Cytokine alterations are more strongly correlated with illness duration than with measures of illness severity.
Keywords: myalgic encephalomyelitis/chronic fatigue syndrome, immune signatures, cytokine regulation, illness duration, biomarkers
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is an unexplained incapacitating illness that may affect up to 4 million people in the United States alone. There are no validated laboratory tests for diagnosis or management despite global efforts to find biomarkers of disease. We considered the possibility that inability to identify such biomarkers reflected variations in diagnostic criteria and laboratory methods as well as the timing of sample collection during the course of the illness. Accordingly, we leveraged two large, multicenter cohort studies of ME/CFS to assess the relationship of immune signatures with diagnosis, illness duration, and other clinical variables. Controls were frequency-matched on key variables known to affect immune status, including season of sampling and geographic site, in addition to age and sex. We report here distinct alterations in plasma immune signatures early in the course of ME/CFS (n = 52) relative to healthy controls (n = 348) that are not present in subjects with longer duration of illness (n = 246). Analyses based on disease duration revealed that early ME/CFS cases had a prominent activation of both pro- and anti-inflammatory cytokines as well as dissociation of intercytokine regulatory networks. We found a stronger correlation of cytokine alterations with illness duration than with measures of illness severity, suggesting that the immunopathology of ME/CFS is not static. These findings have critical implications for discovery of interventional strategies and early diagnosis of ME/CFS.
INTRODUCTION
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a disabling disorder with complex features that can include fatigue, memory and concentration deficits, sleep disturbances, headache, joint and muscle pain, postexertional malaise, and gastrointestinal and immune system dysfunction (1–5), lasting for 6 months or more. Elderly patients with ME/CFS are at increased risk for non-Hodgkin’s lymphoma (6). The diagnosis of ME/CFS is largely based on recognition of signs and symptoms by experienced clinicians (1). Although laboratory assays play a role in the diagnostic process, they are used to rule out other conditions that can mimic aspects of ME/CFS, rather than as biomarkers to facilitate early diagnosis. Efforts to identify immune biomarkers in ME/CFS have produced inconsistent results (1, 7–9). Inconsistencies may reflect variations in the use of diagnostic criteria or laboratory methods as well as the timing of sample collection during the course of the illness (10).
To better control for heterogeneity across patients and sampling strategies, in the present study we leveraged the clinical databases and biological specimen repositories of two recent, large, multicenter cohort studies of ME/CFS to assess the relationship of immune signatures (51 cytokines) with diagnosis and other clinical variables: (i) the National Institutes of Health (NIH) study, initially developed to address a specific hypothesis regarding infectious exposure (11) and (ii) the Chronic Fatigue Initiative (CFI) cohort study (12). Controls were frequency-matched on key variables known to affect immune status, including season of sampling and geographic site, in addition to age and sex. We investigated the relationship of diagnosis and clinical phenotype to immune profiles derived from plasma samples collected at the same time of day under controlled conditions of a mild stressor (defined as completion of study forms and procedures for the ME/CFS study population) (13).
RESULTS
Table 1 shows the demographic features of the study group, including 52 subjects with short-duration illness (≤3 years), 246 subjects with long-duration illness (>3 years) (total of 298 cases), and 348 controls. For ME/CFS cases participating in both the NIH and the CFI cohort studies, only the clinical and laboratory data obtained during participation in the earlier NIH study were included in the analyses (the number of control subjects is greater than that of cases because the NIH and CFI control groups have no overlap). As anticipated, subjects with more recent onset of illness were younger (40.5 ± 13.6 years) than ME/CFS cases with a long duration of illness (50.2 ± 11.4 years, in comparison with the short-duration group, P < 0.0001) as well as in comparison to controls (48.5 ± 12.0 years, P < 0.0001). Multidimensional Fatigue Inventory (MFI) mental fatigue subscale scores in the short- and long-duration ME/CFS groups were, as expected, both higher than in the control group (P < 0.0001), but did not differ significantly from each other.
Table 1. Characteristics of study population.
| Variable |
All ME/CFS (n = 298) |
Short-duration ME/CFS (≤3 years) (n = 52) |
Long-duration ME/CFS (>3 years) (n = 246) |
Control (n = 348) |
All ME/CFS versus control |
Short- versus long-duration ME/CFS versus control |
| P* | P | |||||
| Sex, n (%) | 0.80 | 0.95 | ||||
| Female | 220 (73.8) | 39 (75.0) | 181 (73.6) | 260 (74.7) | ||
| Male | 78 (26.2) | 13 (25.0) | 65 (26.4) | 88 (25.3) | ||
| Age [mean (SD)] | 48.5 (12.4) | 40.5 (13.6) | 50.2 (11.4) | 48.5 (12.0) | 0.95 | <0.0001† |
| Illness duration, years [mean (SD)] | 13.2 (9.2) | 1.7 (0.8) | 15.6 (8.2) | — | ||
| MFI mental fatigue subscale score [mean (SD)] | 15.3 (3.6) | 15.2 (3.7) | 15.3 (3.6)‡ | 6.2 (2.7)§ | <0.0001 | <0.0001¶ |
| Race, n (%) | 0.54 | 0.77 | ||||
| White | 292 (98.0) | 52 (100.0) | 240 (97.6) | 337 (96.8) | ||
| African American | 1 (0.3) | 0 (0.0) | 1 (0.4) | 5 (1.4) | ||
| Asian | 3 (1.0) | 0 (0.0) | 3 (1.2) | 4 (1.1) | ||
| Other | 2 (0.7) | 0 (0.0) | 2 (0.8) | 2 (0.6) | ||
| Site, n (%) | 0.80 | 0.22 | ||||
| Boston, MA | 54 (18.1) | 4 (7.7) | 50 (20.3) | 55 (15.8) | ||
| Miami, FL | 57 (19.1) | 9 (17.3) | 48 (19.5) | 67 (19.3) | ||
| New York, NY | 67 (22.5) | 15 (28.8) | 52 (21.1) | 72 (20.7) | ||
| Palo Alto, CA | 23 (7.7) | 1 (1.9) | 22 (8.9) | 23 (6.6) | ||
| Salt Lake City, UT | 46 (15.4) | 11 (21.2) | 35 (14.2) | 65 (18.7) | ||
| Sierra, NV | 51 (17.1) | 12 (23.1) | 39 (15.9) | 66 (19.0) | ||
| Months of sample acquisition, n (%)|| | 0.02 | <0.0001** | ||||
| January to March | 77 (25.9) | 11 (21.2) | 66 (26.9) | 95 (27.3) | ||
| April to June | 72 (24.2) | 17 (32.7) | 55 (22.4) | 90 (25.9) | ||
| July to September | 26 (8.8) | 11 (21.2) | 15 (6.1) | 53 (15.2) | ||
| October to December | 122 (41.1) | 13 (25.0) | 109 (44.5) | 110 (31.6) |
*Sex, race, site, and months of sample acquisition, χ2 test; age and MFI, Kruskal-Wallis test (three-group comparisons) and Mann-Whitney U tests (two-group comparisons).
†Significant intergroup comparisons for age: short versus long duration and short duration versus control, both P < 0.0001.
‡n = 238 (MFI scores missing for 8 long-duration subjects).
§n = 341 (MFI scores missing for 7 control subjects).
¶Significant intergroup comparisons for MFI subscale: short duration versus control and long duration versus control, both P < 0.0001.
||Blood draw date missing for one ME/CFS case.
**Significant intergroup comparisons for months of sample acquisition: short versus long duration, P = 0.001.
Cases versus controls
General linear model (GLM) and t-test follow-up comparison of all ME/CFS cases (short and long duration combined, n = 298) with controls (n = 348) yielded few significant results, with cases showing lower levels for most analytes (tables S1 to S3). Cases had lower plasma levels of the proinflammatory cytokines interleukin (IL)-17A (pg/ml, mean ± SEM; cases: 18.3 ± 0.6; controls: 20.6 ± 0.6; P = 0.0043), CXCL8 (IL-8) (cases: 3.4 ± 0.2; controls: 4.0 ± 0.2; P = 0.0437), CXCL10 [interferon-inducible protein-10 (IP-10)] (cases: 40.5 ± 0.8; controls: 43.5 ± 1.2; P = 0.0444), tumor necrosis factor–β (TNFβ) (cases: 2.4 ± 0.2; controls: 3.3 ± 0.2; P = 0.0028), soluble Fas ligand (sFasL) (cases: 10.2 ± 0.3; controls: 11.4 ± 0.3; P = 0.0129), and IL-6 (cases: 10.2 ± 0.4; controls: 10.9 ± 0.3; P = 0.0401), as well as of the anti-inflammatory cytokine IL-10 (cases: 6.8 ± 0.2; controls: 7.2 ± 0.2; P = 0.0241) and CSF1 [macrophage colony-stimulating factor (M-CSF)], a factor associated with the immunosuppressive M2 phenotype of macrophages/microglia (14) (cases: 7.1 ± 0.3; controls: 7.9 ± 0.3; P = 0.0235). In contrast, the adipose-derived cytokine, leptin, was increased in the larger ME/CFS case group (short and long duration combined) as compared to controls (cases: 1506.4 ± 76.4; controls: 1398.4 ± 96.3; P = 0.0301).
Short-duration versus long-duration ME/CFS versus controls
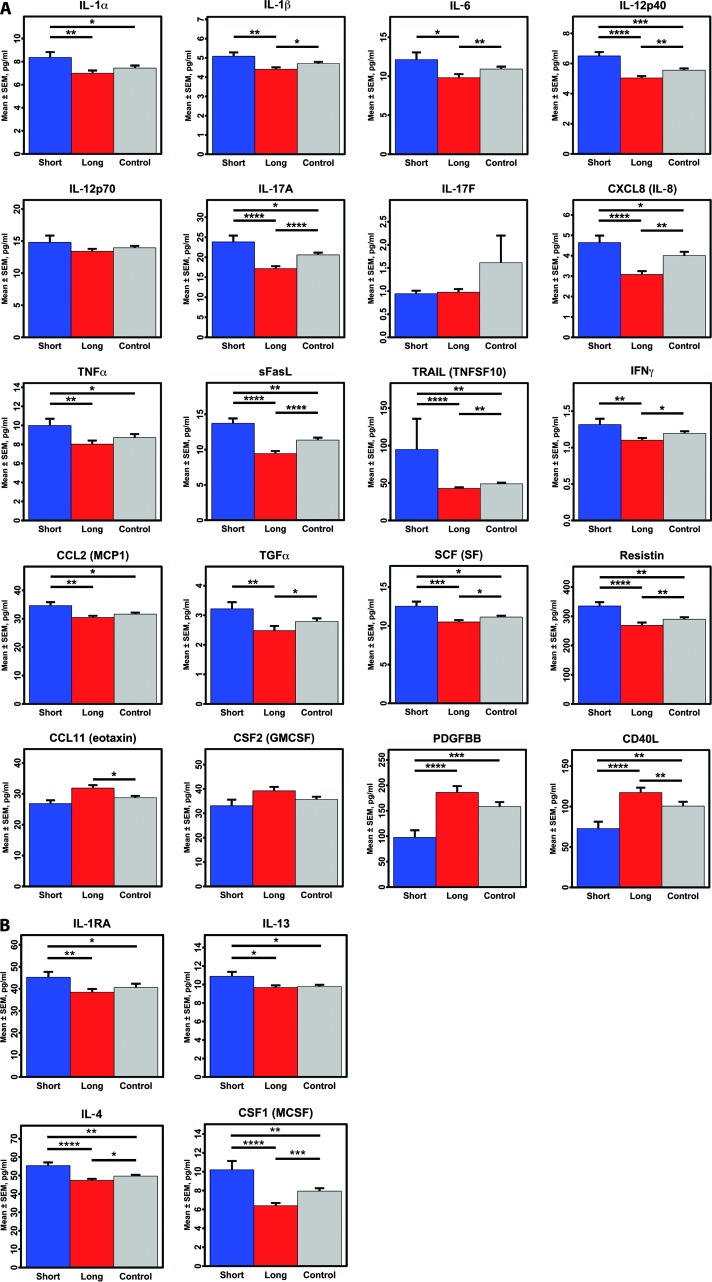
GLM comparison of short-duration ME/CFS cases (≤3 years, n = 52), long-duration ME/CFS cases (>3 years, n = 246), and controls (n = 348) (with and without sex and age included in the models) showed significant differences for more than half of the 51 cytokines (tables S4 to S6). Follow-up t tests showed that short illness duration subjects generally had higher cytokine levels than controls or long-duration cases. Both classical proinflammatory cytokines {short duration versus control: IL-1α, P = 0.0178; CXCL8 (IL-8), P = 0.0112; IL-12p40, P = 0.0009; IL-17A, P = 0.0243; TNFα, P = 0.0261; TRAIL [TNF-related apoptosis-inducing ligand (TNFSF10)], P = 0.0079; CC chemokine ligand (CCL)-2 [monocyte chemoattractant protein 1 (MCP1)], P = 0.0208; stem cell factor (SCF) [steel factor (SF)], P = 0.0110; resistin, P = 0.0024} and anti-inflammatory cytokines [short duration versus control: IL-1 receptor antagonist (IL-1RA), P = 0.0105; IL-4, P = 0.0028; IL-13, P = 0.0198] were elevated (representative cytokines meeting GLM criteria are shown in Fig. 1; all means, SEM, and t-test data are in table S6). In contrast, only 2 of the 51 cytokines were reduced in short-duration subjects as compared with controls: CD40 ligand (CD40L) (P = 0.0037) and platelet-derived growth factor BB (PDGFBB) (P = 0.0004).
Fig. 1. Comparison of plasma cytokine levels in short-duration ME/CFS, long-duration ME/CFS, and control subjects.
(A) Proinflammatory cytokines. (B) Anti-inflammatory cytokines. The means ± SEM for each cytokine are shown. Only cytokines meeting significance criteria (P < 0.05) in either the one-way or the two-way GLM are represented. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by two-sample t-test comparisons.
Similar patterns were obtained when comparing short-duration with long-duration subjects. For cytokines that differed between short- and long-duration groups, levels were correlated with duration of illness, and in the expected direction: inverse correlations with duration of illness for cytokines that were increased in the short-duration group, and positive correlations for the two cytokines with reduced levels in the short-duration group (CD40L and PDGFBB) (table S7). For this same set of cytokines, when long-duration subjects were compared to healthy controls, we observed the opposite pattern to that observed when short-duration cases were compared to controls: the same cytokines that were higher than controls in short-duration cases were lower than controls in long-duration cases, except for levels of CD40L and PDGFBB, which were increased (table S6). The inclusion of the MFI mental fatigue subscale score to GLM analyses as an additional continuous covariate did not change these results (data not shown).
Feature selection and logistic regression model comparing short-duration versus long-duration groups
After feature selection procedures, cytokines and predictor variables meeting either the LASSO (least absolute shrinkage and selection operator) or principal components analysis (PCA)/partial least square (PLS) criteria (table S8) were selected for inclusion in a final logistic regression model with short- versus long-duration ME/CFS as the outcome. Younger age was associated with short-duration ME/CFS subgroup status, as anticipated [odds ratio (OR), 0.953; 95% confidence interval (CI), 0.926 to 0.981; P = 0.001].
Two proinflammatory cytokines had a prominent association with short-duration ME/CFS (Table 2). This association was markedly elevated for interferon-γ (IFNγ), with an OR of 104.77 (95% CI, 6.975 to 1574.021; P = 0.001), and more modestly elevated for IL-12p40 (OR, 1.501; 95% CI, 1.075 to 2.096; P = 0.017). Short-duration subgroup status was also associated with lower plasma levels of TNFα (OR, 0.866; 95% CI, 0.765 to 0.980; P = 0.023), CSF2 [granulocyte-macrophage colony-stimulating factor (GM-CSF)] (OR, 0.970; 95% CI, 0.947 to 0.995; P = 0.017), and IL-12p70 (OR, 0.783; 95% CI, 0.650 to 0.942; P = 0.010).
Table 2. Final logistic regression model for association of plasma cytokines and covariates with short-duration versus long-duration ME/CFS.
Final model includes cytokines meeting LASSO and/or PCA/PLS criteria (see Materials and Methods and Supplementary Materials and Methods). Bold text indicates P values <0.05.
| Variable | OR | 95% CI | P |
| Age | 0.953 | 0.926–0.981 | 0.001 |
| Sex | 0.669 | 0.296–1.512 | 0.334 |
| IL-12p40 | 1.501 | 1.075–2.096 | 0.017 |
| IL-12p70 | 0.783 | 0.650–0.942 | 0.010 |
| IL-17A | 0.988 | 0.866–1.127 | 0.857 |
| IFNγ | 104.777 | 6.975–1574.021 | 0.001 |
| TNFα (TNFSF2) | 0.866 | 0.765–0.980 | 0.023 |
| sFasL | 0.981 | 0.856–1.126 | 0.789 |
| CCL11 (eotaxin) | 0.966 | 0.929–1.004 | 0.075 |
| CSF1 (M-CSF) | 1.068 | 0.901–1.267 | 0.448 |
| CSF2 (GM-CSF) | 0.970 | 0.947–0.995 | 0.017 |
| PDGFBB | 0.998 | 0.994–1.002 | 0.370 |
Network associations
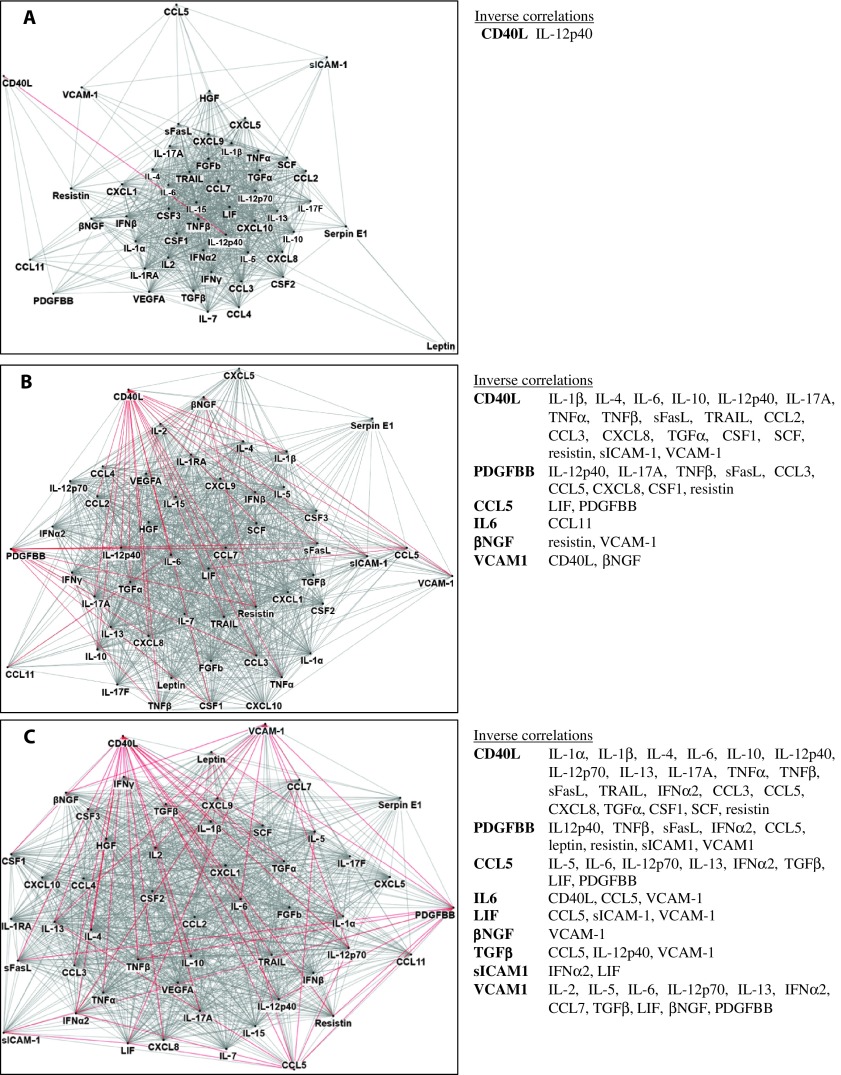
Network diagrams of intercytokine correlations revealed unusual regulatory relationships among cytokines in the short-duration ME/CFS group that were not apparent in either the long-duration case group or healthy controls (Fig. 2). Whereas CD40L drove most of the inverse relationships with other immune molecules in long-duration ME/CFS and controls, CD40L was only related to five other cytokines in the short-duration ME/CFS group; furthermore, only one of these associations, PDGFBB, was negative. PDGFBB was a negative driver of many other cytokines in long-duration ME/CFS subjects and healthy controls, but had no inverse associations with other cytokines within the short-duration subset. Among short-duration subjects, the levels of the adipose-related cytokine leptin were only correlated with IL-12p40, CSF2 (GM-CSF), and serpin E1 [plasminogen activator inhibitor-1 (PAI1)], whereas leptin was tightly related to most of the other 51 cytokines in both the long-duration ME/CFS and the control groups. Leptin also had an inverse relationship to PDGFBB among controls.
Fig. 2. Network cytokine-cytokine associations differ for short-duration versus long-duration ME/CFS versus control subjects.
(A to C) Network diagrams for short-duration ME/CFS subjects (A, n = 52), long-duration ME/CFS subjects (B, n = 246), and healthy controls (C, n = 348). Network diagrams of the 51 measured cytokines were created in NodeXL (http://nodexl.codeplex.com) using a 0.01 family-wise false discovery rate (FDR) to adjust for multiple comparisons (A, short-duration group, P = 0.0065; B, long-duration group, P = 0.0081; C, control group, P = 0.0075). Red lines (edges) indicate negative correlations, and gray lines indicate positive cytokine-cytokine correlations with associated P values that fall below the corrected P value criterion for each group. Note that whereas CD40L drives most of the inverse relationships with other immune molecules in both the long-duration ME/CFS and the control groups, CD40L is only related to five other cytokines in the short-duration ME/CFS group, and only one of these associations is negative (inverse relationship with IL-12p40). Similarly, PDGFBB is a negative driver of many other cytokines in both long-duration ME/CFS and control subjects, but shows no negative correlations with other cytokines in the short-duration subset.
CART analysis
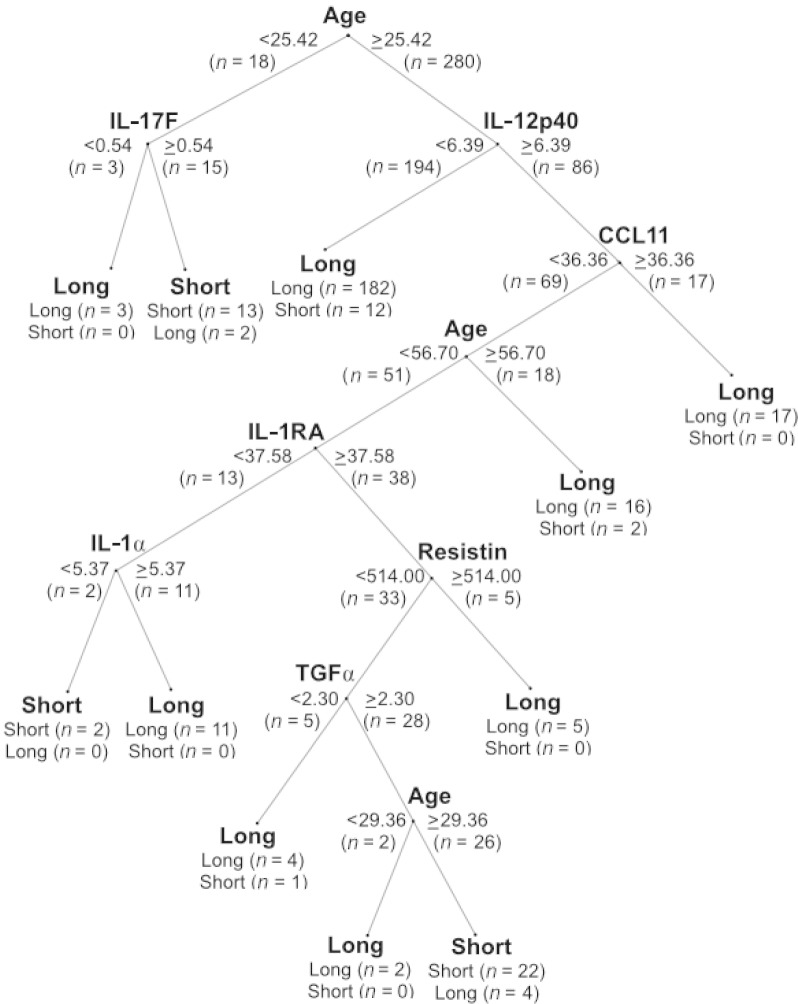
The CART (Classification and Regression Tree) decision tree machine learning method was applied to plasma cytokine and clinical covariate data to find predictors that distinguished ME/CFS cases of short illness duration (≤3 years) from those with a long illness duration (>3 years). Predictor variables and cutoffs at each node in the decision tree are those with the maximum capacity to differentiate between the different levels of the dependent variable (here, the diagnostic subgroups of short-duration versus long-duration ME/CFS cases). Resulting cytokine classifiers are dependent on subject age within both the short-duration and long-duration ME/CFS subgroups, but predictor patterns varied differently with age across different cytokines (Fig. 3). Results show that cytokine differences are not solely due to older mean age of the long-duration ME/CFS subgroup.
Fig. 3. CART analysis of cytokine and clinical predictors in subjects with short- and long-duration ME/CFS.
The CART decision tree machine learning method was applied to plasma cytokine and clinical covariate data to derive predictors associated with ME/CFS of short (≤3 years, n = 52) versus long (>3 years, n = 246) duration. Predictor variables and cutoffs at each of the nodes in the decision tree are those with the maximum capacity to differentiate between the different levels of the dependent variable (here, short versus long duration of illness). Resulting cytokine classifiers are highly dependent on subject age within both the short-duration and long-duration ME/CFS subgroups, but predictor patterns are shown to vary differently with age across different cytokines. These data provide evidence that cytokine differences are not solely due to the older mean age of the long-duration ME/CFS subgroup.
Among subjects between 25.4 and 56.7 years of age, short-duration subjects were more likely than long-duration subjects to have higher plasma levels of IL-12p40 (>6.39 pg/ml) in association with lower levels of CCL11 (eotaxin; <36.36 pg/ml), IL-1RA (<37.58 pg/ml), and IL-1α (<5.37 pg/ml). When restricting to short-duration subjects between 29.3 and 56.7 years of age, the short-duration group was best distinguished from the long-duration group by the combination of elevated IL-12p40 (≥6.39 pg/ml), IL-1RA (≥37.58 pg/ml), and transforming growth factor–α (TGFα) (≥2.3 pg/ml) along with lower levels of CCL11 (<36.36 pg/ml) and resistin (<514 pg/ml).
Cytokine predictors of long-duration ME/CFS status also varied with age. Low levels of IL-17F (<0.54 pg/ml) were characteristic only of younger long-duration subjects (<25.4 years of age). Among subjects >25.4 years, two subsets of long-duration ME/CFS cases were apparent: one distinguished by lower levels of IL-12p40 (<6.39 pg/ml) and another subgroup of long-duration cases who had higher levels of IL-12p40 (>6.39 pg/ml) in combination with higher CCL11 levels (≥36.36 pg/ml).
DISCUSSION
This is the first study to demonstrate altered plasma immune signatures early in the course of ME/CFS that are not present in subjects with longer duration of illness. No substantive differences were found between cases and controls when short- and long-duration cases were combined and compared with healthy control subjects. However, analyses that considered duration of illness revealed that early ME/CFS cases had a prominent activation of both pro- and anti-inflammatory cytokines as well as a dissociation of characteristic intercytokine regulatory networks. Previous studies of immune disturbances in ME/CFS may have missed these findings because they were collected from heterogeneous study populations, without characterization of the duration of illness. We found a stronger correlation of cytokine alterations with duration of illness than with global measures of severity of illness, suggesting that the immunopathology of ME/CFS is not static.
The marked association of IFNγ with the early phase of illness that was detected through logistic regression modeling is consistent with a viral trigger or disrupted immune regulatory networks. IFNγ is a product of activated T cells [particularly CD8+ and CD4+ T helper 1 (TH1) cells], natural killer cells, as well as activated macrophages in the periphery (15, 16) and microglia in the central nervous system (17–19). IFNγ may disrupt immune cell homeostasis, resulting in a TH2-type skew and greater vulnerability toward the development of certain types of autoimmune responses (20–23). Some studies in ME/CFS (7), but not all (24–26), have found plasma levels of IFNγ to be elevated, with effects restricted to males in one study (27). Postviral fatigue in West Nile virus infection has also been reported to be associated with elevations of IFNγ (28). Individuals experiencing more severe fatigue as part of an acute sickness response to infection have been noted to have specific genetic polymorphisms in IFNG (29). IFNγ may accelerate degradation of tryptophan, resulting in depletion of the neurotransmitters serotonin and melatonin in the central nervous system through activation of indoleamine-2,3-dioxygenase, a rate-limiting enzyme in the kynurenine pathway. Activation of this pathway also results in increased levels of quinolinic acid, a neurotoxic compound that acts as an agonist at glutamatergic [NMDA (N-methyl-d-aspartate)] receptors (30–32). Psychomotor changes, including cognitive problems (deficits of memory and attention) as well as affective disturbances, are correlated with changes in the kynurenine pathway in disorders ranging from Alzheimer’s disease (33) to major depression (34). We hypothesize that IFNγ-mediated lesions in kynurenine metabolism may culminate in the depression and psychomotor retardation that contribute to disability in some patients with ME/CFS (34–36).
Our observation that the levels of CD40L were both decreased and dissociated from normal intercytokine regulation early in the course of illness in ME/CFS is intriguing. CD40L (CD154), a TNF superfamily transmembrane protein found on immune (especially CD4+ T cells), endothelial, and smooth muscle cells, is essential for regulation of B cell maturation and isotype switching (37); however, abnormally high levels have been associated in some studies with risk of adverse neurovascular events (38) as well as mild cognitive impairment (39) and Alzheimer’s disease (40). Of potential relevance for ME/CFS, constitutive CD40L deficiency (X-linked hyperimmunoglobulin M syndrome) not only is associated with susceptibility to recurrent infections of the sinuses and pulmonary tracts but also can present with a pattern of cognitive and neurologic decline that is unexplained by the presence of any known pathogen or clear clinical signs of encephalitis (41). Additional immunophenotyping studies, including isotype analyses and in vitro studies comparing peripheral blood mononuclear cells from short- and long-duration subjects, may also reveal immune signaling pathway differences early in the course of illness that may be more amenable to modification and help clarify this pattern of results.
We were surprised to find that the levels of so many cytokines were higher in short-duration cases than in healthy controls. Equally surprising was the observation that these same cytokines were lower in long-duration cases than in healthy controls. We can only speculate as to why that might be. Possibly, as occurs with pancreatic β cell production of insulin in type 2 diabetes (42), or in chronic infections (43), the exuberant stimulation of cytokine-producing cells seen in the first 3 years of the illness leads to an “exhaustion” of the cytokine-producing cells thereafter. Although CD57, a marker of premature immune senescence that is found to increase in later phases of some chronic viral infections such as HIV (44) or hepatitis C virus (HCV) (45), has been shown to be decreased in previous work in ME/CFS (46, 47), studies that control for duration of illness may provide additional clarity as to the potential contribution of mechanisms of immune exhaustion to the immune changes observed later in the course of ME/CFS.
As anticipated, longer-duration ME/CFS subjects were significantly older than those presenting closer to the onset of their illness (short-duration subjects, ≤3 years). However, age was only associated with a handful of cytokines in our GLM analyses (table S2). In addition, CART analysis (Fig. 3) revealed the presence of age-dependent cytokine subgroups within the short- and long-duration ME/CFS subgroups, demonstrating that the predictive value of cytokine levels was not linearly associated with age. Future longitudinal study designs should control for effects of both age and illness severity within subjects to better define potential interactions of aging and the disease process in this patient population. Given the evidence of a sharply increased risk of non-Hodgkin’s lymphoma in elderly subjects with ME/CFS (6), it is of paramount importance to understand the clinical significance of age- and illness-related changes in immune system function in this vulnerable population.
We found significantly different levels of CD40L in the short-duration versus long-duration subgroups, and an uncoupling of CD40L levels with other cytokines in the short-duration subgroup. To our knowledge, only one other study has evaluated CD40L levels in patients with ME/CFS and found low levels (as did we, in the short-duration patients) (48). CD40L plays an important role in B cell maturation (49). There is indirect evidence that B cells play an important role in ME/CFS (50), although not all studies have found abnormalities of B cell phenotype or function (51, 52). Most studies indicate a higher frequency of autoantibodies in the illness (53), and a randomized trial of a monoclonal antibody that targets B cells has demonstrated symptom improvement in some patients (54).
Our study has limitations. First, our strategy was to measure a large panel of cytokines with the rationale that findings might rapidly transition to clinical diagnostic applications. We did not pursue proteomic discovery with the goal of identifying noncytokine biomarkers. Second, the CART analysis was conducted on a training set (the entire study sample) but not then validated on an independent test set. Third, our assays were conducted using only blood samples. Although blood is more easily obtained than cerebrospinal fluid, analysis of the latter may enable unique insights into immune activation in the central nervous system. This is important because many signs and symptoms of ME/CFS are consistent with central nervous system dysfunction (55).
The presence of a specific immune profile early in the course of ME/CFS has important implications for the diagnostic process. First, we were able to define a distinctive immune signature that differed from that of healthy controls. Integration of these immune markers with clinical findings will provide clinicians with a stronger framework for establishing an ME/CFS diagnosis, and, possibly, make it easier to rule out other conditions at an earlier time point. It may also facilitate resolution of some of the clinical heterogeneity observed in this illness (56) by enhancing the ability to distinguish cytokine-associated phenotypes from ME/CFS subsets wherein disease may be triggered by nonimmunologic factors. Second, the restriction of this pattern of immune disturbances to short-duration as opposed to long-duration cases suggests that both the dysregulation of immune cell interactions (for example, faulty CD40L signaling leading to impaired T and B cell interactions) and the opportunities for intervention may be transient. Therapeutic strategies that specifically target abnormalities found in these early immune profiles may present novel but time-limited opportunities not only for remediation but potentially also for staving off the long-term, chronic decline associated with ME/CFS. Prospective analyses that establish the diagnosis of ME/CFS at an early or incipient stage and follow the trajectory of immune responses over the course of illness are required to elucidate the clinical significance of these findings. Delineation of immune profiles in well-characterized subjects with ME/CFS, both early and late in the course of illness, as we have presented here, provides a unique tool for the establishment of clinically relevant phenotypes and discovery of novel treatment targets. If replicated in longitudinal studies, these data may provide a basis for early immunomodulatory intervention to prevent long-term, recalcitrant illness.
MATERIALS AND METHODS
Experimental design
Study population
Cases and controls were derived from two large studies of ME/CFS in the United States: a study supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH (cases, n = 147; controls, n = 146) (11) and another supported by the CFI (cases, n = 203; controls, n = 202) (12). Although inclusion/exclusion criteria varied slightly for the two studies (see below), both required subjects to have previously been diagnosed with ME/CFS at one of the participating clinical sites across the United States (five of six sites participating in the NIH study also participated in the CFI study) to be eligible. Up to 25% of the cases who participated in the NIH study were re-recruited about 1 year later for the CFI study; for these cases (n = 52), clinical and laboratory data from the earlier NIH study were retained in the final, combined data set. None of the control subjects participating in the NIH study were re-recruited for the CFI study; thus, all control data were included in the current analysis. In both studies, controls were frequency-matched on sex, age (±5 years), race/ethnicity, geographic region of residence, and season of case subject blood sampling (±12 weeks). All participants completed screening and assessment instruments, underwent a standardized physical examination, and provided blood samples (11). A diagnosis of ME/CFS requires duration of illness for more than 6 months; hence, acute disease cannot be appreciated except in retrospect. A 3-year cutoff for early ME/CFS cases was used on the basis of feasibility of recruitment and longitudinal changes in immune profiles in persistent infections with West Nile virus, HCV, and HIV (28, 57–61). Illness onset was defined as the date when a patient reported the onset of the clinical features that led to a later diagnosis of ME/CFS illness, rather than the date when a physician made the diagnosis of ME/CFS. Discrepancies were resolved by clinician consensus ratings after review of all available data. Severity of illness scores were based on the mental fatigue subscale of the MFI (62). In all, the study included 298 cases of ME/CFS and 348 matched healthy control subjects.
NIH study case definition
NIH cases were between the ages of 18 and 70 years and met both the 1994 CDC Fukuda criteria (63) and the 2003 Canadian consensus criteria for ME/CFS (2), expanded in 2010 (3). Additionally, all NIH study cases had a history of a viral-like prodrome before the onset of CFS and reduced functional status in two or more areas identified by the RAND36 survey (11).
CFI cohort study case definition
CFI cohort cases were between the ages of 18 and 65 years and met either or both of the 1994 CDC Fukuda criteria (63) and the 2003 Canadian consensus criteria for ME/CFS (2). Twenty-five percent of all CFI cohort study cases were re-recruited from the NIH study.
Informed consent
All participants provided informed, written consent in accordance with protocols approved by the Institutional Review Board (IRB) for research with human subjects associated with each referring institution: (a) NIH study—Columbia University (included approvals for work at clinical sites not associated with an IRB), Stanford University, and Brigham and Women’s Hospital (Partners Healthcare IRB), and (b) CFI study—Columbia University, Western IRB (clinical sites not associated with an IRB), and Massachusetts General Hospital (Partners Healthcare IRB). Only those NIH study subjects who provided written consent to be recontacted were re-recruited for the CFI study.
Biologic samples
Blood samples were obtained from consented subjects immediately after clinical assessments were complete in an effort to simulate a mild stressor. To minimize variation associated with circadian rhythms (64, 65), samples were collected between the hours of 10 a.m. and 2 p.m. Samples were deidentified and shipped overnight at 2° to 4°C for processing at either Columbia University (NIH study) or the CFI Biobank at Duke University (CFI study). Plasma aliquots were then frozen at −80°C until use in immunoassays to avoid additional freeze/thaw cycles. For subjects participating in both the NIH and the CFI cohort studies (n = 52 ME/CFS subjects), only the NIH plasma samples and their associated clinical assessment data were included in the analysis.
Cytokine analyses
The plasma concentrations of the following immune molecules were determined using a magnetic bead–based 51-plex immunoassay: IL-1 superfamily—IL-1α, IL-1β, IL-1RA; type I IL/γ chain family—IL-2, IL-4, IL-7, IL-13, IL-15; type I IL/β chain family—IL-5, GM-CSF (CSF2); IL-6 (gp130) family—IL-6, LIF; IL-12 family—IL-12p40, IL-12p70; IL-10 family—IL-10; IL-17 family—IL-17A, IL-17F; type I IFNs—IFNα2, IFNβ; type II IFN—IFNγ; TNF superfamily—TNFα (TNFSF2), TNFβ (TNFSF1), CD40L, sFasL (TNFSF6), TRAIL (TNFSF10); CC chemokines—CCL2 (MCP1), CCL3 [macrophage inflammatory protein-1α (MIP-1α)], CCL4 (MIP-1β), CCL5 [regulated on activation, normal T cell expressed and secreted (RANTES)], CCL7 (MCP3), CCL11 (eotaxin); CXC chemokines—CXCL1 [growth-regulated oncogene α (GROα)], CXCL5 [epithelial-derived neutrophil-activating peptide 78 (ENA78)], CXCL8 (IL-8), CXCL9 [monokine induced by IFNγ (MIG)], CXCL10 (IP-10); PDGF family/VEGF subfamily—PDGFBB, VEGFA; cell adhesion molecules—sICAM-1 [soluble intercellular adhesion molecule–1 (CD54)], VCAM-1 [vascular cell adhesion molecule–1 (CD106)]; serine protease inhibitor—serpin E1 (PAI1); adipose-derived hormones—leptin, resistin; and neurotrophic/growth/cellular factors—TGFα, TGFβ, FGFb (fibroblast growth factor, basic), βNGF (β-nerve growth factor), HGF (hepatocyte growth factor), SCF, M-CSF (CSF1), G-CSF (granulocyte colony-stimulating factor; CSF3) (customized Procarta immunoassay, Affymetrix).
Cases (both short duration and long duration) and healthy control subject samples were coded and run on the same assay plates in randomized fashion. In addition, all plasma samples were run in duplicate along with serial standards, buffer controls, and in-house human control plasma samples (66). Mean fluorescence intensities of analyte-specific immunoassay bead sets were detected by flow-based Luminex 3D suspension array system (Luminex Corp.) (67). Cytokine concentrations were calculated by xPONENT build 4.0.846.0 and Milliplex Analyst software (v.3.5.5.0) using a standard curve derived from the known reference concentrations supplied by the manufacturer. A five-parameter model was used to calculate final concentrations by interpolation. Values are expressed in picograms per milliliter. Concentrations obtained below the sensitivity limit of detection (LOD) of the method were recoded to the mid-point between zero and the LOD for that analyte for statistical comparisons. Values obtained from the reading of samples that exceeded the upper limit of the sensitivity method were further diluted, and cytokine concentrations were calculated accordingly.
Statistical analysis
Descriptive statistics
Descriptive statistics were computed for demographic variables across diagnostic groups. Sex and age were included as possible confounders. Differences across diagnostic groups and subgroups in proportion of females and males, sex, race, clinic site, and months of sample acquisition were assessed by χ2. Differences in median age, illness duration, and MFI mental fatigue subscale scores were examined using the nonparametric Mann-Whitney U (two-group comparisons) and Kruskal-Wallis (three-group comparisons) test.
Data transformations
Because distributions deviated from normality, raw cytokine levels were first transformed using Box-Cox transformation defined as:
where, for each cytokine variable, the optimal λ was identified to maximize the log-likelihood function (68). After transformation, all variables failed to reject the null hypothesis using the Kolmogorov-Smirnov test at the 0.05 level, confirming at the 95% confidence level that all transformed variables followed a normal distribution.
GLM, t tests, and correlations
GLM analyses were applied to examine both the main effect of diagnosis and the main and interaction effects of different fixed factors including diagnosis (two-level case versus control comparisons and three-level short duration versus long duration versus control analyses) and sex, with age adjusted as a continuous covariate. Because GLM uses the family-wise error rate, no additional adjustments for multiple comparisons were applied. When the main effect of diagnosis [one-way analysis of variance (ANOVA)/GLM or more complex models] or the interaction term of diagnosis*sex was found to be significant (α = 0.05), two-sample t tests, also using a nominal α level of 0.05, were conducted to compare the mean plasma levels of each transformed cytokine between appropriate diagnostic groups: cases versus controls, short-duration cases versus controls, long-duration cases versus controls, and short-duration versus long-duration cases. Spearman correlations were performed to determine the relationship between the level of each cytokine (nontransformed values, in pg/ml) and duration of illness (in years).
Logistic regression modeling
Data for the 51-plex cytokine assays as a whole were also used to develop a logistic regression model for prediction of the binary response variable (case versus control, short versus long disease duration). To eliminate multicollinearity among cytokines, a number of dimensionality reduction methods were used to guide selection of variables for the final logistic regression model. Multiple methods were used to enable capture of factors potentially missed by one individual method. Raw cytokine levels were used in these analyses to allow for computation of ORs.
Dimensionality reduction methods
Feature selection strategies select subsets of relevant features for use in regression model construction based on the assumption that the data contain many redundant or irrelevant features. Feature extraction transforms highly dimensional input data into a reduced, representative set of features by extracting as much relevant information as possible from the input data. We used one of the most widely used feature selection techniques, LASSO (69), and two feature extraction approaches, PCA and the PLS method. Details of the LASSO, PCA, and PLS methods for selection of cytokine variables for inclusion in logistic regression models are described in Supplementary Methods. A nominal α level of 0.05 defined significance at each step of the PCA and PLS feature extraction procedures. For LASSO, features were selected for inclusion in the model if they were associated with a nonzero coefficient.
For the final logistic regression model, a cytokine variable was entered either if it was selected by LASSO or if it met criteria for representing a potential candidate via both the PCA and PLS feature extraction methods. The ORs, their 95% CIs, and P values of the selected cytokines were calculated accordingly.
NodeXL diagrams
The NodeXL platform was used to produce a network diagram of 51-plex assays within each subgroup (70). The platform provides a display of the relationships among the analytes, thereby facilitating discovery of different cytokine-cytokine structures across the different group populations. Bivariate Pearson’s correlations were first conducted between every pairwise combination of cytokine variables using their power-transformed (Box-Cox) values. We then used the Benjamini and Hochberg method to adjust for multiple comparisons with a 0.01 family-wise FDR (71). Significantly correlated cytokine pairs were fed into the algorithm in NodeXL to produce the network diagram. Cytokines are represented by the “nodes” in the diagram, and significantly correlated cytokines are connected by “lines” or edges. Red lines represent negative correlations; gray lines represent positive correlations.
CART analyses
The CART (72) method was used to analyze differences among diagnostic subgroups (short-duration ME/CFS, long-duration ME/CFS, and controls) by examining data from 51-plex cytokine assays from another perspective. CART is a nonparametric decision tree learning technique that produces a classification tree for dependent variables. It works by recursively splitting the feature space (the values of all the independent variables, or “features,” included in the CART analysis) into a set of nonoverlapping regions and then predicting the most likely value of the dependent variable within each region. The decision rule at each node of the tree is determined by searching over all independent variables and all possible values of those independent variables to provide the split that will best differentiate observations on the basis of the dependent variable (here, diagnostic subgroups of short-duration ME/CFS, long-duration ME/CFS, and controls). Once a rule is selected and splits each node into two, the same process is applied to each subsequent “child” node in the classification tree. Partitioning stops when CART detects that there is no further gain to be made: when diagnostic subgroups can be best distinguished by the criterion values associated with specific variables. To maximize predictive accuracy, the tree is pruned to optimize cross-validation. This is accomplished by repetitively resampling two subsets from the overall population, establishing one subset as a training set and allowing the other subset to function as a test set. This resampling procedure is repeated 100 times; the final tree that is selected is based on the algorithm that best fits the data for the population in the test set.
Statistical analyses were run in SPSS version 22.0.0.0, MATLAB version R2013a, and R version 3.0.2.
Supplementary Material
Acknowledgments
We are grateful to W. Wong, N. Pura, and S. Baile for their technical support. Funding: This study was funded by the CFI/Hutchins Family Foundation (W.I.L. and M.H.) and NIH (AI1057158; NBC-WIL). Author contributions: Experimental design and analysis: M.H., W.I.L., A.F.S., and X.C.; review of manuscript before submission for publication: M.H., J.G.M., N.G.K., S.L., D.F., L.B., D.L.P., C.G.G., A.F.S., X.C., M.L.E., A.L.K., and W.I.L.; biostatistical analysis: A.F.S. and X.C.; case and control recruitment and characterization: J.G.M., N.G.K., S.L., D.F., L.B., D.L.P., C.G.G., and A.L.K.; coordination and data and sample management: M.H., C.G.G., M.L.E., and W.I.L.; laboratory analyses: M.H. Competing interests: The authors declare that they have no competing interests.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/1/e1400121/DC1
Materials and Methods
Table S1. Comparison of plasma immune analytes in ME/CFS subjects versus controls.
Table S2. Comparison of plasma immune analytes in ME/CFS subjects versus controls.
Table S3. Comparison of plasma immune markers in ME/CFS subjects versus controls.
Table S4. Comparison of plasma immune analytes in short-duration versus long-duration ME/CFS subjects versus controls.
Table S5. Comparison of plasma immune analytes in short-duration versus long-duration ME/CFS subjects versus controls.
Table S6. Comparison of plasma immune markers in short-duration versus long-duration ME/CFS versus controls.
Table S7. Spearman correlations of levels of plasma immune markers with duration of illness in ME/CFS.
Table S8. Feature selection data for logistic regression predicting short-duration versus long-duration ME/CFS.
Reference (73)
REFERENCES AND NOTES
- 1.Brurberg K. G., Fonhus M. S., Larun L., Flottorp S., Malterud K., Case definitions for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): A systematic review. BMJ Open 4, e003973 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carruthers B. M., Jain A. K., DeMeirleir K. L., Peterson D. L., Klimas N. G., Lerner A. M., Bested A. C., Flor-Henry P., Joshi P., Powles A. C. P., Sherkey J. A., van de Sande M. I., Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatments protocols. J. Chr. Fatigue Synd. 11, 7–115 (2003). [Google Scholar]
- 3.Jason L. A., Evans M., Porter N., Brown M., Brown A., Hunnell J., Anderson V., Lerch A., DeMeirleir K., Friedberg F., The development of a revised Canadian myalgic encephalomyelitis chronic fatigue syndrome case definition. Am. J. Biochem. Biotechnol. 6, 120–135 (2010). [Google Scholar]
- 4.Tan E. M., Sugiura K., Gupta S., The case definition of chronic fatigue syndrome. J. Clin. Immunol. 22, 8–12 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Ravindran M. K., Zheng Y., Timbol C., Merck S. J., Baraniuk J. N., Migraine headaches in chronic fatigue syndrome (CFS): Comparison of two prospective cross-sectional studies. BMC Neurol. 11, 30 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang C. M., Warren J. L., Engels E. A., Chronic fatigue syndrome and subsequent risk of cancer among elderly US adults. Cancer 118, 5929–5936 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenu E. W., van Driel M. L., Staines D. R., Ashton K. J., Ramos S. B., Keane J., Klimas N. G., Marshall-Gradisnik S. M., Immunological abnormalities as potential biomarkers in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. J. Transl. Med. 9, 81 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho H. J., Skowera A., Cleare A., Wessely S., Chronic fatigue syndrome: An update focusing on phenomenology and pathophysiology. Curr. Opin. Psychiatry 19, 67–73 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Lyall M., Peakman M., Wessely S., A systematic review and critical evaluation of the immunology of chronic fatigue syndrome. J. Psychosom. Res. 55, 79–90 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Nijs J., Nees A., Paul L., De Kooning M., Ickmans K., Meeus M., Van Oosterwijck J., Altered immune response to exercise in patients with chronic fatigue syndrome/myalgic encephalomyelitis: A systematic literature review. Exerc. Immunol. Rev. 20, 94–116 (2014). [PubMed] [Google Scholar]
- 11.Alter H. J., Mikovits J. A., Switzer W. M., Ruscetti F. W., Lo S. C., Klimas N., Komaroff A. L., Montoya J. G., Bateman L., Levine S., Peterson D., Levin B., Hanson M. R., Genfi A., Bhat M., Zheng H., Wang R., Li B., Hung G. C., Lee L. L., Sameroff S., Heneine W., Coffin J., Hornig M., Lipkin W. I., A multicenter blinded analysis indicates no association between chronic fatigue syndrome/myalgic encephalomyelitis and either xenotropic murine leukemia virus-related virus or polytropic murine leukemia virus. MBio 3, e00266-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.J. Kaiser, Family puts $10 million into chronic fatigue research. ScienceInsider, 19 September 2011.
- 13.Lattie E. G., Antoni M. H., Fletcher M. A., Czaja S., Perdomo D., Sala A., Nair S., Fu S. H., Penedo F. J., Klimas N., Beyond myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) symptom severity: Stress management skills are related to lower illness burden. Fatigue 1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mia S., Warnecke A., Zhang X. M., Malmstrom V., Harris R. A., An optimized protocol for human M2 macrophages using M-CSF and IL-4/IL-10/TGF-β yields a dominant immunosuppressive phenotype. Scand. J. Immunol. 79, 305–314 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darwich L., Coma G., Pena R., Bellido R., Blanco E. J., Este J. A., Borras F. E., Clotet B., Ruiz L., Rosell A., Andreo F., Parkhouse R. M., Bofill M., Secretion of interferon-γ by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology 126, 386–393 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson C. M., O’Dee D., Hamilton T., Nau G. J., Cytokines involved in interferon-γ production by human macrophages. J. Innate Immun. 2, 56–65 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Simone R., Levi G., Aloisi F., Interferon γ gene expression in rat central nervous system glial cells. Cytokine 10, 418–422 (1998). [DOI] [PubMed] [Google Scholar]
- 18.Kawanokuchi J., Mizuno T., Takeuchi H., Kato H., Wang J., Mitsuma N., Suzumura A., Production of interferon-γ by microglia. Mult. Scler. 12, 558–564 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Makela J., Koivuniemi R., Korhonen L., Lindholm D., Interferon-γ produced by microglia and the neuropeptide PACAP have opposite effects on the viability of neural progenitor cells. PLOS One 5, e11091 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skurkovich S. V., Skurkovich B., Kelly J. A., Anticytokine therapy—New approach to the treatment of autoimmune and cytokine-disturbance diseases. Med. Hypotheses 59, 770–780 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Schoenborn J. R., Wilson C. B., Regulation of interferon-γ during innate and adaptive immune responses. Adv. Immunol. 96, 41–101 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Annunziato F., Romagnani C., Romagnani S., The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. S0091-6749(14)01585-1 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Elenkov I. J., Glucocorticoids and the Th1/Th2 balance. Ann. N. Y. Acad. Sci. 1024, 138–146 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Neu D., Mairesse O., Montana X., Gilson M., Corazza F., Lefevre N., Linkowski P., Le Bon O., Verbanck P., Dimensions of pure chronic fatigue: Psychophysical, cognitive and biological correlates in the chronic fatigue syndrome. Eur. J. Appl. Physiol. 114, 1841–1851 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Broderick G., Fuite J., Kreitz A., Vernon S. D., Klimas N., Fletcher M. A., A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav. Immun. 24, 1209–1217 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fletcher M. A., Zeng X. R., Barnes Z., Levis S., Klimas N. G., Plasma cytokines in women with chronic fatigue syndrome. J. Transl. Med. 7, 96 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smylie A. L., Broderick G., Fernandes H., Razdan S., Barnes Z., Collado F., Sol C., Fletcher M. A., Klimas N., A comparison of sex-specific immune signatures in Gulf War illness and chronic fatigue syndrome. BMC Immunol. 14, 29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia M. N., Hause A. M., Walker C. M., Orange J. S., Hasbun R., Murray K. O., Evaluation of prolonged fatigue post–West Nile virus infection and association of fatigue with elevated antiviral and proinflammatory cytokines. Viral Immunol. 27, 327–333 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piraino B., Vollmer-Conna U., Lloyd A. R., Genetic associations of fatigue and other symptom domains of the acute sickness response to infection. Brain Behav. Immun. 26, 552–558 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller A. H., Haroon E., Raison C. L., Felger J. C., Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depress. Anxiety 30, 297–306 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myint A. M., Kim Y. K., Network beyond IDO in psychiatric disorders: Revisiting neurodegeneration hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 48, 304–313 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Bellmann-Weiler R., Schroecksnadel K., Holzer C., Larcher C., Fuchs D., Weiss G., IFN-gamma mediated pathways in patients with fatigue and chronic active Epstein Barr virus-infection. J. Affect. Disord. 108, 171–176 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Tan L., Yu J. T., Tan L., The kynurenine pathway in neurodegenerative diseases: Mechanistic and therapeutic considerations. J. Neurol. Sci. 323, 1–8 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Myint A. M., Schwarz M. J., Muller N., The role of the kynurenine metabolism in major depression. J. Neural Transm. 119, 245–251 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Anderson G., Berk M., Maes M., Biological phenotypes underpin the physio-somatic symptoms of somatization, depression, and chronic fatigue syndrome. Acta Psychiatr. Scand. 129, 83–97 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Maes M., Mihaylova I., Ruyter M. D., Kubera M., Bosmans E., The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): Relevance for depression - and other conditions characterized by tryptophan depletion induced by inflammation. Neuro Endocrinol. Lett. 28, 826–831 (2007). [PubMed] [Google Scholar]
- 37.Durandy A., De Saint Basile G., Lisowska-Grospierre B., Gauchat J. F., Forveille M., Kroczek R. A., Bonnefoy J. Y., Fischer A., Undetectable CD40 ligand expression on T cells and low B cell responses to CD40 binding agonists in human newborns. J. Immunol. 154, 1560–1568 (1995). [PubMed] [Google Scholar]
- 38.Elkind M. S., Luna J. M., Coffey C. S., McClure L. A., Liu K. M., Spitalnik S., Paik M. C., Roldan A., White C., Hart R., Benavente O., The Levels of Inflammatory Markers in the Treatment of Stroke study (LIMITS): Inflammatory biomarkers as risk predictors after lacunar stroke. Int. J. Stroke 5, 117–125 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross J., Sharma S., Winston J., Nunez M., Bottini G., Franceschi M., Scarpini E., Frigerio E., Fiorentini F., Fernandez M., Sivilia S., Giardino L., Calza L., Norris D., Cicirello H., Casula D., Imbimbo B. P., CHF5074 reduces biomarkers of neuroinflammation in patients with mild cognitive impairment: A 12-week, double-blind, placebo-controlled study. Curr. Alzheimer Res. 10, 742–753 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Buchhave P., Janciauskiene S., Zetterberg H., Blennow K., Minthon L., Hansson O., Elevated plasma levels of soluble CD40 in incipient Alzheimer’s disease. Neurosci. Lett. 450, 56–59 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Bishu S., Madhavan D., Perez P., Civitello L., Liu S., Fessler M., Holland S. M., Jain A., Pao M., CD40 ligand deficiency: Neurologic sequelae with radiographic correlation. Pediatr. Neurol. 41, 419–427 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Festa A., Haffner S. M., Wagenknecht L. E., Lorenzo C., Hanley A. J., Longitudinal decline of β-cell function: Comparison of a direct method vs a fasting surrogate measure: The Insulin Resistance Atherosclerosis Study. J. Clin. Endocrinol. Metab. 98, 4152–4159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Utzschneider D. T., Legat A., Fuertes Marraco S. A., Carrie L., Luescher I., Speiser D. E., Zehn D., T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat. Immunol. 14, 603–610 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Ferrando-Martinez S., Ruiz-Mateos E., Romero-Sanchez M. C., Munoz-Fernandez M. A., Viciana P., Genebat M., Leal M., HIV infection-related premature immunosenescence: High rates of immune exhaustion after short time of infection. Curr. HIV Res. 9, 289–294 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Golden-Mason L., Palmer B., Klarquist J., Mengshol J. A., Castelblanco N., Rosen H. R., Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J. Virol. 81, 9249–9258 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta S., Vayuvegula B., A comprehensive immunological analysis in chronic fatigue syndrome. Scand. J. Immunol. 33, 319–327 (1991). [DOI] [PubMed] [Google Scholar]
- 47.Tirelli U., Marotta G., Improta S., Pinto A., Immunological abnormalities in patients with chronic fatigue syndrome. Scand. J. Immunol. 40, 601–608 (1994). [DOI] [PubMed] [Google Scholar]
- 48.White A. T., Light A. R., Hughen R. W., Bateman L., Martins T. B., Hill H. R., Light K. C., Severity of symptom flare after moderate exercise is linked to cytokine activity in chronic fatigue syndrome. Psychophysiology 47, 615–624 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rickert R. C., Jellusova J., Miletic A. V., Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol. Rev. 244, 115–133 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robertson M. J., Schacterle R. S., Mackin G. A., Wilson S. N., Bloomingdale K. L., Ritz J., Komaroff A. L., Lymphocyte subset differences in patients with chronic fatigue syndrome, multiple sclerosis and major depression. Clin. Exp. Immunol. 141, 326–332 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curriu M., Carrillo J., Massanella M., Rigau J., Alegre J., Puig J., Garcia-Quintana A. M., Castro-Marrero J., Negredo E., Clotet B., Cabrera C., Blanco J., Screening NK-, B- and T-cell phenotype and function in patients suffering from Chronic Fatigue Syndrome. J. Transl. Med. 11, 68 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LaManca J. J., Sisto S. A., Zhou X. D., Ottenweller J. E., Cook S., Peckerman A., Zhang Q., Denny T. N., Gause W. C., Natelson B. H., Immunological response in chronic fatigue syndrome following a graded exercise test to exhaustion. J. Clin. Immunol. 19, 135–142 (1999). [DOI] [PubMed] [Google Scholar]
- 53.Morris G., Maes M., Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med. 11, 205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fluge O., Bruland O., Risa K., Storstein A., Kristoffersen E. K., Sapkota D., Naess H., Dahl O., Nyland H., Mella O., Benefit from B-lymphocyte depletion using the anti-CD20 antibody rituximab in chronic fatigue syndrome. A double-blind and placebo-controlled study. PLOS One 6, e26358 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moss-Morris R., Deary V., Castell B., Chronic fatigue syndrome. Handb. Clin. Neurol. 110, 303–314 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Fischer D. B., William A. H., Strauss A. C., Unger E. R., Jason L., Marshall G. D. Jr, Dimitrakoff J. D., Chronic fatigue syndrome: The current status and future potentials of emerging biomarkers. Fatigue 2, 93–109 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murray K. O., Garcia M. N., Rahbar M. H., Martinez D., Khuwaja S. A., Arafat R. R., Rossmann S., Survival analysis, long-term outcomes, and percentage of recovery up to 8 years post-infection among the Houston West Nile virus cohort. PLOS One 9, e102953 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alberti A., Chemello L., Benvegnu L., Natural history of hepatitis C. J. Hepatol. 31 (Suppl. 1), 17–24 (1999). [DOI] [PubMed] [Google Scholar]
- 59.Khaitan A., Unutmaz D., Revisiting immune exhaustion during HIV infection. Curr. HIV/AIDS Rep. 8, 4–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dustin L. B., Rice C. M., Flying under the radar: The immunobiology of hepatitis C. Annu. Rev. Immunol. 25, 71–99 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Barathan M., Gopal K., Mohamed R., Ellegard R., Saeidi A., Vadivelu J., Ansari A. W., Rothan H. A., Ravishankar Ram M., Zandi K., Chang L. Y., Vignesh R., Che K. F., Kamarulzaman A., Velu V., Larsson M., Kamarul T., Shankar E. M., Chronic hepatitis C virus infection triggers spontaneous differential expression of biosignatures associated with T cell exhaustion and apoptosis signaling in peripheral blood mononucleocytes. Apoptosis 10.1007/s10495-1084-y (2015). [DOI] [PubMed] [Google Scholar]
- 62.Smets E. M., Garssen B., Bonke B., De Haes J. C., The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 39, 315–325 (1995). [DOI] [PubMed] [Google Scholar]
- 63.Fukuda K., Straus S. E., Hickie I., Sharpe M. C., Dobbins J. G., Komaroff A., The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group Ann. Intern. Med. 121, 953–959 (1994). [DOI] [PubMed] [Google Scholar]
- 64.Wipfler P., Heikkinen A., Harrer A., Pilz G., Kunz A., Golaszewski S. M., Reuss R., Oschmann P., Kraus J., Circadian rhythmicity of inflammatory serum parameters: A neglected issue in the search of biomarkers in multiple sclerosis. J. Neurol. 260, 221–227 (2013). [DOI] [PubMed] [Google Scholar]
- 65.Crofford L. J., Kalogeras K. T., Mastorakos G., Magiakou M. A., Wells J., Kanik K. S., Gold P. W., Chrousos G. P., Wilder R. L., Circadian relationships between interleukin (IL)-6 and hypothalamic-pituitary-adrenal axis hormones: Failure of IL-6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis. J. Clin. Endocrinol. Metab. 82, 1279–1283 (1997). [DOI] [PubMed] [Google Scholar]
- 66.Martins T. B., Development of internal controls for the Luminex instrument as part of a multiplex seven-analyte viral respiratory antibody profile. Clin. Diagn. Lab. Immunol. 9, 41–45 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vignali D. A., Multiplexed particle-based flow cytometric assays. J. Immunol. Methods 243, 243–255 (2000). [DOI] [PubMed] [Google Scholar]
- 68.Box G. E. P., Cox D. R., An analysis of transformations. J. R. Stat. Soc. Ser. B Stat. Methodol. 26, 211–252 (1964). [Google Scholar]
- 69.Tibshirani R., Regression shrinkage and selection via the Lasso. J. R. Stat. Soc. Ser. B Stat. Methodol. 58, 267–288 (1996). [Google Scholar]
- 70.M. Smith, N. Milic-Frayling, B. Shneiderman, E. Mendes Rodrigues, J. Leskovec, C. Dunne. NodeXL: A Free and Open Network Overview, Discovery and Exploration Add-in for Excel 2007/2010 (Social Media Research Foundation, Belmont, CA, 2010); www.smrfoundation.org.
- 71.Benjamini Y., Hochberg Y., Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 57, 289–300 (1995). [Google Scholar]
- 72.L. Breiman, J. H. Friedman, R. A. Olshen, C. J. Stone, Classification and Regression Trees (Wadsworth & Brooks/Cole Advanced Books & Software, Monterey, CA, 1984). [Google Scholar]
- 73.Aguilera A. M., Escabias M., Valderrama M. J., Using principal components for estimating logistic regression with high-dimensional multicollinear data. Comput. Stat. Data Anal. 50, 1905–1924 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/1/e1400121/DC1
Materials and Methods
Table S1. Comparison of plasma immune analytes in ME/CFS subjects versus controls.
Table S2. Comparison of plasma immune analytes in ME/CFS subjects versus controls.
Table S3. Comparison of plasma immune markers in ME/CFS subjects versus controls.
Table S4. Comparison of plasma immune analytes in short-duration versus long-duration ME/CFS subjects versus controls.
Table S5. Comparison of plasma immune analytes in short-duration versus long-duration ME/CFS subjects versus controls.
Table S6. Comparison of plasma immune markers in short-duration versus long-duration ME/CFS versus controls.
Table S7. Spearman correlations of levels of plasma immune markers with duration of illness in ME/CFS.
Table S8. Feature selection data for logistic regression predicting short-duration versus long-duration ME/CFS.
Reference (73)