Abstract
Introduction
While systemic therapy for metastatic breast cancer (MBC) continues to evolve, there is little data to guide physicians and patients when symptoms develop. Here we report the frequency and durability of palliative procedures performed in the setting of MBC.
Methods
From 7/02-6/03, 91 patients with MBC underwent 109 palliative procedures (operative n=73; IR n=39, endoscopic n=3). At study entry, patients had received a mean of six prior systemic therapies for metastatic disease. System-specific symptoms included neurologic(33%), thoracic(23%), musculoskeletal(22%) and GI(14%). The most common procedures were thoracostomy +/− pleurodesis(27%), craniotomy with resection + resection(19%) and orthopaedic ORIF(19%).
Results
Symptom improvement at 30dys and 100dys was reported by 91% and 81% of patients respectively, and 70% reported continued benefit for duration of life. At a median interval of 75dys from intervention(range, 8–918), 23 patients(25%) underwent 61 additional procedures for recurrent symptoms. The durability of palliation varied with system-specific symptoms. Patients with neurologic or musculoskeletal symptoms were least likely to require additional maintenance procedures(p<0.0002). The 30dy complication rate was 18% and there were no procedure-related deaths. At a median survival of 37.4mos from MBC diagnosis(range, 1.6–164), and 8.4mos after intervention(range,0.2–73), 7/91 patients remain alive.
Conclusion
Palliative interventions for symptoms of MBC are safe and provide symptom control for the duration of life in 70% of patients. Definitive surgical treatment of neurologic or musculoskeletal symptoms provided the most durable palliation; interventions for other symptoms frequently require subsequent procedures. The longer median survival for patients with MBC highlights the need to optimize symptom control to maintain quality of life.
Keywords: Palliative, interventions, metastatic breast cancer, MBC, surgery
Introduction
In the current era of improved survival with targeted systemic therapy, more patients are living well with metastatic breast cancer (MBC) than ever before. Yet for most, progression of disease will become a reality, and maintaining quality of life will become a foremost priority. Historical data indicate that as patients progress through their illness, a wide variety of clinical issues may develop which can have a negative impact on quality of life.1 Such issues may be directly related to the primary tumor, sites of metastasis, or to the multitude of paraneoplastic syndromes that may develop. Not only can these symptoms affect a patient’s physical quality of life, but they may also have a profound affect on their psychological, social and spiritual well-being, as well as cause suffering to their families and caregivers.2
When caring for a patient with metastatic disease, the goals of treatment are to prolong progression-free survival and to palliate symptoms when they arise. In its purest sense, palliative treatment is undertaken to provide symptom relief and maintain quality of life. Surgical palliation, while not a novel concept, remains poorly defined in MBC. Data detailing clinical progression of disease and the utility of palliative interventions near the end of life are largely absent from the literature. Thus physicians and patients are left to make treatment decisions based on individual expectations, emotion, and anecdotal experience, rather than on well-defined outcome data.3–6
In an attempt to better define the need for palliation in the natural progression of MBC and to evaluate the durability of commonly performed palliative interventions, we prospectively identified patients undergoing palliative procedures in the setting of MBC over a 1-year period at Memorial Sloan-Kettering Cancer Center (MSKCC) and followed them until death.
Methods
A prospective database of all procedures performed to palliate symptoms of advanced malignancy was established at MSKCC in July 2002.7 The initial intent of this database was to capture all operative, endoscopic, bedside, and interventional radiology (IR) procedures performed primarily for palliative intent over a 12-month period (July 2002 – June 2003). Within this context, a palliative procedure was defined as any procedure that was undertaken with the primary intention of symptom control in patients with metastatic disease. To distinguish between palliative versus non-palliative procedures, indications for each intervention were ascertained either by review of the clinical record and/or by interviewing the physician responsible for the care of the patient. Drawing from this experience, we initiated a focused analysis of palliative interventions in the setting of MBC.
All patients with MBC undergoing a scheduled palliative procedure between July 2002 and June 2003 were entered into a prospective database. The database was designed to capture patient-reported/subjective symptoms, thus palliative interventions were classified as dictated by the presenting/chief complaint of the patient. The nature and duration of the presenting symptom requiring palliation, as well as standard clinical parameters, including medical co-morbidities and breast cancer history, were recorded. For those patients who presented with pain, clinically significant pain was defined by either (1) persistence of pain over 2 clinic visits; (2) pain requiring narcotic analgesia for > 30 days; or (3) pain necessitating referral to a pain specialist. Patterns of metastatic disease were categorized by the involved system at first diagnosis of metastatic disease and again at time of entry into the palliative interventions database. For patients presenting with de novo stage IV disease, staging was based on either the 5th or 6th editions of the AJCC Cancer Staging Manual as dictated by the date of diagnosis. All patients entered into the database were followed prospectively until last follow-up (January, 2009) or death.
During the study period, palliative procedures were further defined by symptom order and progression. Primary palliative procedures were those performed to achieve control of the first presenting symptom at the time of entry into the database. Maintenance procedures were those performed for recurrence of the first presenting symptom after a period of palliation. Alternate palliative procedures were those performed to palliate a new symptom developing during the follow-up period. Outcome was assessed in terms of both the first presenting symptom and the organ system involved, with respect to durability of symptom control, requirement for additional procedures, hospital admissions, length of stay, and procedure-related morbidity and mortality. Symptom control endpoints included symptom relief at or within a 30-day period from the primary palliative intervention, and continued symptom control at 100 days and/or for duration of life as documented in the medical record. Complications within 30 days of the procedure were recorded and classified based on maximum grade of complication: (I) local/bedside non-invasive therapy; (II) Invasive monitoring or medication; (III) Operation, IR, intubation or therapeutic endoscopy; (IV) Persistent disability or major organ resection; (V) Death.8 All data were collected prospectively from the electronic medical record with the approval of our institutional review board. Analyses were done with SPSS software Version 11 (SPSS Inc, Chicago, IL).
Results
Over the 12-month period, 91 patients with MBC underwent a total of 109 primary palliative procedures to control the symptoms of their disease and were entered into the database (mean: 1.2 procedures/patient; range, 1–6). This represented 5% of an estimated 1,950 patients with stage IV breast cancer being managed at our institution during the same time period. Among these 91 patients, 71 (78%) had been treated for an earlier stage breast cancer (0–III) before developing metastatic disease (median time to progression, 47.7 months; range, 1 – 323.6 months), and the remaining 20 patients (22%) presented with stage IV disease at diagnosis. At the time of stage IV diagnosis, 24/91 patients (26%) had in-breast disease (including all 20 patients with de novo stage IV disease and 4 patients with synchronous local recurrence and distant disease), and 82/91 patients (90%) had only a single organ site of metastatic disease. The most common sites of initial metastatic disease included: bone (34/91, 37%); extensive local/regional disease (15 of 91, 17%) (see footnote on Table 2); and pulmonary/pleural involvement (14 of 91, 15%).
The clinical characteristics of patients at the time of entry into the database are illustrated in Table 1. The median time interval from stage IV diagnosis to the palliative intervention captured by this study was 16.6 months (range, 0.2 – 156.8). Prior to the time period selected for this study, patients had received a mean of 6 prior therapies (systemic/radiation) for metastatic disease, and 13 patients (14%) had undergone a mean of 1.8 prior palliative procedures for symptom control. Among the 13 patients who had undergone a prior palliative procedure, the prior symptom requiring palliation was the same symptom which led to entry into our study in 4 patients, and was a different symptom in 9 patients. Among these 13 patients, the median time interval from the first palliative intervention to that which was captured in this study was 16.1 months (range, 8.7 – 49.4). Evidence of disease progression among all patients is depicted by the increasing sites of metastatic disease at study entry as compared to those at the time of stage IV diagnosis (Table 2).
Table 1.
Clinical characteristics of patients at the time of study entry
| N (%) | |
|---|---|
|
| |
| Median age, years (range) | 56.9 years (range 27.4 – 91.4) |
|
| |
| Gender | |
| Female | 90 (99%) |
| Male | 1 (1%) |
|
| |
| Prior treatment for stage IV disease, (%) | |
| Chemotherapy | 60 (66%) |
| Radiotherapy | 23 (25%) |
| Hormonal therapy | 65 (71%) |
| Trastuzumab | 37 (41%) |
|
| |
| Solitary site of metastatic disease | 7 (8%) |
|
| |
| Median time interval from Stage IV diagnosis, months (range) | 16.6 (0.2 – 156.8) |
Table 2.
Sites of metastatic disease
| At time of Stage IV diagnosis | At study entry | |
|---|---|---|
| Bone | 34 (37%) | 50 (55%) |
| Local/regional* | 15 (17%) | 23 (25%) |
| Thoracic / Mediastinal | 14 (15%) | 54 (59%) |
| Brain | 9 (10%) | 24 (26%) |
| Liver | 5 (5%) | 46 (50%) |
| Other abdominal/pelvic disease | 1 (1%) | 14 (15%) |
| Retroperitoneum | – | 12 (13%) |
| Cerebrospinal fluid | – | 8 (8%) |
| Other | 1 (1%) | 7 (7%) |
| Unknown | 1 (1%) | – |
| 1 site of metastatic disease >1 site of metastatic disease |
80 (88%) 11 (12%) |
7 (8%) 84 (92%) |
8 patients with supraclavicular lymph node disease were classified as stage IV prior to 2002 as dictated by the 5th edition of the AJCC Cancer Staging Manual. The remaining local/regional sites at time of stage IV diagnosis included: brachial plexus disease, cervical node involvement, and contralateral intramammary node disease.
The 3 most common patient complaints were neurologic symptoms (30/91, 33%), pain (22/91, 24%), and dyspnea (20/91, 22%) (Table 3). Categorizing symptoms by the affected organ system demonstrates a similar distribution and illustrates that symptoms of pain were primarily attributable to skeletal disease (Figure 1). Surgical interventions were the most common primary palliative procedures performed (67/109, 61%), with neurosurgical and orthopedic interventions being the most frequent (Table 4).
Table 3.
Presenting symptoms at time of first palliative procedure
| All Patients N=91 |
|
|---|---|
|
| |
| Presenting Complaint: | |
| • Neurologic | 30 (33%) |
| • Pain | 22 (24%) |
| • Dyspnea | 20 (22%) |
| • Abdominal Distention | 5 (6%) |
| • GI obstruction | 3 (3%) |
| • Wound | 3 (3%) |
| • Hoarseness | 2 (2%) |
| • Jaundice | 2 (2%) |
| • Urinary Obstruction | 2 (2%) |
| • Dysphagia | 1 (1%) |
| • Pruritus | 1 (1%) |
Figure 1.
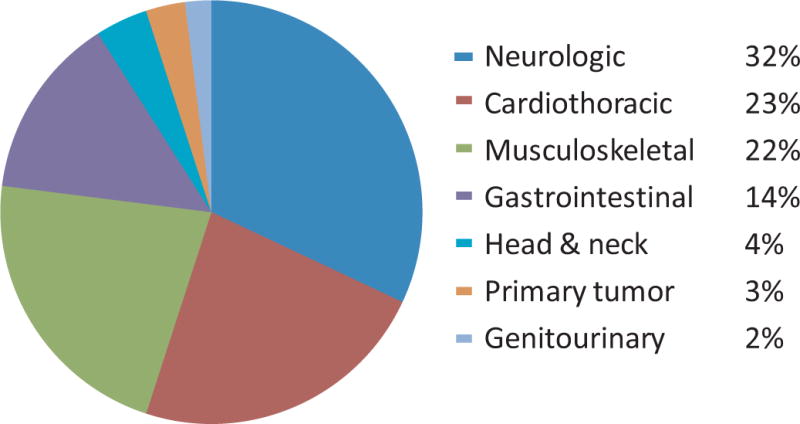
Symptomatic organ system at time of first palliative procedure
Table 4.
Primary palliative procedures
| All primary palliative procedures N=109 |
|
|---|---|
|
| |
| Surgical Procedures, n=67 (61%) | |
| • Craniotomy +/− resection | 19 (17%) |
| • Orthopaedic ORIF | 19 (17%) |
| • Creation of VP shunt | 5 (5%) |
| • Spinal decompression + fixation | 5 (5%) |
| • VATS +/− pleuradhesis | 4 (4%) |
| • Mastectomy +/− reconstruction | 3 (3%) |
| • Laparotomy +/− diversion | 3 (3%) |
| • Laryngoplasty | 3 (3%) |
| • Cystoscopy and insertion of ureteric stents | 2 (2%) |
| • VATS + Cardiac window | 2 (2%) |
| • Creation of Eloesser flap | 1 (1%) |
| • Excision of abdominal wall lesion + reconstruction | 1 (1%) |
|
| |
| Interventional radiology, n=39 (36%) | |
| • Placement of thoracostomy tube +/− pleuradhesis | 29 (27%) |
| • Paracentesis +/− placement of denver shunt / Tenkoff catheter | 5 (5%) |
| • Biliary Drainage | 5 (5%) |
|
| |
| Endoscopic procedures, n=3 (3%) | 3 (3%) |
| • PEG tube insertion | |
ORIF = open reduction, internal fixation, VP = Ventriculoperitoneal shunt, VATS Video Assisted Thorascopic Surgery, PEG = percutaneous endoscopic gastrostomy
Interventional radiology procedures (39/109, 36%) were more commonly performed as primary palliation for dyspnea. Overall, primary palliative procedures provided symptom improvement or resolution at or within 30 days for 83 patients (91%), and of 72 patients remaining alive at 100 days post intervention, 58 (81%) reported ongoing palliation. However, at a median interval of 75 days (range, 8–918), 60 maintenance procedures were performed for recurrent symptoms in 23/91 patients (25%) (Table 5). In contrast to primary palliative procedures, the majority of the maintenance procedures were performed by the interventional radiology service (26/60, 43%).
Table 5.
Outcome of palliative intervention by organ system involved
| All Patients n=91 |
Neurologic n=29 |
CTS n=21 |
MSK n=20 |
GI n=13 |
Other* n=8 |
|
|---|---|---|---|---|---|---|
|
| ||||||
| Palliative Procedures, n=109 | ||||||
| # Procedures (mean) | 109 (1.2) | 29 (1) | 36 (1.7) | 20 (1) | 16 (1.2) | 8 (3) |
| Symptom improvement, 30 days, % | 91% | 90% | 90% | 100% | 92% | 75% |
| Alive at 100 days, n | 72 | 25 | 16 | 17 | 7 | 7 |
| Symptom control, 100 days, % | 81% | 92% | 69% | 100% | 29% | 71% |
| Symptom control, duration of life % | 70% | 72% | 57% | 100% | 38% | 75% |
|
| ||||||
| Maintenance Procedures (MP), n=60 | ||||||
| # Patients (%) | 23 (25%) | 5 (17%) | 12 (57%) | 0 | 5 (38%) | 1 (13%) |
| Median time to first MP, days (range) | 75 (8–918) | 91 (12–577) | 60 (8–918) | – | 22 (9–69) | 105 |
| Total # MP | 60 | 6 | 28 | – | 25 | 1 |
| Mean # MP (range) | 2.7 (1–11) | 1.2 (1–2) | 2.3 (1–6) | – | 5.2 (1–11) | 1 |
|
| ||||||
| Total procedures per symptom (mean) | 169 (1.9) | 35 (1.2) | 64 (1.8) | 20 (1) | 41 (3.2) | 9 (1.1) |
|
| ||||||
| Median Survival | ||||||
| From stage IV diagnosis, months (range) | 37.4 (1.6–164) | 47.3 (17.7–78) | 22.3 (1.6–113) | 38 (2–164) | 46.7 (17.7–78) | 57.3 vd(19.1–78) |
| From first intervention, months (range) | 8.4 (.2–73) | 11.2 (.2–70) | 7.1 (.5–36) | 29 (2.4–73) | 1.9 (.4–41) | 8.4 (2.9–71) |
Including: Primary tumor, Head and Neck, genitourinary
CTS = cardiothoracic, MSK = musculoskeletal, GI – gastrointestinal
The durability of palliation and frequency of maintenance procedures varied with organ system involvement. Specifically, definitive surgical treatment of neurologic and musculoskeletal symptoms provided the most durable short term (90% and 100%, respectively) and long-term/duration of life (72% and 100%, respectively) palliation, with low requirements for additional maintenance procedures (17% and 0%, respectively). Patients presenting with GI or cardiothoracic symptoms achieved good short-term palliation (92% and 90%, respectively), but had a high requirement for maintenance procedures (38% and 57%, respectively) and were less likely to benefit from the intervention for the duration of life (57% and 38%, respectively) (Table 5).
In summary, these 91 patients underwent a total of 169 procedures (primary palliative + maintenance) to palliate their first presenting symptom (mean, 1.9 procedures/patient). Overall median survival from initial intervention was 8.4 months (range, 0.2 – 73 months), and 64 patients (70%) reported ongoing palliation for duration of life (Table 5). In addition, 15 patients (16%) underwent a total of 69 alternate procedures (mean, 4.6 procedures; range, 1–44) for new symptoms related to progression of disease at a median time of 3.2 months from study entry (range, 0.2–50.6 months). These 15 patients were among those presenting initially with either neurologic symptoms, pain, or dyspnea. The 2 most common organ systems involved with disease progression in this setting were GI (41/69, 59%) and GU (16/69, 23%), and led to the frequent need for beside paracentheses and urologic stent placement and/or exchange, respectively. Combining primary palliative, maintenance, and alternate procedures, these 91 patients underwent a total of 238 procedures (mean, 2.6 procedures/patient; range, 1–44) to palliate symptoms of MBC during the course of this study (76 months).
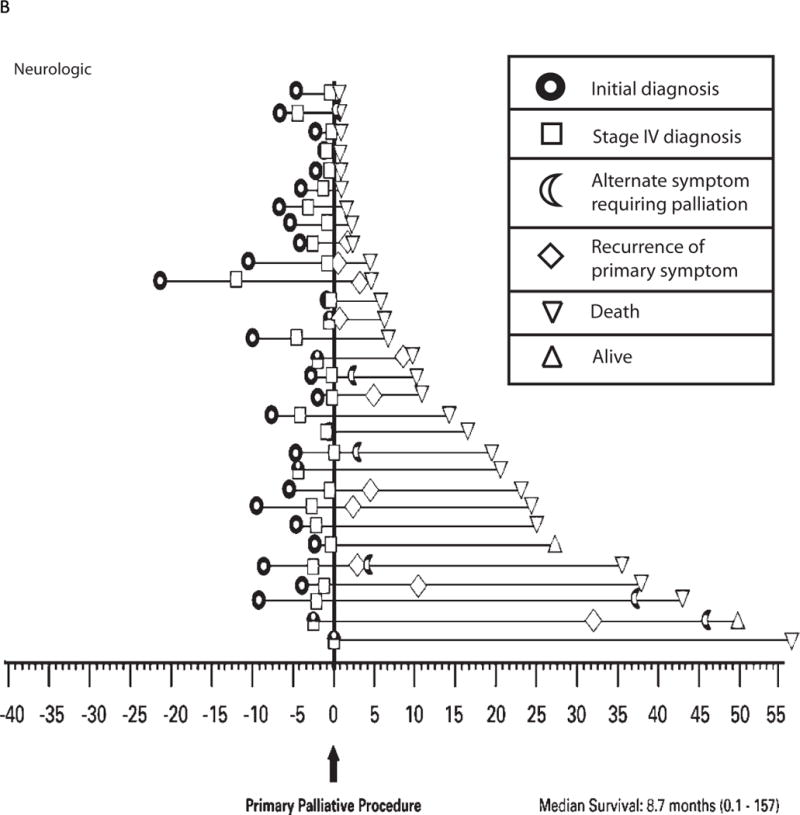
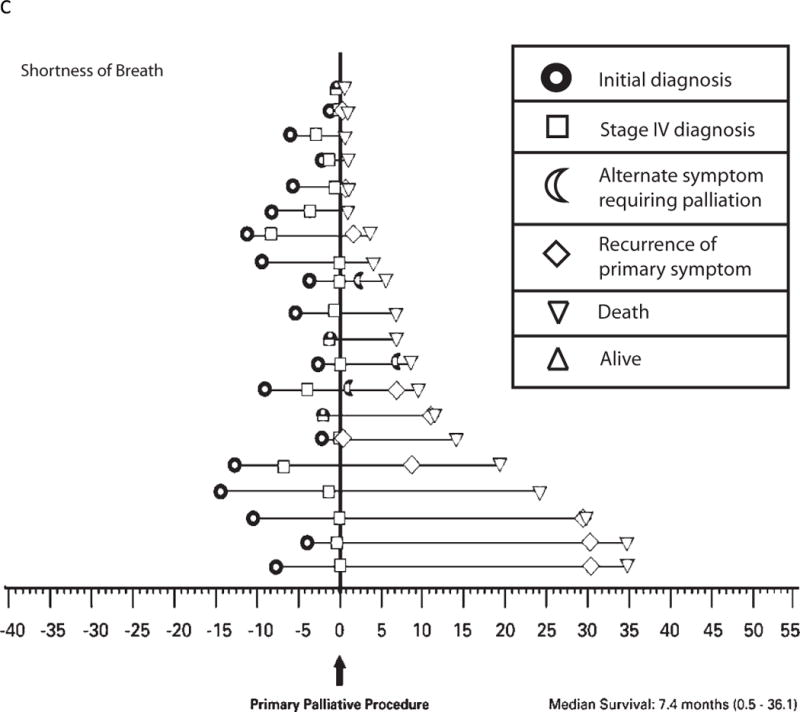
The natural progression of disease and the frequency of palliative intervention as experienced by the individual patient are illustrated for the 3 most common symptoms in Figure 2A–C. Patients presenting with a chief complaint of “pain” reported the greatest frequency of short-term benefit (96%) with minimal repeat interventions for recurrence of the primary symptom, and 95% of patients reported ongoing benefit for duration of life (Figure 2A). In contrast, patients presenting with a chief complaint of “neurologic symptoms” reported a similar frequency of short-term benefit (87%); however, at a median interval of 3 months from first intervention (range, 0.5–19.2 months), 17% of patients required maintenance procedures, and only 70% of patients reported ongoing palliation for duration of life (Figure 2B). Similarly, of 20 patients presenting with a chief complaint of “shortness of breath,” although 80% reported good short-term benefit, at a median interval of 8 months from first intervention (range, 0.4–35.3 months), 60% of patients required maintenance procedures (mean, 2.3 maintenance procedures/patient), and only 50% reported ongoing palliation for duration of life (Figure 2C).
Figure 2.
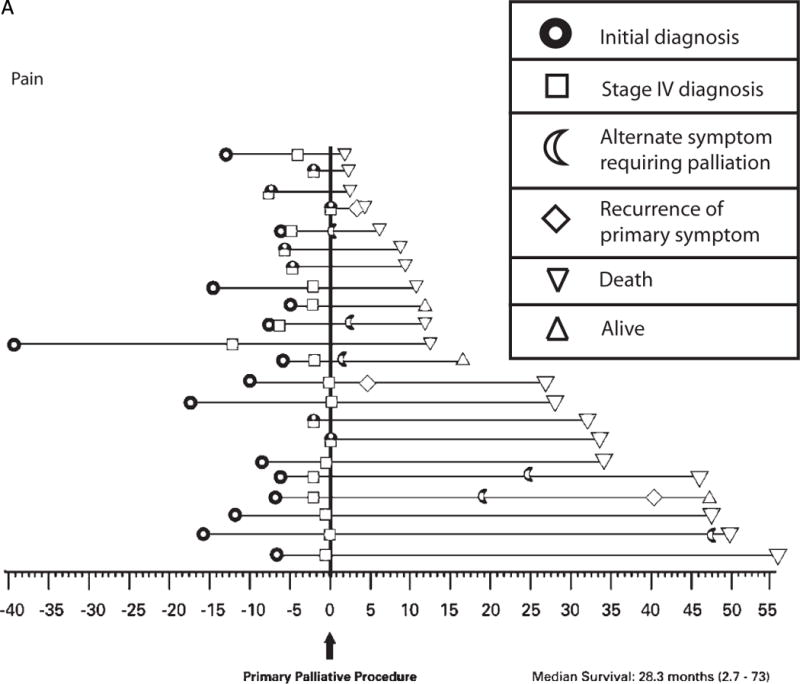
a: Time-course of disease progression and the need for palliative intervention as captured by this study for individual patients presenting with pain. Each horizontal line on the graph represents a single patient (n = 22). Time point “0” represents the first palliative procedure at study entry, and subsequent events are noted along the time course of their disease as denoted in the figure legend. Two of these 22 patients underwent a palliative procedure for pain prior to the time period selected for this study.
b: Time-course of disease progression and the need for palliative intervention as captured by this study for individual patients presenting with neurologic symptoms. Each horizontal line on the graph represents a single patient (n = 30). Time point “0” represents the first palliative procedure at study entry, and subsequent events are noted along the time course of their disease as denoted in the figure legend. Seven of these 30 patients underwent a palliative procedure prior to the time period selected for this study, 2/7 prior palliative procedures were also performed for neurologic symptoms.
c: Time-course of disease progression and the need for palliative intervention as captured by this study for individual patients presenting with shortness of breath. Each horizontal line on the graph represents a single patient (n = 20). Time point “0” represents the first palliative procedure at study entry, and subsequent events are noted along the time course of their disease as denoted in the figure legend. Two of these 20 patients underwent a palliative procedure for unrelated symptoms prior to the time period selected for this study
The associated impact of the palliative intervention on quality of life was measured by the morbidity and mortality of the procedures. A total of 18 patients (20%) experienced a procedure-related complication within 30 days of the intervention. Complications were graded in terms of maximum morbidity (Grade I–V). In order of increasing severity, recorded complications included displaced stent (n = 1), transient dysrhythmia (n = 1), pain (n = 2), blood transfusion (n = 1), infection (n = 4), DVT/PE (n = 3), re-intubation for respiratory failure (n = 1), and persistent/worsened neurologic disability (n = 4). There were no procedure-related deaths. The impact on quality of life from the individual patient perspective is also illustrated by total number of procedures, number of hospital admissions, and total number of hospital days for patients presenting with the 3 most common symptoms (Table 6). Overall, palliation in this cohort accounted for a total of 359 hospital admissions and a mean of 36.4 hospital days as patients approached the end of life.
Table 6.
Outcome for patients presenting with the 3 most common symptoms
| Neurologic N=30 |
Pain N=22 |
Dyspnea N=20 |
|
|---|---|---|---|
|
| |||
| Mean number of primary palliative procedures | 1.2 | 1 | 1.7 |
| Number of patients requiring maintenance procedures, (%) | 5 (17%) | – | 12 (60%) |
| Mean number of maintenance procedures | 1.2 | – | 2.3 |
| Number of patients requiring alternate procedures, (%) | 6 (20%) | 6 (27%) | 3 (15%) |
| Mean number of alternate procedures | 8.5 | 1.2 | 1.7 |
| Total number of procedures (mean) | 93 (3.1) | 29 (1.3) | 67 (3.4) |
| Mean number of hospital admissions | 4.5 | 3.3 | 4.1 |
| Mean number of hospital days | 40.9 | 34.8 | 44.3 |
| Median survival from stage IV diagnosis, months (range) | 32.8 (2–162) | 58 (5–164) | 33.7 (5.9–109) |
| Median survival from first intervention, months (range) | 8.7 (0.1–157) | 28.3 (2.4–73) | 7.4 (0.5–36.1) |
| Number of patient(s) alive at last follow-up (%) | 2 (7%) | 3 (14%) | 0 |
In total, these 91 patients underwent 238 procedures to palliate symptoms of their disease during the course of this study, with good short-term results across all organ systems involved. Overall median survival from diagnosis of metastatic disease was 37.4 months (range, 1.6–164), and median survival from first palliative intervention at study entry was 8.4 months (range, 0.2 – 73). Patients with primarily skeletal involvement frequently present with pain and have the longest median survival (Figure 3) with excellent symptom control for the duration of life.
Figure 3.
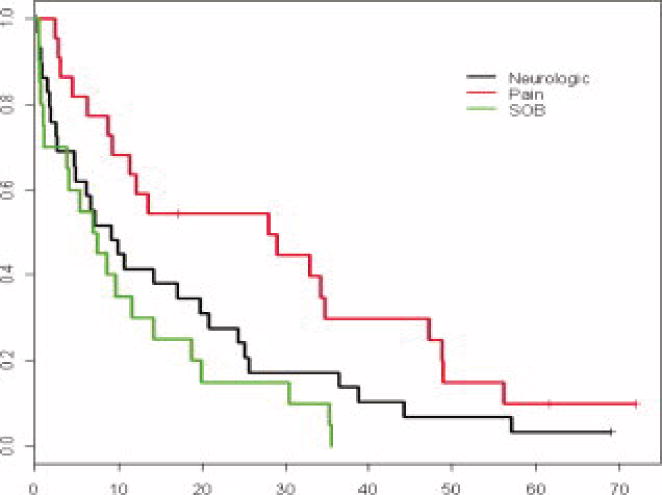
Survival from the time of initial palliative procedure for patients presenting with the 3 most common symptoms (Median survival from first intervention: 8.7 [0.1 – 156.8])
Discussion
Although the last 15–20 years have seen significant developments in both the philosophy and practice of palliative care in the United States, and indeed a rediscovery of palliation as a surgical tradition9,10, there is little data to guide palliative surgical decisions in patients with MBC. Current strategies require an individualized approach based on factors such as patient age, co-morbidities, prior treatment history and response, tumor biology, pattern of metastatic disease, and risk of toxicity. The patient’s needs and expectations are also an essential component of decision making and treatment planning. The use of operative or non-operative procedures in this setting may provide symptom relief; however, any potential morbidity associated with such procedures and the natural history of the individual disease course must be taken into consideration.11 It is fundamental, however, that anticipated survival time is not the most important decision-making factor when considering palliative intervention. Selection for intervention should be based on the anticipated impact on quality of life with minimal risk from the procedure, while also considering the anticipated survival time.
In this select group of 91 patients with MBC, the median overall survival (from stage IV diagnosis) was 37.4 months (range, 1.6–164 months). Patients presented with symptomatic disease at a median interval of 16.6 months from stage IV diagnosis (range, 0–156.8 months) and after receiving a mean of 6 prior therapies. Median survival following palliative intervention in this cohort was 8.4 months (range, 0.2–73 months), with shortest median survival for patients undergoing palliation of GI-related symptoms (1.9 months, range .4–41) and longest for patients undergoing palliation of MSK-related symptoms (29 months, range 2.4–73). The NCCN guidelines for chemotherapy in MBC support treatment until disease progression, cessation of clinical benefit, or development of unacceptable toxicities.12 In the setting of symptomatic disease progression, the role of systemic therapy is to palliate cancer-related symptoms by achieving disease stability, yet the inevitability of disease progression and limited ability of systemic therapies to manage symptoms emphasizes the need to identify alternate, reliable means of palliation. The longer median survival for breast cancer patients as compared with other cancers further emphasizes the need to select durable treatment options and to treat new symptoms as they arise.
In the absence of curative therapy, appropriate endpoints of intervention for patients with symptomatic disease progression should optimize quality of life. In this study, we measured symptom relief as a surrogate for improved quality of life within the context of number of procedures performed, time spent (hospital admissions), and perioperative morbidity/mortality risks. Among 91 patients with symptomatic MBC, surgical and non-surgical means of intervention provided short-term (30 days) symptom improvement in 91% of patients, and although 25% of patients required additional intervention for recurrent symptoms and 16% underwent additional intervention for “new/alternate” symptoms, overall, 70% of patients reported ongoing palliation for the duration of life. Our data also demonstrate a difference in outcome based on the organ system involved and the nature of the presenting complaint, allowing for an appreciation of specific patterns of disease progression. For example, patients who present with neurological symptoms or shortness of breath are likely to progress over a shorter period of time, requiring repeat palliation for recurrent or additional symptoms over extended hospital admissions, and ultimately have a shorter median survival. In contrast, patients presenting with a chief complaint of pain are most likely to experience long-term benefit from palliative intervention which is maintained throughout an extended median survival. The observation that the initial symptom may recur, or that new symptoms may arise, which may require additional hospital admissions or extended hospital stays, is important and allows for a more informed discussion of the expected disease course with patients and their families.
Further, since effective palliation depends on the ability of the treatment to optimize QOL without subjecting the patient to procedure-related complications, it is important to evaluate the potential benefit and risks of the procedure in light of the disease course. In this study, palliative intervention was associated with a 20% risk of 30-day procedure-related morbidity, and there were no treatment-related deaths. Specifically, 15% of patients experienced a grade I/II complication’ (i.e., a complication that required either temporary local or intravenous therapy, or invasive monitoring), and 5% experienced a grade 3/4 complication. While this 30-day morbidity may seem high, it is worth noting that of those experiencing a grade I/II complication, all but 2 patients reported ongoing symptom palliation for the duration of life, demonstrating that a minor complication may not negate the potential to achieve palliation. Other studies addressing palliative intervention in mixed populations of cancer patients report perioperative morbidity and mortality of up to 40% and 11%, respectively.2,3,7,16,17,18
As a surgical community, we are limited by the lack of outcome data to allow for evidence-based surgical decision making in the setting of advanced metastatic disease. While we recognize our role in symptom palliation, there is wide variation in practice patterns. In a survey of 419 practicing members of the Society of Surgical Oncology (79% completed fellowship training in an oncology-related specialty), it was estimated that 21% of surgeries performed were palliative in nature. The majority of surgeons considered palliative procedures to be interventions to treat general illnesses arising secondary to the natural progression of disease (84%) or complications of cancer surgery (76%), yet there were 2 different views as to what defined surgical palliation. Specifically, 43% of respondents felt that palliative surgery was defined based on pre-operative intent, while 57% defined palliative surgery based on patient progress or postoperative factors. Importantly, symptom relief and pain relief were identified as the 2 most common goals in palliative surgery, with the majority of the group reporting that survival time was not an important factor in surgical decision making.13 Because of their appreciation of the disease process, as well as their knowledge of the risks and benefits of the procedures available, surgeons can provide valuable insight into the role of palliative intervention.14 Yet data suggest that surgeons themselves may underestimate the degree of palliation possible with operative intervention.15 The potential for surgical palliation is further limited by the reality that surgeons are rarely the primary physicians involved in day-to-day treatment planning for patients with metastatic disease. As such, we rely on the assessment of other members of the multidisciplinary team to select patients who may benefit from palliative surgery, and it is possible that many patients who could benefit from intervention may not be referred to the surgical service.
Our study demonstrates that exhaustive review of all procedures performed in the operating room, and in the endoscopy and interventional radiology suites over a 1-year period only identified 5% of patients with stage IV breast cancer being managed at our institution during the same time period. Considering that almost all patients with stage IV disease will ultimately develop symptoms, this is likely to represent only a subset of the patients considered for palliative intervention during that time period. This dataset therefore represents the disease course of those patients pre-selected for palliative intervention, and cannot address the selection criteria used by the primary physicians, the surgeons, or indeed the patients/family members. Evaluation of all patients considered for palliation would allow for greater appreciation of the selection criteria and treatment decision-making processes, as well as expected and achieved outcomes. Our dataset also includes 13 patients who underwent their first palliative procedure prior to the beginning of our study and entry into our database. Collectively these 13 patients are likely to represent a unique subset with a prolonged disease course as they experienced a median time of 16.1 months from first palliative intervention to entry into our study. As the purpose of our study was to prospectively evaluate the durability of commonly performed procedures, these patients were analyzed by their symptoms at presentation to our study. Finally, we acknowledge that outcome data may be biased by subsequent palliative procedures that may have been performed at outside institutions but were not captured by our database. However, since most patients were followed in our hospital until time of death, we believe the likelihood of unreported subsequent interventions is low.
In summary, while current systemic treatments for metastatic disease may improve progression-free survival, the majority of patients will ultimately require alternate strategies for the management of symptomatic disease. As patients progress though their illness, they and their families will seek reliable information to properly frame difficult treatment choices. Patient-reported outcomes of symptom relief such as those illustrated in this dataset are invaluable in assessing the efficacy of intervention from the patient’s perspective. Here we report that for patients with MBC, both surgical and non-surgical palliative interventions are safe and provide durable control of symptoms. More specifically, definitive surgical treatment of neurologic or musculoskeletal symptoms can provide the most durable palliation, while interventions for other symptoms frequently require additional maintenance procedures. Importantly, although intervention was associated with a 20% morbidity rate, the vast majority of minor complications were not associated with compromised palliation. A true understanding of the efficacy of palliative interventions among all members of the multidisciplinary team is fundamental. While we await prospective trials that focus on quality of life using patient-reported outcomes, for now, open discussion among the multidisciplinary team members, patients, and their families must be encouraged to optimize patient care and quality of life.
Acknowledgments
This project was supported by an Exceptional Project Award from the Breast Cancer Alliance.
Footnotes
None of the authors have any financial disclosures.
References
- 1.Bloom HJ. The natural history of untreated breast cancer. Acad Ann NY Sci. 1964;114:747–754. [PubMed] [Google Scholar]
- 2.Podnos YD, Juarez G, Pameijer C, et al. Impact of Surgical Palliation on Quality of Life in Patients with Advanced Malignancy: Results of the Decisions and Outcomes in Palliative Surgery (DOPS) Trial. Ann Surg Oncol. 2007;14:922–8. doi: 10.1245/s10434-006-9238-y. [DOI] [PubMed] [Google Scholar]
- 3.Miner TJ, Jaques DP, Shriver CD, et al. Palliative surgery for advanced cancer: lessons learned in patient selection and outcome assessment. Am J Clin Oncol. 2005;28(4):411–4. doi: 10.1097/01.coc.0000158489.82482.2b. [DOI] [PubMed] [Google Scholar]
- 4.McCahill LE, Smith DD, Borneman T, et al. A prospective evaluation of palliative outcomes for surgery of advanced malignancies. Ann Surg Oncol. 2003;10(6):654–63. doi: 10.1245/aso.2003.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Miner TJ, Jaques DP, Tavaf-Motamen H, et al. Decision making on surgical palliation based on patient outcome data. Am J Surg. 1999;177(2):150–4. doi: 10.1016/s0002-9610(98)00323-7. [DOI] [PubMed] [Google Scholar]
- 6.Miner TJ, Jaques DP, Shriver CD, et al. A prospective evaluation of patients undergoing surgery for the palliation of an advanced malignancy. Ann Surg Oncol. 2002;9(7):696–703. doi: 10.1007/BF02574487. [DOI] [PubMed] [Google Scholar]
- 7.Miner TJ, Brennan MF, Jaques DP, et al. A prospective, symptom related, outcomes analysis of 1022 palliative procedures for advanced cancer. Ann Surg. 2004;240(4):719–26. doi: 10.1097/01.sla.0000141707.09312.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin RC, Jaques DP, Brennan MF, et al. Achieving R0 resection for locally advanced gastric cancer: is it worth the risk of multiorgan resection? J Am Coll Surg. 2002;195:411–422. doi: 10.1016/s1072-7515(02)01116-x. [DOI] [PubMed] [Google Scholar]
- 9.Dunn JP. The surgeon and palliative care: an evolving perspective. Surg Oncol Clin N Am. 2001;10(1):7–24. [PubMed] [Google Scholar]
- 10.Dunn GP, Milch RA. Introduction and historical background of palliative care: where does the surgeon fit in? J Am Coll Surg. 2001;193(3):325–8. doi: 10.1016/s1072-7515(01)01019-5. [DOI] [PubMed] [Google Scholar]
- 11.Stebbing J, Crane J, Gaya A, et al. Breast cancer (metastatic) Clin Evid. 2006;(15):2331–59. [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network: Clinical practice guidelines in oncology: Breast cancer. Version. 3:2003. [Google Scholar]
- 13.McCahill LE, Krouse R, Chu D, et al. Indications and use of palliative surgery-results of Society of Surgical Oncology survey. Ann Surg Oncol. 2002;9(1):104–12. doi: 10.1245/aso.2002.9.1.104. [DOI] [PubMed] [Google Scholar]
- 14.Markus PM, Martell J, Leister I, et al. Predicting postoperative morbidity by clinical assessment. Br J Surg. 2005;92(1):101–6. doi: 10.1002/bjs.4608. [DOI] [PubMed] [Google Scholar]
- 15.Smith DD, McCahill LE. Predicting life expectancy and symptom relief following surgery for advanced malignancy. Ann Surg Oncol. 2008;15(12):3335–41. doi: 10.1245/s10434-008-0162-1. [DOI] [PubMed] [Google Scholar]


