Abstract
BACKGROUND
The Diabetes Control and Complications Trial (DCCT) showed a beneficial effect of 6.5 years of intensive glycemic control on retinopathy in patients with type 1 diabetes.
METHODS
Between 1983 and 1989, a total of 1441 patients with type 1 diabetes in the DCCT were randomly assigned to receive either intensive diabetes therapy or conventional therapy aimed at preventing hyperglycemic symptoms. They were treated and followed until 1993. Subsequently, 1375 of these patients were followed in the observational Epidemiology of Diabetes Interventions and Complications (EDIC) study. The self-reported history of ocular surgical procedures was obtained annually. We evaluated the effect of intensive therapy as compared with conventional therapy on the incidence and cost of ocular surgery during these two studies.
RESULTS
Over a median follow-up of 23 years, 130 ocular operations were performed in 63 of 711 patients assigned to intensive therapy (8.9%) and 189 ocular operations in 98 of 730 patients assigned to conventional therapy (13.4%) (P<0.001). After adjustment for DCCT baseline factors, intensive therapy was associated with a reduction in the risk of any diabetes-related ocular surgery by 48% (95% confidence interval [CI], 29 to 63; P<0.001) and a reduction in the risk of all such ocular procedures by 37% (95% CI, 12 to 55; P = 0.01). Forty-two patients who received intensive therapy and 61 who received conventional therapy underwent cataract extraction (adjusted risk reduction with intensive therapy, 48%; 95% CI, 23 to 65; P = 0.002); 29 patients who received intensive therapy and 50 who received conventional therapy underwent vitrectomy, retinal-detachment surgery, or both (adjusted risk reduction, 45%; 95% CI, 12 to 66; P = 0.01). The costs of surgery were 32% lower in the intensive-therapy group. The beneficial effects of intensive therapy were fully attenuated after adjustment for mean glycated hemoglobin levels over the entire follow-up.
CONCLUSIONS
Intensive therapy in patients with type 1 diabetes was associated with a substantial reduction in the long-term risk of ocular surgery. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; DCCT/EDIC ClinicalTrials.gov numbers, NCT00360893 and NCT00360815.)
Retinopathy, A Common Microvascular complication of type 1 diabetes, is a leading cause of vision loss worldwide.1 In the Diabetes Control and Complications Trial (DCCT),2 6.5 years of intensive therapy aimed at achieving glycemia as close to the nondiabetic range as safely possible, as compared with conventional therapy at the time, was associated with a 76% reduction in the onset of retinopathy and a 52% reduction in disease progression. In the subsequent long-term observational follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) study,3 the patients in the intensive-therapy group had a durable reduction in progression of microvascular and macrovascular complications despite similar levels of glycemia in the two groups. This reduction persisted for 18 years in a phenomenon that has been called metabolic memory.4–8 Maintenance of near-normal glycemia as safely as possible has become the primary therapeutic goal in patients with type 1 diabetes.
Diabetes can cause vision loss by promoting sight-threatening conditions such as severe retinopathy, cataracts, and glaucoma.9,10 Ocular surgery may preserve vision or prevent loss of vision in patients with these conditions. However, surgery may be associated with increased morbidity and substantial societal, economic, and health care burdens. Given the currently estimated 382 million persons with diabetes worldwide, 19 million to 38 million of whom have type 1 diabetes,11 a reduction in the rate of ocular surgery would have a substantial salutary effect.
We now report the incidence and costs of ocular surgery among patients in the intensive-therapy and conventional-therapy groups for up to 27 years after the start of the original DCCT.
Methods
Study Oversight
The methods of both studies have been described in detail previously.2,3,7 The DCCT and EDIC studies were designed by their respective research groups. The data were collected at the Biostatistics Center, George Washington University. The members of the writing committee analyzed the data, wrote the manuscript, and vouch for the accuracy and completeness of the data and analyses and for the fidelity of the study to the protocols (available with the full text of this article at NEJM.org). The DCCT/EDIC Research Group made the decision to submit the manuscript for publication.
The institutional review board or ethics committee at each participating center approved both study protocols, and all patients provided written informed consent.
Patients
From 1983 through 1989, the original, randomized trial (DCCT) enrolled 1441 patients with type 1 diabetes who were 13 to 39 years of age. A total of 711 patients were randomly assigned to receive intensive therapy (aimed at lowering glycemia as close to the nondiabetic range as safely possible), and 730 patients were assigned to receive conventional therapy (aimed at preventing symptoms of hyperglycemia and hypoglycemia with no specific glucose targets).2
The primary-prevention cohort (726 patients) had diabetes for 1 to 5 years, an albumin excretion rate of less than 40 mg per 24 hours, and no retinopathy detected by means of stereoscopic fundus photography. The secondary-intervention cohort (715 patients) had diabetes for 1 to 15 years, mild-to-moderate nonproliferative diabetic retinopathy, and an albumin excretion rate of up to 200 mg per 24 hours.
The DCCT ended in 1993, after an average of 6.5 years of treatment. The patients in the conventional-therapy group were offered intensive therapy and were instructed in its use, and all patients were referred to their health care providers for diabetes care.2 In 1994, a total of 1375 of the 1428 surviving patients (96%) enrolled in the observational study (EDIC), with annual follow-up examinations and periodic evaluation of complications of diabetes.3 Here, we present data obtained over a total of up to 27 years (through 2010), with a median of 23 years of follow-up.
Ocular Operations
Ocular surgical procedures were self-reported annually. Surgical details were acquired with the use of structured interviews by the study coordinator and were reviewed by the site principal investigator.
Ophthalmologists who were unaware of the DCCT treatment assignments and the glycated hemoglobin levels of the patients later reviewed the surgical reports to classify all operations as being either substantially diabetes-related (e.g., cataract extraction) or not substantially diabetes-related (e.g., elective refractive operations or oculoplastic procedures). Diabetes-related operations were further classified as being cataract extraction; vitrectomy, retinal-detachment surgery, or both (these two procedures are often performed together); glaucoma-related surgery (including laser treatment, filtering surgery, cyclocryotherapy, and other operative procedures to lower intraocular pressure); cornea-related or lens-related surgery (including corneal transplantation or yttrium– aluminum–garnet [YAG] posterior capsulotomy); or enucleation. Previous studies showed that intensive therapy significantly reduced the risks of laser therapy or anti–vascular endothelial growth factor injections for macular disease or proliferative retinopathy7; these treatments were not included as “surgery” here.
Retinopathy and Visual Acuity
Standardized seven-field fundus photographs were obtained every 6 months during the DCCT and every fourth year during the EDIC study, with approximately 25% of the patients having photographs obtained each year (see the Supplementary Appendix, available at NEJM.org). In addition, photographs were obtained in the complete cohort during years 4 and 10 of the EDIC study.
Photographs were graded centrally with the use of the Early Treatment Diabetic Retinopathy Study (ETDRS) scale12 and DCCT methods.2 Graders were unaware of the therapy assignments of the patients. Visual acuity was measured by certified examiners on the basis of the ETDRS charts and procedures.12
Biomedical Evaluations
Each patient underwent an annual examination that assessed demographic characteristics and clinical history. Blood pressure and glycated hemoglobin levels13 were measured quarterly during the DCCT and annually thereafter. The albumin excretion rate and plasma lipid concentrations were measured yearly during the DCCT and every other year thereafter.8 The estimated glomerular filtration rate (GFR) was calculated from serum creatinine levels14 that were measured annually. A central biochemistry laboratory performed all laboratory measurements.
Classification of nephropathy was based on an impaired GFR of less than 60 ml per minute per 1.73 m2 of body-surface area on two or more consecutive visits, microalbuminuria (albumin excretion rate, ≥30 mg per 24 hours on two or more consecutive visits), or macroalbuminuria (single albumin excretion rate, ≥300 mg per 24 hours).15 Confirmed clinical neuropathy, defined as the presence of both definite clinical neuropathy and abnormal nerve conduction, was assessed at baseline and at 5 years in the DCCT and at 13 years or 14 years in the EDIC study.16
Statistical Analysis
The primary outcome was the time from randomization in the DCCT to the annual visit at which a diabetes-related ocular operation was first reported or the time to the last study visit or death, if no surgery was performed. For the purpose of these analyses, patients who had one or more ocular operations were categorized as patients with “any” surgery. In addition, the total number of operations (“all”) were analyzed.
Cataract extraction and vitrectomy, retinal-detachment surgery, or both were analyzed separately owing to their high frequency. The cumulative incidence of each outcome was estimated by means of Gray’s method, accounting for death as a competing risk.17
Cox proportional-hazards models were used to assess the effects of the covariates on the cause-specific hazard of ocular surgery.18 The robust estimation of the covariance matrix according to the method of Lin and Wei was used to compute confidence limits and P values that are valid when proportional-hazards assumptions are violated.19 Poisson regression models with the robust covariance matrix estimate assessed the risk of all operations, allowing for multiple operations for each patient.18
The cost of ocular surgery was calculated in 2010 U.S. dollars with the use of the medical care Consumer Price Index20,21 and with the use of discounted dollars to account for a yearly inflation rate of 3%. Costs were available only for cataract extraction, vitrectomy, and glaucoma-related operations.
All analyses were conducted with the use of SAS software, version 9.2 (SAS Institute).22
Results
Characteristics of the Patients
Baseline characteristics of the patients in the DCCT are provided in Table S1 in the Supplementary Appendix. The mean age of the patients was 27 years, the mean duration of diabetes was 6 years, and the mean glycated hemoglobin level was 9.1%. More than 80% of the patients had corrected visual acuity of 20/20 or better.
Ocular Operations
In the combined studies, with a median follow-up of 23 years (interquartile range, 22 to 25), a total of 161 patients reported 319 diabetes-related ocular operations (Table 1), 1132 completed follow-up without undergoing ocular surgery, 72 were lost to follow-up, and 76 died. Only 6 of the operations occurred during the DCCT (1 glaucoma-related procedure, 2 cataract extractions, 2 vitrectomies, and 1 enucleation).
Table 1.
Ocular Operations and Risk Reduction with Intensive Therapy among the Patients during Follow-up.*
| Operation | Intensive-Therapy Group (N = 711) |
Conventional-Therapy Group (N = 730) |
Risk Reduction with Intensive Therapy† |
P Value | ||
|---|---|---|---|---|---|---|
| no. (%) |
operations/ 1000 patient-yr‡ |
no. (%) |
operations/ 1000 patient-yr‡ |
% (95% CI) | ||
| Diabetes-related ocular surgery§ | ||||||
| Any (patients with ≥1 operations) | 63 (8.9) | 3.95 | 98 (13.4) | 6.24 | 48.5 (28.8 to 62.7) | <0.001 |
| All (total operations) | 130 | 8.01 | 189 | 11.64 | 37.3 (11.9 to 55.4) | 0.01 |
| Type of surgery | ||||||
| Cataract extraction¶ | ||||||
| Patients with operations | 42 (5.9) | 2.61 | 61 (8.4) | 3.80 | 48.5 (23.2 to 65.4) | 0.002 |
| Patients with operations in both eyes | 27 (3.8) | 29 (4.0) | ||||
| All operations | 69 | 4.25 | 90 | 5.54 | 35.3 (4.8 to 56.0) | 0.03 |
| Vitrectomy, retinal detachment, or both║ | ||||||
| Patients with operations | 29 (4.1) | 1.80 | 50 (6.8) | 3.14 | 45.4 (12.5 to 65.9) | 0.01 |
| Patients with vitrectomy only | 26 (3.7) | 45 (6.2) | ||||
| Patients with operations in both eyes | 12 (1.7) | 13 (1.8) | ||||
| All operations | 42 | 2.59 | 75 | 4.62 | 44.7 (9.5 to 66.2) | 0.02 |
| Glaucoma-related operation | ||||||
| Patients with operations** | 9 (1.3) | 0.55 | 14 (1.9) | 0.86 | ||
| Patients with operations in both eyes | 6 (0.8) | 9 (1.2) | ||||
| All operations | 15 | 0.92 | 23 | 1.42 | ||
| Cornea-related operations | ||||||
| Patients with operations | 2 (0.3) | 0.12 | 3 (0.4) | 0.18 | ||
| Patients with operations in both eyes | 1 (0.1) | 1 (0.1) | ||||
| All operations | 3 | 0.18 | 4 | 0.25 | ||
| Enucleation | ||||||
| Patients with operations | 1 (0.1) | 0.06 | 1 (0.1) | 0.06 | ||
| Patients with operations in both eyes | 0 | 1 (0.1) | ||||
| YAG posterior capsulotomy | ||||||
| Patients with operations | 3 (0.4) | 0.18 | 4 (0.5) | 0.25 | ||
| Patients with operations in both eyes | 2 (0.3) | 1 (0.1) | ||||
| All operations | 5 | 0.31 | 5 | 0.31 | ||
| Surgery not related to diabetes | ||||||
| Refractive error surgery | ||||||
| Patients with operations | 24 (3.4) | 1.49 | 22 (3.0) | 1.36 | −13.5 (−103.3 to 36.6) | 0.67 |
| Patients with operations in both eyes | 23 (3.2) | 17 (2.3) | ||||
| All operations | 47 | 2.89 | 44 | 2.71 | −11.3 (−102.3 to 38.7) | 0.73 |
| Oculoplastic operations | ||||||
| Patients with operations | 7 (1.0) | 0.43 | 7 (1.0) | 0.43 | ||
| Patients with operations in both eyes | 2 | 5 | ||||
YAG denotes yttrium–aluminum–garnet.
In evaluations of patients, relative risk was obtained from a Cox proportional-hazards model, and in analyses of all operations, relative risk was obtained from a Poisson regression model; in both cases, robust variance estimates were used. All the models were adjusted for age, sex, duration of diabetes, glycated hemoglobin level, secondary versus primary cohort, and visual acuity level at baseline in the Diabetes Control and Complications Trial (DCCT). The percentage risk reduction associated with intensive diabetes therapy was calculated as (1 - hazard ratio with intensive versus conventional diabetes therapy) × 100. In outcomes with less than 60 operations, no relative risk estimates are provided because of insufficient precision and power.
In analyses involving patients who underwent any operation, the incidence rate was calculated as 1000 times the ratio of the number of patients who underwent an operation over total patient-years when patients were at risk (i.e., from baseline in the DCCT up to the first event time in patients with events, or year 17 in the Epidemiology of Diabetes Interventions and Complications [EDIC] study for administrative censoring, or censoring time for loss to follow-up or death). In analyses of the total number of operations, the incidence rate was calculated as 1000 times the ratio of the total number of operations over total patient-years up to year 17, censoring, or death.
The time to the first of any diabetes-related ocular surgery was the primary study outcome. In patients who underwent multiple types of ocular surgery, the time to the onset of the first surgery of each type was used. All the operations occurred in the EDIC study except for six during the DCCT, including one glaucoma-related surgery (in the intensive-therapy group), two cataract extractions (in the conventional-therapy group), two vitrectomy operations (in the conventional-therapy group), and one enucleation (in the conventional-therapy group).
Cataract-extraction surgery was performed in conjunction with another ocular surgery in two patients in the intensive-therapy group and in one patient in the conventional-therapy group.
There were four retinal-detachment operations without vitrectomy in patients in the intensive-therapy group and six in patients in the conventional-therapy group. One patient underwent vitrectomy in one eye and retinal-detachment surgery in the other eye. Another patient underwent vitrectomy in an earlier year and retinal detachment without vitrectomy in a later year.
Surgery for narrow-angle glaucoma was performed in four patients in the conventional-therapy group.
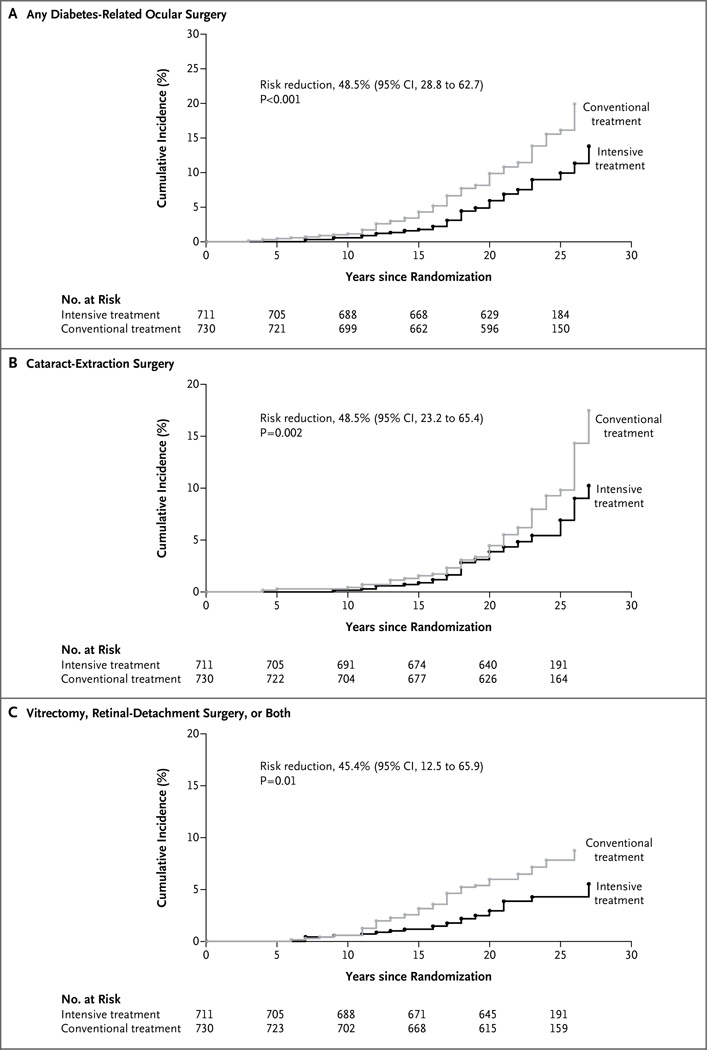
Sixty-three patients in the former intensive-therapy group and 98 patients in the former conventional-therapy group underwent at least 1 diabetes-related ocular operation (3.95 operations vs. 6.24 operations per 1000 patient-years), corresponding to a risk reduction of 48% (95% confidence interval [CI], 29 to 63; P<0.001 in the intensive-therapy group). The 20-year cumulative incidence of diabetes-related ocular surgery was 5.9% in the intensive-therapy group and 9.8% in the conventional-therapy group (Fig. 1A). Fewer total operations were performed in patients in the intensive-therapy group than in patients in the conventional-therapy group (130 vs. 189 operations), corresponding to a risk reduction of 37% (95% CI, 12 to 55; P = 0.01 in the intensive-therapy group) (Table 1).
Figure 1. Cumulative Incidence of Diabetes-Related Ocular Surgery, According to Treatment Group and Type of Surgery.
CI denotes confidence interval.
A total of 42 patients in the intensive-therapy group and 61 in the conventional-therapy group underwent cataract extraction, the most frequent type of ocular surgery (risk reduction with intensive therapy, 48%; 95% CI, 23 to 65; P = 0.002) (Table 1 and Fig. 1B). Of these patients, 27 in the intensive-therapy group and 29 in the conventional-therapy group underwent cataract extraction in both eyes, and 2 in the intensive-therapy group and 1 in the conventional-therapy group concurrently had other ocular operations. Overall, 69 cataract extractions were performed in the intensive-therapy group as compared with 90 in the conventional-therapy group (risk reduction with intensive therapy, 35%; 95% CI, 5 to 56; P = 0.03).
The second most frequently reported operations were categorized as “vitrectomy, retinal-detachment surgery, or both.” A total of 29 patients in the intensive-therapy group and 50 patients in the conventional-therapy group underwent at least one of these procedures; this corresponds to a risk reduction of 45% (95% CI, 12 to 66; P = 0.01) in the intensive-therapy group (Table 1 and Fig. 1C). Overall, 42 such operations were performed in the intensive-therapy group, versus 75 operations in the conventional-therapy group (risk reduction with intensive therapy, 45%; 95% CI, 10 to 66; P = 0.02).
Less frequently performed operations included glaucoma-related procedures (in 9 patients in the intensive-therapy group and 14 in the conventional-therapy group), cornea-related operations (2 patients vs. 3 patients), enucleation (1 patient in each group), and YAG posterior capsulotomy (3 patients vs. 4 patients). There were no between-group differences in non–diabetes-related ocular operations, including elective surgery to correct refractive error (in 24 patients vs. 22 patients) and oculoplastic surgery (7 patients in each group) (Table 1).
Costs
The inflation-adjusted costs in 2010 dollars for all cataract extraction, vitrectomy, and glaucoma-related operations were 32% lower in the intensive-therapy group than in the conventional-therapy group ($429,469 vs. $634,925). The surgery-specific and total unadjusted and adjusted costs are provided in Table S2 in the Supplementary Appendix.
Baseline Factors
In separate Cox proportional-hazard models adjusted only for treatment group, the variables of female sex, increasing age, longer duration of diabetes, increasing levels of glycated hemoglobin, secondary versus primary cohort, and visual acuity worse than 20/20 (in 16% of patients) versus 20/20 or better were each strongly associated with a higher risk of any surgery (Table 2). However, sex and visual acuity were not associated with the risk of vitrectomy, retinal-detachment surgery, or both. In a joint multivariate model, similar associations were observed except that primary versus secondary cohort had no effect after adjustment for the other factors (principally, the duration of diabetes that differed according to design between the cohorts) (Table 2).
Table 2.
Association of Baseline Factors with the Risk of Diabetes-Related Ocular Operations.
| Variable | Any Surgery | Cataract Extraction | Vitrectomy Retinal Detachment or Both |
|||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI)* |
P Value | Hazard Ratio (95% CI)* |
P Value | Hazard Ratio (95% CI)* |
P Value | |
| Separate models† | ||||||
| Female vs. male sex | 1.42 (1.04–1.93) | 0.03 | 1.59 (1.08–2.35) | 0.02 | 1.19 (0.77–1.85) | 0.44 |
| Age, per 10-yr increase | 1.48 (1.18–1.86) | 0.003 | 2.49 (1.84–3.38) | <0.001 | 0.64 (0.47–0.87) | 0.006 |
| Duration of diabetes, per 10-yr increase | 2.66 (1.88–3.75) | <0.001 | 3.08 (1.99–4.75) | <0.001 | 1.97 (1.20–3.25) | 0.003 |
| Glycated hemoglobin level, per 10% increase | 1.29 (1.19–1.41) | <0.001 | 1.31 (1.18–1.45) | <0.001 | 1.32 (1.17–1.48) | <0.001 |
| Secondary vs. primary cohort | 1.97 (1.42–2.74) | <0.001 | 2.25 (1.47–3.44) | <0.001 | 1.90 (1.18–3.04) | 0.008 |
| Visual acuity worse than 20/20 vs. 20/20 or better | 1.63 (1.14–2.35) | 0.008 | 1.61 (1.02–2.55) | 0.05 | 1.19 (0.67–2.11) | 0.56 |
| Joint baseline multivariate model | ||||||
| Female vs. male | 1.33 (0.97–1.81) | 0.07 | 1.58 (1.07–2.33) | 0.03 | 0.99 (0.64–1.54) | 0.99 |
| Age, per 10-yr increase | 1.60 (1.24–2.06) | <0.001 | 2.71 (2.00–3.67) | <0.001 | 0.68 (0.49–0.93) | 0.03 |
| Duration of diabetes, per 10-yr increase | 2.89 (1.66–5.03) | <0.001 | 3.20 (1.67–6.13) | <0.001 | 1.72 (0.82–3.61) | 0.17 |
| Glycated hemoglobin level, per 10% increase | 1.35 (1.24–1.48) | <0.001 | 1.39 (1.23–1.56) | <0.001 | 1.31 (1.15–1.49) | <0.001 |
| Secondary vs. primary cohort | 0.96 (0.56–1.62) | 0.87 | 0.99 (0.50–1.95) | 0.98 | 1.41 (0.69–2.86) | 0.35 |
| Visual acuity worse than 20/20 vs. 20/20 or better | 1.52 (1.06–2.18) | 0.02 | 1.57 (1.00–2.47) | 0.06 | 1.06 (0.60–1.90) | 0.84 |
The hazard ratio was evaluated according to every 10% increase in the baseline glycated hemoglobin level, computed as 1.1β, where β is the coefficient for the natural logarithm of the glycated hemoglobin level. The hazard ratio was also evaluated according to every 10-year increase in age and duration of diabetes and for yes versus no for qualitative covariates.
A Cox proportional-hazards model adjusted for treatment group with the robust covariance estimation method of Lin and Wei was used.
The incidence of operations in the secondary cohort was approximately twice that in the primary cohort (Table S3 in the Supplementary Appendix), but the risk reductions with intensive therapy versus conventional therapy were not significantly different between the cohorts (P = 0.65). For all operations and each specific type, the risk reduction with intensive therapy versus conventional therapy was nominally greater among men than among women (Table S4 in the Supplementary Appendix); however, the difference between the sexes was only significant for vitrectomy, retinal-detachment surgery, or both (P = 0.02).
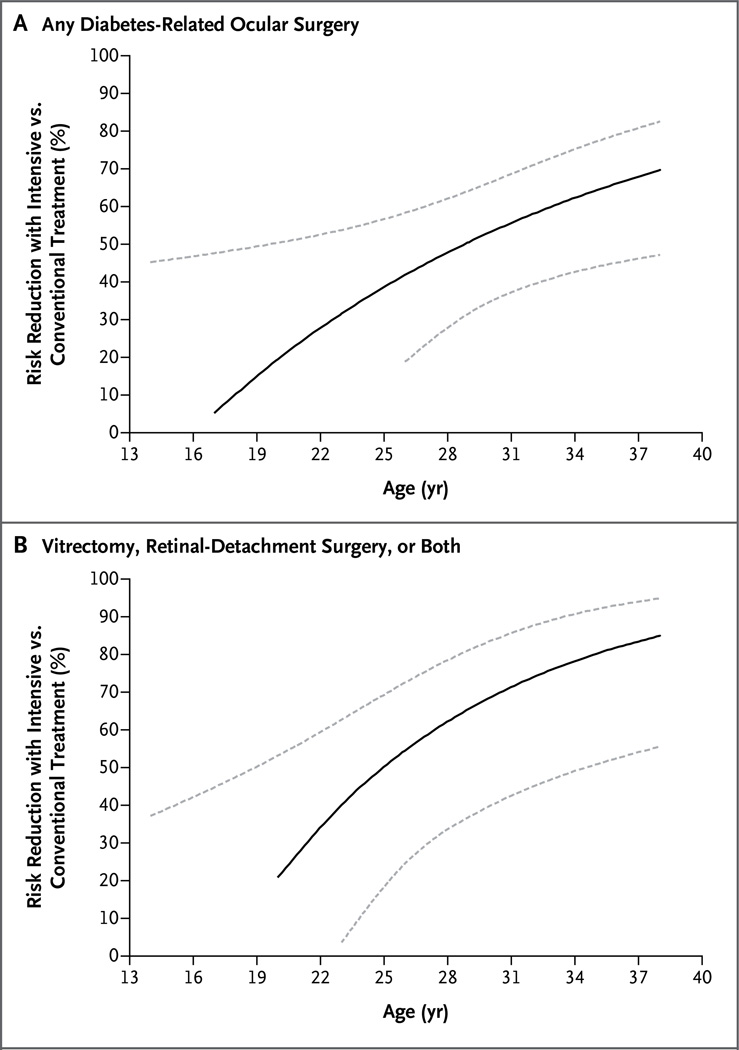
There was also a significant treatment-group interaction with baseline age for any surgery (P = 0.04), such that as age increased, the risk reduction with intensive therapy increased (Fig. 2A). This effect was largely driven by a stronger interaction with age for vitrectomy, retinal-detachment surgery, or both (P = 0.006) (Fig. 2B).
Figure 2. Risk Reduction in the Intensive-Therapy Group versus the Conventional-Therapy Group, as a Function of Age at Baseline in the DCCT, According to Type of Surgery.
Dashed lines indicate 95% confidence intervals.
Role of Glycemia and Other Complications
Table 3 shows the association of the updated time-weighted DCCT/EDIC mean glycated hemoglobin level (i.e., the mean level up to the current year of follow-up) and progression of diabetes complications (as time-varying covariates) with the risk of any surgery, the risk reduction with intensive treatment after adjustment for each factor, and the extent to which the between-group difference in the factor explains the between-group difference in the risk of surgery. The risk of any surgery was 1.88 times higher for each 10% increase in the DCCT/EDIC mean glycated hemoglobin level, and the risk reduction with intensive therapy was eliminated completely after adjustment for the glycated hemoglobin level. This indicates that differences in the mean glycated hemoglobin levels during the study explained virtually all the benefit of intensive therapy.
Table 3.
Association of Glycated Hemoglobin Level and Microvascular Complications over Time with the Risk of Any Diabetes-Related Ocular Surgery, the Covariate-Adjusted Treatment-Group Effect, and the Proportion of the Treatment Effect Explained by Group Differences in the Covariate.
| Model* | Time-Dependent Covariate |
Risk Reduction with Intensive Diabetes Therapy |
Proportion of Treatment Effect Explained by Each Covariate§ |
|||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI)† |
P Value | Percent (95% CI)‡ |
P Value | Percent | ||
| No time-dependent covariate | — | — | 48.5 (28.8 to 62.7) | <0.001 | ||
| Updated DCCT/EDIC mean glycated hemoglobin level, per 10% increase¶ | 1.88 (1.64 to 2.15) | <0.001 | 0.0 (−42.5 to 29.8) | 0.99 | 100.0 | |
| Retinopathy: moderate NPDR or worse vs. mild NPDR or better | 4.53 (2.98 to 6.90) | <0.001 | 21.4 (−11.1 to 44.5) | 0.18 | 88.5 | |
| CSME | 3.89 (2.64 to 5.72) | <0.001 | 34.8 (8.2 to 53.7) | 0.01 | 62.9 | |
| Visual acuity worse than 20/20 vs. 20/20 or better | 4.61 (3.24 to 6.57) | <0.001 | 40.9 (18.4 to 57.2) | 0.001 | 37.0 | |
| Albumin excretion rate | ||||||
| Per mg/24 hr | 1.03 (1.02 to 1.04) | <0.001 | 38.1 (13.1 to 55.9) | 0.006 | 52.5 | |
| Sustained rate ≥30 mg/24 hr | 2.89 (1.98 to 4.21) | <0.001 | 39.1 (14.8 to 56.5) | 0.004 | 48.1 | |
| ≥300 mg/24 hr | 3.51 (2.16 to 5.68) | <0.001 | 42.9 (19.9 to 59.2) | 0.002 | 34.8 | |
| Hypertension | 3.29 (2.18 to 4.98) | <0.001 | 43.1 (21.4 to 58.8) | <0.001 | 27.5 | |
| Use of RAAS inhibitor | 2.19 (1.53 to 3.14) | <0.001 | 45.1 (24.2 to 60.3) | <0.001 | 17.9 | |
| Glomerular filtration rate | ||||||
| Estimated per 1 SD (18 ml/ min/1.73 m2) increase | 0.75 (0.66 to 0.85) | <0.001 | 45.2 (23.5 to 60.7) | <0.001 | 22.4 | |
| Sustained estimated <60 ml/min/1.73 m2 | 3.02 (1.54 to 5.91) | 0.001 | 46.4 (26.1 to 61.2) | <0.001 | 10.7 | |
| Confirmed clinical neuropathy | 2.26 (1.54 to 3.31) | <0.001 | 46.1 (24.9 to 61.2) | <0.001 | 17.1 | |
All models used the Cox proportional-hazards model to adjust for age, sex, duration of diabetes, glycated hemoglobin level, secondary versus primary cohort, and visual acuity level at baseline in the DCCT, plus treatment group and the specific time-dependent covariate. In addition, all models used the robust estimation of the covariance matrix according to the method of Lin and Wei. CSME denotes clinically significant macular edema, GFR glomerular filtration rate, NPDR nonproliferative diabetic retinopathy, and RAAS renin–angiotensin–aldosterone system.
The hazard ratio was evaluated per 10% increase in the updated mean glycated hemoglobin level, calculated as 1.1β, where β is the coefficient for the natural logarithm of the glycated hemoglobin level. The hazard ratio was also evaluated for a 1-SD increase in the estimated GFR (18 ml per minute per 1.73 m2 of body-surface area), or for yes versus no for qualitative covariates.
The percentage risk reduction associated with intensive diabetes therapy was calculated as (1 - hazard ratio of intensive versus conventional diabetes therapy) × 100.
The proportion of the treatment-group effect explained is the percentage change in the treatment-group chi-square test value in the Cox model without and then with adjustment for the covariate.
The time-weighted mean values of all glycated hemoglobin values that were obtained every 3 months during DCCT (weighted by one quarter) and annual glycated hemoglobin values (weighted by 1) during EDIC were updated up to the time of each annual visit at which ocular operations were recorded.
The risk of any surgery with the presence of at least moderate nonproliferative diabetic retinopathy was 4.5 times higher than the risk of surgery with mild nonproliferative retinopathy or no diabetic retinopathy. The between-group difference in the incidence of nonproliferative diabetic retinopathy explained 88.5% of the between-group difference in the risk of surgery, and the risk reduction with intensive therapy became insignificant after adjustment for nonproliferative retinopathy. The development of clinically significant macular edema, nephropathy, neuropathy, and hypertension and the use of inhibitors of the renin–angiotensin–aldosterone system were all significantly associated with the risk of surgery, but the difference between treatment groups remained significant after adjustment for each of these factors (i.e., none of them alone explained the between-group difference in risk) (Table 3).
Nearly identical results were observed in similar analyses of cataract-extraction surgery (Table S5 in the Supplementary Appendix), with the glycated hemoglobin level or development of nonproliferative diabetic retinopathy explaining most of the between-group difference. All factors were also associated with the risk of vitrectomy, retinal-detachment surgery, or both, and the between-group difference was no longer significant after adjustment for many of these factors owing to the smaller number of such operations (Table S5 in the Supplementary Appendix).
Discussion
Over a median of 23 years of follow-up in patients with type 1 diabetes, an initial average period of 6.5 years of intensive, as compared with conventional, diabetes therapy was associated with a 48% reduction in the long-term risk of any patient undergoing one or more diabetes-related ocular procedure (P<0.001) and a 37% reduction in all ocular procedures (total operations in either eye) (P=0.01). These effects were related to improved glycemic control with intensive therapy. The long-term benefit of early implementation of intensive therapy with respect to the risk of ocular surgery is similar to previously reported long-term benefits with respect to the risk of nephropathy8,23 and is another manifestation of metabolic memory.
Separate (but not joint) models showed that the risk of ocular surgery was associated with the development of hypertension and worsening of retinopathy, neuropathy, and nephropathy. In a joint model, this risk was associated with baseline age, duration of diabetes, and glycated hemoglobin level, but not sex. The primary determinant of risk was the mean glycated hemoglobin level since randomization, which was associated with a risk that was 1.88 times higher for each 10% increase in the mean glycated hemoglobin level. The treatment-group differences in glycated hemoglobin levels completely explained the between-group differences in the risk of surgery. Differences in the severity of nonproliferative diabetic retinopathy also explained most, but not all, of the between-group difference in risk.
With intensive therapy, the risk of any cataract surgery was significantly reduced by 48%, and the risk of vitrectomy, retinal-detachment surgery, or both was significantly reduced by 45%; the risks of all such operations were reduced by 35% and 45%, respectively. Vitrectomy is performed in patients with diabetes primarily for advanced indications, such as vitreous hemorrhage and retinal detachment.
Cataract extraction is the most common ophthalmologic surgery performed in the United States and many other countries worldwide.24 Between 2009 and 2010, 3.1 million cataract extractions were performed in the United States. This procedure was the top surgical expenditure for Medicare, with charges exceeding $2 billion.25 Although the development of cataracts and the need for cataract surgery are not specific to diabetes, they are more frequent in patients with diabetes,26 and surgery for cataracts occurs at a younger age in patients with this condition.27 In the Wisconsin Epidemiologic Study of Diabetic Retinopathy,28 elevated glycated hemoglobin levels were associated with an increased risk of cataract surgery among persons with type 1 diabetes. In a case–control study involving patients with type 2 diabetes,29 elevated glycated hemoglobin levels were associated with the prevalence of posterior subcapsular cataract and in a third study with an increased incidence of lens opacities and cataract progression.30
Cataract progression in patients with diabetes has been linked to the presence of advanced glycation end products.31–33 Levels of these end products are increased in human cataractous lenses, and they may be involved in the pathogenesis of cataracts.32,34 Thus, a potential mechanism underlying the new finding that improved glycemic control reduces the risk of cataract surgery might involve differing concentrations of advanced glycation end products as a result of the initial 6.5 years of intensive therapy versus conventional therapy. Indeed, in a small DCCT ancillary study, skin collagen glycation was strongly associated with progression of retinopathy and nephropathy.35,36
Given that the worldwide prevalence of type 1 diabetes is approaching 38 million persons, the potential benefits of intensive therapy to reduce morbidity and health care costs are substantial. Indeed, in the current study, the inflation-adjusted costs of cataract extraction, vitrectomy, and glaucoma-related operations were 32% lower in the intensive-therapy group than in the conventional-therapy group.
A limitation of our study is that the incidence of ocular surgery was self-reported by patients. Annual follow-up with structured data acquisition, however, allowed repeated review of prior responses to elicit previously omitted information. Furthermore, a panel of ophthalmologists who were unaware of the treatment-group assignments reviewed reports of ocular operations, and investigators at study sites were contacted whenever additional information was needed for clarification. Finally, we had previously found that the DCCT/EDIC cohort was extremely accurate in self-reporting other eye procedures, such as photocoagulation.37
This study was also limited to the evaluation of patients with type 1 diabetes. Thus, it remains unknown whether similar benefits of an intensive-therapy regimen would accrue in patients with type 2 diabetes, which has a worldwide prevalence that is 10 to 20 times as high as that of type 1 diabetes.
In conclusion, intensive insulin therapy during the DCCT was associated with a substantial reduction in the long-term risk of ocular surgery among patients with type 1 diabetes. Reduced glycated hemoglobin levels account for essentially all the benefits of intensive therapy versus conventional therapy, and these results highlight the importance of early, intensive diabetes control. Intensive diabetes therapy has the potential to reduce substantially the morbidity and the extensive societal, economic, and health care burdens of ocular surgery among patients with type 1 diabetes.
Supplementary Material
Acknowledgments
Supported by cooperative agreement grants (1982–1993, 2012–2017) and contracts (1982–2012) with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (current grant numbers, U01 DK094176 and U01 DK094157), and by the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the General Clinical Research Center Program (1993–2007), and the Clinical and Translational Science Center Awards Program (2006–present). Free or discounted supplies or equipment were contributed by Abbott Diabetes Care, Animas, Becton Dickinson, Diabetes Care by Bayer, Eli Lilly, Extend Nutrition, Insulet, LifeScan, Medtronic Diabetes, Nipro Home Diagnostics, Nova Diabetes Care, Omron, Perrigo Diabetes Care, Roche Diabetes Care, and Sanofi-Aventis.
Appendix
The members of the writing committee (Lloyd Paul Aiello, M.D., Ph.D., Beetham Eye Institute, Joslin Diabetes Center, and Harvard Medical School, Boston; Wanjie Sun, Ph.D., the Biostatistics Center, George Washington University, Rockville, MD; Arup Das, M.D., Ph.D., University of New Mexico, Albuquerque; Sapna Gangaputra, M.D., University of Wisconsin School of Medicine and Public Health, Madison; Szilard Kiss, M.D., Weill Cornell Medical College and New York–Presbyterian Hospital, New York; Ronald Klein, M.D., University of Wisconsin School of Medicine and Public Health, Madison; Patricia A. Cleary, M.S., and John M. Lachin, Sc.D., the Biostatistics Center, George Washington University, Rockville, MD; and David M. Nathan, M.D., Massachusetts General Hospital and Harvard Medical School, Boston) assume responsibility for the content and integrity of this article. Address reprint requests to Dr. Lachin at the Biostatistics Center, George Washington University, 6110 Executive Blvd., Rockville, MD 20852, or at jml@bsc.gwu.edu
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta: Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.The DCCT/EDIC Research Group. Epidemiology of diabetes interventions and complications (EDIC): design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA, Nathan DM. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes. 2015;64:631–642. doi: 10.2337/db14-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Boer IH, Afkarian M, Rue TC, et al. Renal outcomes in patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol. 2014;25:2342–2350. doi: 10.1681/ASN.2013091004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman-Casey PA, Talwar N, Nan B, Musch DC, Stein JD. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology. 2011;118:1318–1326. doi: 10.1016/j.ophtha.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein BE, Klein R, Moss SE. Prevalence of cataracts in a population-based study of persons with diabetes mellitus. Ophthalmology. 1985;92:1191–1196. doi: 10.1016/s0161-6420(85)33877-0. [DOI] [PubMed] [Google Scholar]
- 11.International Diabetes Federation. IDF Diabetes Atlas. 6th ed. Brussels: International Diabetes Federation; 2013. [PubMed] [Google Scholar]
- 12.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagu-lation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991;1991;98(Suppl):766–785. [PubMed] [Google Scholar]
- 13.Steffes M, Cleary P, Goldstein D, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem. 2005;51:753–758. doi: 10.1373/clinchem.2004.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Boer IH, Sun W, Cleary PA, et al. Intensive diabetes therapy and glomeru-lar filtration rate in type 1 diabetes. N Engl J Med. 2011;365:2366–2376. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albers JW, Herman WH, Pop-Busui R, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33:1090–1096. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 18.Lachin JM. Biostatistical methods: the assessment of relative risks. 2nd ed. New York: Wiley; 2011. [Google Scholar]
- 19.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 20.Busbee BG, Brown MM, Brown GC, Sharma S. Cost-utility analysis of cataract surgery in the second eye. Ophthalmology. 2003;110:2310–2317. doi: 10.1016/S0161-6420(03)00796-6. [DOI] [PubMed] [Google Scholar]
- 21.Schmier JK, Covert DW, Lau EC, Robin AL. Trends in annual Medicare expenditures for glaucoma surgical procedures from 1997 to 2006. Arch Ophthalmol. 2009;127:900–905. doi: 10.1001/archophthalmol.2009.122. [DOI] [PubMed] [Google Scholar]
- 22.SAS Institute. SAS/STAT 9.2 User’s guide. Cary, NC: SAS Institute; 2008. [Google Scholar]
- 23.DCCT/EDIC Research Group. Intensive therapy and GFR in type 1 diabetes. N Engl J Med. 2011;365:2366–2376. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. U.S. outpatient surgeries on the rise. Press release: January 28, 2009. http://www.cdc.gov/media/pressrel/2009/r090128.htm.
- 25.Centers for Medicare and Medicaid Services. Current Procedural Terminology (CPT) (Fourth Edition) 2010 https://www.cms.gov/apps/ama/license.asp?file=/DataCompendium/Downloads/2011Utilization.zip.
- 26.Dowler J, Hykin PG. Cataract surgery in diabetes. Curr Opin Ophthalmol. 2001;12:175–178. doi: 10.1097/00055735-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Haddad NM, Sun JK, Abujaber S, Schlossman DK, Silva PS. Cataract surgery and its complications in diabetic patients. Semin Ophthalmol. 2014;29:329–337. doi: 10.3109/08820538.2014.959197. [DOI] [PubMed] [Google Scholar]
- 28.Klein BE, Klein R, Moss SE. Incidence of cataract surgery in the Wisconsin Epi-demiologic Study of Diabetic Retinopa-thy. Am J Ophthalmol. 1995;119:295–300. doi: 10.1016/s0002-9394(14)71170-5. [DOI] [PubMed] [Google Scholar]
- 29.Olafsdottir E, Andersson DK, Stefáns-son E. The prevalence of cataract in a population with and without type 2 diabetes mellitus. Acta Ophthalmol. 2012;90:334–340. doi: 10.1111/j.1755-3768.2011.02326.x. [DOI] [PubMed] [Google Scholar]
- 30.Klein BE, Klein R, Lee KE. Diabetes, cardiovascular disease, selected cardiovascular disease risk factors, and the 5-year incidence of age-related cataract and progression of lens opacities: the Beaver Dam Eye Study. Am J Ophthalmol. 1998;126:782–790. doi: 10.1016/s0002-9394(98)00280-3. [DOI] [PubMed] [Google Scholar]
- 31.Hashim Z, Zarina S. Advanced glycation end products in diabetic and non-diabetic human subjects suffering from cataract. Age (Dordr) 2011;33:377–384. doi: 10.1007/s11357-010-9177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sensi M, Pricci F, Pugliese G, et al. Role of advanced glycation end-products (AGE) in late diabetic complications. Diabetes Res Clin Pract. 1995;28:9–17. doi: 10.1016/0168-8227(94)01061-4. [DOI] [PubMed] [Google Scholar]
- 33.Ramalho J, Marques C, Pereira P, Mota MC. Crystallin composition of human cataractous lens may be modulated by protein glycation. Graefes Arch Clin Exp Ophthalmol. 1996;234(Suppl 1):S232–S238. doi: 10.1007/BF02343078. [DOI] [PubMed] [Google Scholar]
- 34.Nagaraj RH, Linetsky M, Stitt AW. The pathogenic role of Maillard reaction in the aging eye. Amino Acids. 2012;42:1205–1220. doi: 10.1007/s00726-010-0778-x. [DOI] [PubMed] [Google Scholar]
- 35.Monnier VM, Sell DR, Strauch C, et al. The association between skin collagen glucosepane and past progression of microvascular and neuropathic complications in type 1 diabetes. J Diabetes Complications. 2013;27:141–149. doi: 10.1016/j.jdiacomp.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monnier VM, Bautista O, Kenny D, et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes. 1999;48:870–880. doi: 10.2337/diabetes.48.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grassi MA, Sun W, Gangaputra S, et al. Validity of self-report in type 1 diabetic subjects for laser treatment of retinopathy. Ophthalmology. 2013;120:2580–256. doi: 10.1016/j.ophtha.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.