Abstract
Effective management of chronic diseases involves sustained changes in health behavior, which often requires substantial effort and patient burden. As treatment burden is associated with reduced adherence across several chronic conditions, its assessment and treatment are important clinical priorities. The balance between patient demands and capacity (e.g., coping resources) may be indexed by patients’ subjective experience of treatment fatigue. We present a modified workload-capacity model that incorporates evidence that treatment fatigue may 1) be caused by increased workload due to treatment burden (e.g., intensity, complications) and 2) undermine adherence. Emerging technology-based interventions may be well-suited to reduce treatment burden, prevent treatment fatigue, and increase treatment adherence.
Chronic Disease and Health Behaviors
The leading causes of chronic disease and preventable death are attributed to modifiable risk behaviors [1], such as minimal physical activity, poor nutrition, tobacco use, and overconsumption of alcohol. Although numerous interventions have been found to promote health behaviors within clinical trials, these often fail to translate into sustained, real world effectiveness. This disconnect has been attributed, in part, to poor adherence to self-administered treatments (e.g., medication, behavioral strategies). Despite 182 randomized controlled trials (RCTs) of interventions designed to increase medication adherence, there is no clear solution [2]. To further complicate matters, medication adherence is typically only one component of an extensive set of treatment recommendations. For example, proper management of diabetes can require approximately two hours of daily care activities [3]. Similar levels of effort, sustained over time, are a necessary component of chronic care management for most health behaviors. Thus, treatment adherence in and of itself is a major health behavior.
Recognizing the substantial costs of developing treatments that patients will not adopt, clinical researchers have proposed frameworks to evaluate optimal dosing parameters (e.g., duration, frequency, amount) for behavioral interventions [4, 5]. Behavioral interventions that are too burdensome, required too frequently, or incur too much effort will result in non-compliance. More is not always better, and increases in patient burden are a primary determinant of reduced adherence and effectiveness. Thus, the optimal dose for any behavioral intervention is that which results in maximum adherence. Adaptive treatment strategies (also called dynamic treatment regimens) are innovative personalized medicine approaches that tailor treatment delivery dynamically, to meet patients’ changing needs [6, 7]. Guided by the assumption that optimal dosing may change over time, and unnecessary treatment provision can lead to patient overburden and treatment fatigue, one simulation study demonstrated that adaptive interventions can enhance effectiveness through less treatment [8].
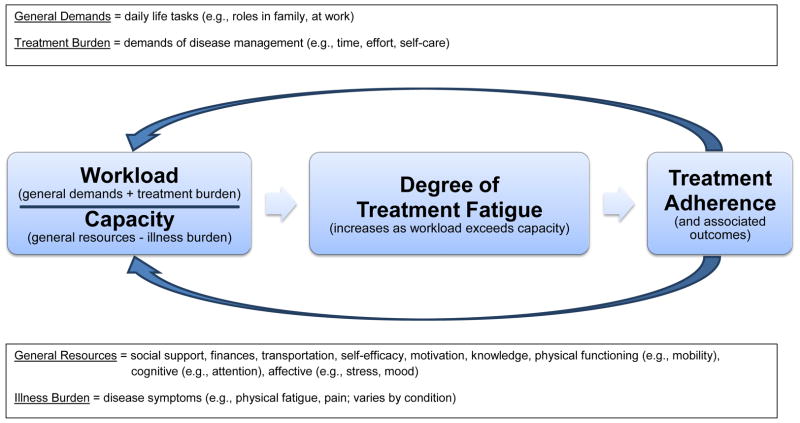
The aforementioned studies suggest that treatment burden and treatment fatigue must be better understood and incorporated into healthcare if we hope to realize the full potential of rigorously tested interventions, and the future of behavioral medicine. Unfortunately, how best to conceptualize and quantify these constructs is unclear. Though the literature on adherence is vast, we herein review recent studies (since 2012) that address newer concepts that underlie adherence: treatment burden and treatment fatigue. We aim to characterize how these constructs are defined, measured, and influence health behaviors. Findings are integrated within an adapted model that views treatment adherence to depend on the balance between patient workload and capacity (Figure 1, discussed further below) [9]. Demands associated with disease management represent treatment burden. Together with general life demands (e.g., job, family), they comprise overall patient workload. When workload exceeds capacity (e.g., resources, abilities, readiness, and disease symptoms or illness burden), this potentiates treatment fatigue, and ultimately disengagement from recommended health behaviors (i.e., non-adherence).
Figure 1.
Modified workload-capacity model.
Treatment Burden
The science of treatment burden has advanced substantially since 2012. Several qualitative studies have been conducted to conceptualize patient concerns [10, 11], and examine how well these are addressed by primary care providers [12]. These studies are complemented by systematic reviews of studies that examined treatment burden both qualitatively [13, 14] and quantitatively [15, 16]. Studies reviewed rarely focused on treatment burden specifically, but a priori definitions of burden were used to identify relevant patient responses. As indicated in Table 1, these studies are inclusive of different patient populations and assessment methods, but several common themes were observed. There is clear evidence that treatment burden is experienced by patients with at least one chronic health condition, and this affects many aspects of their lives.
Table 1.
Characteristics of treatment burden reviews.
| First Author | Publication Year | Chronic Condition(s) | Type of Studies | Number of Studies | Themes Identified |
|---|---|---|---|---|---|
| Eton | 2013 | diabetes, kidney disease, heart failure | quantitative | 98 | emotional impact/regimen-related distress, family conflict/unsupportive behavior, treatment convenience, self-care convenience, monitoring burden, lifestyle impact (social, work interference), scheduling flexibility, medication side effects, diet/food-related problems, device function/bother, economic burden, and overall treatment burden |
| Gallacher | 2013 | stroke | qualitative | 54 | making sense of treatment and planning care, interacting with others, enacting management strategies (institutional admissions, managing stroke in the community, reintegrating into society, adjusting to life after stroke), and reflecting on management |
| Gallacher | 2013 | stroke | qualitative | 69 | making sense of treatments, planning recovery and care, interacting with others, institutional admissions, managing stroke in the community, reintegrating into society, adjusting to life after diagnosis, and reflecting on management |
| Sav | 2013 | asthma, diabetes, cardiovascular disease, musculoskeletal illness, cancer, and mental health | qualitative and quantitative | 30 | physical (side effects), financial (care/travel), temporal, and psychosocial time demands (roles, caregiving) |
Measures of treatment burden are often specific to a single medical condition, although patients are often diagnosed with several. The Treatment Burden Questionnaire was designed to capture a broader assessment of burden across any medical condition, or set of conditions [17, 18]. A global score is derived by summing 0 (not a problem/not applicable) to 10 (large problem) ratings for 15 items. Four items address taking medicine, and the remaining assess self-monitoring, laboratory tests, doctor visits, need for organization, administrative tasks, following advice on diet and physical activity, social impact of treatment, and financial burden. Global treatment scores are negatively correlated with adherence and quality of life. However, this measure fails to address the emotional distress that is so often associated with treatment adherence directly.
Treatment Fatigue
Beyond assessment of treatment burden (i.e., how much effort is required for a given health behavior), a growing area of interest focuses on the impact of that burden. There is extensive research on physical fatigue caused by specific interventions (e.g., chemotherapy among cancer patients), but we focus on the psychological fatigue associated with treatment engagement, herein called treatment fatigue. This nascent literature is mostly restricted to diabetes and human immunodeficiency virus (HIV) management.
In a qualitative study of adults with type 1 diabetes patients attributed non-adherence to treatment fatigue [19]. Known also as “diabetes overwhelmus,” “diabetes emotional distress,” and “diabetes burnout” [20], patients describe treatment fatigue as feeling overwhelmed by the cumulative effort of disease management. Such themes are consistent with item content within diabetes-specific questionnaires that assess burden of disease management. For example, the emotional burden subscale of the Diabetes Distress Scale includes items such as “feelings that diabetes is taking too much of my mental and physical energy” and “feeling overwhelmed by the demands of living with diabetes” [21]. Elevated fatigue is observed among those who experienced complications or had more intensive regimens. Even in cases where patients engage in proper disease management, fatigue may still result if such patients perceive negligible benefits (e.g., “I’m doing all the right things, but it isn’t doing any good”).
A systematic review identified 17 studies that addressed fatigue associated with chronic disease management among people living with HIV [22]. To consolidate the vast terminology (e.g., pill-, medication-, treatment-, regimen-, dosing-, drug-, and injection-fatigue), the authors recommended future use of treatment regimen fatigue, which they defined as decreased desire and motivation to maintain vigilance adhering to prescribed treatment regimens. Consistent with the diabetes literature, fatigue was positively associated with treatment intensity and complications (e.g., side effects), and negatively associated with adherence.
Integrative Model and Clinical Implications
The treatment fatigue literature within diabetes and HIV both point toward the need for common terminology, definition, and measurement tools. A broader conceptualization of fatigue, across a range of chronic health behaviors, would facilitate a transdiagnostic understanding. Our workload-capacity model (Figure 1) incorporates the evidence that treatment fatigue may 1) be caused by increased workload due to treatment burden (e.g., intensity, complications) and 2) undermine adherence. This is consistent with limited resource models, such that expending effort on a task can lead to subjective fatigue and diminished performance on subsequent tasks [23]. However, limited resource theory cannot address why fatigue occurs, for example, among diabetic patients whose workload remains constant, who experience minimal treatment benefits. This observation is better explained by motivational control theories of fatigue [24–28], where fatigue serves as an indicator of inefficient resource allocation. In other words, without tangible benefits (i.e., symptom reduction), the patient is likely to decrease adherence and allocate capacity elsewhere, even when demands are unchanged.
We could only find one study to examine the temporal dynamics of treatment fatigue, but results support the importance of noticeable benefits from adherence. Within a clinical trial of 1504 daily smokers (each randomized to placebo, monotherapy, or combination therapy) [29], a single-item measure of cessation fatigue (“I am tired of trying to quit smoking”) was assessed daily for two weeks post-quit. Fatigue increased across time, was negatively associated with abstinence at 6-month follow-up, and was positively correlated with daily ratings of nicotine withdrawal (craving and negative affect). As treatment dose/intensity increased, both withdrawal symptoms and treatment fatigue were reduced. This suggests active treatments increased capacity (e.g., via withdrawal reduction) to a greater degree than they increased treatment burden, which created a balance in favor of abstinence.
The integrated model makes clear predictions that fatigue (and subsequently non-adherence), can result from at least one of four pathways: 1. increased workload, via general demands; 2. increased workload, via treatment burden; 3. decreased capacity, via general resources; and 4. decreased capacity, via illness burden. This model is in need of further testing, especially given the recency of studies that address fatigue/burden. There are many unanswered questions within this model, including the threshold of burden or fatigue that has bearing on clinical outcome, the longitudinal course of fatigue, and the degree to which these constructs are disease-specific vs. general. As treatment fatigue assessments are further developed, they could serve as triage tools, with elevated fatigue indicating the need for a comprehensive evaluation of workload and capacity, and specifically the four pathways noted above. Fortunately, these pathways also offer potential intervention targets when fatigue is observed.
Our adapted model also recognizes that treatment adherence results in reciprocal feedback that adjusts both workload and capacity, and this too may determine sustainability of health behaviors. Intuitively, treatment recommendations will have direct effects on treatment and illness burden, but they can also influence general demands and capacity. For example, practicing cognitive-behavioral techniques to help curb smoking (e.g., cognitive restructuring, relaxation, problem solving) may generalize and build capacity in other areas of patients’ lives. Therefore, interventions that improve capacity broadly may be more sustainable than those of equivalent workload that focus on illness alone.
Potential Applications
With the exception of one study [29], the concepts of treatment burden and fatigue have not been applied to understand the most common sources of morbidity and mortality: obesity, nicotine dependence, or alcohol dependence. These are chronic and relapsing conditions that require substantial effort to change, and may be susceptible to the same patterns described above. It is unclear if burden and fatigue will manifest differently when attempting to change behaviors that are hard to reduce (e.g., smoking), hard to sustain (e.g., exercise, medication adherence), or both (e.g., diet) [30].
Additionally, the burden and fatigue associated with adhering to psychosocial therapies has yet to be explored. Patients report the use of psychosocial skills to overcome treatment burden, for example: problem-focused strategies (e.g., routines, planning, using technology); emotion-focused coping strategies (e.g., positive attitude, focusing on other life priorities, and spirituality/faith); and cognitive restructuring (e.g., recognizing and challenging beliefs about treatment burden) [31]. Use of these strategies to overcome other forms of treatment burden suggests they enhance capacity and impose minimal burden, but this remains an empirical question.
We are now at the forefront of a new wave of behavioral health care [32], one that encompasses technological innovation, to meet and treat patients at times and places where they are most susceptible to treatment fatigue. Such technologic strategies could ultimately have a very large impact on adherence behaviors, thereby augmenting chronic care treatment for health. Predictions from the workload-capacity model presented herein (Figure 1) could help guide how these technologies are applied to meet their full potential.
Future of Chronic Care
Behavioral intervention technologies offer scalable approaches to reduce patient efforts [33–36], and are evolving rapidly [37–39]. Mobile phones are owned by 90% of American adults [40], representing a platform to increase accessibility to chronic care. Mobile health interventions (mHealth) can facilitate communication between health care providers and patients, thereby reducing burden associated with travel (e.g., transportation costs, time). Although mHealth has typically been limited to those that deploy static messages at fixed schedules [41, 42], recent applications have been more interactive and may allow for treatment delivery when capacity is compromised [43]. Patients can self-report the need for treatment delivery in real time, and patient history can even be used to proactively offer interventions [44].
The next generation of mHealth is working towards automatic treatment delivery by leveraging sensors that are part of smartphones (e.g., global positioning system [GPS], Bluetooth, accelerometers, magnetometers, gyroscopes, cameras and microphones) or can be integrated with phones (e.g., biosensors) [43]. Data from these sensors can be paired with self-report to predict contextual factors that undermine or promote health behaviors (e.g., location, physical activity, driving, social interaction, stress). This information can be used to inform machine learner models, such that sensor data alone can predict contexts and trigger intervention delivery. Patients may be willing to train models early in treatment, when fatigue is low, which would facilitate automatic treatment delivery at later times when fatigue increases, ultimately preventing burden and optimizing treatment effectiveness.
Context sensing technologies are also beginning to show potential. For example, one study demonstrated that a smartphone application incorporated with GPS prevented relapse among alcohol dependent patients leaving residential treatment [45]. Patients registered locations where they previously obtained or consumed alcohol, and were provided treatment support when they approached high-risk locations. This automatic yet personalized treatment component allows patients to prepare for high-risk situations before they arise, or to avoid them altogether. That is, it can reduce the amount of effort typically needed to monitor high-risk situations, and to resist them. These are but a few examples of the many innovative strategies that might sustain patient engagement (i.e., protect against fatigue) over the long term.
Conclusions
Chronic disease management requires substantial effort, and is associated with both positive and negative consequences. The balance between patient demands and capacity may be indexed by treatment fatigue, and will determine the sustainability of the behavioral change (i.e., adherence). This suggests the need for ongoing efforts to reduce treatment burden and/or increase patient capacity to undertake necessary health behaviors. Innovations in technology-based interventions (e.g., mHealth) are well positioned to meet this need.
Highlights.
Burden associated with chronic care management may undermine treatment adherence.
Treatment fatigue may index when patient demands exceed coping capacity.
Treatments should aim to minimize treatment burden and increase patient capacity.
Technological innovations offer promising tools to reduce patient burden.
Additional research is needed to better understand treatment burden and fatigue.
Acknowledgments
Funding for this research was provided by National Institute on Drug Abuse awards T32 DA007288 (BWH), and F32 DA036947 (ARM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ford ES, Zhao G, Tsai J, Li C. Low-risk lifestyle behaviors and all-cause mortality: findings from the National Health and Nutrition Examination Survey III Mortality Study. Am J Public Health. 2011;101:1922–1929. doi: 10.2105/AJPH.2011.300167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, Agoritsas T, Mistry N, Iorio A, Jack S, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;11:Cd000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jowsey T, Yen L, WPM Time spent on health related activities associated with chronic illness: a scoping literature review. BMC Public Health. 2012;12:1044. doi: 10.1186/1471-2458-12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voils CI, King HA, Maciejewski ML, Allen KD, Yancy WS, Jr, Shaffer JA. Approaches for informing optimal dose of behavioral interventions. Ann Behav Med. 2014;48:392–401. doi: 10.1007/s12160-014-9618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voils CI, Chang Y, Crandell J, Leeman J, Sandelowski M, Maciejewski ML. Informing the dosing of interventions in randomized trials. Contemp Clin Trials. 2012;33:1225–1230. doi: 10.1016/j.cct.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty B, Murphy SA. Dynamic Treatment Regimes. Annu Rev Stat Appl. 2014;1:447–464. doi: 10.1146/annurev-statistics-022513-115553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laber EB, Lizotte DJ, Qian M, Pelham WE, Murphy SA. Dynamic treatment regimes: technical challenges and applications. Electron J Stat. 2014;8:1225–1272. doi: 10.1214/14-ejs920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *8.Lagoa CM, Bekiroglu K, Lanza ST, Murphy SA. Designing adaptive intensive interventions using methods from engineering. J Consult Clin Psychol. 2014;82:868–878. doi: 10.1037/a0037736. This paper provides a detailed step-by-step explanation of how control engineering methods can be used with intensive longitudinal data to design an adaptive intensive intervention. Simulation results illustrate how the designed adaptive intensive intervention can result in improved outcomes with less treatment by providing treatment only when it is needed. These new methods can be used to design adaptive interventions that are effective yet reduce participant burden. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **9.Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2012;65:1041–1051. doi: 10.1016/j.jclinepi.2012.05.005. This functional, patient-centered model of patient complexity serves as a guiding framework for understanding how patient workload of demands and patient capacity to address demands impacts treatment adherence. With its components largely supported by existing literature, the model has implications for analytic design, clinical epidemiology, and clinical practice. [DOI] [PubMed] [Google Scholar]
- 10.Eton DT, Ramalho de Oliveira D, Egginton JS, Ridgeway JL, Odell L, May CR, Montori VM. Building a measurement framework of burden of treatment in complex patients with chronic conditions: a qualitative study. Patient Relat Outcome Meas. 2012;3:39–49. doi: 10.2147/PROM.S34681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sav A, Kendall E, McMillan SS, Kelly F, Whitty JA, King MA, Wheeler AJ. ‘You say treatment, I say hard work’: treatment burden among people with chronic illness and their carers in Australia. Health Soc Care Community. 2013;21:665–674. doi: 10.1111/hsc.12052. [DOI] [PubMed] [Google Scholar]
- 12.Bohlen K, Scoville E, Shippee ND, May CR, Montori VM. Overwhelmed patients: a videographic analysis of how patients with type 2 diabetes and clinicians articulate and address treatment burden during clinical encounters. Diabetes Care. 2012;35:47–49. doi: 10.2337/dc11-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallacher K, Jani B, Morrison D, Macdonald S, Blane D, Erwin P, May CR, Montori VM, Eton DT, Smith F, et al. Qualitative systematic reviews of treatment burden in stroke, heart failure and diabetes - methodological challenges and solutions. BMC Med Res Methodol. 2013;13:10. doi: 10.1186/1471-2288-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallacher K, Morrison D, Jani B, Macdonald S, May CR, Montori VM, Erwin PJ, Batty GD, Eton DT, Langhorne P, et al. Uncovering treatment burden as a key concept for stroke care: a systematic review of qualitative research. PLoS Med. 2013;10:e1001473. doi: 10.1371/journal.pmed.1001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Eton DT, Elraiyah TA, Yost KJ, Ridgeway JL, Johnson A, Egginton JS, Mullan RJ, Murad MH, Erwin PJ, Montori VM. A systematic review of patient-reported measures of burden of treatment in three chronic diseases. Patient Relat Outcome Meas. 2013;4:7–20. doi: 10.2147/PROM.S44694. Authors performed a review of patient-reported measures that assess aspects of treatment burden in three chronic diseases: diabetes, chronic kidney disease, and heart failure. 57 patient-reported measures of treatment burden were identified, with 12 content domains common across measures and disease types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sav A, King MA, Whitty JA, Kendall E, McMillan SS, Kelly F, Hunter B, Wheeler AJ. Burden of treatment for chronic illness: a concept analysis and review of the literature. Health Expect. 2013 doi: 10.1111/hex.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Tran VT, Harrington M, Montori VM, Barnes C, Wicks P, Ravaud P. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Med. 2014;12:109. doi: 10.1186/1741-7015-12-109. This paper presents the validity and reliability of an English version of the Treatment Burden Questionnaire (TBQ), an assessment of treatment burden in different condition and treatment contexts. The measure showed a unidimensional structure with good internal consistency and support for construct validity in assessing treatment burden for patients with one or more chronic conditions in English-speaking countries. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran VT, Montori VM, Eton DT, Baruch D, Falissard B, Ravaud P. Development and description of measurement properties of an instrument to assess treatment burden among patients with multiple chronic conditions. BMC Med. 2012;10:68. doi: 10.1186/1741-7015-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pyatak EA, Florindez D, Weigensberg MJ. Adherence decision making in the everyday lives of emerging adults with type 1 diabetes. Patient Prefer Adherence. 2013;7:709–718. doi: 10.2147/PPA.S47577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritschi C, Quinn L. Fatigue in patients with diabetes: a review. J Psychosom Res. 2010;69:33–41. doi: 10.1016/j.jpsychores.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, Jackson RA. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 22.Claborn KR, Meier E, Miller MB, Leffingwell TR. A systematic review of treatment fatigue among HIV-infected patients prescribed antiretroviral therapy. Psychol Health Med. 2014:1–11. doi: 10.1080/13548506.2014.945601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Baumeister RF. Self-regulation, ego depletion, and inhibition. Neuropsychologia. 2014;65:313–319. doi: 10.1016/j.neuropsychologia.2014.08.012. This paper presents a key conceptual model of self-regulation. Inhibition is a type of self-regulation presented as a pervasive feature of everyday life that is prosocial and adaptive in nature. Inhibition and other forms of self-regulation can be undermined by ego depletion (i.e., state fluctuations in willpower resources) caused by exertion of self-control. [DOI] [PubMed] [Google Scholar]
- 24.Beedie CJ, Lane AM. The role of glucose in self-control: another look at the evidence and an alternative conceptualization. Personality and Social Psychology Review. 2012;16:143–153. doi: 10.1177/1088868311419817. [DOI] [PubMed] [Google Scholar]
- 25.Inzlicht M, Schmeichel BJ, Macrae CN. Why self-control seems (but may not be) limited. Trends in cognitive sciences. 2014 doi: 10.1016/j.tics.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Molden DC, Hui CM, Scholer AA, Meier BP, Noreen EE, D’Agostino PR, Martin V. Motivational versus metabolic effects of carbohydrates on self-control. Psychological Science. 2012;23:1137–1144. doi: 10.1177/0956797612439069. [DOI] [PubMed] [Google Scholar]
- **27.Hockey R. The psychology of fatigue: work, effort, and control. Cambridge: Cambridge University Press; 2013. This book reviews researh on fatigue that has emerged across disciplines (social history, neuroscience, energetics, exercise physiology, clinical). Evidence is integrated to support a motivational control theory of fatigue, such that fatigue is an emotion that has the adaptive role of managing goals. [Google Scholar]
- 28.Kurzban R, Duckworth A, Kable JW, Myers J. An opportunity cost model of subjective effort and task performance. Behav Brain Sci. 2013;36:661–679. doi: 10.1017/S0140525X12003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.Liu X, Li R, Lanza ST, Vasilenko SA, Piper M. Understanding the role of cessation fatigue in the smoking cessation process. Drug Alcohol Depend. 2013;133:548–555. doi: 10.1016/j.drugalcdep.2013.07.025. This is the first empirical study to examine treatment fatigue in the smoking cessation process (termed cessation fatigue) in relation to intervention type and cessation outcomes. Cessation fatigue reduced the likelihood of 6-month post-quit abstinence (OR=0.97, 95% CI (0.95, 0.99)), and was positively associated with craving and negative affect. Cessation fatigue was significantly reduced by combined pharmacotherapy (t=−13.4, p<0.0001), as well as monotherapy (t=−6.2, p<0.0001), showing that active treatments appeared to reduce fatigue and weaken its relations with craving and negative affect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Borland R. Understanding hard to maintain behaviour change: a dual process approach. Hoboken, New Jersey: John Wiley & Sons Inc; 2014. This book describes CEOS theory, which explains behavior as a function of Context, Executive, and Operational Systems. CEOS theory is applied to behaviors that are hard-to-reduce (e.g., smoking), and hard-to-sustain (e.g., exercise). Implications for clinical practice and public health are discussed. [Google Scholar]
- 31.Ridgeway JL, Egginton JS, Tiedje K, Linzer M, Boehm D, Poplau S, de Oliveira DR, Odell L, Montori VM, Eton DT. Factors that lessen the burden of treatment in complex patients with chronic conditions: a qualitative study. Patient Prefer Adherence. 2014;8:339–351. doi: 10.2147/PPA.S58014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsch LA, Lord SE, Dallery J. Behavioral healthcare and technology: using science-based innovations to transform practice. Oxford; New York: Oxford University Press; 2015. [Google Scholar]
- 33.Mohr DC, Burns MN, Schueller SM, Clarke G, Klinkman M. Behavioral intervention technologies: evidence review and recommendations for future research in mental health. Gen Hosp Psychiatry. 2013;35:332–338. doi: 10.1016/j.genhosppsych.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohr DC, Cheung K, Schueller SM, Hendricks Brown C, Duan N. Continuous evaluation of evolving behavioral intervention technologies. Am J Prev Med. 2013;45:517–523. doi: 10.1016/j.amepre.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohr DC, Schueller SM, Montague E, Burns MN, Rashidi P. The behavioral intervention technology model: an integrated conceptual and technological framework for eHealth and mHealth interventions. J Med Internet Res. 2014;16:e146. doi: 10.2196/jmir.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsch LA, Carroll KM, Kiluk BD. Technology-based interventions for the treatment and recovery management of substance use disorders: a JSAT special issue. J Subst Abuse Treat. 2014;46:1–4. doi: 10.1016/j.jsat.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker TB, Gustafson DH, Shah D. How can research keep up with eHealth? Ten strategies for increasing the timeliness and usefulness of eHealth research. J Med Internet Res. 2014;16:e36. doi: 10.2196/jmir.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker S, Miron-Shatz T, Schumacher N, Krocza J, Diamantidis C, Albrecht UV. mHealth 2. 0: Experiences, Possibilities, and Perspectives. JMIR Mhealth Uhealth. 2014;2:e24. doi: 10.2196/mhealth.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riley WT, Glasgow RE, Etheredge L, Abernethy AP. Rapid, responsive, relevant (R3) research: a call for a rapid learning health research enterprise. Clin Transl Med. 2013;2:10. doi: 10.1186/2001-1326-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pew Research Center Internet Survey Project. Washington, DC: Pew Research Center; 2014. [accessed January 30, 2015]. Available from: http://www.pewinternet.org/data-trend/mobile/cell-phone-and-smartphone-ownership-demographics/ [Google Scholar]
- 41.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, Patel V, Haines A. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10:e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiordelli M, Diviani N, Schulz PJ. Mapping mHealth research: a decade of evolution. J Med Internet Res. 2013;15:e95. doi: 10.2196/jmir.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **43.Kumar S, Nilsen WJ, Abernethy A, Atienza A, Patrick K, Pavel M, Riley WT, Shar A, Spring B, Spruijt-Metz D, et al. Mobile health technology evaluation: the mHealth evidence workshop. Am J Prev Med. 2013;45:228–236. doi: 10.1016/j.amepre.2013.03.017. This paper outlines an approach to evidence generation in the field of mHealth to examine the potential, as well as the challenges, of utilizing mobile technologies to improve health outcomes. The areas covered can be categorized broadly into three concepts: (1) evaluating assessments; (2) evaluating interventions; and (3) reshaping evidence generation using mHealth. This paper brings these concepts together to describe current evaluation standards, discuss future possibilities, and set a grand goal for the emerging field of mHealth research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chih MY, Patton T, McTavish FM, Isham AJ, Judkins-Fisher CL, Atwood AK, Gustafson DH. Predictive modeling of addiction lapses in a mobile health application. J Subst Abuse Treat. 2014;46:29–35. doi: 10.1016/j.jsat.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *45.Gustafson DH, McTavish FM, Chih MY, Atwood AK, Johnson RA, Boyle MG, Levy MS, Driscoll H, Chisholm SM, Dillenburg L, et al. A smartphone application to support recovery from alcoholism: a randomized clinical trial. JAMA Psychiatry. 2014;71:566–572. doi: 10.1001/jamapsychiatry.2013.4642. Authors tested whether patients leaving residential treatment for alcohol use disorders with a smartphone application to support recovery (the Addiction-Comprehensive Health Enhancement Support System or A-CHESS) have fewer risky drinking days than control patients. A-CHESS provides monitoring, information, communication, and support services to patients, including ways for patients and counselors to stay in contact. For the 8 months of the intervention and 4 months of follow-up, patients in the A-CHESS group reported significantly fewer risky drinking days than did patients in the control group. [DOI] [PMC free article] [PubMed] [Google Scholar]