---|---|---|
| NMDA-induced responses | |||
| Enalapril treatment | F=4.7 (p=0.010) | F=6.2 (p=0.003) | F=5.8 (p=0.004) |
| Losartan treatment | F=8.2 (p=0.001) | F=6.7 (p=0.002) | F=5.5 (p=0.005) |
| Tempol treatment | F=6.4 (p=0.003) | F=4.2 (p=0.016) | F=6.0 (p=0.003) |
|
| |||
| SNP-induced responses | |||
| Enalapril treatment | F=5.4 (p=0.006) | F=6.0 (p=0.003) | F=5.2 (p=0.067) |
| Losartan treatment | F=3.9 (p=0.020) | F=5.9 (p=0.004) | F=4.1 (p=0.018) |
| Tempol treatment | F=5.0 (p=0.008) | F=6.0 (p=0.003) | F=6.6 (p=0.002) |
Main Outcome Measure
After 2 weeks of treatment, in freely moving conscious rats, penile erections induced by NMDA or SNP into the PVN were determined. nNOS expression and O2•− production in the PVN was also measured by using Western blot and DHE staining approaches.
RESULTS
General Data
After a total of 5 weeks, induction and treatment, rats with T1D showed significantly higher glucose level compared to the control group (398 ± 51 mg/dl vs. 112 ± 14 mg/dl, P < 0.05). Body weight was significantly lower in T1D rats compared with control rats (249 ± 28 g vs. 398 ± 37 g, P < 0.05). Enalapril, losartan or tempol treatments had no significant effects on the blood glucose levels or body weight in either control or T1D groups (Table 1).
Table 1.
Characteristics of control and diabetic rats
| Body weight (g) | Blood glucose (mg/dl) | |
|---|---|---|
|
| ||
| Control+vehicle (n=12) | 398 ± 37 | 112 ± 14 |
| T1D+vehicle (n=12) | 249 ± 28* | 398 ± 51* |
| Control+enalapril (n=12) | 379 ± 26 | 106 ± 14 |
| T1D+enalapril (n=12) | 255 ± 36* | 374 ± 33* |
| Control+losartan (n=12) | 377 ± 29 | 101 ± 9 |
| T1D+losartan (n=12) | 257 ± 29* | 357 ± 44* |
| Control+tempol (n=12) | 377 ± 24 | 108 ± 7 |
| T1D+tempol (n=12) | 248 ± 29* | 355 ± 19* |
Values are presented as mean±SE;
indicates P < 0.05 vs. control rats
Behavioral Responses to NMDA Administration
Three weeks after induction of diabetes plus 2 weeks of enalapril, losartan or tempol treatment, NMDA was microinjected into the PVN of conscious, freely moving rats. The erectile responses to central microinjection of NMDA in the PVN in the conscious animals were significantly improved in T1D rats (T1D+ENL: 1.7 ± 0.6, T1D+LOS: 2.0 ± 0.3, T1D+TEM: 2.0 ± 0.6 vs. T1D+VE: 0.6 ± 0.3 penile erection/rat in the first 20 min, P < 0.05; Figure 1). In T1D rats, the erectile responses to central microinjection of NMDA in the PVN were still lower than in control rats with treatments (P < 0.05). Concurrent behavioral responses including yawning and stretching, induced by central NMDA microinjection were also significantly increased in the first 20 min in T1D rats after enalapril, losartan or tempol treatments (Figure 2–3, compared to T1D+VE group P < 0.05). There was no significant difference between treated and untreated groups in gross behaviour, such as spontaneous locomotor activity.
Figure 1.
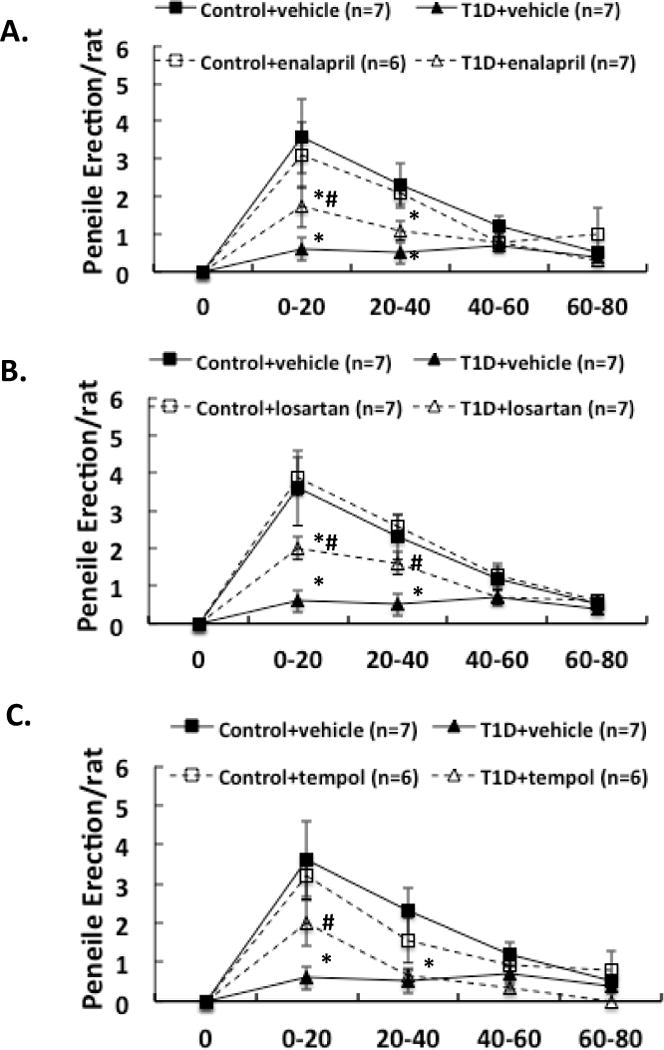
Effect of NMDA microinjections in the PVN on penile erection in the enalapril (A), losartan (B) and tempol (C) treatment groups of rats. Each value is the mean ± SE. *P < 0.05 with respect to control+vehicle group. #P<0.05 with respect to T1D+vehicle group.
Figure 2.
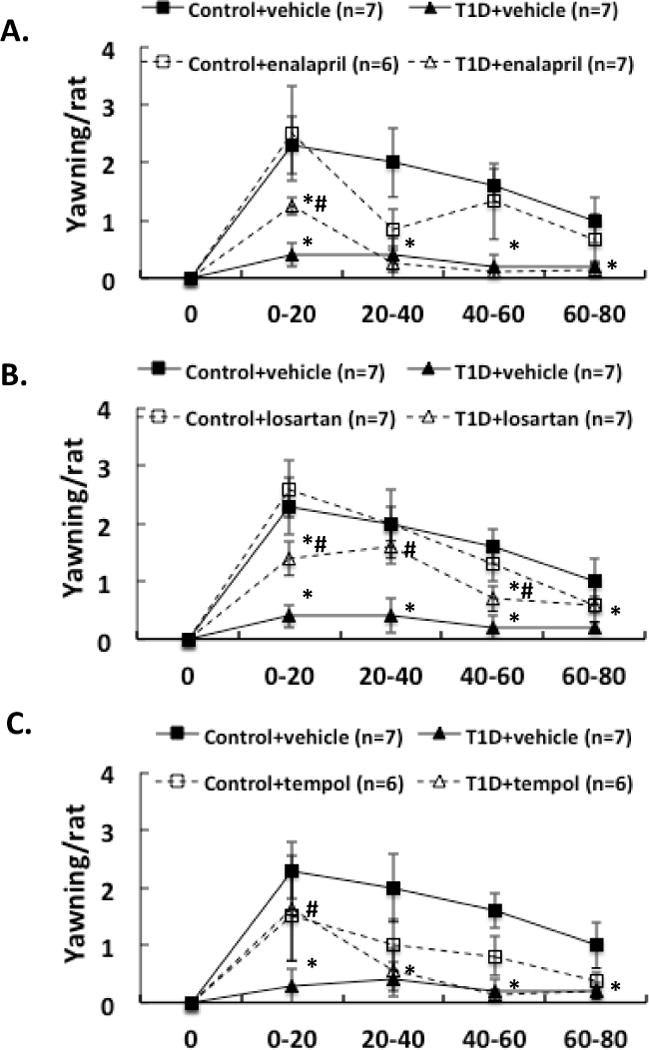
Effect of NMDA microinjections in the PVN on yawning in the enalapril (A), losartan (B) and tempol (C) treatment groups of rats. Each value is the mean±SE. *P < 0.05 with respect to control+vehicle group. #P < 0.05 with respect to T1D+vehicle group.
Figure 3.
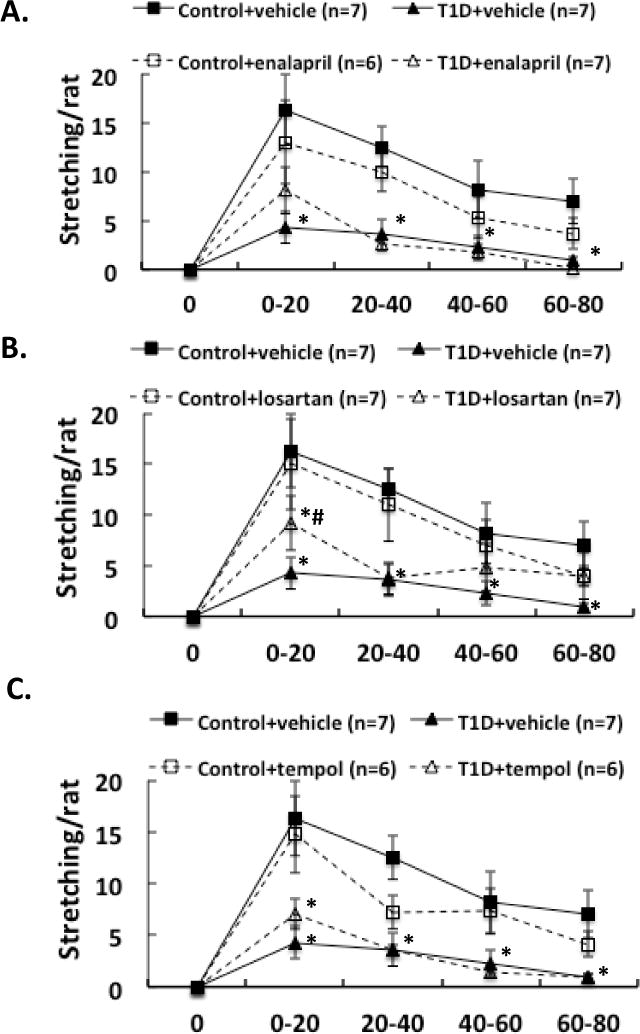
Effect of NMDA microinjections in the PVN on stretching in the enalapril (A), losartan (B) and tempol (C) treatment groups of rats. Each value is the mean ± SE. *P < 0.05 with respect to control+vehicle group. #P < 0.05 with respect to T1D+vehicle group.
Behavioral Responses to SNP Administration
We also compared the erectile, yawning and stretching responses to administration of an NO donor, SNP, into the PVN in these 8 groups of rats. After 2 weeks of enalapril, losartan or tempol treatment, the erectile responses to central microinjection of SNP in the PVN in conscious animals were significantly improved in T1D rats (T1D+ENL: 0.9 ± 0.3, T1D+LOS: 1.3 ± 0.3, T1D+TEM: 1.4 ± 0.4 vs. T1D+VE: 0.4 ± 0.2 penile erection/rat at 0-20 min, compared to T1D+VE group P < 0.05) (Figure 4). In T1D rats, the erectile responses to central microinjection of SNP in the PVN were still lower than in control rats with treatments (P < 0.05). Concurrent behavioral responses such as yawning and stretching, induced by central SNP microinjection were also significantly increased in the first 20 min of observation in T1D rats after enalapril, losartan or tempol treatments (Figure 5–6, compared to T1D+VE group P < 0.05).
Figure 4.
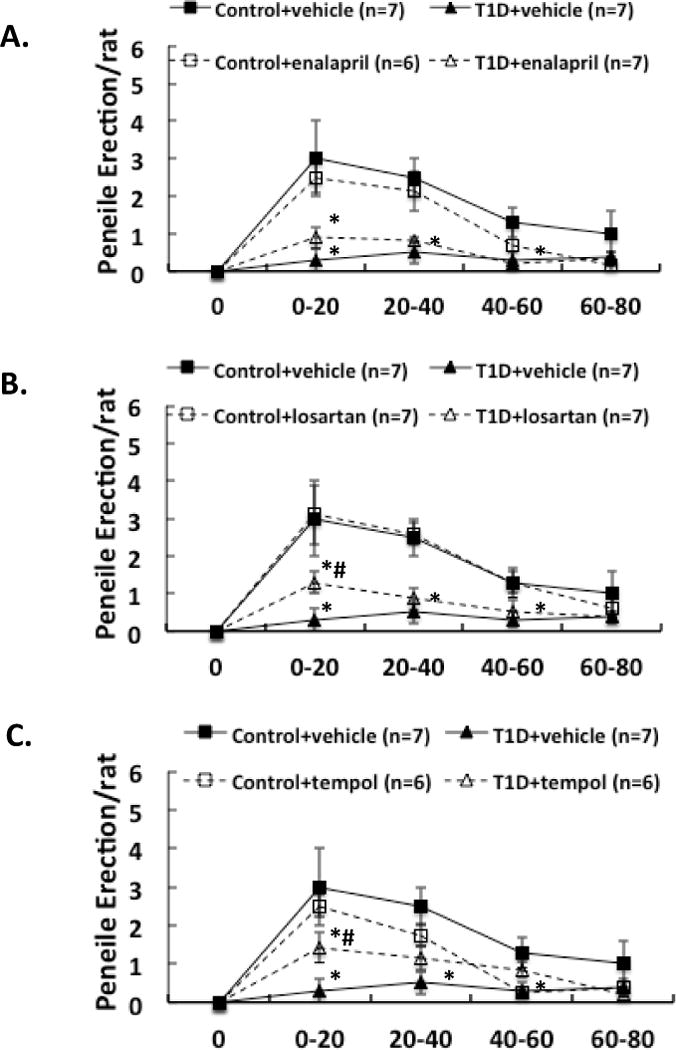
Effect of SNP microinjections in the PVN on penile erection in the enalapril (A), losartan (B) and tempol (C) treatment groups of rats. Each value is the meanSE. *P < 0.05 with respect to control+vehicle group. #P < 0.05 with respect to T1D+vehicle group.
Figure 5.
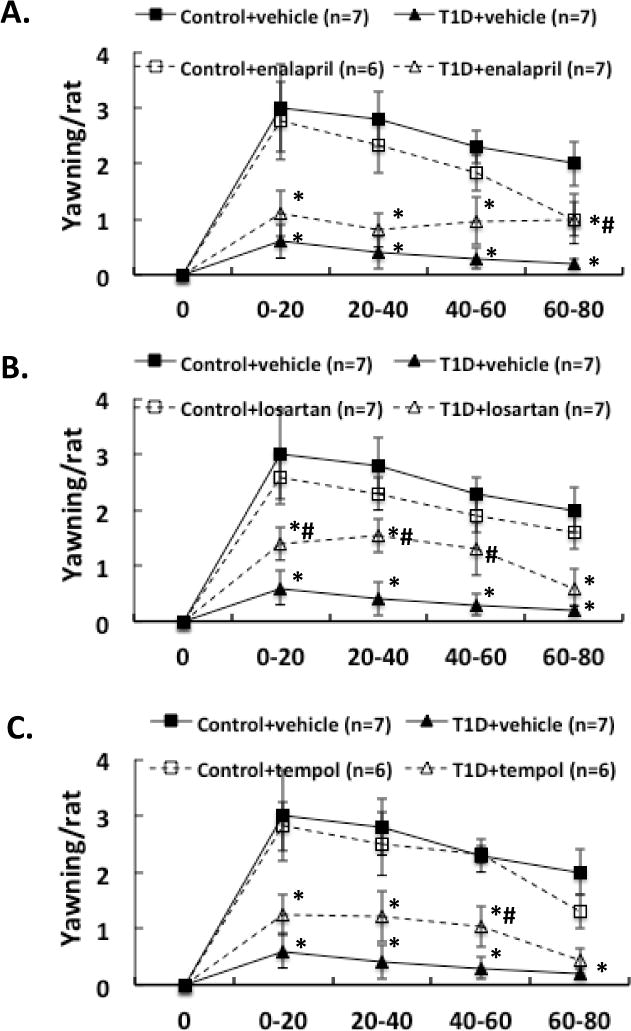
Effect of NMDA microinjections in the PVN on yawning in the enalapril (A), losartan (B) and tempol (C) treatment groups of rats. Each value is the mean±SE. *P < 0.05 with respect to control+vehicle group. #P < 0.05 with respect to T1D+vehicle group.
Figure 6.
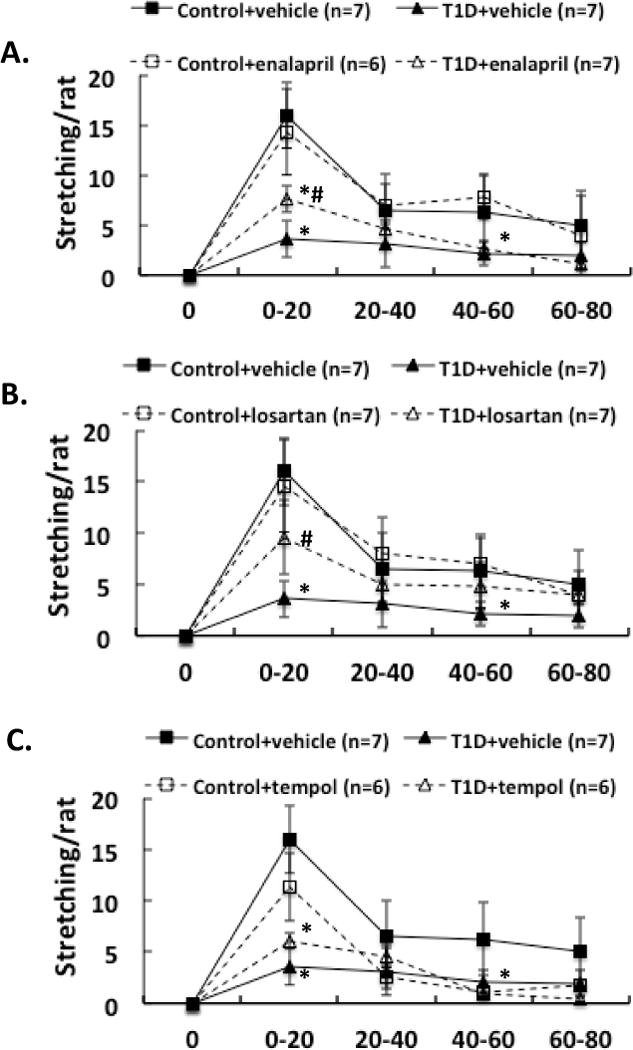
A. Effect of NMDA microinjections in the PVN on stretching in the enalapril (A), losartan (B) and tempol (C) treatment groups of rats. Each value is the mean±SE. *P < 0.05 with respect to control+vehicle group. # P < 0.05 with respect to T1D+vehicle group.
Expression of nNOS Protein within the PVN
nNOS protein expression, measured by Western blot, is shown in Figure 7. Sample gels showing nNOS and β tubulin protein in the eight experimental groups are presented in Figure 7A. The level of nNOS protein expression in the T1D+VE group was significantly lower than in the control+VE group (relative protein level 0.39 ± 0.10 vs. 0.76 ± 0.13, P < 0.05). In the T1D+ENL (0.63 ± 0.08), T1D+LOS (0.72 ± 0.20), T1D+TEM (0.76 ± 0.11) group, nNOS protein expression was significantly higher than in the T1D+VE group (0.39 ± 0.10, P < 0.05) and was not significantly different from the control group (0.76 ± 0.13, P > 0.05) (Figure 7).
Figure 7.
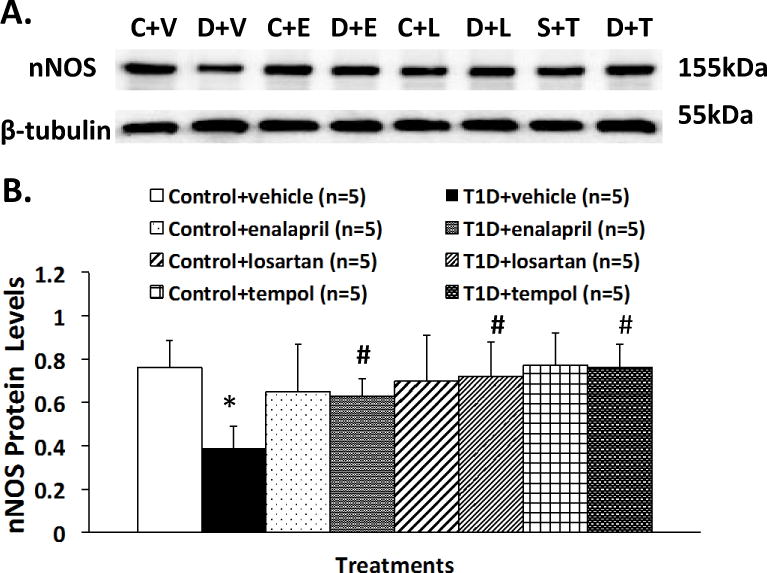
Representative Western blotting of nNOS protein (A) and composite data of nNOS protein expression (B) of PVN in the eight groups of rats. *P < 0.05 with respect to control+vehicle group. #P < 0.05 with respect to T1D+vehicle group. C: control; V: vehicle; E: enalapril; L: losartan; T: tempol.
O2•− Production in the PVN
To provide direct evidence that there was a decrease in intracellular O2•− levels in the central nervous system of diabetic rats after the three treatments, DHE fluorescence staining was used to estimate O2•− levels in the PVN of control and diabetic rats, with/without treatment (Figure 8). Quantitative analyses (Figure 8B) showed that there was significant decrease in T1D+ENL (relative DHE density 2.0 ± 0.2), T1D+LOS (1.8 ± 0.4) and T1D+TEM (1.2 ± 0.4) relative to basal fluorescence observed in T1D+VE group (3.2 ± 0.7, P < 0.05) and was not different from the control group (1.2 ± 0.4, P > 0.05) (Figure 8).
Figure 8.
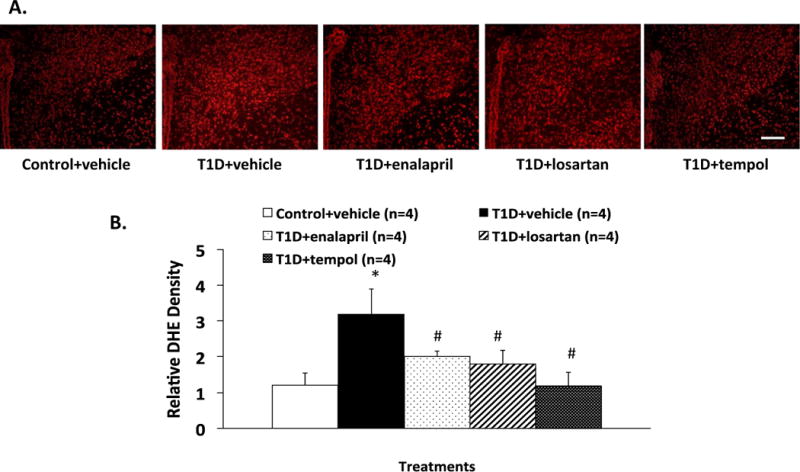
Representative DHE staining of PVN (A) and composite data of (B) in the five groups of rats. *P < 0.05 with respect to control+vehicle group. #P < 0.05 with respect to T1D+vehicle group. Scale bar=100 μm.
Discussion
The present study shows that 2 weeks of central administration of enalapril, losartan or tempol treatment, significantly improved the erectile responses to microinjection of NMDA and SNP in the PVN in conscious T1D rats. Accompanying behavioral responses such as yawning and stretching induced by central NMDA and SNP microinjections were also significantly increased in T1D rats after central enalapril, losartan or tempol treatments. Concomitant with these behavioral changes, expression of nNOS protein within the PVN was also significantly increased in T1D rats after enalapril, losartan or tempol treatments. The O2•− production within the PVN was also significantly reduced in T1D rats after central enalapril, losartan or tempol treatments. These data support the contention that altered nNOS mechanism/s within the PVN, possibly mediated by an enhanced ANG II AT1 receptor mechanism, by stimulation of O2•−, contributes to centrally-induced erectile dysfunction in T1D rats.
As the main active product of the renin-angiotensin system, in the periphery, ANG II has been shown to play a role in the regulation of cavernosal tone. An intracavernosal injection of ANG II can terminate erection in anesthetized dogs28. It has been suggested that specificity in systemic hemodynamic control by ANG II is also critical in establishing the optimal erectile environment in rats29. Furthermore, ANG II regulates vascular tone by counteracting the effects of NO, the principal mediator of penile erection. Recent results from clinical and experimental studies, show that treatment with ACE inhibitors in hypertensive patients and rats is able to recover/improve erectile function30.
Our previous study suggested that there is an enhanced ANG II system in T1D17. T1D rats have higher plasma ANG II compared to non-T1D rats. In the PVN, T1D rats show an increased AT1 receptor mRNA and protein expression17. This provides evidence indicating that the central ANG II system is altered in T1D rats. There is a positive relationship between ANG II and NO. Acute administration of ANG II has been shown to activate NOS and increase NO production in various systems31, 32 including the PVN33. However, chronically the interaction between ANG II and NO appears to be organized at the cellular level in such a way as to constitute a mutually inhibitory influence, in which, ANG II causes a decrease in nNOS gene expression and vice versa34, 35. Using a neuronal cell line, we have previously shown that ANG II down-regulates nNOS expression in vitro18. In the present study, we compared the effects of chronic central administration of the ACE inhibitor, enalapril and the ANG II AT1 receptor inhibitor, losartan on the NMDA-induced erectile responses between control and T1D groups. These chronic treatments improved the erectile as well as the behavioral responses in TID rats. We have further shown that ACE and ANG II AT1 receptor inhibitors restored the endogenous levels of nNOS expression in the PVN of T1D rats. The results confirm the potential influence of the ANG II system (via ACE, ANG II AT1 receptors) on erectile dysfunction and NO mechanism/s within the PVN of T1D rats.
It has been known for the past several years that ANG II is a potent stimulator of ROS36. In the periphery, at the level of the penis, it is thought that one of the mechanisms for restoration of erectile dysfunction in diabetes by ANG II blockers, is the alleviation of endothelial dysfunction. Further, ANG II is known to induce endothelial dysfunction via the increased production of ROS. The increased generation of ROS is thought to be responsible for NO degradation because it mediates the uncoupling of NO synthesis and inactivates NO via peroxynitrite formation. In the brain, it has been shown that ANG II signaling is mediated by ROS, particularly O2•−, produced from NADPH oxidase37. However, virtually nothing is known regarding ANG II-induced activation of NAD(P)H oxidase and subsequent O2•− production on the neural control of erectile dysfunction. In the present study, we found ACE and ANG II AT1 receptor inhibitors reduced O2•− production in the PVN, suggesting ANG II via AT1 receptors mediate O2•− signaling which may be involved in the erectile dysfunction observed in diabetes.
Previously, we observed an increase in NAD(P)H oxidase and O2•− levels with concomitant decrease in CuZnSOD levels in the PVN of rats that were diabetic for 6 weeks17. Further, the AT1 receptor antagonist losartan reduced NAD(P)H oxidase subunit expression, and O2•− in the PVN of T1D rats. Considering O2•− reacts with NO in a diffusion-limited fashion, we speculated that increased O2•− levels might contribute to the impaired erectile function in T1D by diminishing the bioavailability of NO. We also found that CuZnSOD protein in the PVN was less in T1D than in non-T1D rats, suggesting that this antioxidant enzyme is deficient in the PVN from T1D rats17. As a SOD mimetic agent, tempol has been shown to decrease excitatory effect of ANG II in the PVN. In the present study, we have shown that tempol has similar effects on the erectile response. This is because tempol, a SOD by mimetic agent, enhances O2•− scavenging and reduces the excitatory effects of ANG II in the PVN. These data provide crucial evidence for the involvement of O2•− in the erectile dysfunction during T1D. Increased ANG II levels in diabetes increases O2•− levels in the PVN, that may contribute to the reduced bioavailable NO leading to impaired centrally mediated erectile responses in T1D. These data provide a novel mechanistic insight for erectile dysfunction commonly seen in diabetes.
Although the present data suggest that in T1D rats ANG II activity is increased leading in turn to an increased O2•− production that decreases NO content in the PVN, other possibilities cannot be ruled out. For instance, the improvements in penile erection and yawning induced by NMDA injected into the PVN after the chronic administration of enalapril, losartan or tempol may be due to a direct effect of these drugs on NMDA receptors, i.e., changes in the number or affinity of NMDA receptors in the PVN. Previously we have observed that ANG II treatment stimulated NMDA NR1 protein expression and losartan significantly ameliorated the NR1 expression induced by ANG II in a neuronal culture model38. This would support the hypothesis that chronic administration of enalapril, losartan or tempol may have their effect due to a direct effect of these ANG II blockers on NMDA receptors.
Furthermore, excitatory amino acids are not the only substances that induce penile erection when injected into the PVN, there are several other neurotransmitters that could also change after enalapril, losartan or tempol treatments in diabetes, such as oxytocin, dopamine, GABA, NPY. All these neurotransmitters influence the pro-erectile activity and could change the penile erection39–41. It would be of interest to examine if enalapril, losartan or tempol influence other neurotransmitters involved in the pro-erectile activity in this model of diabetes in the future.
Long-term complications of diabetes have been ascribed to both the effects of prolonged hyperglycemia and increased oxidative stress42, 43. The Diabetic Control and Complications Trial (DCCT) has established the importance of hyperglycemia and other consequences of insulin deficiency in the pathogenesis of diabetic autonomic dysfunction. Hyperglycemia has been implicated in the etiology of diabetic neuropathy. It has become apparent that in insulin-deficient conditions, such as T1D, both insulin and insulinomimetic C-peptide must be replaced in order to gain hyperglycemic control and to combat complications44, 45. It has been found that in response to chronic hyperglycemia, the activity of ANG II is significantly up-regulated in the peripheral tissue46. High glucose stimulates angiotensinogen synthesis and ROS generation in renal tubular cells47. In light of this evidence, and the data presented in this manuscript, it is important to amalgamate the changes in the central nervous system with those in the periphery by further investigations.
Conclusion
Previously, we have demonstrated that erectile dysfunction in diabetes is partially due to a selective defect in the central nNOS mechanism, specifically, within the PVN7. The present set of experiments validate and support the hypothesis that enhanced ANG II AT1 mechanisms via stimulation of O2•− contributes to centrally induced erectile dysfunction in T1D rats. Thus, any and all mechanisms that up-regulate nNOS within the PVN, such as ANG II inhibitors and superoxide dismutase may be used as therapeutic modalities to consequently improve the central component of the erectile dysfunction in diabetes mellitus in humans.
Acknowledgments
This work is supported by NIH grant RO1 DK082956-03. The technical assistance of Lirong Xu is greatly appreciated.
References
- 1.Schiavi RC, Stimmel BB, Mandeli J, Rayfield EJ. Diabetes mellitus and male sexual function: a controlled study. Diabetologia. 1993;36:745–51. doi: 10.1007/BF00401146. [DOI] [PubMed] [Google Scholar]
- 2.Schiavi RC, Schanzer H, Sozio G, Setacci C, Stimmel B, Rayfield EJ. Erectile function and penile blood pressure in diabetes mellitus. J Sex Marital Ther. 1994;20:119–24. doi: 10.1080/00926239408403422. [DOI] [PubMed] [Google Scholar]
- 3.McCulloch DK, Campbell IW, Wu FC, Prescott RJ, Clarke BF. The prevalence of diabetic impotence. Diabetologia. 1980;18:279–83. doi: 10.1007/BF00251005. [DOI] [PubMed] [Google Scholar]
- 4.Sweni S, Meenakshisundaram R, Senthikumaran S, Thirumalaikolundusubramanian P. Propofol’s derivative: a potential drug for erectile dysfunction? Med Hypotheses. 2011;77:668–70. doi: 10.1016/j.mehy.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Melis MR, Succu S, Iannucci U, Argiolas A. N-methyl-D-aspartic acid-induced penile erection and yawning: Role of hypothalamic paraventricular nitric oxide. EurJPharmacol. 1997;328:115–23. doi: 10.1016/s0014-2999(97)83037-3. [DOI] [PubMed] [Google Scholar]
- 6.Melis MR, Stancampiano R, Argiolas A. Nitric oxide synthase inhibitors prevent N-methyl-D-aspartic acid-induced penile erection and yawning in male rats. Neurosci Lett. 1994;179:9–12. doi: 10.1016/0304-3940(94)90922-9. [DOI] [PubMed] [Google Scholar]
- 7.Zheng H, Bidasee KR, Mayhan WG, Patel KP. Lack of central nitric oxide triggers erectile dysfunction in diabetes. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1158–64. doi: 10.1152/ajpregu.00429.2006. [DOI] [PubMed] [Google Scholar]
- 8.Reid IA. Interaction between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol. 1992;262:E763–E78. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- 9.Matsukawa S, Keil LC, Reid IA. Role of endogenous angiotensin II in the control of vasopressin secretion during hypervolemia and hypotension in conscious rabbits. Endocrinology. 1998;128:204–10. doi: 10.1210/endo-128-1-204. [DOI] [PubMed] [Google Scholar]
- 10.Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central intergration. Front Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson AV, Washburn DLS. Angiotensin II: A peptidergic neurotransmitter in central autonomic pathways. Prog Neurobiol. 1998;54:169–92. doi: 10.1016/s0301-0082(97)00065-8. [DOI] [PubMed] [Google Scholar]
- 12.Davisson RL, Yang GY, Beltz TG, Cassell MD, Johnson AK, Sigmund CD. The brain renin-angiotensin system contributes to the hypertension in mice containing both the human renin and human angiotensinogen transgenes. Circ Res. 1998;83:1047–58. doi: 10.1161/01.res.83.10.1047. [DOI] [PubMed] [Google Scholar]
- 13.Phillips MI, Shen L, Richards EM, Raizada MK. Immunohistochemical mapping of angiotensin AT1 receptors in the brain. Regul Pept. 1993;44:95–107. doi: 10.1016/0167-0115(93)90233-x. [DOI] [PubMed] [Google Scholar]
- 14.Lenkei Z, Corvol P, Llorens-Cortes C. The angiotensin receptor subtype AT1A predominates in rat forebrain areas involved in blood pressure, body fluid homeostasis and neuroendocrine control. Brain Res Mol Brain Res. 1995;30:53–60. doi: 10.1016/0169-328x(94)00272-g. [DOI] [PubMed] [Google Scholar]
- 15.Tagawa T, Dampney RAL. AT1 receptors mediate excitatory inputs to rostral ventrolateral medulla presser neurons from hypothalamus. Hypertension. 1999;34:1301–07. doi: 10.1161/01.hyp.34.6.1301. [DOI] [PubMed] [Google Scholar]
- 16.Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci. 2003;23:5041–9. doi: 10.1523/JNEUROSCI.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel KP, Mayhan WG, Bidasee KR, Zheng H. Enhanced angiotensin II-mediated central sympathoexcitation in streptozotocin-induced diabetes: role of superoxide anion. Am J Physiol Regul Integr Comp Physiol. 2011;300:R311–20. doi: 10.1152/ajpregu.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma NM, Zheng H, M PP, Li YF, Patel KP. Decreased nNOS in the PVN leads to increased sympathoexcitation in chronic heart failure: role for CAPON and Ang II. Cardivasc Res. 2011;92:348–57. doi: 10.1093/cvr217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angulo J, Cuevas P, Fernandez A, Gabancho S, Allona A, Martin-Morales A, Moncada I, Videla S, Saenz de Tejada I. Diabetes impairs endothelium-dependent relaxation of human penile vascular tissues mediated by NO and EDHF. Biochem Biophys Res Commun. 2003;312:1202–8. doi: 10.1016/j.bbrc.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Jeremy JY, Angelini GD, Khan M, Mikhailidis DP, Morgan RJ, Thompson CS, Bruckdorfer KR, Naseem KM. Platelets, oxidant stress and erectile dysfunction: an hypothesis. Cardiovasc Res. 2000;46:50–54. doi: 10.1016/s0008-6363(00)00009-2. [DOI] [PubMed] [Google Scholar]
- 21.Bivalacqua TJ, Usta MF, Kendirci M, Pradhan L, Alvarez X, Champion HC, Kadowitz PJ, Hellstrom WJ. Superoxide anion production in the rat penis impairs erectile function in diabetes: influence of in vivo extracellular superoxide dismutase gene therapy. J Sex Med. 2005;2:187–97. doi: 10.1111/j.1743-6109.2005.20228_1.x. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Zhuan L, Wang T, Rao K, Yang J, Yang J, Quan W, Liu J, Ye Z. Apocynin improves erectile function in diabetic rats through regulation of NADPH oxidase expression. J Sex Med. 2012;9:3041–50. doi: 10.1111/j.1743-6109.2012.02960.x. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Orlando: Academic Press; 1986. [Google Scholar]
- 24.Francis J, Wei SG, Weiss RM, Felder RB. Brain angiotensin-converting enzyme activity and autonomic regulation in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H2138–46. doi: 10.1152/ajpheart.00112.2004. [DOI] [PubMed] [Google Scholar]
- 25.Porter JP, Phillips A, Rich J, Wright D. Effect of chronic stress on the cardiac baroreflex in the post-weanling rat. Life Sci. 2004;75:1595–607. doi: 10.1016/j.lfs.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone Acts Centrally to Increase Brain Renin-Angiotensin System Activity and Oxidative Stress in Normal Rats. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.01131.2007. [DOI] [PubMed] [Google Scholar]
- 27.Palkovits M, Brownstein M. Brain microdissection techniques. In: Cuello AE, editor. Brain microdissection techniques. Chichester: John Wiley & Sons; 1983. [Google Scholar]
- 28.Kifor I, Williams GH, Vickers MA, Sullivan MP, Jodbert P, Dluhy RG. Tissue angiotensin II as a modulator of erectile function. I. Angiotensin peptide content, secretion and effects in the corpus cavernosum. J Urol. 1997;157:1920. [PubMed] [Google Scholar]
- 29.MacKenzie LD, Heaton JP, Adams MA. Impact of systemically active neurohumoral factors on the erectile response of the rat. J Sex Med. 2011;8:2461–71. doi: 10.1111/j.1743-6109.2011.02333.x. [DOI] [PubMed] [Google Scholar]
- 30.Hale TM, Okabe H, Bushfield TL, Heaton JPW, Adams MA. Recovery of erectile function after brief aggressive antihypertensive therapy. J Urol. 2002;168:348–54. [PubMed] [Google Scholar]
- 31.Pueyo ME, Arnal JF, Rami J, Michel JB. Angiotensin II stimulates the production of NO and peroxynitrite in endothelial cells. Am J Physiol Cell Physiol. 1998;274:C214–C20. doi: 10.1152/ajpcell.1998.274.1.C214. [DOI] [PubMed] [Google Scholar]
- 32.Thorup C, Kornfeld M, Winaver JM, Goligorsky MS, Moore LC. Angiotensin II stimulates nitric oxide release in isolated perfused renal resistance arteries. Pflugers Arch. 1998;435:432–34. doi: 10.1007/s004240050535. [DOI] [PubMed] [Google Scholar]
- 33.Li YF, Wang W, Mayhan WG, Patel KP. Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1035–43. doi: 10.1152/ajpregu.00338.2004. [DOI] [PubMed] [Google Scholar]
- 34.Khara M, Umemura A, Kadota T, Yabana M, Tamura K, Nyuui N, Ogawa N, Murakami K, Fukamizu A, Ishii M. The neuronal isoform of constituative nitric oxide synthase is up-regulated in the macula densa of angiotensinogen gene-knockout mice. Lab Invest. 1997;76:285–94. [PubMed] [Google Scholar]
- 35.Iwai N, Hanai K, Tooyama I, kitamura Y, Kinoshita M. Regulation of neuronal nitric oxide synthase in rat adrenal medulla. Hypertension. 1995;25:431–36. doi: 10.1161/01.hyp.25.3.431. [DOI] [PubMed] [Google Scholar]
- 36.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–48. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–45. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 38.Kleiber AC, Zheng H, Sharma NM, Patel KP. Chronic AT1 receptor blockade normalizes NMDA-mediated changes in renal sympathetic nerve activity and NR1 expression within the PVN in rats with heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1546–55. doi: 10.1152/ajpheart.01006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melis MR, Stancampiano R, Gessa GL, Argiolas A. Prevention by morphine of apomorphine- and oxytocin-induced penile erection and yawning: site of action in the brain. Neuropsychopahrmacology. 1992;6:17–21. [PubMed] [Google Scholar]
- 40.Melis MR, Argiolas A. Reduction of drug-induced yawning and penile erection and of noncontact erections in male rats by the activation of GABAA receptors in the paraventricular nucleus: involvement of nitric oxide. The European journal of neuroscience. 2002;15:852–60. doi: 10.1046/j.1460-9568.2002.01922.x. [DOI] [PubMed] [Google Scholar]
- 41.Melis MR, Mascia MS, S S, Torsello A, Muller EE, Deghenghi R, Argiolas A. Ghrelin injected into the paraventricular nucleus of the hypothalamus of male rats induces feeding but not penile erection. Neurosci Lett. 2002;329:339–43. doi: 10.1016/s0304-3940(02)00673-0. [DOI] [PubMed] [Google Scholar]
- 42.Manuel-Y-Keenoy B, Vertommen J, De Leeuw I. Divergent effects of different oxidant on glutathione homeostasis and protein damage in erythrocytes from diabetic patients: effects of high glucose. Mol Cell Biochem. 2001;225:59–73. doi: 10.1023/a:1012268807728. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL, Hofmann SM, Vlassara H, Shi Y. Diabetes-induced oxidative stress and long-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472–8. doi: 10.1161/01.CIR.0000080378.96063.23. [DOI] [PubMed] [Google Scholar]
- 44.Marques RG, Fontaine MJ, Rogers P. C-peptide: much more than a byproduct of insulin biosynthesis. Pancreas. 2004;29:231–8. doi: 10.1097/00006676-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Li ZG, Sima AA. C-peptide and central nervous system complications in diabetes. Exp Diabesity Res. 2004;5:79–90. doi: 10.1080/15438600490424550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. Improved islet morphology after blockade of the renin-angiotensin system in the ZDF rat. Diabetes. 2004;53:989–97. doi: 10.2337/diabetes.53.4.989. [DOI] [PubMed] [Google Scholar]
- 47.Zhang SL, Filep JG, Hohman TC, Tang SS, Ingelfinger JR, Chan JSD. Molecular mechanisms of glucose action on angiotensinogen gene expression in rat proximal tubular cells. Kidney Int. 1999;55:454–64. doi: 10.1046/j.1523-1755.1999.00271.x. [DOI] [PubMed] [Google Scholar]


