Abstract
Aims
To investigate the correlation between the ‘perfusion index’ (PI) and other commonly used estimates of cutaneous blood flow [heart rate (HR), surface temperatures (ST) and central-to-peripheral thermal gradients (C-P grad)] and to use this new non-invasive tool to compare differences between prone and supine sleep position in low birth weight (LBW) infants.
Methods
Six-hour continuous recordings of pulse oximetry, cardiac activity and absolute ST from three sites (flank, forearm and leg), along with minute-to-minute assessment of behavioural states were performed in 31 LBW infants. Infants were randomly assigned to the prone or supine position for the first 3 h and then reversed for the second 3 h. PI data were correlated with HR and C-P grad, and compared across sleep positions during quiet sleep (QS) and active sleep (AS).
Results
Perfusion index correlated significantly with HR (r2 = 0.40) and flank-to-forearm thermal gradient (r2 = 0.28). In the prone position during QS, infants exhibited higher PI (3.7 ± 0.9 vs. 3.1 ± 0.7), HR (158.4 ± 8.9 vs. 154.1 ± 8.8 bpm), SpO2 (95.8 ± 2.6 vs. 95.2 ± 2.6%), flank (36.7 ± 0.4 vs. 36.5 ± 0.4°C), forearm (36.1 ± 0.6 vs. 35.5 ± 0.4°C) and leg (35.4 ± 0.7 vs. 34.7 ± 0.7°C) temperatures and narrower flank-to-forearm (0.6 ± 0.4 vs. 0.9 ± 0.3°C) and flank-to-leg (1.3 ± 0.6 vs. 1.8 ± 0.7°C) gradients, compared to those of the supine position. Similar differences were observed during AS.
Conclusion
Perfusion index is a good non-invasive estimate of tissue perfusion. Prone sleeping position is associated with a higher PI, possibly reflecting thermoregulatory adjustments in cardiovascular control. The effects of these position-related changes may have important implications for the increased risk for sudden infant death syndrome in prone position.
Keywords: Active sleep, Prone, Quiet sleep, Sudden infant death syndrome, Supine
INTRODUCTION
Sudden infant death syndrome (SIDS) is a major cause of death in early infancy (1). Epidemiological data relate SIDS to prone body positioning during sleep (2,3) and the incidence of SIDS has decreased coincident with public health measures to reduce the incidence of prone positioning during sleep (4). Numerous physiological differences related to body position have been reported (5–9), and several hypotheses have been formulated to explain how these differences might render infants more vulnerable to SIDS. One hypothesis relates prone-sleep vulnerability to relative hyperthermia (10–12). Sleeping in the prone position impairs heat loss and leads to increased heat storage. The cardiovascular response to increased heat storage is an increase in cardiac output [increased heart rate (HR)] and tissue perfusion resulting from thermoregulatory cutaneous vasodilatation. Alterations in tissue perfusion are difficult to assess. Clinical evaluation (warmth and coolness of skin, and capillary refill time) and central-to-peripheral temperature gradients (C-P grad) have been used as indirect estimates of alterations in cutaneous blood flow, but as with other variables, conclusions regarding peripheral vasodilatation have not been validated by direct measurements (13–18).
The perfusion index (PI), a non-invasive estimate of tissue perfusion, is obtained by comparing the pulsatile signal from pulse oximetry with the non-pulsatile signal. The non-pulsatile signal, which emanates from skin, other tissues and venous or non-pulsatile blood is expressed as percentage of the pulsatile signal and can be useful in assessing local cutaneous vasomotor changes (19,20). Its measurement is influenced primarily by the amount of blood at the monitoring site and not by the level of oxygen saturation (SpO2) of the arterial blood. The PI reflects the real-time changes in peripheral blood flow and would be expected to be affected by changes in the arterial circulation.
A detailed study of the interactions among peripheral tissue perfusion, cardiac activity, surface temperature (ST) profiles, temperature gradients and body position during different sleep states in low birth weight (LBW) infants may provide important information concerning physiological disturbances that underlie SIDS, a condition to which LBW infants are especially susceptible as they grow into early infancy. Having previously observed higher HR, and SpO2 and lower variability in HR in the prone position compared with that in the supine position in LBW infants (6,7,9), we hypothesized that peripheral perfusion would be similarly sensitive to changes in body position. To test this hypothesis, we evaluated a new pulse oximetry-derived non-invasive estimate of tissue perfusion, the ‘perfusion index’. The objectives of this study were to correlate PI with other indirect estimates of cutaneous blood flow [cardiac activity (HR) and C-P grad] and compare PI, SpO2, HR, STs and C-P grad in prone and supine positions during quiet sleep (QS) and active sleep (AS) states in LBW infants.
PATIENTS AND METHODS
Patient population
The patient population was comprised of 31 growing LBW infants, ranging in birth weight from 625 to 1230 (mean 966 ± 247) g and in gestational age from 24 to 29 (mean 26.4 ± 1.6) weeks. All subjects were enrolled in a prospective, double-blind, randomized, controlled, nutritional study. The study was approved by the Institutional Review Board at Columbia University Medical Center and written consent was obtained from parents of all infants. All infants were being maintained in room air, were free of apnoea of prematurity and were receiving no cardiac or respiratory medications. None had sonographic evidence of central nervous system pathology. Infants ranged in weight from 1075 to 2310 (mean 1677 ± 371) g and 29–38 (mean 33.0 ± 2.9) weeks in post-conceptional age at the time of the study,
Experimental design
The infants were part of a prospective study of the effects of aggressive parenteral amino acid supplementation and were randomized to one of two different nutritional regimens. As a part of the study, the infants were subjected to 6-h measurements of cardiorespiratory activity, and thermal profile along with behavioural sleep state assessments. These studies were performed in the Infant Physiology Laboratory at Children’s Hospital of New York, beginning when the infant reached full enteral intake. Each 6-h study was comprised of two sequential 3-h periods of observation separated by a feeding period. Infants were randomly assigned to prone or supine position for the first 3 h of the study starting after 8:00 AM feed and the position was reversed for the second 3 h of the study which began after the 11:00 AM feed. The volume and composition of the two feeds were identical. The infants remained in their assigned positions throughout the inter-feeding period, and no further manipulations were performed.
Experimental protocol
After they had been on full enteral intakes for a minimum of 3 days, infants were brought to the laboratory, located adjacent to the nursery, at approximately 7:30 AM for 6-h measurements of cardiorespiratory activity, thermal profile and behavioural state assessments. They were placed in a Plexiglas isolette, and pulse oximeter sensor, electrodes for recording electrocardiogram and thermistors for ST were attached. No physical constraints such as swaddling were employed during the study period. The infants were clothed in diapers and undershirts and no supplemental heat was provided.
Measurement of pulse oximetry data
Pulse oximetry-derived PI, instantaneous heart rate (HR-P) and SpO2 were measured using the Masimo Set Radical oximeter (Masimo Corporation, Irvine, CA, USA). The disposable adhesive pulse oximeter sensor (Masimo low-noise optical probe Neo) was placed on the left foot and connected to the oximeter to verify that the pulse wave was artefact free. The pulse oximeter data were collected electronically using an RS-232 serial communication port and recorded digitally once per second throughout the entire study.
Measurement of heart rate
The electrocardiogram was obtained from a standard heart rate monitor (Hewlett Packard 3680) and the intervals between the RR-waves were processed using a special purpose RR-interval preprocessor. The preprocessor detects R-waves, measures the RR-interval (±1 msec) and passes these data to the computer over a parallel port.
Measurement of surface temperatures
Surface temperatures were recorded from 3 body sites, i.e. right abdominal flank, right forearm and right leg, using Incutemp® thermistors (Mallinckrodt, St. Louis, MO, USA). The thermistor on the flank was insulated and care was taken to ensure that it was not sandwiched between the infant and the mattress. Environmental temperatures, i.e. chamber temperature and room temperature, were also recorded using the same Incutemp® sensors. Temperatures from all sites were recorded every 8 sec. The temperature data were collected using a custom-made, multiplexed, self-calibrating device that logged measurements from each thermistor to a dedicated computer. The computer was, in turn, linked via a common clock to a sister computer that recorded continuous measurements of the other physiological variables.
Coding of behavioural state
Each minute of the study was coded for behavioural state. Coding began 10 min after the 8:00 AM feeding, continued until 11:00 AM feeding, resumed 10 min after the 11:00 AM feeding and terminated just prior to the 2:00 AM feeding. Behaviour codes were assigned by direct observation each minute using a scoring system developed and validated in our laboratory (21). Briefly, AS was coded whenever at least one rapid eye movement was observed during the minute. In addition to small body movements typical of AS, movements of whole extremities and the torso were seen in this state. Stretching, yawning, whimpering, sucking and grimacing were also present occasionally. QS was designated when the infant was asleep without any rapid eye movement. The use of 1-min epochs without the use of smoothing algorithms leads to more minute-to-minute variability in state assignment, but has the advantage of maintaining a tighter relationship between the assigned state and the simultaneous changes in physiological variables.
Data analysis
At the termination of each study, a computer file was constructed which contained minute-by-minute sleep state codes as well as averages of pulse oximetry (PI, HR-P, SpO2), RR-interval and temperature data. Mean heart rate (HR-M, in bpm) was computed as 60 times the inverse of the mean of RR-intervals measured during each block of time. RR-intervals exceeding 667 msec (<90 bpm) or shorter than 300 msec (>200 bpm), which were generally associated with brief periods of motion artefact, were excluded from the analyses. The average number of excluded RR-intervals was extremely small, <1% per minute. The temperature data from the abdominal flank were designated as central temperature. The forearm and the leg temperatures were referred to as peripheral temperatures. From these temperature data, C-P grad, i.e. flank-to-forearm and flank-to-leg temperature gradients, was computed for each minute. The PI was correlated with heart rate (HR-M and HR-P) and C-P grad, the other indirect estimates of tissue perfusion. Data were then averaged for prone and supine positions and for QS and AS. Differences between prone and supine data were analysed by within-subject, paired t-tests, within each of the two sleep states.
RESULTS
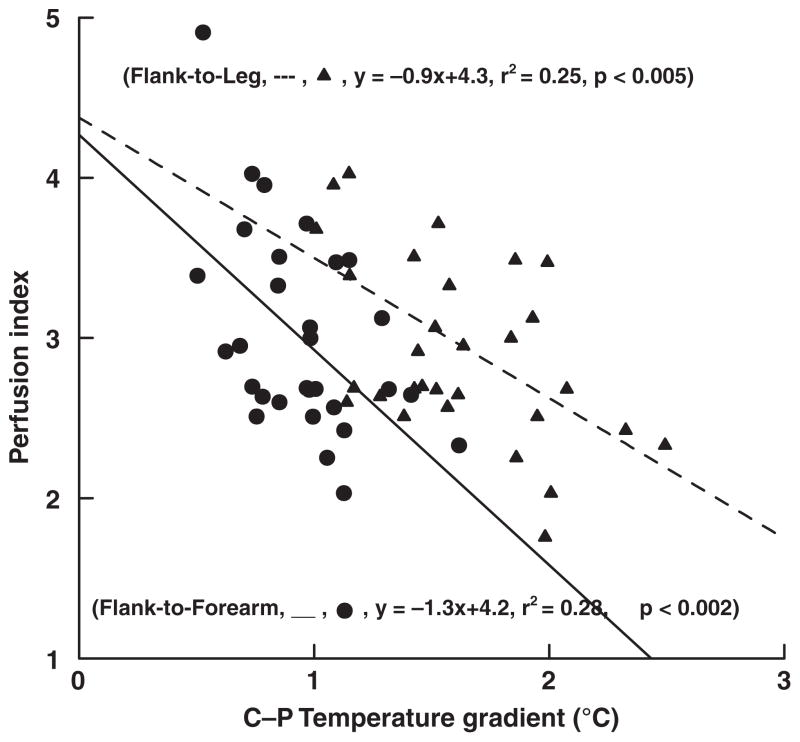
Perfusion index was positively correlated with both HR obtained from the pulse oximeter, HR-P (r2 = 0.39, p < 0.0001), and from the ECG, HR-M (r2 = 0.40, p < 0.0005), see Figure 1. By contrast, PI was negatively correlated with the C-P grad indices of tissue perfusion; differences between flank and forearm temperature (r2 = 0.28, p < 0.002) and differences between flank and leg temperature (r2 = 0.25, p < 0.005), see Figure 2.
Figure 1.
Relationship between perfusion index and RR-interval heart rate (HR-M) and instantaneous pulse oximeter heart rate (HR-P).
Figure 2.
Relationship between perfusion index and central-to-peripheral (C-P: flank-to-forearm and flank-to-leg) temperature gradients.
In prone position during QS, infants exhibited higher PI (3.7 ± 0.9 vs. 3.1 ± 0.7, p < 0.0005), HR-P (157.0 ± 7.9 vs. 153.1 ± 9.1 bmp, p < 0.0001), HR-M (158.4 ± 8.9 vs.154.1 ± 8.8 bpm, p < 0.005), SpO2 (95.8 ± 2.6 vs. 95.2 ± 2.6 %, p < 0.03), flank (36.7 ± 0.4 vs. 36.5 ± 0.4°C, p < 0.05), forearm (36.1 ± 0.6 vs. 35.5 ± 0.4°C, p < 0.0001) and leg (35.4 ± 0.7 vs. 34.7 ± 0.7, p < 0.001) STs and narrower C-P grad, i.e. flank-to-forearm (0.6 ± 0.4 vs. 0.9 ± 0.3°C, p < 0.005) and flank-to-leg (1.3 ± 0.6 vs. 1.8 ± 0.7°C, p < 0.005), compared to those of the supine sleeping position. There were no positional differences in the environmental temperature (27.1 ± 1.0 vs. 27.2 ± 0.9, NS). Similar prone–supine differences were observed during AS, as shown in Table 1.
Table 1.
Prone-supine differences in perfusion index, instantaneous pulse oximeter heart rate (HR-P); RR-interval heart rate (HR-M); oxygen saturation (SpO2); flank, forearm, leg and environment temperatures; and central-to-peripheral (flank-to-forearm and flank-to-leg) temperature gradients (mean ± SD)
| Variables | Quiet sleep
|
Active sleep
|
||||
|---|---|---|---|---|---|---|
| Prone | Supine | p | Prone | Supine | p | |
| Perfusion index | 3.7 ± 0.9 | 3.1 ± 0.7 | <0.0005 | 2.9 ± 0.9 | 2.3 ± 0.9 | <0.0001 |
| HR-P (bpm) | 157.0 ± 7.9 | 153.1 ± 9.1 | <0.0001 | 162.5 ± 9.5 | 159.5 ± 8.1 | <0.001 |
| HR-M (bpm) | 158.4 ± 8.9 | 154.1 ± 8.8 | <0.005 | 162.7 ± 9.5 | 160.2 ± 7.7 | 0.007 |
| SpO2 (%) | 95.8 ± 2.6 | 95.2 ± 2.6 | <0.03 | 94.8 ± 2.4 | 94.2 ± 2.5 | <0.02 |
| Flank temperature (°C) | 36.7 ± 0.4 | 36.5 ± 0.4 | <0.05 | 36.6 ± 0.3 | 36.5 ± 0.4 | NS |
| Forearm temperature (°C) | 36.1 ± 0.6 | 35.5 ± 0.4 | <0.0001 | 35.9 ± 0.3 | 35.4 ± 0.5 | <0.0001 |
| Leg temperature (°C) | 35.4 ± 0.7 | 34.7 ± 0.7 | <0.001 | 35.2 ± 0.4 | 34.6 ± 0.7 | <0.0001 |
| Environment temperature (°C) | 27.1 ± 1.0 | 27.2 ± 0.9 | NS | 27.1 ± 1.1 | 27.2 ± 1.0 | NS |
| Flank-to-forearm gradient (°C) | 0.6 ± 0.4 | 0.9 ± 0.3 | <0.005 | 0.7 ± 0.3 | 1.0 ± 0.4 | <0.005 |
| Flank-to-leg gradient (°C) | 1.3 ± 0.6 | 1.8 ± 0.7 | <0.005 | 1.4 ± 0.4 | 1.9 ± 0.5 | <0.0001 |
DISCUSSION
Our study addressed two issues: (i) whether the ‘Perfusion Index’ derived non-invasively from pulse oximetry was a reliable estimate of tissue perfusion and (ii) whether peripheral perfusion estimated PI was sensitive to changes in body position. We found that PI correlated significantly with other indirect estimates of cutaneous blood flow, i.e. cardiac activity (HR) and C-P grad, suggesting that it can be a useful tool in estimating peripheral perfusion non-invasively and continuously. In addition, infants sleeping in prone position during QS and AS exhibited higher PI, HR, SpO2, flank, forearm and leg temperatures and narrower C-P grad.
Skin perfusion is frequently used to assess adequacy of global blood flow. Clinical signs (skin temperature, pallor and texture, and capillary refill time) (13) and central-to-peripheral temperature difference (13,15) have been utilized to assess peripheral perfusion and alterations in flow under different physiological states. Although these estimates may be altered during poor perfusion states, the interpretation still remains subjective. Assessment of capillary refill time is difficult during critical medical conditions, and application of peripheral temperature measurements is often limited in emergency situations (22). Furthermore, the practical application of these indices and the relationship with central hemodynamics or tissue oxygenation are not well studied. In adults with cardiogenic shock and in cardiac surgery patients, a crude correlation between central-to-toe temperature difference and cardiac output has been reported (14,23). In paediatric post-cardiac surgical patients, both capillary refill time and temperature gradients do not correlate well with the hemodynamic variables (13). However, in general intensive care paediatric patients, most of whom had septic shock, these indices of peripheral perfusion did correlate significantly with global hemodynamics and blood lactate concentrations (13). With recent developments in pulse oximetry, it is now possible to evaluate tissue perfusion using PI, which is a numerical value that correlates with the strength of the infrared signal returning from the monitored site and is determined by the local blood flow (19,20). Furthermore, as tissue PI varies with the quantity of red blood cells in the skin microvasculature, it has been used as an indicator of alterations in cutaneous blood flow in both humans and animals (24). Recent studies suggest that PI may be an accurate predictor of illness severity (25–27) and a useful tool for early detection of critical left heart obstruction in neonates (28), and tissue perfusion in adults (29). Also in neonates, continuous measurement of PI over the foot by pulse oximetry is more feasible for peripheral perfusion monitoring than spot measurements of the calf blood flow and oxygen consumption by indirect near infrared spectroscopy (30). Data from our study also indicate that PI obtained from the pulse oximeter probe placed over the foot correlates well with other indirect estimates of cutaneous blood flow, i.e. cardiac activity (HR) and C-P grad, suggesting that it can be a useful tool in estimating peripheral perfusion non-invasively and continuously.
The second goal of the study was to evaluate whether the peripheral perfusion (PI) was sensitive to changes in body position. We and others have previously reported that LBW infants sleeping prone are known to exhibit many physiological differences from those sleeping supine (5–9), including lower metabolic rates in the prone position (9). Our study demonstrates that in the prone position, infants exhibit higher PI, HR, absolute central and peripheral temperatures and narrower gradients between the central and peripheral sites, despite the known reduction in heat production. These observations are consistent with the following unifying hypothesis. Sleeping in the prone position impairs heat loss and leads to increased heat storage. The thermoregulatory cardiovascular response to increased heat storage is cutaneous vasodilatation (higher perfusion index and narrowed C-P grads), which, in turn, is associated with an increased cardiac output (increased HR). Despite these thermoregulatory and cardiovascular adjustments, a small increase in body ST in the prone position occurs. It is not known if this modest hyperthermia represents thermal stress resulting from relative failure of thermoregulation in the prone position or a change in thermal set points. It appears that in the prone position, the cardiorespiratory system is being driven by thermoregulatory inputs and not primarily by metabolic needs. We speculate that similar postural differences in thermal profile and cardiac activity are seen in older infants and may contribute to increased risk for SIDS when infants sleep in the prone position.
In conclusion, PI is a promising new non-invasive monitoring tool that can estimate cutaneous blood flow across all age groups. It has application in multiple clinical settings where monitoring of peripheral perfusion, circulatory status and thermoregulatory control is essential. It is very likely that PI will prove to be not only useful in monitoring progress but also helpful in evaluating outcomes under these conditions. Potential areas for future investigation of its utility include estimation of volume status in trauma patient, restoration of peripheral perfusion after major cardiac and non-cardiac surgery, prediction of the success of re-implanted body parts and in SIDS research.
Acknowledgments
This work was supported by United States Public Health Service Grants HD 13065, HD 27564, HD 32774 and UL1 RR024156.
References
- 1.Hamilton BE, Minino AM, Martin JA, Kochanek KD, Strobino DM, Guyer B. Annual summary of vital statistics: 2005. Pediatrics. 2007;119:345–60. doi: 10.1542/peds.2006-3226. [DOI] [PubMed] [Google Scholar]
- 2.Bayes BJ. Prone infants and SIDS. N Engl J Med. 1974;290:693–4. doi: 10.1056/nejm197403212901223. [DOI] [PubMed] [Google Scholar]
- 3.Ponsonby AL, Dwyer T, Gibbons LE, Cochrane JA, Wang YG. Factors potentiating the risk of sudden infant death syndrome associated with the prone position. N Engl J Med. 1993;329:377–82. doi: 10.1056/NEJM199308053290601. [DOI] [PubMed] [Google Scholar]
- 4.Malloy MH, Freeman DH., Jr Birth weight- and gestational age-specific sudden infant death syndrome mortality: United States, 1991 versus 1995. Pediatrics. 2000;105:1227–31. doi: 10.1542/peds.105.6.1227. [DOI] [PubMed] [Google Scholar]
- 5.Martin RJ, Herrell N, Rubin D, Fanaroff A. Effect of supine and prone positions on arterial oxygen tension in the preterm infant. Pediatrics. 1979;63:528–31. [PubMed] [Google Scholar]
- 6.Sahni R, Schulze KF, Kashyap S, Ohira-Kist K, Fifer WP, Myers MM. Postural differences in cardiac dynamics during quiet and active sleep in low birth weight infants. Acta Paediatr. 1999;88:1396–401. doi: 10.1080/080352599750030158. [DOI] [PubMed] [Google Scholar]
- 7.Sahni R, Schulze KF, Kashyap S, Ohira-Kist K, Myers MM, Fifer WP. Body position; sleep states, and cardiorespiratory activity in developing low birth weight infants. Early Hum Dev. 1999;54:197–206. doi: 10.1016/s0378-3782(98)00104-2. [DOI] [PubMed] [Google Scholar]
- 8.Chong A, Murphy N, Matthews T. Effect of prone sleeping on circulatory control in infants. Arch Dis Child. 2000;82:253–6. doi: 10.1136/adc.82.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ammari A, Schulze KF, Ohira-Kist K, Kashyap S, Fifer WP, Myers MM, et al. Effects of body position on thermal, cardiorespiratory and metabolic activity in low birth weight infants. Early Hum Dev. 2009;85:497–501. doi: 10.1016/j.earlhumdev.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox GP, Matthews TG. Autonomic dysfunction at different ambient temperatures in infants at risk of sudden infant death syndrome. Lancet. 1989;2:1065–7. doi: 10.1016/s0140-6736(89)91080-5. [DOI] [PubMed] [Google Scholar]
- 11.Nelson EA, Taylor BJ, Weatherall IL. Sleeping position and infant bedding may predispose to hyperthermia and the sudden infant death syndrome. Lancet. 1989;1:199–200. doi: 10.1016/s0140-6736(89)91211-7. [DOI] [PubMed] [Google Scholar]
- 12.Tuffnell CS, Petersen SA, Wailoo MP. Prone sleeping infants have a reduced ability to lose heat. Early Hum Dev. 1995;43:109–16. doi: 10.1016/0378-3782(95)01659-7. [DOI] [PubMed] [Google Scholar]
- 13.Tibby SM, Hatherill M, Murdoch IA. Capillary refill and core-peripheral temperature gap as indicators of haemodynamic status in paediatric intensive care patients. Arch Dis Child. 1999;80:163–6. doi: 10.1136/adc.80.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey JM, Levy JH, Kopel MA, Tobia V, Grabenkort WR. Relationship between clinical evaluation of peripheral perfusion and global hemodynamics in adults after cardiac surgery. Crit Care Med. 1990;18:1353–6. doi: 10.1097/00003246-199012000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Brock L, Skinner JM, Manders JT. Observations on peripheral and central temperatures with particular reference to the occurrence of vasoconstriction. Br J Surg. 1975;62:589–95. doi: 10.1002/bjs.1800620802. [DOI] [PubMed] [Google Scholar]
- 16.Ryan CA, Soder CM. Relationship between core / peripheral temperature gradient and central hemodynamics in children after open heart surgery. Crit Care Med. 1989;17:638–40. doi: 10.1097/00003246-198907000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Murdoch IA, Qureshi SA, Mitchell A, Huggon IC. Core-peripheral temperature gradient in children: does it reflect clinically important changes in circulatory haemodynamics? Acta Paediatr. 1983;82:773–6. doi: 10.1111/j.1651-2227.1993.tb12556.x. [DOI] [PubMed] [Google Scholar]
- 18.Butt W, Shann F. Core-peripheral temperature gradient does not predict cardiac output or systemic vascular resistance in children. Anaesth Intensive Care. 1991;19:84–7. doi: 10.1177/0310057X9101900115. [DOI] [PubMed] [Google Scholar]
- 19.Lima AP, Beelen P, Bakker J. Use of a peripheral perfusion index derived from the pulse oximetry signal as a noninvasive indicator of perfusion. Crit Care Med. 2002;30:1210–3. doi: 10.1097/00003246-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Lima A, Bakker J. The peripheral perfusion index in reactive hyperemia in critically ill patients. Crit Care. 2004;8:S27–P53. [Google Scholar]
- 21.Stefanski M, Schulze K, Bateman D, Kairam R, Pedley TA, Masterson J, et al. A scoring system for states of sleep and wakefulness in term and preterm infants. Pediatr Res. 1984;18:58–62. [PubMed] [Google Scholar]
- 22.Champion HR, Sacco WJ, Copes WS, Gann DS, Gennarelli TA, Flanagan ME. A revision of the trauma score. J Trauma. 1989;29:623–9. doi: 10.1097/00005373-198905000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Vincent JL, Moraine JJ, van der LP. Toe temperature versus transcutaneous oxygen tension monitoring during acute circulatory failure. Intensive Care Med. 1988;14:64–8. doi: 10.1007/BF00254125. [DOI] [PubMed] [Google Scholar]
- 24.Hales JR, Stephens FR, Fawcett AA, Daniel K, Sheahan J, Westerman RA, et al. Observations on a new non-invasive monitor of skin blood flow. Clin Exp Pharmacol Physiol. 1989;16:403–15. doi: 10.1111/j.1440-1681.1989.tb01578.x. [DOI] [PubMed] [Google Scholar]
- 25.De Felice C, Latini G, Vacca P, Kopotic RJ. The pulse oximeter perfusion index as a predictor for high illness severity in neonates. Eur J Pediatr. 2002;161:561–2. doi: 10.1007/s00431-002-1042-5. [DOI] [PubMed] [Google Scholar]
- 26.De Felice C, Del Vecchio A, Criscuolo M, Lozupone A, Parrini S, Latini G. Early postnatal changes in the perfusion index in term newborns with subclinical chorioamnionitis. Arch Dis Child Fetal Neonatal Ed. 2005;90:F411–4. doi: 10.1136/adc.2004.068882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Felice C, Goldstein MR, Parrini S, Verrotti A, Criscuolo M, Latini G. Early dynamic changes in pulse oximetry signals in preterm newborns with histologic chorioamnionitis. Pediatr Crit Care Med. 2006;7:138–42. doi: 10.1097/01.PCC.0000201002.50708.62. [DOI] [PubMed] [Google Scholar]
- 28.Granelli AW, Ostman-Smith I. Noninvasive peripheral perfusion index as a possible tool for screening for critical left heart obstruction. Acta Paediatr. 2007;96:1455–9. doi: 10.1111/j.1651-2227.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 29.Partridge BL. Use of pulse oximetry as a noninvasive indicator of intravascular volume status. J Clin Monit. 1987;3:263–8. doi: 10.1007/BF03337381. [DOI] [PubMed] [Google Scholar]
- 30.Zaramella P, Freato F, Quaresima V, Ferrari M, Vianello A, Giongo D, et al. Foot pulse oximeter perfusion index correlates with calf muscle perfusion measured by near-infrared spectroscopy in healthy neonates. J Perinatol. 2005;25:417–22. doi: 10.1038/sj.jp.7211328. [DOI] [PubMed] [Google Scholar]