Abstract
This review summarizes patent applications in the past 5 years for the management of brain tumors and metastases. Most of the recent patents discuss one of the following strategies: the development of new drug entities that specifically target the brain cells, the blood–brain barrier and the tumor cells, tailor-designing a novel carrier system that is able to perform multitasks and multifunction as a drug carrier, targeting vehicle and even as a diagnostic tool, direct conjugation of a US FDA approved drug with a targeting moiety, diagnostic moiety or PK modifying moiety, or the use of innovative nontraditional approaches such as genetic engineering, stem cells and vaccinations. Until now, there has been no optimal strategy to deliver therapeutic agents to the CNS for the treatment of brain tumors and metastases. Intensive research efforts are actively ongoing to take brain tumor targeting, and novel and targeted CNS delivery systems to potential clinical application.
Background
“The brain, the masterpiece of creation, is almost unknown to us.” as quoted by Nicolaus Steno in 1669 [1]. After centuries, the mysteries of this amazingly complex organ still need to be unraveled. The word ‘brain’ appeared for the first time in any language in the Ancient Egyptian medical text entitled Edwin Smith Papyrus [2–4]. It is dated 1500 years before the Christian era during the 16th and the 17th dynasties of the second intermediate period in ancient Egypt. In this papyrus, the first known description of the CNS was detailed [5,6].
The CNS is a delicate and central system, which controls every aspect of our biological routines and interaction with our surroundings. The brain plays a central role in the regulation and control of most of the body functions, including responsiveness, movements, sensations, thoughts, speech, emotion, learning and memory [7,8].
Many patents have been filed in recent years relating to the CNS system in all aspects. This review will focus on the patents of models, diagnosis and treatment, targeting strategies, and novel drug delivery systems that researchers designed to combat the brain tumors and metastases.
The blood–brain barrier: the optimal natural brain self-defense mechanism
The blood–brain barrier (BBB) is a physical and functional barrier limiting passive diffusion of extrinsic agents into the brain [9]. The unique vasculature nature of the BBB compared with blood vessels in the rest of the body represents a major hindrance in the successful delivery of CNS drugs to the brain [10].
Globally, the CNS drug market represents one of the largest, high-selling therapeutic sectors, with sales exceeding almost US$90 billion in 2007 [11]. In the USA alone, the CNS drug market exceeded $55 billion as recorded in 2005 [12]. The CNS drug market is expected to be over $60 billion now in the USA alone [13–15]. In spite of such huge potential, the striving CNS drug development represents a main hurdle against the successful transformation of CNS drugs from bench to bedside. This could be attributed to poor drug delivery techniques and failure of the therapeutic active pharmaceutical ingredients to cross the BBB. Pardridge shed the light on an important common misconception, which is, small molecules can readily cross the BBB [16,17]. In fact, more than 98% of all small molecules and almost 100% of large molecules do not cross the BBB. On the other hand, biologics such as proteins, peptides, genes and oligonucleotides are newer drugs with tremendous potential for treating CNS disorders. However, their transport across the BBB from the systemic circulation is often very much restricted.
The unique hallmark structure of the tight junctions that sealed the brain endothelial cells form the BBB was revealed by electron microscopy more than four decades ago. Tight junctions are highly specific paracellular clefts between cells of the choroid plexus and arachnoid epithelia. The tight junctions, along with the associated astrocytes and neuronal input, create a barrier that significantly hinders paracellular diffusion of polar and/or large molecules into the brain through the BBB (Figure 1).
Figure 1. Cross-section of a brain capillary, which represents the structure of the blood–brain barrier and brain–tumor barrier.
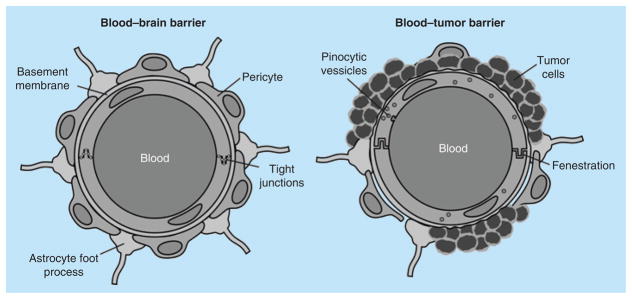
The blood–brain barrier does not represent a distinct organ, but rather is a functional concept.
Other than the physical barrier properties of the tight junctions, functional barriers also exist to protect the brain against the passage of unwanted endogenous and exogenous agents. Tight junctions perform two major functions: gate function to prevent paracellular diffusion of macromolecules, and fence function to separate apical and basolateral fractions of plasma membranes [18]. Astrocytes and pericytes also make the BBB more specific. The astrocytes regulate homeostasis of brain, while pericytes control the formation, differentiation and maintain the integrity of the BBB [19].
Efflux transporters are also considered main functional barriers of the BBB. They significantly limit brain accumulation of several classes of drugs (e.g., anticancer, antiviral, antibacterial, antiepileptic, analgesics and HIV-protease inhibitors) [20–24]. Some of these efflux transporters are P-glycoprotein (P-gp; ABCB1), breast cancer resistant protein (BCRP; ABCG2), multidrug resistance associated proteins (MRP; ABCC1–6), organic anion transporters, equilibrative nucleoside transporter and concentrative nucleoside transporter (CNT-2) [25]. Doxorubicin and paclitaxel are some of the most significantly subjected chemotherapeutics to the active efflux transport mechanisms including p-gp, BCRP and the family of multi-drug resistance proteins, most of which are expressed on the luminal (blood side) BBB surface [22,26]. The major hurdles presented by these efflux transporters against the brain delivery of drugs have been a focal point of research in the last decade.
Another functional barrier is the numerous degradative enzymes present in the BBB, such as phosphatase enzymes. These enzymes aid the biotransformation or inactivation of several molecules, including peptides and neuropeptides [27–29], as they attempt to cross the BBB into the brain parenchyma.
In spite of these physical and functional barriers that protect the brain from the entry of any unwanted agents, the brain vasculature presents other pathways that facilitate the penetration of many wanted compounds, including polar and large molecules, across the BBB. These pathways are essential for the uptake of required nutrients and minerals to the brain to maintain its normal and healthy status. The first major pathway is the influx transporters that translocate various polar nutrients (glucose, amino acids and monocarboxylic acids), vitamins or hormones from the blood into the brain across the BBB [30–32]. Indispensable compounds such as glucose and amino acids utilize carrier-mediated transporters, such as GLUT1 (glucose transmembrane transporter 1), LAT (L-amino acid transporter), cationic amino acid transporter and lactate transporter, to enter the brain parenchyma [33]. The second major pathway is the receptor-mediated transport, which transports various macromolecules (e.g., insulin, leptin, transferrin and so on) across the BBB [34,35]. For example, insulin is transported by the insulin receptor [36] and iron utilizes the transferrin receptor [37], both of which are highly expressed on the capillary endothelium of the brain.
Brain tumors & metastases
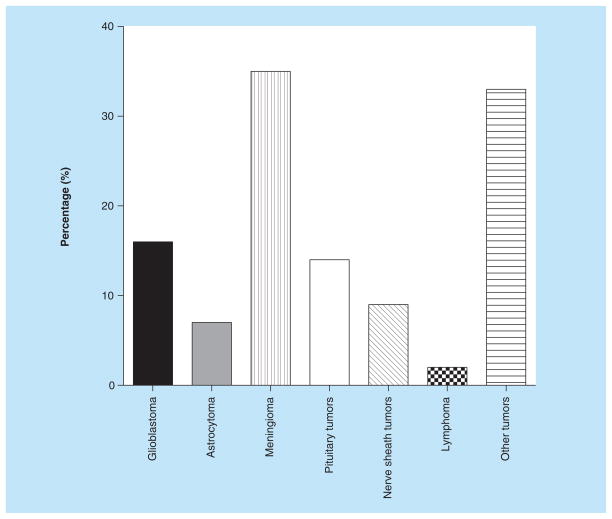
Cancer is a common cause of death in the world; about 10 million new cases occur each year. Moreover, cancer is responsible for 12% of deaths worldwide, making it the third leading cause of death [38]. Brain tumors are generally either a primary brain tumor or a secondary metastasis from a distant primary tumor in another organ. Meningioma, a diverse set of tumors originating from the membranous layers surrounding the CNS (meninges), represent 35% of all primary brain tumors [39]. Glioblastoma and pituitary tumors are the most frequently occurring primary brain tumors aside of meningioma, representing 16 and 14% of primary brain tumors, respectively (Figure 2) [39].
Figure 2. Primary brain tumor types.
Reproduced with permission from [39] © Oxford University Press (2013).
According to the The Central Brain Tumor Registry of the United States statistical report in 2012, an estimate of over 688,000 patients in the USA are diagnosed with a primary brain or CNS tumors; 37% of these tumors are malignant [39]. Primary brain tumors represent about 2% of all cancers and about 25% of pediatric cancers, with an estimated 43,800 new cases of benign and malignant brain tumors diagnosed annually in the USA. The mortality rate from malignant primary brain tumors is very high. Malignant primary brain tumors are the leading cause of death from solid tumors in children and the third leading cause of death from cancer in adolescents and adults aged 15 to 34 years [39]. Glioblastoma multiforme, also known as glioblastoma, is the most common malignant brain tumor in adults, and accounts for approximately 40% of all malignant brain tumors and approximately 50% of gliomas. It is ranked at the highest malignancy level (grade IV) among all gliomas. Its lethal rate is very high, the average life expectancy is 9 to 12 months after first diagnosis. The 5-year survival rate during the observation period from 1986 to 1990 was 8.0%. To date, the 5-year survival rate following aggressive therapy, including gross tumor resection, is still less than 10% [40–44].
Metastatic brain tumors are the most common intracranial neoplasm in adults. Although the exact incidence is unknown, it has been estimated to be as high as 200,000 cases per year in the USA alone. An alarming fact is that approximately 20–40% of all cancers develop brain metastases [39]. This accounts for 98,000 to 170,000 new metastatic brain tumor cases each year in the USA alone. Approximately 30% of all cancer patients have detectable metastases at the moment of clinical diagnosis and a further 30% of patients have occult metastases [39]. Approximately 20% of all brain metastases are single tumors.
Brain metastases usually originate from lung, breast, melanocytes, colon/colorectal and/or kidney primary tumors [45,46]. Brain metastases of lung cancer are the most common type in both men and women. It is usually diagnosed either before or at the same time as the primary lung cancer (6 to 9 months on average) [47,48]. In 2009, an estimated 219,000 cases of lung cancer were diagnosed in the USA. It is the leading cause of cancer deaths and approximately 80% of patients with primary lung malignancy have nonsmall cell lung cancer (NSCLC). Brain metastasis can affect up to 50% of primary lung malignancy patients during their lifetime [47,48].
Brain metastasis of breast cancer is the second common type of brain metastases in women leading to high mortality rates and deteriorated quality of life. Breast cancer is the second leading cause of brain metastasis along with lung cancer, exceeded only by melanoma [49–52]. In spite of being second in line after melanoma in brain metastasis, breast cancer is considered by far the most common due to their significantly greater incidence [52,53]. Brain metastases are found in about 30% of breast cancer patients as reported by Tsukada et al. [54] and other investigators [55–64] via autopsy samples. Recent clinical treatment has focused on developing antagonists for the estrogen receptor, progesterone receptor and human epidermal growth factor receptor to target breast cancer. However, approximately 25% of all patients with breast cancer lack these three receptors and do not respond to standard chemotherapy [52,53].
The brain–tumor barrier: the unpredictable obstacle
The presence of an intracranial primary cancer metastasis modifies the vascular integrity of the BBB both within and around the lesion. The brain tumor vasculature is no longer having the normal BBB characteristics and is generally termed as the blood–tumor barrier (BTB) [65]. In comparison to the normal BBB, the BTB is generally thought to have increased permeability, reduced blood flow and an increased or decreased expression of influx or efflux transporters [66–68].
The changes in BTB vascular permeability are usually not homogenous throughout the lesion. Tumor vascular permeability is variable both within and in between the preclinical metastatic models. Passive permeability changes can moderately predict the uptake of drug into a metastasis, which suggests a continuation of inadequate chemotherapeutic outcomes [65]. Vascular permeability is also not homogenous between metastatic lesions in the same brain. Recently, it was demonstrated that permeability of brain metastases of breast cancer in two experimental model systems are highly heterogeneous, ranging nearly 30-fold between various lesions [65].
However, the vasculature structure within brain tumors is both anatomically and functionally different (Figure 1). On the anatomical level, tumors promote the formation of new deformed blood vessels that lack the classic BBB tight junction structure [69]. The new deformed blood vessels have no astrocytic proper contact, with increased vesicular density [70] and fenestrations or pores that allow the free paracellular passage of molecules into the brain [71–75]. On the functional level, the vasculature within a primary brain tumor typically has reduced expression of tight junction proteins such as ZO-1, which acts as scaffolding protein to anchor another tight junction protein, occludin, to the endothelial membrane [9,76], which results in increased vascular permeability [77,78]. Moreover, during the growth of primary brain tumors or metastases, the cancerous tissues become hypoxic as they grow beyond their blood supply. In this case, the cancerous lesions tend to increase vascular endothelial growth factor (VEGF) production and secretion to initiate new blood vessel formation (or elongate existing vessels) to obtain adequate oxygen and nutrient supplies [79]. The increased VEGF secretion results in the turnover of endothelial cells, the growth of new blood vessels and, ultimately, an increase in permeability [79]. Such VEGF secretion is so concentrated and immediate that the newly created vascular bed is generally tortuous and ineffective [79].
Current methodologies to treat brain metastasis
A large amount of world population is affected by many different types of CNS diseases such as brain tumors, HIV encephalopathy, epilepsy, cerebrovascular diseases, Parkinson’s disease, Alzheimer disease and other neurodegenerative disorders. Surprisingly, despite the advanced ongoing research, the number of patients dying of these fatal CNS diseases far exceeds the number of patients suffering from all types of systemic cancers or cardiovascular diseases [80,81]. The development of successful techniques, which can enhance drug delivery to the brain across the BBB, is essential for the treatment of different CNS disorders.
There are two general strategies adopted to facilitate drug molecules crossing the BBB, invasive and noninvasive techniques, bearing in mind that the BBB is the most challenging hurdle that prevents efficient delivery of therapeutic agents into the brain [13,15,82]. The invasive methods rely primarily on disrupting the BBB integrity by direct intracranial drug delivery through intracerebroventricular, intracerebral or intrathecal administration, osmotic pumps or biochemical means. On the other hand, noninvasive techniques include chemical drug delivery, carrier-mediated drug delivery, receptor/vector mediated drug delivery, intranasal drug delivery, prodrug approach and modification of drug moieties into lipophilic analogs [83].
The design of a successful prodrug for circumventing the BBB involves either lipidization strategy, the use of endogenous transporters in the CNS prodrug design, the use of receptor-mediated prodrug delivery or the use of antibodies in CNS prodrug design [84–85]. The commonly used lipidization strategy incorporate a lipophilic bio-removable targeting moiety that may increase the lipophilicity of drugs to facilitate crossing the BBB followed by locking drugs in the brain preventing them from effluxing out of the BBB. One of the best examples of this lipidization approach is the diacetylated form of morphine and heroin. Heroin is the lipophilic prodrug of morphine that crosses the BBB with about 100-fold higher concentration than morphine [85].
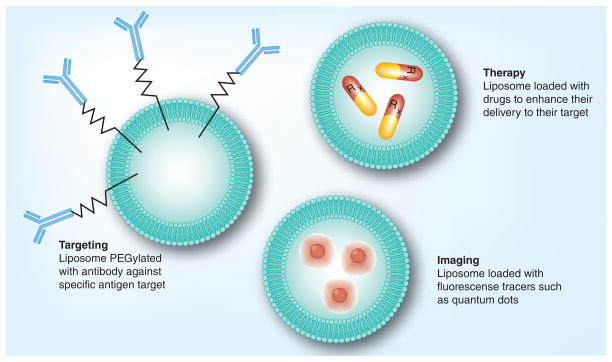
Colloidal based novel drug delivery systems have been widely experimented in brain drug delivery. Such systems include liposomes, nanoparticles, microspheres, nanospheres, nanotubes, micelles and nanoconjugates. These systems might be lipid-, peptide- or polymer-based. Each one of them has its own advantages and disadvantages when it comes to its formulation techniques, stability, versatility, ability to scale-up the formulation and cost. The common advantages of all of these novel carrier systems are their ability to change the physicochemical parameters of the active drug, their ability to enhance tissue specificity and targetability through the use of the proper targeting moieties, and their ability to protect the active drug from degradation and enzymatic inactivation [86]. Most of the current novel colloidal drug delivery systems are nano-engineered and tailor-designed to interact with the target tissues on the molecular level. The potential behind the nano-engineered colloidal drug delivery system is due to the fact that they exhibit totally different physicochemical properties distinct from the original chemical moiety and unique to the new colloidal system. Such new physicochemical characteristics can be tailored based on the proper design of the colloidal carrier system (Figure 3). Such tailor-designed carrier systems can be deployed for diagnosis and/or treatment purposes.
Figure 3. Liposomes as a novel colloidal drug delivery system.
Liposomes can be tailor-designed adopting diverse grafting strategies on its surface or modifications within the liposomal composition to provide tissue specificity, diagnosis and targetability.
Various targeting ligands have been deployed to target the brain such as transferrin, RI7217 (Transferrin Receptor Antibody), COG133 (Apolipoprotein E), angiopep-2 (Peptide sequence TFFYGGSRGKRN-NFKTEEY) and CRM197 (nontoxic mutant of diphtheria toxin). These brain targeting ligands have been tested with different nanoparticles and lipid vesicular systems. The main mechanism of cell entry for targeted delivery systems is receptor-mediated endocytosis, which uses targeting ligands that specifically bind to receptors expressed on the brain endothelial cells. The drug of interest can be either directly conjugated to the ligand or encapsulated into nanoparticles coupled to the ligand on the outside either with or without the use of a spacer [87,88].
Treatment & diagnosis of brain tumors & metastases: current patents overview
The current patents status is always a significant indicator on the forthcoming technologies that will mainstream products in the future. A scan of the last 5 years (2008–2013) registered patents portfolio discussing brain tumors and metastases revealed the broad outlines of future anticipated products and technologies proposed by various research investigators and major drug companies, such as Galaxosmithkline Inc., Genentech Inc., Merck Inc., biOasis Inc., Nektar therapeutics and Alza Corporation. The Supplementary Information lists the major patents discussing brain tumors and metastases, which fall in six broad different categories: diagnosis of brain tumors and metastases, new drug entities for the treatment of brain tumors and brain metastases, vaccination against brain tumors and metastases, targeting strategies for the treatment of brain tumors and metastases, novel combination therapies and, finally, novel drug delivery systems for the treatment of brain tumors and metastases. Some of these patents fall within more than one of these categories due to the overlapping nature of the mechanism of action and design language.
Diagnosis of brain tumors & metastases
Sukumar et al. studied claudins as methods for diagnosis and treatment of breast cancer metastasizing to the brain or the bones [89]. Claudins are a group of proteins, some of which are over expressed (claudins 3 and 4), while others are under expressed (claudins 1 and 7) in the breast cancer metastatic cells. Sukumar et al. proposed immunohistochemical detection of these proteins as a means of diagnosing metastatic breast cancer [89]. Diagnosis, along with treatment, strategies include enterotoxins that detect the over-expressed claudins 3 and 4, bind to them and cause cell lyses (Clostridium perfringens enterotoxin), antibodies that bind to claudins 3 and 4 and trigger immunological reactions leading to destruction of the cancerous cells by the cytotoxic T-cells, and antibodies attached to cytotoxic agents, which bind to claudins 3 and 4 found in the metastatic cancer cells but not in the normal brain cells. As hypothesized by the patent investigators, re-introduction of claudin 7 into the breast cancer cells diminishes their ability to metastasize, because claudin 7 is a main sealing protein in the tight junctions between the cells, which stick them together and prevent their detachment from the tumor. Claudin 7 can be introduced via gene therapy or protein delivery as proposed by the patent’s investigator [89].
In another patent, Arap et al. studied targeting aminopeptidase A (APA) in treatment and diagnosis of cancer [90]. In malignant gliomas and metastatic brain tumors, APA is over expressed and enzymatically active. APA is a proteolytic enzyme involved in pathological angiogenesis. It is found in tumor vascular endothelium, while absent in the normal blood vessels. As proposed by the patent’s investigator, peptides and antibodies specific to APA, coupled with diagnostics and/or therapeutic agents, can be used to diagnose the cancer and its metastasis, together with delivering the therapeutic agent specifically into the tumor. Therapeutic agents can be chemotherapeutics, radio therapeutics or viral vectors targeting gene therapy [90].
In another patent owned by Genentech, Inc. (a member of F. Hoffmann-La Roche group), Patel et al. investigated the use of cancer biomarker (C-met) in diagnosis and treatment of NSCLC and its metastases [91]. According to the American Cancer Society, there were expected to be approximately 185,000 Americans diagnosed with NSCLC in 2007. Around 80% of all patients with NSCLC surviving for more than 2 years will have brain metastases. The investigators performed randomized Phase II clinical trials using a combination of C-met antibody (Met MAb) (onartuzumab) and erlotinib (TARCEVA®) (epidermal growth factor receptor-EGFR antagonist) on patients with second and third line NSCLC. Studies showed that in patients with high levels of C-met biomarker, the treatment was useful with a doubled survival rate when compared with erlotinib alone. However, the case worsened and death occurred in patients with low levels of C-met biomarker, when compared with erlotinib alone. As concluded by the investigators, the detection of the level of C-met biomarker by immunehistochemistry is an essential pretreatment step to predict the susceptibility of the patient to the treatment [91].
In another patent, a method and a kit for the identification of NSCLC patients at higher risk of brain metastasis were described using miRNA expression. Weiss et al. isolated specific miRNA from patient serum sample, which is over expressed in NSCLC patients at higher risk of brain metastasis [92]. The sample is then added to a reagent, such as a stem loop oligonucleotide, capable of specific binding to a marker that includes a certain sequence on the sample miRNA. A reverse transcriptase is then used to form a DNA template comprising the marker, then a second and a third oligonucleotide that bind to opposite strands of the DNA template are added [92].
Marchetti et al. developed a method for diagnosing, and hence treating, brain metastatic breast cancer using a sample from the individual circulating tumor cells (EpCAM negative peripheral blood mononuclear cells compromising one or more of the following genetic markers HER2/neu; EGFR; uPAR; ALDH1; cytokeratins; CD44high/CD24low; vimentin; and CD45) [93].
Another patent described a method for diagnosing brain tumor by detecting calcitonin receptor in brain cells, where higher levels of calcitonin receptor is diagnostic, prognostic and/or predictive of brain tumor as claimed by Wookey et al. [94]. This invention also provides a method for treating brain tumors by a compound that binds to calcitonin receptor and is conjugated to a biological response modifier; a lymphokine, a cytokine or a growth factor or a cytotoxic agent; a toxin, a chemotherapeutic agent or a radioactive agent [94].
Wong et al. provided a noninvasive molecular diagnostic tool, performed on a previously excised tumor biopsy sample, that can predict the likelihood of brain metastasis of breast cancer and, hence, tailor a customized treatment plan unique for a particular tumor [95]. Proteins are extracted from the tissue sample to determine the expression of one or more genetic markers, a 31 protein signature, that have been predictive of brain metastasis of breast cancer [95].
Badve et al. provided a method for diagnosing patients with HER2+ cancers who are at an elevated risk for developing brain metastasis within 3 years of having been diagnosed with HER2+ cancer [96]. The patent described collecting a sample of the tumor tissue from patients with probes that selectively bind to an expression product of at least one gene selected from a group of 13 genes, that are expressed in these patients at markedly higher levels than in control patients who are not at a heightened risk for developing brain metastasis within this 3-year window. The sample is processed by extracting the total RNA from the primary tumor, which is reverse transcribed to form a set of cDNA molecules corresponding to the RNA in the tumor sample, amplifying the cDNA and contacting the amplified cDNA with a set of probes that selectively bind to the expression products of the following set of genes: MYC, CDK4, CCNC, PTK2, BARDl, RAD 51, FANCG, PCNA, PRCC, TPR, EMS1, DSP and HDGF [96].
Massague et al. in another patent provided a method for predicting the risk of brain metastatic breast cancer and, hence, identifying the suitable treatment using two sets of genes and their encoded proteins, one set of 17 genes/proteins and the other of 18 genes/proteins [97]. These genes/proteins are found to be differentially expressed relative to a control value and hence are suitable targets for therapy [97].
Pamplona et al. provided a method for determining the risk of developing brain metastatic breast cancer [98]. The method involves isolating a sample from the breast tumor, determining the level of expression of GRP94, FN14 or both in the sample and comparing the determined level with the level of these genes in a control sample. An over expression of these genes, in respect of the control sample, would be indicative of the risk of developing brain metastasis [98].
New drug entities for the treatment of brain tumors & brain metastases
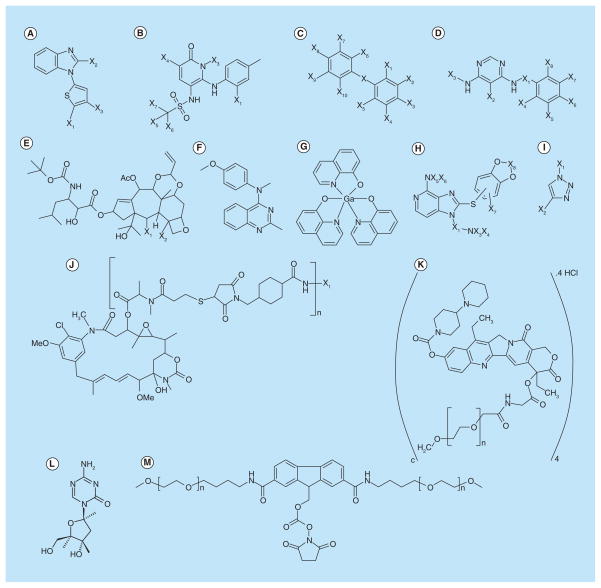
In a patent owned by Smithkline Beecham Corporation, Mohammed Dar suggested a novel method for the treatment of primary and/or secondary (metastatic) CNS tumors using benzimidazole thiophene compounds having the general formula illustrated in Figure 4A [99].
Figure 4. Novel compounds for the treatment of brain tumors and metastases.
(A) Benzimidazole thiophene compounds; (B) Di-hydro pyridine sulphonamides and di-hydro pyridine sulphamides derivatives; (C) Diphenylmethane derivatives; (D) Substituted pyrimidine compounds; (E) (4-Methoxy-phenyl)-methyl-(2-methyl-quinazolin-4-yl)-amine hydrochloride; (F) P97-Trastuzumab monoclonal antibody conjugate; (G) Trastuzumab-MCC-DM1 antibody-drug conjugate; (H) NKTR-102; (I) Taxane derivative; (J) KISS-1 polypeptides and nonpeptide water-soluble polymer conjugate (n = 2 to 4000); (K) HSP 90 inhibitor(s) containing fused amino pyridine core; (L) Cytidine analogs 5-aza-2′-deoxycytidine (decitabine); and (M) Tris(8-quinolinolato)gallium(III)).
As proposed by the inventor, Benzimidazole thiophene compounds have the ability to inhibit a series of serine/threonine kinases, named polo-like kinases, especially polo-like kinase-1. These kinases play a crucial role in the entry and exit of the cell into and from mitosis. Once inhibited, the normal entry, normal progression and normal exit of the cell through mitosis (M-phase) is inhibited, thus preventing the tumor growth. Another advantage of the benzimidazole thiophene compounds, as hypothesized by the inventor, is their ability to penetrate the CNS and cross the intact BBB [99].
Dilea et al., in a patent owned by Novartis Pharma GmbH, investigated the use epothilones A and B in the treatment of primary and metastatic cancers, especially those refractory or resistant to TAXOL® and 5-fluorouracil [100]. Epothilones are microtubule stabilizing agents, given in a loading dose followed by maintenance doses, and proved to show activity against multi-drug resistant cancer cells, especially those of the lung, breast, colon, rectum, prostate, ovaries, head and neck [100].
Bleam et al. investigated the use of BRaf inhibitor in the treatment of V600 mutant melanoma and its metastases including brain metastasis [101]. Mutations in the BRaf enzyme, which is a key enzyme in Ras signaling pathway, result in abnormal cellular growth and proliferation leading to melanoma. As proposed by the inventors, BRaf inhibitors can be used in combination with mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) inhibitors to overcome the resistance that can be built against BRaf inhibitors [101]. This patent is owned by Glaxosmithkline Corporation.
Evans-Freke investigated the use of corticotrophin releasing factor (CRF) for the treatment of cancer [102]. The inventor found that CRF can prevent tumor growth and development, and can reduce the size of the tumor, mainly brain tumors. Also patients with metastatic tumors were responsive to this treatment. CRF acts by altering the biological activity of the tumors including growth, metabolism, cell division and apoptosis. The inventor also suggested that CRF can be used alone or in combination with angiogenesis inhibitors such as bevacizumab [102].
Another US patent filled by Bonny investigated the inhibition of JNK transduction pathway in the treatment of cancers [103]. JNK belongs to the stress activated kinases of the mitogen activated protein (MAP) kinases. These kinases affect the oncogenic transformation of the cells, together with apoptosis. Inhibitors of the JNK transduction pathway include upstream kinase inhibitors (e.g., CEP-1347), small chemical inhibitors (e.g., AS600125 and AS601245, which compete with the ATP binding site), peptide inhibitors, which compete with the JNK substrate, and compounds that encode inhibitory amino acid sequences, for example, D-JNK1 inhibitor, chimeric peptides or nucleic acids [103].
Vernier et al. studied the use of di-hydro pyridine sulphonamides and di-hydro pyridine sulphamides in treatment of cancers (Figure 4B) [104]. They act as MEK inhibitors. MEK enzymes are serine/threonine and tyrosine kinases that play an important role in MAP kinase (MAP-K) activation resulting in increased cellular proliferation and enhanced expression of proto-oncogenes (such as c-fos), leading to tumor development. The inventors suggested that those chemical compounds act by allosteric inhibition at the MAP-K binding site, rather than by blocking the ATP binding site [104].
A US patent applied by inventors Park et al. described the use of miRNA as a method for the treatment of brain, cervical and breast cancers and their metastases, mainly concerned with brain cancers and their recurrence due to metastasis [105]. Mir-125, as described by the inventors, are miRNA molecules that down-regulate the secretion of VEGF and, thus, act as antiangiogenic factors. These miRNAs are down-expressed in hypoxic cancers. Therapeutic mir-125 are introduced into the cells via transfection using a vector, usually a viral vector (lentivirus), although sometimes a nonviral vector can be used [105].
In a US patent owned by Kinex Pharmaceuticals, Hangauer et al. provided a method for treating brain cancer, most specifically glioblastoma, using certain pharmaceuticals as illustrated in Figure 4C [106]. Treatment may include a reduction in tumor size or a reduction in metastatic extent of cancer cells [106].
Moskal et al. provided a method for treating neurological diseases, specifically brain cancer, where sialylation and/or glycosylation of proteins are involved [107]. It comprises transfection of an isolated nucleic acid molecule encoding a protein having sialyl- or glycosyltransferase (‘glyco-enzyme’) activity into a target cell. This alters the pattern of glycosylation or sialylation and accordingly can decrease the ability of the cell to proliferate or function or increase the ability of the host immune system to recognize the target cell [107].
Struck proposed a method for treating primary and metastatic malignant brain tumors using 4-per-oxycyclophosphamide (PeroxyCPA) [108]. As claimed by the inventor, PeroxyCPA is superior to cycolphosphamide due to its higher lipophilicity and, hence, greater transport across the BBB [108].
In another US patent, Battista et al. suggested the use of substituted pyrimidine compounds as tyrosine kinase inhibitors for treating cancer, including primary or metastatic brain tumors (Figure 4D) [109]. The tyrosine kinase family, such as EGFR, HER-1 and HER-2 kinase proteins, is overexpressed in primary and metastatic brain tumors [109].
McChesney et al. used a taxane derivative to treat brain cancer (Figure 4E) [110]. This novel taxane drevative can cross BBB, stabilize tubulin dimers and microtubules at G2-M interface during cell division, and is not a substrate of multidrug resistance protein (P-glycoprotein). Multidrug resistance protein (P-glycoprotein) genetic code encodes the expression of the efflux transporter P-gp, which pumps drugs such as docetaxel and paclitaxel outside of brain cells [110].
In a US patent registered by O’Neill et al. [111], the inventors provided a method for treating metastatic cancers using cholorotoxin, a peptide found in the venom of deathstalker, a yellow scorpion of species Leiurus Quinqestriatus. They claimed that cholorotoxin can inhibit neovascularization and/or cause regression of existing newly formed vasculature that are crucial for nurturing metastases [111]. This patent was owned by TransMolecular, Inc. until 2011. TransMolecular, Inc. advertized its oncology product pipeline based on chlorotoxin sequences as a revolutionary adjuvant therapy for cancer patients. As of March 2011, TransMolecular, Inc. was acquired by Morphotek, Inc., along with all their proposed products and patents portfolio. TransMolecular, Inc. was founded in 1996 and was based in Cambridge (MA, USA). The strength of the TransMolecular, Inc. patent portfolio was the driving force for Morphotek, Inc. to acquire it.
Laughlin registered a method for treatment and/or prophylaxis of brain and spinal cord tumors by using (4-Methoxy-phenyl)-methyl-(2-methyl-quinazolin-4-yl) amine hydrochloride as a potent tubulin inhibitor and cytotoxic agent (Figure 4F) [112]. The inventors claim that these novel compounds can achieve adequate concentrations in the brain and CNS [112].
Remarkably, the same proposed (4-Methoxy-phenyl)-methyl-(2-methyl-quinazolin-4-yl)-amine hydrochloride is also mentioned in another patent by Laughlin [113]. These sets of patents are owned by Myriad Genetics, which was founded in 1991 as one of the first genomic companies developing novel molecular diagnostic and therapeutic products to improve patient healthcare.
Sheshbaradaran et al. proposed methods for treating or preventing brain cancer using a novel pharmaceutical compound (tris(8-quinolinolato)gallium(III), Figure 4G) in an acceptable salt form [114]. The inventors claimed superior antitumor activity for the proposed compound. This patent was owned by Niiki Pharma, which was acquired along with its patents portfolio by Intezyne Technologies Inc.
Sodium meta arsenite was proposed by Jo et al. in a US patent for the treatment and/or prophylaxis of brain tumor [115]. This compound is able to cross the BBB, therefore eliminating the need to use osmotic BBB disruption, commonly used with chemotherapeutic agents, to treat brain tumors [115]. This patent is owned by the Korean biotechnology pharmaceutical company, Kominox, Inc.
Cai et al. registered a method for treating brain and lung related cancers by using certain HSP 90 inhibitors containing fused amino pyridine core [116]. The inventors claimed these compounds have good to excellent brain tissue deposition (Figure 4H) [116].
Targeting strategies for the treatment of brain tumors & metastases
The use of targeting moieties for the BBB and/or brain tumors has huge potential in increasing the efficacy of brain chemotherapeutics. Zhang investigated the potential to treat breast cancer brain metastasis by targeting 1,6 fructose biphosphatase enzyme (FBP) [117]. The inventor found that the breast cancer metastatic cell line survived glucose free medium as opposed to non-metastatic cells. The brain interstitial spaces are low in glucose and high in glutamine and branched chain ketoacids. For cancer cells to survive this low glucose medium, they undergo gluconeogenesis, by using branched chain ketoacids as a source of energy as well as a source of nucleotides required for cell proliferation. FBP is a rate limiting step enzyme in gluconeogenesis. It converts 1,6 fructose biphosphate into fructose 6 phosphate, needed in energy production (via the conversion to glucose 6 phosphate and glycolysis) and in nucleotide production (via pentose phosphate pathway). By using FBP inhibitors, the metastatic cells lose the ability to produce both energy and nucleotides, and, thus, they are unable to survive. FBP inhibitors include benzimidazole derivatives, aminopyridines, indole and azaindole inhibitors, sulfonyl ureido thiazoles, tricyclic thiazoles, usnic acid and 5’adenosine monophosphate mimics [117].
Wolfe et al. proposed the use of humanized Tetanus Toxin C fragment (TTC) as a carrier for the delivery of drugs into the CNS [118]. TTC is the tetanus non-toxic fragment responsible for the attachment to the neurons. In this patent, the inventors suggested the coupling of the TTC amino acid sequence and a fusion protein (the protein that has the therapeutic effect), or the administration of the nucleic acids encoding both TTC and the therapeutic fusion protein in a vector (mainly a viral vector or nanoparticles) to result in high accumulation in the brain [118].
In a US patent owned by biOasis Inc., Hutchison et al. investigated the benefits of coupling P97 polypeptide to antibodies against HER2/neu and Her1/EGFR in treatment of cancers metastasizing to the brain (Figure 4I) [119]. Trastuzumab monoclonal antibody (anti HER2) is already used in treating breast cancer, but it is unable to cross the BBB, so it was unable to treat metastasis to the brain. The systemic administration of trastuzumab conjugate with P97 showed 1000-times higher therapeutic efficacy in treating metastatic brain tumors compared with trastuzumab alone and reduction in the cardio-toxic side effects of trastuzumab [119].
In another patent owned by Genentech Corporation, the anti-HER2 antibody trastuzumab was used as a targeting moiety. Agresta et al., in this patent, developed a method for treating breast cancer patients with antibody-drug conjugate, trastuzumab-MCC-DM1 (Figure 4J) [120]. It’s a novel antibody-drug conjugate specifically designed for the treatment of HER2-positive breast cancer. It is composed of the cytotoxic agent DM1 (a thiol-containing maytansinoid anti-microtubule agent) conjugated to trastuzumab via lysine side chains. The inventors hypothesized that, after binding to HER2, T-DM1 undergoes receptor-mediated internalization, resulting in intracellular release of DM1 and subsequent cell death [120]. As of August 2013, the Swiss global health-care company F. Hoffmann-La Roche AG completed the full acquisition of Genentech Corporation after completing its purchase on March 26, 2009 for approximately $46.8 billion.
Genentech owns another US patent, which suggested the use of the anti-HER2 antibody trastuzumab as a targeting moiety in a novel combination therapy. In this patent, Paton et al. investigated the treatment of HER-2 positive metastatic breast cancer in patients who did not receive chemotherapy or biotherapy/immunotherapy [121]. The HER receptor family plays an important role in cellular differentiation and survival. In the case of HER-2 positive metastatic breast cancer, HER-2 receptors are over-expressed, leading to uncontrolled proliferation. HER-2 activation occurs in two steps: stimulation by extracellular signals and hetero-dimerization with HER-1 under the influence of epidermal growth factor. Treatment, as described by the inventors, depends on a combination of growth inhibitory HER-2 antibody (trastuzumab, an anti 4D5 epitope, stimulates the destruction of cancer cells by the immune cells), HER2 dimerization inhibitor antibody (pertuzumab, which is an anti 2C4 epitope) and Taxane (Decetaxel®) [121].
Mueller-Hermelink et al. [122], in a US patent owned by Patrys Limited, studied the benefits of PM-2 antibody and its related functional fragments in reduction or inhibition of metastasis. The functional fragments retain one or more of the actions of PM-2 antibody, together with having about 1–5000-fold binding affinity higher than PM-2 antibody, thus giving better treatment outcomes. PM-2 antibody has multiple actions including inhibition of tumor cell growth, induction of apoptosis, reduction of disseminated tumor cells and inhibition of metastasis [122].
In another patent, also owned by Patrys Limited, Vollmers et al. described the use of another targeting antibody, LM-1, and its functional peptide fragments in treating cancer metastasis [123]. LM-1 target antigens are expressed in various tumor cells. Those antigens include NONO/nmt 55 protein fragments, whose presence indicates the presence of neoplasma. LM-1 antibodies and the functional fragments inhibit the tumor growth, induce apoptosis of the cancer cells and reduce metastasis. The functional fragments have the advantage that they have a higher binding affinity than the original LM-1 antibody. These peptides can also be provided via nucleic acid sequences that encode them [123].
Nektar therapeutics proposed a passive targeting strategy through enlarging the therapeutic brain chemotherapeutic agent [124,125]. The same concept was introduced in other patents owned by Nektar therapeutics [126–129]. Nektar therapeutics developed a novel irinotecan (topoisomerase I inhibitor) pro-drug conjugate in a polymer form, Etirinotecan Pegol (NKTR-102, Figure 4K). As claimed by the company, NKTR-102 has two main advantages over irinotecan. The first merit is enlarging irinotecan molecular size to prevent its passage through the normal endothelia, but enabling its penetration through the leaky tumor endothelia. NKTR-102 would, afterwards, hydrolyze and release its drug load. This represents an innovative way offering a passive targeting strategy. The second advantage of this novel NKTR-102 prodrug is its ability to modify the distribution of irinotecan, decreasing the Cmax while prolonging the plasma circulation time (when compared with irinotecan). Nektar therapeutics performed Phase II clinical trials on Etirinotecan Pegol (NKTR-102). Data from Phase II clinical trials showed that NKTR-102 is highly active against metastatic breast cancer [125].
A novel target for cancer, particularly breast cancer and glioma, and malignant secondary tumors resulting therefrom is the calcitonin gene related peptide receptor (and its component protein subunits) as described by Dickerson et al. [127]. In this patent, the inventors described a method of treating breast cancer using a modulator of calcitonin gene related peptide receptor activity, which kills or inhibits cancer cell growth and, hence, prevents metastasis [127].
Novel drug delivery systems for the treatment of brain tumors & metastases
As discussed earlier, the use of lipid-based or polymeric novel carrier systems to aid shuttling drugs across the BBB is a promising tool in CNS drug delivery. It is even surprising that not only the nature of the carrier system is critical for the optimal delivery to the CNS, but also the design language of every aspect of the delivery system starting from the nature of the polymer or lipid to external coating material [128,129].
Engbers et al. [130], in a US patent owned by Alza Corporation, proposed a method for treating primary brain cancer, using at least one topoisomerase inhibitor, camptothecin or camptothecin derivative (CKD-602) of about 0.10 μM drug per μM lipid concentration, entrapped in a liposomal vesicular system. The proposed liposomal formulation composed of at least about 20 mole% of vesicle forming lipid and 1 mole% of a vesicle forming lipid derivatized with a hydrophilic polymer, polyethylene glycol of Mwt between 500–5000 daltons, that is distributed on both sides of liposome bilayer. The main aim of the use of liposomal carrier system is to enhance the drug and/or formulation PK parameters, and hence its efficacy. CKD602 camptothecin derivative showed PK advantages in plasma and tumors when compared with the nonliposomal formulation of CKD-602 at 1/30 of the original dose. In addition, the results of the study were consistent with the improved antitumor efficacy and therapeutic index of CKD602 compared with nonliposomal CKD-602. The ideal pharmacologic characteristics of a liposomal, nanoparticle or conjugated anticancer agent are prolonged circulation of the encapsulated drug in the blood or plasma, high tumor delivery and the release of drug from the carrier into the tumor extracellular fluids. As claimed by the inventors, CKD602 meets all of these pharmacologic and PK criteria [130].
Sinko et al. [131], in a US patent owned by Rutgers University, proposed the use of Gel Microparticles that contain nanoparticles containing a cytotoxic agent, an agent that enhances cellular uptake (targeting agent) and an agent that enhances the pro-apoptotic action of chemotherapeutics (chemo potentiator) as a synergistic combination in treatment of NSCLC and its metastasis. Gel Microparticles, being large in size, become retained and accumulated within the lung (entrapped about 90%). The targeting moiety used is DV3, a peptide sequence that is known to interact with CXCR4, which binds to pro-metastatic chemokine receptors, delivering the drug into the cell, together with preventing the metastatic cascade (dual action, targeting and therapeutic). The expression of CXCR4 is associated with distant NSCLC metastases and shorter survival times [131].
In another US patent owned by the Berlin-based German nanomedicine company Magforce Nano-technologies AG, Thiesen et al. proposed the use of paramagnetic or super paramagnetic nanoparticles contained within an implantable biodegradable carrier matrix introduced post-surgical at the operative field (site of surgery) in thermo-therapeutic treatment of cancers, ensuring the destruction of any remaining cancer cells and inhibiting tumor re-growth or metastasis [132]. The paramagnetic nanoparticles, under the effect of an external magnetic field, start to generate heat. This heat is sufficient to kill the neighboring cancer cells. Also, cytotoxic agents can be incorporated in the matrix to give a dual action (both thermo- and chemo-therapy). Such formulation have many advantages including decreased recurrence of the cancer, limited side effects, more precise drug targeting and lower doses of the chemotherapeutic agent [132].
Novel combination therapies for the treatment of brain tumors & metastases
Over the course of the continuous fight against cancer, combinational therapy has shown great potential in enhancing anticancer activity via decreasing cytotoxic drug dose, resistance and side effects, despite the initial higher cost [133].
In a US patent owned by the French National Institute of Health and Medical Research (Institut National de la Santé et de la Recherche Médicale), Barthomeuf et al. described a method for enhancing the clinical efficacy of Docetaxel (Taxotere®, DTX) in treating Pgp-resistant primary and metastatic brain tumors [134]. The inventors proposed the use of Docetaxel (Taxotere, DTX) in combination with a selected curcuminoid. The selected group of curcuminoids could be curcumin, demethoxycurcumin or bisdemethoxycurcumin [134].
In a US patent owned by Celgene Corporation, Nguyen et al. proposed methods for treating, preventing or managing NSCLC using a combination of two or more active agents, including 5-azacytidine [135]. The proposed cytidine analogs are 5-azacytidine (azacitidine) and 5-aza-2′-deoxycytidine (decitabine) (Figure 4L).
Cao et al. developed a method for treating brain tumors by a compound demonstrating one or more of these activities: selective inhibition of the pathological production of human VEGF, inhibition of tumor angiogenesis, tumor related inflammation, tumor related edema and/or tumor growth or prolongation of the G1/S phase of cell cycle [136]. The compound would be administered in combination with one or more additional therapies to a human in need of such treatment [136].
In another US patent owned by HoffMan La Roche Ltd., Swamy et al. investigated the effect of antiangiogenesis therapy on patients with previously treated metastatic HER2-negative breast cancer [137]. They performed randomized Phase III clinical trials using a combination of anti-VEGF (bevacizumab, Avastin®) and chemotherapeutic regimens (mainly of taxane, gemcitabine, vinorelbine and/or capecitabine). The study showed that the combination therapy resulted in better progression free survival than the chemotherapy alone [137]. Remarkably, on the same time frame of the approval of this patent (November, 2011), the US FDA announced that breast cancer indication for Avastin (bevacizumab) had been withdrawn after concluding that the drug had not been shown to be safe and effective for the treatment of breast cancer [138].
Vaccination against brain tumors & metastases
A patent for treating a variety of cancers using a vaccine medication was registered by Immatics Biotechnologies GmbH. In this patent, Weinschenk et al. suggested the use of immunotherapeutic peptides in immunotherapy of cancer [139]. The invention detailed the isolation and use of tumor specific and associated T-helper cell peptide epitopes, alone or in combination with other tumor-associated peptides that serve as active pharmaceutical ingredients of vaccine compositions, which stimulate antitumor immune responses. This vaccine comprises either a peptide of a certain sequence that induces T-cells cross-reacting with variant peptide with a human leukocyte antigen subtype, a nucleic acid encoding this peptide, an antigen presenting host cell, a dendritic cell comprising the nucleic acid, activated cytotoxic T lymphocytes contacting in vitro cytotoxic T lymphocytes with antigen loaded human major histocompatibility complex molecules or an artificial construct mimicking an antigen-presenting cell [139].
Miscellaneous patents
Minamitani et al. investigated the effect of altering the PK parameters of brain chemotherapeutic agents on brain uptake kinetics, which is registered as a US patent owned by Nektar therapeutics [140]. The inventors explored the effect of coupling KISS-1 polypeptides with a non-peptide water-soluble polymer in increasing the circulating half-life of KISS-1 peptides and thus prolonging its action (Figure 4M). KISS-1 peptide is a 54 amino acids peptide that suppresses the metastasis of melanoma and breast carcinoma. The main problem is its very short half-life, which limits its therapeutic benefits. Covalent coupling with 1, 2, 3 or 4 water-soluble nonpeptide polymers such as polyethylene glycol and polypropylene glycol was shown to increase the circulating half life of KISS-1 polypeptide, together with the advantage of being biodegradable and nonimmunogenic [140].
Miyazono et al. [141], in a US patent owned by the University of Tokyo, proposed a method for reducing the tumorigenicity/cellularity of brain tumor stem cells by promoting differentiation, using a TGF-β receptor antagonist. TGF-β pathway maintains the stem cellularity of brain tumor stem cells. This invention proposed the use of an active pharmaceutical ingredient that can reduce the tumorigenicity of glioma stem cells or raise sensitivity to anti-cancer drugs and radiation. This invention suggested the induction of the TGF-β-Sox4-Sox2 pathway in glioma cancer stem cells and, thus, supplying a differentiation promoter for them [141].
The South Korean company, Biomedics, owns another interesting novel US patent. The inventor, Kim, presented the use of genetically engineered human stem cells encoding therapeutic genes for treating an individual suffering from primary brain tumors (glioma and medulloblastoma) and brain metastases of extracranial cancers [142]. The inventor proposed the use of a pharmaceutical formula containing a suitable carrier together with neural stem cells, which migrate selectively to the site of the tumor and there they express two genes: a cytokine gene (interferone B gene) or a suicide gene, expressing cytosine deaminase enzyme. Upon the concomitant administration of 5-fluorocytosine (a non-cytotoxic compound), it is selectively metabolized in the cancer cells by cytosine deaminase to the cytotoxic metabolite 5-fluorouracil, resulting in tumor cell death [142].
Conclusion & future perspective
The global CNS pharmaceutical market is expected to grow by over 500%, overtaking the cardiovascular therapeutics market in the next era [13,143]. During the past 5 years, there has been a huge surge in the development of novel strategies for management of brain tumors and metastases, as illustrated by the set of patents discussed in this review article.
90% of the current patents’ portfolio falls in one of the following broad categories:
Development of new drug entities that specifically target the brain cells, the BBB and/or the tumor cells. This is usually tailored through cracking the code of these cells’ various cellular and biomolecular mechanisms. The applied development and design of these new chemical entities are fueled by the understanding of the special and selective biochemical nature of the target cells, either brain or tumor, and selectively reverse-engineering and tailor-designing of novel drug moieties that target the selective differences these cells have. These new chemical entities can be either therapeutic or diagnostic;
Tailor-design a novel carrier system that can multitask and multifunction as a drug carrier, targeting vehicle and even as a diagnostic tool;
Direct conjugation of an FDA approved drug with a targeting, diagnostic or PK modifying moiety. Such bioconjugation would enhance the drug efficacy, safety and targetability. Moreover, the bio-conjugation would aid decreasing the dose of the drug, enhancing the efficacy and diminishing the side effects;
The use of innovative nontraditional approaches such as genetic engineering and stem cells.
It is expected that the global CNS pharmaceutical market would grow significantly overtaking the cardiovascular therapeutics market in the next 10 years [13,143]. While there has been much work in trying to help facilitate a drugs uptake across the BBB into the brain, there has been no single approach that has worked for all drugs. The lack of proper technology to effectively cross the BBB or the blood–tumor barrier prevents researchers from providing the proper effective therapeutics for the majority of patients with brain disorders. Further research is required to take brain targeting and CNS delivery systems from bench to bedside and to overcome the hurdles facing the successful development of an effective formulation. There are challenges ahead to resolve the question of delivery of the drugs to CNS. More research is required, and is currently in progress, to address these challenges and to resolve the question of drug delivery across the BBB. A long way of optimization and evaluation is still needed before potential clinical applications.
The intellectual properties of the majority of the patents enlisted in this review article belong to big and startup pharmaceutical and nanomedical companies from all around the globe. The strength of the patent portfolio of each pharmaceutical firm represents its main wealth and value. The number and importance of the patents owned by small startup pharmaceutical firms are the driving force for big pharmaceutical companies to acquire them to gain access to their patents, future products, clinical trials and all associated research with such proposed products. Over the course of the last decade, many acquisitions have taken place in this critical field of research in the pharmaceutical industry. For these reasons, startup pharmaceutical and nanotechnology companies try to increase their patent portfolio to increase their commercial value for possible buyouts by big pharmaceutical firms such as Hoffman La Roche, Bayer, Genentech and so on. Such acquisitions show the importance of this area of research and, most importantly, highlight the global need for effective and safe pharmaceutical chemotherapeutic agents that have the potential to target the brain tumors and metastases with minimal toxicity and side effects. Furthermore, the benefits of such inventions would go far beyond cancer therapy, to aid treatment of complicated CNS diseases such as Alzheimer’s disease, Parkinson’s disease, epilepsy, meningitis, encephalitis, Huntington’s disease, multiple sclerosis, infections and many others.
On the other hand, the revocation of Avastin (Bevacizumab, Genentech, Inc.) FDA approval on November 2011 is an alarming red light in this field, and highlights the need for a careful clinical trial methodology for safety and efficacy. The FDA approval revocation was due to the lack of efficacy significance in postmarketing clinical trials and the reported adverse effects. The FDA commissioner, Dr. Margaret Hambury, stated in the revocation alert that there was no significant clinical proof that Avastin would help patients live longer or bring enough significant benefits to outweigh the therapy’s dangerous adverse effects. The Avastin therapy generated some life-threatening risks such as high blood pressure, massive bleeding, heart attack, heart failure, and tears in the stomach and intestine, as concluded by the FDA. Genentech, part of the Swiss giant drug-maker Roche Group, was severely affected by such decision. The company is still undergoing research regarding Avastin efficacy and safety for resubmission to the FDA for possible reconsideration. Furthermore, this case highlights that the fast-track approval and marketing of a pharmaceutical product before careful clinical trials could yield a huge financial loss and negative publicity to the inventor pharmaceutical firm. This case is considered as an inflection point in the FDA approval process for formulations with targeting moieties and antibodies. In spite of the discouraging outcomes of the Avastin revocation by the FDA, such action might be motivating for the pharmaceutical companies to perform further research in order to improve the drug safety/efficacy and/or find other promising potentials, especially for a commercial perspective. In 2011, Chi-not et al. described a protocol for the AVAglio Phase III registration trial [144], which is designed to evaluate the efficacy and safety of bevacizumab in patients with newly diagnosed glioblastoma. Besides, in the 2013 ASCO Annual Meeting, Gilbert et al. presented an abstract indicating that the addition of Avastin for newly diagnosed glioblastoma did not improve survival, but did improve the progression-free survival [145]. They declared that further interpretation of the progression-free survival results incorporating symptom burden, quality of life and neurocognitive function is ongoing.
Executive summary.
Background
The lack of proper technology to effectively cross the blood–brain barrier or the blood–tumor barrier prevents researchers from providing the proper effective therapeutics for the majority of patients with brain disorders.
During the past 5 years, there has been a huge surge in the development of novel strategies for management of brain tumors and metastases, as illustrated by the set of patents discussed in this review article.
Current patents’ categories
Development of new drug entities that specifically target the brain cells, the blood–brain barrier and/or the tumor cells. This is usually tailored through cracking the code of these cells’ various cellular and biomolecular mechanisms. The applied development and design of these new chemical entities is fuelled by the understanding of the special and selective biochemical nature of the target cells, either brain or tumor, and selectively reverse-engineering and tailor-designing novel drug moieties that target the selective differences these cells have. These new chemical entities can be either therapeutic or diagnostic.
Tailor-design a novel carrier system that can multitask and multifunction as a drug carrier, targeting vehicle and even as a diagnostic tool.
Direct conjugation of a US FDA approved drug with a targeting, diagnostic or PK modifying moiety. Such bioconjugation would enhance the drug efficacy, safety and targetability. Moreover, the bioconjugation would aid decreasing the dose of the drug, enhancing the efficacy and diminishing the side effects.
The use of innovative nontraditional approaches such as genetic engineering and stem cells.
Conclusion
There are challenges ahead to resolve the question of delivery of drugs to the CNS.
Current patents’ portfolio shows the importance of this area of research and, most importantly, highlights the global need for effective and safe pharmaceutical chemotherapeutic agents that have the potential to target the brain tumors and metastases with minimal toxicity and side effects.
Further research is required to take brain targeting and CNS delivery systems from bench to bedside and to overcome the hurdles facing the successful development of an effective formulation.
Key terms
- Astrocytes
Specialized star-shaped glial cells that provide physical and nut ritional functions to neurons and clean up debris within the brain
- Brain metastasis
Tumor that has metastasized to the brain from other distant locations in the body. It usually originates from lung, breast, melanocytes, colon/colorectal and/or kidney primary tumors
- Human epidermal growth factor receptor
Human epidermal growth factor family normally controls different cellular effects such as growth, proliferation and survival. Their inappropriate activation or increased expression is strongly related to the spread of cancer. They include 4 tyrosine kinases: HER1, HER2, HER3 and HER4
- Blood–tumor barrier
Modified blood–brain barrier vascular integrity in the presence of an intracranial primary cancer metastasis, within and around the lesion, including increased permeability, reduced blood flow and an increased or decreased expression of influx or efflux transporters
- Vascular endothelial growth factor
Protein that represents the key regulator of physiological angiogenesis
- Brain targeting ligands
Compounds that are conjugated to the drug of interest, either directly or on the outside of nanoparticles encapsulating the drug, with or without a spacer. They specifically bind to receptors expressed on the brain endothelial cells and internalized mainly by receptor-mediated endocytosis. They include transferrin, RI7217, COG133, angiopep-2 and CRM197
- Proto-oncogenes
Normal genes that can be transformed into oncogenes upon mutation, causing cells to divide in an uncontrollable way. They code for proteins that help to regulate cell growth and differentiation
- Calcitonin gene related peptide receptor
37 amino acid neuropeptide, widely distributed in the sensory nerves and acts as a potent vasodilator at the microvascular level. It regulates the blood flow in physiological and patho-physiological situations
Footnotes
For reprint orders, please contact reprints@future-science.com
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.future-science.com/doi/full/10.4155/PPA.14.19
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.BBC Radio 4. In Our Time. The Brain. www.bbc.co.uk/programmes/b00b54yx.
- 2.Kamp MA, Tahsim-Oglou Y, Steiger HJ, Hanggi D. Traumatic brain injuries in the ancient Egypt: insights from the Edwin Smith Papyrus. J Neurol Surg A Cent Eur Neurosurg. 2012;73(4):230–237. doi: 10.1055/s-0032-1313635. [DOI] [PubMed] [Google Scholar]
- 3.Stiefel M, Shaner A, Schaefer SD. The Edwin Smith Papyrus: the birth of analytical thinking in medicine and otolaryngology. Laryngoscope. 2006;116(2):182–188. doi: 10.1097/01.mlg.0000191461.08542.a3. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan R. The identity and work of the ancient Egyptian surgeon. J R Soc Med. 1996;89(8):467–473. doi: 10.1177/014107689608900813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jex HS. The Edwin Smith Surgical Papyrus: first milestone in the march of medicine. Merck Rep. 1951;60(2):20–22. [PubMed] [Google Scholar]
- 6.Cunha F. The Edwin Smith surgical papyrus. Am J Surg. 1949;78(2):277. doi: 10.1016/0002-9610(49)90346-3. [DOI] [PubMed] [Google Scholar]
- 7.Packer RJ, Macdonald T, Vezina G. Central nervous system tumors. Hematol Oncol Clin North Am. 2010;24(1):87–108. doi: 10.1016/j.hoc.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Mineta K, Nakazawa M, Cebria F, Ikeo K, Agata K, Gojobori T. Origin and evolutionary process of the CNS elucidated by comparative genomics analysis of planarian ESTs. Proc Natl Acad Sci USA. 2003;100(13):7666–7671. doi: 10.1073/pnas.1332513100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Toth A, Veszelka S, Nakagawa S, Niwa M, Deli MA. Patented in vitro blood-brain barrier models in CNS drug discovery. Recent Pat CNS Drug Discov. 2011;6(2):107–118. doi: 10.2174/157488911795933910. [DOI] [PubMed] [Google Scholar]
- 11.Celanire S, Campo B. Recent advances in the drug discovery of metabotropic glutamate receptor 4 (mGluR4) activators for the treatment of CNS and non-CNS disorders. Expert Opin Drug Discov. 2012;7(3):261–280. doi: 10.1517/17460441.2012.660914. [DOI] [PubMed] [Google Scholar]
- 12.Boado RJ, Zhou QH, Lu JZ, Hui EK, Pardridge WM. Pharmacokinetics and brain uptake of a genetically engineered bifunctional fusion antibody targeting the mouse transferrin receptor. Mol Pharm. 2010;7(1):237–244. doi: 10.1021/mp900235k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pathan SA, Iqbal Z, Zaidi SM, et al. CNS drug delivery systems: novel approaches. Recent Pat Drug Deliv Formul. 2009;3(1):71–89. doi: 10.2174/187221109787158355. [DOI] [PubMed] [Google Scholar]
- 14•.Li Y, He H, Jia X, Lu WL, Lou J, Wei Y. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials. 2012;33(15):3899–3908. doi: 10.1016/j.biomaterials.2012.02.004. Highlights the importance of tailor-designing the drug carrier system to bypass the blood–brain barrier (BBB) defense mechanisms. The authors used bioresponsive dendritic nanocarrier system decorated with dual targeting moieties to effectively treat brain gliomas. [DOI] [PubMed] [Google Scholar]
- 15••.Costantino L, Tosi G, Ruozi B, Bondioli L, Vandelli MA, Forni F. Chapter 3 - colloidal systems for CNS drug delivery. Prog Brain Res. 2009;180:35–69. doi: 10.1016/S0079-6123(08)80003-9. Highlights the novel colloidal carrier systems used to effectively deliver molecular therapeutics across the BBB into the brain. It provides a comprehensive overview of the novel pharmaceutical nano-formulations in efficiently targeting the problematic CNS. [DOI] [PubMed] [Google Scholar]
- 16.Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12(1–2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Curr Opin Pharmacol. 2006;6(5):494–500. doi: 10.1016/j.coph.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Gloor SM, Wachtel M, Bolliger MF, Ishihara H, Landmann R, Frei K. Molecular and cellular permeability control at the blood-brain barrier. Brain Res Brain Res Rev. 2001;36(2–3):258–264. doi: 10.1016/s0165-0173(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 19.Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59(6):454–477. doi: 10.1016/j.addr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 20••.Smith QR. Drug delivery to brain and the role of carrier-mediated transport. Adv Exp Med Biol. 1993;331:83–93. doi: 10.1007/978-1-4615-2920-0_14. One of the first studies to highlight the importance of carrier mediated transport to efficiently shuttle therapeutic agents across the BBB. The results reported in this study show the benefits of carrier-mediated transport in enhancing brain delivery of a wide variety of hydrophilic therapeutic agents. [DOI] [PubMed] [Google Scholar]
- 21.Shen H, Damcott CM, Rampersaud E, et al. Familial defective apolipoprotein B-100 and increased low-density lipoprotein cholesterol and coronary artery calcification in the old order amish. Arch Intern Med. 2010;170(20):1850–1855. doi: 10.1001/archinternmed.2010.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76(1):22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13(6):1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 24.Begley DJ. ABC transporters and the blood-brain barrier. Curr Pharm Des. 2004;10(12):1295–1312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- 25.Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res. 2003;61:39–78. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- 26.Thomas FC, Taskar K, Rudraraju V, et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res. 2009;26(11):2486–2494. doi: 10.1007/s11095-009-9964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witt KA, Gillespie TJ, Huber JD, Egleton RD, Davis TP. Peptide drug modifications to enhance bioavailability and blood-brain barrier permeability. Peptides. 2001;22(12):2329–2343. doi: 10.1016/s0196-9781(01)00537-x. [DOI] [PubMed] [Google Scholar]
- 28.Minn A, Ghersi-Egea JF, Perrin R, Leininger B, Siest G. Drug metabolizing enzymes in the brain and cerebral microvessels. Brain Res Brain Res Rev. 1991;16(1):65–82. doi: 10.1016/0165-0173(91)90020-9. [DOI] [PubMed] [Google Scholar]
- 29.Brownlees J, Williams CH. Peptidases, peptides, and the mammalian blood-brain barrier. J Neurochem. 1993;60(3):793–803. doi: 10.1111/j.1471-4159.1993.tb03223.x. [DOI] [PubMed] [Google Scholar]
- 30.Tsuji A, Tamai II. Carrier-mediated or specialized transport of drugs across the blood-brain barrier. Adv Drug Deliv Rev. 1999;36(2–3):277–290. doi: 10.1016/s0169-409x(98)00084-2. [DOI] [PubMed] [Google Scholar]
- 31.Tamai I, Tsuji A. Transporter-mediated permeation of drugs across the blood-brain barrier. J Pharm Sci. 2000;89(11):1371–1388. doi: 10.1002/1520-6017(200011)89:11<1371::aid-jps1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 32.Oldendorf WH. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971;221(6):1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- 33.Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3(2):90–105. 151. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- 34.Pardridge WM. Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjug Chem. 2008;19(7):1327–1338. doi: 10.1021/bc800148t. [DOI] [PubMed] [Google Scholar]
- 35.Karkan D, Pfeifer C, Vitalis TZ, et al. A unique carrier for delivery of therapeutic compounds beyond the blood-brain barrier. PLoS ONE. 2008;3(6):e2469. doi: 10.1371/journal.pone.0002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laron Z. Insulin and the brain. Arch Physiol Biochem. 2009;115(2):112–116. doi: 10.1080/13813450902949012. [DOI] [PubMed] [Google Scholar]
- 37.Boado RJ, Zhang Y, Wang Y, Pardridge WM. Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse. Biotechnol Bioeng. 2009;102(4):1251–1258. doi: 10.1002/bit.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cancer statistics. JAMA. 2013;310(9):982. doi: 10.1001/jama.2013.5289. [DOI] [PubMed] [Google Scholar]
- 39.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhan C, Meng Q, Li Q, Feng L, Zhu J, Lu W. Cyclic RGD-polyethylene glycol-polyethylenimine for intracranial glioblastoma-targeted gene delivery. Chem Asian J. 2012;7(1):91–96. doi: 10.1002/asia.201100570. [DOI] [PubMed] [Google Scholar]
- 41.Verhoeff JJ, Van Tellingen O, Claes A, et al. Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme. BMC cancer. 2009;9:444. doi: 10.1186/1471-2407-9-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Server A, Orheim TE, Graff BA, Josefsen R, Kumar T, Nakstad PH. Diagnostic examination performance by using microvascular leakage, cerebral blood volume, and blood flow derived from 3-T dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in the differentiation of glioblastoma multiforme and brain metastasis. Neuroradiology. 2011;53(5):319–330. doi: 10.1007/s00234-010-0740-3. [DOI] [PubMed] [Google Scholar]
- 43.Giese A, Kucinski T, Knopp U, et al. Pattern of recurrence following local chemotherapy with biodegradable carmustine (BCNU) implants in patients with glioblastoma. J Neurooncol. 2004;66(3):351–360. doi: 10.1023/b:neon.0000014539.90077.db. [DOI] [PubMed] [Google Scholar]
- 44.Ahluwalia MS, De Groot J, Liu WM, Gladson CL. Targeting SRC in glioblastoma tumors and brain metastases: rationale and preclinical studies. Cancer Lett. 2010;298(2):139–149. doi: 10.1016/j.canlet.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peereboom DM. Chemotherapy in brain metastases. Neurosurgery. 2005;57(5 Suppl):S54–S65. doi: 10.1227/01.neu.0000182740.39014.9a. discusssion S51–54. [DOI] [PubMed] [Google Scholar]
- 46.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13(6):1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 47•.American Brain Tumor Association. Metastatic brain tumors. www.abta.org/secure/metastatic-brain-tumor.pdf This online and print booklet, published by the American Brain Tumor Association, represents a comprehensive and frequently updated guide for metastatic brain tumors to patients and caregivers. The American Brain Tumor Association was founded in 1973 as the first national nonprofit organization committed merely to support brain tumor research and patients.
- 48.American Brain Tumor Association Offers “Top Ten” Brain Tumor Facts. www.abta.org/news/press-releases/american-brain-tumor-6.html.
- 49.Rocco N, Rispoli C, Pagano G, et al. Undertreatment of breast cancer in the elderly. BMC Surg. 2013;13(Suppl 2):S26. doi: 10.1186/1471-2482-13-S2-S26. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Palmieri D, Smith QR, Lockman PR, et al. Brain metastases of breast cancer. Breast disease. 2006;26:139–147. doi: 10.3233/bd-2007-26112. [DOI] [PubMed] [Google Scholar]
- 51.Lin NU, Amiri-Kordestani L, Palmieri D, Liewehr DJ, Steeg PS. CNS metastases in breast cancer: old challenge, new frontiers. Clin Cancer Res. 2013;19(23):6404–6418. doi: 10.1158/1078-0432.CCR-13-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang EL, Lo S. Diagnosis and management of central nervous system metastases from breast cancer. Oncologist. 2003;8(5):398–410. doi: 10.1634/theoncologist.8-5-398. [DOI] [PubMed] [Google Scholar]
- 53.Dawood S, Gonzalez-Angulo AM. Progress in the biological understanding and management of breast cancer-associated central nervous system metastases. Oncologist. 2013;18(6):675–684. doi: 10.1634/theoncologist.2012-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52(12):2349–2354. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 55.Schonberg MA, Marcantonio ER, Ngo L, Li D, Silliman RA, Mccarthy EP. Causes of death and relative survival of older women after a breast cancer diagnosis. J Clin Oncol. 2011;29(12):1570–1577. doi: 10.1200/JCO.2010.33.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riihimaki M, Thomsen H, Brandt A, Sundquist J, Hemminki K. Death causes in breast cancer patients. Ann Oncol. 2012;23(3):604–610. doi: 10.1093/annonc/mdr160. [DOI] [PubMed] [Google Scholar]
- 57.Mueller CB, Ames F, Anderson GD. Breast cancer in 3,558 women: age as a significant determinant in the rate of dying and causes of death. Surgery. 1978;83(2):123–132. [PubMed] [Google Scholar]
- 58.Mueller CB, Jeffries W. Cancer of the breast: its outcome as measured by the rate of dying and causes of death. Ann Surg. 1975;182(3):334–341. doi: 10.1097/00000658-197509000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hagemeister FB, Jr, Buzdar AU, Luna MA, Blumenschein GR. Causes of death in breast cancer: a clinicopathologic study. Cancer. 1980;46(1):162–167. doi: 10.1002/1097-0142(19800701)46:1<162::aid-cncr2820460127>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 60.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 61.Gottwald G, Szakolczai I, Szemenyei K. Metastases and causes of death in breast cancer. Radiobiol Radiother. 1983;24(4):501–506. [PubMed] [Google Scholar]
- 62.Fish EB, Chapman JA, Link MA. Competing causes of death for primary breast cancer. Ann Surg Oncol. 1998;5(4):368–375. doi: 10.1007/BF02303502. [DOI] [PubMed] [Google Scholar]
- 63.Du XL, Fox EE, Lai D. Competing causes of death for women with breast cancer and change over time from 1975 to 2003. Am J Clin Oncol. 2008;31(2):105–116. doi: 10.1097/COC.0b013e318142c865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belous TA. Causes of death of patients with breast cancer (according to the materials of the Oncological Research Center of the USSR AMS for the period 1953–1975) Arkh Patol. 1976;38(10):76–79. [PubMed] [Google Scholar]
- 65•.Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–5678. doi: 10.1158/1078-0432.CCR-10-1564. Revealed important findings about the blood–tumor barrier (BTB). It showed that the BTB remains sufficiently intact (not uniformly permeable as it thought it was) in most experimental brain metastases. Therefore, the BTB hinders drug delivery in a comparable way to the BBB, which highlights the need for brain-permeable molecular therapeutics. This article was the cover story of the Clinical Cancer Research Journal (Volume 16, Issue 23, 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hiesiger EM, Voorhies RM, Basler GA, Lipschutz LE, Posner JB, Shapiro WR. Opening the blood-brain and blood-tumor barriers in experimental rat brain tumors: the effect of intracarotid hyperosmolar mannitol on capillary permeability and blood flow. Ann Neurol. 1986;19(1):50–59. doi: 10.1002/ana.410190110. [DOI] [PubMed] [Google Scholar]
- 67.Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol. 2007;25(16):2306–2312. doi: 10.1200/JCO.2006.10.0677. [DOI] [PubMed] [Google Scholar]
- 68.Bronger H, Konig J, Kopplow K, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res. 2005;65(24):11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- 69.Front D, Israel O, Kohn S, Nir I. The blood-tissue barrier of human brain tumors: correlation of scintigraphic and ultrastructural findings: concise communication. J Nucl Med. 1984;25(4):461–465. [PubMed] [Google Scholar]
- 70.Bertossi M, Virgintino D, Maiorano E, Occhiogrosso M, Roncali L. Ultrastructural and morphometric investigation of human brain capillaries in normal and peritumoral tissues. Ultrastruct Pathol. 1997;21(1):41–49. doi: 10.3109/01913129709023246. [DOI] [PubMed] [Google Scholar]
- 71.Ningaraj NS, Rao MK, Black KL. Adenosine 5′-triphosphate-sensitive potassium channel-mediated blood-brain tumor barrier permeability increase in a rat brain tumor model. Cancer Res. 2003;63(24):8899–8911. [PubMed] [Google Scholar]
- 72.Hirano A, Matsui T. Vascular structures in brain tumors. Hum Pathol. 1975;6(5):611–621. doi: 10.1016/s0046-8177(75)80045-1. [DOI] [PubMed] [Google Scholar]
- 73.Hasegawa H, Ushio Y, Hayakawa T, Yamada K, Mogami H. Changes of the blood-brain barrier in experimental metastatic brain tumors. J Neurosurg. 1983;59(2):304–310. doi: 10.3171/jns.1983.59.2.0304. [DOI] [PubMed] [Google Scholar]
- 74.Groothuis DR, Fischer JM, Pasternak JF, Blasberg RG, Vick NA, Bigner DD. Regional measurements of blood-to-tissue transport in experimental RG-2 rat gliomas. Cancer Res. 1983;43(7):3368–3373. [PubMed] [Google Scholar]
- 75.Blasberg RG, Gazendam J, Patlak CS, Shapiro WS, Fenstermacher JD. Changes in blood-brain transfer parameters induced by hyperosmolar intracarotid infusion and by metastatic tumor growth. Adv Exp Med Biol. 1980;131:307–319. doi: 10.1007/978-1-4684-3752-2_24. [DOI] [PubMed] [Google Scholar]
- 76.Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 77.Liu LB, Xue YX, Liu YH, Wang YB. Bradykinin increases blood-tumor barrier permeability by down-regulating the expression levels of ZO-1, occludin, and claudin-5 and rearranging actin cytoskeleton. J Neurosci Res. 2008;86(5):1153–1168. doi: 10.1002/jnr.21558. [DOI] [PubMed] [Google Scholar]
- 78.Gardner TW, Lesher T, Khin S, Vu C, Barber AJ, Brennan WA., Jr Histamine reduces ZO-1 tight-junction protein expression in cultured retinal microvascular endothelial cells. Biochem J. 1996;320(Pt 3):717–721. doi: 10.1042/bj3200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 80.Soni V, Kohli DV, Jain SK. Transferrin coupled liposomes as drug delivery carriers for brain targeting of 5-florouracil. J Drug Target. 2005;13(4):245–250. doi: 10.1080/10611860500107401. [DOI] [PubMed] [Google Scholar]
- 81.Patel T, Zhou J, Piepmeier JM, Saltzman WM. Polymeric nanoparticles for drug delivery to the central nervous system. Adv Drug Deliv Rev. 2012;64(7):701–705. doi: 10.1016/j.addr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuo Y-C, Lin P-I, Wang C-C. Targeting nevirapine delivery across human brain microvascular endothelial cells using transferrin-grafted poly(lactide-co-glycolide) nanoparticles. Nanomedicine (Lond) 2011;6(6):1011–1026. doi: 10.2217/nnm.11.25. [DOI] [PubMed] [Google Scholar]
- 83.Kazantsev AG, Outeiro TF. Drug discovery for CNS disorders: from bench to bedside. CNS Neurol Disord Drug Targets. 2010;9(6):668. doi: 10.2174/187152710793237395. [DOI] [PubMed] [Google Scholar]
- 84.Zanotti I, Pedrelli M, Potì F, et al. Macrophage, but not systemic, apolipoprotein E is necessary for macrophage reverse cholesterol transport in vivo. Arterioscler Thromb Vasc Biol. 2011;31(1):74–80. doi: 10.1161/ATVBAHA.110.213892. [DOI] [PubMed] [Google Scholar]
- 85.Krikken JA, Dallinga-Thie GM, Navis G, Dullaart RP. Short term dietary sodium restriction decreases HDL cholesterol, apolipoprotein A-I and high molecular weight adiponectin in healthy young men: relationships with renal hemodynamics and RAAS activation. Nutr Metab Cardiovasc Dis. 2012;22(1):35–41. doi: 10.1016/j.numecd.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 86.Salvatore A, Cigliano L, Carlucci A, Bucci EM, Abrescia P. Haptoglobin binds apolipoprotein E and influences cholesterol esterification in the cerebrospinal fluid. J Neurochem. 2009;110(1):255–263. doi: 10.1111/j.1471-4159.2009.06121.x. [DOI] [PubMed] [Google Scholar]
- 87.Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv Drug Deliv Rev. 2013 doi: 10.1016/j.addr.2013.08.008. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 88.Tosi G, Costantino L, Ruozi B, Forni F, Vandelli MA. Polymeric nanoparticles for the drug delivery to the central nervous system. Expert Opin Drug Deliv. 2008;5(2):155–174. doi: 10.1517/17425247.5.2.155. [DOI] [PubMed] [Google Scholar]
- 89.Johns Hopkins University. US8093013. 2012
- 90.University of Texas. US7914780. 2011
- 91.Genentech Inc. US2012/0089541. 2012
- 92.The Translational Genomics Research Institute. US2012/0178093. 2012
- 93.Baylor College Medicine. PCT/US2012/066868. 2013
- 94.Welcome Receptor Antibodies Pty Ltd. US2013/0230453. 2013
- 95.The Methodist Hospital Research Institute. US2012/0184560. 2012
- 96.Indiana University Res & Tech Corp. US2011/0269636. 2011
- 97.Sloan-kettering Institute For Cancer Research. US2010/0029748. 2010
- 98.Institut Catala D’oncologia, Fundacio Privada Institut D’investigacio Biomedica De Bellvitge (idibell), Universitat Pompeu Fabra, Universitat De Barcelona. US2012/0157338. 2012
- 99.Smithkline Beecham Corporation. US2009/0005398. 2009
- 100.Dilea C, McSheehy PM, Maira S, Tanaka C, Wartmann MR. US2009/0069394. 2009
- 101.Glaxosmithkline Limited. US2013/0231347. 2013
- 102.Evans-Freke S. US2012/0183536. 2012
- 103.Xigen S.a. and Xigen Inflammation Ltd. US2011/0183888. 2011
- 104.Ardea Biosciences Inc. US2012/0107307. 2012
- 105.National Cancer Center. US2011/0124712. 2011
- 106.Kinex Pharmaceuticals Llc. US2011/0281872. 2011
- 107.Naurex Inc. US2004/0204381. 2004
- 108.Struck RF. US2010/0234325. 2010
- 109.Battista KA, Bignan GC, Xu G, et al. US2012/0157412. 2012
- 110.Tapestry Pharmaceuticals Inc. US2011/0318334. 2011
- 111.Transmolecular Inc. US20128227439. 2012
- 112.Myriad Pharmaceuticals Inc. US2010/0129470. 2010
- 113.Myriad Pharmaceuticals Inc. US2010/0261739. 2010
- 114.Niiki Pharma. US20130090321. 2011
- 115.Kominox Usa Inc. US2010/048314. 2011
- 116.Curis Inc. US2010/0184801. 2010
- 117.Zhang W. US2013/0143910. 2013
- 118.The Children’s Hospital Of Philadelphia. US2013/01439519. 2013
- 119.Bioasis Technologies, Inc. US2013/0183368. 2013
- 120.Genentech Inc. US2011/0165155. 2011
- 121.Genentech Inc. US2009/0317387. 2009
- 122.Patrys Limited. US2009/0202570. 2009
- 123.Patrys Limited. US2010/0322936. 2010
- 124.Nektar Therapeutics. WO2013063286. 2013
- 125.Nektar Therapeutics. Etirinotecan Pegol (NKTR-102) A Next-Generation Topoisomerase I Inhibitor Being Developed in Breast, Ovarian and Colorectal Cancers. www.nektar.com/pdf/pipeline/NKTR-102/NKTR-102_Product_Fact_Sheet.pdf.
- 126.Nektar Therapeutics. US20110269789. 2010
- 127.University Of Rochester. US 2011/0189205. 2011
- 128.Ramge P, Unger RE, Oltrogge JB, et al. Polysorbate-80 coating enhances uptake of polybutylcyanoacrylate (PBCA)-nanoparticles by human and bovine primary brain capillary endothelial cells. Eur J Neurosci. 2000;12(6):1931–1940. doi: 10.1046/j.1460-9568.2000.00078.x. [DOI] [PubMed] [Google Scholar]
- 129.Sun W, Xie C, Wang H, Hu Y. Specific role of polysorbate 80 coating on the targeting of nanoparticles to the brain. Biomaterials. 2004;25(15):3065–3071. doi: 10.1016/j.biomaterials.2003.09.087. [DOI] [PubMed] [Google Scholar]
- 130.Alza Corporation. US2007/006518. 2007
- 131.Rutgers University. US 2012/0183621. 2012
- 132.Magforce Nanotechnologies AG. US2011/0223255. 2011
- 133.Bozic I, Reiter JG, Allen B, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. eLife. 2013;2:e00747. doi: 10.7554/eLife.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Institut National De La Sante Et De La Recherche Medicale. US2012/0022148. 2012
- 135.Celgene Corporation. US20130274219. 2013
- 136.Ptc Therapeutics Inc. US2012/0157402. 2012
- 137.Genentech Inc., F. Hoffmann-la Roche Ag. US2011/0047103. 2011
- 138.Sasich LD, Sukkari SR. The US FDAs withdrawal of the breast cancer indication for Avastin (bevacizumab) Saudi Pharm J. 2012;20(4):381–385. doi: 10.1016/j.jsps.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Immatics Biotechnologies Gmbh. US2011/0002963. 2011
- 140.Nektar Therapeutics. US2011/0171160. 2011
- 141.The University of Tokyo. US2012/0039808. 2012
- 142.S. Biomedics Co. Ltd. US2012/0107282. 2012
- 143.Olivier JC. Drug targeting to brain with targted nanoparticles. NeuroRx. 2005;2:108–119. doi: 10.1602/neurorx.2.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chinot OL, De La Motte Rouge T, Moore N, et al. AVAglio. Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther. 2011;28(4):334–340. doi: 10.1007/s12325-011-0007-3. [DOI] [PubMed] [Google Scholar]
- 145.Gilbert MR, Dignam J, Won M, et al. RTOG 0825: phase III double-blind placebo-controlled trial evaluating bevacizumab (Bev) in patients (Pts) with newly diagnosed glioblastoma (GBM) J Clin Oncol. 2013;31(Suppl, abstract 1):1. [Google Scholar]
- 146.Onat A, Can G, Cicek G, Ayhan E, Dogan Y. Predictive value of serum apolipoprotein B/LDL-cholesterol ratio in cardiometabolic risk: population-based cohort study. Clin Biochem. 2010;43(18):1381–1386. doi: 10.1016/j.clinbiochem.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 147.Mickle JD., Jr Re: Superiority of apolipoprotein B (apoB) over low-density lipoprotein cholesterol (LDL-C) in treatment of hyperlipidemia. J Clin Lipidol. 2010;4(4):317. doi: 10.1016/j.jacl.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 148.Nektar Therapeutics. US20060182692. 2011
- 149.Nektar Therapeutics. US20130158062. 2013