Abstract
Dominance hierarchies are ubiquitous in social species. Social status is established initially through physical conflict between individuals and then communicated directly by a variety of signals. Social interactions depend critically on the relative social status of those interacting. But how do individuals acquire the information they need to modulate their behaviour and how do they use that information to decide what to do? What brain mechanisms might underlie such animal cognition? Using a particularly suitable fish model system that depends on complex social interactions, we report how the social context of behaviour shapes the brain and, in turn, alters the behaviour of animals as they interact. Animals observe social interactions carefully to gather information vicariously that then guides their future behaviour. Social opportunities produce rapid changes in gene expression in key nuclei in the brain and these genomic responses may prepare the individual to modify its behaviour to move into a different social niche. Both social success and failure produce changes in neuronal cell size and connectivity in key nuclei. Understanding mechanisms through which social information is transduced into cellular and molecular changes will provide a deeper understanding of the brain systems responsible for animal cognition.
Keywords: animal observers, Astatotilapia burtoni, brain changes, mate choice, social behaviour, transitive inference
In all social systems, animals must interact to survive and thrive in their social and physical environments. Remarkably diverse social systems have evolved repeatedly across phylogeny during the course of evolution, reflecting adaptations to the environment constrained by intrinsic capacities of the species. Perhaps animal groups initially arose from times when animals aggregated around food sources and from these caloric encounters came organized social interactions. All such social groups are believed to continue in a population because individuals derive a genetic benefit for themselves by being members of a group. Since behaviour is the key interface between an animal and its environment, animals respond to novel situations first through behavioural change, whereas adaptations in morphology, physiology and life history take longer. While ethologists have tended to focus on the mechanisms and development of behaviour, behavioural ecologists have concentrated generally on the causes and consequences of social behaviour. In this review, I will show how integrating these ways of thinking in a single system allows a mechanistic understanding of an animal’s behaviour from its ecosystem to its social brain (Robinson et al., 2008). I will also discuss the cognitive challenges of living socially and some examples of how social behaviour influences the brain to shape cognitive skills.
In his prolific and prescient writing, Aristotle identified four causes for behaviour that should be studied (Hladky & Havlicek, 2013). About 2300 years later, Tinbergen in a classic paper ‘rediscovered’ Aristotle’s four causes, situating them in two more modern categories: proximate explanations that were directly causal such as hormones and neural activity, and ultimate, or evolutionary explanations such as adaptations that conferred fitness and the phylogenetic trajectory of the species (Tinbergen, 1963). In this review, we have used the Aristotle/Tinbergen level of explanations to understand social behaviour and in particular to gain insight into the cognitive demands social living places on animals.
To understand social behaviour in a naturalistic context, I wanted a model organism that allows natural conditions to be replicated in a laboratory setting with sufficient fidelity to assure realistic results. Fish species are a natural choice because they allow construction of a semi-naturalistic setting in which careful experimentation can be done without compromising the behaviour of the animal. More specifically, because social information is essential, we need to allow animals to live in normal social groups. Fish make up ~50% of all vertebrate species and are increasingly appreciated as models for understanding the complexities of social behaviour (reviewed in Brown et al., 2011). Moreover, they represent more than 400 million years of vertebrate evolution, and their taxonomic dimensions exceed the phylogenetic distance from frogs to humans (Romer, 1959). Fish species have evolved sensory systems exquisitely tuned to their particular environment, including the usual suspects (e.g. vision, olfaction, taste and hearing), but also mechanosensory detection (e.g. lateral line), external taste buds and numerous electroreceptor systems that have driven evolution of specialized brain structures (reviewed in Collin & Marshall, 2003). It is also known that among fish species, every known kind of social system has evolved from monogamy to harems to sex-changing animals (Desjardins & Fernald, 2009; Keenleyside, 1979).
Cognitive skills in various fish species have been shown in several domains including acquisition of foraging skills (Brännäs & Eriksson, 1999), tool use (Timms & Keenleyside, 1975; Paśko, 2010), spatial memory and manipulation of the environment (Hughes & Blight, 1999). Examples of social intelligence in fish have been measured by how they interact in group-living environments (Balshine-Earn et al., 1998), enhance offspring survival with biparental care (Alonzo et al., 2001; Van den Berghe & McKaye, 2001; Gross & Sargent, 1985; Hourigan, 1989), cooperate in hunting (Vail et al., 2013; Diamant & Shpigel, 1985) and share information about predator inspection (Pitcher et al., 1986).
Among fish species, the cichlid species flocks in the rift valley lakes of East Africa offer an unparalleled adaptive radiation of species with many different social systems represented. The ~2000 species have diversified into widely different ecological systems in a relatively short time (Brawand et al., 2014). African cichlids have been studied since the end of the 19th century, most notably in Lake Tanganyika by Boulenger (1898), who published four volumes cataloguing the freshwater fishes of Africa. The colonization of Africa by European countries led to further exploration focused on fish as a potential resource and were particularly well studied by Max Poll (1946, 1986), who performed a comprehensive analysis of cichlid fish species and other organisms in Lake Tanganyika and wrote several definitive volumes describing his findings. The radiations in some East African lakes have the highest rates of speciation known in vertebrates (McCune, 1997); cichlid phenotypic diversity includes variation in behaviour, body shape, colour and trophic specialization. Exactly how cichlids evolved their highly varied phenotypes remains unexplained, but close examination of one species described here suggests that unique adaptations to highly social lives might be a partial explanation.
I study the social behaviour of a cichlid species from Lake Tanganyika, Astatotilapia burtoni (formerly Haplochromis burtoni). While developing this species as a model organism, it became clear that the male hierarchial social system required particular social skills and, furthermore, that social interactions could change the brain. Astatotilapia burtoni offers unique opportunities for discovering how social behaviour changes the brain because (1) the social system, based on resource guarding, can be reliably and accurately replicated in the laboratory, (2) male status is signalled phenotypically via bright coloration, including a dark bar through the eye making animals easy to distinguish and behaviour readily quantifiable, (3) in this species, as in all vertebrates, GnRH1 neurons in the brain ultimately control reproduction, but in A. burtoni, are directly regulated by male social status, (4) A. burtoni allows measurement of behaviour, circulating hormones, tissues, cells and molecular expression and (5) the A. burtoni genome has been sequenced (Brawand et al., 2014), enabling experiments at the genetic level not previously possible.
SOCIAL SYSTEM OF A. BURTONI
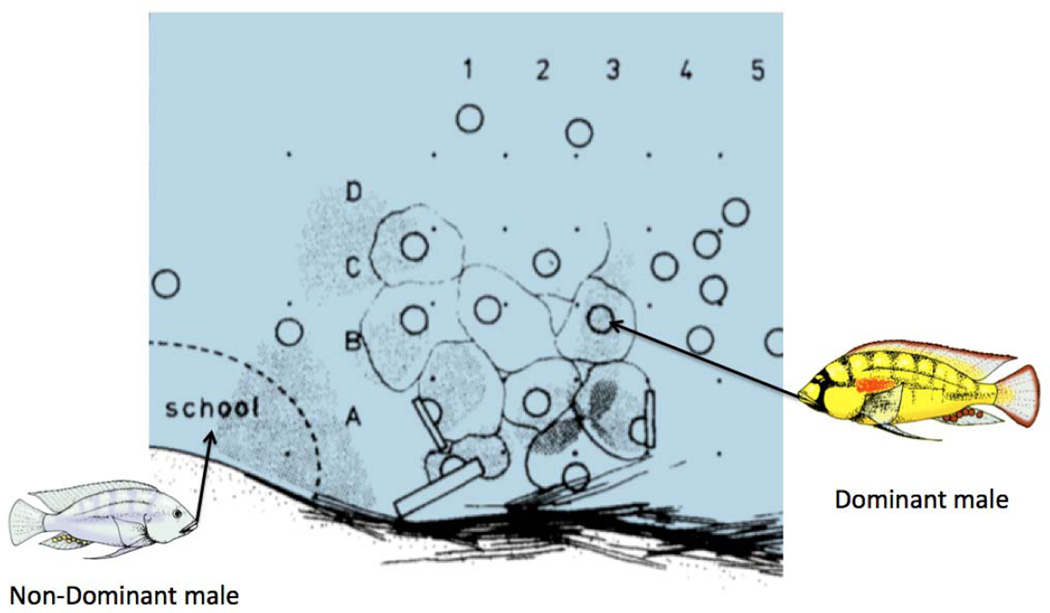
Astatotilapia burtoni males live as one of two quickly reversible, socially controlled phenotypes: reproductively competent dominant males and reproductively incompetent nondominant males (see Fig. 1). Dominant males are brightly coloured, aggressively defend territories and actively court females (Fernald & Hirata, 1977). In striking contrast, nondominant males have a dull coloration, mimic female behaviour and school with females and other nondominant males, except when fleeing from an attacking dominant male.
Figure 1.
Sketch of an observation area in Lake Tanganyika, Burundi, Africa. Solid dots are grid stakes spaced ~50 cm and labelled (1–4; A–D) for identification. Circles represent spawning pit locations of dominant males. Lighter coloured outlines circumscribe the territories of individuals. Nondominant males and females school near the territorial area. (Based on Fernald & Hirata, 1977)
These obvious external differences reflect major physiological differences due to social status. As animals transition from one phenotype to the other, some changes including expression of the black bar through the eye, brightening of the body colour and switch in behaviours expressed occur in minutes.
A nondominant male that previously performed only two behaviours begins to express 17 distinct behaviours rapidly upon social ascent (Fernald & Hirata, 1977; Burmeister et al., 2005). Over a few days, the reproductive system is remodelled as can be observed at several levels along the hypothalamic–pituitary–gonadal (HPG) axis (Maruska & Fernald, 2014). In A. burtoni, as in all vertebrates, reproduction is controlled by gonadotropin-releasing hormone (GnRH) containing neurons in the hypothalamus that deliver the eponymously named GnRH decapeptide to the pituitary. When a male ascends (nondominant→dominant), delivery of this molecule sets in motion a cascade of actions ultimately resulting in reproductive competence. The GnRH neurons increase in volume by eight-fold (Davis & Fernald, 1990), extend their dendrites (Fernald, 2012) and rapidly increase production of GnRH mRNA (Burmeister et al., 2007) and GnRH peptide (White et al., 2002). However, when a dominant male is moved into a social system with larger dominant males (>5% longer), it abruptly loses its colour (<1 min) and joins other nondominant males and females in a school. Its GnRH-containing neurons in the preoptic area (POA) shrink to one-eighth their volume and produce less GnRH mRNA and peptide, causing hypogonadism and loss of reproductive competence (2 weeks) (Davis & Fernald, 1990, Francis et al., 1993). Similarly, androgen, oestrogen and GnRH receptor mRNA expression levels depend on social status (Au et al., 2006; Burmeister et al., 2007; Harbott et al., 2007), as do electrical properties of the GnRH neurons themselves (Greenwood & Fernald, 2004).
CHANGES IN THE BRAIN
While the changes in GnRH neuron size and concomitant changes in GnRH production and in hormone receptors are part and parcel of a response to new reproductive opportunity, other brain changes caused by social status are more subtle. As is typical of many social hierarchies, dominant males have high levels of testosterone and low levels of the stress hormone cortisol, and these levels are reversed in nondominant males (Fox et al., 1997). Among other changes caused by social subordination, the stress response inhibits the reproductive axis in many species and results in chronic elevation of stress hormones, which, if sustained, is known to be detrimental (Webster et al., 2008; Kaplan & Manuck, 2004; Kaplan, 2008). Thus, it is a puzzle how socially suppressed nondominant A. burtoni cope with sustained levels of elevated cortisol levels. One possible mechanism for modulating the responsiveness of nondominant individuals to stress would be to change the sensitivity of the cortisol receptors that mediate its potentially damaging effects on the body (Avitsur et al., 2001) and brain (Sapolsky, 1996).
I first showed that A. burtoni has three receptor genes for cortisol: two glucocorticoid receptors (GR1 and GR2) and a mineralocorticoid receptor (MR) (Greenwood et al., 2003). Of these genes, GR1 has two splice variants (GR1a and GR1b) that differ by a nine-amino-acid insertion in the DNA-binding domain in GR1b that greatly reduces binding to cortisol, and has been shown to act as a dominant negative inhibitor of transcription in zebrafish (Schaaf et al., 2008). I have recently shown that when A. burtoni males become nondominant, they replace the high sensitive receptor with this less sensitive receptor in the hypothalamic/pre-optic area, the site of the GnRH neurons (Korzan et al., 2014). This modulation of cortisol receptor subtype expression could mitigate the consequences of socially induced increases in cortisol levels in nondominant males. Just how external social information about the change in social status is transduced into this molecular change in the cortisol receptor remains a mystery.
DOMAINS OF A. BURTONI SOCIAL COGNITION
Living in a colony makes A. burtoni dependent on other animals in multiple ways, competing for food and mates as well as avoiding predators. As males try to find defensible territories and willing mates, they observe and respond to other individuals that regulate their behaviour and their interactions. It is as if they are ‘swimming ethologists’, collecting information on individuals that may figure in their future as competitors or mates. Below I detail how we have assessed how animals collect and use information and where this information is processed in the brain.
Can Males Be Deceptive?
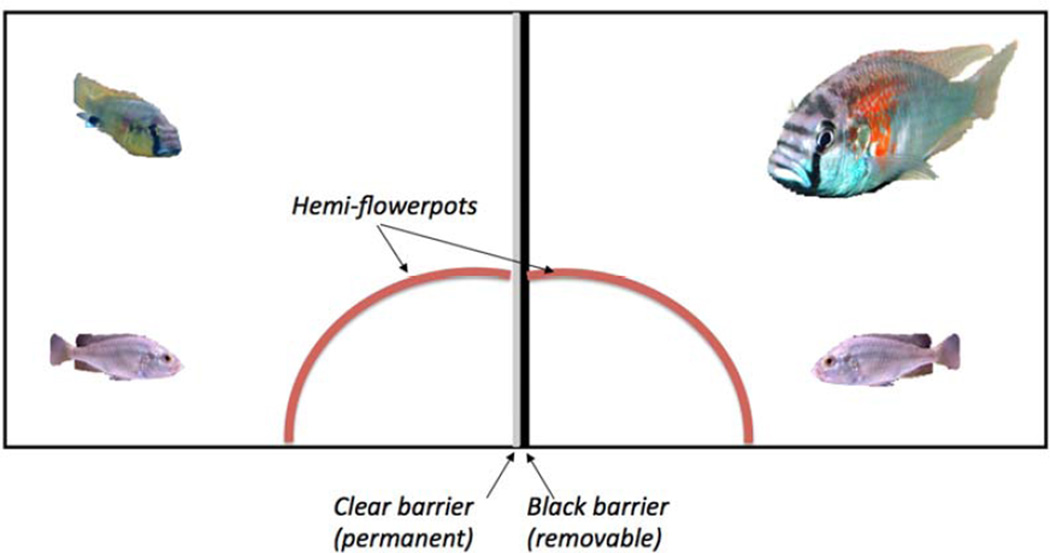
One of the outstanding questions in behaviour is whether animals can be deceptive. I asked whether male A. burtoni could or would deceive one another and under what circumstances. I posed this question using a novel paradigm in which two differently sized males share a tank, divided in half by a clear, watertight divider and a black removable divider (Chen & Fernald, 2011). A half terra-cotta pot was provided, cut in half lengthwise and placed so that it allowed the males on each side to occupy this ‘shared’ shelter, although with the black divider in place, neither animal knew the other animal was present (Fig. 2). One male was about four times larger than the other and each male had an appropriately sized female in their hemi-tank.
Figure 2.
Front view of the aquarium (45 litres) divided in half with a watertight, clear divider (grey midline) and a removable opaque barrier (black midline). There is a small male fish (left compartment) and a large male fish (~4× larger; right compartment). The half terra-cotta pot was cut so that both fish ‘shared’ the same shelter, although they were not aware of each other’s presence. This ‘shared’ shelter was hemi-sected by both centre dividers. A layer of gravel covered the bottom of the tank.
Both the small and large fish were habituated to the condition with the opaque barrier in place for 2 days, during which time each behaved like a normal dominant male in its territory: excavating gravel from their hemi-pot, courting the female in their half of the tank, leading the female back to the shelter and performing typical courtship and territorial male behaviours, all of which were quantified. On the third day, the opaque barrier was lifted, and although there was no physical or chemical contact possible, the larger male made several ‘attacks’ towards the small male, which quickly lost its coloration, including the eyebar. This is typical behaviour for a male losing his territorial status and was confirmed by quantifying the male’s behaviour. Indeed, the smaller males essentially abandoned their part of the shelter, digging a new pit remote from that corner. Interestingly, this suppression of dominance, which is based entirely on visual signals, as reflected by the behavioural quantification (Chen & Fernald, 2011) also reduced the expression of androgen hormones, but only for the first 3 days. Seven days after the black barrier was lifted, the smaller animals recovered their hormone expression levels and other brain markers of dominance while maintaining the coloration of a nondominant male. Moreover, they could be seen courting their females when out of view of the dominant male. So the effects of the visual suppression resulted in changes in the expression of aggressive, territorial behavioural responses by the smaller male but did not result in sustained physiological changes. This suggests that the smaller males uncoupled changes in circulating hormones from their effects on outward appearance, seemingly presenting a false outward appearance not consonant with internal changes. This appears to be an example of deceptive behaviour on the part of the male, allowing him to continue his courtship but not be influenced by the larger male. One can assume that the smaller animal learned that the clear barrier prevented the large male from actual physical attacks and this recognition led the smaller animals to a novel strategy. The small male attended carefully to the dominant male when carrying out his behaviour. The attention that the nondominant male paid to the dominant male is a more general feature that we find in an attention hierarchy these animals have.
Attention Hierarchy Amongst Male Fish
In many instances, individual animals monitor the behaviour of other conspecifics in their group. In particular, subordinate animals attend to the behaviour of dominant animals in what has been called an ‘attention hierarchy’ (Chance & Larson, 1976). Attention hierarchies have been identified in humans, particularly in groups of children (Boulton & Smith, 1990), where individuals modulate their behaviour depending on their own status relative to that of others. Within a hierarchy, when a high-ranking individual attacks a lower-ranking individual, the lower-ranking individual often then subsequently attacks an individual lower in rank (Vaughn & Waters, 1981). In addition to humans, attention hierarchies have been described in baboons and mandrills (Emory, 1976) as well as in reptiles (Summers et al., 2005) and fish (Overli et al., 2004).
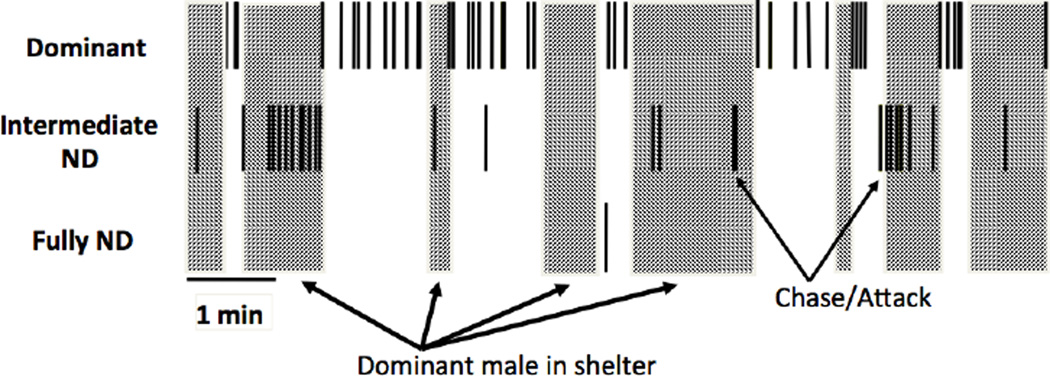
In A. burtoni, we videotaped groups of individually marked animals (N = 20/group; four replicates) and quantified interactions between dominant and nondominant males (Desjardins et al., 2012). We found that dominant and nondominant males never behaved aggressively at the same time. Even more interesting was that when the dominant male was out of view in a shelter, the nondominant males that were larger and attempting to ascend, behaved aggressively and even courted females, behaviours that never occurred when the dominant male could see the nondominant male (Fig. 3).
Figure 3.
Schematic illustration of typical dominant male behaviour in the presence of an intermediate nondominant (ND) male trying to ascend to dominance. Large rectangles represent the dominant male in his shelter and small black bars show when an individual attacks. Note that intermediate males only attack other males when they cannot be seen because the dominant male is in his shelter.
In the example shown in Fig. 3, each time the dominant animal is out of view, the intermediate male attacks another nondominant male until the dominant male reappears. When the dominant male returns to the scene, he attacks within a few seconds but does not specifically target fish that have been aggressive to others in his absence (Desjardins et al., 2012).
Our data show that the nondominant males are attending to the dominant males and altering their behaviour by acting aggressively, which is not possible when the dominant male is present. In addition, these males on occasion will approach and court females when the dominant male cannot see them, another behaviour not possible when a dominant male is present. These behaviours reflect a sophisticated social calculus in which nondominant males are doing the most they can to increase their chances of becoming dominant. It is possible that they are also learning about being dominant through watching, a skill we have also reported elsewhere (Alcazar et al., 2014).
When Being Observed, Males Change Their Behaviour
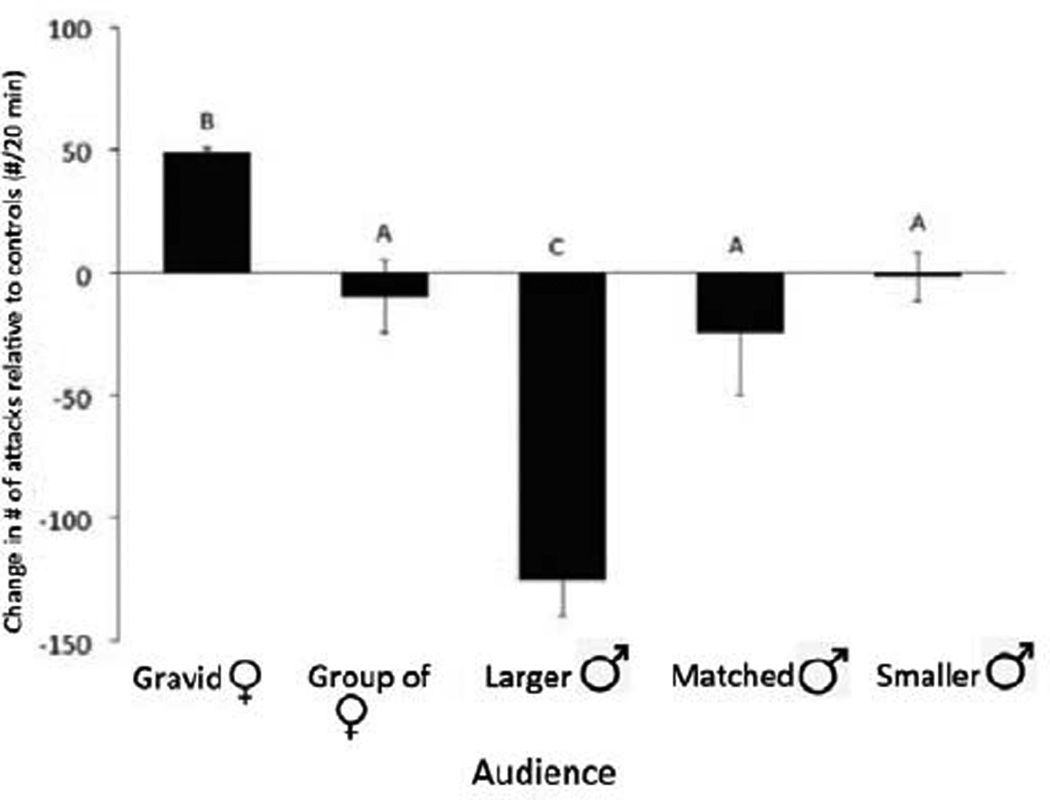
During their lifetimes, like all animals, A. burtoni must continually make important choices about reproduction and survival (Darwin, 1858). Their choices are essential to their reproductive success because, as described above, territorial ownership and defence is the key to reproduction. Both physical and social changes can drastically reorganize social hierarchies by changing the number and location of available territories as animals are preyed upon, exposing fish to new social environments. Storms, intruders and other disruptions of the substrate also contribute to the instability of the social system, so continual maintenance of territory is a critical social behaviour (Fernald & Hirata, 1977). Based on the attention hierarchy described above, we reasoned that animals might alter their behaviour when being observed by conspecifics, consistent with the notion that they could deceive other individuals that are watching them. To test this notion, we designed experiments in which two dominant male fish were in a tank, separated by a clear, watertight barrier and both could be observed from a third compartment. When two size-matched dominant males are placed in these compartments, they will fight through the transparent barrier for the 20 min duration of the experiments while they are observed from a noninteracting audience. We asked how the behaviour of the fighting animals would vary as a function of the composition of the audience and found that the animals that were fighting altered their behaviour depending on the audience (Fig. 4). Aggression is energetically costly and also increases visibility and hence danger from predation, so animals may assess opponents or likely opponents to evaluate competitive ability before engaging in a fight (Enquist & Leimar, 1983; Maynard Smith & Harper, 1988; Parker, 1974).
Figure 4.
Aggressive acts (biting and ramming) displayed by a pair of males being watched by different audiences. Note that males were significantly (α = 0.05) more aggressive when being watched by a gravid female, and significantly less aggressive when being watched by a larger male (marked with B and C, respectively). In contrast, differences in aggression when being watched by a group of females, a matched-size male or smaller males did not differ from control conditions (marked by A). (Redrawn from Desjardins et al., 2012.)
How might audience members interpret such differences in fighting intensities? Fish decreasing their aggressive intensity when being watched by a larger male may allow the audience male to infer that the displaying males are not a threat to him in his future attempts to reproduce. Similarly, increasing aggression in the presence of gravid females might allow females to infer that the fighting males are more dominant than they actually are, increasing the displaying male’s chances for reproduction. This change in aggressiveness could be seen as social manipulation (Getty, 1999), which has been reported in primates (White & Byrne, 1985, 2010), children (Wimmer & Perner, 1983) and fish (Plath et al., 2008). These data extend the view that bystanders are gathering information that may be useful in future encounters.
Transitive Inference by Males
An ongoing goal of nondominant A. burtoni males is to ascend in the social hierarchy to become a dominant, reproductively active male and then to find mates using behaviours evolved for this purpose. In Lake Tanganyika, colonies of burtoni range in size from a few dozen animals to over 100, depending on how many animals the local feeding substrate can support. Males are faced with the challenge of engaging in fights with other individuals to identify which one(s) might be likely targets for a take-over of their territories. However, fighting with tens of animals in order to find a territory holder weak enough to beat would require energetic and physical demands that are prohibitive. Our previous studies revealed that animals paid attention to one another during social encounters, and we wondered whether their observational skills might allow them to predict the outcome of male–male encounters, allowing them to engage in fights they had a chance of winning. That is, could males infer their chances of winning a fight simply from watching other animals fight? The logic of this process, known as transitive inference, is that if you know that A is taller than B and B is taller than C, you can infer that A is taller than C by constructing a virtual cognitive hierarchy without needing to see A, B and C lined up for comparison. This ability was one of the developmental milestones described by Piaget (1928) and has since been identified in humans older than 3 years of age as well as in nonhuman primates (Gillian, 1981; McGonigle & Chalmers, 1977; Rapp et al., 1996), rats (Davis, 1992; Roberts & Phelps, 1994) and birds (Bond et al., 2003; Steirn et al.,1995; von Fersen et al., 1991; Weiss et al., 2010).
To discover whether A. burtoni had the ability to infer fighting abilities of other fish, we tested whether bystander males could synthesize information from observing pairwise fights into an implied hierarchy of male fighting abilities. We tested bystander fish by having them observe staged fights between five size-matched males (A–E) in which A>B, B>C, C>D, D>E, which has the implied hierarchy of A>B>C>D>E (Grosnick et al., 2007). In a specially built aquarium, fights were staged by moving one rival into another rival’s territory, which resulted in the intruder animal losing. For the control animals, there was no implied hierarchy (e.g. A=B=C=D=E). The bystander males were trained on pairwise fights, and we tested their preference between rivals they had never seen together before. When we tested whether the animal chose B or D as a winner, they consistently chose D as the weaker animal based on the prior data (Oliveria et al., 1998; Clement et al., 2005), showing that animals will indicate a choice by moving towards the rival they perceive to be weaker.
The fact that these fish can perform transitive inference in an important situation, choosing which male to attack, is consistent with the behavioural needs and ecological context in their natural habitat. In the temporary shore-pools and estuaries of their native habitat, there is regular disruption of established territories by movement of hippopotamuses, wind and predation (Fernald & Hirata, 1977). So being able to judge their rivals based on featural representations, independent of context would be invaluable for them to increase their chances of reproductive success. We have shown that social ascent upon gaining a territory is swift and activates many behavioural, physiological and molecular processes allowing the ascending male a chance at reproductive success (Burmeister et al., 2005; Maruska & Fernald, 2014). It seems likely that transitive inference could be found in other colony-living animals that face similar constraints on reproduction. This would require designing experimental tests that exploit a natural context and behavioural elements related to behavioural acts in the animal’s natural life.
Genomic Consequences of Female Mate Choice
In females of many species, information about potential mates can change reproductive physiology, including gene expression. Since female mate choice is a large and active field of research in behaviour, it is important to understand how a female brain responds to social information. This specific experimental question depends on deciding both how and where to look for a signal in the brain that reflects female mate choice. We chose to look for changes in brain activity marked by gene expression using an immediate early gene (IEG), egr-1, following a behavioural mate choice paradigm. IEGs are inducible transcription factors that make up part of the first wave of gene expression induced in neurons. Prior work showed that IEG expression is induced by a range of natural experiences including sensory stimuli and, consequently, it has been used extensively in mammals and birds (e.g. Mello et al., 1992; Rusak et al., 1990). More recently, we showed that egr-1 is highly conserved in A. burtoni and that functionally it responds robustly within 30 min of stimulation (Burmeister & Fernald, 2004). As for the brain location, we hypothesized that the conserved vertebrate social behaviour network (SBN) would be a logical place to look. The SBN was originally described by Newman (1999) as a collection of brain nuclei implicated in a variety of social behaviours including male mating behaviour, female sexual behaviour, parental behaviour and aggressive behaviour. Anatomical homologues to this network have subsequently been identified in birds and fish (Goodson, 2005; Goodson & Bass, 2002) and used in a variety of experiments. It was unknown, however, whether they might also respond to social information as well as to behavioural actions. Our previous experiments on female choice had demonstrated that reproductively ready (i.e. gravid) females prefer to associate with dominant, reproductively active males while nongravid females prefer nondominant, nonreproductive males (Clement, 2005).
Using a similar paradigm, we placed females in a tank with one male at each end, behind a clear Plexiglas barrier that was watertight to eliminate chemical cues so the female received only visual information. The female indicated her preferred male by interacting with that male during a 20 min period. Following this, we staged a fight between the two males and at random allowed the chosen male to win or lose that fight. Our control condition was for the female to choose between two males and not see a subsequent fight. I hypothesized that this different outcome would produce distinct patterns of brain gene expression. I then examined the brain gene expression patterns, comparing the mRNA levels of egr-1 and another IEG, cfos, in six brain nuclei constituting the SBN. Surprisingly, females seeing their selected male win or lose a fight produced dramatically different brain IEG expression patterns. Females who saw their preferred male win a fight activated brain nuclei associated with reproduction and reproductive behaviour. Specifically, the anterior hypothalamus, ventromedial hypothalamus, preoptic area and the periaqueductal grey. In striking contrast, females who saw their preferred male lose a fight had a much higher expression of the IEGs that were also in a part of the brain associated with anxiety, the lateral septum (Desjardins et al., 2010). A remarkable aspect of these results is that the female brains were responding to visual information alone. It is important to remember that the IEG expression we measured is surely only a very small part of the total brain response and hence is just a glimpse of the genomic response to social information, but its differential effect on specific brain areas shows that females are activating their brains based on visual information and may use this to guide rapid decisions about what to do.
One additional question is how this information might inform the female’s choice of a mate. In a separate experiment, we performed the same protocol, but after the female had chosen and seen the staged fight, she had to choose again. In this second choice, if she had seen her preferred male lose, she almost always switched her choice, and if he won, she rarely switched her choice (Table 1).
Table 1.
Tabulation of female A. burtoni choices after seeing their preferred male win or lose a fight as compared to their choices after not seeing a fight
| Number of switches/total choices | ||
|---|---|---|
| Preferred male wins | Preferred male loses | |
| Female saw a fight | 2/13 | 11/12 |
| Female did not see a fight | 0/10 | 1/9 |
After seeing her preferred male win a fight, the female rarely switched her choice (Fisher’s exact test: P = 0.0002), but after seeing her preferred male lose, she nearly always switched her choice (Fisher’s exact test: P = 0.0004; Klausner, Desjardins, & Fernald, n.d.).
Summary
This review of research about the social behaviour in A. burtoni has highlighted the important role of social status in regulating the social life in this species. Since A. burtoni is a resource-guarding species with a hierarchy of males, this feature has been the focus of much of the research. Our observations in the field and experiments in the laboratory reveal that these animals collect information on conspecifics and use that information to decide what to do in particular circumstances. Both males and females use information gathered through observation to guide their behaviour. We have followed this information into the brain to identify where it acts using immediate early genes to mark active areas. As expected, males can change quickly what they do depending on what they perceive is happening in their surroundings and that these changes cause corresponding changes in the brain in specific cells, receptors and circuits, preparing the brain of the animal for a phase of life in a new status. How social information is transduced into cell and molecular changes in the brain, however, remains a mystery.
Highlights.
Tanganyikan male cichlids are either dominant (defend, court, reproduce) or nondominant.
Male phenotypes differ in appearance and behaviour and in many features of their brains.
Neurons of males gaining dominance enlarge and those of males losing dominance shrink.
Several other receptors, cell processes and circuits also change.
When females choose a mate, their brain expression patterns reflect that change.
Acknowledgments
I thank the members of my laboratory for their inspired contributions. This research was supported by the National Science Foundation (IOS-0923588), the National Institutes of Mental Health (grant number 087930) and the National Institute of Neurological Disease and Stroke (grant number 034950).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins-Regan E. Hormonal mechanisms of mate choice. Am. Zool. 1998;38:166–178. [Google Scholar]
- Alcazar RM, Hilliard AT, Becker L, Bernaba M, Fernald RD. Brains over brawn: experience overcomes a size disadvantage in fish social hierarchies. Journal of Experimental Biology. 2014;217:1462–1468. doi: 10.1242/jeb.097527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo JJ, McKaye KR, van den Berghe EP. Parental defense of young by the convict cichlid, Archocentrus nigrofasciatus, in Lake Xiloa, Nicaragua. J. Aquaricult. Aquat. Sci. 2001;9:208–228. [Google Scholar]
- Argiolas A. Neuropeptides and sexual behaviour. Neuroscience and Biobehavioral Reviews. 1999;23(8):1127–1142. doi: 10.1016/s0149-7634(99)00068-8. [DOI] [PubMed] [Google Scholar]
- Au TM, Greenwood AK, Fernald RD. Differential social regulation of two pituitary gonadotropin-releasing hormone receptors. Behavioural Brain Research. 2006;170(2):342–346. doi: 10.1016/j.bbr.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Hormones and Behavior. 2001;39(4):247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Balshine-Earn S, Neat FC, Reid H, Taborsky M. Paying to stay or paying to breed? Field evidence for direct benefits of helping behavior in a cooperatively breeding fish. Behav. Ecol. 1998;9:432–438. [Google Scholar]
- Bond AB, Kamil AC, Balda RP. Social complexity and transitive inference in corvids. Anim. Behav. 2003;65:479–487. [Google Scholar]
- Boulton M, Smith PK. Affective bias in children’s perceptions of dominance relationships. Child Dev. 1990;61:221–229. doi: 10.1111/j.1467-8624.1990.tb02774.x. [DOI] [PubMed] [Google Scholar]
- Brännäs E, Eriksson T. Floating, switching, or nonswitching as different behaviours when Arctic charr (Salvelinus alpinus) are visiting two feeding tanks. Can. J. Fish. Aquat. Sci. 1999;56:1068–1077. [Google Scholar]
- Brawand D, Wagner CE, Li YI, Malinsky M, Keller I, Fan S, et al. The genomic substrate for adaptive radiation in African cichlid fish. Nature. 2014;513(7518):375–381. doi: 10.1038/nature13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Laland K, Krause J. Fish cognition and behavior. Chichester: Wiley-Blackwell; 2011. [Google Scholar]
- Burmeister SS, Jarvis ED, Fernald RD. Rapid behavioral and genomic responses to social opportunity. PLoS Biology. 2005;3(11):e363. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Kailasanath V, Fernald RD. Social dominance regulates androgen and estrogen receptor gene expression. Hormones and Behavior. 2007;51(1):164–170. doi: 10.1016/j.yhbeh.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance MRA. Social structure of attention. New York, NY: J. Wiley; 1976. [Google Scholar]
- Clayton DF. The genomic action potential. Neurobiology of Learning and Memory. 2000;74(3):185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- Clement TS, Grens KE, Fernald RD. Female affiliative preference depends on reproductive state in the African cichlid fish, Astatotilapia burtoni. Behav Ecol. 2005;16:83–88. [Google Scholar]
- Clement TS, Parikh V, Schrumpf M, Fernald RD. Behavioral coping strategies in a cichlid fish: the role of social status and acute stress response in direct and displaced aggression. Hormones and Behavior. 2005;47(3):336–342. doi: 10.1016/j.yhbeh.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Collin SP, Marshall NJ, editors. Sensory processing in aquatic environments. Berlin, Germany: Springer Verlag; 2003. [Google Scholar]
- Davis MR, Fernald RD. Social control of neuronal soma size. Journal of Neurobiology. 1990;21(8):1180–1188. doi: 10.1002/neu.480210804. [DOI] [PubMed] [Google Scholar]
- Davis H. Transitive inference in rats (Rattus norvegicus) Journal of Comparative Psychology. 1992;106(4):342–349. doi: 10.1037/0735-7036.106.4.342. [DOI] [PubMed] [Google Scholar]
- Desjardins JK, Fernald RD. Fish sex: why so diverse? Current Opinion in Neurobilogy. 2009;19:648–652. doi: 10.1016/j.conb.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins JK, Klausner JQ, Fernald RD. Female genomic response to mate information. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(49):21176–21180. doi: 10.1073/pnas.1010442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins JK, Hofmann HA, Fernald RD. Social context influences aggressive and courtship behavior in a cichlid fish. PLoS One. 2012;7(7):e32781. doi: 10.1371/journal.pone.0032781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant A, Shpigel M. Interspecific feeding associations of groupers (Teleostei: Serranidae) with octopuses and moray eels in the Gulf of Eilat (Aqaba) Environ. Biol. Fishes. 1985;13:153–159. [Google Scholar]
- Doutrelant C, McGregor PK. Eavesdropping and mate choice in female fighting fish. Behaviour. 2000;137:1655–1669. [Google Scholar]
- Earley RL, Dugatkin LA. Three poeciliid pillars: fighting, mating and networking. In: McGregor PK, editor. Animal communication networks. Cambridge, U.K.: Cambridge University Press; 2005. pp. 84–113. [Google Scholar]
- Emory GR. Aspects of attention, orientation, and status hierarchy in mandrills (mandrillus sphinx) and gelada baboons (Theropithecus gelada) Behaviour. 1976;59:70–87. [Google Scholar]
- Enquist M, Leimar O. Evolution of fighting behaviour: decision rules and assessment strategies. J Theor Biol. 1983;102:387. [Google Scholar]
- Fernald RD. Social control of the brain. Annual Review of Neuroscience. 2012;35:133–151. doi: 10.1146/annurev-neuro-062111-150520. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Communication about social status. Current Opinion in Neurobiology. 2014;28:1–4. doi: 10.1016/j.conb.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald RD, Hirata NR. Field study of Haplochromis burtoni: quantitative behavioral observations. Animal Behaviour. 1977;25:964–975. [Google Scholar]
- Fox HE, White SA, Kao MH, Fernald RD. Stress and dominance in a social fish. Journal of Neuroscience. 1997;17(16):6463–6469. doi: 10.1523/JNEUROSCI.17-16-06463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis RC, Soma K, Fernald RD. Social regulation of the brain–pituitary–gonadal axis. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(16):7794–7798. doi: 10.1073/pnas.90.16.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getty T. Deception: the correct path to enlightenment? Trends in Ecology & Evolution. 1999;12:159–161. doi: 10.1016/s0169-5347(97)89783-2. [DOI] [PubMed] [Google Scholar]
- Gillian DJ. Reasoning in the chimpanzee: II. Transitive inference. J. Exp. Psychol.: Anim. Behav. Process. 1981;7:87–108. [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Hormones and Behavior. 2005;48(1):11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood AK, Fernald RD. Social regulation of the electrical properties of gonadotropin-releasing hormone neurons in a cichlid fish (Astatotilapia burtoni) Biology of Reproduction. 2004;71(3):909–918. doi: 10.1095/biolreprod.104.030072. [DOI] [PubMed] [Google Scholar]
- Greenwood AK, Butler PC, White RB, DeMarco U, Pearce D, Fernald RD. Multiple corticosteroid receptors in a teleost fish: distinct sequences, expression patterns, and transcriptional activities. Endocrinology. 2003;144(10):4226–4236. doi: 10.1210/en.2003-0566. [DOI] [PubMed] [Google Scholar]
- Gross MR, Sargent RC. The evolution of male and female parental care in fishes. Am. Zool. 1985;25:807–822. [Google Scholar]
- Harbott LK, Burmeister SS, White RB, Vagell M, Fernald RD. Androgen receptors in a cichlid fish, Astatotilapia burtoni: structure, localization, and expression levels. Journal of Comparative Neurology. 2007;504(1):57–73. doi: 10.1002/cne.21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladký V, Havlíček J. Was Tinbergen an Aristotelian? Comparison of Tinbergen’s four whys and Aristotle’s four causes. Human Ethology Bulletin. 2013;28(4):3–11. [Google Scholar]
- Hourigan TF. Environmental determinants of butterflyfish social systems. Environ. Biol. Fishes. 1989;25:61–78. [Google Scholar]
- Hughes RN, Blight CM. Algorithmic behaviour and spatial memory are used by two intertidal fish species to solve the radial maze. Animal Behaviour. 1999;58(3):601–613. doi: 10.1006/anbe.1999.1193. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease: a primate continuum. Institute of Laboratory Animal Resources. 2004;45(2):89–115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- Kaplan JR. Origins and health consequences of stress-induced ovarian dysfunction. Interdisciplinary Topics in Gerontology. 2008;36:162–185. doi: 10.1159/000137709. [DOI] [PubMed] [Google Scholar]
- Keenleyside MHA. Diversity and adaptation in fish behavior. Berlin, Germany: Springer-Verlag; 1979. [Google Scholar]
- Klausner JK, Desjardins JK, Fernald RD. Female second choice. (n.d.). Unpublished raw data. [Google Scholar]
- Korzan WJ, Grone BP, Fernald RD. Social regulation of cortisol receptor gene expression. Journal of Experimental Biology. 2014;217(18):3221–3228. doi: 10.1242/jeb.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. Social regulation of gene expression in the African cichlid fish Astatotilapia burtoni. Oxford Handbook of Molecular Psychology. 2014 [Google Scholar]
- Maynard Smith J, Harper DGC. Animal Signals: models and terminology. J. Theor. Biol. 1995;177:305–311. [Google Scholar]
- Givnish TJ, Sytsma KJ, McCune A. How fast is speciation? Molecular, geological and phylogenetic evidence from adaptive radiations of fishes. In: Givnish TJ, Sytsma KJ, editors. Molecular evolution and adaptive radiation. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 585–610. [Google Scholar]
- McGonigle BO, Chalmers M. Are monkeys logical? Nature. 1977;267(5613):694–696. doi: 10.1038/267694a0. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennill DJ, Ratcliffe LM, Boag PT. Female eavesdropping on male song contests in songbirds. Science. 2002;296(5569):873. doi: 10.1126/science.296.5569.873. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior: a node in the mammalian social behavior network. Annals of the New York Academy of Sciences. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, M PK, Burford FRL, CustOdio MR, Latruffe C. Functions of mudballing behaviour in the European fiddler crab Uca tangeri. Anim Behav. 1998;55:1299–1309. doi: 10.1006/anbe.1997.0685. [DOI] [PubMed] [Google Scholar]
- Otter K, McGregor PK, Terry AMR, Burford FRL, Peake TM, Dabelsteen T. Do female great tits (Parus major) assess males by eavesdropping? A field study using interactive song playback. Proc. Biol. Sci. 1999;266:1305–1309. [Google Scholar]
- Øverli ØKW, Höglund E, Winberg S, Bollig H, et al. Stress coping style predicts aggression and social dominance in rainbow trout. Horm Behav. 2004;45:235–241. doi: 10.1016/j.yhbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Pasko L. Tool-like behavior in the sixbar wrasse, Thalassoma hardwicke (Bennett, 1830) Zoo Biology. 2010;29(6):767–773. doi: 10.1002/zoo.20307. [DOI] [PubMed] [Google Scholar]
- Piaget J. Judgement and reasoning in the child. London, U.K.: Kegan, Paul, Trench & Trubner; 1928. [Google Scholar]
- Pitcher TJ, Green DA, Magurran AE. Dicing with death: predator inspection behavior in minnow Phoxinus phoxinus shoals. J. Fish Biol. 1986;28:439–448. [Google Scholar]
- Plath MRS, Tiedemann R, Schlupp I. Male fish deceive competitors about mating preferences. Curr Biol. 2008;18:1138–1141. doi: 10.1016/j.cub.2008.06.067. [DOI] [PubMed] [Google Scholar]
- Plath MSI. Misleading mollies: the effect of an audience on the expression of mating preferences. Comm Int Biol. 2008;1:199–203. doi: 10.4161/cib.1.2.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath M, Richter S, Schlupp I, Tiedemann R. Misleading mollies: surface but not cave dwelling Poecilia mexicana males deceive competitors about mating preferences. Acta Eth. 2010;13:49–56. [Google Scholar]
- Rapp PR, Kansky MT, Eichenbaum H. Learning and memory for hierarchical relationships in the monkey: effects of aging. Behavioral Neuroscience. 1996;110(5):887–897. doi: 10.1037//0735-7044.110.5.887. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Phelps MT. Transitive inference in rats: a test of the spatial coding hypothesis. Psychol. Sci. 1994;5:368–374. [Google Scholar]
- Robinson GE, Fernald RD, Clayton DF. Genes and social behavior. Science. 2008;322(5903):896–900. doi: 10.1126/science.1159277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer AS. The vertebrate story. Chicago, IL: Univ. of Chicago Press; 1959. [Google Scholar]
- Rusak B, Robertson HA, Wisden W, Hunt SP. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;248:1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. The physiological relevance of glucocorticoid endangerment of the hippocampus. Ann NY Acad Sci. 1994;746:294–304. doi: 10.1111/j.1749-6632.1994.tb39247.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress. 1996;1(1):1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Champagne D, van Laanen IH, van Wijk DC, Meijer AH, Meijer OC, et al. Discovery of a functional glucocorticoid receptor beta-isoform in zebrafish. Endocrinology. 2008;149(4):1591–1599. doi: 10.1210/en.2007-1364. [DOI] [PubMed] [Google Scholar]
- Steirn JN, Weaver JE, Zentall TR. Transitive inference in pigeons: simplified procedures and a test of value transfer theory. Anim. Learn. Behav. 1995;23:76–82. [Google Scholar]
- Summers CH, Forster GL, Korzan WJ, Watt MJ, Larson ET, Overli O, et al. Dynamics and mechanics of social rank reversal. J Comp Phys A. 2005;191:241–252. doi: 10.1007/s00359-004-0554-z. [DOI] [PubMed] [Google Scholar]
- Timms AM, Keenleyside MH. The reproductive behavior of Aequidens paraguayensis (Pisces, Cichlidae) Zeitschrift für Tierpsychologie. 1975;39:8–23. doi: 10.1111/j.1439-0310.1975.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. On aims and methods of ethology. Zeitschrift für Tierpsychologie. 1963;20:410–433. [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Chicago, IL: Aldine; 1972. pp. 136–179. [Google Scholar]
- Vail AL, Manica A, Bshary R. Referential gestures in fish collaborative hunting. Nature Communications. 2013;4:1765. doi: 10.1038/ncomms2781. [DOI] [PubMed] [Google Scholar]
- Van den Berghe EP, McKaye KR. Reproductive success of maternal and biparental care in a Nicaraguan cichlid fish, Parachromis dovii. J. Aquaricult. Aquat. Sci. 2001;9:49–65. [Google Scholar]
- Vaughn BEWE. Attention structure, sociometric status and dominance: interrelations, behavioral correlates and relationships to social competence. Dev Psy. 1981;17:275–288. [Google Scholar]
- von Fersen L, Wynne CDL, Delius JD, Staddon JER. Transitive inference formation in pigeons. J. Exp. Psychol. Anim. Behav. Process. 1991;17:334–341. [Google Scholar]
- Webster RI, Majnemer A, Platt RW, Shevell MI. Child health and parental stress in school-age children with a preschool diagnosis of developmental delay. Journal of Child Neurology. 2008;23(1):32–38. doi: 10.1177/0883073807307977. [DOI] [PubMed] [Google Scholar]
- Weiss BM, Kehmeier S, Schloegl C. Transitive inference in free-living greylag geese, Anser anser. Animal Behaviour. 2010;79:1277–1283. [Google Scholar]
- White SA, Nguyen T, Fernald RD. Social regulation of gonadotropin-releasing hormone. Journal of Experimental Biology. 2002;205(17):2567–2581. doi: 10.1242/jeb.205.17.2567. [DOI] [PubMed] [Google Scholar]
- Whiten A, Byrne RW. Machiavellian intelligence: Social expertise and the evolution of intellect. Oxford, U.K.: Oxford University Press; 1985. [Google Scholar]
- Whiten A, Byrne RW. Tactical deception by primates. Behav Brain Sci. 2010;11:233–244. [Google Scholar]
- Wimmer H, Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children. Cognition. 1983;1:103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]