Abstract
Objective
Antibiotic-associated diarrhea (AAD) and Clostridium difficile infection (CDI) are well-known outcomes from antibiotic administration. Because emergency department (ED) visits frequently result in antibiotic use, we evaluated the frequency of AAD/CDI in adults treated and discharged home with new prescriptions for antibiotics to identify risk factors for acquiring AAD/CDI.
Methods
This prospective multicenter cohort study enrolled adult patients who received antibiotics in the ED and were discharged with a new prescription for antibiotics. Antibiotic-associated diarrhea was defined as 3 or more loose stools for 2 days or more within 30 days of starting the antibiotic. C difficile infection was defined by the detection of toxin A or B within this same period. We used multivariate logistic regression to assess predictors of developing AAD.
Results
We enrolled and followed 247 patients; 45 (18%) developed AAD, and 2 (1%) developed CDI. Patients who received intravenous (IV) antibiotics in the ED were more likely to develop AAD/CDI than patients who did not: 25.7% (95% confidence interval [CI], 17.4–34.0) vs 12.3% (95% CI, 6.8–17.9). Intravenous antibiotics had adjusted odds ratio of 2.73 (95% CI, 1.38–5.43), and Hispanic ethnicity had adjusted odds ratio of 3.04 (95% CI, 1.40–6.58). Both patients with CDI had received IV doses of broad-spectrum antibiotics.
Conclusion
Intravenous antibiotic therapy administered to ED patients before discharge was associated with higher rates of AAD and with 2 cases of CDI. Care should be taken when deciding to use broad-spectrum IV antibiotics to treat ED patients before discharge home.
1. Introduction
Antibiotic-associated diarrhea (AAD), a common side effect of antibiotic administration, complicates between 5% and 39% of treatment regimens [1]. The frequency of AAD is influenced by physician factors, such as antibiotic selection, and by patient characteristics, including comorbidities and age [2]. The widespread use of antibiotics has led to increases in the incidence of AAD and Clostridium difficile infection (CDI) [3,4]. The pathophysiology of CDI causes a pseudomembranous colitis that ranges from mild diarrhea to fulminant colitis [5]. Since its emergence, CDI is the leading cause of gastroenterologic hospitalizations and deaths [6]. United States annual direct costs for CDI are estimated at nearly $3.4 billion [7]. Both the incidence and the severity of CDI have increased over the last decade [8]. This high economic and public health cost justifies the use of additional resources for CDI prevention and control [9].
C difficile infection is responsible for 10 to 20% of all AAD cases [1]. Although virtually all antibiotics have been implicated in AAD and CDI, the cephalosporins, clindamycin, and broad-spectrum penicillins are more frequently involved [10]. Prolonged courses of antibiotic treatment, administration of multiple antibiotics, patient age more than 65 years, or history of diarrhea after antibiotic use impart additional risk [1,11,12]. The use of broad-spectrum intravenous (IV) antibiotics as an initial treatment for outpatient emergency department (ED) patients has not been clearly shown to affect the rates of AAD and CDI, but a single dose of perioperative IV antibiotics has been shown to place patients at greater risk [13]. Most patients who develop CDI do so within the first 2 weeks of antibiotic exposure [14], but AAD can occur at any time, including up to 2 to 3 weeks after cessation of antibiotic therapy [11].
Both AAD and CDI are important clinical problems because they contribute to morbidity, mortality, antibiotic noncompliance, and increased health care costs [15]. The widespread use of antibiotics for nonbacterial infections contributes directly to rates of AAD and CDI [4]. By conservative estimates, at least one quarter of the 160 million antibiotic prescriptions written annually in the United States are unnecessary [16]. Although most of the antibiotics prescribed originate from acute care settings, little is known about the rates and risk factors for AAD and CDI in the ED outpatient population. To our knowledge, the impact of AAD and CDI on patients who are seen in the ED and treated as outpatients has not been evaluated. In addition, it is not known what effect the use of IV antibiotics before discharge on oral antibiotics has on the rates of AAD and CDI. A better understanding of risk factors for AAD and CDI in the ED outpatient setting may lead to safer practices for prescribing antibiotics in the ED and may spur interventions to reduce unnecessary use of antibiotics.
The main purposes of this study were to prospectively determine the frequency of AAD in a sample of adult patients treated and discharged home from the ED and to identify risk factors for acquiring AAD, such as type of antibiotic, initial route of administration, and patient demographic characteristics.
2. Methods
2.1. Study design and setting
This prospective observational study was conducted over a 13-month period from September 2012 to October 2013 at 2 large urban academic EDs and 1 smaller community ED located in the states of Rhode Island and Massachusetts. The hospitals' institutional review boards (IRBs) approved the study (IRB dockets #H-14491 and #4061-13), and all participants gave written informed consent and signed a release of pertinent medical records.
2.2. Selection of participants
Patients were screened for enrollment during 6-hour time blocks chosen randomly from the hours of 7 am through 1:00 am, 7 days a week. The principal investigator, co-investigators, and trained research assistants identified eligible patients through real-time review of ED tracking boards. Patients were eligible if they were English speaking, were 18 years or older, and were subsequently discharged home from the ED on antibiotics. Patients were excluded if they had diarrhea on presentation or in the preceding 4 weeks, had taken an antibiotic in the preceding 4 weeks, were unable to cooperate with the questionnaire or follow-up, had a history of CDI in the preceding 4 weeks, or were admitted to the hospital.
2.3. Data collection
At the time of enrollment, data were collected on the participants' demographic characteristics, medical history, allergies, and contact information. Antibiotic data were obtained from medical records. Participants were contacted in a follow-up survey 4 weeks after completion of antibiotic therapy. This follow-up period covers the time during which diarrhea symptoms typically begin after taking antibiotics [17]. The survey collected information on antibiotic compliance, occurrence of AAD or CDI, and subsequent health care visits. Patients who could not be contacted within 7 days were considered lost to follow-up. Diarrhea was defined as any loose stools that the patient reported. Antibiotic-associated diarrhea was defined as 3 or more loose stools per day for 2 or more consecutive days [11,18]. Mild diarrheal illness (MDI) was defined as diarrheal symptoms not fulfilling the criteria for AAD. C difficile infection was defined as AAD that led to a diagnosis of CDI and/or treatment of diarrhea consistent with treatment for CDI. When possible, CDI was confirmed through stool studies by reviewing hospital or outpatient records. Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at the University of Massachusetts Medical School [19]. REDCap is a secure, Web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for importing data from external sources.
2.4. Outcomes
The primary outcome was the presence of diarrheal symptoms. The secondary outcome was the presence of CDI, confirmed by a positive C difficile toxin A assay in combination with CDI diagnosis.
2.5. Analysis
The analysis used χ2 tests to compare rates of AAD among categories of single variables and logistic regression analysis to assess variables' effects, adjusted for the contributions of other variables. The software used for the analyses was Stata, Release 13 (StataCorp LP, College Station, TX) and Prism Release 6 (GraphPad Software Inc, La Jolla, CA).
3. Results
3.1. Characteristics of the study subjects
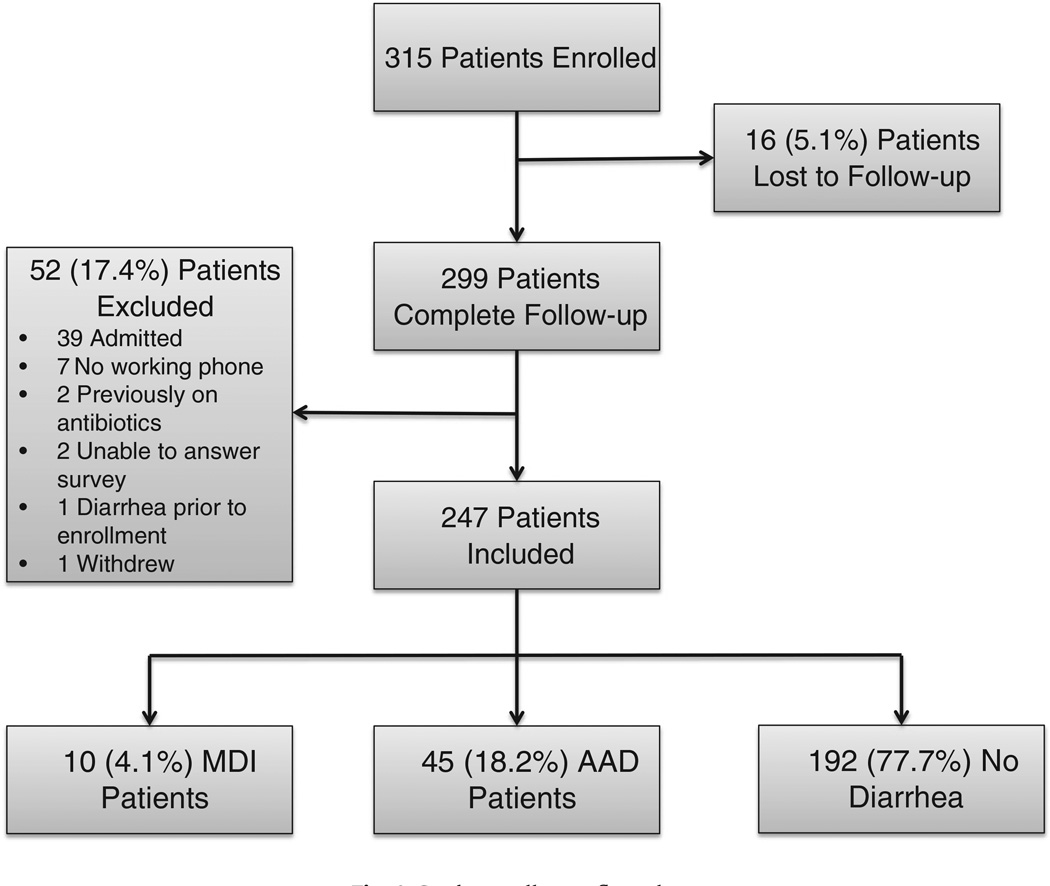
Over the 13-month period, we enrolled 315 ED patients who met inclusion/exclusion criteria and were discharged home on antibiotics from the 3 ED sites (Fig. 1). We were unable to contact 16 (5.1%) patients. Of the remaining 299 patients, 52 (17.4%) were excluded for the following reasons: 39 patients were found to be admitted to the hospital for care during or within 24 hours of the initial visit, 7 did not have a working phone to complete follow-up, 2 were found to be on antibiotics before enrollment, 2 were unable to complete the survey, 1 had diarrhea just before enrollment, and 1 withdrew from the study. The final analysis used data from a total of 247 subjects. The average age of subjects enrolled was 40.9 (SD, 17.2); 47.8% were female, 74.1% Caucasian, 7.7% African American, and 17.4% Hispanic. The patients were generally healthy: 61.5% reported no medical history, and 25.1% had only one medical condition (Table 1). The most common treatment targets were skin/soft tissue infections, with 34.8% cellulitis and 7.7% abscess. The remaining subjects were treated for dental infections (10.9%); ear, nose, or throat infections (13.8%); respiratory tract infections (6.1%); or urinary tract infections (15.0%); or were given antibiotics prophylactically (11.7%).
Fig. 1.
Study enrollment flow chart.
Table 1.
Enrolled patients' treatment targets and medical history, and the percentage who had AAD, MDI, or no diarrhea
| Treatment Target | Total number (%) |
Percentage with AAD |
Percentage with MDI |
Percentage with no diarrhea |
|---|---|---|---|---|
| Cellulitis | 86 (34.8) | 17.4 | 2.3 | 80.2 |
| Abscess | 19 (7.7) | 10.5 | 10.5 | 78.9 |
| Dental | 27 (10.9) | 29.6 | 7.4 | 63.0 |
| ENT | 34 (13.8) | 20.6 | 5.9 | 73.5 |
| Respiratory | 15 (6.1) | 20.0 | 6.7 | 73.3 |
| UTI | 37 (15.0) | 16.2 | 0.0 | 83.8 |
| Prophylaxis | 29 (11.7) | 13.8 | 3.4 | 82.8 |
| Medical history | ||||
| No disease | 152 (61.5) | 19.7 | 4.6 | 75.7 |
| One disease | 62 (25.1) | 16.1 | 4.8 | 79.0 |
| Two or more | 33 (13.4) | 15.2 | 0.0 | 84.8 |
| Diabetes | 22 (8.9) | 18.2 | 0.0 | 81.8 |
| IBS | 12 (4.9) | 25.0 | 0.0 | 75.0 |
| Hx diarrhea | 37 (15.0) | 24.3 | 8.1 | 67.6 |
Abbreviations: IBS, irritable bowel syndrome; Hx, history; ENT, ear, nose, and throat infection; UTI, urinary tract infection.
3.2. Main results
Forty-five patients (18.2%) developed AAD, 10 patients (4.0%) had MDI, and 192 (77.7%) had no gastrointestinal side effects. Median time to onset of diarrheal symptoms was 1.5 days into therapy (range, 0–10 days; quartiles 1 and 2 days), and the median duration of symptoms was 4 days (range, 1–14 days; quartiles 3 and 7.5 days). Two patients in the AAD group went on to develop CDI (4.4%). The rates of AAD were similar in the 3 study sites. Of note, Hispanic patients were at greater risk of developing AAD than Caucasians or African Americans (32.6% vs 15.3% and 15.8%; relative risk, 2.122, vs Caucasians and African Americans combined; 95% confidence interval [CI], 1.238–3.635).
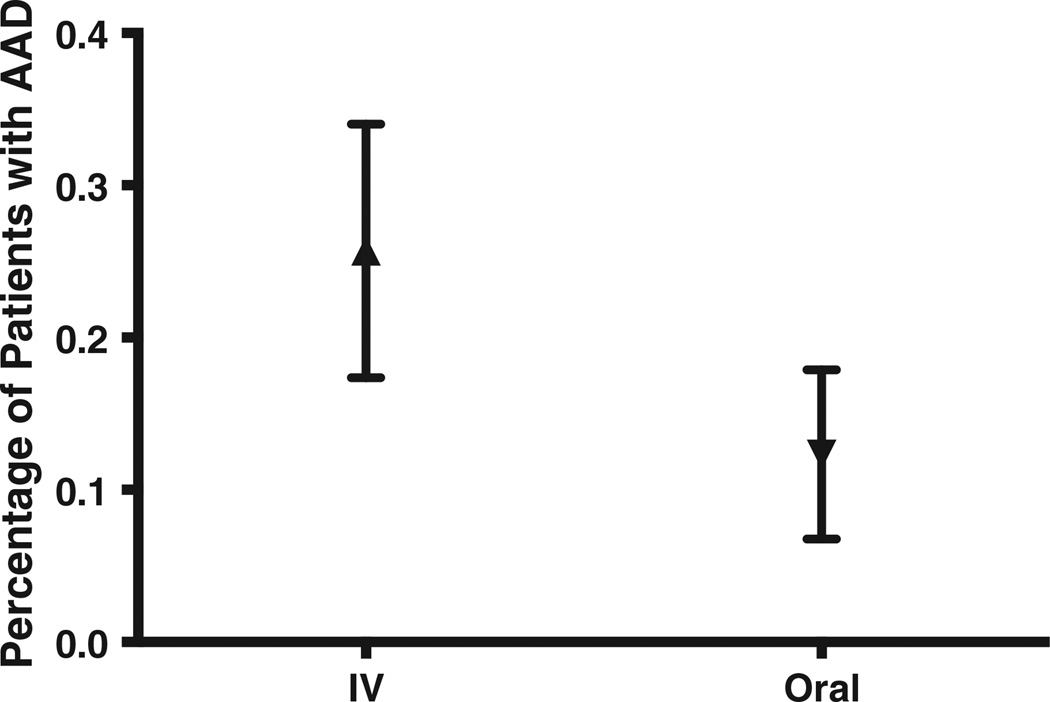
Higher rates of AAD were noted among the 109 patients who received IV antibiotics as part of their ED care (Fig. 2) than among the138 patients given only oral antibiotics. Of the IV group, 25.7% (95% CI, 17.4–34.0) developed AAD, compared with 12.3% (95% CI, 6.8–17.9) of the oral-alone group. Receiving IV antibiotics carried an increased risk of developing AAD: relative risk, 2.085 (95% CI, 1.206–3.606). Among the patients who received IV antibiotics, 70% received a single dose, and 14% received 2 doses. The largest number of IV doses administered in the ED was six. The number of IV doses appeared not to have a significant effect on the risk of developing AAD (the numbers of patients receiving specific numbers of multiple doses were too small to indicate a consistent pattern).
Fig. 2.
Rates of AAD among patients treated with IV antibiotics and then discharged home on oral antibiotics compared with patients given only oral antibiotics. Data are presented as means with 95% CI.
The IV and oral-alone groups had similar emergency severity index (ESI) scores and similar percentages of patients presenting as tachycardic or as febrile (Table 2). The 2 groups also had similar demographic characteristics and medical histories. A greater percentage of patients with cellulitis were treated with initial IV antibiotics (P < .001), whereas patients with urinary tract infections were primarily treated with oral antibiotics alone (P < .001). In the follow-up survey, 10.2% of the IV group and 9.1% of the oral-alone group felt that the initial therapy did not improve their condition, and 3.1% of the IV group and 4.0% of the oral-alone group failed antibiotic therapy (the differences are not statistically significant).
Table 2.
Comparison of initial IV antibiotic treatment (IV) group with the oral antibiotic alone group
| Presentation | IV group | Oral alone |
|---|---|---|
| ESI | 3.44 (3.30–3.57) | 3.59 (3.42–3.77) |
| Tachycardic | 22.1 (13.5–32.6) | 20.3 (13.5–32.6) |
| Febrile | 14.1 (6.2–22.0) | 5.8 (0.1–11.5) |
| Demographics | ||
| Age | 43.1 (39.8–46.4) | 39.1 (36.3–42.0) |
| Female | 46.8 (37.3–56.3) | 48.6 (40.1–57.0) |
| Caucasian | 77.1 (69.1–85.1) | 71.7 (64.1–79.4) |
| Hispanic | 14.7 (7.9–21.4) | 19.6 (12.9–26.3) |
| Black | 7.3 (2.4–12.3) | 8.0 (3.4–12.6) |
| Treatment Target | ||
| Cellulitisa | 46.8 (37.3–56.3) | 25.4 (18.0–32.7) |
| Abscess | 8.3 (3.0–13.5) | 7.2 (2.9–11.6) |
| Dental | 7.3 (2.4–12.3) | 13.8 (7.9–19.6) |
| ENT | 14.7 (7.9–21.4) | 13.0 (7.4–18.7) |
| Respiratory | 4.6 (0.6–8.6) | 7.2 (2.9–11.6) |
| UTIb | 5.5 (1.2–9.9) | 22.5 (15.4–29.5) |
| Prophylaxis | 12.8 (6.5–19.2) | 10.9 (5.6–16.1) |
| Medical history | ||
| Hx diarrhea | 15.6 (8.7–22.5) | 14.5 (8.5–20.4) |
| No disease | 59.6 (50.3–69.0) | 63.0 (54.9–71.2) |
| One disease | 26.6 (18.2–35.0) | 23.9 (16.7–31.1) |
| Two or more | 13.8 (7.2–20.3) | 13.0 (7.4–18.7) |
Means (for ESI and age) and percentages with 95% CIs. Abbreviations: ENT, ear, nose, and throat infection; UTI, urinary tract infection; Hx, history.
P = .0005.
P= .0002.
The rate of AAD showed a tendency to increase with the total duration of antibiotic therapy (Table 3). The mean duration of therapy was higher in the AAD group (9.5 days) than in the group with no diarrheal symptoms (8.4 days). Rates of AAD varied among the antibiotic classes (Table 4). Clindamycin had the highest rates of AAD (30.0%), both as monotherapy (26.5%) and in combination with other antibiotics (37.5%). Other classes with relatively high rates of AAD were vancomycin (21.4%), cephalosporins (19.8%), penicillins (19.6%), and macrolides (19.0%). The lowest rates occurred with quinolones (6.9%) and with doxycycline combined with another antibiotic (doxycycline plus) (8.3%). No AAD was observed in the 4 patients treated with doxycycline alone.
Table 3.
Duration of antibiotic therapy and development of AAD
| Duration of antibiotic therapy |
Number treated (%) |
Percentage with AAD (95% CI) |
|---|---|---|
| ≤ 5 d | 55 (22.3) | 14.6 (4.9, 24.6) |
| 6–7 d | 55 (22.3) | 12.7 (3.6, 21.8) |
| 8–13 d | 109 (44.1) | 19.3 (11.7, 26.8) |
| ≥ 14 d | 28 (11.3) | 32.1 (13.7, 50.6) |
Table 4.
Rates of AAD among patients who received the classes of antibiotics in the ED
| Antibiotic | Number treated (%) | Percentage with AAD (95% CI) |
|---|---|---|
| Cephalosporin | 91 (36.8) | 19.8 (11.4–28.1) |
| Cephalosporin alone | 47 (19.0) | 14.9 (4.3–25.5) |
| Cephalosporin + other | 44 (17.8) | 25.0 (11.7–38.3) |
| Bactrima | 40 (16.2) | 15.0 (3.4–26.6) |
| Vancomycinb | 42 (17.0) | 21.4 (8.5–34.4) |
| Clindamycin | 50 (20.2) | 30.0 (16.8–43.2) |
| Clindamycin alone | 34 (13.8) | 26.5 (10.9–42.1) |
| Clindamycin + other | 16 (6.5) | 37.5 (10.9–64.1) |
| Macrolide | 21 (8.5) | 19.0 (0.7–37.4) |
| Macrolide alone | 13 (5.3) | 7.7 (0.0–24.5) |
| Macrolide + other | 8 (3.2) | 37.5 (0.0–80.7) |
| All penicillinsc | 46 (18.6) | 19.6 (7.7–31.5) |
| Penicillin | 20 (8.1) | 25.0 (4.2–45.8) |
| Penicillin alone | 16 (6.5) | 18.8 (0.0–40.2) |
| Penicillin + other | 4 (1.6) | 50.0 (0.0–100) |
| Penicillin/I | 26 (10.5) | 15.4 (0.5–30.3) |
| Penicillin/I alone | 19 (7.7) | 21.1 (0.8–41.2) |
| Penicillin/I + other | 7 (2.8) | 0.0 |
| Quinolone | 29 (11.7) | 6.9 (0.0–16.7) |
| Quinolone alone | 21 (8.5) | 4.8 (0.0–14.7) |
| Quinolone + other | 8 (3.2) | 12.5 (0.0–42.1) |
| Doxycycline + otherd | 12 (8.5) | 8.3 (0.0–26.7) |
Quinolone = fluoroquinolones, penicillin/I= penicillin inhibitor combination antibiotic.
No difference when used as monotherapy or in combination.
Used only in combination with other antibiotics.
Combining the penicillin and PCN Combo groups.
All AAD in combination therapy group; 4 patients in monotherapy did not develop AAD.
Because 34% of patients received antibiotics in more than 1 class (72 patients received 19 distinct pairs of classes, 11 received 6 distinct triples, and 1 received 4 classes) and because the classes and other variables are interrelated in the data, the rates of AAD in Table 4 give only a partial summary. We used multivariable logistic regression models to examine the joint contribution of factors potentially associated with AAD. An initial model accounted for the 10 classes, age of patient (in 11 categories, mainly 5-year intervals), race/ ethnicity, whether the patient received IV antibiotics, and duration of antibiotic therapy. The classes of antibiotics and age did not have a statically significant contribution to the rate of AAD (after adjusting for the contributions of the other variables). After further simplification, the final logistic regression model contained 2 dichotomous variables: IV antibiotics, adjusted odds ratio of 2.73 (95% CI, 1.38–5.43); and Hispanic ethnicity, adjusted odds ratio (vs white and African American combined) of 3.04 (95% CI, 1.40–6.58).
Among patients who developed AAD, 2 (4.4%) went on to develop CDI. These 2 patients were older (ages 54 and 60), and both were treated for a skin infection; 1 patient had no past medical history, and the other had had hepatitis C and a seizure disorder. Both patients had received IV antibiotics in the ED (one vancomycin, the other clindamycin). Of the subjects who developed diarrhea while on antibiotics, 27.9% stopped taking the antibiotic because of this side effect, and 16.3% needed a health care visit to address the diarrheal symptoms; the remaining patients had self-limiting symptoms.
4. Limitations
Similar to most of the literature on AAD, this report attempts to relate risk factors for AAD to CDI. Although this rationale is logical, our observational data can only indicate associations; they are not able to establish that factors actually influence CDI (or AAD). In addition, because this disease is rare in the general population given antibiotics, a much larger number of patients would be needed to estimate associations with reasonable precision. Care should be taken when generalizing these results to non-ED patients because ED patients typically have a higher acuity of illness than those seen in outpatient settings. In addition, although we report that Hispanic patients had a higher risk of AAD, only 17.4% of this study's patients were Hispanic. Further, recall bias may have affected subjects' responses on the follow-up survey (4 weeks after completion of antibiotic therapy).
5. Discussion
We found that 22% of patients discharged to home from the ED on antibiotics developed either mild diarrhea or AAD. Our major finding, that the use of IV antibiotics before discharge was associated with increased rates of AAD, did not change with multiple antibiotic classes or IV doses. IV antibiotic therapy was also linked to 2 cases of CDI. There is a lack of data on rates of AAD in the ambulatory setting, but the rates of AAD in this study fall within the range 13% to 29% frequently reported for hospitalized patients, with patients in the IV group being toward the higher end of this range [20].
Antibiotic-associated diarrhea is a well-known complication of antibiotic therapy [1].We did not observe a connection between AAD and age or medical history, but this study included primarily younger patients, average age of 41 years (only 21 patients were more than 65 years of age), who had fewer medical problems, with most reporting no past medical history. Hispanic patients had a greater risk of AAD on antibiotics. The implications of this result remain unclear, but it is unexpected, not previously reported, and warrants further investigation. Other patient characteristics such as history of diarrhea on antibiotics were associated with higher rates of MDI but not with AAD.
Our findings have implications for the growing trend of treatment delivered in observation units. Over the past decade, the use of ED observation units (EDOU) has risen sharply, with over one third of all EDs having an EDOU [21]. Patients are commonly placed in the EDOU for a short course of IV antibiotics before discharge home [22,23]. This is the first report on ED patients treated as outpatients and risk factors for AAD/CDI. Initially considered to be limited to hospital environment, CDI is increasingly common in the outpatient setting [24]. The 2 patients who developed CDI had received IV antibiotics and had been prescribed broad-spectrum antibiotics. There is no evidence that the treatment of ED patients in observation units reduces outpatient failure on oral antibiotics; given the findings of this investigation, antibiotics given in observation units may paradoxically increase the risk of CDI.
Intravenous antibiotics have been associated with CDI. In the surgical literature, a one-time perioperative prophylactic IV dose of antibiotics has been associated with increased rates of CDI [13]. The use of IV cephalosporins, more than other antibiotic classes, leads to C difficile overgrowth [25]. In our investigation, the cephalosporin class was most frequently associated with cases of AAD (40.0%), followed by clindamycin (33.3%). Fluoroquinolones, tetracyclines, and macrolides were less frequently associated with AAD, a finding consistent with other reports [15]. Clindamycin given IV or only oral carried an increased risk of developing AAD; more than a third of patients (37.5%) given clindamycin along with another class of antibiotic went on to report AAD symptoms. We did not investigate the decision to treat patients with IV antibiotics before discharge, but the IV group and the oral-alone group did not seem to differ in the ESI or presenting vital signs. Thus, physicians were not using IV antibiotics on “sicker” patients, which might have led to higher rates of AAD. We did not observe any differences in treatment failures between the IV and the oral-alone treatment groups and have not found evidence in the literature recommending the use of an initial IV dose of antibiotics before discharge. We believe the decision to treat initially with IV antibiotics may reflect variations in clinical practice not guided by evidence and not based on patient presentation.
In conclusion, over one fifth of patients discharged from the ED on antibiotics develop diarrheal symptoms as a result of their treatment, and more than 1 in 6 develop AAD. The physician's decision to use IV antibiotics in the treatment of an infectious process in outpatients had a substantial influence on the development of AAD. Physicians should be careful when deciding on a treatment plan that involves the use of IV antibiotics, as more is not necessarily better. More-liberal uses of broader-spectrum antibiotics delivered intravenously may increase risk of CDI without providing benefit in treatment of the infectious process.
Footnotes
Meetings: Presented at the Society for Academic Emergency Medicine (SAEM) Annual Meeting, Atlanta GA, May 16, 2013 (Poster Presentation).
Presented at Society for Academic Emergency Medicine New England Emergency Medicine Research Directors Regional Meeting, Providence, RI, April 3, 2013 (Oral Presentation).
Grant: This study was designed and carried out at the University of Massachusetts Medical School/University of Massachusetts Medical Centers, Rhode Island Hospital/Brown University, and was supported by intradepartmental grants through each institution's Department of Emergency Medicine.
Conflicts of interest: None of the authors listed have any conflict of interest.
Author contributions: JPH, EWB, and SL conceived the study, designed the trial, and obtained research funding. JPH and CM supervised the conduct of the trial and data collection. JPH, GH, SS, and CM recruited participating centers and patients and managed the data, including collection and quality control. JPH and DCH provided statistical advice on study design and analyzed the data; JPH drafted the manuscript; and all authors contributed substantially to its revision. JPH takes responsibility for the article as a whole.
References
- 1.Bartlett JG. Antibiotic-associated diarrhea. N Engl J Med. 2002;346(5):334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 2.McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis. 1998;16(5):292–307. doi: 10.1159/000016879. [DOI] [PubMed] [Google Scholar]
- 3.Gonzales R, Camargo CA, Jr, MacKenzie T, et al. Antibiotic treatment of acute respiratory infections in acute care settings. Acad Emerg Med. 2006;13(3):288–294. doi: 10.1197/j.aem.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Ghose C. Clostridium difficile infection in the twenty-first century. Emerg Microbes Infec. 2013;2(9):1–8. doi: 10.1038/emi.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693–1703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 6.Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis. 2012;55(2):216–223. doi: 10.1093/cid/cis386. [DOI] [PubMed] [Google Scholar]
- 7.Dubberke ER, Wertheimer AI. Review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2009;30(1):57–66. doi: 10.1086/592981. [DOI] [PubMed] [Google Scholar]
- 8.Jones AM, Kuijper EJ, Wilcox MH. Clostridium difficile: a European perspective. J Infect. 2013;66(2):115–128. doi: 10.1016/j.jinf.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Ghantoji SS, Sail K, Lairson DR, DuPont HL, Garey KW. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010;74(4):309–318. doi: 10.1016/j.jhin.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Graul T, Cain AM, Karpa KD. Lactobacillus and bifidobacteria combinations a strategy to reduce hospital-acquired Clostridium difficile diarrhea incidence and mortality. Med Hypotheses. 2009;73(2):194–198. doi: 10.1016/j.mehy.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Wistrom J, Norrby SR, Myhre EB, et al. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients a prospective study. J Antimicrob Chemother. 2001;47:43–50. doi: 10.1093/jac/47.1.43. [DOI] [PubMed] [Google Scholar]
- 12.Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40(1):1–15. doi: 10.1016/s0195-6701(98)90019-6. [DOI] [PubMed] [Google Scholar]
- 13.Westh H, Iversen JT, Gyrtrup HJ. Clostridium difficile in faecal flora after perioperative prophylaxis with ampicillin or ceftriaxone. J Infect. 1991;23(3):347–350. doi: 10.1016/0163-4453(91)93656-w. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett JG. Antibiotic-associated diarrhea. Clin Infect Dis. 1992;15(4):573–579. doi: 10.1093/clind/15.4.573. [DOI] [PubMed] [Google Scholar]
- 15.Spencer RC. The role of antimicrobial agents in the aetiology of Clostridium-difficile–associated disease. J Antimicrob Chemother. 1998;41(Suppl C):21–27. doi: 10.1093/jac/41.suppl_3.21. [DOI] [PubMed] [Google Scholar]
- 16.Karras D. Antibiotic misuse in the emergency department. Acad Emerg Med. 2006;13(3):331–333. doi: 10.1197/j.aem.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 17.Probiotics MH. in the prevention of antibiotic-associated diarrhoea and Clostridium difficile infection. Therap Adv Gastroenterol. 2011;4(3):185–197. doi: 10.1177/1756283X11399115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium Difficile infection. N Engl J Med. 1989;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101(4):812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 21.Venkatesh AK, Geisler BP, Gibson Chambers JJ, Baugh CW, Bohan JS, Schuur JD. Use of observation care in US emergency departments, 2001 to 2008. PLoS One. 2011;6(9):1–10. doi: 10.1371/journal.pone.0024326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moseley MG, Hawley MP, Caterino JM. Emergency department observation units and the older patient. Clin Geriatr Med. 2013;29(1):71–89. doi: 10.1016/j.cger.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volz KA, Canham L, Kaplan E, Sanchez LD, Shapiro NI, Grossman SA. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31(2):360–364. doi: 10.1016/j.ajem.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Kelly CP, LaMont JT. Clostridium difficile—more difficlut than ever. N Engl J Med. 2008;359(18):1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 25.Ambrose NS, Johnson M, Burdon DW, Keighley MRB. The influence of single dose intravenous antibiotics on faecal flora and emergence of Clostridium difficile. J Antimicrob Chemother. 1985;15(3):319–326. doi: 10.1093/jac/15.3.319. [DOI] [PubMed] [Google Scholar]