Abstract
Background
Acute myeloid leukemia (AML) is treated with conventional induction chemotherapy shortly after diagnosis for most patients ≤ 65 years old. A recent report suggested a substantial decline in the early, or one-month, mortality rate in patients treated on clinical trials over the past 2 decades. It is unknown if a similar improvement has been observed in the general population.
Methods
We examined the one-month mortality in a large population-based series of 9,380 AML patients ≤ age 65 diagnosed and treated with chemotherapy between 1973 and 2010.
Results
We observed a significant decline in the one-month mortality rate from 18.7% among patients diagnosed from 1973–1977 (95% CI 16.4–21.2%) to 5.8% for those diagnosed in 2008–2010 (95% CI 4.5–7.6%) (p-value < 0.001). Median overall survival (OS) improved significantly from 6 months (95% CI 5–7) in 1973–1977 to 23 months (95% CI 16–20) in 2008–2010 (p-value < 0.001). Though age and geographic variation significantly influenced one-month mortality in 1973–1977, these differences in one-month mortality were no longer significant in AML patients treated more recently (2008–2010).
Conclusions
Over the past four decades, early mortality has become uncommon in younger patients (≤ 65 years) with newly diagnosed AML undergoing induction chemotherapy. It is encouraging that the improvements seen in one-month mortality in a selective cohort of clinical trial patients are also observed in a population-based analysis.
Keywords: Leukemia, epidemiology, outcomes, early death, treatment-related mortality
Background
Without treatment, acute myeloid leukemia (AML) is uniformly fatal in weeks to months.1 For the past 40 years, most younger (≤ 65 years) patients with AML receive treatment combining infusional cytarabine with an anthracycline (most commonly daunorubicin, using the 7+3 regimen) shortly after diagnosis.2, 3 With this combination, the complete remission (CR) rate in patients younger than age 60 ranges between 60 and 80%.4, 5 The CR rates in patients over age 60 are significantly lower.6, 7 Primary refractory disease is reported in approximately 15 to 30% of younger patients, depending on the series and type of induction therapy. The remaining AML patients experience a fatal complication within one month of diagnosis (known as early death or treatment-related mortality).8–10 Death most commonly occurs from infectious or bleeding complications related to cytopenias. The risk of mortality decreases significantly four weeks from the time of treatment initiation.10
Retrospective data combined from two large cohorts of patients treated on clinical trials indicate that one-month mortality has declined significantly over the past two decades.11 Reasons for this decrease may relate to improved supportive measures during the period of marrow aplasia, including empiric initiation of potent, broad-spectrum antibiotics and antifungals, strict adherence to guidelines regarding management of neutropenic fever, and improved transfusion support prior to count recovery.
It remains unknown, however, whether the decline in early mortality rates reported among highly selected patients treated on clinical trials and in tertiary care centers has also been observed in the general population. It has been estimated that only 5 to 10% of AML patients participate in clinical trials.12 In this study, we set out to examine the one-month mortality in a large population-based series of patients with AML undergoing chemotherapy using a representative national sample.
Methods
Study Population
We obtained from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI) information regarding all 10,940 patients diagnosed with a first primary AML [International Classification of Disease for Oncology, 3rd Edition, (ICD-O-3) histology codes 9840, 9861, 9865, 9867, 9870–9874, 9891, 9895–9897, 9910, 9920, 9930, 9931] between the age of 18 and 65 during the period January 1, 1973 through December 31, 2011 in 9 SEER regions [San Francisco-Oakland, Connecticut, Detroit (Metropolitan), Hawaii, Iowa, New Mexico, Seattle (Puget Sound), Utah, Atlanta (Metropolitan)] for which data was available since 1973. We excluded patients with acute promyelocytic leukemia (ICD-O-3 code 9866). The cancer registrar recorded AML cases based on the interpreting pathologist’s documentation. We excluded, in a hierarchical manner, 54 cases who were diagnosed by death certificate only, 81 who had zero days of survival, and 1,425 who did not have a record of ever having received chemotherapy. The final study population included 9,380 patients. For each AML case, we obtained information routinely abstracted from the medical record regarding patient age at diagnosis, race/ethnicity, stage of diagnosis, as well as treatment within the first 12 months after diagnosis. We also obtained vital status as of December 31st, 2011 and the underlying cause of death from the death certificate.
For analysis of effect of time period on one-month mortality, we divided the cohort into 5-year increments based on date of diagnosis. For analysis of effect of geographical location, we divided the 9 SEER regions into three categories based on one-month mortality from 1973–1977: lowest early death rate (Detroit Metropolitan); intermediate early death [Atlanta (Metropolitan), Connecticut, Hawaii, Iowa, San Francisco-Oakland SMSA, Seattle (Puget Sound), and Utah]; and highest early death rate (New Mexico). We assessed the one-month mortality in these three cohorts over time, using the same five-year increments.
Statistical analysis
We used the software program SEER*Stat (version 6.7, NCI, Bethesda, MD) to calculate overall survival (OS) rates, median OS, one-month mortality rate (and associated 95% confidence intervals) from the vital status recorded in SEER with follow-up through December 31, 2010 by year of diagnosis. We calculated the one-month mortality rate event as death occurring within one month of the recorded date of AML diagnosis.
Linear regression weighted on standard error was used to measure and test for statistical significance of the annual trend in one-month mortality rates across time periods. Regressions and calculations of R2 and β statistics were carried out using SAS software version 9.3 (SAS Institute, Cary, North Carolina). All P values reported were two-sided, and those that were <0.05 were considered to be statistically significant. Statistical analysis was performed by L.T. This project was overseen by the institutional review board of the Cancer Prevention Institute of California.
Results
Baseline Characteristics
The baseline characteristics of the 9,380 patients who met the inclusion criteria between 1973 and 2010 are presented in Table 1. As expected, there was an elevated male to female ratio (1.23:1), and non-Hispanic whites represented the majority of patients (75.4%). The incidence of AML increased with advancing age, while the median age at diagnosis over the study period remained unchanged (50 years for entire cohort). The incidence rate of AML remained stable when measured in five-year increments from 1973 to 2010. The one-month mortality for the entire cohort was 10.4% (95% CI 9.8–11.0).
Table 1.
Demographic and clinical characteristics for patients diagnosed with AML, SEER 9 registry, 1973–2010.
| Baseline characteristic | Population (n = 9,380) |
|---|---|
| Age at diagnosis (≥18 to ≤65): Median | 50 |
| 18–25 | 815 (8.7%) |
| 26–35 | 1233 (13.1%) |
| 36–45 | 1673 (17.8%) |
| 46–55 | 2406 (25.7%) |
| 56–65 | 3253 (34.7%) |
| SEER registry | |
| Atlanta (Metropolitan) - 1975+ | 830 (8.8%) |
| Connecticut - 1973+ | 1304 (13.9%) |
| Detroit (Metropolitan) - 1973+ | 1652 (17.6%) |
| Hawaii - 1973+ | 480 (5.1%) |
| Iowa - 1973+ | 1117 (11.9%) |
| New Mexico - 1973+ | 493 (5.3%) |
| San Francisco-Oakland SMSA - 1973+ | 1506 (16.1%) |
| Seattle (Puget Sound) - 1974+ | 1390 (14.8%) |
| Utah - 1973+ | 608 (6.5%) |
| Sex | |
| Female | 4206 (44.8%) |
| Male | 5174 (55.2%) |
| Race/ethnicity | |
| Non-Hispanic White | 7077 (75.4%) |
| Non-Hispanic Black | 815 (8.7%) |
| Hispanic | 599 (6.4%) |
| Non-Hispanic Asian or Pacific Islander | 820 (8.7%) |
| Non-Hispanic American Indian/Alaska Native | 57 (0.6%) |
| Unknown | 12 (0.1%) |
| Rural-Urban Continuum | |
| Nonmetropolitan | 1291 (13.8%) |
| Metropolitan | 8089 (86.2%) |
| Year of diagnosis | |
| 1973–1977 | 1011 (10.8%) |
| 1978–1982 | 1033 (11.0%) |
| 1983–1987 | 1113 (11.9%) |
| 1988–1992 | 1120 (11.9%) |
| 1993–1997 | 1294 (13.8%) |
| 1998–2002 | 1425 (15.2%) |
| 2003–2007 | 1475 (15.7%) |
| 2008–2010 | 909 (9.7%) |
We observed changes in the composition of AML diagnoses with respect to histologic classification over time. The introduction of the cytogenetic-based World Health Organization classification in 2001 and the associated change in diagnostic codes to ICD-O-3 provided more granularity in AML subtyping (Supporting Table 1). The new classification of AML entities led to a decrease in the percentage of patients coded as 9861/3 (acute myeloid leukemia) from 78.6% in 1973–2000 to 46.4% in 2001–2010. For those entities where cytogenetic-based classification is not relevant, such as 9840/3 (acute erythroid leukemia), the percentage of patients reported remained stable (2.1% from 1973–2000 and 2.1% from 2001–2010).
Effect of time period of diagnosis on median OS and one-month mortality
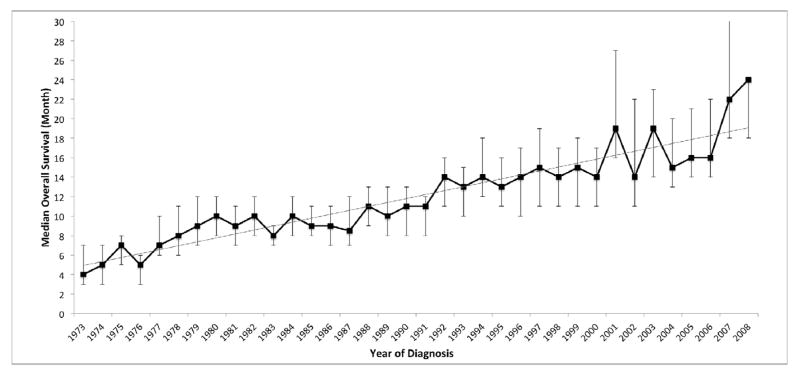
We assessed whether time period of diagnosis affected the median OS. Throughout the study period, the median age at diagnosis ranged between 50 and 52 years (Table 2). During this time, however, we observed a significant improvement in the median OS from 6 months (95% CI 5–7) in patients diagnosed between 1973 and 1977 to 18 months (95% CI 16–20) in patients diagnosed between 2003 and 2007. At the time of analysis, patients diagnosed in 2009 and 2010 did not have adequate follow up to calculate their median OS, but the median OS for patients diagnosed in 2008 had improved further to 23 months (95% CI 20–31; Table 2 and Figure 1) (p-value < 0.001). Linear regression analysis confirmed a strong linear trend for improvement in median OS over time (Figure 1; R2 = 0.86).
Table 2.
One-month mortality after diagnosis of AML, by period of diagnosis, SEER 9 registry, 1973–2010. CI = confidence interval.
| Period | Median Age at Diagnosis | Median Overall Survival (months, 95% CI) | 1 Month Mortality (%, 95% CI) |
|---|---|---|---|
| 1973–1977 | 52 | 6 (5–7) | 18.7 (16.4–21.2) |
| 1978–1982 | 51 | 10 (8–10) | 15.5 (13.4–17.8) |
| 1983–1987 | 51 | 9 (8–10) | 12.5 (10.7–14.6) |
| 1988–1992 | 50 | 11 (10–12) | 9.6 (8.1–11.5) |
| 1993–1997 | 50 | 13 (13–15) | 8.3 (7.0–10.0) |
| 1998–2002 | 50 | 16 (14–17) | 7.9 (6.6–9.5) |
| 2003–2007 | 52 | 18 (16–20) | 7.2 (6.0–8.6) |
| 2008–2010 | 52 | 23 (20–31) | 5.8 (4.5–7.6) |
Figure 1.
Median overall survival (months, %95 CI) for AML patients, by year of diagnosis, SEER 9 Registries, 1973–2010. Median survival was not available for patients diagnosed in 2009 and 2010. Test for linear trend: R2 = 0.86. β = 0.4042.
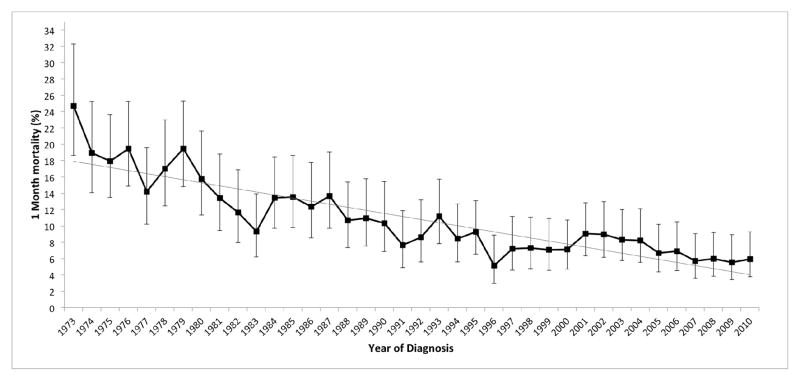
Next we determined the one-month mortality rate for AML patients who received chemotherapy (Table 2 and Figure 2). Our analysis revealed a significant temporal decline in the one-month mortality from 18.7% (95% CI 16.4–21.2) between 1973 and 1977 to 7.2% (95% CI 6.0–8.6) between 2003 and 2007. The lowest one-month mortality was noted in patients diagnosed between 2008 and 2010 (5.8%, 95% CI 4.5–7.6). Weighted linear regression analysis revealed a strong linear trend in the one-month mortality rate (Figure 2; R2 = 0.78), with a steady annual decrease in the rate of approximately 0.4% per year (β = 0.3755). Notably, we observed a similar decline in two-month mortality over the study period (data not shown). Together, in our cohort over time, we observed a parallel increase in median OS and decrease in one-month mortality.
Figure 2.
One-month mortality (%, 95% CI) for AML patients, by year of diagnosis, SEER 9 Registries, 1973–2010. Test for linear trend by weighted linear regression analysis: R2 = 0.78, p < 0.001. β = −0.3755.
Impact of age on one-month mortality rates
To examine the effect of age on the one-month mortality rates of AML patients, we divided the cohort into three age groups spanning approximately 15 years each (Supporting Table 2). For all three age groups, the one-month mortality was highest in 1973–1977, with older patients experiencing the highest early death rates (Supporting Table 2 and Figure 3). Over the study period, a significant improvement in the one-month mortality rates was observed in all age cohorts. The largest absolute improvements were noted in older patients (approximately 15% absolute decrease in one-month mortality for patients aged 51–65 over entire time period). Linear regression analysis showed a strong trend for decrease in one-month mortality in all age cohorts (Figure 3). For all age cohorts, one-month mortality rate was lowest in the most recent time period (2008–2010), without significant differences in one-month mortality among age cohorts (p-value = 0.3347 between the 51–65 year cohort and 18–35 year cohort; p-value = 0.2531 between 36–50 year cohort and 18–35 year cohort). In summary, one-month mortality rates have improved among AML patients ≤ 65 years, with particular benefit noted in patients between the ages of 51 and 65 years at diagnosis.
Figure 3.
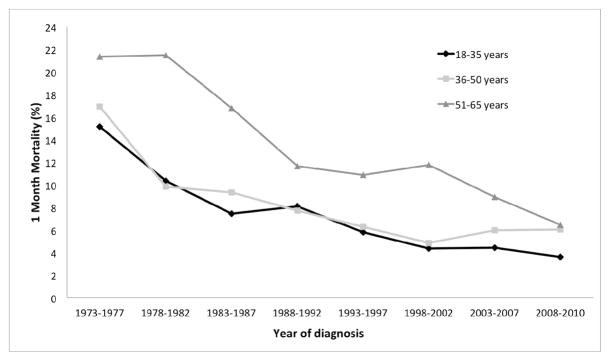
One-month mortality (%) for AML patients, by year of diagnosis and age at diagnosis, SEER 9 Registries, 1973–2010. Statistics for 18–35 years: R2 = 0.8500 and β = −1.4507; for 36–50 years, R2 = 0.6934 and β = −1.3138; for 51–65 years, R2 = 0.9063 and β = −2.1761.
Geographic variation in one-month mortality rates
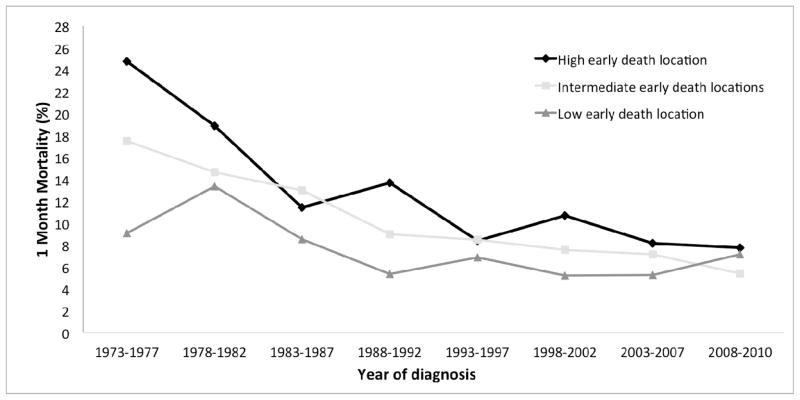
We next examined whether location at diagnosis had an effect on one-month mortality. We examined the one-month mortality in the nine SEER regions, and found improvement over time in each region (Supporting Figure 1). We further divided the nine SEER regions into 3 groups based on one-month mortality in the earliest time period included in our analysis (1973–1977); then the one-month mortality in these 3 groups was evaluated over time. Little absolute improvement in the one-month mortality rate was observed in the group with initial low early death, decreasing minimally from 9.1% (95% CI 3.5–22.4%) between 1973 and 1977 to 7.1% (95% CI 2.7–17.9%) between 2008 and 2010 (Supporting Table 3 and Figure 4). Conversely, our analyses revealed significant declines in one-month mortality in the cohorts of patients with high and intermediate early death rates. This mismatched improvement of the one-month mortality resulted in a convergence of the early death rates observed among AML patients from all geographic areas in the 2008–2010 period (Figure 4). As such, our results demonstrated a lack of significant differences in one-month mortality in the 2008–2010 period (p = 0.4616 for intermediate compared to high early death locations; p = 0.9119 for low compared to high early death locations).
Figure 4.
One-month mortality (%) for AML patients, by year of diagnosis and location, SEER 9 Registries, 1973–2010. Rate of early death was determined in each location during earliest time period (1973–1978). Statistics for high early death location, R2 = 0.7626 and β = −2.1482; for intermediate early death locations, R2 = 0.923 and β = −1.6536; for low early death location, R2 = 0.4381 and β = −0.7434.
Discussion
Though induction chemotherapy for patients with AML has not changed substantially since its introduction in 1973, our population-based study demonstrates that one-month mortality in 9,380 patients ≤ 65 years undergoing initial chemotherapy has decreased by nearly 70% over the same time frame. This improvement in one-month mortality was noted in all patient cohorts, independent of age or geographic region at diagnosis. Our results also reveal that one-month mortality has particularly improved in patients between 51 and 65 years, and from certain geographic areas with high and intermediate initial one-month mortality rates.
The reasons for the decreases in one-month mortality remain largely unexplained, as there is no clear inflection point in the observed improvement over time. Recently, significant improvements in survival attributable to supportive care were reported in patients with hematologic malignancies after allogeneic transplant, and similar mechanisms likely account for the improvements observed here in AML patients.13 Bleeding and infections are the most common issues leading to treatment-related early death; relevant advances in the management of AML patients undergoing aggressive chemotherapy over this time period include the use of rigorous prophylactic platelet transfusions and breakthroughs in the management of infectious complications, particularly fungal disease.14–16
Our observations are particularly powerful since the study dataset was derived from a large, representative, national cohort identified from the high-quality SEER program. The program covers 28% of new cancer diagnoses in the United States, though the coverage was lower in 1973 when SEER was established and when the data for this study began collection. Additionally, the patient characteristics in our cohort have generally remained similar over time (Table 1). Although improvements in early death rates have been reported in patients participating in AML clinical trials,11 such beneficial effects in outcomes are frequently not observed in subjects receiving therapy in the “real world.”17 Additionally, only 5–10% of patients with AML in the United States are estimated to participate in clinical trials, potentially limiting the generalizability of those findings.12 In fact, in a highly curable subtype of AML, acute promyelocytic leukemia (APL), a clear disparity exists between the low early death rates observed on clinical trials (approximately 10%) compared to the much higher rates seen in population-based analyses (ranging from approximately 15–30%).18–21 These differences in outcomes exist even though recent data indicate that over 70% of newly diagnosed APL patients participate in research protocols.22 However, in contrast to the findings in patients with APL, our results in a cohort of AML patients drawn from the general population find improvements over time in one-month mortality comparable to those observed in a large clinical trial-based population.11 An unusual finding in our study was the large variability in one-month mortality based on geographic location in the earliest time periods; further study should be devoted to answering this question.
Our study had the benefit of being large and representative, but has several important limitations. SEER data do not contain details regarding what type of chemotherapy regimen patients received (including potential need for re-induction chemotherapy), compliance to therapy, nor when chemotherapy was started. Therefore, if delays existed in diagnosis or initiation of treatment, the one-month survival rate we reported might instead be a less meaningful 2 or 3-week survival rate for some patients; however, we did also observe a decrease in 2-month mortality over the same time period, which suggests that the trend in improvement is valid regardless of variations in timing of initiation of chemotherapy. The SEER dataset also does not include standard baseline characteristics often used to risk-stratify AML patients such as performance status, white blood cell count, percentage of bone marrow blasts, and cytogenetics, though it seems unlikely that these characteristics would vary significantly over time in the population.5 During the study period, the percentage of blasts needed to make the diagnosis of AML changed from 30 to 20%; however, this change in classification would affect any longitudinal study of AML patients.
Although the median age at the time of AML diagnosis is 67, we chose to limit our study population to patients between the ages of 18 to 65 years.23 First, we performed our analyses only in patients confirmed to have received at least one dose of chemotherapy, which is not universally considered in patients over the age of 65 at diagnosis. In fact, a recent analysis of a SEER-Medicare linked database of patients over the age of 65 with newly diagnosed AML demonstrates only 40% of these patients receive chemotherapy (Medeiros, BC, personal communication). Also, the cohort of 9,380 patients we selected likely received relatively homogenous therapies, as population-based registries have previously shown that only 5% of patients younger than age 65 are not treated with induction chemotherapy.3, 24 Finally, it has been previously demonstrated that advanced age in AML is associated with disproportionately high early mortality, decreased treatment rates, and decreased use of conventional induction chemotherapy with preference for lower-intensity regimens, such as low-dose cytarabine or hypomethylating agents.25–28 The goal of our analysis was to examine patients likely to have undergone standard induction chemotherapy, and patients over 65 likely did not meet that criterion.29 We would expect that only a small minority of patients in our cohort would have been treated upfront with hypomethylating agents since azacitidine was only FDA-approved in 2004 and is typically used in patients over the age of 60.
Overall, the improvements in early mortality and OS observed in our population-based cohort of AML patients are reassuring and consistent with clinical trial results.11 Previous analyses have determined that age and performance status are the two most important variables influencing treatment-related mortality in AML.10 Our results fail to demonstrate a significant inflection point in the early death rates or OS for younger AML patients, suggesting that advances in routine clinical care during the study period are responsible for the progressive and continuous improvements we observed. Finally, our results suggest that concerns regarding increased early mortality should not be a deterrent to induction chemotherapy in patients under the age of 65 with AML.
Supplementary Material
Acknowledgments
Funding: Stanford Cancer Institute (C.A.C.); Conquer Cancer Foundation of ASCO Young Investigator Award (M.-E.M.P.); KL2 Mentored Career Development Award (M.-E.M.P.) of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR 001083)
This work was conducted with the support of the Stanford Cancer Institute (C.A.C.), and additionally funded by a Conquer Cancer Foundation of ASCO Young Investigator Award (M.-E.M.P.) and a KL2 Mentored Career Development Award (M.-E.M.P.) of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR 001083). The ideas and opinions expressed herein are those of the authors and endorsement by the state of California, Department of Public Health or the NCI or their Contractors or Subcontractors is not intended nor should be inferred. Any opinions, findings, and conclusions expressed in this material are those of the authors and do not necessarily reflect those of the American Society of Clinical Oncology® or the Conquer Cancer Foundation.
Footnotes
Authorship
Contribution: All authors designed the study, analyzed and interpreted data, and participated in writing the manuscript; L.T. performed data collection and statistical analysis.
Conflict-of-interest disclosure: The authors have no relevant conflict of interest to disclose.
References
- 1.Kantarjian H, O’Brien S, Cortes J, Wierda W, Faderl S, Garcia-Manero G, et al. Therapeutic advances in leukemia and myelodysplastic syndrome over the past 40 years. Cancer. 2008;113(7 Suppl):1933–52. doi: 10.1002/cncr.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yates JW, Wallace HJ, Jr, Ellison RR, Holland JF. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer chemotherapy reports Part 1. 1973;57(4):485–8. [PubMed] [Google Scholar]
- 3.Juliusson G, Antunovic P, Derolf A, Lehmann S, Mollgard L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179–87. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 4.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368(9550):1894–907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 5.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 6.Thein MS, Ershler WB, Jemal A, Yates JW, Baer MR. Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer. 2013;119(15):2720–7. doi: 10.1002/cncr.28129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krug U, Rollig C, Koschmieder A, Heinecke A, Sauerland MC, Schaich M, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet. 2010;376(9757):2000–8. doi: 10.1016/S0140-6736(10)62105-8. [DOI] [PubMed] [Google Scholar]
- 8.Schlenk RF, Benner A, Hartmann F, del Valle F, Weber C, Pralle H, et al. Risk-adapted postremission therapy in acute myeloid leukemia: results of the German multicenter AML HD93 treatment trial. Leukemia. 2003;17(8):1521–8. doi: 10.1038/sj.leu.2403009. [DOI] [PubMed] [Google Scholar]
- 9.Jabbour E, Daver N, Champlin R, Mathisen M, Oran B, Ciurea S, et al. Allogeneic stem cell transplantation as initial salvage for patients with acute myeloid leukemia refractory to high-dose cytarabine-based induction chemotherapy. American journal of hematology. 2013 doi: 10.1002/ajh.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter RB, Othus M, Borthakur G, Ravandi F, Cortes JE, Pierce SA, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29 (33):4417–23. doi: 10.1200/JCO.2011.35.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Othus M, Kantarjian H, Petersdorf S, Ravandi F, Godwin J, Cortes J, et al. Declining rates of treatment-related mortality in patients with newly diagnosed AML given ‘intense’ induction regimens: a report from SWOG and MD Anderson. Leukemia. 2013 doi: 10.1038/leu.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe JM. Closer to the truth in AML. Blood. 2009;113(18):4129–30. doi: 10.1182/blood-2008-12-192856. [DOI] [PubMed] [Google Scholar]
- 13.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. The New England journal of medicine. 2010;363(22):2091–101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higby DJ, Cohen E, Holland JF, Sinks L. The prophylactic treatment of thrombocytopenic leukemic patients with platelets: a double blind study. Transfusion. 1974;14(5):440–6. doi: 10.1111/j.1537-2995.1974.tb04558.x. [DOI] [PubMed] [Google Scholar]
- 15.Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. The New England journal of medicine. 1999;340(10):764–71. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- 16.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. The New England journal of medicine. 2007;356(4):348–59. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 17.Elting LS, Cooksley C, Bekele BN, Frumovitz M, Avritscher EB, Sun C, et al. Generalizability of cancer clinical trial results: prognostic differences between participants and nonparticipants. Cancer. 2006;106(11):2452–8. doi: 10.1002/cncr.21907. [DOI] [PubMed] [Google Scholar]
- 18.Mandelli F, Diverio D, Avvisati G, Luciano A, Barbui T, Bernasconi C, et al. Molecular remission in PML/RAR alpha-positive acute promyelocytic leukemia by combined all-trans retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano-Malattie Ematologiche Maligne dell’Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood. 1997;90(3):1014–21. [PubMed] [Google Scholar]
- 19.McClellan JS, Kohrt HE, Coutre S, Gotlib JR, Majeti R, Alizadeh AA, et al. Treatment advances have not improved the early death rate in acute promyelocytic leukemia. Haematologica. 2012;97(1):133–6. doi: 10.3324/haematol.2011.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann S, Ravn A, Carlsson L, Antunovic P, Deneberg S, Mollgard L, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia. 2011;25 (7):1128–34. doi: 10.1038/leu.2011.78. [DOI] [PubMed] [Google Scholar]
- 21.Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118(5):1248–54. doi: 10.1182/blood-2011-04-346437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Micol JB, Raffoux E, Boissel N, Lengline E, Canet E, Daniel MT, et al. Management and treatment results in patients with acute promyelocytic leukaemia (APL) not enrolled in clinical trials. European journal of cancer. 2014;50(6):1159–68. doi: 10.1016/j.ejca.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Pollyea DA, Kohrt HE, Medeiros BC. Acute myeloid leukaemia in the elderly: a review. British journal of haematology. 2011;152(5):524–42. doi: 10.1111/j.1365-2141.2010.08470.x. [DOI] [PubMed] [Google Scholar]
- 24.Juliusson G. Older patients with acute myeloid leukemia benefit from intensive chemotherapy: an update from the Swedish Acute Leukemia Registry. Clinical lymphoma, myeloma & leukemia. 2011;11 (Suppl 1):S54–9. doi: 10.1016/j.clml.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Blum W, Garzon R, Klisovic RB, Schwind S, Walker A, Geyer S, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(16):7473–8. doi: 10.1073/pnas.1002650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28 (4):562–9. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 27.Lang K, Earle CC, Foster T, Dixon D, Van Gool R, Menzin J. Trends in the treatment of acute myeloid leukaemia in the elderly. Drugs & aging. 2005;22(11):943–55. doi: 10.2165/00002512-200522110-00004. [DOI] [PubMed] [Google Scholar]
- 28.Burnett AK, Milligan D, Goldstone A, Prentice A, McMullin MF, Dennis M, et al. The impact of dose escalation and resistance modulation in older patients with acute myeloid leukaemia and high risk myelodysplastic syndrome: the results of the LRF AML14 trial. British journal of haematology. 2009;145(3):318–32. doi: 10.1111/j.1365-2141.2009.07604.x. [DOI] [PubMed] [Google Scholar]
- 29.Menzin J, Lang K, Earle CC, Kerney D, Mallick R. The outcomes and costs of acute myeloid leukemia among the elderly. Archives of internal medicine. 2002;162(14):1597–603. doi: 10.1001/archinte.162.14.1597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.