Abstract
Since the discovery of Th1 and Th2 cells in the late 80’s, the family of effector CD4+ helper T (Th) cell subsets has expanded. The differentiation of naïve CD4+ T cells is largely determined when they interact with dendritic cells in lymphoid organs, and cytokines play a major role in the regulation of Th differentiation in the early stages. Recent studies show that the developmental mechanism of certain Th subsets is not fully shared between mice and humans. Here we will review recent discoveries on the roles of cytokines in the regulation of Th differentiation in humans, and discuss the differences between mice and humans in the developmental mechanisms of several Th subsets, including Th17 cells and T follicular helper (Tfh) cells. We propose that the differentiation of human Th subsets is largely regulated by the three cytokines, IL-12, IL-23, and TGF-β.
Introduction
The family of Th subsets has expanded during the past two decades, and currently includes regulatory T cells (Treg), Th17, Tfh, Th9, and Th22 cells. These subsets are largely defined by the cytokines that each subset expresses, except FoxP3+ thymus-derived Tregs and T follicular helper (Tfh) cells. Thymus-derived Tregs are defined by the expression of the transcription factor FoxP3 and their suppressive functions. Tfh cells are often defined by the combination of markers (such as CXCR5, ICOS, PD-1, and Bcl-6) and their follicular localization in vivo, although they function by secreting IL-21 and IL-4 (and IL-10 in some cases) [1].
Studies with in vivo mouse models have significantly contributed to understand the developmental mechanism of each Th subsets. However, significant differences have been introduced in the immune system of humans and mice during more than 60 million years of independent evolution, and conclusions demonstrated in mouse studies are sometimes not fully translated to humans [2]. In the context of Th differentiation, it is becoming clear that the developmental mechanism is not fully shared between mice and humans in certain subsets. In this review, we will summarize the current knowledge on the cytokine conditions promoting the development of each Th subset in humans. We classify the Th subsets into two groups according to the similarities in the developmental mechanism between mice and humans: one with large similarities (Th1, Th2, Th9, and Th22) and the other with some differences (induced Treg, Th17, and Tfh). Then we will discuss how cytokines regulate Th differentiation programs in humans.
Th subsets with similar developmental mechanisms between mice and humans
Th1
IL-12 was discovered in the early 90’s to play the major role for the generation of Th1 cells in both mice and humans [3,4]. In 1995, STAT4 was identified as the major transcription factor mediating the IL-12 signals, and in 2000, the transcription factor T-bet was discovered to be essential for Th1 development [5]. IFN-γ also contributes to the expression of IFN-γ and T-bet via STAT1 activation [5]. These major pathways associated with the generation of Th1 cells are largely shared between mice and humans. For example, Th1 generation is severely impaired in subjects who lack the expression of functional IL-12 and/or IL-12 receptor, due to mutations of IL12B (encoding IL-12p40 subunit common to IL-12 and IL-23), IL12RB1 (encoding the β1 chain for the receptors of IL-12 and IL-23), IRF8 (associated with the development of IL-12-producing dendritic cells (DCs)), and ISG15 (a molecule that acts in synergy with IL-12) [6].
Th2
In early 90’s, IL-4 was discovered as critical cytokines for the generation of Th2 cells in vitro in mice. STAT6 was identified as the main transcription factors downstream of IL-4 signals in 1996, and the transcription factor Gata3 was discovered to be essential for in vivo Th2 development in 1997 [7]. In addition to the IL-4-STAT6, low signals via T cell receptor (TCR) were found to play an important role for the initial expression of Gata3 in activated CD4+ T cells [8]. These mechanisms associated with Th2 development are also largely shared between mice and humans [9]. A recent study identified a set of candidate transcription factors associated with the generation of human Th2 cells through genome-wide profiling of histone modifications in human blood CCR4+ CD4+ T cells (that are enriched with Th2 cells) [10]. The set of the identified transcription factors contains Gata3 and Stat5, but also includes many transcription factors previously not implicated in Th2 cell differentiation. Whether and how these newly identified transcription factors contribute to Th2 cell differentiation in humans and/or mice remain to be determined. It is still possible that eventually the transcriptional network regulating Th2 cell differentiation turns out to be somewhat different between mice and humans.
Th9
Early studies performed in the 90’s demonstrated that IL-9 secretion was largely associated with Th2 cells [11]. However, it was also shown that the cytokine combination of IL-4, TGF-β, and IL-2 can induce naïve CD4+ T cells in vitro to become producers of IL-9, but no other Th2 cytokines [12]. The in vivo presence of CD4+ T cells producing primarily IL-9 was eventually demonstrated in 2008 (termed as Th9 cells), and Th9 cells are found to be associated with antitumor immunity, allergy, and autoimmune diseases [13]. Current evidence shows that both in mice and humans, IL-4-STAT6 and TGF-β play a key role for the generation of Th9 cells as well as IL-9 expression by other Th subsets [14,15]. Furthermore, the transcription factors Gata3, IRF-4, and PU.1. induced by the combination of IL-4-STAT6 and TGF-β signals were shown to be important for IL-9 expression in both mouse and human CD4+ T cells [11]. However, it is of note that recent studies show differences in the regulation of IL-9 expression between mice and humans. For example, in humans, IL-21 promotes IL-9 expression by naïve CD4+ T cells primed in the presence of IL-4 and TGF-β [16]. However, in mice, IL-21 strongly inhibits IL-4 and TGF-β-driven IL-9 expression by promoting the expression of transcriptional repressor Bcl-6 [17]. Thus, the magnitude and the quality of Th9 response is likely regulated in a different fashion between mice and humans due to differences in the biological activity of certain cytokines on CD4+ T cells.
Th22
There is ample evidence that CD4+ T cells that primarily secrete IL-22 but no IL-17 or IFN-γ constitute a subset distinct from Th17 and Th1 cells (termed Th22 cells), in particular in humans. First, Th22 cells express only low amounts of RORγt [18], the transcription factor essential for Th17 cell generation [19], and conversely transfection of RORγt does not promote human CD4+ T cells to express IL-22 [20]. Second, while TGF-β contributes to the generation of Th17 cells (as discussed later), IL-22 expression is inhibited by TGF-β in both humans and mice. This effect (at least in mice) is attributed to the repressor function of the transcription factor c-Maf activated by TGF-β [21]. Third, Th22 clones established from patients with psoriasis were remarkably stable and demonstrated gene profiles distinct from Th1, Th2, and Th17 clones [22]. Yet, a recent mouse study using an IL-22 fate reporter questioned the existence of stable Th22 cells, in contrast to the presence of γδ cells and innate immune lymphocytes that constitutively produce IL-22 [23]. Thus, the stability of Th22 cells might be different between mice and humans.
In humans, IL-22 expression is promoted by IL-23, IL-12, and IL-6+TNFα and inhibited by TGF-β [18,20,24]. However, IL-22+ cells induced in vitro by culturing human naïve CD4+ T cells contain cells co-expressing IFN-γ and/or IL-17A, and the precise mechanism that induces bona fide Th22 cells remains to be established. Given that IL-22 expression is promoted by agonists of aryl hydrocarbon receptor (AhR) and AhR expression is associated with IL-22 expression in both mice and humans [25], AhR signals are likely necessary in addition to cytokine signals for the generation of Th22 cells.
Th subsets that show different developmental mechanisms in humans
Induced Treg
While mouse naïve CD4+ T cells primed in the presence of IL-2 and TGF-β express high levels of Foxp3, and acquire the capacity to suppress T cell response (termed induced Tregs), human naïve CD4+ T cells primed in the same condition do not suppress T cell response despite high expression of Foxp3 [26]. While there is evidence that the addition of retinoic acid or rapamycin to the combination of IL-2 and TGF-β renders human naive CD4+ T cells to become FoxP3+ suppressors [27,28], their stability remains unknown. Whether the conversion of naïve CD4+ T cells into FoxP3+ Tregs can happen in vivo in humans also remains unclear. Human blood FoxP3+ Tregs contain CD45RA+CD25+ “resting” subset and CD45RA− CD25++ “activated” subset [29]. Transcriptional analysis of CD45RA− CD25++ “activated” Tregs at a single cell level might reveal heterogeneous subpopulations which might contain converted Tregs at periphery.
Th17
The observation that mouse CD4+ T cells express IL-17 in response to the combination of IL-6 and TGF-β was first reported in 2006 [19]. Shortly after, however, it was found that this cytokine combination does not induce human CD4+ T cells to express IL-17 [30]. Early studies demonstrated that IL-1β and IL-23 were important to drive the differentiation of human naïve CD4+ T cells into IL-17-producers, and that TGF-β inhibited their generation [31,32]. In contrast, later studies showed that TGF-β is indeed essential for the development of human Th17 cells, and acts together with other cytokines such as IL-1β, IL-6, IL-21, and IL-23, but not IL-12 [20,33]. Indeed, among the human Th17-promoting cytokines, TGF-β is the most potent to induce human cord-blood derived naïve CD4+ T cells to express RORγt (the transcription factor essential for Th17 development [19]) and CCR6 (the chemokine receptor expressed by human Th17 cells [31]) [20,34]. The discrepancy regarding the impact of TGF-β in human Th17 cell generation seem to be derived from the differences in the purity of truly naïve CD4+ T cells and the differences in the culture media used in the experiments. In particular, some serum components including TGF-β and AhR ligand were found to largely affect the expression of IL-17 in cultured human CD4+ T cells [20,35]. Importantly, similar to observations in mice [19], stimulation with TGF-β by itself is insufficient to induce human naïve CD4+ T cells to express IL-17, and needs to co-operate with STAT3-activating cytokines such as IL-21 and IL-23 [20,34,36]. This is further supported by the observation that patients with hyper IgE syndrome who lack the expression of functional STAT3 are completely devoid of Th17 cells [37].
Another line of evidence that TGF-β is important for the generation of human Th17 cells was obtained from studies on CD161+ CD4+ T cells, a cell population proposed to be the direct precursor of Th17 cells in humans [38]. CD161+ CD4+ T cells are already found in newborn thymus and cord blood, and these cells isolated from cord blood constructively express RORγt, IL23R, CCR6, but not IL17. The expression of IL-17 by these cells requires stimulation with a combination of IL-1β and IL-23 together with anti-CD3 and CD28, but not TGF-β [38]. However, after stimulation with IL-1β and IL-23, a majority of CD161+ CD4+ T cells become Th1 cells expressing T-bet and IFN-γ. Supplementation of TGF-β into the culture strongly increases the generation of IL-17+IFN-γ− cells by inhibiting the generation of IFN-γ [39]. Thus, TGF-β plays an important role in skewing the differentiation of human naïve CD4+ T cells towards the Th17 lineage and away from the Th1 lineage [34], even of cells pre-committed to the Th17 lineage.
There are several differences in the type of cytokines regulating the early differentiation stage of Th17 cells between mice and humans. Among STAT3-activating cytokines, while IL-6 plays a dominant role in mice, IL-6 seems to play only a supportive role in humans [36]. In contrast, while IL-23 plays the major role in the differentiation of Th17 cells in humans, IL-23 is more associated in mice with the maturation of generated Th17 cells into inflammatory cells [19]. The difference of the importance of IL-23 in the early generation of Th17 cells is due to the differences in the regulation of IL-23 receptor expression by naïve CD4+ T cells. While mouse naïve CD4+ T cells express IL-23 receptor only after priming in the presence of IL-6 and IL-21 [19], human naïve CD4+ T cells can immediately respond to IL-23 (even those in cord blood), and the IL-23 receptor expression is further enhanced by IL-23 signals [20,30].
Another puzzling difference between humans and mice is the role of IL-2. While IL-2 suppresses IL-17 expression by mouse CD4+ T cells both in vitro and in vivo [40], IL-2 promotes the generation of human Th17 cells in vitro [20] and IL-2 treatment did not affect the number of blood Th17 cells in HIV-infected subjects [41]. Multiple mechanisms are involved in the suppression of mouse Th17 cell generation by IL-2-STAT5: 1) STAT5 competes with STAT3 for binding to the sites in the IL17a locus, 2) IL-2 inhibits the expression of receptors for IL-6, and 3) IL-2 promotes the expression of T-bet, which suppresses the expression of RORγt [42]. Why IL-2 does not inhibit the generation of human Th17 cells remains unknown.
Tfh
In the early 2000s, studies on CD4+ T cells in human tonsils demonstrated that the cells expressing CXCR5 display a superior capacity to induce B cells to produce immunoglobulins in vitro as compared to CD4+ T cells lacking CXCR5 expression. Based on their localization and functions, tonsillar CD4+ T cells were designated Tfh cells [1]. In 2009, the transcription repressor Bcl-6 was discovered as an essential factor for Tfh cell generation in vivo in mice, and TFH cells were established as an independent Th lineage [1].
The differentiation and maturation process of Tfh cells is more complicated than that of other subsets, and composed of three phases: 1. Initial differentiation towards the Tfh-lineage, 2. Migration into B cell follicles and interaction with B cells, and 3. Maturation in germinal centers. Therefore, as compared to mouse models where the generation of Tfh cells can be assessed in vivo, determining the developmental mechanism of Tfh cells in humans has been a challenge. Importantly, however, multiple mouse studies have demonstrated that the differentiation process of Tfh cells initiates when naïve CD4+ T cells interact with DCs loaded with antigens in secondary lymphoid organs [43]. Programming towards the Tfh cell lineage occurs as early as the first few divisions [44–47]. Therefore, to determine the type of cytokines promoting human Tfh cell generation, it is a reasonable strategy to analyze the expression of multiple Tfh molecules (such as CXCR5, PD-1, ICOS, CD40L, Bcl-6, and IL-21) by naïve CD4+ T cells stimulated for a few days in vitro (by anti-CD3 and anti-CD28) in the presence of different cytokines.
Recent studies show that the dominant cytokines associated with the development of Tfh cells differ between mice and humans. In mice, IL-6 and IL-21 (which activate STAT3) have dominant roles [48,49], while IL-12 (which mainly activates STAT4) can also participate in the early phase [50]. In contrast, in humans, IL-12 induces higher expression of IL-21, ICOS, CXCR5 and Bcl-6 on activated naive helper T cells than IL-6, IL-21, and IL-27 [34,51,52]. Consistently, the subjects that lack the expression of functional IL-12 receptor β1 chain (IL-12Rβ1), a common receptor for IL-12 and IL-23, display reduced blood memory Tfh cells and memory B cells, and altered GC formation in lymph nodes [51]. Furthermore, the affinity of IgG against tetanus toxoid in these subjects was significantly lower than that in controls, supporting altered GC responses [51]. Our recent study further identified that TGF-β plays an important role for Tfh cell generation in humans [34]. In the presence of TGF-β, IL-12 and in particular IL-23 promote the expression of multiple Tfh molecules including CXCR5, IL-21 and Bcl-6 on activated naïve CD4+ T cells [34]. TGF-β also contributes to the generation of Tfh cells by strongly suppressing the expression of Blimp-1 [34], a transcription repressor that inhibits the function of Bcl-6 [48]. These cytokine combinations also induce human naïve CD4+ T cells to express multiple other transcription factors such as c-Maf and Batf that are essential for Tfh development. This is in stark contrast to mouse CD4+ T cells, in which TGF-β signals suppress expression of Tfh molecules including IL-21, ICOS and Bcl-6 [34,53–55].
Furthermore, the role of IL-2 might be different between mice and humans. In mice, IL-2-STAT5 strongly inhibits the generation of Tfh cells by promoting the expression of Blimp-1 [56,57] and activating mammalian target of rapamycin (mTOR) [58], while inhibiting the expression of Bcl-6 [59]. Consistently, while CXCR5+Bcl-6+ CD4+ Tfh precursors generated shortly after the interactions with DCs express IL-2Rα only at low amounts, CXCR5−Bcl-6− CD4+ T cells highly express IL-2Rα [45]. Intriguingly, a recent study showed that TGF-β contributes to the generation of Tfh cells in mice infected with influenza virus. Yet, such positive regulation by TGF-β seems dependent on the suppression of the expression of IL-2Rα by primed CD4+ T cells, providing further evidence demonstrating the significance of IL-2 signals in Tfh cell generation in mice [58]. However, in humans at least in vitro, there is no difference in the expression of IL-2Rα between CXCR5+ and CXCR5− cells developed from naïve CD4+ T cells (Schmitt et al, unpublished observations). Whether and to which extent IL-2-STAT5 regulates human Tfh cell generation remains to be determined.
Regulation of the differentiation of human Th subsets
We propose that the development of distinct Th subsets in humans is largely regulated by the three cytokines: IL-12, IL-23, and TGF-β. In this context, the differentiation of Th subsets can be viewed as vectors starting from the origin in a three dimensional model (Figure 1). The three cytokines IL-12, IL-23, and TGF-β define the three axes (herein defined as X, Y, Z axis respectively). First, human Th subsets can be largely split into two groups according to the role of TGF-β in their differentiation: one group promoted by TGF-β (Tfh, Th17, and Th9) and another inhibited by TGF-β (Th1, Th2, and Th22). Second, another two groups can be defined by the role of IL-12 and IL-23: one promoted by IL-12 (Th1, Tfh, and Th22), another promoted by IL-23 (Th17, Tfh), and the other inhibited by IL-12 or IL-23 (Th2 and Th9). In this 3D model, many human Th subsets can be placed on the vertex of the cube.
Figure 1. Regulation of human Th differentiation by IL-12, IL-23, and TGF-β.
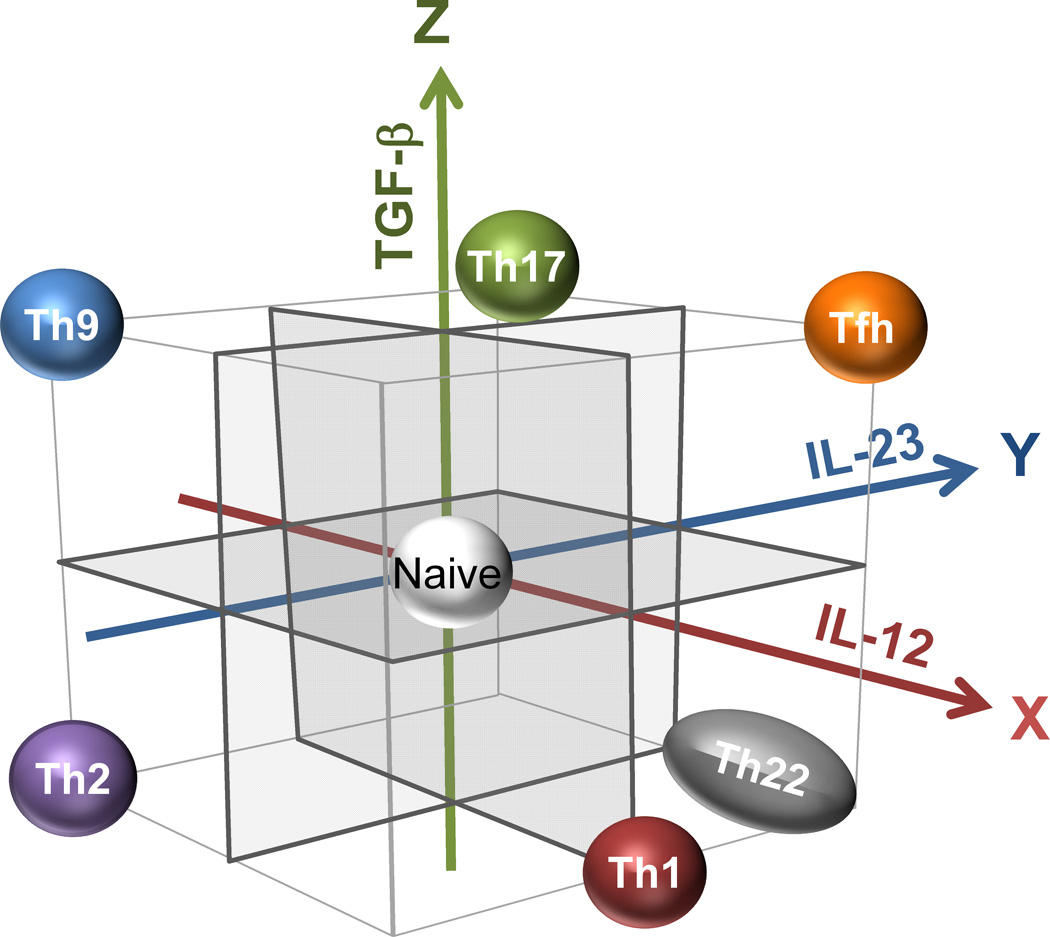
The differentiation of Th subsets can be viewed as vectors starting from the origin in a three dimensional model. The three cytokines IL-12, IL-23, and TGF-β define the three axes. With this model, many Th subsets are placed on the vertex of the cube. Human Th subsets can be largely split into two groups according to the role of TGF-β in their differentiation: one group promoted by TGF-β (Tfh, Th17, and Th9) and another inhibited by TGF-β (Th1, Th2, and Th22). Second, other groups can be defined by the role of IL-12 and IL-23: one promoted by IL-12 (Th1, Tfh, and Th22) or by IL-23 (Th17, Tfh, and Th22), and another inhibited by IL-12 or IL-23 (Th2 and Th9).
This model provides several important concepts. First, the developmental vector is similar between Tfh cells (X+Y+Z+) and Th17 (X−Y+Z+) cells. With this regard, we recently demonstrated that human Th17 cells generated by culturing naïve CD4+ T cells (by the combination of TGF-β, IL-1β, IL-6, and IL-23) were found to express multiple Tfh molecules including CXCR5, ICOS, PD-1, IL-21, and Bcl-6. Furthermore, in human tonsillar germinal centers, some Tfh cells were found to co-express Bcl-6 and RORγt [34]. These observations suggest that the developmental mechanism is largely shared between Tfh and Th17 cells in humans. Whether the common precursors differentiate into mature Th17 cells and Tfh cells might be dependent on the amount of IL-12 exposed during the priming and/or whether the common precursors subsequently interact with B cells or not.
Second, the development of Tfh cells (X+Y+Z+) and Th2 cells (X−Y−Z−) is regulated in totally opposite directions. This is ironic as both subsets are/had been considered as the major Th subsets promoting antibody response. While both subsets can produce IL-4, the molecular mechanism associated with IL-4 production is different at least in mice: The conserved noncoding sequence 2 is an essential enhancer element for IL-4 expression in Tfh cells, but not in Th2 cells [60]. In germinal centers of human tonsils, Tfh cells do not express GATA3, while some co-express RORγt or T-bet together with Bcl-6 [34]. These observations suggest that Th1 and Th17-rich environment inhibits the generation of Tfh cells expressing GATA3.Despite totally different developmental mechanisms, there is evidence that some Th cells display properties of both Tfh and Th2 cells. First, in mice infected with N. brasiliensis, GATA3+ Tfh cells are present in the draining lymph nodes [61]. Second, blood memory Tfh cells in humans contain a subpopulation expressing Th2 cytokines (IL-4, IL-5, and IL-13) and GATA3 [62], although the origin of blood memory Tfh cells remains to be established [63]. However, how Th2-type Tfh cells develop in humans remains unknown. Given a Th2-prone cytokine IL-4 strongly inhibits the expression of IL-21 by human CD4+ T cells [62], probably the their development is regulated by other factors, such as the strength and the duration of signals via T cell receptor during the priming [64], and/or various co-stimulatory molecules expressed by DCs [65].
Conclusions
Some differences have been discovered in the regulation of Th differentiation between mice and humans. There is little doubt that it will be increasingly important to analyze human samples (blood, tissues, urine, body fluids etc) and assess whether the findings in mouse models can be translated to humans. Nonetheless, many mechanisms in Th differentiation are remarkably shared between mice and humans, and thus mouse studies will continue to provide important templates to test the pathways in humans. The differences in the Th development between the species seem due in part to the differences in the regulation of cytokine receptor expression and the differences in the biological impact of cytokines on T cell biology. These differences might be also associated with distinct epigenetic modifications. Yet, the molecular mechanisms that regulate the Th differentiation programs in humans have just started getting revealed. Thorough re-evaluation of the cytokine effect on human Th cells including epigenetic modifications will be useful to understand the regulatory mechanisms of human Th differentiation in depth and beneficial to develop novel therapeutic approaches in diseases.
Highlights.
Development of Th1, Th2, Th9, and Th22 is largely similar between mice and humans.
TGF-β and STAT3 signals promote Th17 differentiation in both species.
Some differences exist in the development of induced Tregs, Th17, and Tfh cells.
Human Th differentiation is largely regulated by IL-12, IL-23, and TGF-β.
Acknowledgements
Supported by the US National Institutes of Health (U19-AI057234, U19-AI082715 and U19- AI089987), the Alliance for Lupus Research, Lupus Research Institute, and the Baylor Health Care System.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. 2015;16:142–152. doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri G, Wysocka M, D'Andrea A, Rengaraju M, Aste-Amezaga M, Kubin M, Valiante NM, Chehimi J. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4:355–368. doi: 10.1016/0955-2235(92)90016-b. [DOI] [PubMed] [Google Scholar]
- 5.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 6.Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol. 2014;26:454–470. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 8.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cousins DJ, Lee TH, Staynov DZ. Cytokine coexpression during human Th1/Th2 cell differentiation: direct evidence for coordinated expression of Th2 cytokines. J Immunol. 2002;169:2498–2506. doi: 10.4049/jimmunol.169.5.2498. [DOI] [PubMed] [Google Scholar]
- 10.Seumois G, Chavez L, Gerasimova A, Lienhard M, Omran N, Kalinke L, Vedanayagam M, Ganesan AP, Chawla A, Djukanovic R, et al. Epigenomic analysis of primary human T cells reveals enhancers associated with TH2 memory cell differentiation and asthma susceptibility. Nat Immunol. 2014;15:777–788. doi: 10.1038/ni.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186:3283–3288. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kuhn R, Muller W, Palm N, Rude E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153:3989–3996. [PubMed] [Google Scholar]
- 13.Schmitt E, Klein M, Bopp T. Th9 cells, new players in adaptive immunity. Trends Immunol. 2014;35:61–68. doi: 10.1016/j.it.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Putheti P, Awasthi A, Popoola J, Gao W, Strom TB. Human CD4 memory T cells can become CD4+IL-9+ T cells. PLoS One. 2010;5:e8706. doi: 10.1371/journal.pone.0008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beriou G, Bradshaw EM, Lozano E, Costantino CM, Hastings WD, Orban T, Elyaman W, Khoury SJ, Kuchroo VK, Baecher-Allan C, et al. TGF-beta induces IL-9 production from human Th17 cells. J Immunol. 2010;185:46–54. doi: 10.4049/jimmunol.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong MT, Ye JJ, Alonso MN, Landrigan A, Cheung RK, Engleman E, Utz PJ. Regulation of human Th9 differentiation by type I interferons and IL-21. Immunol Cell Biol. 2010;88:624–631. doi: 10.1038/icb.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao W, Spolski R, Li P, Du N, West EE, Ren M, Mitra S, Leonard WJ. Opposing actions of IL-2 and IL-21 on Th9 differentiation correlate with their differential regulation of BCL6 expression. Proc Natl Acad Sci U S A. 2014;111:3508–3513. doi: 10.1073/pnas.1301138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. This is the first study describing the presence of human Th22 cells.
- 19.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 20. Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. This study convincingly demonstrates that TGF-β is essential for the generation of human Th17 cells. This study also demonstrates that components contained in culture media largely affect the cytokine expression profiles of cultured CD4+ T cells.
- 21.Rutz S, Noubade R, Eidenschenk C, Ota N, Zeng W, Zheng Y, Hackney J, Ding J, Singh H, Ouyang W. Transcription factor c-Maf mediates the TGF-beta-dependent suppression of IL-22 production in T(H)17 cells. Nat Immunol. 2011;12:1238–1245. doi: 10.1038/ni.2134. [DOI] [PubMed] [Google Scholar]
- 22.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahlfors H, Morrison PJ, Duarte JH, Li Y, Biro J, Tolaini M, Di Meglio P, Potocnik AJ, Stockinger B. IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J Immunol. 2014;193:4602–4613. doi: 10.4049/jimmunol.1401244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpe E, Touzot M, Servant N, Marloie-Provost MA, Hupe P, Barillot E, Soumelis V. Multiparametric analysis of cytokine-driven human Th17 differentiation reveals a differential regulation of IL-17 and IL-22 production. Blood. 2009;114:3610–3614. doi: 10.1182/blood-2009-05-223768. [DOI] [PubMed] [Google Scholar]
- 25.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 26.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu L, Zhou X, Wang J, Zheng SG, Horwitz DA. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-beta and retinoic acid. PLoS One. 2010;5:e15150. doi: 10.1371/journal.pone.0015150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, June CH, Miller JS, Wagner JE, Blazar BR. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11:1148–1157. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Tato CM, Muul L, Laurence A, O'Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 32.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 33. Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. This study provides evidence that TGF-β is important for the generation of human Th17 cells by a multiparametric analysis.
- 34. Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, Banchereau J, Ueno H. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014;15:856–865. doi: 10.1038/ni.2947. This study describes that TGF-β play a positive role in the differentiation of human Tfh cells. An unbiased systematic approach was taken to draw the conclusions
- 35.Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008 doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santarlasci V, Maggi L, Capone M, Frosali F, Querci V, De Palma R, Liotta F, Cosmi L, Maggi E, Romagnani S, et al. TGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur J Immunol. 2009;39:207–215. doi: 10.1002/eji.200838748. [DOI] [PubMed] [Google Scholar]
- 40.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Ndhlovu LC, Sinclair E, Epling L, Tan QX, Ho T, Jha AR, Eccles-James I, Tincati C, Levy JA, Nixon DF, et al. IL-2 immunotherapy to recently HIV-1 infected adults maintains the numbers of IL-17 expressing CD4+ T (T(H)17) cells in the periphery. J Clin Immunol. 2010;30:681–692. doi: 10.1007/s10875-010-9432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011;35:671–680. doi: 10.1016/j.immuni.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, Okada T. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 45.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baumjohann D, Okada T, Ansel KM. Cutting Edge: Distinct Waves of BCL6 Expression during T Follicular Helper Cell Development. Journal of immunology. 2011;187:2089–2092. doi: 10.4049/jimmunol.1101393. [DOI] [PubMed] [Google Scholar]
- 47.Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, Haberman AM. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34:947–960. doi: 10.1016/j.immuni.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 49.Sweet RA, Lee SK, Vinuesa CG. Developing connections amongst key cytokines and dysregulated germinal centers in autoimmunity. Current opinion in immunology. 2012;24:658–664. doi: 10.1016/j.coi.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, Sun HW, Vahedi G, Hakim O, Handon R, et al. Early Th1 Cell Differentiation Is Marked by a Tfh Cell-like Transition. Immunity. 2011;35:919–931. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schmitt N, Bustamante J, Bourdery L, Bentebibel SE, Boisson-Dupuis S, Hamlin F, Tran MV, Blankenship D, Pascual V, Savino DA, et al. IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood. 2013;121:3375–3385. doi: 10.1182/blood-2012-08-448902. This study provides in vivo evidence that IL-12 and IL-23 are important for the generation of human Tfh and GC responses.
- 52. Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. This is the first study demonstrating that IL-12-STAT4 strongly promotes the human Th differentiation towards the Tfh lineage.
- 53.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suto A, Kashiwakuma D, Kagami S, Hirose K, Watanabe N, Yokote K, Saito Y, Nakayama T, Grusby MJ, Iwamoto I, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–1379. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. The Journal of experimental medicine. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marshall HD, Ray JP, Laidlaw BJ, Zhang N, Gawande D, Staron MM, Craft J, Kaech SM. The tumor growth factor beta signaling pathway is critical for the formation of CD4 T follicular helper cells and isotype-switched antibody responses in the lung mucosa. Elife. 2014;4 doi: 10.7554/eLife.04851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat Immunol. 2012;13:405–411. doi: 10.1038/ni.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harada Y, Tanaka S, Motomura Y, Harada Y, Ohno S, Ohno S, Yanagi Y, Inoue H, Kubo M. The 3' enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity. 2012;36:188–200. doi: 10.1016/j.immuni.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35:436–442. doi: 10.1016/j.it.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tubo NJ, Jenkins MK. TCR signal quantity and quality in CD4 T cell differentiation. Trends Immunol. 2014;35:591–596. doi: 10.1016/j.it.2014.09.008. This is an excellent review summarizing the recent findings on the regulation of Th differentiation by TCR signals.
- 65.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012;209:1241–1253. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]


